Summary:
It is not clear why some children have life-threatening RSV disease. We found RSV and any viral coinfection compared to RSV monoinfection is not associated with more severe disease. Increased life-threatening disease in RSV-ADV and RSV-Influenza coinfection warrants further study.
Keywords: respiratory syncytial virus, viral coinfection, lower respiratory tract infection disease severity
Abstract
Background.
Molecular diagnostics enable sensitive detection of respiratory viruses, but their clinical significance remains unclear in pediatric lower respiratory tract infection (LRTI). We aimed to determine whether viral coinfections increased life-threatening disease in a large cohort.
Methods.
Molecular testing was performed for respiratory viruses in nasopharyngeal aspirates collected from children aged <5 years within 24 hours of hospital admission during sentinel surveillance for severe acute respiratory illness (SARI) hospitalization conducted in South Africa during February 2009–December 2013. The primary outcome was life-threatening disease, defined as mechanical ventilation, intensive care unit admission, or death.
Results.
Of 2322 HIV-uninfected children with respiratory syncytial virus (RSV)–associated LRTI, 1330 (57.3%) had RSV monoinfection, 38 (1.6%) had life-threatening disease, 575 (24.8%) had rhinovirus, 347 (14.9%) had adenovirus (ADV), and 30 (1.3%) had influenza virus. RSV and any other viral coinfection was not associated with severe disease (odds ratio [OR], 1.4; 95% confidence interval [CI], OR, 0.74; 95% CI, .39–1.4), ADV coinfection had increased odds of life-threatening disease (adjusted OR, 3.4; 95% CI, 1.6–7.2; P = .001), and influenza coinfection had increased odds of life-threatening disease and prolonged length of stay (adjusted OR, 2.1; 95% CI, 1.0–4.5; P = .05) compared with RSV monoinfection.
Conclusions.
RSV coinfection with any respiratory virus is not associated with more severe disease when compared to RSV alone in this study. However, increased life-threatening disease in RSV-ADV and RSV-influenza coinfection warrants further study.
Respiratory syncytial virus (RSV) is a global health problem, causing an estimated 66000–199000 deaths per year globally in children <5 years of age [1]. The clinical manifestations of RSV infection range widely from a mild, self-limiting upper respiratory tract infection (URTI) to severe lower respiratory tract infection (LRTI), which may lead to death. Risk factors for severe disease include premature birth, low birth weight, immunocompromised status, chronic lung disease, congenital heart disease, human immunodeficiency virus (HIV) infection, and Down syndrome [2–8]; however, the majority of infants hospitalized for RSV LRTI are previously healthy children [9].
Currently, management options for RSV-associated disease are limited, with supportive treatment as the cornerstone of clinical care [10]. Therefore, it is essential to gain insight into factors contributing to disease severity to effectively direct future preventive and therapeutic interventions.
The development of sensitive molecular diagnostics for the detection of respiratory viruses has given insight into the viral respiratory dynamics during severe respiratory infection [11]. There are conflicting data on whether viral coinfection results in more severe RSV-associated LRTI. Whereas some studies report an association for RSV–human metapneumovirus (HMPV) coinfection and less severe disease [12–14], others report more severe disease associated with RSV-HMPV, RSV-rhinovirus (RV), RSV-adenovirus (ADV), and any coinfection compared to identification of RSV alone [15–21]. Furthermore, no association with disease severity for RSV-RV, RSV-HMPV, and any viral coinfection has been reported by others [13, 22–25]. The majority of these studies are limited by assessment over a single season [15, 18, 26], lack of adjustment for confounders [13,15,20,22–25], and small sample size (38–666 RSV cases), all of which could bias the interpretation of the results.
The aim of this study was to evaluate the effect of respiratory viral coinfection on disease severity among children hospitalized with RSV-associated LRTI.
METHODS
Study Site, Design, and Population
Children <5 years of age hospitalized with severe acute respiratory illness (SARI) were enrolled in a prospective, hospital-based, sentinel surveillance study conducted at 6 sites in 4 provinces in South Africa from February 2009 through December 2013 as described elsewhere [27]. Four rural, periurban, and urban hospital sites enrolled children in 3 provinces (Gauteng, Mpumalanga, KwaZulu-Natal), and 2 sites were added in a fourth province (North West) in June 2010. There were a total of 24 pediatric intensive care unit (ICU) beds available across all sites.
Data Collection and Case Definition
SARI was defined among hospitalized children as follows: physician-diagnosed sepsis or LRTI in children aged 2 days to 3 months; or physician-diagnosed LRTI in children aged 3 months to 5 years, presenting within 7 days of symptom onset. Exclusion criteria were transfer from another hospital, neonates who were never discharged after delivery, and children residing outside of the hospital catchment area. A nasopharyngeal aspirate (NPA) in 4 mL of normal saline and a blood sample were collected from the child, ideally within 24 hours of admission but up to 7 days after onset of symptoms. Specimens were transported within 72 hours of collection to the National Institute for Communicable Diseases in Johannesburg for viral and bacterial analysis. Demographic and hospitalization data were collected by interview and record review, and children were followed up to hospital discharge.
Laboratory Testing
RSV infection was confirmed via multiplex real-time reverse-transcription polymerase chain reaction (PCR) assay performed on collected NPAs. NPAs were also tested for 9 other viruses: ADV, parainfluenza viruses 1, 2, and 3 (PIV1–3), influenza A and B viruses, HMPV, RV, and enterovirus (EV) with the same molecular testing technique [28]. RV clades A, B, and C were detected in the primer set utilized [29]. ADV testing was not done from August to October 2009 due to limited availability of reagents [28]. PCR data were semiquantitative and specimens with a cycle threshold (Ct) value <37 were considered positive. To detect pneumococcal infection, both blood culture for Streptococcus pneumoniae and whole blood lytA PCR were performed on blood specimens, although blood cultures were not systematically performed on all patients [30]. HIV testing was performed on a whole blood specimen or dried blood spot using an HIV PCR assay for children <18 months of age and HIV enzyme-linked immunosorbent assay for children ≥18 months of age. Quality Control for Molecular Diagnostics external quality assessments for all viruses in the panel were performed as well as annual World Health Organization panels for influenza alongside live and post hoc data quality checks.
Outcomes
The primary outcome of this study, life-threatening disease, was defined as a composite outcome of mechanical ventilation, ICU admission, or death. The secondary outcome was life-threatening disease or prolonged length of hospital stay ≥5 days.
Statistical Analyses
Continuous variables were described using mean (standard deviation) or median (interquartile range [IQR]). Differences in mean/median of continuous variables were tested with the 2-sided t test or a nonparametric Mann-Whitney test when appropriate. Categorical variables were described with frequencies and percentages and compared between groups using χ2 test or Fisher exact test if there were <5 observations in one group.
Logistic regression was used to assess the association between any viral coinfection (at least one of the following viruses detected: HMPV, RV, ADV, EV, influenza, PIV1, PIV2, PIV3) and virus-specific coinfections on the study outcomes as described above among RSV-positive children. In addition, we compared ADV-RSV and influenza-RSV coinfections to ADV and influenza monoinfection, as coinfection with these pathogens among RSV-positive children was found to be associated with increased risk of life-threatening disease. This analysis was implemented to assess whether ADV and influenza monoinfection were the driver of severe disease. Results were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Multivariate logistic regression was performed by use of the manual forward stepwise procedure including variables with a P value < .2 in univariate analyses. The analysis was adjusted for age using the subgroups <6 months and ≥6 months. The primary analysis was conducted on the HIV-uninfected population; subsequently, a separate analysis was performed for the HIV-infected population due to significantly elevated mortality rate and altered immune status of this subgroup.
We considered P < .05 to be significant for all analyses. Statistical analysis was performed using STATA/SE software, version 13.1 (StataCorp, College Station, Texas).
Ethical Considerations
The study protocol was approved by the University of the Witwatersrand Ethics Committee and the KwaZulu-Natal Human Biomedical Research Ethics Committee (protocol M081042 and BF157/08). Details of consenting, which included written informed consent from the parent or primary caregiver of the child, have been described [30]. This surveillance was deemed nonresearch by the US Centers for Disease Control and Prevention (NRD 2012 6197).
RESULTS
Study Population
During February 2009 to December 2013, 10128 children <5 years of age were enrolled, including 2404 (23.7%) with RSV-associated LRTI. Our total HIV-uninfected population with RSV-associated LRTI was 2322 children. We performed a sensitivity analysis to validate HIV status and found that the untested and HIV-negative population did not differ in baseline characteristics or underlying conditions, and both had a similar mean RSV Ct value of 25.1 (SD, 5.1) and 25.7 (SD, 4.6) (P = .004), respectively (Supplementary Table 1).
Prevalence of Viral Coinfection
Table 1 details the prevalence of respiratory virus coinfections among children hospitalized for RSV-associated LRTI, including stratification by age groups <6 months and ≥6 months of age. The prevalence of any respiratory viral coinfection was more common among children aged ≥6 months (529 [51.1%]) compared with those aged <6 months (463 [36.0%]) (P < .001). The prevalence of RSV-PIV1, RSV-PIV2, and RSV-PIV3 coinfection was <1% in both groups. Rhinovirus was the most prevalent coinfecting virus, found among 23.5% of children aged <6 months and 26.4% of children aged ≥6 months; followed by RSV-ADV coinfection (8.3% and 23.2% in children aged <6 months and ≥6 months, respectively; P < .001) and RSV-EV coinfection (5.6% and 11.5% in children aged <6 months and ≥6 months, respectively; P < .001). The different permutations of viral coinfections in the RSV-infected population are elucidated in a coinfection matrix (Supplementary Table 2).
Table 1.
Respiratory Viral Coinfections, Stratified by Age Group, in HIV-Uninfected Children Aged <5 Years With Respiratory Syncytial Virus–Associated Lower Respiratory Tract Infection at 6 Sentinel Sites in South Africa, 2009–2013
| Infection | All Ages (N = 2322) | <6 mo (n = 1287) | ≥6 mo (n = 1035) | P Valuea | |||
|---|---|---|---|---|---|---|---|
| Frequency | (%) | Frequency | (%) | Frequency | (%) | ||
| RSV monoinfection | 1330 | (57.3) | 824 | (64.0) | 506 | (48.9) | <.001 |
| RSV + any coinfectionb | 992 | (42.7) | 463 | (36.0) | 529 | (51.1) | <.001 |
| RSV-HMPV | 26 | (1.1) | 16 | (1.2) | 10 | (0.97) | .53 |
| RSV-RV | 575 | (24.8) | 302 | (23.5) | 273 | (26.4) | .11 |
| RSV-ADV | 347 | (14.9) | 107 | (8.3) | 240 | (23.2) | <.001 |
| RSV-EV | 191 | (8.2) | 72 | (5.6) | 119 | (11.5) | <.001 |
| RSV-Influenza | 30 | (1.3) | 11 | (0.85) | 19 | (1.8) | .04 |
| RSV-PIV1 | 12 | (0.52) | 2 | (0.16) | 10 | (0.97) | .01 |
| RSV-PIV2 | 12 | (0.52) | 4 | (0.31) | 8 | (0.77) | .12 |
| RSV-PIV3 | 20 | (0.86) | 12 | (0.93) | 8 | (0.77) | .70 |
Abbreviations: ADV, adenovirus; EV, enterovirus; HIV, human immunodeficiency virus; HMPV, human metapneumovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RV, rhinovirus.
The P value is given for a comparison of the prevalence of a certain coinfection in the age group <6 mo and ≥6 mo.
Any viral respiratory coinfection with HMPV, RV, ADV, EV, influenza, PIV1, PIV2, or PIV3.
We compared the prevalence of viruses in the presence (n = 2404) or absence of RSV (n = 7447) and found that the presence of RSV was associated with a lower prevalence of all other respiratory viruses during RSV season (Supplementary Figure 1). In the RSV-negative population, 19.3% (1436/7447) of children hospitalized for LRTI had 2 or more viruses detected in the respiratory tract, with the most prevalent viruses being RV and ADV, respectively.
Demographic and Clinical Characteristics
We examined the prevalence of demographic and clinical characteristics among RSV monoinfection cases and those with any respiratory virus coinfection, stratified by age <6 months and ≥6 months. A total of 1287 children were aged <6 months and 1035 were aged ≥6 months. The median age for RSV monoinfection was 4.2 months (IQR, 1.9–9.6 months), and 6.6 months (IQR, 3.0–14.7 months) for RSV with any viral coinfection. Age was associated with RSV and any viral coinfection in both children <6 months of age (P < .001) and aged ≥6 months (P = .05; Table 2).
Table 2.
Demographic and Clinical Characteristics, Stratified by Age Group, for Any Coinfection or Respiratory Syncytial Virus Monoinfection in HIV-Uninfected Children <5 Years of Age at 6 Sentinel Sites in South Africa, 2009–2013
| Characteristic | <6 mo | ≥6 mo | ||||
|---|---|---|---|---|---|---|
| RSV Monoinfection (n = 824) |
Any Coinfectiona (n = 463) |
P Value | RSV Monoinfection (n = 506) |
Any Coinfectiona (n = 529) |
P Value | |
| Demographics | ||||||
| Age, mo, median (IQR) | 2.4 (1.3–3.8) | 2.8 (1.9–4.0) | <.001 | 12.8 (8.1–21.0) | 14.0 (9.0–22.7) | .05 |
| Female sex | 354/824 (43.0) | 199/463 (43.0) | .99 | 223/506 (44.1) | 227/529 (42.9) | .71 |
| Race, black | 810/823 (98.4) | 454/463 (98.1) | .63 | 493/504 (97.8) | 519/528 (98.3) | .58 |
| Duration of symptoms, d, median (IQR) | 2 (1–3) | 2 (1–3) | .44 | 2 (1–3) | 2 (1–3) | .16 |
| Premature birthb | 16/822 (2.0) | 12/463 (2.6) | .45 | 4/503 (0.80) | 5/528 (0.95) | .99 |
| DOB within 10 wk of start of RSV season | 524/824 (63.6) | 295/463 (63.7) | .97 | 182/506 (36.0) | 210/529 (39.7) | .22 |
| RSV Ct value, mean (SD) | 24.8 (4.5) | 25.6 (4.6) | .002 | 25.8 (4.6) | 26.3 (5.3) | .15 |
| Crowding (≥5 people in the household) | 82/813 (10.1) | 57/455 (12.5) | .18 | 31/499 (6.2) | 56/525 (10.7) | .01 |
| Underlying conditionsc | ||||||
| Underlying illness | 20/823 (2.4) | 16/463 (3.5) | .29 | 15/505 (3.0) | 21/528 (4.0) | .38 |
| Whole blood PCR + Streptococcus pneumoniae | 29/453 (6.4) | 6/254 (2.4) | .02 | 13/297 (4.4) | 18/277 (6.5) | .26 |
| Outcome | ||||||
| Primary outcome | 17/810 (2.1) | 12/460 (2.6) | .56 | 2/496 (0.4) | 7/511 (1.4) | .18 |
| Secondary outcome | 363/811 (44.8) | 189/458 (41.3) | .23 | 115/499 (23.1) | 100/523 (19.1) | .12 |
Data are presented as no./No. (%) unless otherwise indicated.
Abbreviations: Ct, cycle threshold; DOB, date of birth; IQR, interquartile range; PCR, polymerase chain reaction; RSV, respiratory syncytial virus; SD, standard deviation.
Any viral respiratory coinfection with HMPV, RV, ADV, EV, influenza, PIV1, PIV2, or PIV3.
Born at <37 weeks gestation age.
Underlying conditions included asthma, chronic renal failure, splenectomy/asplenia, autoimmune disease, seizure disorders, malignancy, chronic lung disease, heart failure, organ transplant, diabetes, kwashiorkor/marasmus, prematurity, valvular heart disease, immunosupressive therapy, burns, nephrotic syndrome, obesity, cirrhosis/liver failure, coronary artery disease, sickle cell, immunoglobulin deficiency, spinal cord injuries, chronic obstructive pulmonary disease/emphysema, or other as specified by parent(s).
We described the demographics and underlying conditions of RSV monoinfection and coinfections in Table 2. Underlying conditions were not more prevalent in viral coinfection than in RSV monoinfection (P = .29 for children aged <6 months; P = .38 for children aged ≥6 months).
Respiratory Viral Coinfections and Disease Severity
Within the RSV-positive population <5 years old, 26 children (1.1%) were admitted to the ICU, 21 children (0.90%) needed mechanical ventilation, and 8 children died (0.34%). Seventeen of the 21 children (81%) who received mechanical ventilation were admitted to the ICU. Sixty-seven percent of children were hospitalized for <5 days. When comparing RSV with any respiratory viral coinfection to RSV monoinfection, we found no overall association between any viral infection and life-threatening disease (OR, 0.74; 95% CI, .39–1.4; P = .36; Table 3). We found the same to be true for our secondary outcome, including extended length of stay (adjusted OR [aOR], 0.83; 95% CI, .69–1.0; P = .05) Table 4. After adjusting for confounders, RSV-ADV coinfection had a 3.4 increased odds of life-threatening disease compared with RSV monoinfection (95% CI, 1.6–7.2; P = .001; Table 3). RSV-ADV coinfection was not associated with the secondary outcome (aOR, 1.0; 95% CI, 0.77–1.3; P = .77, Table 4). When we compared RSV-ADV coinfection to ADV monoinfection, we found no relation to life-threatening disease (aOR, 0.78; 95% CI, .37–1.6; P = .51) and decreased life-threatening disease and extended length of stay (aOR, 0.51; 95% CI, .38–.70; P < .001). The median ADV Ct value was significantly lower in ADV monoinfection (29.7; IQR, 20.8–34.3) compared with RSV-ADV infection (33.2; IQR, 30.1–35.5) (P < .001). Finally, RSV-influenza showed increased odds for our secondary outcome including prolonged length of stay (aOR, 2.1; 95% CI 1.0–4.5; P = .05) Table 4. We identified an increased odds of our secondary outcome for RSV-influenza when compared to influenza alone (aOR, 2.1; 95% CI, 1.0–4.4; P = .04). No other viral coinfections showed increased odds of severe disease compared with RSV monoinfection.
Table 3.
Primary Outcome in Univariate and Multivariate Analyses of Respiratory Syncytial Virus Viral Coinfection and Life-threatening Disease in HIV-Uninfected Children <5 Years of Age at 6 Sentinel Sites in South Africa, 2009–2013
| Coinfection | Life-threatening Disease | MV, ICU, Death, no./No. (%) |
OR (95% CI) |
P Value | aOR (95% CI) |
P Value |
|---|---|---|---|---|---|---|
| Anya | No Yes |
19/1306 (1.5) 19/971 (2.0) |
0.74 (.39–1.4) |
.36 | ||
| HMPV | No Yes |
38/2251 (1.7) 0/26 (0.0) |
… | … | ||
| RV | No Yes |
31/1715 (1.8) 7/562 (1.3) |
0.69 (.30–1.6) |
.37 | ||
| ADV | No Yes |
27/1937 (1.4) 11/340 (3.2) |
2.4 (1.2–4.8) |
.02 | 3.4 (1.6–7.2) |
.001 |
| EV | No Yes |
35/2091 (1.7) 3/186 (1.6) |
0.96 (.29–3.2) |
.95 | ||
| Influenza | No Yes |
38/2248 (1.7) 0/29 (0.0) |
… | … | ||
| PIV1 | No Yes |
37/2266 (1.6) 1/11 (9.1) |
6.0 (.75–48.3) |
.09 | ||
| PIV2 | No Yes |
38/2265 (1.7) 0/12 (0.0) |
… | … | ||
| PIV3 | No Yes |
37/2257 (1.6) 1/20 (5.0) |
3.2 (.41–24.2) |
.27 |
Univariate analysis: All factors with P < .20 were entered into the multivariate model. Multivariate analysis: Only factors with P < .05 are shown. Multivariate analysis was adjusted for the covariates prematurity and age, which were found to be significant in univariate analysis and subsequently in multivariate analysis using the manual forward stepwise procedure. Primary outcome data were missing for 45 RSV–infected children.
Abbreviations: ADV, adenovirus; aOR, adjusted odds ratio; CI, confidence interval; EV, enterovirus; HMPV, human metapneumovirus; ICU, intensive care unit; MV, mechanical ventilation, OR, odds ratio; PIV, parainfluenza virus; RV, rhinovirus, RSV: respiratory syncitial virus.
Any viral respiratory coinfection with HMPV, RV, ADV, EV, influenza, PIV1, PIV2, or PIV3.
Table 4.
Secondary Outcome in Univariate and Multivariate Analyses of Respiratory Syncytial Virus Viral Coinfection and Life-threatening Disease or Length of Stay of ≥5 Days in HIV-Uninfected Children <5 Years of Age at 6 Sentinel Sites in South Africa, 2009–2013
| Coinfection | Secondary Outcome | MV, ICU, Death, or LOS ≥5 d, no./No. (%) | OR (95% CI) |
P Value | aOR (95% CI) |
P Value |
|---|---|---|---|---|---|---|
| Anya | No Yes |
478/1310 (36.5) 289/981 (29.5) |
1.3 (1.2–1.6) |
<.001 | ||
| HMPV | No Yes |
758/2265 (33.5) 9/26 (34.6) |
1.1 (.47–2.4) |
.90 | ||
| RV | No Yes |
599/1724 (34.7) 168/567 (29.6) |
0.79 (.64–.97) |
.03 | ||
| ADV | No Yes |
671/1946 (34.5) 96/345 (27.8) |
0.73 (.57–.94) |
.02 | ||
| EV | No Yes |
721/2101 (34.3) 46/190 (24.2) |
0.61 (.43–.86) |
.005 | ||
| Influenza | No Yes |
753/2261 (33.3) 14/30 (46.7) |
1.8 (.85–3.6) |
.13 | 2.1 (1.0–4.5) |
.05 |
| PIV1 | No Yes |
765/2280 (33.6) 2/11 (18.2) |
0.44 (.09–2.0) |
.30 | ||
| PIV2 | No Yes |
765/2279 (33.6) 2/12 (16.7) |
0.40 (.09–1.8) |
.23 | ||
| PIV3 | No Yes |
761/2271 (33.5) 6/20 (30.0) |
0.85 (.33–2.2) |
.74 |
Univariate analysis: All factors with P < .20 were entered into the multivariate model. Multivariate analysis: Only factors with P < .05 are shown. Multivariate analysis was adjusted for the covariates prematurity and age, which were found to be significant in univariate analysis and subsequently in multivariate analysis using the manual forward stepwise procedure. Secondary outcome was missing for 31 RSV–infected children.
Abbreviations: ADV, adenovirus; aOR, adjusted odds ratio; CI, confidence interval; EV, enterovirus; HMPV, human metapneumovirus; ICU, intensive care unit; LOS, hospital length of stay; MV, mechanical ventilation, OR, odds ratio; PIV, parainfluenza virus; RV, rhinovirus, RSV: respiratory syncitial virus.
Any viral respiratory coinfection with HMPV, RV, ADV, EV, influenza, PIV1, PIV2, or PIV3.
In the HIV-infected population, 6.3% (n = 5) of children had life-threatening disease. Mean RSV Ct value was significantly lower in the HIV-infected population than the HIV-uninfected population (RSV Ct value 27.1 [SD, 5.0] vs 25.5 [SD, 4.8]; P = .003). Similarly, in this population, we found no association between any viral coinfection and more severe disease compared with RSV monoinfection [Supplementary Table 4].
RSV Viral Load and Disease Severity
We found a mean Ct value of 25.1 (SD, 4.6) for children aged <6 months and 26.0 (SD, 5.0) for children aged ≥6 months (P < .001). RSV viral load was not associated with life-threatening disease in children with RSV monoinfection or children with RSV with any coinfection. When included in our multivariate model, RSV Ct values were not found to be associated with life-threatening disease (aOR, 1.0; 95% CI, .94–1.1) or the secondary outcome including increased length of stay (aOR, 1.0; 95% CI, .98–1.0).
DISCUSSION
In general, our study did not corroborate the findings from previous smaller studies, that children hospitalized with LRTI characterized by RSV coinfection with respiratory viruses had more severe disease compared with children with RSV monoinfection [15, 31, 32]. We did, however, identify an association between RSV-ADV coinfection and life-threatening disease, which may be indicative of synergistic pathogenesis leading to respiratory failure or that severe disease in these children was largely driven by coinfection with ADV. The association of RSV-ADV coinfection with severe disease was, however, not evident when we assessed prolonged hospitalization. Our data are supported by findings of another study in which RSV-ADV coinfection showed statistically significant increases in hospital length of stay, days with supplemental oxygen use, ICU admission, and mechanical ventilation compared with RSV monoinfection in children hospitalized for LRTI, although no comparison was made with ADV monoinfection [33]. In a study of mixed RSV-ADV infection, RSV-ADV coinfection was not found to be more severe than ADV alone when examining duration of fever, oxygen requirement, and length of hospital stay [34]. Another study of 9 RSV-confirmed infants found that 75% of children with RSV-ADV coinfection died despite mechanical ventilation [35]. Even though ADV alone may be responsible for more severe disease, clinical features such as hospital stay were not found to differ between RSV and ADV hospitalized LRTI [36]. However, increased pathogenicity may be explained by distinctly different immunological responses produced by RSV and ADV. ADV induces interferon-γ production activating the classical antiviral defense mechanism and heightened mononuclear cell activation compared with RSV, possibly leading to more severe disease with coinfection [37].
The increased odds of severe disease for RSV-ADV coinfection may warrant further exploration on a host and pathogen level. Virus–virus interactions can be classified into 3 categories [1]: viral genes or gene products interacting directly [2], host environment changes that result in indirect interaction, or [3] immunological interactions [38]. It is plausible that similar mechanisms that enhance bacterial superinfection may also enhance viral superinfection, namely, depletion of host defenses due to initial viral infection [39].
We found that coinfection of RSV and any other virus was not related to disease severity. This is in line with a retrospective study which found that clinical severity did not differ between RSV monoinfection and viral coinfection with 17 different respiratory viruses [40]. A recent meta-analysis of clinical disease severity and viral coinfection vs monoinfection found no clinical difference in severity between these 2 groups even when constrained to more pathogenic respiratory viruses (influenza, RSV, HMPV, PIV) [19]. Another meta-analysis of single and multiple virus respiratory infections (influenza, RV, ADV, HMPV, coronavirus, bocavirus, PIV1–3) and severity of disease concluded that the influence of coinfection on disease severity remains unclear due to the heterogeneity of results [41].
In our study, the highest prevalence of viral coinfection was detected in HIV-uninfected children aged ≥6 months hospitalized for LRTI. This is in accordance with findings from a number of studies that found multiple viral respiratory infection to be associated with older age [21, 33, 42, 43] when compared to RSV monoinfection. Increased rates of virus infection with increasing age have been described previously in this surveillance population [30]. The increased prevalence of respiratory viral coinfections among children aged ≥6 months may be explained by increased exposure to respiratory viruses, an increased immune response during primary infection that discourages viral coinfection, or increased susceptibility due to waning maternal antibodies [44].
In the presence of RSV, our data show lower prevalence of non-RSV viruses in children hospitalized for viral respiratory illnesses during the RSV season (Supplementary Figure 1). This could be indicative of viral interference in which the presence of RSV in the community inhibits infection by or circulation of other viruses. Evidence of viral interference has been found in studies of children who received influenza vaccine [45, 46] and among children receiving immunoprophylaxis for RSV [47]. In both groups, the prevalence of nonpreventively targeted viruses was higher than among comparison groups that did not receive vaccination or immunoprophylaxis. However, these speculations and the clinical relevance of some of these identified viruses need further exploration with a more suitable study design.
The strength of this study lies in the large sample size, which allowed us to look at different permutations of coinfection within the RSV population and compare them to RSV monoinfection only and to draw conclusions about an infrequent, yet important, outcome. Furthermore, we were able to control for important confounders of disease severity including age and prematurity. Finally, we did not limit our assessment of respiratory viral coinfection to a single season.
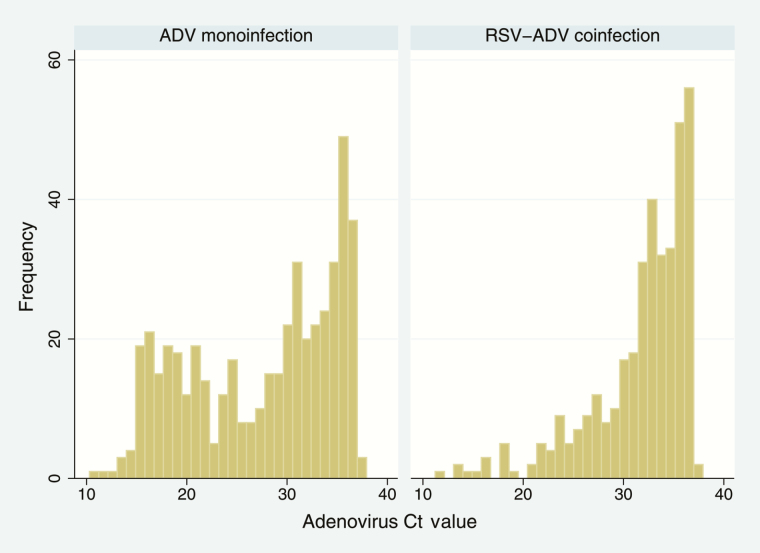
There were some limitations to our study. Given that viral data were collected at one time point after disease onset, it is difficult to link viral detection to etiology of LRTI. Some respiratory viruses are frequently detected in asymptomatic children and infants. RSV, HMPV, influenza, and ADV are significantly more prevalent in symptomatic children, whereas RV is commonly found in asymptomatic individuals [48]. Another study of infants up to 12 months of age found that detection of RSV, RV, influenza, ADV, and HMPV are highly associated with symptoms, with an OR >4 for presence of symptoms, whereas for EV, detection is not significantly associated with symptoms [49]. In South Africa, ADV was only moderately associated with severe disease as it was commonly identified in controls; the attributable fraction of ADV detection was 10.1% [50]. Viral detection may also be an artefact of prolonged viral shedding: ADV, for example, is known to exhibit longer low-level shedding [51]. In the RSV-ADV coinfection population, we found more frequent low-level virus than in the population with ADV monoinfection, which may be indicative of prolonged viral shedding and acute infection, respectively (Figure 1). The multiplex PCR used was limited in its ability to discriminate between RV and EV due to cross-reactivity; therefore, these coinfections are not optimally characterized within this population. Furthermore, the definition of any viral coinfection is limited by the respiratory viruses we did not test for, although the clinical significance of many of those (eg, human coronavirus and human bocavirus) as LRTI etiological agents also remain to be fully elucidated. Another limitation was that we only had semiquantitative data for viral load.
Figure 1.
Histogram of adenovirus cycle threshold (Ct) values in human immunodeficiency virus-uninfected children <5 years of age with adenovirus (ADV) monoinfection and those with respiratory syncytial virus (RSV)–ADV coinfection at 6 sentinel sites in South Africa, 2009–2013.
CONCLUSIONS
The present study contributes to a better understanding of the role of viral coinfection in children hospitalized for RSV-associated LRTI. Molecular diagnostics for respiratory viruses may serve as an important diagnostic tool in pediatric LRTI, but the possible synergy of multiple viruses in the respiratory tract is an area with no clear consensus. In our study, we found that RSV and any respiratory viral coinfection was not associated with more severe disease. The association between RSV-ADV coinfection and life-threatening disease in hospitalized children <5 years of age warrants further exploration and may be explained by enhanced ADV disease alone.
Supplementary Material
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention or the National Institute for Communicable Diseases, South Africa.
Potential conflicts of interest. Authors certify no potential conflicts of interest. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011; 378:1917–30. [DOI] [PubMed] [Google Scholar]
- 2. Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr 1995; 126:212–9. [DOI] [PubMed] [Google Scholar]
- 3. Holman RC, Shay DK, Curns AT, Lingappa JR, Anderson LJ. Risk factors for bronchiolitis-associated deaths among infants in the United States. Pediatr Infect Dis J 2003; 22:483–90. [DOI] [PubMed] [Google Scholar]
- 4. Hall CB, Powell KR, MacDonald NE, et al. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med 1986; 315:77–81. [DOI] [PubMed] [Google Scholar]
- 5. Groothuis JR, Gutierrez KM, Lauer BA. Respiratory syncytial virus infection in children with bronchopulmonary dysplasia. Pediatrics 1988; 82:199–203. [PubMed] [Google Scholar]
- 6. MacDonald NE, Hall CB, Suffin SC, Alexson C, Harris PJ, Manning JA. Respiratory syncytial viral infection in infants with congenital heart disease. N Engl J Med 1982; 307:397–400. [DOI] [PubMed] [Google Scholar]
- 7. Moyes J, Cohen C, Pretorius M, et al. ; South African Severe Acute Respiratory Illness Surveillance Group Epidemiology of respiratory syncytial virus-associated acute lower respiratory tract infection hospitalizations among HIV-infected and HIV-uninfected South African children, 2010–2011. J Infect Dis 2013; 208:S217–26. [DOI] [PubMed] [Google Scholar]
- 8. Bloemers BL, van Furth AM, Weijerman ME, et al. Down syndrome: a novel risk factor for respiratory syncytial virus bronchiolitis—a prospective birth-cohort study. Pediatrics 2007; 120:e1076–81. [DOI] [PubMed] [Google Scholar]
- 9. Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 2013; 132:e341–8. [DOI] [PubMed] [Google Scholar]
- 10. Mazur NI, Martinón-Torres F, Baraldi E, et al. ; Respiratory Syncytial Virus Network (ReSViNET) Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med 2015; 3:888–900. [DOI] [PubMed] [Google Scholar]
- 11. Henrickson KJ. Advances in the laboratory diagnosis of viral respiratory disease. Pediatr Infect Dis J 2004; 23:S6–10. [DOI] [PubMed] [Google Scholar]
- 12. Canducci F, Debiaggi M, Sampaolo M, et al. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J Med Virol 2008; 80:716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marguet C, Lubrano M, Gueudin M, et al. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS One 2009; 4:e4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papenburg J, Hamelin MÈ, Ouhoummane N, et al. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis 2012; 206:178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Semple MG, Cowell A, Dove W, et al. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis 2005; 191:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papadopoulos NG, Moustaki M, Tsolia M, et al. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med 2002; 165:1285–9. [DOI] [PubMed] [Google Scholar]
- 17. Franz A, Adams O, Willems R, et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol 2010; 48:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harada Y, Kinoshita F, Yoshida LM, et al. Does respiratory virus coinfection increases the clinical severity of acute respiratory infection among children infected with respiratory syncytial virus? Pediatr Infect Dis J 2013; 32:441–5. [DOI] [PubMed] [Google Scholar]
- 19. Asner SA, Science ME, Tran D, Smieja M, Merglen A, Mertz D. Clinical disease severity of respiratory viral co-infection versus single viral infection: a systematic review and meta-analysis. PLoS One 2014; 9:e99392. doi: 10.1371/journal.pone.0099392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foulongne V, Guyon G, Rodière M, Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J 2006; 25:354–9. [DOI] [PubMed] [Google Scholar]
- 21. Calvo C, García-García ML, Blanco C, et al. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J Clin Virol 2008; 42:268–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lazar I, Weibel C, Dziura J, Ferguson D, Landry ML, Kahn JS. Human metapneumovirus and severity of respiratory syncytial virus disease. Emerg Infect Dis 2004; 10:1318–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xepapadaki P, Psarras S, Bossios A, et al. Human metapneumovirus as a causative agent of acute bronchiolitis in infants. J Clin Virol 2004; 30:267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McNamara PS, Flanagan BF, Smyth RL, Hart CA. Impact of human metapneumovirus and respiratory syncytial virus co-infection in severe bronchiolitis. Pediatr Pulmonol 2007; 42:740–3. [DOI] [PubMed] [Google Scholar]
- 25. Subbarao EK, Griffis J, Waner JL. Detection of multiple viral agents in nasopharyngeal specimens yielding respiratory syncytial virus (RSV). An assessment of diagnostic strategy and clinical significance. Diagn Microbiol Infect Dis 1989; 12:327–32. [DOI] [PubMed] [Google Scholar]
- 26. Ferraro AA, Ferronato AE, Sacramento PR Do, et al. Severity of viral coinfection in hospitalized infants with respiratory syncytial virus infection. J Pediatr (Rio J) 2011; 87:307–13. [DOI] [PubMed] [Google Scholar]
- 27. Pretorius MA, Madhi SA, Cohen C, et al. Respiratory viral coinfections identified by a polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness—South Africa, 2009–2010. J Infect Dis 2012; 206:S159–65. [DOI] [PubMed] [Google Scholar]
- 28. Pretorius MA, Madhi SA, Cohen C, et al. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness—South Africa, 2009–2010. J Infect Dis 2012; 206:S159–65. [DOI] [PubMed] [Google Scholar]
- 29. Brittain-Long R, Nord S, Olofsson S, Westin J, Anderson LM, Lindh M. Multiplex real-time PCR for detection of respiratory tract infections. J Clin Virol 2008; 41:53–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen C, Walaza S, Moyes J, et al. Epidemiology of viral-associated acute lower respiratory tract infection among children <5 years of age in a high HIV prevalence setting, South Africa, 2009-2012. Pediatr Infect Dis J 2015; 34:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. König B, König W, Arnold R, Werchau H, Ihorst G, Forster J. Prospective study of human metapneumovirus infection in children less than 3 years of age. J Clin Microbiol 2004; 42:4632–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greensill J, McNamara PS, Dove W, Flanagan B, Smyth RL, Hart CA. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis 2003; 9:372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodríguez DA, Rodríguez-Martínez CE, Cárdenas AC, et al. Predictors of severity and mortality in children hospitalized with respiratory syncytial virus infection in a tropical region. Pediatr Pulmonol 2014; 49:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palomino MA, Larrañaga C, Villagra E, Camacho J, Avendaño LF. Adenovirus and respiratory syncytial virus-adenovirus mixed acute lower respiratory infections in Chilean infants. Pediatr Infect Dis J 2004; 23:337–41. [DOI] [PubMed] [Google Scholar]
- 35. Tristram DA, Miller RW, McMillan JA, Weiner LB. Simultaneous infection with respiratory syncytial virus and other respiratory pathogens. Am J Dis Child 1988; 142:834–6. [DOI] [PubMed] [Google Scholar]
- 36. Barberi S, Barreto M, La Penna F, et al. Respiratory syncytial virus and adenovirus in acute lower respiratory infections in hospitalized infants and children. Open J Pediatr 2012; 2:31–7. [Google Scholar]
- 37. Díaz PV, Calhoun WJ, Hinton KL, et al. Differential effects of respiratory syncytial virus and adenovirus on mononuclear cell cytokine responses. Am J Respir Crit Care Med 1999; 160:1157–64. [DOI] [PubMed] [Google Scholar]
- 38. DaPalma T, Doonan BP, Trager NM, Kasman LM. A systematic approach to virus-virus interactions. Virus Res 2010; 149:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol 2014; 12:252–62. [DOI] [PubMed] [Google Scholar]
- 40. Asner SA, Rose W, Petrich A, Richardson S, Tran DJ. Is virus coinfection a predictor of severity in children with viral respiratory infections? Clin Microbiol Infect 2015; 21:264.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goka EA, Vallely PJ, Mutton KJ, Klapper PE. Single and multiple respiratory virus infections and severity of respiratory disease: a systematic review. Pediatr Respir Rev 2014; 15:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stempel HE, Martin ET, Kuypers J, Englund JA, Zerr DM. Multiple viral respiratory pathogens in children with bronchiolitis. Acta Paediatr 2009; 98:123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diaz J, Morales-Romero J, Pérez-Gil G, et al. Viral coinfection in acute respiratory infection in Mexican children treated by the emergency service: a cross-sectional study. Ital J Pediatr 2015; 41:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martin ET, Kuypers J, Wald A, Englund JA. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses 2012; 6:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54:1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Belshe RB, Mendelman PM, Treanor J, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med 1998; 338:1405–12. [DOI] [PubMed] [Google Scholar]
- 47. Blanken MO, Rovers MM, Molenaar JM, et al. ; Dutch RSV Neonatal Network Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–9. [DOI] [PubMed] [Google Scholar]
- 48. Jansen RR, Wieringa J, Koekkoek SM, et al. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol 2011; 49:2631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chonmaitree T, Alvarez-Fernandez P, Jennings K, et al. Symptomatic and asymptomatic respiratory viral infections in the first year of life: association with acute otitis media development. Clin Infect Dis 2015; 60:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pretorius MA, Tempia S, Walaza S, et al. The role of influenza, RSV and other common respiratory viruses in severe acute respiratory infections and influenza-like illness in a population with a high HIV sero-prevalence, South Africa 2012–2015. J Clin Virol 2016; 75:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kalu SU, Loeffelholz M, Beck E, et al. Persistence of adenovirus nucleic acids in nasopharyngeal secretions: a diagnostic conundrum. Pediatr Infect Dis J 2010; 29:746–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.