Abstract
Since the early work of August Krogh in 1919, capillaries have been assumed to be the sole supplier of oxygen for tissue. Recent studies provide convincing evidence that other microvessels also contribute to tissue oxygenation and that capillaries play a much more complex role than originally proposed by Krogh.
August Krogh (7), a Danish physiologist, provided the first insights into the role of the smallest microvessels in the supply of oxygen to striated muscle. His studies, carried out in the early part of the 20th century, focused on the capillaries as the sole site of diffusive oxygen transfer from the blood to the tissue, a concept that still has wide acceptance. In the half century that followed, little additional experimental information was obtained, due largely to the lack of the technology required to critically evaluate Krogh’s concepts. In the past 25 years, the necessary methodology has been developed and, as a consequence, much new research has been undertaken in this area. The results of these studies have provided new insights into the complex nature of oxygen delivery to tissue, results which suggest that capillaries may not be unique in their ability to supply oxygen to tissue.
Krogh’s studies were confined to the capillary network, since he presumed the capillaries to be the unique supplier of oxygen to the tissue. This selection of capillaries as the oxygen providers was due largely to their large surface area-to-volume ratio, close proximity to parenchymal cells, and low velocity of red blood cells passing through them. In addition, he proposed that each capillary obtained all of its oxygen by convection (bulk flow) from the terminal arterioles. Each capillary, in turn, served as an independent diffusive source delivering oxygen to a single distinct volume of tissue with homogeneous oxygen consumption. Thus Krogh’s concept predicted a linear fall in hemoglobin oxygen content along each capillary.
These basic ideas are illustrated in Fig. 1. For an extensive discussion of Krogh’s model and its assumptions, refer to Kreuzer (6). The mathematical descriptions of oxygen transport based on Krogh’s tissue cylinder model were recently reviewed by Popel (9).
FIGURE 1.
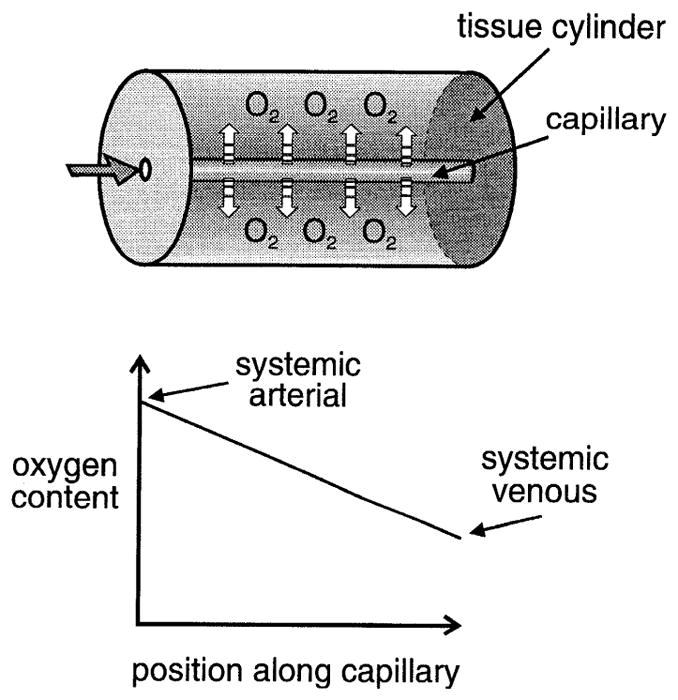
Top: Krogh’s model of oxygen supply to tissue considers each capillary to be the unique supplier of oxygen to a tissue cylinder. Bottom: predicted oxygen content as a function of distance along capillary. Because model assumes that all oxygen exchange occurs in capillary network, oxygen content at entrance to capillary must be equal to that in systemic arterial blood. Oxygen diffuses out along length of capillary, resulting in a linear decrease in oxygen content that reaches a value at the end of the capillary equal to that found in systemic venous blood.
Acceptance of Krogh’s concept has several important consequences. 1) Because Krogh considered each capillary to be the source of oxygen for a unique volume of tissue, the distance between capillaries, or a related quantity, capillary density, within a specific tissue must be an important determinant of tissue oxygenation. Increased oxygen demand requires a higher capillary density than in a resting tissue, hence the concept of “capillary recruitment” in muscle during exercise. 2) Because oxygen is being released at a uniform rate from red blood cells as they travel through the capillary, their transit time from arteriole to venule must determine whether the tissue will be adequately supplied with oxygen, i.e., there must be sufficient time for oxygen to be released from hemoglobin, the primary oxygen carrier. Each of these ideas has significantly affected the interpretation of investigations of tissue oxygenation.
Critical evaluation of Krogh’s predictions required the collection of extensive experimental data on different aspects of the oxygen delivery process. These studies were undertaken in different animals and tissues, thus requiring the establishment of a consistent nomenclature for classifying individual vessels based on their location within the microvascular network.
The classification scheme widely used by those interested in hemodynamics and oxygen transport designates the largest vessels that can be considered part of the microvasculature to be first order (1°). The vessels that branch from these vessels are classified as second order (2°), and so on. The vessels continue to diverge, forming a network composed of progressively smaller diameter and more numerous vessels, whose branch lengths become shorter. The capillaries represent the terminal branches of the network with single-file red cell flow and diameters less than that of an undeformed red blood cell. The 1° and 2° arterioles usually run in parallel with venules. The smaller arterioles are frequently found crossing above or below groups of capillaries. With the development of a uniform nomenclature, information about red cell dynamics and oxygenation in different animals and tissues could be organized and compared with the expectation that a consistent picture would emerge.
One of the first studies to evaluate oxygen exchange in the microvasculature was published by Duling and Berne in 1970 (1). In a study carried out in superfused rat and hamster cremaster muscles, and the hamster cheek pouch, they provided evidence that oxygen can diffuse from precapillary vessels. They determined the Po2 on the surface of arterioles from four consecutive branching orders using Po2 microelectrodes positioned adjacent to the vessel wall. In the hamster cheek pouch, they observed a 15-mmHg drop in Po2 between a large 80-μm arteriole and small terminal arterioles having diameters of 5–10 μm. This coincides with an estimated decrease in oxygen saturation (So2) of almost 40% So2, with So2 being defined as the percentage of oxygen binding sites on hemoglobin that have oxygen molecules bound to them. This decrease in saturation represents >50% of the entire arterial-venous So2 difference for this tissue.
Similar evidence of precapillary oxygen losses has been observed in the cat and rabbit pial circulations (2, 5). The presence of the superfusion solution may be partly responsible for the magnitude of the precapillary losses. However, similar findings in the Saran-covered retractor muscle (8) indicate that the loss of oxygen from the arteriolar network is not an artifact introduced by the presence of the superfusion solution.
These results raise several important issues. If 50% of the arterial-venous So2 difference occurs in arterioles, where has the oxygen gone and what role do capillaries play in tissue oxygenation? Because oxygen is a readily diffusible substance and arterioles have relatively thin oxygen-permeable walls, any location having an oxygen tension lower than that of the arteriole should be considered as a possible destination.
Thus possible “sinks” for oxygen include 1) the oxygen-consuming tissue immediately surrounding the arteriole, including the arteriolar wall itself; 2) the venule running in parallel with the arteriole, causing the oxygen to effectively bypass the capillary network; and 3) nearby capillaries either crossing or running in parallel with the arteriole, Supplementing the oxygen that enters the capillaries by convection. most recent studies have focused on determining the relative importance of the various sinks for oxygen and characterizing oxygen transport in capillaries in an attempt to understand how these vessels contribute to tissue oxygenation.
Among the proposed destinations for oxygen that diffused out of the arterioles, the most obvious sink was its consumption by the tissue immediately surrounding each arteriole. An evaluation of the amount of oxygen that could be accounted for by this mechanism was made using data obtained by Kuo and Pittman (8) on oxygen losses in the largest four orders of arterioles in the hamster retractor muscle. They determined hemoglobin oxygen saturation spectrophotometrically in conjunction with red cell velocity and microvessel hematocrit and computed the longitudinal So2 gradient (difference in So2 per unit length of arteriole) in each vessel.
Kuo and Pittman (8) found that oxygen saturation decreased from 78% in systemic arterial blood to 67% in large 1° arterioles having an average internal diameter of 62 μm, then fell gradually to 51% in 4° arterioles with an internal diameter of 23 μm. Using these data, they calculated what portion of the lost oxygen could be accounted for by tissue oxygen consumption.
In their calculation, Kuo and Pittman (8) adapted Krogh’s cylinder model of oxygen exchange for arterioles, assumed literature values for both tissue oxygen consumption and the permeability of the tissue for oxygen, the latter defined as the product of the diffusion and solubility coefficients, and assumed a maximum size for the “Krogh cylinder” based on the arteriolar Po2 estimated from the So2 measurement. They concluded that only ~10–15% of the lost oxygen could be directly utilized by the retractor muscle. Thus it is clear that a significant amount of the oxygen must diffuse to other locations such as the paired venules, in the case of the 1° and 2° arterioles, or to the capillaries.
The diffusive shunting of oxygen from arterioles to venules, the first of these two remaining possible sinks, would be primarily countercurrent in nature, with the amount of shunted oxygen presumably affected by relative Po2 values, red cell velocity in the paired arterioles and venules, the distance separating them, and the total surface area available for exchange.
Sharan and Popel (11) mathematically modeled oxygen exchange between an arteriole and venule, incorporating a range of possible values for these parameters. They determined that only under very unusual circumstances, e.g., very low blood flow, could the diffusive shunting of oxygen between arterioles and venules be of any major importance.
Recent experimental data obtained by Stein et al. (12) in the hamster retractor muscle showed that Po2 can increase by an average of 10 mmHg (19% So2) between the end of the capillary network and the large l° venule when animals inspired 30% O2 and by 4 mmHg (6% So2) when animals inspired room air, with no significant difference observed when animals inspired 10% O2. This oxygenation of venular blood under non-hypoxic conditions indicates that the tissue Po2 surrounding the venular network is, on average, greater than the end-capillary Po2. To what extent this elevated Po2 is a result of diffusive losses from arterioles vs. a generalized loss from the capillary network remains to be ascertained. However, even in the presence of a significant diffusive shunt between paired arterioles and venules, a significant amount of the lost oxygen remains unaccounted for. The only obvious sink remaining is the capillary.
Anatomically, capillaries primarily run parallel to the muscle fibers, frequently running either parallel to or crossing above or below larger microvessels. Therefore, it is not unreasonable to presume that the relatively slowly moving red blood cells within the thin-walled capillaries can pick up oxygen by diffusion from these vessels. However, Krogh’s concept of the role of the capillary as a supplier of oxygen to tissue conflicts with the potential role of the capillary as a sink for oxygen from other vessels.
What evidence do we have that capillaries can have a dual role, acting as both a supplier of oxygen to tissue and a diffusive sink for oxygen from other capillaries and larger microvessels? Initial studies evaluating oxygen transport in capillaries focused on determinations of red cell dynamics, since it was presumed that one could evaluate oxygen supply to tissue based on these determinations. Measurements were made of red cell velocity, the number of red blood cells per unit length of capillary, and the number of red blood cells per unit time passing through the capillary.
It soon became obvious that all of these parameters were markedly heterogeneous when evaluated at the capillary-to-capillary level, within each capillary network, and on a larger scale between networks and between different regions of the tissue (4, 9). Subsequent determinations of So2 and oxygen tension indicated that each of these quantities is likewise nonuniform (4).
However, one result was clear; on average, So2 at the arteriolar end of the capillary network is higher than So2 at the venular end. To this extent, Krogh’s concept was correct. Capillaries supply oxygen to tissue. However, the So2 gradient along the length of a capillary was found to be independent of the number of red blood cells passing through it per unit time, a result that is in contrast to what Krogh’s model would predict. One possible explanation for the lack of a relationship would be that the capillaries interact diffusively with one another. If this is the case, then each capillary is no longer the unique supplier of oxygen to a distinct tissue volume, the basic premise of Krogh’s model.
Further evidence for the presence of diffusive interactions among capillaries came from a study in the hamster retractor muscle (4). In this study, So2 and red cell dynamics were determined at the two ends of the capillary network. It was found that the average longitudinal So2 gradient (amount of oxygen lost per unit length of vessel) obtained from measurements in single capillaries was 0.11 %/μm, with no statistical difference between the arteriolar and venular ends. However, computation of the average longitudinal So2 gradient obtained by dividing the difference in mean So2 determined at the two ends of the network (60.8–39.9%) by the average anatomic capillary length (412 μm) resulted in a value of 0.05%/μm, which is smaller by a factor of two.
One explanation for this result would be that there is diffusive shunting of oxygen between adjacent capillaries. Further examination of these data revealed that in capillaries at both ends of the network, approximately one–third of all capillaries gained oxygen. In pairs of capillaries in which one capillary lost oxygen while the other gained it, the average loss was about two times the average gain, a result consistent with a portion of the lost oxygen being used by the tissue immediately surrounding the capillary.
Subsequently, these data were utilized in model calculations evaluating the influence of the observed heterogeneity in the hemodynamic and oxygenation parameters at the arteriolar end of the capillary network on the variability in So2 at the venular end of the network. If Krogh’s model was correct, there should be a strong dependence on this nonuniformity. Surprisingly, heterogeneities in intercapillary distances, red cell supply rate, and capillary inlet So2 had little impact on the variability in So2 at the venular end of the network in resting striated muscle. However, variability in capillary flow path length was found to have an important effect.
This result is consistent with the premise derived from the experimental results that the loss of oxygen from capillaries is influenced by the presence of neighboring capillaries and that oxygen can enter capillaries by diffusion. The extent of such diffusive exchange should be affected by red cell velocity. If, as in the case of exercise, red cell velocity increases, the extent of diffusive exchange should decrease, due to a decrease in red cell residence time, and the heterogeneities in the hemodynamic and oxygenation parameters could become more critical leading to regions of tissue hypoxia.
This question has yet to be addressed experimentally. However, the theoretical analysis described above was extended to exercising muscle, using estimates of the increase in red cell flow and oxygen consumption expected at each of two different levels of exercise. Under these conditions, the model predicts that heterogeneities in the hemodynamic parameters will markedly affect oxygen transport in this muscle. This would again support the supposition that diffusive shunting of oxygen between adjacent capillaries is at least partially responsible for the result in resting muscle. Thus experimental and theoretical evidence supports the idea that the decrease in So2 along the length of capillaries in resting muscle is neither linear nor unidirectional.
If oxygen can be diffusively picked up by the red blood cells within the capillaries, are other capillaries the only potential source? In 1990, Ellsworth and Pittman (3) provided data to suggest that other capillaries are not the only diffusive source of oxygen for capillaries. They determined So2 at discrete locations along the length of individual capillaries and again found that the amount of oxygen lost from a single capillary per unit length was independent of red cell supply rate.
However, of greater importance was their observation that the amount of oxygen lost was strongly influenced by the presence of larger microvessels in the vicinity of capillaries that either supply oxygen (primarily arterioles) or act as either sources or sinks (venules) depending on a variety of factors. A direct consequence of such diffusive interaction is the potential for a more uniform distribution of oxygen than might result if all oxygen arrived via convection. These findings add a further degree of complexity to the oxygen exchange picture, since one cannot use hemodynamic results to predict changes in oxygen exchange and cannot easily extrapolate whole organ data to the microvasculature.
To what extent such diffusive exchange between large microvessels and capillaries occurs in circumstances other than resting muscle is just beginning to be investigated. Theoretical evaluation of diffusive oxygen exchange between arterioles and capillaries by Secomb and Hsu (10) suggests that 40–50% of the lost oxygen is delivered to the capillaries by diffusion from the arterioles in resting muscle. Under conditions of elevated red cell supply rate and oxygen consumption, as would occur in exercising muscle, the amount of oxygen delivered to the capillaries from the arterioles by diffusion is reduced while that delivered by convection is enhanced. However, the diffusive contribution might still be significant. Experimental verification of the role of diffusive exchange between large microvessels and capillaries in tissues other than resting muscle remains to be ascertained.
Numerous other very important issues of oxygen transport have emerged in recent years, many of which have been discussed in detail by Popel (9). Theoretical work has suggested that a major part of the resistance to oxygen transport is confined within the capillaries due to the particulate nature of blood. This resistance was subsequently quantified for different values of capillary hematocrit and intererythrocyte spacing. Although direct experimental measurements of intracapillary and transendothelial Po2 gradients are not available, substantial indirect evidence for the role of intracapillary resistance from whole organ studies has been accumulated.
Myoglobin-facilitated diffusion of oxygen in skeletal muscle and heart is another important factor that needs to be considered, especially under conditions of high metabolic rate or low oxygen delivery. Both experimental and theoretical studies indicate that myoglobin-facilitated diffusion in combination with high intracapillary transport resistance causes Po2 gradients within muscle cells to be small.
It is clear from the data obtained to date that a significant amount of oxygen can be lost from the precapillary vessels. The destination of the lost oxygen remains unclear, although some conclusions can be drawn as illustrated in Figs. 2 and 3. It is probable that a significant portion of the lost oxygen is used by the tissue for metabolism rather than being shunted through “nonnutritive flow paths” or short-circuited to the venule via a diffusive shunt.
FIGURE 2.
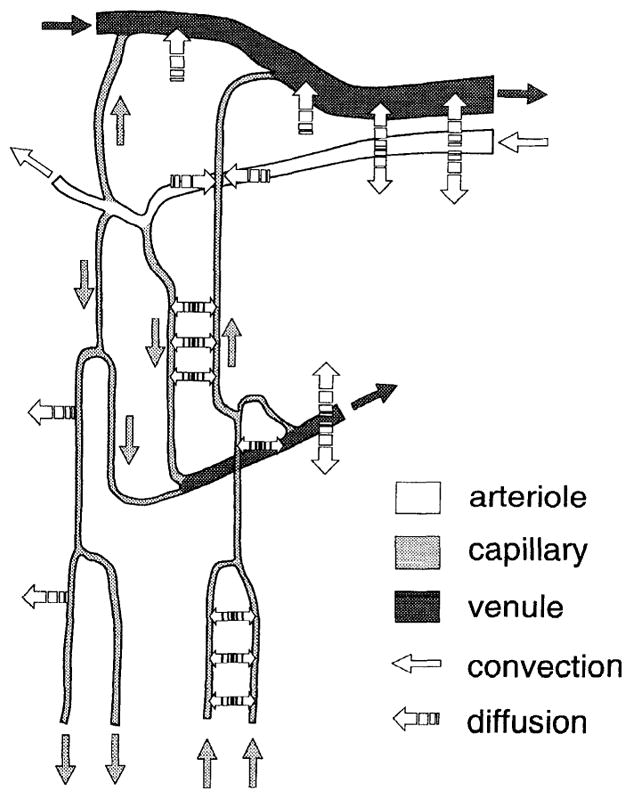
Oxygen exchange among microvessels. Our current understanding of oxygen supply to tissue requires incorporation of extensive diffusive interactions among microvessels. A vascular network is shown containing arterioles, venules, and capillaries, with diffusion of oxygen indicated by interrupted arrows and convection of oxygen in arterioles, capillaries, and venules indicated by appropriately shaded solid arrows. A significant amount of oxygen has been shown to diffuse out of arterioles. Some of the oxygen that leaves arterioles is consumed by tissue immediately surrounding them, some is shunted to nearby venules, while the remaining portion is picked up by capillaries and subsequently distributed to tissue. Exchange of oxygen within capillary network is affected by oxygen tension in adjacent capillaries as well as nearby arterioles and venules. Thus consideration of various diffusive interactions among all types of microvessels is key to understanding oxygen supply to tissue.
FIGURE 3.
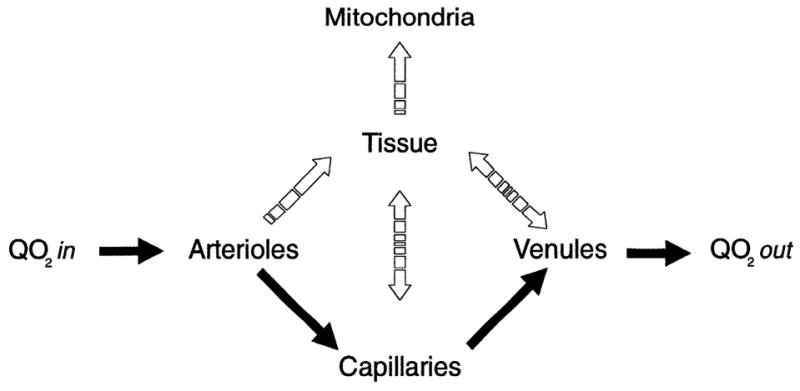
Various pathways oxygen takes once it enters microvasculature by convection (Qo2 in) are presented as a flow diagram. Solid arrows indicate convective flow, and interrupted arrows indicate diffusive pathways. Diffusive pathway that oxygen takes is determined by oxygen tension gradient present at a particular location. Oxygen that does not diffuse out of microvessels is returned to systemic circulation by convection (Qo2 out).
The portion of oxygen consumed by the tissue arrives either directly from the arteriole, which would account for consumption in a localized region surrounding a vessel, or by first diffusing into the capillaries to be dispersed to a much wider tissue region. Some of it is likely shunted to the venules. With the experimental data currently available, we still cannot explain all of the arteriolar oxygen loss, nor can we mathematically model the emerging picture of oxygen transport using the currently available values for variables such as tissue oxygen permeability, a variable whose direct measurement under in vivo conditions is problematic.
While much has been learned from the microcirculatory approach, one must keep in mind that conditions in the intact undisturbed tissue may differ from those that exist in the preparations that have be en used to date. The exteriorization of a tissue clearly alters its environment. However, researchers have worked hard to minimize the differences between the experimental and intact conditions and to ascertain how such differences might affect our interpretation of the data. Therefore, although it is important to keep in mind the experimental situation, it is likely that what we have learned about the complex nature of oxygen supply to tissue reflects the situation one would expect to observe in an intact undisturbed tissue.
There is a great deal more to be learned. However, one thing is clear: the supply of oxygen to tissue is a complex matter. Krogh’s early concepts were focused on a single capillary and, to the extent that such a condition exists in a tissue, much of what he proposed is likely to be correct. However, the experimental data obtained in the past quarter century indicate that understanding oxygen supply requires a change in focus. No longer can one deal with just a single capillary. Rather, one must consider groups of capillaries supplying a finite volume of tissue and the diffusive exchange among these vessels and the larger microvessels. Only when all of these facets of oxygen exchange are taken into account can one hope to obtain a realistic picture of how oxygen is supplied to tissue.
Contributor Information
Mary L. Ellsworth, Dept. of Pharmacological and Physiological Science, St. Louis University School of Medicine, 1402 South Grand Blvd., St. Louis, MO 63104, USA
Christopher G. Ellis, Dept. of Medical Biophysics, Univ. of Western Ontario, London, Ontario N6A 5A5, Canada
Aleksander S. Popel, Dept. of Biomedical Engineering, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA
Roland N. Pittman, Dept. of Physiology, Medical College of Virginia, Richmond, VA 23298, USA
References
- 1.Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension: a possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res. 1970;27:669–678. doi: 10.1161/01.res.27.5.669. [DOI] [PubMed] [Google Scholar]
- 2.Duling BR, Kuschinsky W, Wahl M. Measurements of the perivascular Po2 in the vicinity of the pial vessels in the cat. Pfluegers Arch. 1979;383:29–34. doi: 10.1007/BF00584471. [DOI] [PubMed] [Google Scholar]
- 3.Ellsworth ML, Pittman RN. Arterioles supply oxygen to capillaries by diffusion as well as by convection. Am J Physiol. 1990;258:H1240–H1243. doi: 10.1152/ajpheart.1990.258.4.H1240. (Heart Circ. Physiol. 27) [DOI] [PubMed] [Google Scholar]
- 4.Ellsworth ML, Popel AS, Pittman RN. Assessment and impact of heterogeneities of convective oxygen transport parameters in capillaries of striated muscle. Microvasc Res. 1988;35:341–362. doi: 10.1016/0026-2862(88)90089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivanov KP, Derii AN, Samoilov MO, Semenov DG. Diffusion of oxygen from the smallest arteries of the brain. Pfluegers Arch. 1982;393:118–120. [Google Scholar]
- 6.Kreuzer F. Oxygen supply to tissues: the Krogh model and its assumptions. Experientia Basel. 1982;38:1415–1426. doi: 10.1007/BF01955753. [DOI] [PubMed] [Google Scholar]
- 7.Krogh A. The Anatomy and Physiology of Capillaries. New York: Hatner; 1959. [Google Scholar]
- 8.Kuo L, Pittman RN. Effect of hemodilution on oxygen transport in arteriolar networks of hamster striated muscle. Am J Physiol. 1988;254:H331–H339. doi: 10.1152/ajpheart.1988.254.2.H331. (Heart Circ. Physiol. 23) [DOI] [PubMed] [Google Scholar]
- 9.Popel AS. Theory of oxygen transport to tissue. Crit Rev Biomed Eng. 1989;17:257–321. [PMC free article] [PubMed] [Google Scholar]
- 10.Secomb TW, Hsu R. Simulation of oxygen transport in skeletal muscle: diffusive exchange between arterioles and capillaries. FASEB J. 1993;6:A902. doi: 10.1152/ajpheart.1994.267.3.H1214. [DOI] [PubMed] [Google Scholar]
- 11.Sharan M, Popel AS. A mathematical model of countercurrent exchange of oxygen between paired arterioles and venules. Math Biosci. 1988;91:17–34. doi: 10.1016/0025-5564(88)90022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein JC, Ellis CG, Ellsworth ML. Relationship between capillary and systemic venous Po2 during nonhypoxic and hypoxic ventilation. Am J Physiol. 1993;265:H537–H542. doi: 10.1152/ajpheart.1993.265.2.H537. (Heart Circ. Physiol 34) [DOI] [PubMed] [Google Scholar]


