Abstract
Background
We recently reported that the skin sympathetic nerve activity (SKNA) can be used to estimate the sympathetic tone in humans. In animal models, vagal nerve stimulation (VNS) can damage the stellate ganglion, reduce stellate ganglion nerve activity and suppress cardiac arrhythmia. Whether or not VNS can suppress sympathetic tone in humans remain unclear.
Objective
To test the hypothesis that VNS suppresses SKNA in patients with drug resistant epilepsy.
Methods
We used electrocardiogram (ECG) patch electrodes to continuously record SKNA in 26 patients with drug resistant epilepsy who were admitted for video electroencephalographic monitoring. Among them, 6 (2 men, age 40±11 years) were previously treated with VNS and 20 (7 men, age 37±8 years) did not. The signals from ECG leads I and II were filtered to detect SKNA.
Results
The VNS had an ON-time of 30 s and OFF-time of 158±72 s, with output of 1.92±0.42 mA at 24.17±2.01 Hz. The average SKNA (aSKNA) during VNS OFF-time was 1.06 μV [95% confidence interval, CI, 0.93 to 1.18] in lead I and 1.13 μV [CI, 0.99 to 1.27] in lead II, which was significantly lower than 1.38 μV [CI, 1.01 to 1.75, p=0.036] and 1.38 μV [CI, 0.98 to 1.78, p=0.035] of the control Group, respectively. The heart rate was 65 bpm [CI, 59 to 71] in VNS group, significantly lower than 77 bpm [95% CI, 71 to 83] in control group.
Conclusion
Patients with VNS had significantly lower SKNA than patients without VNS.
Keywords: Autonomic nervous system, neuromodulation, sudden unexpected death in epilepsy
Introduction
Vagal nerve stimulation (VNS) is commonly used to manage patients with drug resistant epilepsy and has been reported to reduce the incidence of sudden unexpected death in epilepsy (SUDEP).1, 2 Recent studies showed that there is significant interictal cardiac electrical instability (elevated T wave alternans) in patients with drug-resistant epilepsy, which was effectively suppressed by VNS.3 Because T wave alternans is enhanced by sympathetic activation,4 these findings suggest that VNS might be associated with reduced sympathetic tone. Consistent with that hypothesis, Clancy et al5 reported that non-invasive stimulation of the auricular branch of the vagal nerve suppressed muscle sympathetic nerve activity in normal human volunteers. However, as far as we know, there was no study that demonstrated reduced sympathetic nerve activity in patients with chronically implanted vagal nerve stimulators. The cervical and thoracic vagal nerves contain significant sympathetic components.6, 7 In ambulatory dogs, left cervical VNS could be associated with high rate of activation of the left stellate ganglion.8 Repeated high rate of excitation might cause neurotoxicity and promote neuronal cell death.9 Consistent with the neurotoxicity hypothesis, we recently reported that there was significant damage to the left stellate ganglion in dogs receiving chronic VNS.10 The stellate ganglion damage was associated with a significant reduction of the stellate ganglion nerve activity and ventricular rate in atrial fibrillation (AF). However, it is difficult to record the stellate ganglion nerve activity in humans. We recently developed a new method (neuECG) to simultaneously record the skin sympathetic nerve activity (SKNA) and electrocardiogram (ECG) in ambulatory dogs.11, 12, 13 and in humans.14 The purpose of the present study was to record SKNA from patients with drug resistant epilepsy to test the hypothesis that the SKNA is lower in patients with VNS than in patients without VNS.
Methods
This research protocol was approved by the Institutional Review Boards of the Indiana University (IU) School of Medicine. We prospectively enrolled 26 patients with drug resistant epilepsy to participate in the study. All patients were recruited after they were admitted to the Epilepsy Monitoring Unit of the IU-Methodist Hospital to record video electroencephalogram. As part of the standard care, anti-epileptic medications were completely or partially withdrawn while the patients were confined to bed with bathroom privileges. The recordings were made continuously except when the patients went to the bathroom. The neuECG was recorded using standard ECG patch electrodes connected to a modified ME6000 (Biomation, Almonte, Ontario) recorder with a wide bandwidth (2 KHz) and high sampling rate (10 KHz). All 26 patients had at least 24-hr of recording (Table 1). Among them, six had previously underwent VNS implantation, while 20 did not receive it. The standard VNS protocol was 30 s on and 3–5 min off.15 Because this is an observational study, the off-time was variable based on the clinical needs. The baseline SKNA of 19 patients in the latter group was included in a previous report.14 A total of 8 patients had seizure during the recording period. We excluded the data recorded 40-min before to 40-min after the seizure from analyses.
Table 1.
Clinical characteristics of the study population.
| Pt No. | Age (yrs) |
Gender | Group | Epilepsy History (yrs) |
Seizure type | Hypertension | Diabetes | Organic Heart disease |
|---|---|---|---|---|---|---|---|---|
| 1 | 25 | M | VNS | 21 | Generalized | No | No | No |
| 2 | 40 | F | Control | 24 | Complex Partial | No | No | No |
| 3 | 47 | F | Control | 27 | Complex Partial with secondary generalization | No | No | No |
| 4 | 53 | M | VNS | 41 | Complex Partial with secondary generalization | Yes | No | No |
| 5 | 44 | M | VNS | 30 | Complex Partial with secondary generalization | No | No | No |
| 6 | 30 | F | Control | 17 | Generalized | No | No | No |
| 7 | 41 | F | Control | 2 | Complex Partial | Yes | No | No |
| 8 | 36 | F | VNS | 12 | Complex Partial | No | No | No |
| 9 | 24 | F | Control | 1 | Complex Partial | No | No | No |
| 10 | 37 | F | Control | 5 | Complex Partial | No | No | No |
| 11 | 26 | F | Control | 2 | Generalized | No | Yes | No |
| 12 | 68 | M | Control | 48 | Complex Partial with secondary generalization | No | No | No |
| 13 | 40 | F | Control | 1 | Complex Partial | Yes | Yes | No |
| 14 | 18 | M | Control | 15 | Generalized | No | No | No |
| 15 | 38 | F | Control | 18 | Complex Partial with secondary generalization | No | No | No |
| 16 | 34 | F | Control | 7 | Complex Partial | No | No | No |
| 17 | 29 | M | Control | 26 | Generalized | Yes | No | No |
| 18 | 27 | M | Control | 21 | Complex Partial with secondary generalization | No | No | No |
| 19 | 36 | M | Control | 6 | Complex Partial with secondary generalization | Yes | No | No |
| 20 | 25 | M | Control | 10 | Generalized | No | No | No |
| 21 | 29 | F | VNS | 16 | Complex Partial | No | No | No |
| 22 | 44 | F | Control | 1 | Complex Partial | Yes | No | No |
| 23 | 46 | F | Control | 27 | Complex Partial with secondary generalization | No | No | No |
| 24 | 50 | F | VNS | 36 | Complex Partial | No | No | No |
| 25 | 53 | F | Control | 33 | Complex Partial | Yes | Yes | No |
| 26 | 38 | M | Control | 37 | Generalized | No | No | No |
The patient (pt) numbers correspond to the sequence of recruitment. F, female; M, male; sec, secondary; VNS, vagal nerve stimulation; Yrs, years
Data Analysis
Because VNS causes large artifacts on the neuECG recording, we only analyzed data during the VNS-off time. The data were analyzed with a custom written software which selected the R waves and calculated the RR intervals and heart rate automatically. All selections were then confirmed by manual examination to exclude artifacts. The average SKNA (aSKNA) and heart rate were determined for 2 min at the beginning, 20-min past and 40-min past the hour for 24 hours, resulting in 72 data points per patient. If the patient went to the bathroom during the analyses window, we used the data acquired after the resumption of recording for analyses. We bandpass filtered the recorded neuECG signals between 200 Hz to 1000 Hz and also between 500 Hz to 1000 Hz to display SKNA. As shown in our previous report,14 the sensitivity of detecting SKNA is higher with the former filter setting but the specificity is higher with the latter filter setting. The same signals were bandpass filtered between 0.5 Hz and 150 Hz to display the surface ECG. For quantitative analyses, we integrated all digitized SKNA signals over each 1-min window and divided the results (in μV) by the number of samples in that window (10,000 × 60, or 60,000 samples) to obtain the aSKNA. The quantitative analyses were done for both filter settings. The aSKNA included both the signal and noise. The noise level of the recording device (recorded when the two poles were shorted) is 1.17 μV (bandpass 200–1000 Hz) and 0.74 μV (bandpass 500–1000 Hz). Because vagal nerve stimulation is unlikely to change the noise level, the differences between VNS group and control group are due to the differences of nerve activity. Heart rate was determined in the same 2-min windows, resulting in 72 data points per patient. The average heart rate of these 72 data points were used as the heart rate for that patient.
Statistical Analysis
Linear regression was used to study the relationship between heart rate and SKNA activity for each patient. Linear mixed-effects models were used to compare SKNA activity between control and VNS stimulation groups where patients were treated as random effects. Generalized additive mixed-effects models were used to characterize the circadian pattern. T test was used to compare average SKNA activities and heart rate (over 24-hour period), and the correlation coefficients between SKNA and heart rate. Two-sided p values ≤0.05 were considered statistically significant.
Results
The VNS group included 6 patients (2 men, age 40±11 years). The control group included 20 patients (7 men, age 37±8 years) (Table 1). None of the patients had cardiovascular diseases. The admission diagnoses were generalized tonic-clonic seizure (N=1), complex partial seizure with secondarily generalized tonic-clonic seizure (N=2) and complex partial seizure (N=3) in VNS group; generalized tonic-clonic seizure (N=6), complex partial seizure with secondarily generalized tonic-clonic seizure (N=6) and complex partial seizure (N=8) in control group. Patients in the VNS group had received VNS treatment for an average of 81±39 months (range 20 to 121 months). VNS was programmed to an ON-time of 30 s and OFF-time of 158±72 s. The output was 1.92±0.42 mA with a frequency of 24.17±2.01 Hz.
Patterns of SKNA in control group
Figure 1 shows a typical example of SKNA episodes recorded in a patient without VNS. The SKNA can be either prolonged or episodic. Panel A shows a prolonged nerve discharge episode associated with heart rate acceleration. The data from the red rectangles are enlarged and shown in Panel B. Panel C shows short bursts of SKNA which were also associated with heart rate elevation. The data from the red rectangles were enlarged and shown in Panel D. These SKNA episodes were not pulse synchronous and were morphologically similar to that recorded from the superficial branches of radial nerves with microneurography techniques.16 The artifacts on surface ECG (red dots) suggest that some of the SKNA episodes occurred coincidentally with the physical activity.
Figure 1.
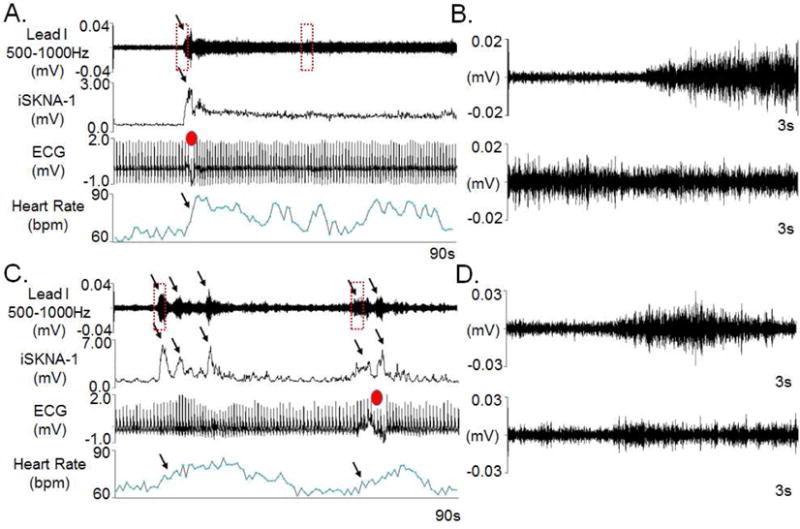
Patterns of baseline SKNA. All data were obtained from ECG Lead I. A shows persistent SKNA associated with heart rate acceleration. The digitized signals were bandpass between 500–1000 Hz to display the high frequency nerve activities. The integrated SKNA of Lead I (iSKNA-I) display the sum of SKNA over each 100 ms window, which is a common method to display nerve activities in microneurography studies.35 The same signals from ECG Lead I were bandpass filtered from 0.5 to 150 Hz to display surface ECG. Note the artifact on ECG (red dot) suggests physical activity at the beginning of the episode. B shows the data in the two red boxes of A in greater detail. C shows short bursts of SKNA associated with corresponding heart rate elevation (arrows). D shows the data in the two red boxes of C in greater detail. (SKNA=skin sympathetic nerve activity, iSKNA= integrated skin sympathetic nerve activity, ECG= electrocardiogram.)
Patterns of SKNA in VNS group
As compared with patients in control group, the VNS group had reduced SKNA episodes. Figure 2A shows SKNA and heart rate in control patient who had an active SKNA and corresponding heart rate elevation. Similar episodes were rare in VNS patients. When present, the SKNA were also associated with heart rate changes. The VNS episodes typically interrupts the SKNA episode and reduces the heart rate variations (Figure 2B). In most patients with VNS, the SKNA episodes were infrequently observed. Supplement Figure 1 shows a longer term (5-min) recording from a VNS patient (Panel A) and a control patient (Panel B). In VNS Patient, there were very low amplitudes of SKNA during the VNS ON and OFF times, with only little heart rate variations. In comparison, a control patient had high SKNA activity and robust heart responses to SKNA.
Figure 2.
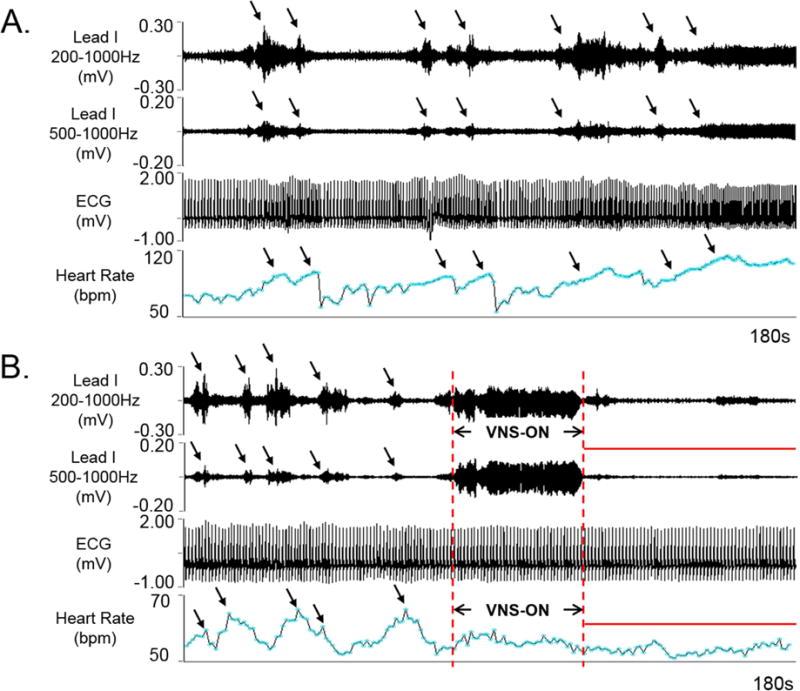
Effects of VNS on nerve activities. A shows recording made on ECG Lead I in a control patient. There were nerve discharges associated with episodic heart rate elevation (arrows). Persistent SKNA occurred towards the end of the recording. B shows a recording from VNS patient. Note that there were intermittent episodic SKNA prior to VNS, but no SKNA after VNS. These intermittent heart rate elevations were noted prior to VNS, corresponding to the SKNA episodes. Little heart rate variations were noted after VNS (red bar). (SKNA=skin sympathetic nerve activity, iSKNA= integrated skin sympathetic nerve activity, ECG= electrocardiogram.)
Correlation between heart rate and SKNA
There was a positive correlation between heart rate and SKNA in all patients studied. We tested the correlation using two different filter settings for SKNA detection. For all patients studied, the average Pearson’s correlation coefficients between aSKNA (200 Hz–1000 Hz bandpass filter) and heart rate was 0.52 [CI, 0.39 to 0.65] in VNS group, and 0.72 [CI, 0.65 to 0.79, p=0.024] in control group. The correlation coefficients were 0.45 [CI, 0.35 to 0.56] in VNS group, and 0.60 [CI, 0.43 to 0.66, p=0.019] in control group after 500 Hz–1000 Hz bandpass filtering. Supplement Figure 2 shows the typical examples of the correlation between heart rate and aSKNA in both groups of patients. A total of 144 dots were in each graph. Panel A and B were obtained a after bandpass filtering between 200 Hz and 1000 Hz. Panel C and D were obtained a after bandpass filtering between 500 Hz and 1000 Hz. Each dot represents the average with a 1-min window.
Circadian variation of SKNA
Figure 3 summarizes hourly averaged aSKNA over a 24-hour period from lead I (Panels A and B) and lead II (Panels C and D) in all patients studied. With either filter setting, the aSKNA of patients in the VNS group for both filter settings was significantly (p<0.00001) lower than the aSKNA in patients of the control group. In both VNS and control groups, aSKNA showed significant (p<0.00001) circadian variations.
Figure 3.
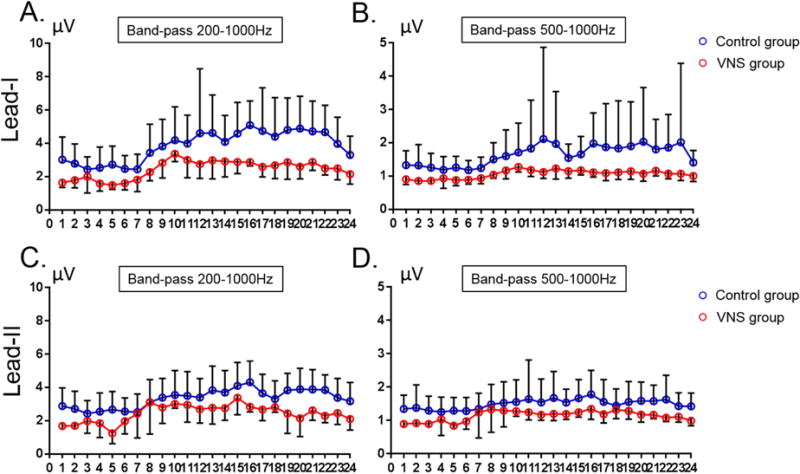
Hourly averaged nerve activities from Lead I and II in all patients studied. A and B show SKNA from Lead I while C and D show SKNA from Lead II. The filter settings are labeled in each panel. With either filter setting, the aSKNA was higher in the control group than in the VNS group. A significant circadian variation was noted in each graph. (Lead I: average SKNA at ECG Lead I. Lead II: average SKNA at ECG Lead II).
Comparison of aSKNA between control group and VNS group
Figure 4 shows the range of aSKNA in Lead I, Lead II and heart rate of all patients studied. The control group had significantly higher aSKNA and heart rate than the VNS group. When bandpass filtered between 200 Hz and 1000 Hz, the aSKNA during VNS OFF-time was 2.44 μV [95% confidence interval, CI, 2.13 to 2.74] in lead I and 2.49 μV [CI, 2.05 to 2.94] in lead II in the VNS group. These aSKNA values were significantly lower than 3.72 μV [CI, 2.58 to 4.87, p=0.012] and 3.26 μV [CI, 2.14 to 4.39, p=0.014], respectively, of the control Group. When filtered between 500 Hz and 1000 Hz, the aSKNA during VNS OFF-time was 1.06 μV [CI, 0.93 to 1.18] in lead I and 1.13 μV [CI, 0.99 to 1.27] in lead II, which were significantly lower than 1.38 μV [CI, 1.01 to 1.75, p=0.036] and 1.38 μV [CI, 0.98 to 1.78, p=0.035], respectively, of the control Group. The average heart rate was 65 bpm [CI, 59 to 71] in VNS group and 77 bpm [CI, 72 to 83] in the control group (p=0.013). The average systolic blood pressure of VNS patients and non-VNS patients were 116±10 mmHg and 127±16 mmHg, respectively (p=0.1369). The average diastolic blood pressure of VNS patients and non-VNS patients were 69±6 mmHg and 75±10 mmHg, respectively (p=0.1807).
Figure 4.
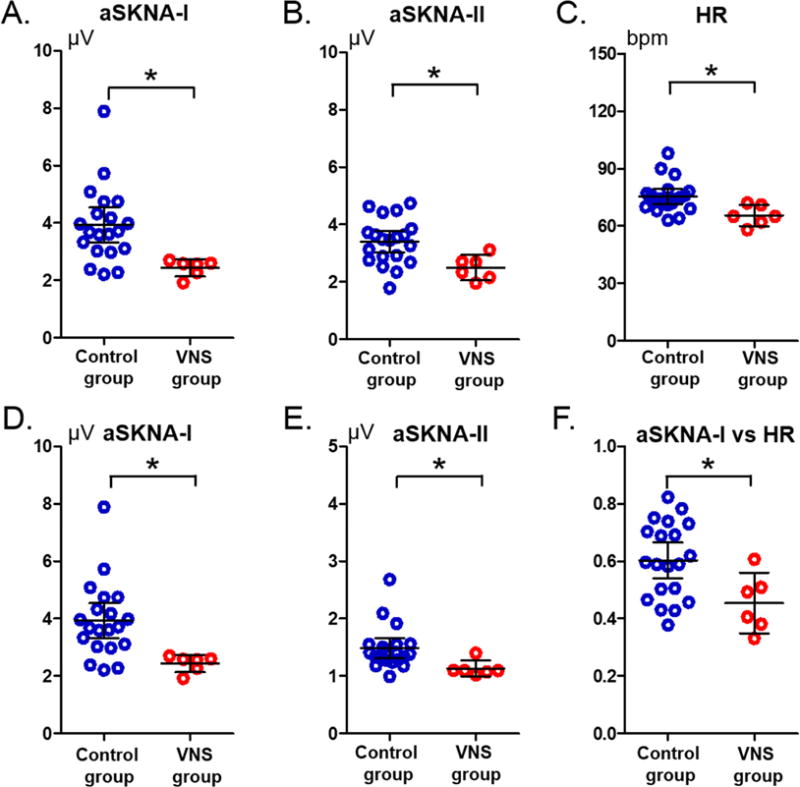
Average skin sympathetic nerve activities (aSKNA) and heart rate recorded of all patients studied. Each dot represents the aSKNA of a patient over a 24-hr period. A and B: The SKNA was bandpass filtered between 200 Hz to 1000 Hz. D and E: The SKNA was bandpass filtered between 500 Hz to 1000 Hz. C: Heart rate of both groups. F: Correlation coefficients of the Pearson’s correlation test of both groups. Horizontal bars represent mean and 95% confidence interval. *: p<0.05. (aSKNA-I: average skin sympathetic nerve activity at ECG Lead I. aSKNA-II: average skin sympathetic nerve activity at ECG Lead II)
Because patients with hypertension may have heightened sympathetic tone,17 we removed patients with hypertension and repeated the analyses of the remaining patients (5 with VNS and 14 without VNS). Using 200–1000 Hz filter setting, the aSKNA in Lead I was 2.41 μV (CI 2.01 to 2.80) in VNS group and 3.81 μV (CI 3.36 to 4.27) in non-VNS group (p=0.023). The aSKNA in Lead II was 2.37 μV (CI 1.96 to 2.78) in VNS group and 3.64 μV (CI 2.79 to 4.49) in non-VNS group (p=0.001). Using 500–1000 Hz filter setting, the aSKNA in Lead I was 1.02 μV (CI 0.92 to 1.12) in VNS group and 1.69 μV (CI 1.29 to 2.09) in non-VNS group (p=0.049). The aSKNA in Lead II was 1.08 μV (CI 1.04 to 1.12) in VNS group and 1.57 μV (CI 1.34 to 1.81) in non-VNS group (p=0.015). Therefore, excluding hypertensive patients did not change the conclusion of the study.
Discussion
The results of the present study show that patients with left VNS had lower heart rate and aSKNA than patients without VNS. These findings suggest that chronic intermittent VNS can reduce sympathetic tone in humans.
Vagal nerve stimulation and cardiovascular diseases
VNS is a well-established therapeutic option for patients with drug resistant epilepsy18, 19 but also had significant effects on cardiovascular function. Studies in animal models showed that VNS might be helpful in managing acute ischemia and in prolonging survival in heart failure,20–23 although recent clinical trials failed to confirm those findings.24 Another potential application of VNS in heart diseases is in the rate and rhythm control of AF.10, 25, 26 VNS has been reported to reduce the incidence of sudden unexpected death in epilepsy (SUDEP),1, 2 but others failed to confirm that finding.27
Mechanisms of VNS
The vagal nerve is a complex anatomical structure. It is generally accepted that parasympathetic activation is responsible for some of the therapeutic effects of VNS. However, if parasympathetic activation is responsible for heart rate control, then the therapeutic effects of the VNS should be limited only to the time when VNS is turned on. When VNS is turned off, ventricular conduction should accelerate, leading to a loss of therapeutic benefit. Recordings in ambulatory dogs showed that the ventricular responses during AF was reduced even when VNS was off.10 One possibility for this carry over effect is that stimulation of the sympathetic component in the vagal nerve may activate the ganglion cells in the SG at high rates,8 which may cause neurotoxicity,9 damages stellate ganglion to reduce the stellate ganglion nerve activity. In addition to interacting with the stellate ganglion, VNS also targets convergent local circuit neurons of the intrinsic cardiac nervous system to mitigate the potential for neurally induced AF.28
Skin sympathetic nerve recording
The skin is well innervated by sympathetic nerve fibers.29, 30 The somata of the nerves in the upper extremities and thorax resides in the cervical and stellate ganglia.31 Our recent studies showed that SKNA could be detected in humans using conventional patch electrodes using a recording system with high sampling rate and wide frequency bandwidth.32, 33 High pass filtering could eliminate the ECG signals and retain high frequency signals that included both muscle and nerve activities. Like most diagnostic tests, specificity could be increased only at the cost of reduction in sensitivity and vice versa. A wider bandpass filter setting (200 Hz to 1000 Hz) allowed us to increase the sensitivity of detecting SKNA but reduced specificity because the frequency of muscle activities could reach 400 Hz.34 A narrow bandpass filter setting (500 Hz to 1000 Hz) was more specific but might be less sensitive in detecting the SKNA. We have therefore used both filter settings to detect nerve activity in the present study. With either filter setting, we were able to document robust SKNA in control patients and reduced SKNA in patients with VNS. These findings suggest that, similar to VNS in ambulatory dogs,10 VNS in humans also suppresses sympathetic tone and maybe potentially useful in cardiac arrhythmia control.
SKNA and heart rate
The heart rate is controlled by both sympathetic and parasympathetic nerve activities. While the SKNA is a useful measure of sympathetic tone, it does not adequately measure all nerve activities that control the sinus node. Therefore, the average correlation coefficient between aSKNA and heart rate was around 0.45–0.72 in different groups and using different filter settings. Similar correlation coefficients were also observed between sympathetic nerve activities and heart rate in ambulatory dogs.11 It is unclear why VNS patients had reduced heart rate responses to SKNA. However, because VNS may damage the stellate ganglion and reduce sympathetic outflow,10 the damaged stellate ganglion might not be able to generate enough sympathetic tone to overcome the parasympathetic influence of the sinus node.
Limitations of the study
We do not have the histological sections or direct recordings of the stellate ganglion in patients with VNS. Therefore, it is not possible to determine if VNS has caused stellate ganglion damage or a reduction of stellate ganglion nerve activity. None of the patients died suddenly or had significant arrhythmias during the recording. Therefore, the relationship between SKNA and SUDEP remains unclear. The electrode impedance was not measured in the present study. However, we have previously reported that the average impedance of the electrodes as the one used in the present study was 37 ± 8 kΩ (range 28–52 kΩ).32
Supplementary Material
Acknowledgments
We thank David Wagner, MSEE, for his assistance.
Sources of Funding
This study was supported in part by an American Heart Association Greater Midwest Affiliate fellowship award (Dr Yuan), NIH Grants R42DA043391 (Dr Everett), P01HL78931, R01HL71140 (Dr Chen), a Charles Fisch Cardiovascular Research Award endowed by Dr Suzanne B. Knoebel of the Krannert Institute of Cardiology (Dr Everett), a Medtronic-Zipes Endowment and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative (Dr Chen).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Shien-Fong Lin and Peng-Sheng Chen have equity interest in Arrhythmotech, LLC. Drs. Yuan, Hassel, Doytchinova, Meshberger, LS Chen, Guerra, Shen, Everett and Salanova report no disclosures. Mr. Adams and Mr. Wright also report no disclosures.
References
- 1.Terra VC, Nisyiama MA, Abrao J, Sakamoto AC, Machado HR, Arida RM, Cavalheiro EA, Scorza FA. Epileptologists probe vagus nerve stimulation in children with refractory epilepsy: a promise against sudden unexpected death in epilepsy. Arq Neuropsiquiatr. 2012;70:953–955. doi: 10.1590/s0004-282x2012001200010. [DOI] [PubMed] [Google Scholar]
- 2.Schachter SC. Therapeutic effects of vagus nerve stimulation in epilepsy and implications for sudden unexpected death in epilepsy. Clin Auton Res. 2006;16:29–32. doi: 10.1007/s10286-006-0275-1. [DOI] [PubMed] [Google Scholar]
- 3.Verrier RL, Nearing BD, Olin B, Boon P, Schachter SC. Baseline elevation and reduction in cardiac electrical instability assessed by quantitative T-wave alternans in patients with drug-resistant epilepsy treated with vagus nerve stimulation in the AspireSR E-36 trial. Epilepsy Behav. 2016;62:85–89. doi: 10.1016/j.yebeh.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Rizas KD, Nieminen T, Barthel P, et al. Sympathetic activity-associated periodic repolarization dynamics predict mortality following myocardial infarction. J Clin Invest. 2014;124:1770–1780. doi: 10.1172/JCI70085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non-invasive Vagus Nerve Stimulation in Healthy Humans Reduces Sympathetic Nerve Activity. Brain Stimul. 2014;7:871–877. doi: 10.1016/j.brs.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Seki A, Green HR, Lee TD, Hong L, Tan J, Vinters HV, Chen PS, Fishbein MC. Sympathetic nerve fibers in human cervical and thoracic vagus nerves. Heart Rhythm. 2014;11:1411–1417. doi: 10.1016/j.hrthm.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onkka P, Maskoun W, Rhee KS, Hellyer J, Patel J, Tan J, Chen LS, Vinters HV, Fishbein MC, Chen PS. Sympathetic nerve fibers and ganglia in canine cervical vagus nerves: Localization and quantitation. Heart Rhythm. 2013;10:585–591. doi: 10.1016/j.hrthm.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee KS, Hsueh CH, Hellyer JA, et al. Cervical vagal nerve stimulation activates the stellate ganglion in ambulatory dogs. Korean Circ J. 2015;45:149–157. doi: 10.4070/kcj.2015.45.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olney JW. Excitotoxicity, apoptosis and neuropsychiatric disorders. Curr Opin Pharmacol. 2003;3:101–109. [PubMed] [Google Scholar]
- 10.Chinda K, Tsai WC, Chan YH, et al. Intermittent Left Cervical Vagal Nerve Stimulation Damages the Stellate Ganglia and Reduces Ventricular Rate During Sustained Atrial Fibrillation in Ambulatory Dogs. Heart Rhythm. 2016;13:771–780. doi: 10.1016/j.hrthm.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson EA, Rhee KS, Doytchinova A, et al. Estimating sympathetic tone by recording subcutaneous nerve activity in ambulatory dogs. J Cardiovasc Electrophysiol. 2015;26:70–78. doi: 10.1111/jce.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doytchinova A, Patel J, Zhou S, Chen H, Lin S-F, Shen C, Everett TH, IV, Lin SF, Chen P-S. Subcutaneous nerve activity and spontaneous ventricular arrhythmias in ambulatory dogs. Heart Rhythm. 2015;122:612–620. doi: 10.1016/j.hrthm.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Z, Zhao Y, Doytchinova A, et al. Using skin sympathetic nerve activity to estimate stellate ganglion nerve activity in dogs. Heart Rhythm. 2015;12:1324–1332. doi: 10.1016/j.hrthm.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doytchinova A, Hassel JL, Yuan Y, et al. Simultaneous noninvasive recording of skin sympathetic nerve activity and electrocardiogram. Heart Rhythm. 2017;14:25–33. doi: 10.1016/j.hrthm.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1:477–482. doi: 10.1016/s1474-4422(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 16.Muzi M, Ebert TJ, Hope WG, Robinson BJ, Bell LB. Site(s) mediating sympathetic activation with desflurane. Anesthesiology. 1996;85:737–747. doi: 10.1097/00000542-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 18.Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 19.Fisher RS, Handforth A. Reassessment: vagus nerve stimulation for epilepsy: a report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1999;53:666–669. doi: 10.1212/wnl.53.4.666. [DOI] [PubMed] [Google Scholar]
- 20.Myers RW, Pearlman AS, Hyman RM, Goldstein RA, Kent KM, Goldstein RE, Epstein SE. Beneficial effects of vagal stimulation and bradycardia during experimental acute myocardial ischemia. Circulation. 1974;49:943–947. doi: 10.1161/01.cir.49.5.943. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 22.Shinlapawittayatorn K, Chinda K, Palee S, Surinkaew S, Thunsiri K, Weerateerangkul P, Chattipakorn S, Kenknight BH, Chattipakorn N. Low-amplitude, left vagus nerve stimulation significantly attenuates ventricular dysfunction and infarct size through prevention of mitochondrial dysfunction during acute ischemia-reperfusion injury. Heart Rhythm. 2013;10:1700–1707. doi: 10.1016/j.hrthm.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Shinlapawittayatorn K, Chinda K, Palee S, Surinkaew S, Kumfu S, Kumphune S, Chattipakorn S, KenKnight BH, Chattipakorn N. Vagus nerve stimulation initiated late during ischemia, but not reperfusion, exerts cardioprotection via amelioration of cardiac mitochondrial dysfunction. Heart Rhythm. 2014;11:2278–2287. doi: 10.1016/j.hrthm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Gold MR, Van Veldhuisen DJ, Hauptman PJ, et al. Vagus Nerve Stimulation for the Treatment of Heart Failure: The INOVATE-HF Trial. J Am Coll Cardiol. 2016;68:149–158. doi: 10.1016/j.jacc.2016.03.525. [DOI] [PubMed] [Google Scholar]
- 25.Shen MJ, Hao-Che Chang X, Park HW, et al. Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart Rhythm. 2013;10:910–915. doi: 10.1016/j.hrthm.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol. 2015;65:867–875. doi: 10.1016/j.jacc.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granbichler CA, Nashef L, Selway R, Polkey CE. Mortality and SUDEP in epilepsy patients treated with vagus nerve stimulation. Epilepsia. 2015;56:291–296. doi: 10.1111/epi.12888. [DOI] [PubMed] [Google Scholar]
- 28.Salavatian S, Beaumont E, Longpre JP, Armour JA, Vinet A, Jacquemet V, Shivkumar K, Ardell JL. Vagal stimulation targets select populations of intrinsic cardiac neurons to control neurally induced atrial fibrillation. Am J Physiol Heart Circ Physiol. 2016;311:H1311–H1320. doi: 10.1152/ajpheart.00443.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baron R, Janig W, With H. Sympathetic and afferent neurones projecting into forelimb and trunk nerves and the anatomical organization of the thoracic sympathetic outflow of the rat. J Auton Nerv Syst. 1995;53:205–214. doi: 10.1016/0165-1838(94)00171-f. [DOI] [PubMed] [Google Scholar]
- 30.Taniguchi T, Morimoto M, Taniguchi Y, Takasaki M, Totoki T. Cutaneous distribution of sympathetic postganglionic fibers from stellate ganglion: A retrograde axonal tracing study using wheat germ agglutinin conjugated with horseradish peroxidase. J Anesth. 1994;8:441–449. doi: 10.1007/BF02514624. [DOI] [PubMed] [Google Scholar]
- 31.Pather N, Partab P, Singh B, Satyapal KS. Cervico-thoracic ganglion: its clinical implications. Clin Anat. 2006;19:323–326. doi: 10.1002/ca.20214. [DOI] [PubMed] [Google Scholar]
- 32.Doytchinova A, J H, Y Y, et al. Simultaneous non-Invasive Recording of skin sympathetic nerve activity and electrocardiogram. Heart Rhythm. 2017;14:25–33. doi: 10.1016/j.hrthm.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uradu A, Wan J, Doytchinova A, Wright KC, Lin AY, Chen LS, Shen C, Lin SF, Everett Tt, Chen PS. Skin Sympathetic Nerve Activity Precedes the Onset and Termination of Paroxysmal Atrial Tachycardia and Fibrillation. Heart Rhythm. 2017;14:964–971. doi: 10.1016/j.hrthm.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komi PV, Tesch P. EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. Eur J Appl Physiol Occup Physiol. 1979;42:41–50. doi: 10.1007/BF00421103. [DOI] [PubMed] [Google Scholar]
- 35.White DW, Shoemaker JK, Raven PB. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Auton Neurosci. 2015;193:12–21. doi: 10.1016/j.autneu.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


