Abstract
Background
Growing evidence suggests that movement abnormalities occur prior to the onset of psychosis. Innovations in technology and software provide the opportunity for a fine-tuned and sensitive measurement of observable behavior that may be particularly useful to detecting the subtle movement aberrations present during the prodromal period.
Methods
In the present study, 54 youth at ultrahigh risk (UHR) for psychosis and 62 healthy controls participated in structured clinical interviews to assess for an UHR syndrome. The initial 15 minutes of the baseline clinical interview was assessed using Motion Energy Analysis (MEA) providing frame-by-frame measures of total movement, amplitude, speed, and variability of both head and body movement separately.
Results
Result showed region-specific group differences such that there were no differences in head movement but significant differences in body movement. Specifically, the UHR group showed greater total body movement and speed of body movements, and lower variation in body movement compared to healthy controls. However, there were no significant associations with positive, negative or disorganized symptom domains.
Conclusion
This study represents an innovative perspective on gross motor function in the UHR group. Importantly, the automated approach used in this study provides a sensitive and objective measure of body movement abnormalities, potentially guiding novel assessment and prevention of symptom development in those at risk for psychosis.
Keywords: movement abnormalities, motion energy analysis, ultrahigh risk, psychosis, gross motor
1. Introduction
Signs of altered motor development are increasingly recognized as an important marker of risk for psychosis (Bernard and Mittal, 2015; Mittal, 2016). A growing body of literature suggests that movement abnormalities are present long before the first signs of thought disorder and prospective studies of youth at risk for psychosis show that movement abnormalities may predict eventual transition to psychosis (Callaway et al., 2014; Mittal et al., 2008; Mittal et al., 2010b).
Assessing youth during the ultrahigh risk (UHR) period immediately prior to psychosis is important as these individuals are experiencing moderate subthreshold psychotic symptoms and a decline in functioning (Cannon et al., 2008). Current research is focused on developing innovative calculators designed to organize or weight various risk markers for psychosis (Cannon et al., 2016). These efforts are important because 10–35% of UHR cases will go on to develop a psychotic disorder within 2–3 years (Fusar-Poli et al., 2012). In addition, with relatively little experience on neuroleptic medication or long term history of illicit drug abuse compared to patients with psychosis, research with UHR individuals is potentially valuable for understanding etiological factors and markers of increased risk for the disorder.
The assessment of movement abnormalities in individuals developing psychosis has gone through exciting developments in recent years (Hirjak et al., 2015; Mittal, 2016; Mittal and Wakschlag, 2016). However, much of this research has been based on observer ratings of movement abnormalities, which require a significant amount of training and time for reliability, are more subject to rater bias, and do not provide continuous data. Developing automated assessment strategies for motor performance has several benefits over traditional methods. First, automated and instrumental measures of movement are sensitive in identifying the same individuals as traditional observer-based methods while also capturing additional individuals showing more subtle aberrations (Mentzel et al., 2016a). Second, these measures are capable of detecting kinematic variables that not readily available with observation methods (e.g., amplitude, speed, variability). Finally, automated and instrumental measures are not subject to bias (Cortese et al., 2005). More recently, there has been a growing interest in using instrumental and automated measures to understand motor performance in UHR and in patients with psychosis (Caligiuri et al., 2009; Caligiuri et al., 2010; Cortese et al., 2005; Dean and Mittal, 2015; Dean et al., 2015b; Dean et al., 2013). Furthermore, instrumental and automated measures may detect a larger proportion of movement variation than traditional observer based measures (Pappa and Dazzan, 2009). Taken together, motor performance assessment may be helpful for early detection and intervention efforts in youth at risk for the disorder.
Novel developments in video technology and custom software may allow more fine-tuned measurement of stationary and seated gross motor performance in specific regions of interests. Ramseyer and colleagues have developed Motion Energy Analysis (MEA) to look at changes in grey scale pixel density in order to measure the amount of movement in user-defined regions of interest (Ramseyer and Tschacher, 2011, 2014; Tschacher et al., 2014). Moreover, this technology has been used to study impairment in nonverbal communication in schizophrenia patients, which may be impaired prior to the onset of psychosis (Kupper et al., 2010; Kupper et al., 2015; Walther and Mittal, 2016). In recent years, instrumental and automated procedures have elucidated motor abnormalities in these domains during the UHR period and in formal psychosis. However, our understanding of gross motor movement is more limited and this technology may allow objective quantification of multiple aspects of movement kinematics that are not ratable by an observing clinician including the size (amplitude) of movements, their speed and variability. Examining movement kinematics during seated communication may aid in symptom assessment and therapeutic efforts (Ramseyer and Tschacher, 2011) as well as treatment response (Caligiuri et al., 2009; Caligiuri et al., 2010; Caligiuri et al., 2006).
The current study seeks to examine gross motor behavior using an automated approach in a sample of UHR and healthy control participants. Each participant was recorded during a structured clinical interview. A 15-min segment of the clinical interview footage was subjected to MEA. Data was processed and target variables for total movement, amplitude of movements, speed of movement, and coefficient of variability of movement were extracted for both the head and body separately. Previous work with traditional observer-based scoring of head and body regions from video recordings has noted that at risk individuals and patients with schizophrenia show abnormal movements (Compton et al., 2015; Mentzel et al., 2017; Mittal et al., 2007b). We hypothesized that the UHR group would show more movement in general, greater movement amplitude and speed, and more variability of movement in both the head and body. Because this is the first study to examine gross motor behavior using MEA, exploratory analysis was conducted to examine relationships between movement kinematic variables and positive, negative and disorganized UHR symptoms.
2. Materials and methods
2.1. Participants
Adolescent and young adult UHR and healthy control participants between 12 and 21 years of age (mean age = 18.68) were recruited by Craigslist, email postings, newspaper ads, and community professional referrals. Exclusion criteria consisted of head injury, the presence of a neurological disorder, and lifetime substance dependence. The presence of an Axis I psychotic disorder (e.g., schizophrenia, schizoaffective disorder, schizophreniform) was an exclusion criterion for UHR participants. The presence of any category of Axis I disorder or a psychotic disorder in a 1st degree relative was an exclusion criterion for controls. The protocol and informed consent procedures were approved by the University Institutional Review Board. See Table 1 for the demographic characteristics of the sample.
Table 1.
UHR and healthy controls did not differ in terms of age, education, gender, and parental education. UHR participants were rated significantly higher on positive, negative and disorganized symptom domains at baseline. NS indicates not significant.
| UHR | Control | Statistic | p ≤ | ||
|---|---|---|---|---|---|
| Age | |||||
| Mean (SD) | 18.78 (1.82) | 18.60 (2.35) | t(114) = .46 | NS | |
| Gender | |||||
| Male | 31 | 28 | |||
| Female | 23 | 34 | |||
|
|
|||||
| Total | 54 | 62 | χ2(1, N = 116) = 1.28 | NS | |
| Education (years) | |||||
| Mean (SD) | 12.46 (1.79) | 12.62 (2.43) | t(113) = .40 | NS | |
| Parent Education | |||||
| Mean (SD) | 15.57 (2.48) | 15.56 (2.56) | t(114) = .04 | NS | |
| Symptoms | |||||
| Positive: Mean (SD) | 12.30 (4.79) | .50 (1.22) | t(59.03) = 17.59 | .001 | |
| Negative: Mean (SD) | 9.67 (7.1) | .47 (1.04) | t(54.97) = 9.43 | .001 | |
| Disorganized: Mean (SD) | 5.77 (3.77) | .31 (.71) | t(56.32) = 10.36 | .001 | |
2.2. Clinical Interviews
The Structured Interview for Prodromal Syndromes (SIPS) (Miller et al., 1999) was administered to both UHR and control subjects to diagnose a UHR syndrome (the SIPS was used to rule out UHR symptoms in healthy controls). Participants in the present study met SIPS criteria for a prodromal or high-risk syndrome, defined by moderate to severe but not psychotic levels of positive symptoms (rated from 3 to 5 on a six-point scale) and/or a decline in global functioning accompanying the presence of schizotypal personality disorder and/or a family history of schizophrenia (Miller et al., 2002). The SIPS gages distinct categories of prodromal symptoms including positive and negative domains. A total sum score for each domain is used as an indicator of the respective dimensions of symptomatology.
The Structured Clinical Interview for Axis-I DSM-IV Disorders (SCID) (First et al., 1995) was administered to rule out a psychotic disorder diagnosis. Training of advanced doctoral student interviewers was conducted over a 2-month period, and inter-rater reliabilities exceeded the minimum study criterion of Kappa ≥ .80.
Antipsychotic prescription and dosage information was collected for each participant. The chlorpromazine equivalent (CPZ) dosage was calculated for each participant currently taking antipsychotic medication (n = 7) (Woods, 2003).
2.3. Motion Energy Analysis
Motion energy analysis (MEA) was completed using an automated software program specifically designed to measure movement in predefined regions of interest (ROI) in digital video recordings. This program provides frame-by-frame parameters of grey scale intensity during the video recording (Kupper et al., 2015; Ramseyer and Tschacher, 2011, 2014; Tschacher et al., 2014). Participants provided consent to be videotaped during the clinical interviews and were recorded using a high-resolution video camera (Sanyo VCC-HD4600P). The first 15 minutes of the SIPS clinical interview was trimmed and subjected to MEA based on similar duration of videos in past studies (Kupper et al., 2010; Ramseyer and Tschacher, 2014). This section of the video was chosen in order to maximize consistency of context for the video analysis. Two research assistants specified the head and body ROIs (see Figure 1). These particular ROIs were chosen based on previous studies with UHR, schizophrenia patients, and healthy individuals (Kupper et al., 2015; Mittal et al., 2007b; Ramseyer and Tschacher, 2011). The head ROI included a region a few inches above the head to the bottom of the participant’s chin. The body ROI included the bottom of the head ROI, under the chin, to the horizontal surface of the couch.
Figure 1.

An example of the head and body regions of interest and the measurement of grey scale density in a movie frame. The person in the figure is a research assistant in the lab.
Motion energy parameters were recorded to a text file for each frame of the video at 29 frames per second. Raw data was preprocessed and filtered using a moving average filter of 5s to remove motion artifacts due to video quality using custom software in R based on previous methods (Kupper et al., 2010; Kupper et al., 2015; Ramseyer and Tschacher, 2011, 2014). Separate target variables for head and body ROI were calculated and include:
Total amount of movement was calculated by summing the number of non-zero frames.
Mean amplitude of movement peaks across the entire 15 min segment.
Speed of movement is the median ratio of height to duration of the peaks.
- Head and body movements were characterized by peaks of grey scale density changes. A measure of variability, the coefficient of variability (CV) for movement was calculated by dividing the standard deviation of the height of these peaks (amplitude) by the mean amplitude across all peaks.
2.4. Data Analysis
Group differences for continuous and categorical demographic variables were assessed with independent t-tests and chi-squared tests, respectively. Group differences on movement parameters were first tested using two tailed independent samples t-tests. In order to examine the effect of antipsychotic medication on movement variables, group differences were also examined using analysis of covariance (ANCOVA) controlling for CPZ equivalent doses. The healthy controls reported few positive, negative or disorganized symptoms. Exploratory analysis looking at the relationship between movement target variables and positive negative and disorganized symptoms were examined within the UHR group only.
3. Results
3.1. Participants
There were no significant differences between groups on demographic characteristics including age, education, gender, and parental education. As expected, UHR participants were rated significantly higher than controls on SIPS symptom domains (see Table 1 for information about the participants).
3.2. Group differences in head and body movement
There were no group differences in terms of total amount of head movement, amplitude of head movement, head movement speed, or variation in head movement between UHR and healthy controls (p values > .2).
The UHR group had significantly more total body movement compared to healthy controls t(114) = 2.85, p ≤ .01, d = .53. There were no group differences in terms of mean amplitude of body movements. On average, the UHR group showed greater speed of body movement compared to controls t(114) = 2.43, p ≤ .05, d = .45. The UHR group showed lower CV of body movements compared to healthy controls t(114) = 2.11, p ≤ .05, d = .39 (see Figure 2). Controlling for CPZ equivalency did not change the results for total body movement, amplitude of body movements, or speed of body movement, however, CV of body movement changed from significant to a trend level group difference F(1, 113) = 3.40, p = .06. See Table 2.
Figure 2.
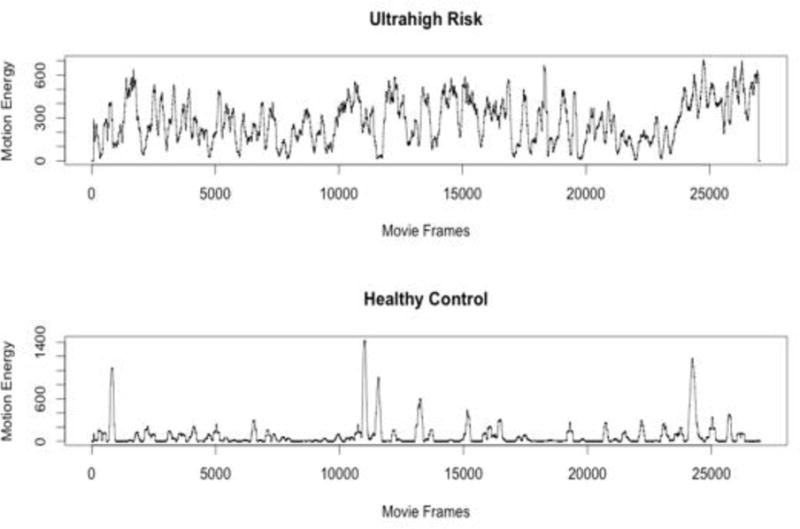
An example body movement time series from an UHR and healthy control participant.
Table 2.
Mean (SD) for MEA variables for the head and body regions of interest.
| UHR | Control | ||
|---|---|---|---|
| Head | Amplitude | 113.04 (86.76) | 131.75 (100.05) |
| Total movement | 5567.43 (3189.43) | 4708.16 (3844.62) | |
| Speed | 3.99 (2.39) | 4.28 (3.26) | |
| CV | 1.12 (.35) | 1.12 (.32) | |
|
| |||
| Body | Amplitude | 289.49 (162.02) | 266.81 (186.76) |
| Total movement | 8595.65 (4849.62) | 6222.48 (4111.67) | |
| Speed | 10.82 (7.33) | 7.97 (5.26) | |
| CV | 1.05 (.36) | 1.23 (.53) | |
3.4. Relationship between positive, negative, and disorganized symptoms and movement
Because the UHR group showed different body movement and not head movement, exploratory within UHR group regressions were run for total body movement, speed of body movement, and CV of body movement separately to predict association with positive, negative and disorganized symptoms. There were no significant relationships between positive, negative and disorganized symptoms and body movement parameters (p values > .1). Controlling for CPZ equivalency did not change the results (p values > .1).
4. Discussion
The current study examined gross motor performance using innovative video analysis software during a standard clinical interview. This technology allowed an investigation of movement behavior from multiple kinematic measurements of the head and body separately. Consistent with our hypotheses the UHR group showed more movement and greater speed of movements. In contrast to our hypothesis, the UHR group showed lower variability in body movements. Head movement appears to be similar across UHR and healthy control groups. The results of this study represent an advance on previous video observer based studies of movement that primarily focused on the frequency and severity of abnormal involuntary movements, indicating a nuanced series of findings, where global measures of body movement (i.e., total movement, speed, and variability) appear to be affected in UHR compared to typically developing young adults. These findings speak to a general motor impairment during the UHR period, and fit within the neural diathesis-stress framework where motor problems may be indicative of altered neurodevelopment in cortical-subcortical networks.
Video based and live observation assessment of motor development and movement abnormalities has a rich history in premorbid and prodromal psychosis literature. Studies of the premorbid period suggest that motor abnormalities may be linked to a broad range of developmental delays, cognitive impairment, and may be an endophenotype for psychosis (Dickson et al., 2012; Schenkel and Silverstein, 2004). Using archival footage of people diagnosed with schizophrenia, Walker and colleagues showed that as children there were delays in motor development and odd posturing (Walker et al., 1994). Erlenmeyer-Kimling and colleagues found that gross motor performance, assessed in children ages 7–12 years old who had a parent with schizophrenia, predicted adult onset schizophrenia with 75% sensitivity (Erlenmeyer-Kimling et al., 2000). In addition, Rosso and colleagues found that unusual gross motor performance in children of patients with schizophrenia at 4 and 7 years of age predicted later development of psychosis (Rosso et al., 2000). In separate studies, Schiffman reviewed videotapes of Danish children eating lunch and found that a number of individuals with genetic risk for psychosis showed greater social and motor function impairment (Schiffman et al., 2009; Schiffman et al., 2004). Recent work suggests that impaired manual dexterity and balance in children at 7 years old may be related to familial risk for schizophrenia but not bipolar disorder (Burton et al., 2017). A population based study found increased occurrence of movement abnormalities in children 8–17 years of age who reported clinical high risk symptoms of psychosis (Kindler et al., 2016). In a series of prospective studies, Mittal and colleagues found evidence that dyskinetic movements in the upper body of schizotypal adolescents were associated with increases in cortisol and markers of prenatal insult (Mittal et al., 2007a; Mittal et al., 2008; Mittal et al., 2007b). A large longitudinal study of prodromal youth suggested that movement abnormalities in the upper body are related to neurocognitive impairment and transition to psychosis (Mittal et al., 2010b). In a multisite study of prodromal youth, Callaway observed that dyskinetic movements predicted transition to psychosis (Callaway et al., 2014). Altogether, this body of evidence suggests that movement abnormalities appear at early stages of development and represent an important behavior tied to risk for psychosis.
The current results suggest that UHR youth show greater movement across several different indices in the upper-body region during clinical interviews. This is consistent with similar movement analysis in schizotypal adolescents and in patients with schizophrenia who show more nonverbal hand and body motions during clinical interviews. Mittal and colleagues (Mittal et al., 2006) found that schizotypal adolescents show more self-stimulatory movements but fewer gestures during clinical interviews. Lavelle and colleagues (Lavelle et al., 2013) note that increased hand gestures by patients with schizophrenia while talking to a clinician may signal anxiety, distress, and produce less rapport between patient and clinician. It is possible that increased body movement in the UHR sample may be a measure of how distressed a person is in regards to their symptoms or how they perceive their symptoms to be problematic; either in an effort to communicate help-seeking behavior or to compensate for difficulties in explaining their symptoms (Holler and Beattie, 2003). Examining the relationship between self-reported anxiety and biological measures of stress would be an important next step to understand the biological underpinnings of participant’s nonverbal movement behavior during the clinical interview.
Using a similar automated MEA process to the current study, Ramseyer and Tschacher found that head movement synchrony between client and therapist predicted therapy outcome whereas body synchrony predicted session-by-session outcome (Ramseyer and Tschacher, 2014). The authors posit that body movement communicates emotional content. Given that the UHR group was discussing troubling positive symptoms during the video, increased body movement and lower variability (i.e., their body movements remained more active but changed less for each topic) may be an indication that the UHR group has altered control over their movement and emotions. This is consistent with current neurodevelopmental and cognitive dysmetria theories for psychosis suggesting that impaired fluidity and coordination of movement and thought is central to the development of psychosis symptoms (Andreasen, 1999; Andreasen and Pierson, 2008).
Movement abnormalities are intimately linked to the pathophysiology of psychosis. Predominant theories and neuroimaging studies suggest that the signs and symptoms of psychosis may be related to altered structure and connectivity within cortical-subcortical motor networks (Tekin and Cummings, 2002; Walther, 2015). Past cross sectional studies suggest that gross motor impairment in patients with schizophrenia is related to abnormal grey matter volume and cerebral blood flow in the inferior frontal gyrus, supplemental motor area, thalamus, and basal ganglia (Bracht et al., 2013; Stegmayer et al., 2014). Work from our research group has noted that individuals showing symptoms of risk for psychosis also have grey matter and functional connectivity abnormalities in both the thalamus and striatum (Bernard et al., 2015; Dean and Mittal, 2015; Lunsford-Avery et al., 2013; Mittal et al., 2013b). Recent neuroimaging studies also suggest that UHR individuals show altered dopaminergic activity in the striatum (Howes et al., 2011; Howes et al., 2009). Taken together, a biological mechanism for the current results may be explained by abnormal neurodevelopment within cortical-thalamo-striatal pathways, thus giving rise to increase body movement overall and greater speed (Hirjak et al., 2015). The finding that antipsychotic medication affected the group differences in body movement variability speaks to changes related to dopaminergic pathways in cortical-striatal networks. Future work examining MEA generated movement variables, along with structural and functional neuroimaging is warranted for understanding the biological underpinnings of movement behavior in UHR individuals.
Exploratory analysis of movement kinematic variables did not show a relationship to the positive, negative or disorganized symptom totals. This result may reflect the differences in how MEA measures gross motor movement and may indicate that the kinematic variables used in the current study are not specific to dyskinesia or Parkinson’s like movements. Hyperkinetic movement such as dyskinesia in the upper limbs has been associated with positive symptoms in youth at risk for psychosis as well as formal psychosis (Docx et al., 2012; Mittal et al., 2010a; Mittal et al., 2011; Mittal et al., 2008; Mittal et al., 2007b; Mittal et al., 2010b; Morrens et al., 2014; van Harten et al., 2014). The lack of association between MEA kinematic variables in this study and UHR positive symptoms may in part reflect a noted limitation of using SIPS symptom ratings and continuous movement parameters. That is, Pruessner and colleagues argue that because of the range of positive symptom scores (i.e., 3 to 5 on a six-point scale) considered for an UHR syndrome using the SIPS, finding associations between biomarkers and symptom ratings may be limited (Pruessner et al., 2017).
In contrast to the finding from Kupper and colleagues who examined MEA in schizophrenia patients and found that slower head and body movement was related to more severe negative symptoms (Kupper et al., 2010), the current results did not find evidence that psychomotor slowing, as the UHR showed greater speed of body movements compared to healthy controls. A possible explanation for this finding is that psychomotor slowing is subtle during the UHR period. For example, Dean and Mittal (2015) found that UHR individuals show worse ability to scale one’s velocity using pen movements on a digital tablet, possibly reflected some rigidity or parkinsonism. This might also suggest that the negative symptoms observed in the UHR sample are not as severe as in formal psychosis, and generation of movement may change once psychosis develops (Walther et al., 2014b).
In addition, we did not find a relationship between body movement variables and disorganized symptoms. This is in contrast to work by Walther and colleagues who found that irregular patterns of activity monitored through actigraphy were related to positive and disorganized symptoms, as well as excitement in patients with schizophrenia (Walther et al., 2014a). The findings from the current study speak to the need for longitudinal data with UHR participants who transition to psychosis and comparisons to patients with psychosis using the same movement parameters to better elucidate the relationship between MEA kinematic variables and psychosis symptom development.
The current study fits in well with a growing body of work to look at movement abnormalities using instrumental measures. As noted, a variety of instruments have been used in both UHR and psychosis samples to measure fine motor performance in UHR and schizophrenia samples including digital tablets for handwriting analysis (Caligiuri et al., 2009; Caligiuri et al., 2010; Caligiuri et al., 2006; Dean and Mittal, 2015; Dean et al., 2015b; Dean et al., 2013), and finger pressure sensors (Cortese et al., 2005; Mittal et al., 2013b; Purdon et al., 2001). In addition to the instrumental measures for fine motor performance, researchers have used actigraph sensors (Mittal et al., 2013a; Walther et al., 2015; Walther et al., 2014b) and postural sway devices (Bernard et al., 2014; Dean et al., 2015a; Kent et al., 2012) to measure standing and ambulatory gross motor performance in UHR and psychosis samples. The current study supports this growing body of literature by hinting at the promise for automatized coding processes. This line of research has already been utilized to supplement traditional coding measures of emotions such as Facial Action Coding System (FACS) (Wolf, 2015). Over time, automated approaches may prove superior because the time and training that traditional coding takes cannot be feasibly incorporated into risk evaluations or calculators.
Most importantly, the results of this study add to the growing literature on motor performance during the UHR period. Data from MEA may provide a holistic approach to understanding motor behavior related to a variety of domains of movement rather than relying on data collected from separate instruments. The footage used for MEA was taken from clinical interviews and may be a helpful addition to assessment of psychosis risk. Indeed, automated and instrumental measures of movement may be able to more quickly assess abnormalities, thus providing data quicker in addition to other measures of risk for psychosis. This may ultimately guide better assessment strategies for psychosis risk.
This study has several strengths and limitations. The MEA used in this study has not classified movements according to traditional observer based measures, such as dyskinesia. This limits our ability to place the current results in a specific context that is rooted in past studies, although this remains a goal for this data. Follow-up studies comparing MEA generated kinematic variables to other instrumental measures of dyskinesia, psychomotor slowing as well as observer based ratings may provide further detail about how this technology may be used to assess movement abnormalities in psychosis populations (Mentzel et al., 2016a; Mentzel et al., 2016b). Validation studies using MEA in larger healthy samples would also help to make stronger conclusions about movement abnormalities during the UHR period. The results did not reveal a relationship to positive, negative or disorganized symptom domains, however, group differences between UHR and healthy controls suggests that altered body movement is related to psychosis risk; future work examining associations to biological and social markers of risk for psychosis may provide insight into how gross motor movement is related to the development of attenuated psychosis symptoms. Finally, the study was cross-sectional and follow-up studies over multiple time points are necessary for examining the relationship between motion energy kinematics and the progression of psychosis.
Acknowledgments
This work was supported by National Institutes of Health Grants R01MH094650 and R21/R33MH103231 to V.A.M.
The authors would like to thank Kim Kramer for her help with data processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Mittal is a consultant with Takeda Pharmaceuticals. No other authors have conflicts to disclose.
Contributors
Authors D.J.D, A.T.S, and V.A.M developed the study concept. V.A.M. obtained funding for the study. All authors contributed to the study design. Testing, data collection as well as data analysis and interpretation were performed by D.J.D, A.T.S and R.E.N under the supervision of V.A.M. D.J.D drafted the paper; A.T.S, R.E.N, and V.A.M provided the critical revisions. All authors approved the final version of the paper for submission.
References
- Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56(9):781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64(2):81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Dean DJ, Kent JS, Orr JM, Pelletier-Baldelli A, Lunsford-Avery JR, Gupta T, Mittal VA. Cerebellar networks in individuals at ultra high-risk of psychosis: Impact on postural sway and symptom severity. Hum Brain Mapp. 2014;35(8):4064–4078. doi: 10.1002/hbm.22458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Mittal VA. Updating the research domain criteria: The utility of a motor dimension. Psychol Med. 2015;45(13):2685–2689. doi: 10.1017/S0033291715000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Orr JM, Mittal VA. Abnormal hippocampal-thalamic white matter tract development and positive symptom course in individuals at ultra-high risk for psychosis. NPJ Schizophr 1. 2015 doi: 10.1038/npjschz.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T, Schnell S, Federspiel A, Razavi N, Horn H, Strik W, Wiest R, Dierks T, Muller TJ, Walther S. Altered cortico-basal ganglia motor pathways reflect reduced volitional motor activity in schizophrenia. Schizophr Res. 2013;143(2–3):269–276. doi: 10.1016/j.schres.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Burton BK, Thorup AA, Jepsen JR, Poulsen G, Ellersgaard D, Spang KS, Christiani CJ, Hemager N, Gantriis D, Greve A, Mors O, Nordentoft M, Plessen KJ. Impairments of motor function among children with a familial risk of schizophrenia or bipolar disorder at 7 years old in denmark: An observational cohort study. Lancet Psychiatry. 2017 doi: 10.1016/S2215-0366(17)30103-7. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Dean CE, Niculescu AB, Lohr J. Handwriting movement analyses for monitoring drug-induced motor side effects in schizophrenia patients treated with risperidone. Human movement science. 2009;28(5):633–642. doi: 10.1016/j.humov.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Dean CE, Niculescu AB, Lohr JB. Handwriting movement kinematics for quantifying extrapyramidal side effects in patients treated with atypical antipsychotics. Psychiatry Res. 2010;177(1–2):77–83. doi: 10.1016/j.psychres.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Filoteo JV, Song D, Lohr JB. Quantitative measurement of handwriting in the assessment of drug-induced parkinsonism. Human movement science. 2006;25(4–5):510–522. doi: 10.1016/j.humov.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Callaway DA, Perkins DO, Woods SW, Liu L, Addington J. Movement abnormalities predict transitioning to psychosis in individuals at clinical high risk for psychosis. Schizophr Res. 2014;159(2):263–266. doi: 10.1016/j.schres.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in north america. Arch Gen Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, Heinssen R, Jeffries CD, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Kattan MW. An individualized risk calculator for research in prodromal psychosis. American Journal of Psychiatry. 2016;173(10) doi: 10.1176/appi.ajp.2016.15070890. appiajp201615070890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton MT, Fantes F, Wan CR, Johnson S, Walker EF. Abnormal movements in first-episode, nonaffective psychosis: Dyskinesias, stereotypies, and catatonic-like signs. Psychiatry Res. 2015;226(1):192–197. doi: 10.1016/j.psychres.2014.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese L, Caligiuri MP, Malla AK, Manchanda R, Takhar J, Haricharan R. Relationship of neuromotor disturbances to psychosis symptoms in first-episode neuroleptic-naive schizophrenia patients. Schizophr Res. 2005;75(1):65–75. doi: 10.1016/j.schres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Dean DJ, Kent JS, Bernard JA, Orr JM, Gupta T, Pelletier-Baldelli A, Carol EE, Mittal VA. Increased postural sway predicts negative symptom progression in youth at ultrahigh risk for psychosis. Schizophr Res. 2015a doi: 10.1016/j.schres.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Mittal VA. Spontaneous parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. npj Schizophrenia 1. 2015 doi: 10.1038/npjschz.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Orr JM, Newberry RE, Mittal VA. Motor behavior reflects reduced hemispheric asymmetry in the psychosis risk period. Schizophr Res. 2015b;170(1):137–142. doi: 10.1016/j.schres.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Teulings HL, Caligiuri M, Mittal VA. Handwriting analysis indicates spontaneous dyskinesias in neuroleptic naive adolescents at high risk for psychosis. Journal of visualized experiments: JoVE. 2013;(81):e50852. doi: 10.3791/50852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med. 2012;42(4):743–755. doi: 10.1017/S0033291711001693. [DOI] [PubMed] [Google Scholar]
- Docx L, Morrens M, Bervoets C, Hulstijn W, Fransen E, De Hert M, Baeken C, Audenaert K, Sabbe B. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatr Scand. 2012;126(4):256–265. doi: 10.1111/j.1600-0447.2012.01846.x. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Rock D, Roberts SA, Janal M, Kestenbaum C, Cornblatt B, Adamo UH, Gottesman II. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: The new york high-risk project. Am J Psychiatry. 2000;157(9):1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for dsm-iv axis i disorders, patient edition, january 1995 final SCID-I/P Version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, Barale F, Caverzasi E, McGuire P. Predicting psychosis: Meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Hirjak D, Thomann PA, Kubera KM, Wolf ND, Sambataro F, Wolf RC. Motor dysfunction within the schizophrenia-spectrum: A dimensional step towards an underappreciated domain. Schizophr Res. 2015;169(1–3):217–233. doi: 10.1016/j.schres.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Holler J, Beattie G. Pragmatic aspects of representational gestures: Do speakers use them to clarify verbal ambiguity for the listener? Gesture. 2003;3(2):127–154. [Google Scholar]
- Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D, Valmaggia L, Allen P, Murray R, McGuire P. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: A pet study. Mol Psychiatry. 2011;16(9):885–886. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66(1):13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Kent JS, Hong SL, Bolbecker AR, Klaunig MJ, Forsyth JK, O’Donnell BF, Hetrick WP. Motor deficits in schizophrenia quantified by nonlinear analysis of postural sway. PLoS One. 2012;7(8):e41808. doi: 10.1371/journal.pone.0041808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler J, Schultze-Lutter F, Michel C, Martz-Irngartinger A, Linder C, Schmidt SJ, Stegmayer K, Schimmelmann BG, Walther S. Abnormal involuntary movements are linked to psychosis-risk in children and adolescents: Results of a population-based study. Schizophr Res. 2016;174(1–3):58–64. doi: 10.1016/j.schres.2016.04.032. [DOI] [PubMed] [Google Scholar]
- Kupper Z, Ramseyer F, Hoffmann H, Kalbermatten S, Tschacher W. Video-based quantification of body movement during social interaction indicates the severity of negative symptoms in patients with schizophrenia. Schizophr Res. 2010;121(1–3):90–100. doi: 10.1016/j.schres.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Kupper Z, Ramseyer F, Hoffmann H, Tschacher W. Nonverbal synchrony in social interactions of patients with schizophrenia indicates socio-communicative deficits. PLoS One. 2015;10(12):e0145882. doi: 10.1371/journal.pone.0145882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle M, Healey PG, McCabe R. Is nonverbal communication disrupted in interactions involving patients with schizophrenia? Schizophr Bull. 2013;39(5):1150–1158. doi: 10.1093/schbul/sbs091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunsford-Avery JR, Orr JM, Gupta T, Pelletier-Baldelli A, Dean DJ, Smith Watts AK, Bernard J, Millman ZB, Mittal VA. Sleep dysfunction and thalamic abnormalities in adolescents at ultra high-risk for psychosis. Schizophr Res. 2013;151(1–3):148–153. doi: 10.1016/j.schres.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzel TQ, Lieverse R, Bloemen O, Viechtbauer W, van Harten PN, Genetic R, Outcome of Psychosis, I High incidence and prevalence of drug-related movement disorders in young patients with psychotic disorders. J Clin Psychopharmacol. 2017;37(2):231–238. doi: 10.1097/JCP.0000000000000666. [DOI] [PubMed] [Google Scholar]
- Mentzel TQ, Lieverse R, Levens A, Mentzel CL, Tenback DE, Bakker PR, Daanen HA, van Harten PN. Reliability and validity of an instrument for the assessment of bradykinesia. Psychiatry Res. 2016a;238:189–195. doi: 10.1016/j.psychres.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Mentzel TQ, Mentzel CL, Mentzel SV, Lieverse R, Daanen HA, van Harten PN. Instrumental assessment of bradykinesia: A comparison between motor tasks. IEEE J Biomed Health Inform. 2016b;20(2):521–526. doi: 10.1109/JBHI.2015.2412656. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the structured interview for prodromal syndromes: Preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159(5):863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Mittal VA. Cross-cutting advancements usher in a new era for motor research in psychosis. Schizophr Bull. 2016;42(6):1322–1325. doi: 10.1093/schbul/sbw123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Daley M, Shiode MF, Bearden CE, O’Neill J, Cannon TD. Striatal volumes and dyskinetic movements in youth at high-risk for psychosis. Schizophr Res. 2010a;123(1):68–70. doi: 10.1016/j.schres.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dean DJ, Pelletier A, Caligiuri M. Associations between spontaneous movement abnormalities and psychotic-like experiences in the general population. Schizophr Res. 2011;132(2–3):194–196. doi: 10.1016/j.schres.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF. The relations among putative biorisk markers in schizotypal adolescents: Minor physical anomalies, movement abnormalities, and salivary cortisol. Biol Psychiatry. 2007a;61(10):1179–1186. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Gupta T, Orr JM, Pelletier-Baldelli A, Dean DJ, Lunsford-Avery JR, Smith AK, Robustelli BL, Leopold DR, Millman ZB. Physical activity level and medial temporal health in youth at ultra high-risk for psychosis. J Abnorm Psychol. 2013a;122(4):1101–1110. doi: 10.1037/a0034085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch Gen Psychiatry. 2008;65(2):165–171. doi: 10.1001/archgenpsychiatry.2007.23. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Orr JM, Turner JA, Pelletier AL, Dean DJ, Lunsford-Avery J, Gupta T. Striatal abnormalities and spontaneous dyskinesias in non-clinical psychosis. Schizophr Res. 2013b;151(1–3):141–147. doi: 10.1016/j.schres.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Tessner KD, McMillan AL, Delawalla Z, Trotman HD, Walker EF. Gesture behavior in unmedicated schizotypal adolescents. J Abnorm Psychol. 2006;115(2):351–358. doi: 10.1037/0021-843X.115.2.351. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Tessner KD, Trottman HD, Esterberg M, Dhrub SH, Simeonova DI, McMillan AL, Murphy E, Saczawa ME, Walker EF. Movement abnormalities and the progression of prodromal symptomatology in adolescents at risk for psychotic disorders. J Abnorm Psychol. 2007b;116(2):260–267. doi: 10.1037/0021-843X.116.2.260. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Wakschlag LS. Research domain criteria (rdoc) grows up: Strengthening neurodevelopment investigation within the rdoc framework. J Affect Disord. 2016 doi: 10.1016/j.jad.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Walker EF, Bearden CE, Walder D, Trottman H, Daley M, Simone A, Cannon TD. Markers of basal ganglia dysfunction and conversion to psychosis: Neurocognitive deficits and dyskinesias in the prodromal period. Biol Psychiatry. 2010b;68(1):93–99. doi: 10.1016/j.biopsych.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrens M, Docx L, Walther S. Beyond boundaries: In search of an integrative view on motor symptoms in schizophrenia. Frontiers in psychiatry. 2014;5:145. doi: 10.3389/fpsyt.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: A systematic review. Psychol Med. 2009;39(7):1065–1076. doi: 10.1017/S0033291708004716. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Cullen AE, Aas M, Walker EF. The neural diathesis-stress model of schizophrenia revisited: An update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci Biobehav Rev. 2017;73:191–218. doi: 10.1016/j.neubiorev.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Purdon SE, Woodward ND, Flor-Henry P. Asymmetrical hand force persistence and neuroleptic treatment in schizophrenia. Journal of the International Neuropsychological Society. 2001;7(5):606–614. doi: 10.1017/s1355617701755087. [DOI] [PubMed] [Google Scholar]
- Ramseyer F, Tschacher W. Nonverbal synchrony in psychotherapy: Coordinated body movement reflects relationship quality and outcome. Journal of Consultation Clinical Psychology. 2011;79(3):284–295. doi: 10.1037/a0023419. [DOI] [PubMed] [Google Scholar]
- Ramseyer F, Tschacher W. Nonverbal synchrony of head- and body-movement in psychotherapy: Different signals have different associations with outcome. Front Psychol. 2014;5:979. doi: 10.3389/fpsyg.2014.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Bearden CE, Hollister JM, Gasperoni TL, Sanchez LE, Hadley T, Cannon TD. Childhood neuromotor dysfunction in schizophrenia patients and their unaffected siblings: A prospective cohort study. Schizophr Bull. 2000;26(2):367–378. doi: 10.1093/oxfordjournals.schbul.a033459. [DOI] [PubMed] [Google Scholar]
- Schenkel LS, Silverstein SM. Dimensions of premorbid functioning in schizophrenia: A review of neuromotor, cognitive, social, and behavioral domains. Genet Soc Gen Psychol Monogr. 2004;130(3):241–270. doi: 10.3200/MONO.130.3.241-272. [DOI] [PubMed] [Google Scholar]
- Schiffman J, Sorensen HJ, Maeda J, Mortensen EL, Victoroff J, Hayashi K, Michelsen NM, Ekstrom M, Mednick S. Childhood motor coordination and adult schizophrenia spectrum disorders. American journal of psychiatry. 2009;166(9):1041–1047. doi: 10.1176/appi.ajp.2009.08091400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman J, Walker E, Ekstrom M, Schulsinger F, Sorensen H, Mednick S. Childhood videotaped social and neuromotor precursors of schizophrenia: A prospective investigation. American journal of psychiatry. 2004;161(11):2021–2027. doi: 10.1176/appi.ajp.161.11.2021. [DOI] [PubMed] [Google Scholar]
- Stegmayer K, Horn H, Federspiel A, Razavi N, Bracht T, Laimbock K, Strik W, Dierks T, Wiest R, Muller TJ, Walther S. Supplementary motor area (sma) volume is associated with psychotic aberrant motor behaviour of patients with schizophrenia. Psychiatry Res. 2014;223(1):49–51. doi: 10.1016/j.pscychresns.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. J Psychosom Res. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Tschacher W, Rees GM, Ramseyer F. Nonverbal synchrony and affect in dyadic interactions. Front Psychol. 2014;5:1323. doi: 10.3389/fpsyg.2014.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harten PN, Bakker PR, Mentzel CL, Tijssen MA, Tenback DE. Movement disorders and psychosis, a complex marriage. Frontiers in psychiatry. 2014;5:190. doi: 10.3389/fpsyt.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20(3):441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- Walther S. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res. 2015;233(3):293–298. doi: 10.1016/j.pscychresns.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Walther S, Mittal VA. Why we should take a closer look at gestures. MPRC. 2016 doi: 10.1093/schbul/sbv229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Ramseyer F, Horn H, Strik W, Tschacher W. Less structured movement patterns predict severity of positive syndrome, excitement, and disorganization. Schizophr Bull. 2014a;40(3):585–591. doi: 10.1093/schbul/sbt038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Stegmayer K, Horn H, Rampa L, Razavi N, Muller TJ, Strik W. The longitudinal course of gross motor activity in schizophrenia - within and between episodes. Frontiers in Psychiatry. 2015;6:10. doi: 10.3389/fpsyt.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Stegmayer K, Horn H, Razavi N, Muller TJ, Strik W. Physical activity in schizophrenia is higher in the first episode than in subsequent ones. Frontiers in Psychiatry. 2014b;5:191. doi: 10.3389/fpsyt.2014.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K. Measuring facial expression of emotion. Dialogues Clin Neurosci. 2015;17(4):457–462. doi: 10.31887/DCNS.2015.17.4/kwolf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]


