Abstract
Background:
Corticosteroid injections are used to treat a variety of orthopedic conditions with the goal of decreasing pain and inflammation. Administration of systemic or local corticosteroids risks temporarily increasing blood glucose levels, especially diabetic patients. The purpose of this study is to quantify the effects of corticosteroid injections on blood glucose levels in diabetic patients with shoulder pathology.
Methods:
Diabetic patients who regularly monitored their blood glucose levels and were indicated for a subacromial corticosteroid injection were included in this prospective investigation. The typical normal morning fasting glucose and most recent hemoglobin A1c level was recorded for each patient. After injection, patients were contacted daily to confirm their fasting morning glucose level for 10 days post-injection.
Results:
Seventeen consecutive patients were enrolled. Patients with hemoglobin A1c of <7% had an average rise in blood glucose of 38 mg/dL compared to 98 mg/dL in the poorly controlled group after injection (P<0.001). Well-controlled patients’ glucose levels returned to near baseline levels around post-injection day 8, while poorly controlled patients levels remained elevated. Similarly, insulin-dependent diabetic patients had an average increase in fasting glucose level of 99 mg/dL versus 50 mg/dL in non-insulin-dependent diabetic patients (P<0.001).
Conclusion:
After corticosteroid injection, patients with well-controlled diabetes experience smaller elevations and faster return to baseline glucose levels than patients with poor control. Insulin dependent diabetics experienced similar findings as patients with poor control. Future studies are needed to evaluate dosing to optimize the risks of blood glucose elevation while maintaining therapeutic benefit.
Keywords: Corticosteroid injection, Diabetic response to corticosteroid injection, Glucose response to corticosteroid, Pain relief with steroid injection, Shoulder injection, Shoulder pain
Introduction
Corticosteroid injections are used to treat a variety of orthopedic conditions with the goal of decreasing pain and inflammation (1-5). In the shoulder, corticosteroid injections are a common non-operative modality to treat both rotator cuff pathology and adhesive capsulitis (1, 4-6). Administration of systemic or local corticosteroid has a known risk of temporarily increasing blood glucose levels (3, 7-9). This risk is especially concerning in diabetic patients, who already have difficulty maintaining blood glucose homeostasis.
Although the risk of elevating blood glucose levels is a known entity following corticosteroid injections, the exact amount and duration of increase still remains unclear (7, 8, 10). A variety of patient and treatment-related factors, including site of injection, dose of corticosteroid, and a patient’s baseline glycemic control, plays into this uncertainty. Specifically for the shoulder, only one case series exists and found no significant effect of intra-articular corticosteroid on diabetic patients’ blood glucose levels (11). The lack of information regarding what effect an injection may have on a patient’s blood glucose control may cause some clinicians to be hesitant to offer corticosteroids to this patient population.
Increased blood glucose levels results in increased irreversible glycosylation of proteins in the blood stream (12). Erythrocytes turnover roughly every 3 months, making the hemoglobin A1c level a clinically useful value to determine a patient’s long-term glucose control and response to diabetic treatment (10, 13). In general, levels of less than 5.6% are considered normal and greater than 6.5% are considered diabetic. The American Diabetic Association recommends routine testing of all diabetic patients. Diabetic patients are advised to keep their hemoglobin A1c level at or below 7% to reduce the potential long-term complications associated with diabetes (14). Additionally, literature suggests in this population, patients at or below hemoglobin A1c of 7% have a reduced risk of post-operative complications (15).
The purpose of this study is to describe the effects of blood glucose levels following a therapeutic dose of corticosteroid injected in the subacromial space of the shoulder in diabetic patients in order to provide better counseling information to this group. Injections in the shoulder typically use higher doses of corticosteroid than the hand, and may theoretically have increased systemic absorption of corticosteroid compared to injections in smaller joints. Our hypothesis was that patients with poorly controlled diabetes (hemoglobin A1c level > 7%) would have a significantly increased blood glucose level following injection and would take longer to return to baseline.
Materials and Methods
Institutional review board approval was obtained for this prospective study. Members of the research team identified patients with a history of diabetes for whom a glenohumeral or subacromial corticosteroid injection was offered as a method of treatment from October 2014 to December 2015. Skeletally mature diabetic patients over the age of 18 who monitor their blood glucose level every morning were included. Patients were excluded if they did not check their blood glucose every morning, did not know their typical fasting glucose (mg/dL) or hemoglobin A1c level (percentage). We did not exclude patients based on their blood glucose control.
Before the injection was administered, informed consent was obtained from each patient. Patients were asked to recall their typical normal morning fasting glucose, their most recent hemoglobin A1c level, and their normal medication regimen, based on their recollection. We designed the study this way to best replicate the normal clinical scenario encountered at our institution. A member of the research team (UMS) contacted patients every morning to record their fasting morning glucose level for a total of 10 days post-injection. Patients were asked to maintain their normal medication regimen or insulin dosing if they used a sliding scale.
Data collected included patient age, body mass index (BMI), years of being treated for diabetes, and diagnosis being treated [Table 1]. All patients received an injection given by a shoulder and elbow fellowship trained surgeon (SN, CLG, or JAA) with a mixture of 8 mL of 1% lidocaine and 2 mL of 40 mg/mL of methylprednisolone acetate (80 mg), which is the standard injection given in our clinical practice. All injections were placed in the subacromial space. Patients were also asked if the injections ultimately provided pain relief at the end of the study period.
Table 1.
Basic Demographic Information
| Average age (years) | 60 (35-80) |
| BMI | 31 (20-41) |
| Years of Being Treated | 17.5 (5-30) |
| A1c > 7% | 9 |
| Insulin Dependent | 7 |
| Average Hemoglobin A1c Level | 7.5% (6 - 12.1) |
The primary outcome measure for this study was the average absolute difference in fasting blood glucose for 10 days following their corticosteroid injection. Patients’ average morning glucose level was also analyzed. Patients’ results were compared to determine if differe-nces existed between patients with well-controlled diabetes versus patients with poorly controlled diabetes. We chose to use patients’ hemoglobin A1c level as one surrogate for glycemic control, using a value of < 7% to define a well-controlled patient versus a value of > 7% to define a poorly controlled patient. Similarly, patients who were insulin dependent were compared to patients who did not require insulin for glycemic control. Additionally, we compared patients’ response to injection based on BMI. Analysis was performed using a student t-test for continuous variables with a p-value of 0.05 set as significant. Based on prior literature examining similar outcomes, our goal sample size was 30 patients (8-11). We did perform statistical analysis midway through enrollment to determine if this sample size was appropriate, or if an adequate number of patients were included based on differences in our primary outcome meausure.
Results
In total, 17 consecutive patients were enrolled and eligible for the study with complete follow-up data [Table 1]. On average, patients were treated for diabetes for 17.5 years and had an average BMI of 31. The average age of our cohort was 60 years (35 – 80), and eleven patients were female. Nine patients had an A1c of > 7%, and 8 patients were insulin dependent. There was no statistically significant difference in the average fasting blood glucose in patients with an A1c level < 7% (123 mg/dL) versus those with a level > 7% (143 mg/dL, P=0.18). Similarly, patients who were insulin dependent (144 mg/dL) did not have a statistically significant difference in fasting blood glucose compared to non-insulin dependent diabetic patients (126 mg/dL, P=0.24). Overall 14 patients experienced pain relief during the study period.
Patients with hemoglobin A1c of <7% and with hemoglobin A1c of >7% experienced increases in blood glucose level during the study period [Figure 1]. Patients with A1c of <7% had an average rise in blood glucose of 38 mg/dL compared to 98 mg/dL in patients with an A1c > 7% (P<0.001). When compared to baseline values, the mean glucose level during the study period was found to be statistically significant in the poorly controlled group (P=0.001), and trended toward significance in the well-controlled group (P=0.06). Mean blood glucose levels were higher in the group with an A1c of >7% at all 10 days after injection [Figure 2].
Figure 1.
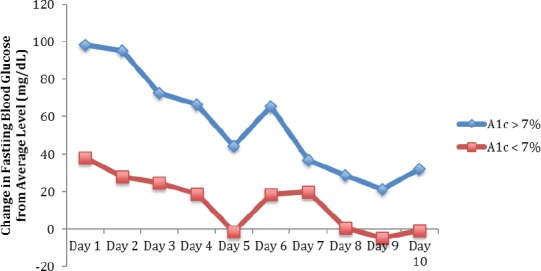
Change in Fasting Blood Glucose Following Injection for Patients with Hemoglobin A1c < 7% Versus > 7%*. *P<0.001
Figure 2.
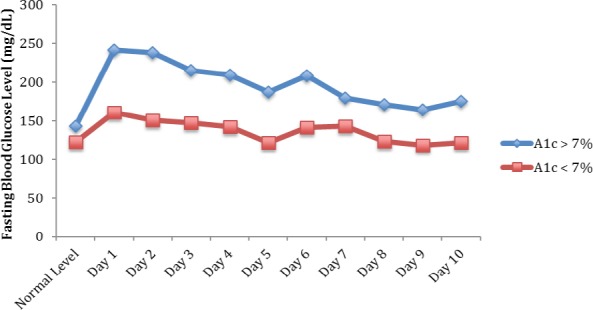
Total Average Fasting Blood Glucose Following Injection for Patients with Hemoglobin A1c < 7% Versus > 7%*. *P<0.001
Patients’ glucose levels showed a gradual decrease over the 10-day period, with well-controlled patients returning close to their baseline levels near post-injection Day 8. At post-injection Day 10, the average blood glucose level for patients with well-controlled diabetes was 122 mg/dL, equivalent to pre-injection levels. Poorly controlled patients still had an elevated, but not statistically significant, glucose of 39 mg/dL higher than normal on post-injection Day 10 (P=0.22).
Poorly controlled diabetic patients were found to have a statistically significant higher mean increase blood glucose levels over the 10 day period following injection compared to patients with a hemoglobin A1c level < 7% (P<0.001).
Similar results were found when using insulin dependence as a surrogate for glycemic control. Figures 3 and 4 illustrate these findings. Overall, patients who use insulin had an average increase in fasting glucose level from baseline on post-injection day 1 of 99 mg/dL versus 50 mg/dL in patients who did not use insulin. Both of these elevations in blood glucose level from baseline were found to be statistically significant (P=0.001 for non-insulin dependent and P=0.003 for insulin dependent). Patients who did not use insulin returned close to their baseline glucose level at post-injection Day 8, while patients who did use insulin still had a significantly increased average increase of fasting glucose of 60 mg/dL (P=0.004 compared to baseline). Insulin-dependent patients showed a statistically significant higher change in blood glucose levels following injection compared to patients who did not require insulin (P<0.001).
Figure 3.
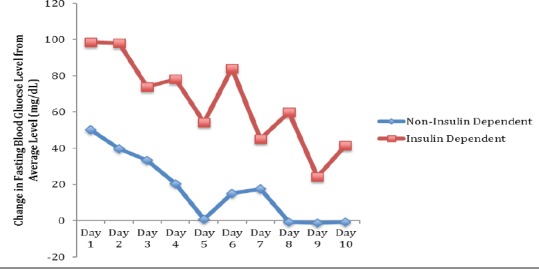
Change in Fasting Blood Glucose Following Injection for Non-Insulin Dependent Patients Versus Insulin Dependent Patients*. *P<0.001
Figure 4.
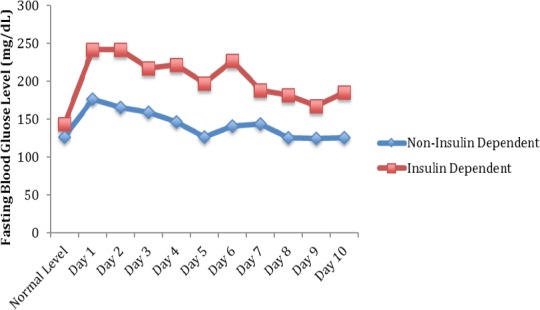
Total Average Fasting Blood Glucose Following Injection for Non-Insulin Dependent Patients Versus Insulin Dependent Patients*. *P<0.001
10 patients in the study group had a BMI > 30 compared to 7 with a BMI of < 30. Typical morning glucose levels averaged 133 mg/dL for both groups. Following corticosteroid injection, both groups of patients experienced statistically significant elevations in their blood glucose with the cohort of patients having a BMI > 30 averaging an increase of 75 mg/dL (P=0.003) and the cohort of patients having a BMI <30 experiencing an elevation of 44 mg/dL (P=0.06). The difference between groups was not statistically significant (P=0.49; Figure 5). Both groups mean glucose levels returned to typical levels by post-injection Day 8.
Figure 5.
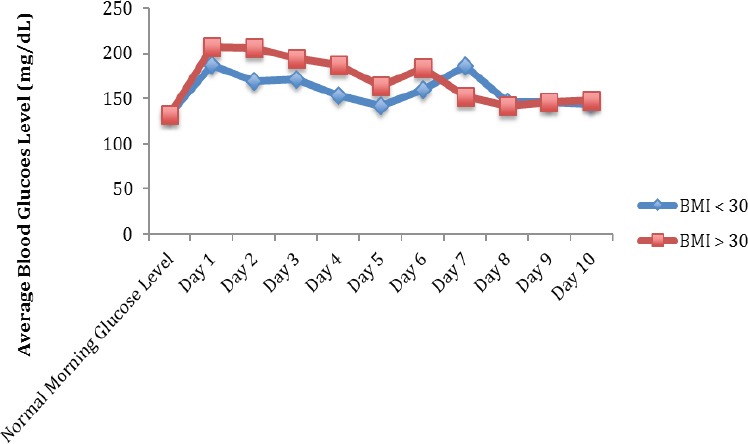
Total Average Fasting Blood Glucose Following Injection Based on BMI*. *P=0.50
Discussion
The purpose of this study was to evaluate the effects of subacromial and glenohumeral corticosteroid injections on diabetic patients in order to better provide counseling to this patient group. We hypothesized that patients with poorly controlled diabetes, defined as a hemoglobin A1c level of > 7%, would respond with higher changes in fasting blood glucose compared to patients with well controlled diabetes. Secondary analysis was also performed comparing patients who require insulin versus those that do not, another surrogate for diabetic control, with similar findings. Additionally, we found that BMI was not associated with differences following injection in mean glucose levels. This study found that all patients experienced a clinically significant increase in their fasting blood glucose level with poorly controlled patients experiencing statistically significant greater changes for a longer time.
Several studies have evaluated the relationship between corticosteroid injections and blood glucose levels (7-11). Most of the recent literature has focused on injections in the hand, and do show varying increases in blood glucose level. Kim et al performed injections in the hand for a variety of conditions in 20 diabetic patients using 10 mg of triamcinolone acetonide. They found that patients with a hemoglobin A1c level > 7% had significantly increased elevation in blood glucose (10). Patients with low hemoglobin A1c levels returned to their baseline blood glucose level 2 days after injection, compared to 5 days for the poorly controlled group. They found no correlation with results based on patient’s insulin use. Stepan et al performed an analysis of 40 consecutive patients with varying doses of methylprednisolone acetate (20 - 120 mg) for the treatment of hand and wrist pathologies (8). They found that blood glucose levels rose significantly on post-injection day 1 and 2 in patients taking insulin or those with type I diabetes. All patients’ levels returned to baseline by day 3. They did report a limited analysis on a subset of patients that reported their hemoglobin A1c level (75% of patients), and reported a poor correlation between glucose elevation and hemoglobin A1c levels.
One case series by Habib et al provides information on the affects of blood glucose level following injections in the shoulder joint (11). In this series, 18 patients were injected with 35 mg of methylprednisolone acetate in the glenohumeral joint for a variety of pathologies. The average hemoglobin A1c level was 7.5%. They found no significant change between average glucose values or average fructosamine values in the patients group. They concluded that intra-articular corticosteroid injections about the shoulder had no significant effect on blood glucose. They did not perform any type of analysis on patients with elevated hemoglobin A1c levels or patients who were insulin dependent, mostly due to the fact that almost none of the patients in their study had any significant increase in blood glucose level.
At our institution, we routinely perform injections for the shoulder with a dose of methylprednisolone acetate of 80 mg, which would theoretically have a greater effect on a patient’s blood glucose level. Our study design attempted to replicate our current clinical practice, in which all patients who present for treatment are offered a standard injection in the shoulder for treatment. Our goal was to find a relationship between common surrogates for long-term glycemic control and a quantitative effect on blood glucose level. Based on the findings of this study, we are able to advise patients with well-controlled diabetes (hemoglobin A1c < 7%) to expect an average rise in their fasting blood glucose level between 30-40 mg/dL for 4 days following an injection with a return to baseline after 8 days. Similarly, patients who do not use insulin can expect similar changes in their blood glucose. Patients with elevated hemoglobin A1c levels, or who use insulin, should be advised to expect changes in their blood glucose of > 80 mg/dL for the first 3-4 days following injection, and they may not return to baseline levels for more than 10 days following injection.
The difference in the results from our study and those of Habib et al demonstrate a dose-dependent response of blood glucose levels based on the amount of corticosteroid injected in the shoulder. This information allows clinicians to potentially alter the dose of corticosteroid given in an injection to balance the treatment benefit against the risk of significantly elevating patients’ blood glucose for a prolonged period of time. The optimal dose of corticosteroid that provides treatment benefit is still not known, but 14 of 17 patients in our study did state that they received benefit from their injection with 80 mg of methylprednisolone acetate.
There are several limitations to our study. Injections were performed with only one type and dose cortico-steroid and cannot be generalized to other corticosteroids or doses. Theoretically, patients with poor glycemic control would experience smaller elevations in blood glucose with smaller doses, but we utilized a dose that is the standard of clinical practice at our institution for shoulder injections. We did not prospectively collect patients’ baseline blood glucose levels, relying on their memory, which may introduce recall bias. Additionally, hemoglobin A1c levels reported did not represent a level done in the 3 months prior to treatment, only their most recent level to date, so may not accurately represent a patient’s blood control. We tried to emulate the normal clinical scenario as best as possible in which patients do not routinely obtain baseline levels of their diabetic control prior to receiving treatment. The total number of patients included in this study is well below the goal sample size of 30. However, our results show clinically meaningful and statistically significant differences in our primary and several secondary outcome measures prior to enrolling all 30 patients. Based on the statistical analysis this study was not underpowered.
The results of this study found that diabetic patients receiving an injection of 80 mg of methylprednisolone for treatment of shoulder pathologies will experience a clinically significant increase in their fasting blood glucose levels. Patients with well-controlled diabetes experience smaller elevations and return to baseline levels faster than patients with poorly controlled diabetes. Poorly controlled diabetic patients can experience significant elevations in blood glucose for more than 10 days following injection. These findings are consistent using both hemoglobin A1c > 7% and use of insulin as surrogates for glycemic control. This study should allow practitioners to give their patients more objective data regarding the glycemic control risks of cortisone injections in diabetic patients. Future studies are needed to determine the optimum dose of corticosteroid for diabetic patients that will provide treatment benefit while minimizing the risk of prolonged elevated blood glucose levels.
References
- 1.Arslan S, Celiker R. Comparison of the efficacy of local corticosteroid injection and physical therapy for the treatment of adhesive capsulitis. Rheumatol Int. 2001;21(1):20–3. doi: 10.1007/s002960100127. [DOI] [PubMed] [Google Scholar]
- 2.Cole BF, Peters KS, Hackett L, Murrell GA. Ultrasound-guided versus blind subacromial corticosteroid injections for subacromial impingement syndrome: a randomized, double-blind clinical trial. Am J Sports Med. 2016;44(3):702–7. doi: 10.1177/0363546515618653. [DOI] [PubMed] [Google Scholar]
- 3.Hart L. Corticosteroid and other injections in the management of tendinopathies: a review. Clin J Sport Med. 2011;21(6):540–1. doi: 10.1097/01.jsm.0000407929.35973.b9. [DOI] [PubMed] [Google Scholar]
- 4.Lorbach O, Kieb M, Scherf C, Seil R, Kohn D, Pape D. Good results after fluoroscopic-guided intra-articular injections in the treatment of adhesive capsulitis of the shoulder. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1435–41. doi: 10.1007/s00167-009-1030-7. [DOI] [PubMed] [Google Scholar]
- 5.Warner JJ. Frozen shoulder: diagnosis and management. J Am Acad Orthop Surg. 1997;5(3):130–40. doi: 10.5435/00124635-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Ranalletta M, Rossi LA, Bongiovanni SL, Tanoira I, Elizondo CM, Maignon GD. Corticosteroid injections accelerate pain relief and recovery of function compared with oral NSAIDs in patients with adhesive capsulitis: a randomized controlled trial. Am J Sports Med. 2016;44(2):474–81. doi: 10.1177/0363546515616238. [DOI] [PubMed] [Google Scholar]
- 7.Habib GS, Miari W. The effect of intra-articular triamcinolone preparations on blood glucose levels in diabetic patients: a controlled study. J Clin Rheumatol. 2011;17(6):302–5. doi: 10.1097/RHU.0b013e31822acd7c. [DOI] [PubMed] [Google Scholar]
- 8.Stepan JG, London DA, Boyer MI, Calfee RP. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39(4):706–12. doi: 10.1016/j.jhsa.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Even JL, Crosby CG, Song Y, McGirt MJ, Devin CJ. Effects of epidural steroid injections on blood glucose levels in patients with diabetes mellitus. Spine (Phila Pa 1976) 2012;37(1):E46–50. doi: 10.1097/BRS.0b013e31821fd21f. [DOI] [PubMed] [Google Scholar]
- 10.Kim N, Schroeder J, Hoffler CE, Matzon JL, Lutsky KF, Beredjiklian PK. Elevated hemoglobin A1C levels correlate with blood glucose elevation in diabetic patients following local corticosteroid injection in the hand: a prospective study. Plast Reconstr Surg. 2015;136(4):474e–9e. doi: 10.1097/PRS.0000000000001624. [DOI] [PubMed] [Google Scholar]
- 11.Habib GS, Abu-Ahmad R. Lack of effect of cortico-steroid injection at the shoulder joint on blood glucose levels in diabetic patients. Clin Rheumatol. 2007;26(4):566–8. doi: 10.1007/s10067-006-0353-8. [DOI] [PubMed] [Google Scholar]
- 12.Lasker RD. The diabetes control and complications trial. Implications for policy and practice. N Engl J Med. 1993;329(14):1035–6. doi: 10.1056/NEJM199309303291410. [DOI] [PubMed] [Google Scholar]
- 13.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed N. Advanced glycation endproducts-role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67(1):3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Giori NJ, Ellerbe LS, Bowe T, Gupta S, Harris AH. Many diabetic total joint arthroplasty candidates are unable to achieve a preoperative hemoglobin A1c goal of 7% or less. J Bone Joint Surg Am. 2014;96(6):500–4. doi: 10.2106/JBJS.L.01631. [DOI] [PubMed] [Google Scholar]


