Abstract
Background:
There is no consensus regarding the best method of reconstruction in pediatric population following the wide resection of malignant bone tumors. More exploration of the complications of osteoarticular reconstruction leads to less existing controversy of this type of reconstruction, which is the main point of this article.
Methods:
Long-term outcomes and complications of osteoarticular allograft reconstruction of primary distal femoral bone sarcomas in 22 children with mean age of 10.7 years old were reviewed in this study. Musculoskeletal Tumor Society (MSTS) scoring system was used for functional evaluation of the allografts.
Results:
With an average follow-up time of 81 months, the outcomes of 16 patients with allografts at the final follow up were evaluated. As expected, Limb length discrepancy (LLD) was observed in all patients (mean LLD= 2.73cm), which was significantly correlated to allograft survival time (P<0.001). Degenerative joint disease (DJD) was also seen in all patients and its grade was also significantly correlated to allograft survival time (P<0.001). The mean MSTS-score was 74% at the latest follow-up, ranging from 60% to 90%. Five and 10 year survival rate of allografts were found to be 93.3% and 62.2%, respectively.
Conclusion:
Osteoarticular allograft reconstruction could result in several complications including DJD. Despite its considerable biologic advantage over endoprosthesis, osteoarticular allograft reconstruction is a long-lasting but still a temporary solution before performing megaprosthesis. This allows patients to preserve their remaining physis for limb growth and become old enough for an adult megaprosthesis.
Keywords: Distal Femoral Tumor, Osteoarticular Allograft, Pediatric
Introduction
Advances in imaging and chemotherapy have made limb salvage surgery increasingly attractive in the treatment of primary malignancies of long bones (1, 2). Distal femur is the most common location of primary malignant bone tumors (3). Wide resection following limb salvage procedure often leaves a large osteoarticular defect, which needs reconstruction. Modular replacement endoproshtesis is a popular option for reconstruction of the resected part in adults (4, 5). However, it is not favorable for growing children who need their physis for elongation of the limb. Although expandable endoprosthesis allows limb lengthening in growing children, it has a high complication rate, as well (6-8). Osteoarticular allograft reconstruction of distal femur has some advantages over other existing methods. These includes salvage of the proximal tibia physis, attachment of the host ligaments to the graft and preservation of the joint motion and bone stocks (9, 10). Like any other technique, this procedure also contains its own shortages including limb length discrepancy, allograft fracture, nonunion, infection and degenerative joint disease (DJD).
Although evaluation of the osteoarticular allograft outcome in children with high-grade tumors have been the core of many investigations, majority of studies have collectively assessed the femoral and tibial allograft outcomes, while separate evaluation of the femoral and tibial osteoarticular allograft outcomes could lead to more uniform results. In this regard, we planned to specifically assess the complications of distal femoral allografts in children with high-grade bone tumors (11-13).
Patients
Using our hospital electronic database, we identified 27 patients under 14 years old who had undergone massive osteoarticular allograft reconstruction treatment for primary malignant bone tumor of distal femur from January 1999- June 2013. Inclusion criteria were defined as implantation of an osteoarticular allograft for treatment of a primary sarcoma of distal femur complete clinical, radiographic and pathologic records and minimum follow-up of 2 years from allograft reconstruction date (1,2). Patients with insufficient clinical information were excluded.
During the study period, five patients discontinued the follow-up sessions. All patients were diagnosed with osteosarcoma except two cases of Ewing sarcoma. Tumors were staged according to American Joint Commission on Cancer (AJCC) staging system (14). All patients received preoperative and postoperative chemotherapy, according to the National Comprehensive Cancer Network (NCCN) protocol (15). Patients’ demographics are shown in table 1. A written consent was obtained from the parents of all patients.
Table 1.
Patients’clinicopathologic characteristics
| Case | Age& gender | Tumor type | Resected Length (cm) | Necrosis (%) | Follow-up (months) | LLD (Cm) | Complication | Reoperation same leg | DJD grade | MSTS (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9,M | IIB osteosarcoma | 15 | 80 | 132 | 4.5 | None | yes | 4 | 66.6 |
| 2 | 8,F | IIB osteosarcoma | 12 | 90 | 60 | 3.5 | Dislocation | yes | 3 | 60 |
| 3 | 13,M | IIB osteosarcoma | 20 | 25 | 36 | - | Lung metastasis | no | - | Death |
| 4 | 11,M | IIB osteosarcoma | 18 | 60 | 24 | 0 | Infection | no | 1 | 90 |
| 5 | 13,M | IIB Ewing sarcoma | 17 | 70 | 24 | - | Local recurrence&metastasis | yes | - | Death |
| 6 | 11,F | IIB osteosarcoma | 18 | 30 | 36 | - | Lung metastasis | no | - | Death |
| 7 | 8,M | IIB osteosarcoma | 16 | 90 | 120 | 4.5 | Nonunion | yes | 4 | 63.3 |
| 8 | 10,F | IIB osteosarcoma | 15 | 80 | 120 | 3.5 | None | no | 4 | 76.6 |
| 9 | 11,M | IIB osteosarcoma | 12 | 100 | 132 | 4.5 | None | no | 4 | 73.3 |
| 10 | 8,F | IIB osteosarcoma | 14 | 80 | 48 | 2 | None | no | 3 | 90 |
| 11 | 10,F | IIB Ewing sarcoma | 18 | 90 | 48 | 2 | Nonunion | Yes | 2 | 70 |
| 12 | 12,M | IIB osteosarcoma | 18 | 60 | 36 | - | Lung&bone metastasis | no | - | Death |
| 13 | 12,M | IIB osteosarcoma | 12 | 90 | 48 | - | Fracture | Yes | - | Failure |
| 14 | 11,F | IIB osteosarcoma | 16 | 100 | 36 | 1 | Infection | no | 2 | 80 |
| 15 | 11,F | IIB osteosarcoma | 17 | 80 | 60 | 3.5 | None | no | 3 | 73.3 |
| 16 | 8,M | IIB osteosarcoma | 16 | 90 | 42 | 2.8 | None | no | 2 | 70 |
| 17 | 10,F | IIB osteosarcoma | 18.5 | 80 | 72 | 2 | None | no | 2 | 83.3 |
| 18 | 10,F | IIB osteosarcoma | 16.5 | 90 | 54 | 2 | None | no | 3 | 73.3 |
| 19 | 13,M | IIB osteosarcoma | 14.5 | 95 | 136 | - | Fracture | no | - | Failure |
| 20 | 12,F | IIB osteosarcoma | 14 | 80 | 120 | 4.5 | Nonunion | yes | 4 | 60 |
| 21 | 11,F | IIB osteosarcoma | 14 | 100 | 154 | 2 | None | no | 4 | 73.3 |
| 22 | 13,F | IIB osteosarcoma | 16 | 80 | 160 | 1.5 | None | no | 4 | 80 |
M= male; F= female; LLD= limb length discrepancy; DJD= degenerative joint disease;
Surgical technique
The appropriate length of femur containing the tumor, the involved surrounding soft-tissue and the biopsy tracts were excised by intra-articular resection through anteromedial or anterolateral longitudinal incision depending on biopsy location. Excision of femur was performed at least 3cm beyond the proximal point of involvement as determined by preoperative imaging including MRI. Intra-operative frozen section analysis was employed for all patients to ensure the presence of no residual disease at surgical margins.
Fresh-frozen osteoarticular distal femur allografts with matched size were obtained from our university tissue bank which harvests and stores allografts according to standard tissue banking protocol of the allograft preparation and processing (16). In general, we used anteroposterior and lateral radiograph of the contralateral distal femur for diameter matching. In the operating room, the allograft was thawed and sized usually 2cm longer than the due bone defect in order to diminish the extent of subsequent limb-length discrepancy. The medullary canal of the allograft was reamed and filled with low viscosity cement to reinforce the allograft. Allograft was fixed to the host bone in appropriate rotation by a broad 4.5 dynamic compression plate (DCP). The correspondent DCP was selected as to cover the entire length of the allograft, while holding at least 4 extra holes to be fixed to the host bone. Medial and lateral collateral ligaments of the allograft were sutured to the remnant of the host with non-absorbable suture.
Post-op and follow-up
Post-operatively, patients used a knee brace to keep the leg immobilized for two weeks and then straight leg raise with range of motion exercises were started. Patients were asked to have non-weight bearing ambulation until radiologic union. Follow-ups for all patients were performed every 3 months in the first 2years, every 6 months for the third year, and annually thereafter. In each follow-up, clinical examination was performed and anteroposterior and lateral radiographs of the surgical site and of the knee joint were obtained. If any sign of local recurrence was seen further diagnostic work-ups like CT guided core needle biopsy were administered. In addition, In order to evaluate the lung metastasis in the first 2 years, chest X-ray and CT were also performed in 3 and 6 months intervals, respectively. Union was regarded as healing of at least three cortices in anteroposterior and lateral views in graphs. In case of any complication the frequency of follow-ups returned to the initial state. Complications were categorized into allograft and oncological types. Allograft complications included infection, fracture, nonunion, limb length discrepancy, (LLD) and degenerative joint disease (DJD). Death, metastasis, and local recurrence were defined as oncological complications. In the last follow-up, functional evaluation of the allograft was performed according to the Tumor Society Scoring system (MSTS) for the patients who still had their allograft “in-situ” (17). The procedure was regarded as failure when the allograft was discarded and replaced by another one or prosthesis.
Statistical analysis
Chi-square, Fisher’s exact test, Pearson’s and Spearman’s correlation coefficient tests were used for univariate analysis of the variables. Kaplan-Meier survival test was applied in order to measure the survival of implanted allograft for a certain amount of time after surgery. IBM SPSS Statistics 22 was used for all statistical analysis and a P<0.05 was considered as statistically significant.
Results
Totally, 12 females and 10 males with average age of 10.7±1.6 years, ranging from 8 to13 years, were included in the study. All patients were at stage IIB at the time of surgery. Overall, four patients (18%) died of oncologic complications. The average time to death for the four deaths was 33 months. The average follow-up time for the remaining patients was 81 months, ranging from 24 to 157 months.
There was one local recurrence (4.5%) leading to the corresponding limb amputation; however, the patient died of lung metastasis six months later.
Two early infections were seen in our patients, both of which were superficial and treated with antibiotics therapy. There also were three cases of nonunion with average duration of 12 months postoperatively; two of which had plate fracture, whereas in the third case the plate was bended at the site of nonunion. They successfully responded to re-surgery with application of simultaneous new double-plate and auto graft from ipsilateral ilium [Figure 1].
Figure 1.
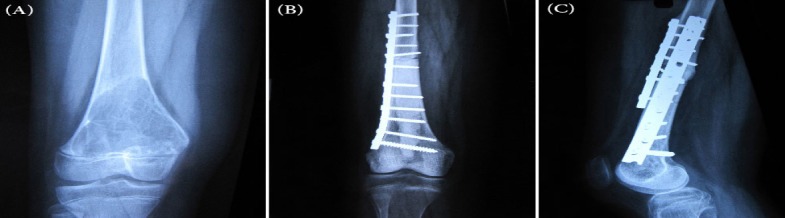
(A) Radiograph of distal femur showing telangectatic osteosarcoma in a 12 year old girl (case 20); (B) Radiograph acquired 10 months after surgery showing nonunion of the allograft; (C) Radiograph taken 9 months after double-plating and autogenous-bone-graft surgery showing complete union of the allograft.
Allograft fracture occurred in two patients (Cases 13 and 19). A low-energy trauma 9 months postoperatively was the cause of a condyle fracture in one of them which was reconstructed with megaprosthesis. A long oblique fracture of the allograft with failure of fixation (4 distal screws was pulled out from allograft) was happened during walking almost 15 months after surgery in the second case. The patient was managed by removal of the fractured allograft and insertion of a new one. Dislocation also occurred in two patients, both caused by mismatched allograft reconstruction (Condyles of femur were larger than the tibia) which were imposed by lack of appropriately sized bone graft. Actually, the applied allografts were the smallest size among the skeletally mature donors of our bone bank, which seemed to be not small enough. They were managed by closed reduction, pinning and immobilization. The patients were also recommended to use knee support for the rest of their lives.
Limb-length discrepancy occurred in all patients of our study. The average shortening was 2.7± 1.5 cm, ranging from 1 cm to 4.5cm. Knee joint range of motion limitations were recorded in 4 patients. Mild knee instability was observed in 14 cases as well.
According to Kallgren and Lawrence osteoarthritis grading system all of our patients experienced DJD at some point after receiving allograft. The mean MSTS-score of 16 survived allografts at the latest follow-up was 74 ± 7.8% ranging from 60% to 90%.
Considering the limited number of patients, analysis of significance has only been performed whenever possible. In this regard, our analysis showed no significant correlation between the functional results and variables such as age (r=0.068, P=0.31, 95% CI), sex (r=0.035, P=0.43, 95% CI) and resected length (r=0.101, P=0.12, 95% CI). In addition, no significant correlation was seen between the allograft survival time (follow-up time) and variables such as age(r=0.117, P=0.28, 95% CI), sex (r= -0.034, P=0.26, 95% CI), resected length (r=0.052, P=0.38, 95% CI). As expected, a significant correlation between LLD and allograft survival was observed (r=0.772, P<0.001, 95% CI). All of our patients experienced degenerative joint disease to some extents. Our statistical analysis showed a strong significant correlation between the allograft survival time and deterioration of joint, leading to a higher grade of DJD in patients with higher follow-up time (r= 0.892, P<0.001, 95% CI) [Table 1]. Patients who lived longer showed higher degrees of DJD [Figure 2].
Figure 2.
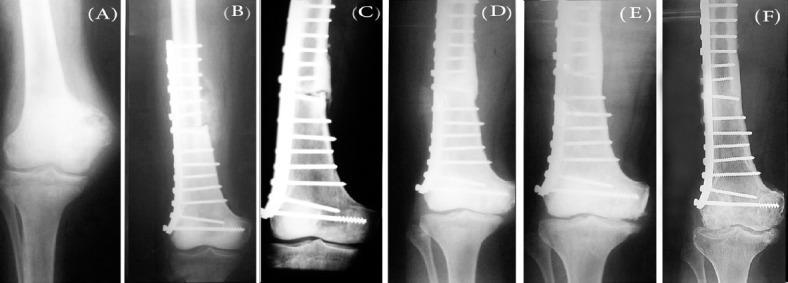
Proceeding trend of DJD in massive osteoarticular allograft in children with distal femoral tumors (Case 22). (A) Radiograph of before allograft procedure showing no DJD; (B) Radiograph of immediately after allograft procedure showing grade 0 DJD; (C) Radiograph of 1 year after allograft procedure showing grade 1 DJD (D) Radiograph of 2 years after allograft procedure showing grade 2 DJD; (E) Radiograph of 4 year after allograft procedure showing grade 3 DJD; (F) Radiograph of 13 years after allograft procedure showing grade 4 DJD.(Kallegren & Lawrence osteoarthritis grading system).
In order to evaluate the allograft survival, Kaplan-Meier cumulative survival curve was depicted [Figure 3]. In this regard, the 5 and 10 years survival rates of our allografts were 93.3 and 62.2 %, respectively.
Figure 3.
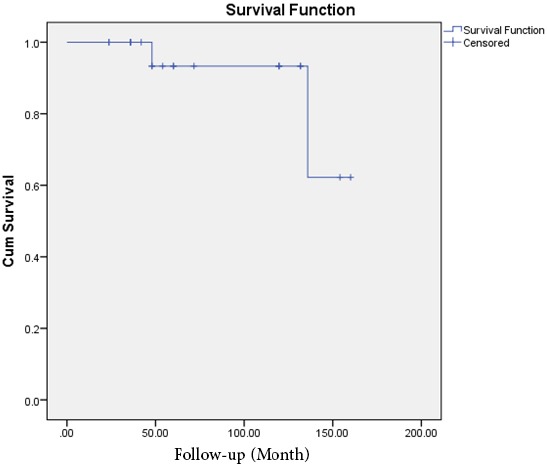
Kaplan-Meier survival curve of allograft survival.
Discussion
Although osteoarticular allograft reconstruction after extremity tumor resection has been a promising procedure in children who need their physis for limb elongation, it has shown a high complication rate (12, 13). Nevertheless, the dimensions of these complications are controversial due to some limitations including short follow-up time and low number of patients. In addition, limited patient number also hinders the evaluation of site-specific complications in femur or tibia. Separate evaluations of bone tumors of different location could lead to a better understanding of the relevant complications. In this regard, we evaluated such complications in 22 pediatric distal femoral sarcomas.
To date, very few articles have reported the complications of pediatric osteoarticular allograft, while most of them did not separate patients based on tumor location. Muscolo et al reported the results of allograft reconstruction in 22 under 10-year-old patients after bone sarcoma resection with a mean fallow-up of 4 years. In their series only 5-osteoarticular allograft of distal femur was evaluated. Given that they did not categorized patients based on their tumor site, they reported 4 allograft failures at an average 13 months. They also reported eight complications, which needed second surgery, out of which, four allografts were removed (one infection, one recurrence and two fractures) and four were preserved (two recurrence, one fracture and one nonunion). An average functional score of 27 point was observed in18 retained limbs according to MSTS score (11).
Exner et al evaluated the outcome of tibial allograft reconstruction following tumor resection in 19 patients of 7-17 years old, among whom 14 patients had good or excellent function at a mean follow-up time of 59 month. However, they did not include complication rate in their report (13).
Companacci et al assessed the long-term results of pediatric osteoarticular allografts after massive resection in 13 distal femoral and 12 proximal tibial tumors. They concluded that allograft mechanical failure was the most important complication, which occurred in 12 cases (60%). While analyzing the complications in 12 distal femoral allografts separately, they reported superior results for this area and only four fractures (33.3%) happened during follow up (12).
Allograft fracture was also the most important complication in our study, which led to allograft discard in two out of 22 patients (9%). The low incidence of allograft fracture in our study can be attributed to the coverage of the allograft entire length by the correspondent plate and augmentation of the allograft with cement.
The most common complication after degenerative joint disease in our series was nonunion with plate failure, which occurred in 3 patients (13%). Adding autogenous cancellous graft to the allograft-host bone junction might have decrease the rate of nonunion. According to Companacci et al, two out of 13 patients with distal femur reconstructions experienced delayed union and successfully treated with autogenous bone graft apposition (12).
The infection rate in our study was also lower compared to similar studiesbut higher than some reports in which antibiotic loaded cement were used (9, 16, 18). In addition, some studies have reported irradiation of allograft to reduce the infection rate (19). In conclusion, antibiotic loaded cements and irradiation could be used in order to further reduce the infection rate, yet attenuate the strength of the allograft as well (16). We ascribed the low rate of infection of our patients to the good coverage of allografts with soft tissue in distal femur.
LLD was observed in all our patients. We performed reconstruction with allograft 2cm longer than the bone defect and as expected LLD in our patients was less than Companacci’s report. Operative treatment for length discrepancy was considered when estimated discrepancy at the time of maturity was 3 cm or more. This included epiphysiodesis of the contralateral limb (20). Our patients preferred wearing high top shoe with compensated lift rather than having surgery.
Tumor recurrence rate was 3.7% in our study. Although margin was declared as tumor-free by pathologist, recurrence occurred in the site of surgery. Consequently, cautions should be taken about the adequacy of resection to reduce the possibility of tumor recurrence, as reported by others (9).
The last and most common complication in our study, which has been underestimated in the majority of similar studies, is the progressive secondary DJD which significantly correlate with implant survival. According to our slides, DJD was observed in all patients and its grade was significantly higher in longer survived allografts. Adding to the existing controversy of osteoarticular allograft procedure, certainty of DJD may lead to the re-consideration in the therapeutic choices.
Osteoarticular femoral allograft following massive tumor resection in children who need their physis for limb elongation has been acknowledged as a promising approach. However, considering the patients’ young age and the subsequent complications of this method such as inevitable DJD, it must be admitted that at the moment, this reconstruction method is a long-lasting but still a temporary solution before performing megaprosthesis. It allows patients to buy time to grow old enough for modular replacement megaprosthesis, especially in those who outlive their disease.
References
- 1.Bloem JL, Taminiau AH, Eulderink F, Hermans J, Pauwels EK. Radiologic staging of primary bone sarcoma: MR imaging, scintigraphy, angiography, and CT correlated with pathologic examination. Radiology. 1988;169(3):805–10. doi: 10.1148/radiology.169.3.3055041. [DOI] [PubMed] [Google Scholar]
- 2.Enneking WF. An abbreviated history of orthopaedic oncology in North America. Clin Orthop Relat Res. 2000;374:115–24. doi: 10.1097/00003086-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Ottaviani G, Jaffe N. Pediatric and adolescent osteosarcoma. New York: Springer; 2009. The epidemiology of osteosarcoma; pp. 3–13. [DOI] [PubMed] [Google Scholar]
- 4.Chauhan A, Joshi GR, Chopra BK, Ganguly M, Reddy GR. Limb salvage surgery in bone tumors: a retrospective study of 50 cases in a single center. Indian J Surg Oncol. 2013;4(3):248–54. doi: 10.1007/s13193-013-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orlic D, Smerdelj M, Kolundzic R, Bergovec M. Lower limb salvage surgery: modular endoprosthesis in bone tumour treatment. Int Orthop. 2006;30(6):458–64. doi: 10.1007/s00264-006-0193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cipriano CA, Gruzinova IS, Frank RM, Gitelis S, Virkus WW. Frequent complications and severe bone loss associated with the Repiphysis expandable distal femoral prosthesis. Clin Orthop Relat Res. 2015;473(3):831–8. doi: 10.1007/s11999-014-3564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruggieri P, Mavrogenis AF, Pala E, Romantini M, Manfrini M, Mercuri M. Outcome of expandable prostheses in children. J Pediatr Orthop. 2013;33(3):244–53. doi: 10.1097/BPO.0b013e318286c178. [DOI] [PubMed] [Google Scholar]
- 8.Saghieh S, Abboud MR, Muwakkit SA, Saab R, Rao B, Haidar R. Seven-year experience of using Repiphysis expandable prosthesis in children with bone tumors. Pediatr Blood Cancer. 2010;55(3):457–63. doi: 10.1002/pbc.22598. [DOI] [PubMed] [Google Scholar]
- 9.Alman BA, De Bari A, Krajbich JI. Massive allografts in the treatment of osteosarcoma and Ewing sarcoma in children and adolescents. J Bone Joint Surg Am. 1995;77(1):54–64. doi: 10.2106/00004623-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Mnaymneh W, Malinin TI, Lackman RD, Hornicek FJ, Ghandur-Mnaymneh L. Massive distal femoral osteoarticular allografts after resection of bone tumors. Clin Orthop Relat Res. 1994;303:103–15. [PubMed] [Google Scholar]
- 11.Muscolo DL, Ayerza MA, Aponte-Tinao L, Farfalli G. Allograft reconstruction after sarcoma resection in children younger than 10 years old. Clin Orthop Relat Res. 2008;466(8):1856–62. doi: 10.1007/s11999-008-0303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campanacci L, Manfrini M, Colangeli M, Alì N, Mercuri M. Long-term results in children with massive bone osteoarticular allografts of the knee for high-grade osteosarcoma. J Pediatr Orthop. 2010;30(8):919–27. doi: 10.1097/BPO.0b013e3181fa7981. [DOI] [PubMed] [Google Scholar]
- 13.Exner U. Allograft reconstruction for malignant bone tumors in the growing child. J Bone Joint Surg Br. 2003;85(SUPP III):261. [Google Scholar]
- 14.Frederick L, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. AJCC cancer staging manual. Berlin, Germany: Springer Science & Business Media; 2002. [Google Scholar]
- 15.Demetri GD, Pollock R, Baker L, Balcerzak S, Casper E, Conrad C, et al. NCCN sarcoma practice guidelines. National Comprehensive Cancer Network. Oncology (Williston Park, NY) 1998;12(7A):183–218. [PubMed] [Google Scholar]
- 16.Ozaki T, Hillmann A, Bettin D, Wuisman P, Winkelmann W. Intramedullary, antibiotic-loaded cemented, massive allografts for skeletal reconstruction. 26 cases compared with 19 uncemented allografts. Acta Orthop Scand. 1997;68(4):387–91. doi: 10.3109/17453679708996183. [DOI] [PubMed] [Google Scholar]
- 17.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286(25):241–6. [PubMed] [Google Scholar]
- 18.Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324:86–97. doi: 10.1097/00003086-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Fitch FR, Doolan PT, Dwyer J, Dwyer V, Halls NA, Tallentire A. Towards microbiological quality assurance in radiation sterilization processing: simulation of the radiation inactivation process. J Appl Bacteriol. 1985;58(3):307–13. doi: 10.1111/j.1365-2672.1985.tb03320.x. [DOI] [PubMed] [Google Scholar]
- 20.Anderson M, Green WT, Messner MB. Growth and predictions of growth in the lower extremities. J Bone Joint Surg Am. 1963;45(1):1–14. [PubMed] [Google Scholar]


