Abstract
Background
Uterine-preserving therapy with progesterone may be used in young women with endometrial cancer who desire fertility preservation. Such therapy delays definitive treatment with hysterectomy.
Objective
We examined the use and safety of progestational therapy in young women with endometrial cancer. The primary outcome of the analysis was overall survival.
Study Design
We identified women ≤49 years of age with stage I endometrial cancer in the National Cancer Database from 2004–2014. Women treated with hormonal therapy with or without hysterectomy were compared to women treated with hysterectomy. After propensity score weighting, overall survival was examined using proportional hazards models.
Results
A total of 23,231 patients, including 872 (3.8%) women treated with hormonal therapy were identified. Use of hormonal therapy was 2.4% (95% CI, 1.8–3.3%) in 2004 and increased over time to 5.9% (95% CI, 5.0–6.9%) by 2014 (P<0.0001). Use of hormonal therapy decreased with older age, higher sub-stage and increasing grade. Black women were more likely to receive hormonal therapy while Medicaid recipients were less likely to receive hormonal therapy. The five-year survival for patients treated with hormonal therapy was 96.4% (95% CI, 94.3–98.0%) compared to 97.2% (95% CI, 96.9–97.4%) for hysterectomy. In a multivariable model, women treated with hormonal therapy were 92% (HR=1.92; 95% CI, 1.15–3.19) more likely to die compared to women who underwent primary hysterectomy. When stratified by stage, hormonal therapy was associated with increased mortality in women with stage IB and I-not otherwise specified (INOS) tumors but not for stage IA neoplasms.
Conclusion
Use of progestational therapy is increasing. Its use was associated with decreased survival, particularly in women with stage IB tumors.
Introduction
Hysterectomy in combination with bilateral salpingo-oophorectomy remains the standard of care for endometrial cancer.1 Hysterectomy results in excellent oncologic outcomes, particularly for women with low grade, early stage neoplasms. However, despite the success of surgical treatment for cancer control, removal of the reproductive organs has a number of long-term consequences including loss of fertility.2
As an alternative to hysterectomy, medical management of endometrial cancer with progestational agents has been described for young women.3,4 Endometrioid tumors typically express progesterone receptors; thus, there is a strong rationale for this type of hormonal therapy. Data describing the efficacy of progestin therapy is largely based on small studies with a wide range of response rates reported.3,5–11 A meta-analysis of 45 studies that included 391 subjects reported an overall response rate of 75% with a complete response rate of 48%. Among women with a complete response, over one third ultimately recurred.6 Progestational therapy is administered either orally, or delivered locally to the uterus through an intrauterine device (IUD).
Progestational therapy is typically administered for several months until patients either have regression of the cancer or have persistence of endometrial cancer and undergo hysterectomy. While small studies have suggested that progestin therapy is safe, such therapy delays definitive treatment with hysterectomy for several months in women who do not respond. Further, the optimal duration of therapy, follow-up schedule, and most appropriate formulation of progesterone are uncertain.3,4 Even more importantly, little is known about the frequency of use of progestin therapy in young women, and the comparative effectiveness of hormonal therapy in real world populations is unknown. We performed a population-based study to examine the trends in use of progestational therapy in young women with endometrial cancer and examined the comparative effectiveness of hormonal therapy compared to hysterectomy.
Methods
Data source and cohort selection
The National Cancer Data Base (NCDB) was used for analysis. NCDB is a nationwide oncology outcomes database developed by the American College of Surgeons and the American Cancer Society.12 The database includes more than 1500 hospitals affiliated with the Commission on Cancer in the United States, registering approximately 70% of newly diagnosed cancers in the nation.13 The database includes information on patient demographics, hospital factors, tumor characteristics, first course of therapy, follow-up and survival data. Information is abstracted by trained cancer registrars. Data do not contain patient identifiers and the Columbia University Institutional Review Board deemed this study exempt.
Patients and treatments
We included women <50 years of age diagnosed with stage I endometrioid endometrial cancer between 2004 and 2014. Patients who received preoperative radiotherapy were excluded. The cohort was divided into those who received hormonal therapy (with or without hysterectomy) and those who underwent hysterectomy only. Women not treated with hysterectomy or hormonal therapy were excluded.
Demographic data analyzed include age (<35, 35–39, 40–44, 45–49 years), race and ethnicity (white, black, Hispanic, other, unknown), insurance status (private insurance, Medicaid, Medicare, uninsured, unknown), median household income for patient’s area of residence (<$30,000, $30,000–$35,999, $36,000–$45,999, $46,000, and unknown), and urban/rural area of residence (metropolitan, urban, rural, unknown). Comorbidity was estimated using the Deyo classification of the Charlson comorbidity score (0, 1, 2, unknown).14,15 Tumors were classified as stage IA (tumor confined to the endometrium or <50% of the myometrium), IB (tumor with >50% myoinvasion), and stage INOS if the depth of myoinvasion was not available. Tumor grade was categorized as 1 (well), 2 (moderate), 3 (poorly), or unknown.
Statistical analysis
The trends in use of hormonal therapy over time are reported as rates with 95% confidence intervals. Univariate analyses of factors associated with use of hormonal therapy were performed by χ2 tests. A marginal log-linear Poisson regression model based on generalized estimating equations (GEE) methodology was developed to explore predictors of hormonal therapy while accounting for hospital-level clustering. Results are reported as adjusted rate ratios (RR) with 95% confidence intervals (CI). The model included all of the clinical and demographic factors in the study.
To account for imbalances in the treatment groups, we performed a propensity score analysis. The propensity score (PS) is the predicted probability of treatment, use of progesterone in the current analysis.16–18 The propensity score was estimated using a logistic regression model that included all of the clinical and demographic characteristics. The predicted probability (the propensity score) was estimated for each patient and ranged from 0 to 1.
The primary analytic approach using the propensity score relied on an inverse probability of treatment weighting approach (IPTW).16,19 With an IPTW approach, each patient is assigned a differential weight based on their treatment group and calculated propensity score. The weighting assumptions of the IPTW approach assigned patients treated with progesterone a weight of 1/propensity score and those who underwent primary hysterectomy a weight of 1/(1-propensity score).16,19 To reduce the variability of IPTW weights and give individuals with extreme weights less influence, a stabilization technique that multiplies the treatment and comparison weights by a constant and a trimming technique that trims the stabilized weights within a specified range (≤ 10) were applied.20 After IPTW, we assessed the balance of measured confounders between treatments via a weighted regression approach, in which each covariate was regressed on the treatment variable. The clinically unimportant differences between treatment groups were determined by a threshold value of less than 0.2 for all coefficients in the weighted regression model.21
The primary outcome of the analysis was overall survival. Marginal Cox proportional hazards models were developed to examine the association between hormonal therapy and overall survival while accounting for hospital-level clustering and all of the clinical and demographic characteristics. The assumption of proportionality was assessed visually by plotting scaled Schoenfeld residuals. The linear function of year of diagnosis was also checked via the distribution of the simulated cumulative martingale residual curves.22 Women diagnosed from 2004–2013 were included in the survival analyses. Results from Cox proportional hazards models are reported as hazard ratios with 95% confidence intervals. Observed five-year survival rates with 95% confidence intervals were calculated using Kaplan-Meier curves stratified by stage.
We performed a number of sensitivity analyses to examine the robustness of our findings. Separate models were developed for each substage of women in the cohort and for women with each grade of tumor. Similarly, separate models were developed for each comorbidity class. All hypothesis tests were two-sided. A P-value of 0.05 was considered statistically significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
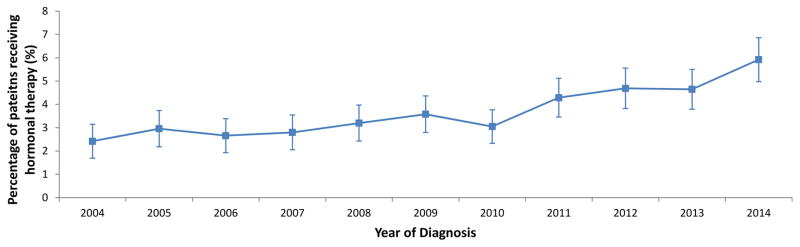
A total of 23,231 subjects, including 872 (3.8%) women treated with hormonal therapy and 22,359 (96.2%) who did not receive hormonal therapy, were identified. Use of hormonal therapy was 2.4% (95% CI, 1.8% to 3.3%) in 2004 and increased over time to 5.9% (95% CI, 5.0% to 6.9%) by 2014 (P<0.0001) (Figure 1).
Figure 1.
Trends in use of progesterone therapy over time in young women with endometrial cancer.
Use of hormonal therapy was associated with younger age, black race, residence in a metropolitan area and more recent year of diagnosis, as well as lower cancer stage and grade (Table 1). Specifically, hormonal therapy was utilized in 17.6% of women <35 years of age, 5.6% of women age 35–39, 1.8% of those age 40–44 and in 0.9% of women age 45–49 years. Among women treated with hormonal therapy, 32.6% underwent hysterectomy. After propensity score weighting, the cohort was better balanced; progesterone-treated women remained younger, were more often non-white, had stage INOS tumors and had tumors of unknown grade.
Table 1.
Demographic and clinical characteristics of women with endometrial cancer stratified by treatment.
| Original Cohort | Inverse Probability of Treatment Weighting* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Progesterone
|
Primary Hysterectomy
|
Progesterone
|
Primary Hysterectomy
|
|||||||
| N | (%) | N | (%) | P † | N | (%) | N | (%) | P ‡ | |
| 872 | 22,359 | 2652 | 22,479 | |||||||
|
| ||||||||||
| Age | <0.0001 | <0.0001 | ||||||||
| <35 | 461 | (52.9) | 2,160 | (9.7) | 439 | (16.6) | 2,536 | (11.3) | ||
| 35–39 | 200 | (22.9) | 3,362 | (15.0) | 586 | (22.1) | 3,445 | (15.3) | ||
| 40–44 | 114 | (13.1) | 6,187 | (27.7) | 797 | (30.1) | 6,098 | (27.1) | ||
| 45–49 | 97 | (11.1) | 10,650 | (47.6) | 830 | (31.3) | 10,400 | (46.3) | ||
| Race | <0.0001 | 0.04 | ||||||||
| White | 489 | (56.1) | 15,330 | (68.6) | 1,734 | (65.4) | 15,302 | (68.1) | ||
| Black | 108 | (12.4) | 1,602 | (7.2) | 285 | (10.8) | 1,660 | (7.4) | ||
| Hispanic | 146 | (16.7) | 2,526 | (11.3) | 344 | (13.0) | 2,587 | (11.5) | ||
| Other | 91 | (10.4) | 1,379 | (6.2) | 167 | (6.3) | 1,421 | (6.3) | ||
| Unknown | 38 | (4.4) | 1,522 | (6.8) | 122 | (4.6) | 1,509 | (6.7) | ||
| Insurance status | 0.26 | 0.36 | ||||||||
| Private Insurance | 671 | (76.9) | 16,909 | (75.6) | 1,951 | (73.5) | 17,003 | (75.6) | ||
| Medicaid | 69 | (7.9) | 2,081 | (9.3) | 239 | (9) | 2,083 | (9.3) | ||
| Medicare | 41 | (4.7) | 1,230 | (5.5) | 212 | (8) | 1,235 | (5.5) | ||
| Uninsured | 72 | (8.3) | 1,565 | (7.0) | 194 | (7.3) | 1,584 | (7.0) | ||
| Other/Unknown | 19 | (2.2) | 574 | (2.6) | 57 | (2.1) | 574 | (2.6) | ||
| Median household income | 0.83 | 0.95 | ||||||||
| < $30,000 | 122 | (14) | 2,969 | (13.3) | 353 | (13.3) | 2,992 | (13.3) | ||
| $30,000 – $35,999 | 156 | (17.9) | 3,812 | (17) | 443 | (16.7) | 3,834 | (17.1) | ||
| $36,000 – $45,999 | 248 | (28.4) | 6,299 | (28.2) | 794 | (29.9) | 6,337 | (28.2) | ||
| >$46,000 | 320 | (36.7) | 8,535 | (38.2) | 970 | (36.6) | 8,569 | (38.1) | ||
| Unknown | 26 | (3.0) | 744 | (3.3) | 91 | (3.4) | 746 | (3.3) | ||
| Urban/rural | . | 0.02 | 0.56 | |||||||
| Metropolitan | 730 | (83.7) | 17,919 | (80.1) | 2,152 | (81.1) | 18,044 | (80.3) | ||
| Urban | 103 | (11.8) | 3,376 | (15.1) | 361 | (13.6) | 3,367 | (15) | ||
| Rural | 10 | (1.1) | 395 | (1.8) | 34 | (1.3) | 392 | (1.7) | ||
| Unknown | 29 | (3.3) | 669 | (3.0) | 105 | (4.0) | 676 | (3.0) | ||
| Comorbidity score | . | 0.07 | 0.43 | |||||||
| 0 | 700 | (80.3) | 17,246 | (77.1) | 2,038 | (76.8) | 17,363 | (77.2) | ||
| 1 | 142 | (16.3) | 4,337 | (19.4) | 546 | (20.6) | 4,336 | (19.3) | ||
| 2 | 30 | (3.4) | 776 | (3.5) | 69 | (2.6) | 780 | (3.5) | ||
| Year of diagnosis | . | <0.0001 | 0.06 | |||||||
| 2004–2006 | 148 | (17.0) | 5,361 | (24.0) | 652 | (24.6) | 5,311 | (23.6) | ||
| 2007–2009 | 196 | (22.5) | 5,909 | (26.4) | 655 | (24.7) | 5,914 | (26.3) | ||
| 2010–2012 | 275 | (31.5) | 6,563 | (29.4) | 676 | (25.5) | 6,655 | (29.6) | ||
| 2013–2014 | 253 | (29) | 4,526 | (20.2) | 670 | (25.2) | 4,600 | (20.5) | ||
| Stage | . | <0.0001 | 0.0005 | |||||||
| IA | 489 | (56.1) | 18,008 | (80.5) | 2,014 | (75.9) | 17,900 | (79.6) | ||
| IB | 19 | (2.2) | 1,436 | (6.4) | 114 | (4.3) | 1,408 | (6.3) | ||
| INOS | 364 | (41.7) | 2,915 | (13.0) | 524 | (19.8) | 3,171 | (14.1) | ||
| Grade | . | <0.0001 | 0.01 | |||||||
| 1 | 589 | (67.5) | 13,670 | (61.1) | 1,597 | (60.2) | 13,792 | (61.4) | ||
| 2 | 121 | (13.9) | 5,099 | (22.8) | 505 | (19.0) | 5,049 | (22.5) | ||
| 3 | 17 | (1.9) | 1,160 | (5.2) | 121 | (4.6) | 1,140 | (5.1) | ||
| Unknown | 145 | (16.6) | 2,430 | (10.9) | 430 | (16.2) | 2,497 | (11.1) | ||
| Histology | . | 0.80 | 0.52 | |||||||
| Endometrioid | 84 | (9.6) | 2,213 | (9.9) | 237 | (8.9) | 2,222 | (9.9) | ||
| Endometrial NOS | 788 | (90.4) | 20,146 | (90.1) | 2,416 | (91.1) | 20,257 | (90.1) | ||
Frequencies were rounded to the nearest integers based on weight.
P values were derived from χ2 tests.
P values were derived from weighted surveylogistic model.
In a multivariable model, the use of hormonal therapy decreased with older age, higher sub-stage and increasing tumor grade (Table 2). Compared to white women, black women (RR=1.48; 95% CI, 1.21–1.80) and women from other races (RR=1.50; 95% CI, 1.15–1.95) were more likely to receive hormonal therapy. Medicaid recipients (RR=0.62; 95% CI, 0.48–0.79 versus commercially insured) were less likely to receive hormonal therapy while women residing in metropolitan areas (RR=1.29; 95% CI, 1.05–1.59) were more likely to receive hormonal therapy compared to women who lived in an urban area. In addition, women who were diagnosed in more recent years were more likely to use hormonal therapy (RR=1.05; 95% CI: 1.03–1.08). Compared to women with stage IA tumors, those with stage IB neoplasms were 49% less likely to receive hormonal therapy (RR=0.51; 95% CI, 0.33–0.79).
Table 2.
Predictors of hormonal therapy use in young women with endometrial carcinoma in the original cohort.
| Adjusted RR (95%CI) | |
|---|---|
| Age | |
| <35 | Referent |
| 35–39 | 0.34 (0.29–0.40)* |
| 40–44 | 0.15 (0.06–0.38)* |
| 45–49 | 0.08 (0.03–0.20)* |
| Race | |
| White | Referent |
| Black | 1.48 (1.21–1.80)* |
| Hispanic | 1.08 (0.90–1.31) |
| Other | 1.50 (1.15–1.95)* |
| Unknown | 0.84 (0.62–1.15) |
| Insurance Status | |
| Private Insurance | Referent |
| Medicaid | 0.62 (0.48–0.79)* |
| Medicare | 0.94 (0.66–1.34) |
| Uninsured | 0.84 (0.66–1.05) |
| Other/Unknown | 0.66 (0.41–1.06) |
| Median Household Income | |
| < $30,000 | Referent |
| $30,000 – $35,999 | 1.09 (0.89–1.35) |
| $36,000 – $45,999 | 1.04 (0.84–1.28) |
| >$46,000 | 1.13 (0.91–1.40) |
| Unknown | 0.91 (0.59–1.41) |
| Urban/rural | |
| Metropolitan | 1.29 (1.05–1.59)* |
| Urban | Referent |
| Rural | 1.07 (0.59–1.94) |
| Unknown | 1.77 (1.24–2.53)* |
| Comorbidity score | |
| 0 | Referent |
| 1 | 0.90 (0.75–1.08) |
| 2 | 1.36 (0.94–1.97) |
| Year of diagnosis# | 1.05 (1.03–1.08)* |
| Stage | |
| IA | Referent |
| IB | 0.51 (0.33–0.79) |
| INOS | 3.37 (2.93–3.88)* |
| Grade | |
| 1 | Referent |
| 2 | 0.73 (0.60–0.88)* |
| 3 | 0.56 (0.35–0.88)* |
| Unknown | 1.22 (1.02–1.45)* |
| Histology | |
| Endometrioid | Referent |
| Endometrial NOS | 1.01 (0.81–1.25) |
Year of diagnosis was fit as continuous variable in multivariable regression model.
P<0.05
Median follow-up time of the cohort was 54 months (IQR: 30–85 months). The five-year survival for patients treated with hormonal therapy was 96.4% (95% CI, 94.3–98.0%) compared to 97.2% (95% CI, 96.9–97.4%) for patients who underwent hysterectomy without hormonal therapy (Table 3). Stratification by sub-stage demonstrated the five-year survival of stage IA patients was similar for hormonally treated patients (97.5%; 95% CI, 94.7–98.8%) versus non-hormonally treated patients at (97.5%; 95% CI, 97.2–97.8%). For stage IB patients, the survival for hormonally treated patients was 75.0% (95% CI, 12.8–96.1%) versus 97.5% (95% CI, 97.2–97.8%) for surgically treated patients. Survival for stage INOS patients was 95.5% (95% CI, 91.8–97.6%) patients treated with hormones compared to 97.3% (95% CI, 96.4–98.0%) for patients who underwent surgery.
Table 3.
Five-year survival rates by stage
| Stage | Treatment | Survival | 95% CI |
|---|---|---|---|
| Overall | Progesterone | 96.4 | (94.3–98.0) |
| Surgery | 97.2 | (96.9–97.4) | |
| IA | Progesterone | 97.5 | (94.7–98.8) |
| Surgery | 97.5 | (97.2–97.8) | |
| IB | Progesterone | 75.0 | (12.8–96.1) |
| Surgery | 97.5 | (97.2–97.8) | |
| I NOS | Progesterone | 95.5 | (91.8–97.6) |
| Surgery | 97.3 | (96.4–98.0) |
In a multivariable model, women treated with hormonal therapy were 92% (HR=1.92; 95% CI, 1.15–3.19) more likely to die compared to women who underwent hysterectomy without receipt of hormonal therapy (Table 4). Higher sub-stage, higher tumor grade, greater comorbidity, advanced age and non-commercial insurance were also associated with increased mortality. When stratified by stage, hormonal therapy was associated with increased mortality in women with stage IB (HR=3.52; 95% CI, 1.57–7.90) and INOS tumors (HR=3.91; 95% CI, 1.41–10.82) but not for women with stage IA neoplasms (HR=1.20; 95% CI, 0.53–2.68).
Table 4.
Multivariable Cox proportional hazards model of predictors of mortality in young women with endometrial carcinoma by stage
| Overall cohort HR (95%CI) | Stage IA HR (95%CI) | Stage IB HR (95%CI) | Stage INOS HR (95%CI) | |
|---|---|---|---|---|
| Hormonal therapy | ||||
| No | Referent | Referent | Referent | Referent |
| Yes | 1.92 (1.15–3.19)* | 1.20 (0.53–2.68) | 3.52 (1.57–7.90)* | 3.91 (1.41–10.82)* |
| Age | ||||
| <35 | Referent | Referent | Referent | Referent |
| 35–39 | 1.23 (0.78–1.92) | 1.11 (0.70–1.77) | 3.61 (0.99–13.23) | 0.52 (0.15–1.81) |
| 40–44 | 1.55 (1.04–2.31)* | 1.44 (0.92–2.26) | 1.91 (0.52–7.06) | 1.52 (0.58–3.93) |
| 45–49 | 2.05 (1.39–3.03)* | 1.71 (1.09–2.69)* | 4.92 (1.44–16.77)* | 1.87 (0.76–4.63) |
| Race | ||||
| White | Referent | Referent | Referent | Referent |
| Black | 1.32 (0.96–1.82) | 1.12 (0.80–1.56) | 0.63 (0.23–1.75) | 3.18 (1.63–6.21)* |
| Hispanic | 0.74 (0.53–1.02) | 0.75 (0.50–1.12) | 0.56 (0.20–1.53) | 0.67 (0.23–1.96) |
| Other | 0.53 (0.31–0.91)* | 0.55 (0.28–1.07) | 0.18 (0.02–1.49) | 0.95 (0.29–3.12) |
| Unknown | 1.45 (1.03–2.02)* | 1.12 (0.77–1.64) | 1.64 (0.87–3.07) | 2.44 (1.28–4.66)* |
| Insurance Status | ||||
| Private Insurance | Referent | Referent | Referent | Referent |
| Medicaid | 2.44 (1.86–3.21)* | 2.39 (1.78–3.22)* | 1.60 (0.75–3.37) | 3.00 (1.62–5.58)* |
| Medicare | 3.08 (2.16–4.40)* | 3.22 (2.07–5.03)* | 2.94 (1.59–5.45)* | 3.78 (1.64–8.73)* |
| Not Insured | 1.41 (1.02–1.96)* | 1.53 (1.03–2.27)* | 1.59 (0.68–3.73) | 0.82 (0.23–2.94) |
| Other/Unknown | 2.29 (1.37–3.82)* | 1.40 (0.79–2.49) | 3.29 (1.34–8.06)* | 2.95 (1.08–8.04)* |
| Median Income | ||||
| < $30,000 | Referent | Referent | Referent | Referent |
| $30,000 – $35,999 | 0.85 (0.62–1.18) | 0.82 (0.58–1.15) | 0.66 (0.31–1.43) | 0.89 (0.37–2.14) |
| $36,000 – $45,999 | 0.91 (0.68–1.23) | 0.73 (0.54–1.00)* | 1.01 (0.52–1.98) | 1.42 (0.65–3.11) |
| $46,000 + | 0.66 (0.47–0.93)* | 0.58 (0.39–0.85)* | 0.55 (0.27–1.12) | 1.31 (0.52–3.30) |
| Unknown | 0.80 (0.48–1.35) | 0.75 (0.44–1.29) | 1.90 (0.63–5.72) | 0.14 (0.01– .23) |
| Urban/Rural | ||||
| Metropolitan | Referent | Referent | Referent | Referent |
| Urban | 0.96 (0.75–1.24) | 0.96 (0.69–1.32) | 1.22 (0.70–2.14) | 0.88 (0.44–1.76) |
| Rural | 1.27 (0.79–2.02) | 1.45 (0.87–2.41) | 0.95 (0.23–3.87) | 0.85 (0.15–4.80) |
| Unknown | 1.38 (0.91–2.10) | 1.38 (0.84–2.29) | 2.24 (0.84–5.97) | 1.12 (0.23–5.54) |
| Comorbidity Score | ||||
| 0 | Referent | Referent | Referent | Referent |
| 1 | 1.79 (1.37–2.34)* | 1.64 (1.23–2.19)* | 1.67 (0.99–2.83) | 2.32 (1.19–4.52)* |
| 2 | 2.60 (1.87–3.63)* | 2.08 (1.39–3.09)* | 3.26 (1.52–7.01)* | 5.56 (2.72–1.40)* |
| Year of diagnosis | 0.98 (0.93–1.04) | 0.96 (0.91–1.02) | 1.07 (0.97–1.19) | 0.99 (0.83–1.17) |
| Stage | ||||
| IA | Referent | NA | NA | NA |
| IB | 2.49 (1.77–3.50)* | NA | NA | NA |
| INOS | 1.29 (0.92–1.82) | NA | NA | NA |
| Grade | ||||
| 1 | Referent | Referent | Referent | Referent |
| 2 | 1.41 (1.09–1.81)* | 3.33 (2.25–4.93)* | 0.98 (0.58–1.66) | 1.57 (0.66–3.76) |
| 3 | 2.79 (2.00–3.87)* | 1.49 (1.14–1.94)* | 1.74 (0.96–3.17) | 2.82 (0.99–8.08) |
| Unknown | 1.14 (0.77–1.70) | 1.24 (0.80–1.92) | 0.87 (0.40–1.87) | 0.77 (0.28–2.11) |
| Histology | ||||
| Endometrioid | Referent | Referent | Referent | Referent |
| EM_NOS | 0.87 (0.67–1.14) | 0.94 (0.70–1.28) | 1.19 (0.66–2.17) | 0.53 (0.23–1.23) |
Cohort derived from propensity score using inverse probability of treatment weighting. #Year of diagnosis was fit as continuous variable in multivariable regression model.
P<0.05
We performed a series of sensitivity analyses. When the cohort was limited to women with grade 1 tumors, hormonal therapy was associated with an increased risk of death (HR=2.26; 95% CI, 1.25–4.10) (Supplemental Tables 1 and 2). However, among women with grade 2 or 3 tumors, there was not a statistically significant association between hormonal therapy use and death (HR=1.43; 95% CI, 0.49–4.18). When stratified by comorbidity, there was no association between hormonal therapy and mortality when separate models for women with no comorbidities or those with a comorbidity score of 2. Progesterone therapy was associated with an increased risk of death (HR=3.30; 95% CI, 1.27–8.62) for those with a comorbidity score of 1.
Comment
Among young women with endometrial cancer, use of hormonal therapy was associated with increased mortality. The adverse outcomes associated with progestin use were most pronounced in women with myoinvasive tumors and not seen in those with stage IA neoplasms. The increased risk of mortality was seen even in women with grade 1 neoplasms.
Although data describing the safety of progestin therapy for endometrial cancer are based predominantly on small studies, its use appears to be rising.3,5–11 One of the largest prospective studies treated 28 women with endometrial cancer with medroxyprogesterone acetate. A complete response rate of 55% was noted.9 Two meta-analyses of progestin therapy reported regression rates of approximately 75%.5,6 However, even women with complete responses are at substantial risk for recurrence, with relapse rates of 35–40% reported.5,6 Given the high recurrence rate, definitive treatment with hysterectomy is typically recommended after completion of childbearing.3 We noted that use of progestin therapy more than doubled from 2.4% in 2004 to 5.9% in 2014. Among women <35 years of age with endometrial cancer, 18% were treated with hormonal therapy.
Several factors may have contributed to the increased mortality associated with hormonal therapy that we noted. The majority of women treated with progestin therapy in the literature have had grade 1 tumors with minimal, if any, myometrial invasion.3 Pretreatment evaluation typically includes uterine curettage to assess grade and magnetic resonance imaging to evaluate the depth of invasion.3 In our cohort, a number of women with more deeply myoinvasive tumors as well as moderately and poorly differentiated cancers received progestin therapy. These tumors may be less responsive to progestational agents. When our analysis was limited to stage IA neoplasms, progestin treatment was not adversely associated with survival.
Second, a major clinical concern with use of hormonal therapy is the delay in definitive treatment with hysterectomy, particularly among women who do not respond. Our results may be in part attributable to delayed hysterectomy in non-responders. Prior studies have reported that the median time to response in women with progesterone therapy of 4–6 months.3 However, how long to treat women who have residual cancer or atypical hyperplasia within the uterus remains uncertain.3,4 While some studies suggest that longer duration treatment is safe, theoretically a prolonged delay in proceeding with hysterectomy in non-responders could allow tumor progression and worsen prognosis.23,24 Currently, the optimal duration of progestin therapy is unknown and in some women who did not achieve a response, the decision to proceed with hysterectomy may have been delayed and negatively impacted prognosis.
Lastly, compliance with hormonal therapy may have been suboptimal. Prior work has suggested that adherence to oral anti-cancer therapies is highly variable.25,26 Progestational therapy often stimulates bothersome side effects, such as increased appetite, which may have limited compliance in young women.9 To help improve compliance and mitigate the side effects associated with oral progesterone, levonorgesterel releasing intra uterine devices have been explored for endometrial cancer and hyperplasia. Limited data have suggested favorable outcomes for women treated with progestin releasing IUDs.27,28 Further studies examining compliance in this population are clearly needed.
Our findings should be interpreted in light of a number of limitations. As many women in the hormonal therapy group did not ultimately undergo surgery, staging data is based on a combination of clinical and surgical staging. Women in the hormonal treatment group who underwent hysterectomy are likely the non-responders and thus more likely to have had deeper myometrial invasion than they would have had at the time of diagnosis. As such, these women may have had stage IA tumors at the time of diagnosis but would have been classified as higher stage at surgery. Accordingly, the precise stage of a relatively large number of patients who received hormonal therapy was reported as stage I without further sub-classification (INOS). While NCDB data utilizes a rigorous process for data capture, we cannot exclude the possibility that hormonal therapy was misclassified in a small number of women. Likewise, for those women treated with progestin therapy we lack data on route of delivery, drug selection, dosing, duration of treatment and compliance with therapy. We also lack data on the primary indication for progestational therapy and acknowledge that some women may have been deemed inoperable and received the drug for that indication and not fertility preservation. Our survival analysis was based on all-cause mortality, and as expected, mortality increased with age. While rare, some of the increase in mortality associated with progestational therapy may have been due to cardiovascular or thromboembolic events. Likewise, survival was favorable for the cohort in general and accordingly, the confidence intervals for some of the groups of women we analyzed were wide due to small sample sizes. While we performed rigorous adjustment for measured confounding factors using a propensity score methodology, we cannot account for the influence of unmeasured confounders on outcome. Finally, while we examined mortality, NCDB does not capture data on other long-term outcomes, including fertility and quality of life.
These data have important implications for young women considering uterine-preserving therapy for endometrial cancer. First, our findings raise concern about the safety of progestational therapy in young women with endometrial cancer. As such, women considering hormonal therapy should be counseled about the potential adverse survival effects of such therapy. Patients and providers must carefully weigh the potential benefits (i.e., fertility) of uterine preservation, particularly in older women, against the small potential risk we documented. Second, our findings suggest that only women with stage IA neoplasms should be considered for hormonal therapy. In these patients pretreatment radiologic assessment of myometrial invasion should be strongly considered. Lastly, women who initiate hormonal treatment should undergo therapy for a limited, well-defined period of time and, if unsuccessful, proceed with hysterectomy. Further, large comparative effectiveness trials examining the long-term outcomes of hormonal therapy as well as patient reported outcomes for young women with endometrial cancer are of great interest.
Supplementary Material
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants from the National Cancer Institute. Dr. Hershman is the recipient of a grant from the Breast Cancer Research Foundation/Conquer Cancer Foundation.
Footnotes
The authors have no conflicts of interest or disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379:1352–60. doi: 10.1016/S0140-6736(12)60442-5. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Buck AM, Shah M, Burke WM, Schiff PB, Herzog TJ. Safety of ovarian preservation in premenopausal women with endometrial cancer. J Clin Oncol. 2009;27:1214–9. doi: 10.1200/JCO.2008.19.8150. [DOI] [PubMed] [Google Scholar]
- 3.Kalogera E, Dowdy SC, Bakkum-Gamez JN. Preserving fertility in young patients with endometrial cancer: current perspectives. Int J Womens Health. 2014;6:691–701. doi: 10.2147/IJWH.S47232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah MM, Wright JD. Management of endometrial cancer in young women. Clin Obstet Gynecol. 2011;54:219–25. doi: 10.1097/GRF.0b013e318218607c. [DOI] [PubMed] [Google Scholar]
- 5.Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012;207:266, e1–12. doi: 10.1016/j.ajog.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012;125:477–82. doi: 10.1016/j.ygyno.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Hahn HS, Yoon SG, Hong JS, et al. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int J Gynecol Cancer. 2009;19:1068–73. doi: 10.1111/IGC.0b013e3181aae1fb. [DOI] [PubMed] [Google Scholar]
- 8.Minaguchi T, Nakagawa S, Takazawa Y, et al. Combined phospho-Akt and PTEN expressions associated with post-treatment hysterectomy after conservative progestin therapy in complex atypical hyperplasia and stage Ia, G1 adenocarcinoma of the endometrium. Cancer Lett. 2007;248:112–22. doi: 10.1016/j.canlet.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Ushijima K, Yahata H, Yoshikawa H, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler DT, Bristow RE, Kurman RJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007;31:988–98. doi: 10.1097/PAS.0b013e31802d68ce. [DOI] [PubMed] [Google Scholar]
- 11.Yu M, Yang JX, Wu M, Lang JH, Huo Z, Shen K. Fertility-preserving treatment in young women with well-differentiated endometrial carcinoma and severe atypical hyperplasia of endometrium. Fertil Steril. 2009;92:2122–4. doi: 10.1016/j.fertnstert.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [Accessed March 10, 2012];The National Cancer Data Base. at http://www.facs.org/cancer/ncdb/index.html.
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Hadley J, Yabroff KR, Barrett MJ, Penson DF, Saigal CS, Potosky AL. Comparative effectiveness of prostate cancer treatments: evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst. 2010;102:1780–93. doi: 10.1093/jnci/djq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemmila MR, Birkmeyer NJ, Arbabi S, Osborne NH, Wahl WL, Dimick JB. Introduction to propensity scores: A case study on the comparative effectiveness of laparoscopic vs open appendectomy. Arch Surg. 2010;145:939–45. doi: 10.1001/archsurg.2010.193. [DOI] [PubMed] [Google Scholar]
- 18.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. Jama. 2007;297:278–85. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. Jama. 2012;307:1611–20. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods. 2010;15:234–49. doi: 10.1037/a0019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross ME, Kreider AR, Huang YS, Matone M, Rubin DM, Localio AR. Propensity Score Methods for Analyzing Observational Data Like Randomized Experiments: Challenges and Solutions for Rare Outcomes and Exposures. Am J Epidemiol. 2015;181:989–95. doi: 10.1093/aje/kwu469. [DOI] [PubMed] [Google Scholar]
- 22.Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among US transplant centers. Jama. 2010;304:53–60. doi: 10.1001/jama.2010.885. [DOI] [PubMed] [Google Scholar]
- 23.Yahata T, Fujita K, Aoki Y, Tanaka K. Long-term conservative therapy for endometrial adenocarcinoma in young women. Hum Reprod. 2006;21:1070–5. doi: 10.1093/humrep/dei434. [DOI] [PubMed] [Google Scholar]
- 24.Perri T, Korach J, Gotlieb WH, et al. Prolonged conservative treatment of endometrial cancer patients: more than 1 pregnancy can be achieved. Int J Gynecol Cancer. 2011;21:72–8. doi: 10.1097/IGC.0b013e31820003de. [DOI] [PubMed] [Google Scholar]
- 25.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–8. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–37. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orbo A, Vereide A, Arnes M, Pettersen I, Straume B. Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: a national multicentre randomised trial. BJOG. 2014;121:477–86. doi: 10.1111/1471-0528.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MK, Seong SJ, Kim YS, et al. Combined medroxyprogesterone acetate/levonorgestrel-intrauterine system treatment in young women with early-stage endometrial cancer. Am J Obstet Gynecol. 2013;209:358, e1–4. doi: 10.1016/j.ajog.2013.06.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.