Abstract
Osteoporosis has become a major medical problem as the aged population of the world rapidly grows. Osteoporosis predisposes patients to fracture, progressive spinal deformities, and stenosis, and is subject to be a major concern before performing spine surgery, especially with bone fusions and instrumentation. Osteoporosis has often been considered a contraindication for spinal surgery, while in some instances patients have undergone limited and inadequate procedures in order to avoid concomitant instrumentation. As the population ages and the expectations of older patients increase, the demand for surgical treatment in older patients with osteoporosis and spinal degenerative diseases becomes progressively more important. Nowadays, advances in surgical and anesthetic technology make it possible to operate successfully on elderly patients who no longer accept disabling physical conditions. This article discusses the biomechanics of the osteoporotic spine, the diagnosis and management of osteoporotic patients with spinal conditions, as well as the novel treatments, recommendations, surgical indications, strategies and instrumentation in patients with osteoporosis who need spine operations.
Keywords: Degenerative scoliosis, Degenerative spondylolisthesis, Fracture, Instrumentation, Osteoporosis, Stenosis
Introduction
Osteoporosis is a skeletal disorder characterized by compromised bone mass accompanied by micro architectural deterioration of bone tissue predisposing a person to an increased risk of fracture (1). Bone strength is based on bone mineral density (BMD) and other factors, such as remodeling frequency (bone turnover), bone size and area, bone microarchitecture, and degree of bone mineralization. According to the World Health Organization, patients are considered osteoporotic when they have a BMD measurement that is 2.5 standard deviations below the typical bone mass of young healthy white woman.
The cellular mechanism of osteoporosis is multifactorial. The cumulative effect of normal aging, the onset of menopause, dietary calcium deficiency, and progressive inactivity are the upregulation of bone resorption and down regulation of bone formation. While it is commonly held that these effects are mediated by stimulation of osteoclasts and inhibition of osteoblasts, the exact mechanisms by which they lead to age-related bone loss is still not well understood (2).
With the rapid growth of the global elderly population, osteoporosis has become one of the most prevalent public-health concerns and a major medical problem for these individuals. The age of patients needing spine surgery is also increasing steadily as the average lifespan increases. Therefore, the number of spine operations in osteoporotic elderly patients is on the rise (3).
Osteoporosis predisposes elderly patients to progressive spinal deformities and potential neurologic compromise, and is subject to be a major concern before performing spine surgery (4). Surgical treatment of osteoporosis is still not widely accepted by orthopedic surgeons. Patients with osteoporosis are difficult to treat surgically because of advanced age and associated risks of anesthesia, making them poor candidates for surgery (4, 5). Furthermore, osteoporosis can complicate spine surgery, especially interventions with bone fusions and instrumentation.
However, spinal surgery in elderly patients with osteoporosis has been gaining support. The recent literature demonstrates that the survival rate after spine surgery for spinal stenosis in the elderly is comparable to the rate after joint replacement surgery (6, 7). Furthermore, the outcomes of surgical treatment are more favorable than conservative treatment, and patients who undergo spine surgery have a better survival rate than a matched general population in each group (8). The advancement of surgical and anesthetic knowledge and technology allows the use of more sophisticated instrumentation and makes it possible to operate successfully on high-risk patients of advanced age who no longer accept disabling physical conditions (5).
Biomechanics of the osteoporotic spine
The human spine has an extensive range of motion and considerable load-carrying capacity required for the physical activities of daily life. The vertebral body and intervertebral disc sustain approximately 80% of the load during axial compression, with the remaining 20% sustained by the facet joints (8). With increasing age, alterations to the form and composition of the individual structures of the spine can increase the risk of injury and can have a profound influence on the quality of life (9).
The architecture of a vertebral body is comprised of porous trabecular bone and dense and solid cortex. Bone density varies between sex, between individuals, between spinal levels, but also as a function of age. Starting in the fourth decade of life, elderly men can lose up to 30% and elderly women up to 50% of bone density (10). Osteoporosis weakens the structural strength of bone to an extent that normal daily activity can exceed the vertebra’s ability to carry this load. Decreased structural strength is not only the result of reduced apparent bone density, but is also due to changes in bone architecture, bone remodeling, and repair rate, resulting in faster damage accumulation for continuous cyclic loading. The increase in bone fragility is due to the replacement of normal trabecular structures by thinner and more open spicules. The more porous appearance of cancellous bone is the result of reduced horizontal cross-linking struts, further reducing the buckling strength of vertically oriented trabeculae (9).
Vertebrae are the most commonly fractured bones among elderly people with osteoporosis. The type of vertebral fracture is related to BMD loss and the pattern of loading, but is also influenced by the position of the spine at the time of injury (2). In osteoporotic vertebrae, the load-bearing capacity of the body changes, since it loses bone faster from trabeculae than from the cortex. Vertebral body trabeculae tend to be denser and in the posterior aspect of the vertebral body compared with the anterior. Likewise, trabeculae are denser in the inferior half compared with the superior half, possibly because they are reinforced by trabecular arcades from the pedicles. The typical osteoporotic vertebral fracture leads to a height loss in the anterior vertebral body, often leaving the posterior vertebral wall intact. This wedge-shaped deformity of a single fractured vertebra usually leads to a local increase in kyphosis.
Fracture risk of adjacent levels has shown to have a fivefold increased fracture risk compared to normal vertebrae, leading to multiple vertebral fractures or “vertebral fracture cascade” (11). Multiple adjacent vertebral fractures lead to a progressive kyphotic deformity with sagittal imbalance and postural disfigurement. A single anterior wedge fracture can increase thoracic kyphosis by 10° or more, and thoracic curves exceeding 70° are common in elderly subjects with multilevel compression fractures (12).
Although the Scoliosis Research Society established that normal thoracic kyphosis can vary from 20º to 40º (measured between T5 and T12), there is an increased range of variability for what can be considered as normal sagittal balance among asymptomatic individuals (13). Normal spinal balance dictates that a weight-bearing plumb line dropped from the base of the occiput should fall through the C7 vertebral body, T12-L1 junction, and caudally within or just anterior to the sacral (S1) promontory. This facilitates even distribution of compressive loads to each of the vertebrae in the spinal column (2). In the presence of osteoporosis, the physiologic thoracic kyphosis and increased stresses at the anterior margins of the thoracic vertebral bodies produce prominent wedging of the mid-thoracic vertebrae, subsequently exacerbating hyperkyphosis by anterior translation of the head and upper torso (12).
The lumbar spine is normally lordotic. If lordosis is maintained at the time of fracture, loads are mainly concentrated within the center of the lumbar endplate. Although anterior wedge compression fractures can occur in this region, fractures more commonly exhibit uniform compression or central (biconcave) types (2, 14).
Prevalence of osteoporosis in patients requiring spine surgery
The aging population is growing at an exponential rate. It has been estimated that up to 50% of this population will require basic nursing care or assistance with activities of daily living. Furthermore, the number of disabled years for these individuals is growing in step with their increasing life expectancy (15).
The older patient has unique characteristics that require differentiation from that of the archetypical adult, such as atypical presentation and response to disease and frailty from comorbidities and chronic disease (15-17).
Patients who need spine operations often have osteoporosis. The number of spine operations in elderly patients is increasing, and so is the incidence of osteoporosis in spine-surgery patients. There are studies on the relationship between degenerative diseases of the lumbar spine in elderly people and lumbar spine BMD. Degenerative diseases of the lumbar spine may have an effect on lumbar spine BMD. Patients with the most severe spinal pathology may have the most severe osteoporosis and are reported to lose bone at a greater than average rate (18). However, most studies indicate that spinal arthropathy and disc degeneration are associated with increased BMD at spinal and appendicular sites. Osteophyte formation, disc-space narrowing, bone sclerosis, spondylolisthesis, and vertebral fractures are associated with increased spinal bone BMD (19, 20). The association between BMD and spinal problems appears to be rather complex, and this discrepancy indicates that degenerative spinal diseases may be associated with increased lumbar spine BMD measurements. Therefore, femoral neck or distal radius BMD may be more appropriate to evaluate for osteoporosis (21, 22).
The age of patients needing spine surgery is slowly increasing because of an increase in the average lifespan. The number of spine surgery patients in their 6th, 7th, or 8th decade of life is growing faster than in other age groups. Chin et al. retrospectively investigated the incidence of osteoporosis in a cohort of 1321 patients who had undergone spine operations (23). The age-adjusted prevalence of osteoporosis for female patients over 50 who had undergone a spine operation was 25.4%, 58.7%, 72.2%, and 86.6% in the 50-59, 60-69, 70-79, and over-80 age groups, respectively. They found a 51.3% rate of osteoporosis for female patients over 50 years of age who had undergone a spine operation, which is higher than that of other reports on osteoporosis in women over 50 years from the general population.
The prevalence of osteoporosis and osteopenia among spinal patients has also been investigated. The prevalence of increased bone fragility was highest in the group made up of female patients with degenerative spondylolisthesis and the group of males with spondylotic stenosis. However, vertebral compression fracture is the most prevalent condition in osteoporotic patients (24).
Instrumentation for the osteoporotic spine
Osteoporosis is subject to be a major concern before performing spine surgery since surgical correction of such patients is difficult. The lack of mineral in the bone and the very porous trabecular bone offer poor pull-out strength. Also, the pedicles widen analogous to the widening of the femur with advanced osteoporosis (4). All that is left are the cortices, with almost no trabecular bone available, offering poor purchase for the instrumentation (25). If we ascertain the existence of osteoporosis before spine surgery, we can choose the most effective spine operation which has the lowest complication rate for osteoporotic spine patients.
In the presence of osteoporosis, the decreased mineral properties of the bone increase the risk of fixation failure. De Wald and Stanley reviewed the postoperative complications observed in a group of 38 patients over the age of 65 years old who had undergone five-level fusions (26). Early complications included pedicle fractures and compression fractures, and the most common late complications were pseud-arthrosis with instrumentation failure and adjacent-level disc degeneration with herniation. Decreased fixation strength in the bone of osteoporotic patients with low BMD leads to an increased incidence of instrument failure. Osteoporosis also decreases the likelihood of successful fusion in these patients. As a consequence, instrumentation that is designed to provide temporary support experiences more prolonged stress loading, thereby increasing the probability of delayed fusion and pseudarthrosis. In the case of posterior fixation with screws, failure is a result of screw pull-out and loosening. Anterior instrumentation failure is secondary to continuous cyclic loading, leading to cutout and subsidence of interbody devices into the osteoporotic bone (27-32).
Many solutions have been proposed to improve the fixation strength of instrumentation and reduce the risk of failure. These include the use of multiple fixation points and a combination of fixation types, screw augmentation with poly methyl methacrylate (PMMA) expandable screws, hydroxyapatite-coated screws, larger diameter screws with bicortical purchase, obtaining adequate sagittal balance, and accepting lesser degrees of coronal correction (17, 27-30, 33-49).
Multiple levels and points of fixation. Combination of fixation types
The use of multiple levels of fixation to dissipate stress and distribute force by increasing the number of fixation points is often considered as a means of improving stability during instrumentation of the osteoporotic spine. Multiple points of fixation can be obtained with segmental constructs, laminar hooks, sub-laminar wires, and, most commonly, pedicle screws (4).
Hooks or wires constructs generally require longer constructs than pedicle screws to provide adequate fixation (50). Current literature provides evidence that supports the use of a combination of fixation types to distribute the stresses on the osteoporotic bone. A combination of pedicle screws and laminar hooks, also known as pediculolaminar fixation, will provide the greatest resistance to pull-out forces. Concurrent use of hooks and pedicle screws can increase pull-out strength up to 100%. Biomechanical studies have shown that laminar hooks and sub-laminar wires used in conjunction with pedicle screws can increase pull-out strength, stiffness, and torsional stability in osteoporotic bone (28-30, 38, 40, 51, 52).
Posterior thoracolumbar instrumentation failure has been shown to correlate with BMD. Coe et al. studied the posterior spine implants modes of failure in osteoporotic thoracic spines (29). Wire fixation failed by cutting through the bone posteriorly. Sub-laminar hooks typically failed by pulling through the lamina posteriorly, although 30% of the failures occurred at the pedicle or the pedicle-body junction. Pedicle screws typically failed by screw pull-out. Overall, sub-laminar hooks showed superior stability compared with wire or pedicle screw constructs (38, 40, 52). These findings were supported by Butler et al. who reported that the performance of hook fixation in the thoracic spine was not adversely affected by osteoporosis (28).
De Wald and Stanley recommended that regardless of the instrumentation used, at least 3 fixation points superior and 3 fixation points inferior to the apex of the spinal deformity should be used to ensure a balanced construct (26). In addition, the level at which to complete the construct is especially critical in osteoporotic patients. It is important that the instrumentation does not end in a kyphotic segment, because progressive sagittal imbalance is a common complication in instrumented osteoporotic spinal deformities, leading to instrumentation failure and adjacent-level fracture. Consequently, instrumented fusion should extend beyond the apex of any deformity and caudal to the thoracic kyphosis (4). Ending the cephalad portion of lumbar constructs at L1 should be avoided. Rather, T10 should be used to avoid kyphotic collapse at the thoracolumbar transition zone (53). Similar issues arise at the caudal side, with lumbar kyphosis and constructs ending at the lumbosacral region. Bilateral iliac screws and bi-cortical sacral screws can be used as additional fixation points (54). Kwon et al. suggested that extension of the fusion to the sacrum or ilium can reduce the potential high risk of failure for constructs ending with pedicle screws at L5 (55). However, the inclusion of additional proximal or caudal levels of fixation must be weighed against the morbidity associated with an extended instrumentation.
Posterior instrumentation. Pedicle screw, techniques and designs
Pedicle screws provide 3 column fixations, and are the most commonly used device with which to achieve posterior fixation in the lumbar and thoracic spine. However, their use is controversial in patients with osteoporosis, since studies have demonstrated that insertional torque, pull-out strength, and fatigue failure are linearly related with BMD, so the weakest link in fixation of the osteoporotic spine is the bone-implant interface (53). In addition, damage caused by such pullouts can complicate revision surgeries and make them more challenging (4). As a result, several new designs have been developed in an effort to improve pedicle-screw fixation in the osteoporotic spine.
Bi-cortical purchase. Increasing screw length does increase screw pull-out strength. The cortex of the vertebral body is stronger than the cancellous bone, so bi-cortical purchase is stronger than uni-cortical-cancellous purchase. Multiple biomechanical studies have demonstrated that bi-cortical purchase of pedicle screws can improve pull-out strength from 20% to 50%, depending on the patient’s vertebrae and the screw dimensions and screw type (56).
Although bi-cortical purchase of pedicle screws can increase fixation in the lumbar, thoracic, and sacral spine, the technique is usually used only at the S1 level because of the potential damage to the aorta and vena cava when used in the lumbar or thoracic spine. However, the use of anteromedial bi-cortical sacral screws also increases the risk of damage to anterior structures like the L5 nerve root, colon, iliac and middle sacral arteries and veins, and sacral sympathetic trunk (57, 58). An alternative technique to the anteromedial approach is to angle a bi-cortical S1 pedicle screw superiorly through the S1 end plate. Luk et al. in a cadaveric biomechanical study, found that bi-cortical screws through the S1 end plate afforded significantly stronger insertion torque and pull-out strength after cyclic loading than did the anteromedially directed bi-cortical screws (59). In addition, this approach avoids the possibility of damaging the neurovascular and visceral structures.
Increasing screw diameter will also increase pull-out strength; however, the dimensions of the instrumented pedicle limits the screw diameter. In the osteoporotic spine, when the screw diameter exceeds 70% of the pedicle diameter, a high risk of pedicle fracture has been reported (60).
Coating. The bone-screw interface may also be improved by hydroxyapatite-coated screws. Improved pedicle screw-bone contact, bone ingrowth and mineralization, and increased screw pull-out strength are the reported advantages of coated pedicle screws (61).
Animal model studies have demonstrated that the pull-out strength and extraction torque of hydroxyapatite-coated screws is significantly greater than that of uncoated screws (62). However, hydroxyapatite coating is not without its disadvantages. Bone-screw interface integration is not expected to happen immediately, so primary stability does not differ much from that of standard screws (33). A non-blinded, prospective, randomized trial compared hydroxyapatite-coated with uncoated pedicle screws and found that, once implanted, the coated screws were difficult to remove even when necessary, such as when infected, requiring significantly more torque for removal during revision surgery than uncoated screws (63).
Expandable screws. Pedicle screws can expand after positioning in the pedicle, providing better fixation in weakened osteoporotic bone and reducing the risk of screw pull-out (4). One biomechanical cadaver study evaluated their use as a revision screw and showed that they had more pull-out strength than an initial pedicle screw but less than that of a PMMA-augmented screw (64). One concern with such implants is that the increasing screw diameter could fracture the pedicle, placing the adjacent nerve root at risk (65, 66).
Cement augmentation of screws. Poly methyl methacrylate augmentation procedures can be achieved through the use of distally fenestrated pedicle screws specifically designed for cement injection. Once PMMA has been extruded though the screw holes, it sets, due to polymerization, creating a continuous mass between the core of the screw and the cancellous bone in the vertebral body. As a result, cement gives immediate restoration of strength and stiffness, and significantly increases pull-out strength in osteoporotic vertebrae compared with non-augmented low-to-normal BMD vertebrae levels (53, 67). Several biomechanical studies have indicated that augmenting pedicle screws with PMMA or other cements can significantly increase screw axial pull-out strength (by 119%-250%) for primary and revision surgeries. These studies have also shown that PMMA augmentation increases mean stiffness, energy absorbed to failure, and initial fixation and fatigue strength of pedicle screws (34, 68).
Distal fenestration allows delivery of the entire volume of cement into the vertebral body around the distal third of the screw, far ventral to the neuro central canal. This distal concentration of cement for screw augmentation promotes a higher force to failure and diminishes the risk of cement extrusion into the spinal canal through an unrecognized pedicle breach (33, 35, 39, 69-79).
Amendola et al. published a study of 21 patients who had a poor bone stock condition due to osteoporosis or tumor and who underwent posterior stabilization by fenestrated pedicle screws and PMMA augmentation (33). All patients were clinically and radiographically followed up for a mean of 36 months. No case of loosening was recorded after a mean follow-up of 36 months. The only clinical complication strictly related to PMMA screw augmentation did not require further surgery. Chang et al. reviewed 291 PMMA-augmented pedicle screws in 41 osteoporotic patients with a median of 22-months of follow-up (35). No screw migration or loosening was reported in the series, and all cement leakages were asymptomatic (Table 1).
Table 1.
Summary of reports of surgical treatment for degenerative lumbar disease using pedicle screws augmented with PMMA
| Author | Year of publication | Number of patients/ Mean age | Mean follow-up months | Diagnosis | Augmentation technique | Cement leakage rate | Complications | Reinterventions |
|---|---|---|---|---|---|---|---|---|
| Singh et al. | 2005 | 9/76.1 years | 11.2 months | Osteoporotic fracture and sponylolisthesis 9 | Through biopsy needle | 22.2% | 1 Screw migration 1 Nonunion fracture 4 AVCFx |
1 cement extravasation removed- laminectomy |
| Frankel et al. | 2007 | 23/ | 21 months | Osteoporotic fracture 8 Sponylolisthesis 5 Spinal malignancy 6 Revision surgery 4 | Through biopsy needle | 39.1% | 1 asymptomatic PMMA pulmonary embolus 2 Superficial wound infection |
None |
| Chang et al. | 2008 | 41/75.1 years | 22.3 months | Osteoporotic fracture 32 Spinal stenosis 5 Spinal malignancy 4 | Through biopsy needle | 26.2% | 1 Stroke 6th day postop. 2 Deep wound infection 1 AVCFx |
None |
| Kim et al. | 2008 | 20/60.5 years | 15 months | Osteoporotic fracture 20 | Through biopsy needle | None | None | 1 Seroma. Debridement and secondary suture |
| Aydogan et al. | 2009 | 36/66 years | 37 months | Osteoporotic fracture 6 Spinal stenosis 26 Sponylolisthesis 3 Spinal malignancy 1 | Through biopsy needle | None | 4 Superficial wound infection | None |
| Moon et al. | 2009 | 37/68.7 years | 33.3 months | Degenerat. spondylolisthesis 6 Spondylolit. spondylolisthesis 5 Spondylotic stenosis 26 | Fenestrated pedicle screws | 5.4% | 1 Pedicle screw loosening | 2 Dural tear repair |
| Hu et al. | 2011 | Group A: 25/73 years Group B: 23/74.6 years | Group A: 16.5 m Group B: 15.9 m | Osteoporotic fracture 31 Sponylolisthesis + stenosis 11 Spinal malignancy 6 | Group A: TBN Group B: FPS | Group A: 18.3% Group B: 13.6% | Group B: 1 post-operative sciatica | None |
| Amendola et al. | 2011 | 21/67.2 years | 36.4 months | Osteoporotic fracture 4 Degenerative disease 2 Spinal malignancy 10 Revision surgery 5 | Fenestrated pedicle screws | 23.8% | 1 nerve root palsy 2 Superficial wound infection 1 DVT 7th day postop. 1 Cauda equina syndrom |
1 PVCF |
| Xie et al. | 2011 | 14/63.14 years | 45.6 months | Degenerative scoliosis | Through biopsy needle | 14.3% | 1 Pneumonia | None |
| Piñera et al. | 2011 | 23/77 years | 32 months | Spinal stenosis 11 Degenerat. sponylolisthesis 12 | Fenestrated pedicle screws | 29.3% | 2 Bronchospasm 4 Adjacent disc degeneration 2 Urinary infection |
3 Debridement for delayed deep wound infection |
| Sawakami et al. | 2012 | 17/73.8 years | 33.6 months | Vertebral pseudarthrosis 17 | Pedicle screws covered with 1.0 mL PMMA | None | 2 Superficial wound infection 5 AVCFx |
2 Fusion extension |
| Zapatowicz et al. | 2012 | 17/69 years | 14.9 months | Osteoporotic fracture 11 Spondylodiscitis 1 Degenerat. sponylolisthesis 4 Spinal malignancy 1 | Through biopsy needle | 45% | 1 Pneumothorax 1 Intraoperative pedicle fracture |
1 Delayed spondylodiscitis reinfection. Removal of loose screws |
| Lubansu et al. | 2012 | 15/71.2 years | 13.3 months | Osteoporotic fracture 4 Degenerat. spondylolisthesis 5 Spinal/foraminal stenosis 6 | Percutaneous fenestrated pedicle screws | 30% | 1 Transient radiculitis due to screw misplacement 1 Superficial wound infection |
None |
TBN:Through biopsy needle; FPS:Fenestrated pedicle screws; AVCFx:Adjacent vertebral compression fracture; DVT:Deep venous thrombosis;
In the authors’ practice, PMMA-augmented pedicle screws have produced very satisfactory results. A previous study conducted by the authors investigated the clinical and radiological outcome of 23 osteoporotic patients (mean age 77 years) with degenerative lumbar instability (stenosis, spondylolisthesis) treated with fusion using fenestrated cemented pedicle screw instrumentation augmented with PMMA (103 screws of 58 cemented vertebrae) (72). Patients were followed for up to 49 months. Pain and function improved at 6 months and were maintained at the final follow-up. Average cement volume injected was 1.8 cc, with cement leakage observed in 29.3% of cemented vertebrae. No clinical complications secondary to PMMA leakage developed. Fusion signs were reviewed in reconstruction images of 6-month control computed tomography scans (CT scan), with no clinical or radiological cases of non-union observed. No fractures occurred in adjacent segments. There were 4 cases of adjacent disc disease. Three deep infections required surgical revision without removal of material and 1 superficial infection, all with complete remission. Widespread use of vertebraplasty has provided experience and consistent data about the low risks of cement leakage when done in a controlled fashion. Cannulated pedicular screws allow for screw augmentation once the screws are inserted, and precisely control the consistency, rhythm, and volume of the cement injected into each screw. Continuous fluoroscopy performed during cementation makes identification of the trabecular pattern of cementation possible and stops cementation at the beginning of cement leakage. The leakage rates found in our series (29.3%) were lower than the vertebroplasty results appearing in the literature (63% to 87%).
PMMA has traditionally been used to augment vertebral screws, but other reinforcing materials include calcium phosphate and injectable bioactive glass ceramic resins (4). Calcium phosphate cements have a compressive strength between that of cancellous and cortical bone but have a shear and tensile strength lower than that of cancellous bone. For this reason, although the use of calcium phosphate augmentation has been reported to increase resistance to screw pull-out, a comparison with PMMA shows that calcium phosphate screws produce a lower increase in pull-out strength (41, 48). Ceramic resins are another class of injectable filler material that have been investigated for vertebral augmentation. There are case reports where this material has been successfully used to augment screws in the distal radius and ankle (80, 81).
The most concerning issues of cement augmentation are probably cement leakage and exothermic reaction, which can lead to surrounding tissue damage with spinal cord and nerve compression. Other disadvantages are its inability to integrate into surrounding bone, and implant removal in case of infection, revision, or other problems.
In case of screw revision, screw extraction continues to be a clinical concern for surgeons considering cement augmentation in osteoporotic vertebrae. Choma et al. in a cadaveric study demonstrated that screws could be easily extracted after cement augmentation with failure occurring at the screw-cement interface in all cases (69). Cho et al. studied the torque required to back out the pedicle screws augmented with PMMA in an osteoporotic model (82). The results of their study showed that the torque required to remove the screws was generally higher than the insertion torque of the primary screws. However, the removal torque for screws augmented was <1N×m and did not cause any bony damage to the osteoporotic vertebrae, demonstrating that safe screw extraction is possible even after cement augmentation of a pedicle screw.
Inter-spinous process spacer
The inter-spinous process distraction device is a less invasive surgical alternative to traditional lumbar laminectomy for patients suffering from lumbar spinal stenosis. The device is implanted between adjacent spinous processes at the affected level reducing lumbar extension, thereby limiting the increase of the lateral recesses and central canal narrowing with lumbar extension [Figure 1]. Inter-spinous devices allow flexion, unrestricted axial rotation and lateral flexion, and preserves much of the anatomy of the lumbar spine. The operation is short and easy to perform, and can even be carried out in lateral decubitus. For some elderly patients with substantial comorbidities, this may be an additional advantage (83, 84).
Figure 1.
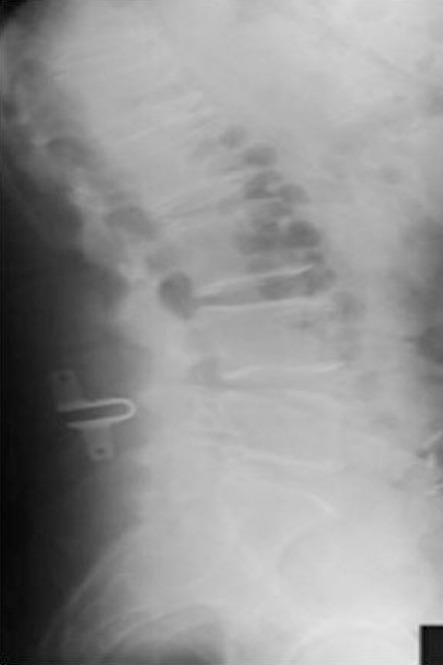
Postoperative lateral radiograph of a 71-year-old male after placement of an interspinous process distraction device at L4-L5. The interspinous process device is a less invasive surgical alternative to traditional lumbar laminectomy for patients suffering from lumbar spinal stenosis.
Kutcha et al. who studied 175 patients with LSS treated with the implantation of an interspinous device, found satisfactory outcomes in the short and long term (85). The visual analog scale score of leg pain was reduced from 6.0 preoperatively to 3.9 at 6 weeks and 3.9 at 2 years postoperatively; Oswestry scores were 32.6, 22.7, and 20.3, respectively. In 8 patients, however, the inter-spinous devices had to be removed and traditional decompression performed. The authors pointed out that the inter-spinous device does not replace microsurgical decompression in patients with massive stenosis and continuous claudication, but offers a safe, effective, and less invasive alternative in selected patients with spinal stenosis.
Efficacy of the inter-spinous device relies on the structural integrity and strength of the spinous process, which is designed to sustain tension as opposed to compression loading. Kim et al. in a study of occult spinous process fractures associated with inter-spinous process implantation, reported a rate of nondisplaced spinous process fractures of 28.9% (86). No patient had reported a traumatic incident. No fractures were identifiable on plain X-rays, and fractures were identified only on postoperative CT scan. Only half of the patients were symptomatic, but the authors indicated that patients with fractures (both symptomatic and asymptomatic) tended toward poorer outcomes. In a later study, Kim et al. related the occurrence of spinous process fracture after inter-spinous spacer implantation to the preoperative presence of spondylolisthesis at the operated level (87).
Spinal osteotomies, vertebrectomy. Anterior approach
Sagittal balance correction and anterior column stabilization can also be performed through a posterior trans pedicle or posterolateral approach. This method avoids the need for sectioning the diaphragm, especially advantageous in elderly patients with respiratory problems (5). Through this approach, anterior column stabilization can be achieved by posterior osteotomy (Smith-Petersen or pedicle substraction osteotomy), vertebrectomy, and bone grafting. The posterior spine is then stabilized using trans-pedicular screw fixation 2 to 3 levels above and below the decompression.
A 360º fusion allows load-shearing and places less strain on the posterior-only fixation, yielding a more stable construct (53). Transforaminal lumbar interbody fusion and posterior lumbar interbody fusion (TLIF, PLIF) are additional techniques to provide greater anterior column support that can be used alone or in combination with a posterior spinal osteotomy to restore sagittal balance and improve fusion rates.
Okuda et al. investigated the clinical and radiological results of posterior lumbar interbody fusion with pedicle screws for the treatment of L4-L5 degenerative spondylolisthesis in patients older than 70 years of age, comparing them with results in younger patients (88). No obvious differences in the clinical results were observed between the age groups. The prevalence of both collapsed union and delayed union in the older group was significantly higher than that in the younger group. However, it did not appear to affect the clinical results of the study.
In osteoporotic patients, careful vertebral endplate preparation and adequate interbody implant size selection are crucial for successful interbody fusion, as interbody devices may subside in the weak osteoporotic bone. Okuda et al. advocated for the preservation of the osseous end plate and the use of a large amount of bone graft for prevention subsidence (88). In their series, all cases of subsidence occurred within 3 months after the surgery, but further progression was not detected. Conversely, other authors have recommended complete removal of the osseous end plate to allow the implant and bone graft to rest on cancellous bone (89).
Anterior instrumentation. An anterior approach to the thoracolumbar spine may be used for decompression of a retro-pulsed bone fragment in patients with neurologic deficit, for anterior release with deformity, and for short segment fixation. Reconstruction and fusion can be achieved either by femoral ring bone allograft, rib struts, iliac bone, cages filled with bone graft, or bone substitutes. Anterior surgery allows placement of bone graft under compression which provides a mechanical advantage over posterior surgery. Stabilization can be accomplished using rigid anterior instrumentation.
In osteoporotic bone, anterior construct failure typically occurs with implant loosening or with subsidence of the interbody implant into the cancellous bone of the adjacent vertebral body. Uchida et al. addressed anterior column reconstruction using expandable strut-cage implants in a group of 28 patients with osteoporotic vertebral fracture (90). The greatest neurologic improvements were seen in patients with wedge-type vertebral body fracture, with middle and posterior columns relatively intact, rather than in flat-type or concave-type collapse. Fusion was confirmed in all cases. However, they found a large mean subsidence of 2.5 mm into adjacent vertebral bodies.
Vertebral interbody implant subsidence constitutes a challenge in vertebral body replacement in patients with osteoporosis. Furthermore, the potential mechanical benefit of using anterior interbody devices must be weighed against the increased risk to this fragile medical population from increased operative time, blood loss, and overall surgical risk (53).
References
- 1.Klibanski A, Adams-Campbell L, Bassford T, Blair SN, Boden SD, Dickersin K, et al. Osteoporosis prevention, diagnosis, and therapy. J Am Med Assoc. 2001;285(6):785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 2.Bono CM, Einhorn TA. Overview of osteoporosis: pathophysiology and determinants of bone strength. Eur Spine J. 2003;12(Suppl 2):S90–6. doi: 10.1007/s00586-003-0603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin DK, Park JY, Yoon YS, Kuh SU, Jin BH, Kim KS, et al. Prevalence of osteoporosis in patients requiring spine surgery: incidence and significance of osteoporosis in spine disease. Osteoporos Int. 2007;18(9):1219–24. doi: 10.1007/s00198-007-0370-8. [DOI] [PubMed] [Google Scholar]
- 4.Ponnusamy KE, Iyer S, Gupta G, Khanna AJ. Instrumentation of the osteoporotic spine: biomechanical and clinical considerations. Spine J. 2011;11(1):54–63. doi: 10.1016/j.spinee.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Hadjipavlou AG, Katonis PG, Tzermiadianos MN, Tsoukas GM, Sapkas G. Principles of management of osteometabolic disorders affecting the aging spine. Eur Spine J. 2003;12(Suppl 2):S113–31. doi: 10.1007/s00586-003-0600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritter MA, Albohm MJ, Keating EM, Faris PM, Meding JB. Life expectancy after total hip arthroplasty. J Arthroplasty. 1998;13(8):874–5. doi: 10.1016/s0883-5403(98)90192-9. [DOI] [PubMed] [Google Scholar]
- 7.Schrøder HM, Kristensen PW, Petersen MB, Nielsen PT. Patient survival after total knee arthroplasty. 5-year data in 926 patients. Acta Orthop Scand. 1998;69(1):35–8. doi: 10.3109/17453679809002353. [DOI] [PubMed] [Google Scholar]
- 8.White A, Panjabi M. Clinical biomechanics of the spine. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins; 1990. [Google Scholar]
- 9.Ferguson SJ, Steffen T. Biomechanics of the aging spine. Eur Spine J. 2003;12(Suppl 2):S97–103. doi: 10.1007/s00586-003-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazess RB. On aging bone loss. Clin Orthop. 1982;165:239–252. [PubMed] [Google Scholar]
- 11.Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285(3):320–23. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 12.Keller TS, Harrison DE, Colloca CJ, Harrison D, Janik TJ. Prediction of osteoporotic spinal deformity. Spine. 2003;28(5):455–62. doi: 10.1097/01.BRS.0000048651.92777.30. [DOI] [PubMed] [Google Scholar]
- 13.Lowe TG. Scheuermann disease. JBJS. 1990;72(6):940–5. [PubMed] [Google Scholar]
- 14.Ismail AA, Cooper C, Felsenberg D, Varlow J, Kanis JA, Silman AJ, et al. Number and type of vertebral deformities: epidemiological characteristics and relation to back pain and height loss. European Vertebral Osteoporosis Study Group. Osteoporosis Int. 1999;9(3):206–13. doi: 10.1007/s001980050138. [DOI] [PubMed] [Google Scholar]
- 15.Kanter AS, Asthagiri AR, Shaffrey CI. Aging spine: challenges and emerging techniques. Clin Neurosurg. 2007;54:10–8. [PubMed] [Google Scholar]
- 16.Benoist M. Natural history of the aging spine. Eur Spine J. 2003;12(2):S86–S89. doi: 10.1007/s00586-003-0593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sengupta DK, Herkowitz HN. Lumbar spinal stenosis: Treatment strategies and indications for surgery. Orthop Clin North Am. 2003;34(2):281–95. doi: 10.1016/s0030-5898(02)00069-x. [DOI] [PubMed] [Google Scholar]
- 18.Margulies JY, Payzer A, Nyska M, Neuwirth MG, Floman Y, Robin GC. The relationship between degenerative changes and osteoporosis in the lumbar spine. Clin Orthop. 1996;324:145–52. doi: 10.1097/00003086-199603000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Paiva LC, Filardi S, Pinto-Neto AM, Samara A, Marques Neto JF. Impact of degenerative radiographic abnormalities and vertebral fractures on spinal bone density of women with osteoporosis. Sao Paulo Med J. 2002;120(1):9–12. doi: 10.1590/S1516-31802002000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muraki S, Yamamoto S, Ishibashi H, Horiuchi T, Hosoi T, Orimo H, et al. Impact of degenerative spinal diseases on bone mineral density of the lumbar spine in elderly women. Osteoporos Int. 2004;15(9):724–8. doi: 10.1007/s00198-004-1600-y. [DOI] [PubMed] [Google Scholar]
- 21.Harada A, Okuizumi H, Miyagi N, Genda E. Correlation between bone mineral density and intervertebral disc degeneration. Spine. 1998;23(8):857–61. doi: 10.1097/00007632-199804150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Nuti R, Righi G, Martini G, Turchetti V, Lepore C, Doretti V. Diagnostic approach to osteoporosis and spondyloarthrosis in post-menopausal women by total body dual-photon absorptiometry. Clin Exp Rheum. 1988;6(1):47–51. [PubMed] [Google Scholar]
- 23.Chin DK, Park JY, Yoon YS, Kuh SU, Jin BH, Kim KS, et al. Prevalence of osteoporosis in patients requiring spine surgery: incidence and significance of osteoporosis in spine disease. Osteoporos Int. 2007;18(9):1219–24. doi: 10.1007/s00198-007-0370-8. [DOI] [PubMed] [Google Scholar]
- 24.Vogt MT, Rubin DA, San Valentin R, Palermo L, Donaldson WF, Nevitt M, et al. Lumbar listhesis and lower back symptoms in elderly white women. The study of osteoporotic fractures. Spine. 1998;23(23):2640–7. doi: 10.1097/00007632-199812010-00020. [DOI] [PubMed] [Google Scholar]
- 25.Bassewitz HL, Herkowitz HN. Osteoporosis of the spine: Medical and surgical strategies. Univ Pennsylvania Orthopaed J. 2000;13:35–42. [Google Scholar]
- 26.De Wald CJ, Stanley T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65 surgical considerations and treatment options in patients with poor bone quality. Spine. 2006;31(Suppl 19):S144–51. doi: 10.1097/01.brs.0000236893.65878.39. [DOI] [PubMed] [Google Scholar]
- 27.Andersen T, Christensen FB, Niedermann B, Helmig P, Høy K, Hansen ES, et al. Impact of instrumentation in lumbar spinal fusion in elderly patients:71 patients followed for 2-7 years. Acta Orthop. 2009;80(4):445–50. doi: 10.3109/17453670903170505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler TE, Jr, Asher MA, Jayaraman G, Nunley PD, Robinson RG. The strength and stiffness of thoracic implant anchors in osteoporotic spines. Spine (Phila Pa 1976) 1994;19(17):1956–62. doi: 10.1097/00007632-199409000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Coe JD, Warden KE, Herzig MA, McAfee PC. Influence of bone mineral density on the fixation of thoracolumbar implants: a comparative study of transpedicular screws, laminar hooks, and spinous process wires. Spine (Phila Pa 1976) 1990;15(9):902–907. doi: 10.1097/00007632-199009000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Greenfield RT, 3rd, Capen DA, Thomas JC, Jr, Nelson R, Nagelberg S, Rimoldi RL, et al. Pedicle screw fixation for arthrodesis of the lumbosacral spine in the elderly. An outcome study. Spine. 1998;23(13):1470–5. doi: 10.1097/00007632-199807010-00008. [DOI] [PubMed] [Google Scholar]
- 31.Cohen DB. Management of scoliosis in the osteoporotic patient. Semin Spine Surg. 2009;21(1):33–40. [Google Scholar]
- 32.Jost B, Cripton PA, Lund T, Oxland TR, Lippuner K, Jaeger P, et al. Compressive strength of interbody cages in the lumbar spine: the effect of cage shape, posterior instrumentation and bone density. Eur Spine J. 1998;7(2):132–41. doi: 10.1007/s005860050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amendola L, Gasbarrini A, Fosco M, Simoes CE, Terzi S, De Iure F, et al. Fenestrated pedicle screws for cement-augmented purchase in patients with bone softening: a review of 21 cases. J Orthop Traumatol. 2011;12(4):193–9. doi: 10.1007/s10195-011-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burval DJ, McLain RF, Milks R, Inceoglu S. Primary pedicle screw augmentation in osteoporotic lumbar vertebrae: biomechanical analysis of pedicle fixation strength. Spine (Phila Pa 1976) 2007;32(10):1077–83. doi: 10.1097/01.brs.0000261566.38422.40. [DOI] [PubMed] [Google Scholar]
- 35.Chang MC, Liu CL, Chen TH. Polymethylmethacrylate augmentation of pedicle screw for osteoporotic spinal surgery: a novel technique. Spine (Phila Pa 1976) 2008;33(10):E317–24. doi: 10.1097/BRS.0b013e31816f6c73. [DOI] [PubMed] [Google Scholar]
- 36.Cho KJ, Suk SI, Park SR, Kim JH, Kim SS, Choi WK, et al. Complications in posterior fusion and instrumentation for degenerative lumbar scoliosis. Spine (Phila Pa 1976) 2007;32(20):2232–7. doi: 10.1097/BRS.0b013e31814b2d3c. [DOI] [PubMed] [Google Scholar]
- 37.Deyo RA, Martin BI, Ching A, Tosteson AN, Jarvik JG, Kreuter W, et al. Interspinous spacers compared to decompression or fusion for lumbar stenosis: complications and repeat operations in the medicare population. Spine (Phila Pa 1976) 2013;38(10):865–72. doi: 10.1097/BRS.0b013e31828631b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hilibrand AS, Moore DC, Graziano GP. The role of pediculolaminar fixation in compromised pedicle bone. Spine (Phila Pa 1976) 1996;21(4):445–51. doi: 10.1097/00007632-199602150-00008. [DOI] [PubMed] [Google Scholar]
- 39.Hu MH, Wu HTH, Chang MC, Yu WK, Wang ST, Liu CL. Polymethylmethacrylate augmentation of the pedicle screw: the cement distribution in the vertebral body. Eur Spine J. 2011;20(8):1281–88. doi: 10.1007/s00586-011-1824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liljenqvist U, Hackenberg L, Link T, Halm H. Pullout strength of pedicle screws versus pedicle and laminar hooks in the thoracic spine. Acta Orthop Belg. 2001;67(2):157–63. [PubMed] [Google Scholar]
- 41.Masaya Y, Akihiro K, Riya K, Kinoshita M, Abe M. Efficacy of novel-concept pedicle screw fixation augmented with calcium phosphate cement in the osteoporotic spine. J Orthop Sci. 2005;10(1):56–61. doi: 10.1007/s00776-004-0862-8. [DOI] [PubMed] [Google Scholar]
- 42.Okuyama K, Abe E, Suzuki T, Tamura Y, Chiba M, Sato K. Influence of bone mineral density on pedicle screw fixation: a study of pedicle screw fixation augmenting posterior lumbar interbody fusion in elderly patients. Spine J. 2001;1(6):402–7. doi: 10.1016/s1529-9430(01)00078-x. [DOI] [PubMed] [Google Scholar]
- 43.Paré PE, Chappuis JL, Rampersaud R, Agarwala AO, Perra JH, Erkan S, et al. Biomechanical evaluation of a novel fenestrated pedicle screw augmented with bone cement in osteoporotic spines. Spine (Phila Pa 1976) 2011;36(18):E1210–4. doi: 10.1097/BRS.0b013e318205e3af. [DOI] [PubMed] [Google Scholar]
- 44.Postacchini F. Surgical management of lubar spinal stenosis. Spine. 1999;24(10):1043–7. doi: 10.1097/00007632-199905150-00020. [DOI] [PubMed] [Google Scholar]
- 45.Tan JS, Bailey CS, Dvorak MF, Fisher CG, Cripton PA, Oxland TR. Cement augmentation of vertebral screws enhances the interface strength between interbody device and vertebral body. Spine (Phila Pa 1976) 2007;32(3):334–41. doi: 10.1097/01.brs.0000253645.24141.21. [DOI] [PubMed] [Google Scholar]
- 46.Tan JS, Kwon BK, Samarasekera D, Dvorak MF, Fisher CG, Oxland TR. Vertebral bone density-a critical element in the performance of spinal implants. In: Melkerson MN, Grififith SL, Kirkpatrick JS, editors. Spinal implants: are we evaluating them appropriately. West Conshohocken, PA: ASTM International; 2003. pp. 217–30. [Google Scholar]
- 47.Vaccaro AR, Scott TB. Indications for instrumentation in degenerative lumbar spinal disorders. Orthopedics. 2000;23(3):260–71. doi: 10.3928/0147-7447-20000301-21. [DOI] [PubMed] [Google Scholar]
- 48.Wuisman PI, Van Dijk M, Staal H, Van Royen BJ. Augmentation of (pedicle) screws with calcium apatite cement in patients with severe progressive osteoporotic spinal deformities: an innovative technique. Eur Spine J. 2000;9(6):528–3. doi: 10.1007/s005860000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yone K, Sakou T, Kawauchi Y, Yamaguchi M, Yanase M. Indication of fusion for lumbar spinal stenosis in elderly patients and its significance. Spine (Phila Pa 1976) 1996;21(2):242–8. doi: 10.1097/00007632-199601150-00016. [DOI] [PubMed] [Google Scholar]
- 50.Hu SS. Internal fixation in the osteoporotic spine. Spine. 1997;22(24):43S–8. doi: 10.1097/00007632-199712151-00008. [DOI] [PubMed] [Google Scholar]
- 51.Hasegawa K, Takahashi HE, Uchiyama S, Hirano T, Hara T, Washio T, et al. An experimental study of a combination method using a pedicle screw and laminar hook for the osteoporotic spine. Spine (Phila Pa 1976) 1997;22(9):958–63. doi: 10.1097/00007632-199705010-00004. [DOI] [PubMed] [Google Scholar]
- 52.Tokuhashi Y, Ajiro Y, Umezawa N. Outcomes of posterior fusion using pedicle screw fixation in patients >70 years with lumbar spinal canal stenosis. Orthopedics. 2008;31(11):1096–8. [PubMed] [Google Scholar]
- 53.Dodwad SN, Khan SN. Surgical stabilization of the spine in the osteoporotic patient. Orthop Clin North Am. 2013;44(2):243–9. doi: 10.1016/j.ocl.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Tsuchiya K, Bridwell KH, Kuklo TR, Lenke LG, Baldus C. Minimum 5-year analysis of L5–S1 fusion using sacropelvic fixation (bilateral S1 and iliac screws) for spinal deformity. Spine (Phila Pa 1976) 2006;31:303–8. doi: 10.1097/01.brs.0000197193.81296.f1. [DOI] [PubMed] [Google Scholar]
- 55.Kwon BK, Elgafy H, Keynan O, Fisher CG, Boyd MC, Paquette SJ, Dvorak MF. Progressive junctional kyphosis at the caudal end of lumbar instrumented fusion: etiology, predictors, and treatment. Spine (Phila Pa 1976) 2006;31(17):1943–51. doi: 10.1097/01.brs.0000229258.83071.db. [DOI] [PubMed] [Google Scholar]
- 56.Zindrick MR, Wiltse LL, Widell EH, Thomas JC, Holland WR, Field BT, et al. A biomechanical study of intrapeduncular screw fixation in the lumbosacral spine. Clin Orthop Relat Res. 1986;203:99–112. [PubMed] [Google Scholar]
- 57.Esses SI, Botsford DJ, Huler RJ, Rauschning W. Surgical anatomy of the sacrum. A guide for rational screw fixation. Spine (Phila Pa 1976) 1991;16(6s):S283–8. doi: 10.1097/00007632-199106001-00021. [DOI] [PubMed] [Google Scholar]
- 58.Licht NJ, Rowe DE, Ross LM. Pitfalls of pedicle screw fixation in the sacrum. A cadaver model. Spine (Phila Pa 1976) 1992;17(8):892–6. doi: 10.1097/00007632-199208000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Luk KD, Chen L, Lu WW. A stronger bicortical sacral pedicle screw fixation through the S1 endplate: an in vitro cyclic loading and pull-out force evaluation. Spine (Phila Pa 1976) 2005;30(5):525–9. doi: 10.1097/01.brs.0000154649.55589.bf. [DOI] [PubMed] [Google Scholar]
- 60.Hirano T, Hasegawa K, Washio T, Hara T, Takahashi H. Fracture risk during pedicle screw insertion in osteoporotic spine. J Spinal Disord. 1998;11(6):493–7. [PubMed] [Google Scholar]
- 61.Yildirim OS, Aksakal B, Hanyaloglu SC, Erdogan F, Okur A. Hydroxyapatite dip coated and uncoated titanium poly-axial pedicle screws: an in vivo bovine model. Spine (Phila Pa 1976) 2006;31(8):E215–20. doi: 10.1097/01.brs.0000210221.00778.c7. [DOI] [PubMed] [Google Scholar]
- 62.Deligianni D, Korovessis P, Porte-Derrieu MC, Amedee J. Fibronectin preadsorbed on hydroxyapatite together with rough surface structure increases osteoblasts'adhesion ''in vitro'': the theoretical usefulness of fibronectin preadsorption on hydroxyapatite to increase permanent stability and longevity in spine implants. J Spinal Disord Tech. 2005;18(3):257–62. [PubMed] [Google Scholar]
- 63.Sandén B, Olerud C, Petrén-Mallmin M, Larsson S. Hydroxyapatite coating improves fixation of pedicle screws. A clinical study. J Bone Joint Surg Br. 2002;84(3):387–91. doi: 10.1302/0301-620x.84b3.12388. [DOI] [PubMed] [Google Scholar]
- 64.Cook SD, Barbera J, Rubi M, Salkeld SL, Whitecloud TS. Lumbosacral fixation using expandable pedicle screws: an alternative in reoperation and osteoporosis. Spine J. 2001;1(2):109–14. doi: 10.1016/s1529-9430(01)00020-1. [DOI] [PubMed] [Google Scholar]
- 65.Hart RA, Prendergast MA. Spine surgery for lumbar degenerative disease in elderly and osteoporotic patients. Instr Course Lect. 2007;56:257–72. [PubMed] [Google Scholar]
- 66.Brantley AG, Mayfield JK, Koeneman JB, Clark KR. The effects of pedicle screw fit: An In Vitro Study. Spine. 1994;19(5):1752–8. doi: 10.1097/00007632-199408000-00016. [DOI] [PubMed] [Google Scholar]
- 67.Pfeifer BA, Krag MH, Johnson C. Repair of failed transpedicle screw fixation. A biomechanical study comparing polymethylmethacrylate, milled bone, and matchstick bone reconstruction. Spine (Phila Pa 1976) 1994;19(3):350–3. doi: 10.1097/00007632-199402000-00017. [DOI] [PubMed] [Google Scholar]
- 68.Cook SD, Salkeld SL, Stanley T, Faciane A, Miller SD. Biomechanical study of pedicle screw fixation in severely osteoporotic bone. Spine J. 2004;4(4):402–8. doi: 10.1016/j.spinee.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Choma TJ, Pfeiffer FM, Swope RW, Hirner JP. Pedicle screw design and cement augmentation in osteoporotic vertebrae: effects of fenestrations and cement viscosity on fixation and extraction. Spine (Phila Pa 1976) 2012;37(26):E1628–32. doi: 10.1097/BRS.0b013e3182740e56. [DOI] [PubMed] [Google Scholar]
- 70.Sawakami K, Yamazaki A, Ishikawa S, Ito T, Watanabe K, Endo N. Polymethylmethacrylate augmentation of pedicle screws increases the initial fixation in osteoporotic spine patients. J Spinal Disord Tech. 2012;25(2):E28–35. doi: 10.1097/BSD.0b013e318228bbed. [DOI] [PubMed] [Google Scholar]
- 71.Aydogan M, Ozturk C, Karatoprak O, Tezer M, Aksu N, Hamzaoglu A. The pedicle screw fixation with vertebroplasty augmentation in the surgical treatment of the severe osteoporotic spines. J Spinal Disord Tech. 2009;22(6):444–7. doi: 10.1097/BSD.0b013e31818e0945. [DOI] [PubMed] [Google Scholar]
- 72.Piñera AR, Duran C, Lopez B, Saez I, Correia E, Alvarez L. Instrumented lumbar arthrodesis in elderly patients: prospective study using cannulated cemented pedicle screw instrumentation. Eur Spine J. 2011;20(Suppl 3):408–14. doi: 10.1007/s00586-011-1907-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frankel BM, Jones T, Wang C. Segmental polymethyl-methacrylate-augmented pedicle screw fixation in patients with bone softening caused by osteoporosis and metastatic tumor involvement: a clinical evaluation. Neurosurgery. 2007;61(3):531–7. doi: 10.1227/01.NEU.0000290899.15567.68. [DOI] [PubMed] [Google Scholar]
- 74.Lubansu A, Rynkowski M, Abeloos L, Appelboom G, Dewitte O. Minimally invasive spinal arthrodesis in osteoporotic population using a cannulated and fenestrated augmented screw: technical description and clinical experience. Minim Invasive Surg. 2012;2012:507826. doi: 10.1155/2012/507826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xie Y, Fu Q, Chen ZQ, Shi ZC, Zhu XD, Wang CF, et al. Comparison between two pedicle screw augmentation instrumentations in adult degenerative scoliosis with osteoporosis. BMC Musculoskelet Disord. 2011;12(1):286. doi: 10.1186/1471-2474-12-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh K, Heller JG, Samartzis D, Price JS, An HS, Yoon ST, et al. Open vertebral cement augmentation combined with lumbar decompression for the operative management of thoracolumbar stenosis secondary to osteoporotic burst fractures. J Spinal Disord Tech. 2005;18(5):413–9. doi: 10.1097/01.bsd.0000173840.59099.06. [DOI] [PubMed] [Google Scholar]
- 77.Zapatowicz K, Godlewski B, Jekimov R, Grochal M. Augmentation of transpedicular screws by intraoperative vertebroplasty. Neurol Neurochir Pol. 2012;46(6):560–8. doi: 10.5114/ninp.2012.32098. [DOI] [PubMed] [Google Scholar]
- 78.Kim HS, Park SK, Joy H, Ryu JK, Kim SW, Ju CI. Bone cement augmentation of short segment fixation for unstable burst fracture in severe osteoporosis. J Korean Neurosurg Soc. 2008;44(1):8–14. doi: 10.3340/jkns.2008.44.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moon BJ, Cho BJ, Choi EY, Zhang HY. Polymethylmethacrylate-augmented screw fixation for stabilization of the osteoporotic spine: a three-year follow-up of 37 patients. J Korean Neurosurg Soc. 2009;46(4):305–11. doi: 10.3340/jkns.2009.46.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Erbe EM, Clineff TD, Gualtieri G. Comparison of a new bisphenola-glycidyl dimethacrylate-based cortical bone void filler with polymethyl methacrylate. Eur Spine J. 2001;10(Suppl 2):S147–52. doi: 10.1007/s005860100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobayashi H, Turner AS, Seim HB, Kawamoto T, Bauer TW. Evaluation of a silicacontaining bone graft substitute in a vertebral defect model. J Biomed Mater Res A. 2010;92(2):596–603. doi: 10.1002/jbm.a.32397. [DOI] [PubMed] [Google Scholar]
- 82.Cho W, Wu C, Zheng X, Erkan S, Suratwala SJ, Mehbod AA, et al. Is it safe to back out pedicle screws after augmentation with polymethyl methacrylate or calcium phosphate cement?A biomechanical study. J Spinal Disord Tech. 2011;24(4):276–9. doi: 10.1097/BSD.0b013e3181f605d0. [DOI] [PubMed] [Google Scholar]
- 83.Gunzburg R, Szpalski M. The conservative surgical treatment of lumbar spinal stenosis in the elderly. Eur Spine J. 2003;12(Suppl 2):S176–80. doi: 10.1007/s00586-003-0611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yi X, McPherson B. Application of X STOP device in the treatment of lumbar spinal stenosis. Pain Physician. 2010;13(5):E327–36. [PubMed] [Google Scholar]
- 85.Kuchta J, Sobottke R, Eysel P, Simons P. Two-year results of interspinous spacer (X-Stop) implantation in 175 patients with neurologic intermittent claudication due to lumbar spinal stenosis. Eur Spine J. 2009;18(6):823–9. doi: 10.1007/s00586-009-0967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim DH, Tantorski M, Shaw J, Martha J, Li L, Shanti N, et al. Occult spinous process fractures associated with interspinous process spacers. Spine (Phila Pa 1976) 2011;36(16):E1080–5. doi: 10.1097/BRS.0b013e318204066a. [DOI] [PubMed] [Google Scholar]
- 87.Kim DH, Shanti N, Tantorski ME, Shaw JD, Li L, Martha JF, et al. Assiciation between degenerative spondylolisthesis and spinous process spacer surgery. Spine J. 2012;12(6):466–72. doi: 10.1016/j.spinee.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 88.Okuda S, Oda T, Miyauchi A, Haku T, Yamamoto T, Iwasaki M. Surgical outcomes of posterior lumbar interbody fusion in elderly patients. J Bone Joint Surg Am. 2007;1(2):310–20. doi: 10.2106/JBJS.G.00307. [DOI] [PubMed] [Google Scholar]
- 89.Steffen T, Tsantrizos A, Aebi M. Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine (Phila Pa 1976) 2000;25(9):1077–84. doi: 10.1097/00007632-200005010-00007. [DOI] [PubMed] [Google Scholar]
- 90.Uchida K, Kobayashi S, Nakajima H, Kokubo Y, Yayama T, Sato R, et al. Anterior expandable strut cage replacement for osteoporotic thoracolumbar vertebral collapse. J Neurosurg Spine. 2006;4(6):454–62. doi: 10.3171/spi.2006.4.6.454. [DOI] [PubMed] [Google Scholar]


