Abstract
Systolic dysfunction was recently described following traumatic brain injury (TBI) and systemic inflammation may be a contributing mechanism. Our aims were to: 1) Examine the association between the early systemic inflammatory response syndrome (SIRS) and systolic cardiac dysfunction following TBI and 2) Describe the longitudinal change in SIRS criteria, cardiac function, and hemodynamic parameters during the first week of hospitalization. We used a secondary analysis of a prospective cohort study examining cardiac function (with transthoracic echocardiography on the first day and serially over the first week of hospitalization) in 32 moderate-severe isolated TBI patients, and quantified the admission and daily SIRS response to injury. We determined the association of admission SIRS and systolic dysfunction following TBI. Admission SIRS was present in 7 (21%) of patients and was associated with systolic dysfunction on multivariable analysis (RR 4.01; 95% 1.16–13.79, p = 0.028). Both SIRS criteria and systolic cardiac function improved over the first week of hospitalization. In conclusion, early SIRS is common among patients with moderate-severe TBI and the presence of SIRS criteria on admission is associated with systolic cardiac dysfunction following TBI.
Keywords: Traumatic brain injury, Systemic inflammatory response syndrome, inflammation, Stress cardiomyopathy, Echocardiography
Introduction
Traumatic brain injury (TBI) is a major worldwide public health problem resulting in significant morbidity and mortality (Hyder et al. 2007). While the primary injury to the brain can render irreversible neuronal loss and cerebral ischemia, reduction in cerebral blood flow from systemic effects such as hypotension further result in secondary brain insults (Chesnut et al. 1993; Jeremitsky et al. 2003; Mascia et al. 2008). Recently, cardiac dysfunction has been detected after TBI (Cheah et al. 2016; Prathep et al. 2014; Qian et al. 2015), which can contribute to cerebral hypoperfusion and secondary injury after TBI. While sympathetic overstimulation may play a role in the pathogenesis of TBI-induced systolic dysfunction (Krishnamoorthy et al. 2016; Mazzeo et al. 2014; Mierzewska-Schmidt and Gawecka 2015; Nguyen and Zaroff 2009), other contributing pathophysiologic mechanisms remain unclear.
Injury to the brain triggers the release of inflammatory mediators into the circulation (Kossmann et al. 1995; Lu et al. 2009; Morganti-Kossman et al. 1997). This condition of systemic inflammation may be clinically manifested as the systemic inflammatory response syndrome (SIRS) (Bone et al. 1992), (Bone 1996; Giannoudis et al. 1998), with hypothermia or hyperthermia, leukocytosis or leukopenia, tachycardia, and tachypnea. (Bone et al. 1992). The systemic inflammatory response syndrome has been described in a variety of acute cerebral insults, including subarachnoid hemorrhage (Yoshimoto et al. 2001), stroke (Audebert et al. 2004; Boehme et al. 2013), intracerebral hemorrhage (Boehme et al. 2013), and TBI (Lou et al. 2013).
Cardiac dysfunction has been linked to inflammation in other critical illness paradigms such as sepsis (Court et al. 2002; Flierl et al. 2008; Flynn et al. 2010; Hochstadt et al. 2011; Pulido et al. 2012), myocardial infarction (van Diepen et al. 2013) and burns (Horton 2007; Li et al. 2013; Niederbichler et al. 2006; Zhang et al. 2007). However, the association between systemic inflammation and cardiac dysfunction has not been investigated either in animal models of TBI or in patients who have TBI. To bridge this gap in knowledge, we conducted a study to: 1) examine the association between the early SIRS response and systolic cardiac dysfunction, and 2) describe the longitudinal change in SIRS criteria, cardiac function, and hemodynamic parameters during the first week of hospitalization following TBI.
Materials and Methods
This was a secondary analysis of a prospective cohort study of cardiac function conducted at Harborview Medical Center (Seattle, WA), a Level 1 trauma center for the states of Washington, Alaska, Montana and Idaho, and affiliated with the University of Washington (Krishnamoorthy et al. 2016). The study was approved by the Institutional Review Board (IRB) at the University of Washington.
Inclusion and Exclusion Criteria
Adult patients with moderate to severe TBI (defined as post-resuscitation Glasgow Coma Scale (GCS) score of ≤ 12) were included. Male and female patients were equally eligible. We excluded patients older than 65 years, those with pre-existing cardiac disease, or having significant systemic disease which could potentially affect systolic function, such as cirrhosis, chronic kidney disease, HIV infection, history of chemotherapy, chronic obstructive pulmonary disease, pulmonary hypertension, or history of cerebrovascular disease. We also excluded patients who had a cardiac arrest prior to evaluation, patients who required more than 2 units of packed red blood cells for initial resuscitation, or patients with polytrauma (having other body regions except head Abbreviated Injury Scale (AIS) > 2).
Echocardiography
Details of TTE methodology are previously described (Krishnamoorthy et al. 2016). After informed consent was obtained from the patient’s legally authorized representatives, a bedside transthoracic echocardiogram (TTE) was performed within the first 24 hours after admission or on hospital day 2 if hemodynamically unstable or undergoing medical procedures Each patient received two more follow-up TTE exams over the first week of hospitalization. All TTE examinations were performed by an echocardiography-certified anesthesiologist-intensivist per the American Society of Echocardiography guidelines (Lang et al. 2006). Patients were placed in a supine position, and systolic function was assessed using endocardial fractional shortening [(left ventricular internal diameter in diastole – left ventricular internal diameter in systole)/left ventricular diameter in diastole from the parasternal long-axis window. Due to the concern of intracranial hypertension, the patient’s head-of-bed was elevated. All TTE images were reviewed offline by both a cardiologist and certified cardiac sonographer separately, both of whom were blinded to patients’ clinical details. Patients with fractional shortening less than 25% were categorized as having systolic cardiac dysfunction (Lang et al. 2006).
Data Collection
Demographic, clinical, and individual SIRS characteristics were abstracted from hospital electronic medical record. Initial head computed tomography (CT) imaging reports were reviewed to classify the type and radiographic characteristics of the TBI (i.e. epidural hematoma, subdural hematoma, subarachnoid hemorrhage, or intraparenchymal hemorrhage). Data on SIRS, hemodynamic parameters, and GCS were recorded from admission and daily over the first week of hospitalization.
Systemic Inflammatory Response Syndrome (SIRS)
We defined SIRS based on the American College of Chest Physicians and Society of Critical Care Medicine Criteria as having 2 or more of the following: 1) body temperature < 36 or > 38°c, 2) heart rate > 90 beats per minute, 3) respiratory rate > 20 per minute, or 4) white blood cell count < 4000 or > 12,000/mm3 (Bone et al. 1992). SIRS on admission was adjudicated when patients first arrived at the emergency department. Daily SIRS was considered present if the patients met SIRS criteria at any time during each daily 24-hour epoch. In patients who were mechanically ventilated, we used the respiratory rate from the patient effort, not the setting of the machine, to evaluate respiratory rate criteria.
Exposure and Outcomes
The primary exposure was the presence of SIRS on admission. The primary outcome was the development of systolic dysfunction among patients with moderate-severe TBI who met SIRS criteria on admission. Secondary exposures included examining individual SIRS criteria, and secondary outcomes included longitudinal changes in SIRS, cardiac function, and hemodynamic parameters over the first week of hospitalization.
Statistical analysis
Descriptive statistics examined the clinical, echocardiographic, and SIRS characteristics of the study cohort. Descriptive statistics were also used to examine the longitudinal changes in SIRS, hemodynamics, and cardiac function over the first week of hospitalization. For comparison of echocardiographic parameters between groups with and without admission SIRS, a 2-sided Student’s t-test or a Fisher’s exact test was used. Univariate and multivariable Poisson regression models with robust standard errors were used to examine the association of the presence of early SIRS with the development of systolic dysfunction. The multivariable models were adjusted for a priori selected confounders, including age, gender, and highest GCS score. Results of the regression analyses are presented as relative risks, with 95% confidence intervals. All analyses were performed using Stata 13.0 (College Station, Texas).
Results
Data from 32 patients were examined. In 24 patients, the first TTE was performed within 24 hours after admission; in 8 patients, the first TTE was performed the next day to avoid interruption in clinical care (resuscitation, clinical procedures, or surgery).
Characteristics of patients with moderate-severe TBI
Of the 32 patients, seven (21.9%) met SIRS criteria on admission. Demographic and clinical characteristics of the patients stratified by the presence of SIRS on admission are described in Table 1. The group with admission SIRS was younger compared to the group without SIRS (mean age of 32.1 and 37.7, respectively). Most patients in both groups were male (71% and 88%, respectively) without major underlying comorbidities. Each patient was tracheally intubated and mechanically ventilated during the first day of hospitalization. Motor vehicle crash was the primary mechanism of injury in the group with SIRS (71%), while falls was the most common mechanism in the group without SIRS (32%). Both groups had high percentage of intracranial hemorrhage from initial computed tomography (CT) findings but median admission GCS was relatively lower in the group with SIRS (3 [3–7]) compared to the group without SIRS (5 [3–7]). Four (12.5%) patients in the study had admission GCS 9–12 (moderate TBI) and none of them had systolic dysfunction. The group with SIRS had more associated injuries but all other injuries were mild (AIS ≤ 2). Admission mean systolic blood pressure was relatively similar in both the group without SIRS (135 ± 24 mmHg) and the group with SIRS (122 ± 21 mmHg). The proportion of patients who experienced hypotension (systolic blood pressure < 90 mmHg) in the first 24 hours was non-significantly higher in the group with SIRS (57%, n = 7) compared to the group without SIRS (28%, n = 25; p = 0.20, Fisher’s exact test). The rates of vasopressor use, ICP monitoring and intracranial surgery were similar in both groups (Table 1). The proportion of patients who died in the group with SIRS (29%, n = 7) was not significantly higher than the group without SIRS (8%, n = 25; p = 0.20, Fisher’s exact test).
Table 1.
Demographic, clinical characteristics, clinical care and outcomes of patients with moderate-severe traumatic brain injury with and without systemic inflammatory response syndrome (SIRS) on admission.
| Without SIRS* (n = 25) |
With SIRS* (n = 7) |
Total* (n = 32) |
|
|---|---|---|---|
| Demographic | |||
| Age (years) | 37.7 (13.5) | 32.1 (12.7) | 36.9 (13.3) |
| Male | 22 (88%) | 5 (71%) | 27 (84%) |
| Clinical characteristics | |||
| Mechanism of injury | |||
| Fall | 8 (32%) | 2 (29%) | 10 (31%) |
| Motor vehicle crash | 5 (20%) | 5 (71%) | 10 (31%) |
| Vehicle versus pedestrian | 5 (20%) | 0 (0%) | 5 (16%) |
| Assault | 3 (12%) | 0 (0%) | 3 (9%) |
| Gunshot | 1 (4%) | 0 (0%) | 1 (3%) |
| Other | 3 (12%) | 0 (0%) | 3 (9%) |
| Initial CT findings | |||
| Subdural hemorrhage | 21 (84%) | 3 (43%) | 24 (75%) |
| Subarachnoid hemorrhage | 19 (76%) | 4 (57%) | 23 (72%) |
| Intraparenchymal hemorrhage and cerebral contusion | 13 (52%) | 2 (29%) | 15 (47%) |
| Epidural hemorrhage | 4 (16%) | 1 (14%) | 5 (16%) |
| Associated injury | |||
| Chest | 8 (32%) | 3 (43%) | 11 (34%) |
| Abdomen | 2 (8%) | 2 (29%) | 4 (13%) |
| Pelvis | 3 (12%) | 2 (29%) | 5 (16%) |
| Extremities | 10 (40%) | 6 (86%) | 16 (50%) |
| Spine | 8 (32%) | 2 (29%) | 10 (31%) |
| Positive serum alcohol | 11 (44%) | 4 (57%) | 15 (47%) |
| Severe TBI (Admission GCS ≤ 8) | 21 (84%) | 7 (100%) | 28 (88%) |
| Admission GCS** | 5 (3–7) | 3 (3–7) | 4 (3–7) |
| Admission systolic blood pressure (SBP) | 135 (24) | 122 (21) | 132 (24) |
| Admission heart rate | 80 (17) | 111 (32) | 87 (24) |
| Admission hematocrit (%) | 38.3 (4.8) | 36.6 (7.2) | 37.9 (5.3) |
| Hypotension (SBP < 90 mmHg) in first 24 hours | 7 (28%) | 4 (57%) | 11 (34%) |
| Hypertension (SBP > 140 mmHg) in first 24 hours | 19 (76%) | 6 (86%) | 25 (78%) |
| Clinical care | |||
| Vasopressor use in first 24 hours | 5 (20%) | 1 (14%) | 6 (19%) |
| ICP monitoring | 13 (52%) | 3 (43%) | 16 (50%) |
| Need for intracranial surgery | 9 (36%) | 3 (43%) | 12 (38%) |
| Any positive bacterial culture within the first 3 days | 1 (4%)a | 0 (0%) | 1 (3%) |
| Outcomes | |||
| ICU length of stay (days) | 12 (12) | 9 (10) | 11 (11) |
| Hospital length of stay (days) | 28 (19) | 13 (11) | 25 (19) |
| Death | 2 (8%) | 2 (29%) | 4 (13%) |
Data are presented as mean (SD) for continuous data or number (%) for categorical data.
Data are presented as median and interquartile range.
Positive culture from bronchoalveolar lavage fluid on day 3
Early echocardiographic findings
Echocardiographic findings from the first TTE evaluation are presented in Table 2. A higher proportion of patients had systolic dysfunction in the group with SIRS compared to the group without SIRS (57% vs. 12%, p = 0.01, Fisher’s exact test). Most diastolic parameters did not differ between groups, including mean E wave, A wave and E to A ratio. The group with SIRS had a significantly lower mean deceleration time (88 ms [n = 5] vs. 130 ms [n = 23]; p = 0.05, t-test) and a higher mitral annular septal tissue velocity [e’(s)] (12.22 cm/s [n = 5] vs. 8.83 cm/s; p = 0.01, t-test).
Table 2.
Early transthoracic echocardiographic findings in patients with moderate to severe traumatic brain injury with and without systemic inflammatory response syndrome (SIRS) on admission. Data are presented as mean (SD) or number (%)
| Without SIRS (n = 25) |
With SIRS (n = 7) |
p | |
|---|---|---|---|
| Systolic function | |||
| Left ventricular area end-diastole (cm2)a | 18.42 (4.44) | 19.38 (7.02) | 0. 70 |
| Left ventricular area end-systole (cm2)a | 8.58 (3.33) | 10.28 (1.64) | 0. 32 |
| Fractional area change (cm2)a | 0.54 (0.09) | 0.46 (0.14) | 0. 11 |
| Left ventricular internal diameter end-diastole (cm) | 4.74 (0.56) | 4.30 (0.71) | 0.09 |
| Left ventricular internal diameter end-systole (cm) | 3.30 (0.61) | 3.20 (0.63) | 0.70 |
| Fractional shortening | 0.31 (0.07) | 0.26 (0.07) | 0.09 |
| Mitral annular septal tissue velocity [S’(s) (cm/s)]a | 9.76 (2.12) | 8.36 (3.21) | 0.24 |
| Systolic dysfunction (Fractional shortening < 0.25) | 3 (12%) | 4 (57%) | 0.01 |
| Diastolic function | |||
| Mitral inflow peak early filling [E wave (cm/s)]b | 66.27 (19.64) | 72.84 (20.92) | 0.51 |
| Mitral inflow peak late filling [A wave (cm/s)]c | 47.75 (13.11) | 39.75 (9.87) | 0.26 |
| E-wave to A wave ratioc | 1.49 (0.54) | 1.84 (0.74) | 0. 27 |
| E-wave to A-wave ratio < 1c | 5 (23 %) | 0 (0 %) | 0.56 |
| E-wave to A-wave ratio > 2c | 3 (14%) | 2 (50%) | 0.16 |
| Mitral inflow E-wave deceleration time (ms)b | 130.0 (43.28) | 88.0 (25.88) | 0.05 |
| Mitral annular septal tissue velocity [e’(s)(cm/s)]d | 8.83 (2.48) | 12.22 (2.20) | 0.01 |
| E-wave to e’(s) ratioe | 7.63 (1.96) | 6.06 (1.87) | 0.12 |
| E-wave to e’(s) ratio > 8e | 10 (48%) | 1 (20 %) | 0.36 |
Data available in 26 patients (21 without SIRS and 5 with SIRS)
Data available in 28 patients (23 without SIRS and 5 with SIRS)
Data available in 26 patients (22 without SIRS and 4 with SIRS)
Data available in 27 patients (22 without SIRS and 5 with SIRS)
Data available in 26 patients (21 without SIRS and 5 with SIRS)
Admission SIRS and cardiac dysfunction
Seven (21.9%) patients met SIRS criteria on admission. The presence of SIRS on admission was associated with an increased risk of developing systolic dysfunction in both univariate (Relative Risk [RR] 4.76; 95% CI 1.35–16.79, p = 0.015, Table 3) and multivariable analysis (RR 4.01; 95% 1.16–13.79, p = 0.028, Table 3). There was no association between individual admission SIRS criteria and systolic dysfunction (Table 3).
Table 3.
Univariate and multivariable analysis of the association between admission SIRS and individual admission SIRS criteria and systolic dysfunction
| Univariate | Multivariable* | ||||
|---|---|---|---|---|---|
| Admission variables | n (%) | Relative risk (95% CI) |
p | Relative risk (95% CI) |
p |
| SIRS | 7 (22%) | 4.76 (1.35–16.79) | 0.02 | 4.01 (1.16–13.79) | 0.03 |
| Body temperature < 36 or > 38°c | 7 (22%) | 1.05 (0.25–4.47) | 0.95 | 0.68 (0.20–2.32) | 0.54 |
| Heart rate > 90 beats per minute | 10 (31%) | 2.93 (0.79–10.96) | 0.11 | 3.35 (0.95–11.78) | 0.06 |
| Respiratory rate > 20 per minute | 2 (6%) | 2.50 (0.51–12.19) | 0.26 | 1.00 (0.28–3.63) | 0.10 |
| White blood cell count <4 or >12×103/mm3 | 18 (56%) | 1.94 (0.43–8.78) | 0.39 | 1.62 (0.45–5.86) | 0.46 |
The final model adjusted for age, gender and highest Glasgow Coma Scale score within 24 hours after admission
Longitudinal change in SIRS criteria, cardiac function and hemodynamic parameters
Figure 1 shows the distribution of SIRS and systolic cardiac function over the first week of hospitalization. Twenty-six patients (81.3%) developed SIRS within the first day of hospitalization. SIRS within the first day of hospitalization was not associated with the development of systolic dysfunction on univariate (RR 1.38, 95% CI 0.20–9.76, p = 0.74) or multivariable (RR 1.61, 95% CI 0.25–10.45, p = 0.62) analysis. The incidence of SIRS was high (71.9–81.3%) in the first 3 days of admission, and SIRS criteria persisted in majority of patients (60.7–65.5%) during days 4 to 7. Overall, the most common positive SIRS criteria were heart rate (62.1–83.9%), followed by respiratory rate (53.1–62.5%) and temperature (41.4–48.4%). White blood cell count criteria was found in 56.3–62.5% of the patients during the first 2 days, decreased to less than 20% in day 4 and 5, and again increased after day 6. Systolic function improved in the entire cohort over the first week of hospitalization, from a median (IQR) fractional shortening of 0.29 (0.25 – 0.34) to 0.33 (0.30 – 0.36).
Figure 1.
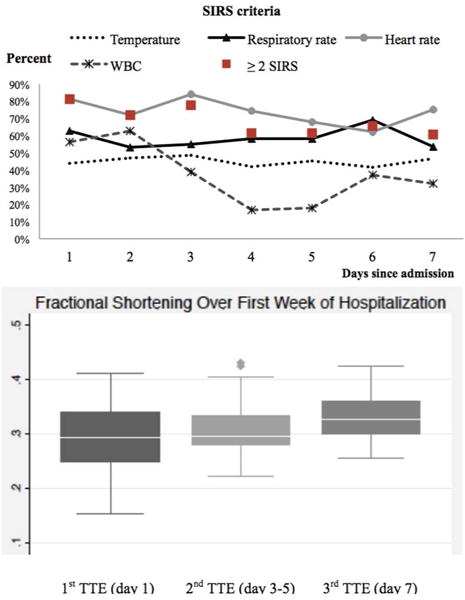
Longitudinal change in SIRS criteria and systolic cardiac function over the first week of hospitalization
Figure 2 shows hemodynamic and neurologic parameters over the first week of hospitalization, grouped by the presence/absence of admission SIRS. Daily highest/lowest systolic blood pressure and highest/lowest mean arterial pressure were similar in the groups with and without admission SIRS. Overall, there was an improvement in GCS over time in most patients in the cohort. In the group with SIRS, median GCS decreased between day 3 and 5, followed by an improvement after day 6 (Figure 2).
Figure 2. Systolic blood pressure (SBP), mean arterial pressure (MAP) and Glasgow Coma Scale (GCS) score trajectory during the first week of hospitalization.
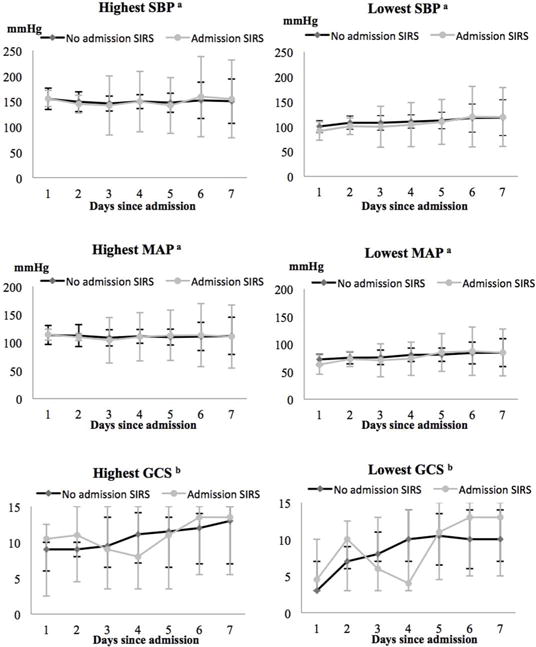
aData are presented as mean and SD.
bData are presented as median and interquartile range.
Discussion
In this preliminary study, we aimed to examine the relationship between SIRS and cardiac dysfunction in patients with moderate-severe TBI. Our study has 3 main findings: 1) A high percentage of patients develop SIRS, 2) The presence of SIRS on admission is associated with systolic cardiac dysfunction following TBI, and 3) Both early SIRS and systolic cardiac function appear to improve over the first week after injury.
SIRS following TBI
Preclinical studies have documented a neuroinflammatory response following TBI (Febinger et al. 2015). Normally, inflammatory mediators are absent in the central nervous system (Kadhim et al. 2008), but following TBI, studies have shown that cerebrospinal fluid (CSF) cytokine levels rapidly elevate in response to brain injury (Helmy et al. 2011; Holmin and Hojeberg 2004; Kushi et al. 2003; Rothwell and Luheshi 2000; Shiozaki et al. 2005; Whalen et al. 2000). This local inflammatory response leaks into the systemic circulation through disruption of blood-brain barrier permeability and triggers acute-phase systemic host responses (Kossmann et al. 1995; Morganti-Kossman et al. 1997). In human studies, higher serum pro-inflammatory cytokines, such as tumor necrosis factor α (TNF α) (Ross et al. 1994) and interleukin-6 (IL-6) (Kossmann et al. 1995; Mazzeo et al. 2014; McKeating et al. 1997), have been observed following TBI. Furthermore, IL-6 may play an important role in the development of the clinical SIRS response (Taniguchi et al. 1999). In our study, a significant proportion of patients (81.3%) demonstrated SIRS within 24 hours following moderate-severe TBI and remained high throughout the first 3 days after injury. Our results are aligned with that of a prospective observational study in 86 patients with severe TBI, in which 60% of the patients had SIRS during the first week of hospitalization and 85% developed early SIRS within 3 days (Lou et al. 2013). Although SIRS can occur as a result of many critical conditions, particularly infection, the early SIRS response demonstrated in our patients were more likely to be due to TBI. The timing which we assessed early SIRS was only hours after injuries and none of the patients were transferred from other hospitals; thus, it is too early for the development of infection or sepsis in previously healthy patients. Moreover, none of the patients included in the study had met criteria for diagnosis of sepsis (having Sequential Organ Failure Assessment (SOFA) score changes > 2) according to the Third International Consensus Definition for Sepsis and Septic Shock (Sepsis-3) guidelines (Rhodes et al. 2017) except for the GCS criteria, which can be explained by TBI.
Relationship between SIRS and Systolic Dysfunction following TBI
A notable stress response phenomenon among critical illness paradigms is catecholamine excess, which has been widely described as the underlying mechanism of cardiac dysfunction following neurological injury (Bybee and Prasad 2008; Masuda et al. 2002; Samuels 2007). Recently, our group reported the distinct hemodynamic pattern in patients with moderate-severe TBI who developed early systolic dysfunction and was suggestive of sympathetic hyper stimulation as one of the underlying mechanisms of cardiac dysfunction following TBI. (Krishnamoorthy et al. 2016). However, the elevated catecholamine levels can also directly affect inflammatory cytokine expression during the acute phase response to injury (Elrifai et al. 1996; Severn et al. 1992; Woiciechowsky et al. 1998). In addition, the release of pro-inflammatory mediators, such as IL-1, can also exaggerate the sympathetic response activity (Ichijo et al. 1994; Severn et al. 1992). In this study, the presence of SIRS on admission was associated with early systolic cardiac dysfunction in both univariate and multivariate analysis. The findings from our pilot study suggest a linkage between the immune response and cardiac dysfunction. Therefore, the underlying mechanism of cardiac dysfunction following TBI may be the combination of both catecholamine excess and systemic inflammatory response.
Longitudinal change in clinical parameters, cardiac function and SIRS
Systolic dysfunction improved in the cohort over the first week following injury while SIRS was still present in more than half of the patients. This is different from patterns of sepsis-induced cardiac dysfunction, which typically begins with a hyperdynamic response, followed by hypokinesis and decreased ventricular function, which remains for 7–10 days (Boissier et al. 2017; Huang et al. 2013), indicating that systolic dysfunction in our patients was likely not due to sepsis. Although admission SIRS was associated with systolic dysfunction, SIRS on the first day was not. Systemic inflammatory response syndrome during the first week of hospitalization can occur as a result of many conditions rather than TBI, such as surgical procedures, critical care interventions and sepsis, unlike SIRS on admission which may directly reflect severity of the injury (Mica et al. 2012). This finding supports the hypothesis that a very early and robust systemic inflammatory response, which may be related to TBI severity, may be more important to the development of systolic dysfunction than the presence of a later inflammatory response. There was no difference in the longitudinal hemodynamic profile between the group with and without SIRS on admission. However, the group with SIRS demonstrated slower recovery in GCS with a drop in median GCS during day 3 to 5. This result might demonstrate a linkage between early SIRS and delayed recovery after TBI secondary to intense early neuroinflammation. However, due to a small sample size, we could not perform any formal statistical testing on this relationship, and our findings should be considered exploratory.
Clinical Relevance of Study Findings
Currently, there is still a major gap in knowledge on the mechanisms and impact of systolic dysfunction following TBI. The findings from our study suggest multifactorial mechanisms of the development of early systolic dysfunction following TBI. Previous studies have demonstrated that the immune-mediated response following TBI is associated with poor clinical outcomes (Arand et al. 2001; Chiaretti et al. 2005; Shiozaki et al. 2005; Winter et al. 2004; Woiciechowsky et al. 2002). Even though SIRS criteria are considered nonspecific for determining the pattern of host response (Marshall 2000), studies have demonstrated SIRS without infection as a predictor of poor outcomes in many patients with acute neurological injury, including subarachnoid hemorrhage (Yoshimoto et al. 2001) and intracerebral hemorrhage (Boehme et al. 2013). While the findings of our pilot study demonstrated an association between admission SIRS and the development of systolic cardiac dysfunction following TBI, future studies should consider clinical SIRS, as well as potential inflammatory biomarkers (i.e. C-reactive protein) and cardiac parameters (i.e. troponin, EKG), in prediction models for cardiac dysfunction and poor outcomes in the TBI population.
Limitations
Some limitations from our study should be noted. First, our study was a single-center pilot study with a small sample size, which may overestimate the true association; therefore, our findings can be considered hypothesis-generating and require confirmation. Second, SIRS criteria are not specific for systemic inflammation, and patients did not have inflammatory biomarkers data for analysis. Third, despite adjustment for possible confounding variables in our analysis, residual confounding may still be present, especially when considering other unmeasured factors that may make the interpretation of SIRS challenging such as pain, undiagnosed infection, and/or genetic susceptibility. Fourth, admission SIRS criteria may be influenced by pre-hospital treatment, such as sedative medications and fluid resuscitation, which were unable to be included in the current analysis. Lastly, for the adjudication of daily SIRS criteria, we defined patients as having individual SIRS criteria by looking at the cross-sectional data at any time point, which may capture patients who only had SIRS criteria transiently.
Conclusion
In summary, early SIRS is common among patients with moderate-severe TBI, and the presence of SIRS criteria on admission is associated with systolic cardiac dysfunction following TBI. Our findings can be considered hypothesis-generating, and future research should focus on the underlying mechanisms and impact of systolic dysfunction, as well as the effect of systemic inflammation and outcomes following TBI.
Significant statement.
Early systolic cardiac dysfunction following traumatic brain injury (TBI) can result in hypotension and inadequate cerebral perfusion. The findings from our study suggest multifactorial mechanisms of the development of early systolic dysfunction following TBI. Our findings can be considered hypothesis-generating, and future research should focus on the underlying mechanisms and impact of systolic dysfunction, as well as the effect of systemic inflammation and outcomes following TBI. Recognition of systolic dysfunction and knowledge of the underlying mechanism will inform optimal hemodynamic support to maintain adequate cerebral blood flow to the injured brain and improve outcomes in TBI.
Acknowledgments
We gratefully acknowledge the support from the Department of Anesthesiology and Pain Medicine, University of Washington and Harborview Injury Prevention and Research Center.
Financial support: No institutional or department funds were used for this study.
Role of Authors: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: NC, VK, MSV. Acquisition of data: NC, VK. Analysis and interpretation of data: NC, VK, QQ. Drafting of the manuscript: NC, VK, AVL, MSV. Critical revision of the manuscript for important intellectual content: NC, VK, AVL, MSV. Statistical analysis: NC, VK, QQ. Obtained funding: None. Administrative, technical, and material support: NC, VK. Study supervision: MSV.”
Footnotes
Name of Associate Editor: Dr. Regino Perez-Polo
Conflict of interest: The authors declare no conflict of interest.
DATA ACCESSIBILITY
Data can be provided per e-mail upon request.
Systolic dysfunction was recently described following traumatic brain injury (TBI) and systemic inflammation may be a contributing mechanism. From this study, early systemic inflammatory response syndrome (SIRS) is common among patients with moderate-severe TBI and the presence of SIRS criteria on admission is associated with systolic cardiac dysfunction following TBI.
References
- Arand M, Melzner H, Kinzl L, Bruckner UB, Gebhard F. Early inflammatory mediator response following isolated traumatic brain injury and other major trauma in humans. Langenbecks Arch Surg. 2001;386(4):241–248. doi: 10.1007/s004230100204. [DOI] [PubMed] [Google Scholar]
- Audebert HJ, Rott MM, Eck T, Haberl RL. Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke. 2004;35(9):2128–2133. doi: 10.1161/01.STR.0000137607.61697.77. [DOI] [PubMed] [Google Scholar]
- Boehme AK, Kapoor N, Albright KC, Lyerly MJ, Rawal PV, Bavarsad Shahripour R, Alvi M, Houston JT, Sisson A, Beasley TM, Alexandrov AW, Alexandrov AV, Miller DW. Systemic inflammatory response syndrome in tissue-type plasminogen activator-treated patients is associated with worse short-term functional outcome. Stroke. 2013;44(8):2321–2323. doi: 10.1161/STROKEAHA.113.001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissier F, Razazi K, Seemann A, Bedet A, Thille AW, de Prost N, Lim P, Brun-Buisson C, Mekontso Dessap A. Left ventricular systolic dysfunction during septic shock: the role of loading conditions. Intensive care medicine. 2017 doi: 10.1007/s00134-017-4698-z. [DOI] [PubMed] [Google Scholar]
- Bone RC. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: what we do and do not know about cytokine regulation. Crit Care Med. 1996;24(1):163–172. doi: 10.1097/00003246-199601000-00026. [DOI] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008;118(4):397–409. doi: 10.1161/CIRCULATIONAHA.106.677625. [DOI] [PubMed] [Google Scholar]
- Cheah CF, Kofler M, Schiefecker AJ, Beer R, Klug G, Pfausler B, Helbok R. Takotsubo Cardiomyopathy in Traumatic Brain Injury Neurocritical care. 2016 doi: 10.1007/s12028-016-0334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Chiaretti A, Genovese O, Aloe L, Antonelli A, Piastra M, Polidori G, Di Rocco C. Interleukin 1beta and interleukin 6 relationship with paediatric head trauma severity and outcome. Childs Nerv Syst. 2005;21(3):185–193. doi: 10.1007/s00381-004-1032-1. discussion 194. [DOI] [PubMed] [Google Scholar]
- Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: Myocardial depression in sepsis and septic shock. Critical care. 2002;6(6):500–508. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrifai AM, Bailes JE, Shih SR, Dianzumba S, Brillman J. Characterization of the cardiac effects of acute subarachnoid hemorrhage in dogs. Stroke. 1996;27(4):737–741. doi: 10.1161/01.str.27.4.737. discussion 741–732. [DOI] [PubMed] [Google Scholar]
- Febinger HY, Thomasy HE, Pavlova MN, Ringgold KM, Barf PR, George AM, Grillo JN, Bachstetter AD, Garcia JA, Cardona AE, Opp MR, Gemma C. Time-dependent effects of CX3CR1 in a mouse model of mild traumatic brain injury. J Neuroinflammation. 2015;12:154. doi: 10.1186/s12974-015-0386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Huber-Lang MS, Sarma JV, Ward PA. Molecular events in the cardiomyopathy of sepsis. Molecular medicine. 2008;14(5–26):327–336. doi: 10.2119/2007-00130.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn A, Chokkalingam Mani B, Mather PJ. Sepsis-induced cardiomyopathy: a review of pathophysiologic mechanisms. Heart failure reviews. 2010;15(6):605–611. doi: 10.1007/s10741-010-9176-4. [DOI] [PubMed] [Google Scholar]
- Giannoudis PV, Smith RM, Banks RE, Windsor AC, Dickson RA, Guillou PJ. Stimulation of inflammatory markers after blunt trauma. The British journal of surgery. 1998;85(7):986–990. doi: 10.1046/j.1365-2168.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- Helmy A, Carpenter KL, Menon DK, Pickard JD, Hutchinson PJ. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab. 2011;31(2):658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstadt A, Meroz Y, Landesberg G. Myocardial dysfunction in severe sepsis and septic shock: more questions than answers? Journal of cardiothoracic and vascular anesthesia. 2011;25(3):526–535. doi: 10.1053/j.jvca.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Holmin S, Hojeberg B. In situ detection of intracerebral cytokine expression after human brain contusion. Neurosci Lett. 2004;369(2):108–114. doi: 10.1016/j.neulet.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Horton JW. A model of myocardial inflammation and dysfunction in burn complicated by sepsis. Shock. 2007;28(3):326–333. doi: 10.1097/01.shk.0000238064.54332.c8. [DOI] [PubMed] [Google Scholar]
- Huang SJ, Nalos M, McLean AS. Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? A meta-analysis. Critical care. 2013;17(3):R96. doi: 10.1186/cc12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. Neuro Rehabilitation. 2007;22(5):341–353. [PubMed] [Google Scholar]
- Ichijo T, Katafuchi T, Hori T. Central interleukin-1 beta enhances splenic sympathetic nerve activity in rats. Brain Res Bull. 1994;34(6):547–553. doi: 10.1016/0361-9230(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Jeremitsky E, Omert L, Dunham CM, Protetch J, Rodriguez A. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J Trauma. 2003;54(2):312–319. doi: 10.1097/01.TA.0000037876.37236.D6. [DOI] [PubMed] [Google Scholar]
- Kadhim HJ, Duchateau J, Sebire G. Cytokines and brain injury: invited review. J Intensive Care Med. 2008;23(4):236–249. doi: 10.1177/0885066608318458. [DOI] [PubMed] [Google Scholar]
- Kossmann T, Hans VH, Imhof HG, Stocker R, Grob P, Trentz O, Morganti-Kossmann C. Intrathecal and serum interleukin-6 and the acute-phase response in patients with severe traumatic brain injuries. Shock. 1995;4(5):311–317. doi: 10.1097/00024382-199511000-00001. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Rowhani-Rahbar A, Chaikittisilpa N, Gibbons EF, Rivara FP, Temkin NR, Quistberg A, Vavilala MS. Association of Early Hemodynamic Profile and the Development of Systolic Dysfunction Following Traumatic Brain Injury. Neurocritical care. 2016 doi: 10.1007/s12028-016-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushi H, Saito T, Makino K, Hayashi N. IL-8 is a key mediator of neuroinflammation in severe traumatic brain injuries. Acta Neurochir Suppl. 2003;86:347–350. doi: 10.1007/978-3-7091-0651-8_74. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W, American Society of Echocardiography’s N, Standards C, Task Force on Chamber Q, American College of Cardiology Echocardiography C, American Heart A, European Association of Echocardiography ESoC Recommendations for chamber quantification. European journal of echocardiography: the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2006;7(2):79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Li Y, Ge S, Peng Y, Chen X. Inflammation and cardiac dysfunction during sepsis, muscular dystrophy, and myocarditis. Burns & trauma. 2013;1(3):109–121. doi: 10.4103/2321-3868.123072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou M, Chen X, Wang K, Xue Y, Cui D, Xue F. Increased intracranial pressure is associated with the development of acute lung injury following severe traumatic brain injury. Clin Neurol Neurosurg. 2013;115(7):904–908. doi: 10.1016/j.clineuro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Lu J, Goh SJ, Tng PY, Deng YY, Ling EA, Moochhala S. Systemic inflammatory response following acute traumatic brain injury. Frontiers in bioscience. 2009;14:3795–3813. doi: 10.2741/3489. [DOI] [PubMed] [Google Scholar]
- Marshall JC. SIRS and MODS: what is their relevance to the science and practice of intensive care? Shock. 2000;14(6):586–589. [PubMed] [Google Scholar]
- Mascia L, Sakr Y, Pasero D, Payen D, Reinhart K, Vincent JL, Sepsis Occurrence in Acutely Ill Patients I Extracranial complications in patients with acute brain injury: a post-hoc analysis of the SOAP study. Intensive care medicine. 2008;34(4):720–727. doi: 10.1007/s00134-007-0974-7. [DOI] [PubMed] [Google Scholar]
- Masuda T, Sato K, Yamamoto S, Matsuyama N, Shimohama T, Matsunaga A, Obuchi S, Shiba Y, Shimizu S, Izumi T. Sympathetic nervous activity and myocardial damage immediately after subarachnoid hemorrhage in a unique animal model. Stroke. 2002;33(6):1671–1676. doi: 10.1161/01.str.0000016327.74392.02. [DOI] [PubMed] [Google Scholar]
- Mazzeo AT, Micalizzi A, Mascia L, Scicolone A, Siracusano L. Brain-heart crosstalk: the many faces of stress-related cardiomyopathy syndromes in anaesthesia and intensive care. Br J Anaesth. 2014;112(5):803–815. doi: 10.1093/bja/aeu046. [DOI] [PubMed] [Google Scholar]
- McKeating EG, Andrews PJ, Signorini DF, Mascia L. Transcranial cytokine gradients in patients requiring intensive care after acute brain injury. Br J Anaesth. 1997;78(5):520–523. doi: 10.1093/bja/78.5.520. [DOI] [PubMed] [Google Scholar]
- Mica L, Furrer E, Keel M, Trentz O. Predictive ability of the ISS, NISS, and APACHE II score for SIRS and sepsis in polytrauma patients. Eur J Trauma Emerg Surg. 2012;38(6):665–671. doi: 10.1007/s00068-012-0227-5. [DOI] [PubMed] [Google Scholar]
- Mierzewska-Schmidt M, Gawecka A. Neurogenic stunned myocardium - do we consider this diagnosis in patients with acute central nervous system injury and acute heart failure? Anaesthesiology intensive therapy. 2015;47(2):175–180. doi: 10.5603/AIT.2015.0017. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossman MC, Lenzlinger PM, Hans V, Stahel P, Csuka E, Ammann E, Stocker R, Trentz O, Kossmann T. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Molecular psychiatry. 1997;2(2):133–136. doi: 10.1038/sj.mp.4000227. [DOI] [PubMed] [Google Scholar]
- Nguyen H, Zaroff JG. Neurogenic stunned myocardium. Current neurology and neuroscience reports. 2009;9(6):486–491. doi: 10.1007/s11910-009-0071-0. [DOI] [PubMed] [Google Scholar]
- Niederbichler AD, Westfall MV, Su GL, Donnerberg J, Usman A, Vogt PM, Ipaktchi KR, Arbabi S, Wang SC, Hemmila MR. Cardiomyocyte function after burn injury and lipopolysaccharide exposure: single-cell contraction analysis and cytokine secretion profile. Shock. 2006;25(2):176–183. doi: 10.1097/01.shk.0000192123.91166.e1. [DOI] [PubMed] [Google Scholar]
- Prathep S, Sharma D, Hallman M, Joffe A, Krishnamoorthy V, Mackensen GB, Vavilala MS. Preliminary report on cardiac dysfunction after isolated traumatic brain injury. Crit Care Med. 2014;42(1):142–147. doi: 10.1097/CCM.0b013e318298a890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido JN, Afessa B, Masaki M, Yuasa T, Gillespie S, Herasevich V, Brown DR, Oh JK. Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clinic proceedings. 2012;87(7):620–628. doi: 10.1016/j.mayocp.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian R, Yang W, Wang X, Xu Z, Liu X, Sun B. Evaluation of cerebral-cardiac syndrome using echocardiography in a canine model of acute traumatic brain injury. American journal of cardiovascular disease. 2015;5(1):72–76. [PMC free article] [PubMed] [Google Scholar]
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive care medicine. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- Ross SA, Halliday MI, Campbell GC, Byrnes DP, Rowlands BJ. The presence of tumour necrosis factor in CSF and plasma after severe head injury. Br J Neurosurg. 1994;8(4):419–425. doi: 10.3109/02688699408995109. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23(12):618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- Samuels MA. The brain-heart connection. Circulation. 2007;116(1):77–84. doi: 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
- Severn A, Rapson NT, Hunter CA, Liew FY. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992;148(11):3441–3445. [PubMed] [Google Scholar]
- Shiozaki T, Hayakata T, Tasaki O, Hosotubo H, Fuijita K, Mouri T, Tajima G, Kajino K, Nakae H, Tanaka H, Shimazu T, Sugimoto H. Cerebrospinal fluid concentrations of anti-inflammatory mediators in early-phase severe traumatic brain injury. Shock. 2005;23(5):406–410. doi: 10.1097/01.shk.0000161385.62758.24. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med. 1999;27(7):1262–1264. doi: 10.1097/00003246-199907000-00005. [DOI] [PubMed] [Google Scholar]
- van Diepen S, Vavalle JP, Newby LK, Clare R, Pieper KS, Ezekowitz JA, Hochman JS, Mahaffey KW, Armstrong PW, Granger CB. The systemic inflammatory response syndrome in patients with ST-segment elevation myocardial infarction. Crit Care Med. 2013;41(9):2080–2087. doi: 10.1097/CCM.0b013e31828a67b2. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Carlos TM, Kochanek PM, Wisniewski SR, Bell MJ, Clark RS, DeKosky ST, Marion DW, Adelson PD. Interleukin-8 is increased in cerebrospinal fluid of children with severe head injury. Crit Care Med. 2000;28(4):929–934. doi: 10.1097/00003246-200004000-00003. [DOI] [PubMed] [Google Scholar]
- Winter CD, Pringle AK, Clough GF, Church MK. Raised parenchymal interleukin-6 levels correlate with improved outcome after traumatic brain injury. Brain. 2004;127(Pt 2):315–320. doi: 10.1093/brain/awh039. [DOI] [PubMed] [Google Scholar]
- Woiciechowsky C, Asadullah K, Nestler D, Eberhardt B, Platzer C, Schoning B, Glockner F, Lanksch WR, Volk HD, Docke WD. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med. 1998;4(7):808–813. doi: 10.1038/nm0798-808. [DOI] [PubMed] [Google Scholar]
- Woiciechowsky C, Schoning B, Cobanov J, Lanksch WR, Volk HD, Docke WD. Early IL-6 plasma concentrations correlate with severity of brain injury and pneumonia in brain–injured patients. J Trauma. 2002;52(2):339–345. doi: 10.1097/00005373-200202000-00021. [DOI] [PubMed] [Google Scholar]
- Yoshimoto Y, Tanaka Y, Hoya K. Acute systemic inflammatory response syndrome in subarachnoid hemorrhage. Stroke. 2001;32(9):1989–1993. doi: 10.1161/hs0901.095646. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang HY, Bassel-Duby R, Maass DL, Johnston WE, Horton JW, Tao W. Role of interleukin-6 in cardiac inflammation and dysfunction after burn complicated by sepsis. American journal of physiology Heart and circulatory physiology. 2007;292(5):H2408–2416. doi: 10.1152/ajpheart.01150.2006. [DOI] [PubMed] [Google Scholar]


