Abstract
BACKGROUND
Cancer patients are often thought to have worse surgical outcomes. There is a growing view that risk models do not adequately predict these outcomes. This study aims to compare the use of common risk models for benign versus malignant gastrointestinal disease.
MATERIALS AND METHODS
The NSQIP 2005–2015 participant use files were queried for patients undergoing elective surgery for benign and malignant diseases with a primary procedure code for major colon, pancreas, or stomach resection. Multivariate logistic regression was performed to identify independent predictors of mortality and morbidity.
RESULTS
We identified 264,401 cases (111,563 malignant). The gastrointestinal cancer population was disproportionately male, older than 65, non-white, and less functionally independent. Comorbidities more common in the cancer population included diabetes, hypertension, dyspnea, and COPD. Cancer patients had a longer length of stay (+0.9 days), higher mortality rate (1.7% vs. 1.1%), and higher complication rate (27.4% vs. 23.2%). NSQIP prediction models for complications in cancer versus non-cancer patients underperformed for predicting mortality (p < 0.001). Multivariate regression demonstrated that a diagnosis of cancer requiring surgery independently conferred an 18% increased odds of death, a 9% increased odds of a complication, and an 8% increased odds of multiple complications compared to patients with benign disease.
CONCLUSIONS
NSQIP prediction models less effectively evaluate the risk of death in cancer patients as compared to patients with benign disease. A diagnosis of cancer is independently associated with an increased risk of surgical complications. Incorporating cancer diagnosis into surgical risk models may better inform patient and surgeon expectations.
Keywords: surgery, morbidity, risk, neoplasms, logistic regression, outcomes assessment
INTRODUCTION
The incidence of cancer continues to rise in the United States with a further expected 45% increase over the next 25 years.1 For surgeons, this trend is particularly important as more patients will require supportive decision making regarding risk for cancer-related surgical care.2 Patients may not fully understand surgical treatment options and are more likely to follow surgeons’ recommendations without effective shared decision making. Risk counseling has been demonstrated to reduce decisional conflict.3–6
Large datasets in surgical quality improvement have proliferated to provide patient-specific surgical risk prediction with an aim to improve outcomes through enhanced decision-making. Early efforts focused on procedure- or specialty-specific perioperative risk,7,8 but standardization and simplification of risk models have been more characteristic of the recent trend.9,10 Within the last decade, the American College of Surgeons’ (ACS) National Surgical Quality Improvement Program (NSQIP) and its associated all-procedure risk calculator have set the standard in terms of both scale and ease of use.8,11 Risk prediction models have been assessed in cancer-only populations, and it has been shown that these models do not perform as well in a highly specific cancer surgery contexts often lacking complete information on cancer type, stage, etc.12,13 Much of the prior work assessing risk prediction has focused on systematic revisions needed in data collection to reduce the risk modeling variability in a cancer surgery patient population (e.g., oncologic omissions such as cancer staging and neoadjuvant chemotherapy).14–17 However, a clinically practical question remains unanswered. What is the comparative performance of risk models in cancer versus benign disease populations? Given the risk models currently available today such as the NSQIP all-procedure risk calculator and their known limitations for cancer surgery patients, understanding this difference may allow for the surgeon to better appreciate the risks in undertaking operative interventions in patients with malignancy.
The purpose of this study was to assess the effect of a cancer diagnosis as a binary variable on the risks associated with major gastrointestinal surgery and to provide practical information for the risk counseling of these patients. We hypothesized that there would be a significant worsening in outcomes for in cancer patients.
MATERIALS AND METHODS
Data source
All data were obtained from the 2005–2015 ACS NSQIP participant use files.18 The development of this national dataset has been previously described.19 All major colon, pancreas, and stomach elective resections were included based on the record’s primary Current Procedural Terminology code (including 44139–41, 44143–7, 44150–1, 44155–8, 44160, 44799, 45121, 44204–8, 44210–3, 43177, 43118, 43121, 43123, 43620, 43621, 43622, 43631, 43632, 43633, 43634, 43635, 43638, 43639, 48150–4, 48140, 48145, 48146, 48155). Surgery for intended gastrointestinal oncologic indication was determined from associated ICD-9 diagnosis code (including 150–159). Emergent cases and those not operated on the same day as admission were excluded. Mirroring the current version of the ACS NSQIP Surgical Risk Calculator (http://riskcalculator.facs.org), we included 17 of the 18 preoperative variables (excluded emergent surgery dummy variable; added patient race variable). Laboratory variables were excluded due to their lack of contribution to predictive models in surgery.20 Predicted mortality and morbidity and all 30-day outcomes (mortality, morbidities, length of stay) were obtained from the same dataset. A complication was defined as the occurrence of any NSQIP-reported postoperative morbidity. The Johns Hopkins University School of Medicine Institutional Review Board approved this study.
Analysis
Individuals were stratified by their binary indication for surgery (e.g., cancer versus benign). Those with and without a surgical cancer diagnosis were directly compared with univariable analysis. Binary and categorical variables were assessed using test and continuous variables’ association were calculated using Wilcoxon-Mann-Whitney tests. Multivariable regression methods were employed in a manner similar to prior prediction model building.8,11 A backward stepwise logistic – and ordered logistic where appropriate – regression was then performed to assess independent predictors of mortality, morbidity, and length of stay. An organ-specific indicator variable was included to independently model for each distinct organ-specific procedure type included in the study population. Heterogeneity in the cancer patient versus benign disease population’s demographics and comorbidities identified during univariable analysis led to post hoc propensity score matching being performed with weighted propensity scores based on the significant univariable findings below (e.g., sex, age, ASA score, specific comorbidities). Subgroup analysis by procedure was also performed to assess for asymmetric effects by procedure type. All statistical analyses were performed in Stata® 14.2 (StataCorp, College Station, TX).
Existing Risk Prediction Models
The ACS NSQIP participant use file includes a predicted risk of mortality and morbidity for each patient. The selection and construction of these risk models has been previously described.21–23 These variables were directly compared to the new multivariable regression described above using goodness-of-fit testing (e.g., c-statistic, Brier score) and observed-to-expected outcomes correlations were compared using Fisher’s r to Z transformation.
RESULTS
We identified 264,401 cases of which 111,563 (42.2%) were for a malignant surgical indication, and 152,838 (57.8%) were for benign disease. A summary and comparison of these populations is provided in Table 1. The two populations were heterogeneous with a greater proportion of the total for the cancer surgery population being male (51.9% versus 45.6%, p < 0.001), older (56.2% versus 36.6% older than 65 years, trend p < 0.001), non-white (23.8% versus 22.2%, p < 0.001), and functionally impaired (3.1% versus 2.9%, p < 0.001). Overall, the malignant disease group was sicker by ASA class (median 3 versus 2, p < 0.001 for mean), and also had more hypertension (55.0% versus 45.3%, p < 0.001), diabetes (20.0% versus 13.5%, p < 0.001), congestive heart failure (0.8% versus 0.5%, p < 0.001), dyspnea on exertion (9.8% versus 6.8%, p < 0.001), and chronic obstructive pulmonary disease (5.3% versus 4.4%, p < 0.001). Comorbidities more common in the benign gastrointestinal surgery group included greater steroid use (9.1% versus 2.5%, p < 0.001), chronic sepsis (3.2% versus 0.3%, p < 0.001), tobacco use (19.6% versus 15.2%, p < 0.001), acute renal failure (0.2% versus 0.1%, p < 0.001), and body mass index (median 27.5 versus 27.2, p <0.001 for mean).
Table 1.
Population preoperative characteristics for gastrointestinal surgery in NSQIP (2005–2015)
| Surgery for GI Cancer (n = 111,563) | Surgery for Benign GI Disease (n = 152,838) | p | |
|---|---|---|---|
| Gender | <0.001 | ||
| Male | 57,879 (51.9%) | 69,624 (45.6%) | |
| Female | 53,684 (48.1%) | 83,214 (54.5%) | |
|
| |||
| Age | <0.001 | ||
| < 65 | 48,945 (43.8%) | 97,000 (63.4%) | |
| 65–74 | 31,024 (27.8%) | 34,923 (22.8%) | |
| 75–84 | 23,876 (21.4%) | 17,669 (11.6%) | |
| > 84 | 7,828 (7.0%) | 3,399 (2.2%) | |
|
| |||
| Race | <0.001 | ||
| White | 79,272 (76.2%) | 115,001 (77.8%) | |
| Black | 10,745 (10.0%) | 12,899 (8.7%) | |
| Hispanic | 11,826 (11.1%) | 16,498 (11.2%) | |
| Other | 5,182 (4.8%) | 3,382 (2.3%) | |
|
| |||
| Functional Status | <0.001 | ||
| Independent | 107,918 (96.9%) | 148,016 (97.1%) | |
| Partially dependent | 2,497 (2.2%) | 2,797 (1.8%) | |
| Totally dependent | 945 (0.9%) | 1,595 (1.1%) | |
|
| |||
| ASA Class, (Mean/Median) | 2.6 / 3 | 2.5 / 2 | <0.001 |
|
| |||
| Comorbidities | |||
| Chronic steroids | 2,916 (2.6%) | 13,890 (9.1%) | <0.001 |
| Ascites | 710 (0.6%) | 879 (0.6%) | 0.044 |
| Systemic sepsis | <0.001 | ||
| SIRS | 1,487 (1.3%) | 3,122 (2.1%) | |
| Sepsis | 310 (0.3%) | 1,671 (1.1%) | |
| Septic shock | 24 (0.0%) | 14 (0.1%) | |
|
| |||
| Ventilator dependent | 41 (0.0%) | 384 (0.3%) | <0.001 |
| Disseminated cancer | 8,128 (7.3%) | 4,372 (2.9%) | <0.001 |
| Diabetes | <0.001 | ||
| Non-insulin dependent | 14,552 (13.0%) | 13,565 (8.9%) | |
| Insulin dependent | 7,785 (7.0%) | 7,004 (4.6%) | |
| Hypertension on meds | 61,442 (55.0%) | 69,329 (45.3%) | <0.001 |
| Congestive heart failure | 909 (0.8%) | 802 (0.5%) | <0.001 |
| Dyspnea | <0.001 | ||
| With activity | 10,309 (9.2%) | 9,581 (6.3%) | |
| At rest | 691 (0.6%) | 831 (0.5%) | |
| Active tobacco use | 17,010 (15.2%) | 29,943 (19.6%) | <0.001 |
| Chr. obstruct. pulm. dis. | 5,924 (5.3%) | 6,695 (4.4%) | <0.001 |
| Dialysis | 460 (0.4%) | 911 (0.6%) | <0.001 |
| Acute renal failure | 128 (0.1%) | 300 (0.2%) | <0.001 |
| BMI (Mean/Median) | 28.1 / 27.2 | 28.5 / 27.5 | <0.001 |
|
| |||
| Procedure Performed | |||
| Colectomy | 83,501 (81.2%) | 131,472 (85.9%) | <0.001 |
| Pancreatectomy | 22,681 (20.3%) | 17,216 (11.3%) | <0.001 |
| Gastrectomy | 5,491 (4.9%) | 4,303 (2.8%) | <0.001 |
These two populations also had heterogeneous outcomes (Table 2) with the cancer surgery population demonstrating greater complication rates (27.4% versus 23.2%, p < 0.001), pneumonia incidence (2.9% versus 2.2%, p < 0.001), failed extubations (2.5% versus 1.7%, p < 0.001), renal failure (1.5% versus 1.1%, p < 0.001), cardiac events (1.5% versus 0.8%, p < 0.001), bleeding (9.4% versus 5.8%, p < 0.001), venous thromboembolism (1.5% versus 1.3%, p < 0.001), and postoperative septic shock (2.1% versus 1.6%, p < 0.001). In addition to an increased complication rate, the cancer surgery group also demonstrated a larger number of complications per patient (mean 0.47 versus 0.39, p < 0.001), length of stay (median 6 versus 5 days, p < 0.001 for mean), and mortality rate (1.7% versus 1.1%, p < 0.001). In the cancer surgery group, the observed-to-expected mortality and morbidity correlations in the cancer surgery group were lower than the benign disease population’s suggesting less correlation between NSQIP risk-adjusted mortality probability estimates and actual mortality and morbidity (respectively, 0.22 versus 0.33, p < 0.001; 0.28 versus 0.29, p < 0.001)).
Table 2.
Postoperative outcomes for gastrointestinal surgery in NSQIP (2005–2015)
| Surgery for GI Cancer (n = 111,563) | Surgery for Benign GI Disease (n = 152,838) | p | |
|---|---|---|---|
| LOS Mean | 8.3 | 7.4 | <0.001 |
| LOS Median | 6 (IQR = 4–9) | 5 (IQR = 4–8) | |
|
| |||
| Death | 1,934 (1.7%) | 1,607 (1.1%) | <0.001 |
|
| |||
| Morbidity | |||
| Any Complication | 30,637 (27.4%) | 35,423 (23.2%) | <0.001 |
| Superficial SSI | 7,342 (6.6%) | 9,821 (6.4%) | 0.109 |
| Deep SSI | 1,497 (1.3%) | 2,173 (1.4%) | 0.083 |
| Organ Space SSI | 5,919 (5.3%) | 7,624 (5.0%) | <0.001 |
| Would Disruption | 1,253 (1.1%) | 1,758 (1.2%) | 0.517 |
| Pneumonia | 3,222 (2.9%) | 3,297 (2.2%) | <0.001 |
| Reintubation | 2,814 (2.5%) | 2,527 (1.7%) | <0.001 |
| Pulmonary Embolism | 925 (0.8%) | 913 (0.6%) | <0.001 |
| Prolonged Mechanical Ventilation (> 48 hours) | 2,514 (2.3%) | 2,834 (1.9%) | <0.001 |
| Progressive Renal Failure | 893 (0.8%) | 934 (0.6%) | <0.001 |
| Acute Renal Failure | 766 (0.7%) | 694 (0.5%) | <0.001 |
| Urinary Tract Infection | 3,609 (3.2%) | 4,544 (3.0%) | <0.001 |
| Cerebrovascular accident | 367 (0.3%) | 285 (0.2%) | <0.001 |
| Cardiac arrest | 762 (0.7%) | 638 (0.4%) | <0.001 |
| Myocardial infarction | 833 (0.8%) | 623 (0.4%) | <0.001 |
| Bleeding w/transfusion | 10,505 (9.4%) | 8,716 (5.7%) | <0.001 |
| Deep vein thrombosis | 1,629 (1.5%) | 1,928 (1.3%) | <0.001 |
| Sepsis | 5,010 (4.5%) | 6,715 (4.4%) | 0.230 |
| Septic Shock | 2,301 (2.1%) | 2,401 (1.6%) | <0.001 |
|
| |||
| Total # Complications, Mean | 0.47 | 0.39 | <0.001 |
| Total # of Complications, Median | 0 (IQR = 0 – 1) | 0 (IQR = 0 – 0) | |
|
| |||
| Any readmission | 6,631 (5.9 %) | 9,098 (6.0%) | 0.924 |
|
| |||
| Observed: Expected Mortality Correlation | 0.22 | 0.33 | <0.001 |
|
| |||
| Observed: Expected Morbidity Correlation | 0.28 | 0.29 | <0.001 |
Tables 3, 4, and 5 summarize the multivariable regression for 30-day mortality, complication rate, and total number of complications. When controlling for other preoperative variables and procedure type, there remained an 18% (OR = 1.18, 95% CI = 1.10–1.28) increase in the odds of death, a 9% (OR = 1.09, 95% CI = 1.07–1.11) increase in the odds of a complication, and an 8% (OR = 1.08, 95% CI = 1.06–1.11) increase in the number of complications in the cancer surgery population versus the benign disease group. Logistic regression was repeated with frequency weights applied based on propensity scoring, and significant variables and effect sizes were identical (Appendix 1). Subgroup analysis by procedure type also demonstrated consistent findings.
Table 3.
Multivariable logistic regression for death within 30 days of operation, controlled for procedure type
| Variables | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Cancer diagnosis | 1.18 | (1.10–1.28) | <0.001 |
| Procedure Control | |||
| Pancreas | Ref | ||
| Colon | 0.53 | (0.48–0.58) | <0.001 |
| Stomach | 0.97 | (0.83–1.14) | 0.746 |
|
| |||
| Male sex | 0.70 | (0.65–0.75) | <0.001 |
|
| |||
| BMI | 0.99 | (0.99–1.00) | 0.026 |
|
| |||
| Age | 1.05 | (1.05–1.05) | <0.001 |
|
| |||
| Functional status | 0.38 | (0.34–0.42) | <0.001 |
|
| |||
| ASA Class | 2.15 | (2.01–2.30) | <0.001 |
|
| |||
| Chronic steroid use | 1.42 | (1.24–1.63) | <0.001 |
|
| |||
| Diabetes | 1.09 | (1.00–1.18) | 0.057 |
|
| |||
| Hypertension | 1.19 | (1.09–1.30) | <0.001 |
|
| |||
| COPD | 1.37 | (1.22–1.53) | <0.001 |
|
| |||
| Ascites | 4.30 | (3.55–5.22) | <0.001 |
|
| |||
| Sepsis | 3.22 | (2.85–3.65) | <0.001 |
|
| |||
| Ventilator dependent | 1.94 | (1.44–2.59) | <0.001 |
|
| |||
| Active smoker | 1.20 | (1.09–1.33) | <0.001 |
|
| |||
| Acute renal failure | 1.88 | (1.35–2.62) | <0.001 |
|
| |||
| Dialysis | 2.27 | (1.81–2.84) | <0.001 |
|
| |||
| Congestive HF | 1.59 | (1.33–1.91) | <0.001 |
|
| |||
| Dyspnea | 1.53 | (1.39–1.68) | <0.001 |
Table 4.
Multivariable logistic regression for any complication within 30 days of operation, controlled for procedure type
| Variables | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Cancer diagnosis | 1.09 | (1.07–1.11) | <0.001 |
| Procedure Control | |||
| Pancreas | Ref | ||
| Colon | 0.45 | (0.44–0.46) | <0.001 |
| Stomach | 0.60 | (0.57–0.63) | <0.001 |
|
| |||
| Male sex | 0.98 | (0.96–0.99) | 0.011 |
|
| |||
| White race | 1.06 | (1.05–1.07) | <0.001 |
|
| |||
| Age | 1.00 | (1.00–1.00) | <0.001 |
|
| |||
| Functional status | 0.59 | (0.56–0.61) | <0.001 |
|
| |||
| ASA Class | 1.56 | (1.53–1.58) | <0.001 |
|
| |||
| Dialysis | 1.13 | (1.00–1.27) | 0.052 |
|
| |||
| Acute renal failure | 1.45 | (1.16–1.82) | 0.001 |
|
| |||
| Ascites | 2.17 | (1.95–2.42) | <0.001 |
|
| |||
| Sepsis | 2.17 | (2.06–2.30) | <0.001 |
|
| |||
| Active smoker | 1.14 | (1.11–1.17) | <0.001 |
|
| |||
| Diabetes | 1.07 | (1.05–1.10) | <0.001 |
|
| |||
| Chronic steroid use | 1.52 | (1.47–1.58) | <0.001 |
|
| |||
| BMI | 1.01 | (1.01–1.01) | <0.001 |
|
| |||
| HTN | 1.04 | (1.02–1.06) | <0.001 |
|
| |||
| Congestive HF | 1.35 | (1.21–1.50) | <0.001 |
|
| |||
| Ventilator dependent | 2.47 | (1.89–3.23) | <0.001 |
|
| |||
| Dyspnea | 1.24 | (1.20–1.29) | <0.001 |
|
| |||
| COPD | 1.24 | (1.19–1.29) | <0.001 |
Table 5.
Multivariable ordered logistic regression for number of complications within 30 days of operation, controlled for procedure type
| Variables | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Cancer diagnosis | 1.08 | (1.06–1.11) | <0.001 |
| Procedure Control | |||
| Pancreas | Ref | ||
| Colon | 0.44 | (0.43–0.45) | <0.001 |
| Stomach | 0.61 | (0.58–0.65) | <0.001 |
|
| |||
| Male sex | 0.96 | (0.94–0.97) | <0.001 |
|
| |||
| White race | 1.06 | (1.05–1.07) | <0.001 |
|
| |||
| Age | 1.00 | (1.00–1.00) | <0.001 |
|
| |||
| Functional status | 0.55 | (0.53–0.58) | <0.001 |
|
| |||
| ASA Class | 1.58 | (1.55–1.60) | <0.001 |
|
| |||
| Ascites | 2.11 | (1.91–2.33) | <0.001 |
|
| |||
| Sepsis | 2.29 | (2.17–2.41) | <0.001 |
|
| |||
| Active smoker | 1.09 | (1.03–1.17) | 0.005 |
|
| |||
| Ventilator dependent | 1.80 | (1.49–2.18) | <0.001 |
|
| |||
| Dialysis | 1.15 | (1.02–1.28) | 0.019 |
|
| |||
| Acute renal failure | 1.42 | (1.17–1.73) | <0.001 |
|
| |||
| Diabetes | 1.06 | (1.03–1.09) | <0.001 |
|
| |||
| Chronic steroids | 1.56 | (1.42–1.71) | <0.001 |
|
| |||
| BMI | 1.01 | (1.01–1.01) | <0.001 |
|
| |||
| Hypertension | 1.05 | (1.02–1.07) | <0.001 |
|
| |||
| Congestive HF | 1.30 | (1.18–1.43) | <0.001 |
|
| |||
| Dyspnea | 1.26 | (1.12–1.17) | <0.001 |
|
| |||
| COPD | 1.28 | (1.23–1.33) | <0.001 |
The morbidity and mortality rate regression models were also compared to the risk predictor variables included in the ACS NSQIP participant use file. Goodness-of-fit testing with Brier scores and c statistics is reproduced in Appendix 2 and demonstrated similar rates of predictive ability between ACS NSQIP models and the models created in this study.
DISCUSSION
The purpose of this study was to assess the utility of risk prediction when comparing surgery for cancer to a benign disease indication. We used short-term surgical outcomes for those undergoing gastrointestinal surgery as a comparable population cohort once controlling for procedure-specific risks. This study represents the first time that national surgical 30-day follow-up data was compared directly between cancer and non-cancer patients to assess the feasibility of risk prediction in these distinct populations. Our findings suggest that a cancer diagnosis confers an independent cancer risk for morbidity that is not addressed in existing risk prediction models.
Although previous work did not use a non-cancer comparison group, our results are consistent with the limited alignment of existing risk models previously observed in cancer populations.12,13 Our results also confirm that cancer and benign disease patients undergoing the same surgery are intrinsically different populations with different postoperative risks. Furthermore, the statistical model building performed here demonstrates that the increased variability, mortality, and morbidity seen in cancer surgery patients cannot be explained away with subset analysis. These findings provide support for the assumptions of proposed changes14–16,24 to current prediction models for cancer operations that there are currently unknown predictive factors that may explain this population’s postoperative variation.
Our findings are also consistent with the evolving understanding of the role solid tumors play systemically at diagnosis. Longstanding clinical observations suggest that systemic metabolic processes underlie cancer pathogenesis,25–27 These theories have more recently been reinforced with increasing biological evidence that cancer alters the body’s immunologic and biosynthetic mechanisms.28,29 Given these systemic alterations in cancer patients and the need for physiologic reserve with invasive procedures, it is not surprising that short-term surgical outcomes are affected. For example, a dysfunctional immune system would be less effective at preventing post-operative sepsis,30 and cancer-induced coagulopathy may increase the risk of hemorrhage.
Although the increased risk variation in cancer surgery using risk models has been recognized, this study is the first to use NSQIP data to compare post-operative outcomes directly between cancer and benign disease surgery patients. These findings represent a very practical “real-world” setting for the surgeon who operates on both malignant and benign surgical disease. With internet access alone, a surgeon today can provide a new patient presenting to clinic with evidence-based, individualized risk predictions of post-operative surgical course that include predicted length of stay, complication-specific morbidity rates, and comparisons to the average population. Our analysis demonstrates that existing risk prediction models may not sufficiently account for the role of a cancer diagnosis. Although proxies for a cancer diagnosis such as NSQIP variables for disseminated malignancy and ascites influence these models, our analysis suggests there is a critical degree of granularity missing when evaluating these patients. Put another way, we have shown that a cancer diagnosis requiring surgery confers as great an increased risk of worse short-term surgical outcomes as other variables included in current prediction models such as hypertension and diabetes. However, cancer is notably absent from these models.
More globally, given the significant differences in health status at baseline for these patients, research is needed to evaluate potential interventions to “normalize” patients with cancer and to understand the possible effectors driving this phenomenon. Efforts are already under way to improve cancer patients’ overall health status to reduce postoperative complications.31 The Lung Cancer Exercise Training Study is currently randomizing patients to various exercise modalities to improve physiologic reserve following partial lung resection.32 Preoperative interventions to “prehabilitate” colorectal patients prior to surgery are currently under investigation with early results demonstrating improved postoperative outcomes following an improvement in preoperative activity levels.33 Additionally, these efforts have also demonstrated the need for continuous improvement of risk prediction models as further data has refined all-procedure risk prediction over time.17
When discussing individualized risks with patients preoperatively, such a distinction will be important in that it recognizes that even the best all-procedure risk models available are unable to fully account for the increased variability and negatively biased outcomes of those with cancer. For surgeons using publicly available risk calculators and their patients, this gap in our current prediction knowledge will be an important caveat. Even for surgeons who provide risk counseling on gestalt alone, these findings can be incorporated into one’s usual heuristic thinking recognizing there is an increased degree of randomness for outcomes of cancer surgery patients.34–36
Although not tested here, an important area of future research is whether other recent simplified risk prediction tools may also be vulnerable to the inherent variability of cancer surgery. For example, simplified clinical review (e.g., the Surgical Risk Preoperative Assessment System), timed stair-climbing, and frailty assessment have been suggested as alternative methods of risk prediction.9,10,37,38 Performance of these other models within cancer patient populations has not been rigorously assessed to date, and the findings here suggest these alternative measures may be equally problematic to predict the inherent excessive variability of cancer surgery outcomes. Many of these risk models advocate for the immediate use of these models at the bedside through incorporation into electronic health record platforms.9,39 However, it remains unresolved how to not only communicate risk but also the degree of uncertainty around that risk. Such an element of risk counseling is intuitively important but is also likely one of the most difficult concepts to convey. Future risk tools may need to incorporate a mechanism to explicitly signal uncertainty and may benefit from delineation of risks by their uncertainty and modifiability.
This study is not without its limitations. This study was not able to address long-term outcomes of the surgeries examined. The information on both patient’s preoperative health as well as post-operative outcomes both come from a national database that is vulnerable to between-institution reporting differences as well as heterogeneity in data capture abilities of the individual clinical reviewers. Fortunately, the lack of complete data capture if occurring should be equally likely across all records and therefore would favor the null hypothesis. Another limitation of this study is the comparison of a de novo multivariable regression model to the ACS NSQIP risk prediction model whose specific methodology is not publicly reported.40
Finally, interpreting diagnostic indication has been simplified for the purpose of this analysis. Both benign and cancer diseases were grouped together without further information about the severity of disease or subtype (e.g., inflammatory versus infectious benign disease). Since operations were grouped by CPT code in this study, it is possible that cancer diagnosis is serving as a proxy measure of residual confounding where cancer operations tend to be more extensive resections than benign disease. Although our own personal experience is not consistent with this consideration (e.g., chronic pancreatitis or severe diverticulitis are no easier than their malignant comparators), even if it were the case that the differences reported here were due to varying intraoperative complexity, it does not negate our conclusions. Additionally, current prediction models do not readily account for planned operative complexity and group by CPT codes in a similar manner to our study methodology. Therefore, for the general surgeon who sees both malignant and benign disease, he or she must account for limitations in current risk prediction models that do not adequately discriminate between these operative indications. Further discerning disease-specific risk factors for a patient will ultimately need to balance increased complexity of the risk models versus practical usability.
CONCLUSIONS
There is increased outcomes variability within the cancer surgery patient population when compared to benign surgical disease patients. This variation cannot be fully explained by current risk prediction model methodologies, and there are likely additional preoperative variables needed to improve the performance of these models in cancer patients. Current prediction models also less effectively evaluate the risk of death and complications in cancer patients. Finally, a diagnosis of cancer independently increases the risk of complications and 30-day mortality from surgical resection, and additional counseling regarding the uncertainty of short-term outcomes for cancer surgery may be needed. Prospective studies are planned to examine this phenomenon further.
Acknowledgments
None
FUNDING SOURCES
I.L. received salary support for the preparation of this manuscript from a National Cancer Institute T32 Institutional Training Grant (5T32CA126607-08) and a Research Foundation of the American Society of Colon and Rectal Surgeons Resident Research Initiation Grant (GSSRIG-031). F.J. received salary support as the primary investigator of an Agency for Healthcare Research and Quality grant (1K08HS024736-01).
APPENDIX 1
Due to the observed heterogeneity of the study population illustrated in Table 1, we performed propensity score matching described below and the repeated the main text’s statistical. This mirrored analysis is described through the series of complementary tables below. Propensity score matching was performed using the teffects package within Stata® 14.2 (StataCorp, College Station, Texas). Covariates matched included sex, race, age, functional status, ASA class, use of chronic steroids, and presence of ascites preoperatively. Patients unable to be matched were excluded from further analysis. A total of 35,839 (23.5%) of benign patients were successfully matched to the cancer patient group.
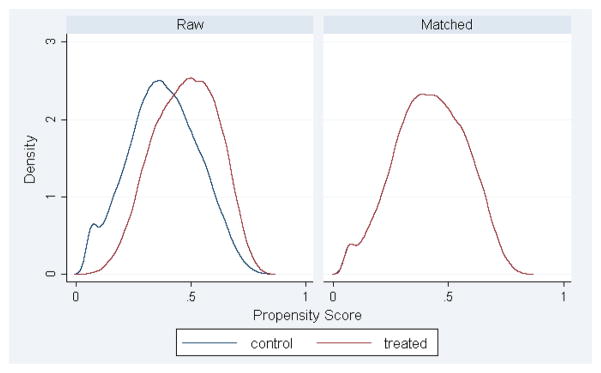
Figure A1. Distribution of aggregate covariate similarity pre- and post-propensity score matching when modeling the risk of death at 30 days.
*By Stata nomenclature, the “treated” group represents those with surgery for a cancer indication.
Figure A2. Distribution of aggregate covariate similarity pre- and post-propensity score matching when modeling the risk of any complication at 30 days.
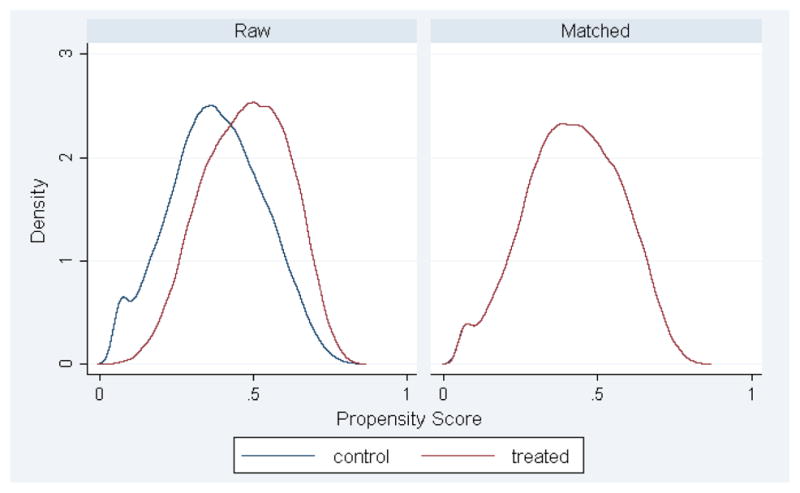
*By Stata nomenclature, the “treated” group represents those with surgery for a cancer indication.
Table A1.
Population preoperative characteristics for gastrointestinal surgery in NSQIP (2005–2015 with propensity score matched benign group)
| Surgery for GI Cancer (n = 111,563) | Surgery for Benign GI Disease (n = 35,839) | p | |
|---|---|---|---|
| Gender | <0.001 | ||
| Male | 57,879 (51.9%) | 16,480 (46.0%) | |
| Female | 53,684 (48.1%) | 19,359 (54.0%) | |
|
| |||
| Age | <0.001 | ||
| < 65 | 48,945 (43.8%) | 22,101 (61.6%) | |
| 65–74 | 31,024 (27.8%) | 8,538 (23.8%) | |
| 75–84 | 23,876 (21.4%) | 4,356 (12.1%) | |
| > 84 | 7,828 (7.0%) | 877 (2.4%) | |
|
| |||
| Race | <0.001 | ||
| White | 79,272 (76.2%) | 26,792 (76.9%) | |
| Black | 10,745 (10.0%) | 2,997 (8.6%) | |
| Hispanic | 11,826 (11.1%) | 4,218 (12.1%) | |
| Other | 5,182 (4.8%) | 816 (2.3%) | |
|
| |||
| Functional Status | <0.001 | ||
| Independent | 107,918 (96.9%) | 34,746 (97.2%) | |
| Partially dependent | 2,497 (2.2%) | 604 (1.7%) | |
| Totally dependent | 945 (0.9%) | 390 (1.1%) | |
|
| |||
| ASA Class, (Mean/Median) | 2.6/3 | 2.5/2 | <0.001 |
|
| |||
| Comorbidities | |||
| Chronic steroids | 2,916 (2.6%) | 3,013 (8.4%) | <0.001 |
| Ascites | 710 (0.6%) | 186 (0.5%) | 0.013 |
| Systemic sepsis | <0.001 | ||
| SIRS | 1,487 (1.3%) | 626 (1.8%) | |
| Sepsis | 310 (0.3%) | 363 (1.0%) | |
| Septic shock | 24 (0.0%) | 78 (0.2%) | |
| Ventilator dependent | 41 (0.0%) | 78 (0.2%) | <0.001 |
| Disseminated cancer | 8,128 (7.3%) | 1,040 (2.9%) | <0.001 |
| Diabetes | <0.001 | ||
| Non-insulin dependent | 14,552 (13.0%) | 3,284 (9.2%) | |
| Insulin dependent | 7,785 (7.0%) | 1,689 (4.7%) | |
| Hypertension on meds | 61,442 (55.0%) | 16,701 (46.6%) | <0.001 |
| Congestive heart failure | 909 (0.8%) | 191 (0.5%) | <0.001 |
| Dyspnea | <0.001 | ||
| With activity | 10,309 (9.2%) | 2,307 (6.4%) | |
| At rest | 691 (0.6%) | 188 (0.5%) | |
| Active tobacco use | 17,010 (15.2%) | 6,818 (19.0%) | <0.001 |
| Chr. obstruct. pulm. dis. | 5,924 (5.3%) | 1,532 (4.3%) | <0.001 |
| Dialysis | 460 (0.4%) | 210 (0.6%) | <0.001 |
| Acute renal failure | 128 (0.1%) | 61 (0.2%) | 0.011 |
| BMI (Mean/Median) | 28.1/27.2 | 28.5/27.6 | <0.001 |
|
| |||
| Procedure Performed | |||
| Colectomy | 83,501 (81.2%) | 30,792 (85.8%) | <0.001 |
| Pancreatectomy | 22,681 (20.3%) | 4,112 (11.5%) | <0.001 |
| Gastrectomy | 5,491 (4.9%) | 968 (2.7%) | <0.001 |
Of note, statistically significant differences are still reflected in Table A1 due to the omission of propensity weighted estimates.
Table A2.
Postoperative outcomes for gastrointestinal surgery in NSQIP (2005–2015 with propensity score matched benign group)
| Surgery for GI Cancer (n = 111,563) | Surgery for Benign GI Disease (n = 35,839) | p | |
|---|---|---|---|
| LOS Mean | 8.3 | 7.4 | <0.001 |
| LOS Median | 6 (IQR = 4 –9) | 5 (IQR = 4–8) | |
|
| |||
| Death | 1,934 (1.7%) | 361 (1.0%) | <0.001 |
|
| |||
| Morbidity | |||
| Any Complication | 30,637 (27.4%) | 8,364 (23.3%) | <0.001 |
| Superficial SSI | 7,342 (6.6%) | 2,253 (6.3%) | 0.050 |
| Deep SSI | 1,497 (1.3%) | 505 (1.4%) | 0.338 |
| Organ Space SSI | 5,919 (5.3%) | 1,843 (5.1%) | 0.230 |
| Would Disruption | 1,253 (1.1%) | 386 (1.1%) | 0.470 |
| Pneumonia | 3,222 (2.9%) | 754 (2.1%) | <0.001 |
| Reintubation | 2,814 (2.5%) | 566 (1.6%) | <0.001 |
| Pulmonary Embolism | 925 (0.8%) | 236 (0.7%) | 0.001 |
| Prolonged Mechanical Ventilation (> 48 hours) | 2,514 (2.3%) | 641 (1.8%) | <0.001 |
| Progressive Renal Failure | 893 (0.8%) | 220 (0.6%) | <0.001 |
| Acute Renal Failure | 766 (0.7%) | 151 (0.4%) | <0.001 |
| Urinary Tract Infection | 3,609 (3.2%) | 1,078 (3.0%) | 0.033 |
| Cerebrovascular accident | 367 (0.3%) | 64 (0.2%) | <0.001 |
| Cardiac arrest | 762 (0.7%) | 141 (0.4%) | <0.001 |
| Myocardial infarction | 833 (0.8%) | 175 (0.5%) | <0.001 |
| Bleeding w/transfusion | 10,505 (9.4%) | 2,185 (6.1%) | <0.001 |
| Deep vein thrombosis | 1,629 (1.5%) | 458 (1.3%) | 0.011 |
| Sepsis | 5,010 (4.5%) | 1,545 (4.3%) | 0.152 |
| Septic Shock | 2,301 (2.1%) | 542 (1.5%) | <0.001 |
|
| |||
| Total # Complications, Mean | 0.47 | 0.39 | <0.001 |
| Total # of Complications, Median | 0 (IQR = 0 – 1) | 0 (IQR = 0 – 0) | |
|
| |||
| Any readmission | 6,631 (5.9 %) | 2,237 (6.2%) | 0.039 |
|
| |||
| Observed Expected Mortality Correlation | 0.22 | 0.32 | <0.001 |
|
| |||
| Observed Expected Morbidity Correlation | 0.28 | 0.29 | <0.001 |
Table 3A.
Multivariable logistic regression with propensity score matching for death within 30 days of operation, controlled for procedure type
| Variables | Odds Ratio 95% | Confidence Interval | P Value |
|---|---|---|---|
| Cancer diagnosis | 1.09 | (1.02–1.17) | 0.015 |
| Procedure Control | |||
| Pancreas | Ref | ||
| Colon | 0.57 | (0.52–0.62) | <0.001 |
| Stomach | 1.01 | (0.86–1.19) | 0.878 |
|
| |||
| Male sex | 0.71 | (0.67–0.77) | <0.001 |
|
| |||
| BMI | 0.99 | (0.98–0.99) | <0.001 |
|
| |||
| Age | 1.05 | (1.05–1.06) | <0.001 |
|
| |||
| Functional status | 0.41 | (0.37–0.45) | <0.001 |
|
| |||
| ASA Class | 2.12 | (1.98–2.26) | <0.001 |
|
| |||
| Chronic steroid use | 1.50 | (1.26–1.77) | <0.001 |
|
| |||
| Hypertension | 1.27 | (1.16–1.38) | <0.001 |
|
| |||
| Diabetes | 1.12 | (1.03–1.21) | 0.007 |
|
| |||
| COPD | 1.35 | (1.21–1.50) | <0.001 |
|
| |||
| Ascites | 5.30 | (4.39–6.40) | <0.001 |
|
| |||
| Sepsis | 2.90 | (2.52–3.33) | <0.001 |
|
| |||
| Ventilator dependent | 2.55 | (1.25–5.22) | 0.010 |
|
| |||
| Active smoker | 1.24 | (1.12–1.38) | <0.001 |
|
| |||
| Acute renal failure | 1.46 | (0.89–2.39) | 0.130 |
|
| |||
| Dialysis | 2.07 | (1.56–2.74) | <0.001 |
|
| |||
| Congestive HF | 1.69 | (1.42–2.02) | <0.001 |
|
| |||
| Dyspnea | 1.65 | (1.51–1.81) | <0.001 |
Table A4.
Multivariable logistic regression with propensity score matching for any complication within 30 days of operation, controlled for procedure type
| Variables | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Cancer diagnosis | 1.07 | (0.72–0.80) | <0.001 |
| Procedure Control | |||
| Stomach | Ref | ||
| Colon | 0.76 | (1.04–1.10) | <0.001 |
| Pancreas | 1.79 | (1.69–1.90) | <0.001 |
|
| |||
| Male sex | 0.95 | (0.93–0.97) | <0.001 |
|
| |||
| White race | 1.07 | (1.05–1.08) | <0.001 |
|
| |||
| Age | 1.00 | (1.00–1.01) | <0.001 |
|
| |||
| Functional status | 0.62 | (0.58–0.66) | <0.001 |
|
| |||
| ASA Class | 1.53 | (1.50–1.56) | <0.001 |
|
| |||
| Ascites | 2.10 | (1.84–2.39) | <0.001 |
|
| |||
| Sepsis | 2.29 | (2.13–2.46) | <0.001 |
|
| |||
| Active smoker | 1.15 | (1.11–1.18) | <0.001 |
|
| |||
| Diabetes | 1.08 | (1.05–1.11) | <0.001 |
|
| |||
| Chronic steroid use | 1.49 | (1.41–1.56) | <0.001 |
|
| |||
| BMI | 1.01 | (1.01–1.01) | <0.001 |
|
| |||
| Hypertension | 1.03 | (1.01–1.07) | 0.004 |
|
| |||
| Congestive HF | 1.35 | (1.19–1.52) | <0.001 |
|
| |||
| Ventilator dependent | 2.04 | (1.40–2.96) | <0.001 |
|
| |||
| Dyspnea | 1.25 | (1.20–1.29) | <0.001 |
|
| |||
| COPD | 1.22 | (1.16–1.28) | <0.001 |
Table A5.
Multivariable ordered logistic regression with propensity score matching for number of complications within 30 days of operation, controlled for procedure type
| Variables | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Cancer diagnosis | 1.06 | (1.03–1.09) | <0.001 |
| Procedure Control | |||
| Stomach | Ref | ||
| Colon | 0.72 | (0.68–0.76) | <0.001 |
| Pancreas | 1.74 | (1.65–1.85) | <0.001 |
|
| |||
| Male sex | 0.93 | (0.91–0.95) | <0.001 |
|
| |||
| White race | 1.07 | (1.05–1.08) | <0.001 |
|
| |||
| Age | 1.00 | (1.00–1.01) | <0.001 |
|
| |||
| Functional status | 0.58 | (0.55–0.61) | <0.001 |
|
| |||
| ASA Class | 1.54 | (1.51–1.58) | <0.001 |
|
| |||
| Ascites | 2.16 | (1.91–2.45) | <0.001 |
|
| |||
| Sepsis | 2.35 | (2.19–2.51) | <0.001 |
|
| |||
| Active smoker | 1.15 | (1.12–1.19) | <0.001 |
|
| |||
| Ventilator dependent | 1.40 | (1.06–1.86) | 0.018 |
|
| |||
| Acute renal failure | 1.23 | (0.95–1.60) | 0.115 |
|
| |||
| Diabetes | 1.07 | (1.04–1.10) | <0.001 |
|
| |||
| Chronic steroids | 1.50 | (1.42–1.57) | <0.001 |
|
| |||
| BMI | 1.01 | (1.01–1.01) | <0.001 |
|
| |||
| Hypertension | 1.04 | (1.02–1.07) | 0.001 |
|
| |||
| Congestive HF | 1.28 | (1.14–1.44) | <0.001 |
|
| |||
| Dyspnea | 1.26 | (1.21–1.31) | <0.001 |
|
| |||
| COPD | 1.25 | (1.19–1.32) | <0.001 |
APPENDIX 2
Table A6.
Goodness-of-fit testing NSQIP Risk Prediction versus Cancer-controlled Logistic Regression within a cancer surgery population
| c-statistic | Brier Score | |
|---|---|---|
| Mortality | ||
| NSQIP | 0.83 | 0.0088 |
| Cancer-controlled | 0.82 | 0.0090 |
|
| ||
| Morbidity | ||
| NSQIP | 0.71 | 0.1710 |
| Cancer-controlled | 0.66 | 0.1694 |
From Bilimoria, NSQIP adjustment, JACS 2014
The c-statistic is a measure of discrimination, that ranges from 0.5 (chance) to 1.0 (perfect), which reflects the extent to which cases are properly classified as having or not having an event. The Brier score describes the averaged squared difference between patients’ predicted probability and the actual outcome (0 for a non-event and 1, for an event). If all patients without an event are assigned a predicted probability of 0, and all patients with an event are assigned a predicted probability of 1, the Brier Score will be 0, indicating perfect prediction. For the null model Brier Score, the overall event rate (say, 0.05 for a 5% mortality rate) is assigned to each patient.
Footnotes
AUTHOR CONTRIBUTIONS
Study conception and design: Leeds, Canner Efron, Ahuja, Haut, Wick, Johnston
Acquisition of data: Leeds, Canner
Analysis and interpretation of data: Leeds, Canner, Efron, Ahuja, Haut, Wick, Johnston
Drafting of manuscript: Leeds, Johnston
Critical revision: Leeds, Canner, Efron, Ahuja, Haut, Wick, Johnston
Meeting presentation: This data was presented at the Academic Surgical Congress in February 2017 in Las Vegas, Nevada.
DATA ACCESS
I. L. had full access to all the data and takes responsibility for the integrity of the data and accuracy of the data analysis.
DISCLOSURES
No author has a financial or personal relationship with other people or organizations that could inappropriately influence the work reported here.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan R, Alatise OI, Anderson BO, et al. Global cancer surgery: Delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015;16(11):1193–1224. doi: 10.1016/S1470-2045(15)00223-5. [DOI] [PubMed] [Google Scholar]
- 3.Knops AM, Legemate Da, Goossens A, Bossuyt PMM, Ubbink DT. Decision aids for patients facing a surgical treatment decision: a systematic review and meta-analysis. Ann Surg. 2013;257(5):860–866. doi: 10.1097/SLA.0b013e3182864fd6. [DOI] [PubMed] [Google Scholar]
- 4.Waljee JF, Rogers MAM, Alderman AK. Decision aids and breast cancer: do they influence choice for surgery and knowledge of treatment options? J Clin Oncol. 2007;25(9):1067–1073. doi: 10.1200/JCO.2006.08.5472. [DOI] [PubMed] [Google Scholar]
- 5.McCaffery K, Irwig L, Bossuyt P. Patient decision aids to support clinical decision making: evaluating the decision or the outcomes of the decision. Med Decis Making. 27(5):619–625. doi: 10.1177/0272989X07306787. [DOI] [PubMed] [Google Scholar]
- 6.Schenker Y, Fernandez A, Sudore R, Schillinger D. Interventions to improve patient comprehension in informed consent for medical and surgical procedures: a systematic review. Med Decis Making. 2011;31(1):151–173. doi: 10.1177/0272989X10364247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin R, Furnary AP, Fine SC, Blackstone EH, Grunkemeier GL. Using Society of Thoracic Surgeons risk models for risk-adjusting cardiac surgery results. Ann Thorac Surg. 2010;89(3):677–682. doi: 10.1016/j.athoracsur.2009.10.078. [DOI] [PubMed] [Google Scholar]
- 8.Cohen ME, Bilimoria KY, Ko CY, Hall BL. Development of an American College of Surgeons National Surgery Quality Improvement Program: Morbidity and Mortality Risk Calculator for Colorectal Surgery. J Am Coll Surg. 2009;208(6):1009–1016. doi: 10.1016/j.jamcollsurg.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Meguid RA, Bronsert MR, Juarez-Colunga E, Hammermeister KE, Henderson WG. Surgical Risk Preoperative Assessment System (SURPAS): III. Accurate Preoperative Prediction of 8 Adverse Outcomes Using 8 Predictor Variables. Ann Surg. 2016;264(1):23–31. doi: 10.1097/SLA.0000000000001678. [DOI] [PubMed] [Google Scholar]
- 10.Reddy S, Contreras CM, Singletary B, et al. Timed Stair Climbing Is the Single Strongest Predictor of Perioperative Complications in Patients Undergoing Abdominal Surgery. J Am Coll Surg. 2016;222(4):559–566. doi: 10.1016/j.jamcollsurg.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-42-3. doi: 10.1016/j.jamcollsurg.2013.07.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borja-Cacho D, Parsons HM, Habermann EB, Rothenberger DA, Henderson WG, Al-Refaie WB. Assessment of ACS NSQIP’s predictive ability for adverse events after major cancer surgery. Ann Surg Oncol. 2010;17(9):2274–2282. doi: 10.1245/s10434-010-1176-z. [DOI] [PubMed] [Google Scholar]
- 13.Parsons HM, Habermann EB, Stain SC, Vickers SM, Al-Refaie WB. What happens to racial and ethnic minorities after cancer surgery at american college of surgeons national surgical quality improvement program hospitals? J Am Coll Surg. 2012;214(4):539–547. doi: 10.1016/j.jamcollsurg.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Merkow RP, Kmiecik TE, Bentrem DJ, et al. Effect of including cancer-specific variables on models examining short-term outcomes. Cancer. 2013;119(7):1412–1419. doi: 10.1002/cncr.27891. [DOI] [PubMed] [Google Scholar]
- 15.Merkow RP, Bentrem DJ, Cohen ME, et al. Effect of cancer surgery complexity on short-term outcomes, risk predictions, and hospital comparisons. J Am Coll Surg. 2013;217(4):685–693. doi: 10.1016/j.jamcollsurg.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Merkow RP, Bentrem DJ, Winchester DP, Stewart AK, Ko CY, Bilimoria KY. Effect of including cancer-specific variables on risk-adjusted hospital surgical quality comparisons. Ann Surg Oncol. 2013;20(6):1766–1773. doi: 10.1245/s10434-013-2867-z. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Cohen ME, Hall BL, Ko CY, Bilimoria KY. Evaluation and Enhancement of Calibration in the American College of Surgeons NSQIP Surgical Risk Calculator. J Am Coll Surg. 2016;223(2):231–239. doi: 10.1016/j.jamcollsurg.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 18.American College of Surgeons. [Accessed October 1, 2016];ACS NSQIP Participant Use Data File. https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use.
- 19.Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: The American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251–267. doi: 10.1016/j.yasu.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Meguid RA, Bronsert MR, Juarez-Colunga E, Hammermeister KE, Henderson WG. Surgical Risk Preoperative Assessment System (SURPAS): II. Parsimonious Risk Models for Postoperative Adverse Outcomes Addressing Need for Laboratory Variables and Surgeon Specialty-specific Models. Ann Surg. 2016;264(1):10–22. doi: 10.1097/SLA.0000000000001677. [DOI] [PubMed] [Google Scholar]
- 21.Cohen ME, Dimick JB, Bilimoria KY, Ko CY, Richards K, Hall BL. Risk adjustment in the American College of Surgeons National Surgical Quality Improvement Program: a comparison of logistic versus hierarchical modeling. J Am Coll Surg. 2009;209(6):687–693. doi: 10.1016/j.jamcollsurg.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: Patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2) doi: 10.1016/j.jamcollsurg.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 23.American College of Surgeons National Surgical Quality Improvement Program. Semi-Annual Report. Chicago: 2008. [Google Scholar]
- 24.Liu JB, Weber SM, Berian JR, et al. Role of Operative Complexity Variables in Risk Adjustment for Patients With Cancer. JAMA Surg. 2016 Aug; doi: 10.1001/jamasurg.2016.2253. [DOI] [PubMed] [Google Scholar]
- 25.Zajicek G. Cancer as a systemic disease. Med Hypotheses. 4(3):193–207. doi: 10.1016/0306-9877(78)90002-6. [DOI] [PubMed] [Google Scholar]
- 26.Fisher B, Redmond C, Fisher ER. The contribution of recent NSABP clinical trials of primary breast cancer therapy to an understanding of tumor biology--an overview of findings 1859. Cancer. 1980;46(4:Suppl):Suppl-25. doi: 10.1002/1097-0142(19800815)46:4+<1009::aid-cncr2820461326>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Sohal DPS, Walsh RM, Ramanathan RK, Khorana AA. Pancreatic adenocarcinoma: Treating a systemic disease with systemic therapy. J Natl Cancer Inst. 2014;106(3) doi: 10.1093/jnci/dju011. [DOI] [PubMed] [Google Scholar]
- 28.Finn OJ. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Annals of Oncology. 2012;23 doi: 10.1093/annonc/mds256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: Biological and clinical aspects. J Thromb Haemost. 2013;11(2):223–233. doi: 10.1111/jth.12075. [DOI] [PubMed] [Google Scholar]
- 30.Williams MD, Braun LA, Cooper LM, et al. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8(5):R291–8. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Carli F, Lee L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27(4):1072–1082. doi: 10.1007/s00464-012-2560-5. [DOI] [PubMed] [Google Scholar]
- 32.Jones L, Eves N, Kraus W, et al. The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design. BMC Cancer. 2010;10(1):155. doi: 10.1186/1471-2407-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carli F, Charlebois P, Stein B, et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. 2010;97(8):1187–1197. doi: 10.1002/bjs.7102. [DOI] [PubMed] [Google Scholar]
- 34.Englesbe MJ, Terjimanian MN, Lee JS, et al. Morphometric age and surgical risk. J Am Coll Surg. 2013;216(5):976–985. doi: 10.1016/j.jamcollsurg.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Englesbe MJ. Frailty: Failing the eyeball test. Am J Transplant. 2014;14:53–54. doi: http://dx.doi.org/10.1111/ajt.12617. [Google Scholar]
- 36.Mack MJ, Holper EM. TAVR Risk Assessment: Does the Eyeball Test Have 20/20 Vision, or Can We Do Better? J Am Coll Cardiol. 2016;68(4):353–355. doi: 10.1016/j.jacc.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 37.Makary Ma, Segev DL, Pronovost PJ, et al. Frailty as a Predictor of Surgical Outcomes in Older Patients. J Am Coll Surg. 2010;210(6):901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 38.Robinson TN, Wu DS, Pointer L, et al. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg. 2013;206(4):544–550. doi: 10.1016/j.amjsurg.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leeds IL, Sadiraj V, Cox JC, et al. Discharge decision-making after complex surgery: Surgeon behaviors compared to predictive modeling to reduce surgical readmissions. Am J Surg. 2017;213(1):112–119. doi: 10.1016/j.amjsurg.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wanderer JP, Ehrenfeld JM. Toward External Validation and Routine Clinical Use of the American College of Surgeons NSQIP Surgical Risk Calculator. J Am Coll Surg. 2016;223(4):674. doi: 10.1016/j.jamcollsurg.2016.06.010. [DOI] [PubMed] [Google Scholar]