Abstract
Background
Limited data is available on the role, and extent of, postchemotherapy lymphadenectomy (PC-LND) in patients with clinical evidence of pelvic (cN1-3) or retroperitoneal (RP) lymph node spread from urothelial bladder carcinoma (UBC).
Objective
To compare the outcomes of operated versus non operated patients after first-line chemotherapy.
Design, Setting, Participants
Data from 34 centers was collected, totalling 522 patients, treated between 01/2000 and 06/2015. Criteria for patient selection were the following: bladder primary tumor, lymph node metastases (pelvic ± RP) only, 1st-line platinum-based chemotherapy given.
Intervention
LND (with cystectomy) versus observation after first-line chemotherapy for metastatic UBC.
Outcome measures and statistical analysis
Overall survival (OS) was the primary endpoint. Multiple propensity score (PS) techniques were adopted, including 1:1 PS matching and inverse probability of treatment weighting (IPTW). Additionally, the IPTW analysis was performed with the inclusion of the covariates, i.e. with “doubly robust” estimation (DREP).
Results and limitations
Overall, 242 (46.4%) patients received PC-LND and 280 (53.6%) observation after chemotherapy. There were 177 (33.9%) and 345 (66.1%) patients with either RP or pelvic LN only, respectively. DREP-adjusted comparison was not significant for improved OS for PC-LND (HR: 0.86, 95%CI: 0.56–1.31, p=0.479), confirmed by matched analysis (HR: 0.91, 95%CI: 0.60–1.36, p=0.628). This was also observed in the RP subgroup (HR: 1.12, 95%CI: 0.68–1.84). The retrospective nature of the data and the heterogeneous patient population were the major limitations.
Conclusions
Although there were substantial differences between the two groups, after accounting for major confounders we report a non-significant OS difference with PC-LND compared to observation only. These findings may be hypothesis-generating for future prospective trials.
Patient summary
We found no differences in survival by adding postchemotherapy lymphadenectomy in patients with pelvic or retroperitoneal lymph node metastatic bladder cancer. The indication to perform postchemotherapy lymphadenectomy in the most suitable patients requires additional studies.
Keywords: Urothelial carcinoma, Postchemotherapy lymphadenectomy, First-line chemotherapy, Propensity-score
Introduction
Patients with metastatic urothelial bladder carcinoma (UBC) have a poor outcome and limited survival.[1] However, a small subgroup of patients with limited metastatic spread, usually limited to the lymph nodes, may still benefit from curative treatment. The therapeutic indication of these patients includes the administration of first-line chemotherapy for 4–6 cycles. After chemotherapy completion, the net benefit obtained from postchemotherapy surgery is hardly evaluable, owing to limited available data. In fact, data exists mostly from few small, retrospective, usually single-center, experiences, with just a few larger analyses.[2–15] All these studies ultimately resulted in similar findings, i.e., significant survival improvement can be obtained in selected patients, in particular when cisplatin-based chemotherapy can be administered, and objective response to chemotherapy is obtained.
In the published series, patients with more extensive metastatic lymph node spread did not usually undergo postchemotherapy lymphadenectomy (PC-LND). However, patients with enlarged pelvic and retroperitoneal lymph nodes without other metastatic sites before starting chemotherapy were usually discussed in multidisciplinary clinics at the time of chemotherapy completion, and radical surgical clearance was sometimes pursued in these patients, mainly in centers offering high surgical expertise. There are few retrospective and long-dated series available about multimodal strategies for these patients, their sample sizes ranging 11–80 patients, generally reporting highly heterogeneous findings.[2–15] Alternative options for such patients are close surveillance or the inclusion in clinical trials of maintenance therapy.
Patients and Methods
Patient selection
The European Association of Urology – Young Academic Urologists (EAU-YAU) and the Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC) groups jointly proposed a retrospective study to compare the outcomes of operated versus non operated patients after they have received first-line chemotherapy for clinically-evident pelvic lymph node metastases, including possible retroperitoneal lymph node involvement. These contemporary databases include data from hospitals in the United States, Europe, Israel, and Canada. Selected patients who fulfilled the following characteristics were analyzed: bladder tumor primary site, predominant UC histology, de novo metastatic UC or relapse after local treatment for muscle-invasive tumor (defined by radical cystectomy or by radical radiotherapy or chemoradiation), presence of radiologically-identified pelvic and/or retroperitoneal lymph node metastases only (visceral metastases were excluded, as well as more distant lymph nodal sites), and administration of cisplatin- or carboplatin-containing chemotherapy in the first-line, metastatic setting.
Statistical methods
Main series characteristics were summarized using conventional statistics, like median and range for continuous variables, and absolute or relative frequencies for categorical data. We used the standardized differences (SD) approach to compare covariates between patients who received PC-LND (i.e., treated cohort) versus those who did not.[16]
The main endpoint of this study was overall survival (OS). The survival time of each patient was computed as the interval between the date of chemotherapy initiation and the date of death for any reason, with censoring at the date of last follow-up in alive patients. The association between OS and treatment group was investigated with the use of Cox regression models using several propensity score (PS) techniques, and summarized with hazard ratios (HRs) and 95% confidence intervals (CIs).
Since the available data did not originate from a randomized clinical trial, the PS was adopted to control for pretreatment imbalances on observed covariates and in order to establish the marginal causal effects of intervention. PS building relied on the inclusion of all available covariates into a generalized boosted model (GBM).[17] This machine learning method has been shown to outperform simple logistic regression in the context of case-mix adjustment. The PS techniques adopted to analyze OS included 1:1 PS matching and inverse probability of treatment weighting (IPTW). Regarding the first approach, we created a matched sample by matching treated and untreated subjects on the logit of the PS using calipers of width equal to 0.2 of the standard deviation of the logit of the PS. A greedy, nearest-neighbour matching algorithm was employed to form pairs of treated and untreated subjects. This sample was then analyzed with a Cox model including treatment as the only covariate. The second approach also consisted in fitting a Cox model including treatment as covariate; a weight derived from the PS was also applied to each observation in order to produce “average treatment effect among the treated” (ATT) estimand.[18] This choice was preferred over the “average treatment effect” (ATE) option for two reasons: to allow for the unavoidable selection of surgical patients and for coherence with PS matching (intrinsically estimating ATT). Since some covariate imbalance remained after weighting, the IPTW analysis was also performed with the inclusion of the covariates, i.e. with “doubly robust” estimation. Given the presence of missing data for some covariates, we resorted to 10-fold multiple imputations and used the Rubin rules for obtaining the doubly robust estimates of Cox regression coefficients. In particular, complete records were available in 62.1% of the overall series, and the proportion of missing data varied between 0.6% (for age) and 23.9% (for objective response to chemotherapy), with a median value of 8.8%.
Other analyses were carried out as complementary, like estimation of adjusted survival curves, as described by Colea and Hernan [19], and 3-month conditional landmark analysis, in order to assess the impact of immortal bias. Furthermore, interaction tests were used to assess the heterogeneity of treatment effect across distinct patient subgroups and a forest plot was drawn thereof. Such an analysis was exploratory, but major interest was a priori focused on extent of lymph node involvement. Statistical analyses were performed using SAS version 9.2, the R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria) and the following R packages, all accessible at http://cran.r-project.org/web/packages/ : twang for PS building, nonrandom for matching, mice for multiple imputation. All tests were two-sided and p values <0.05 were considered statistically significant.
Results
Characteristics of the study groups
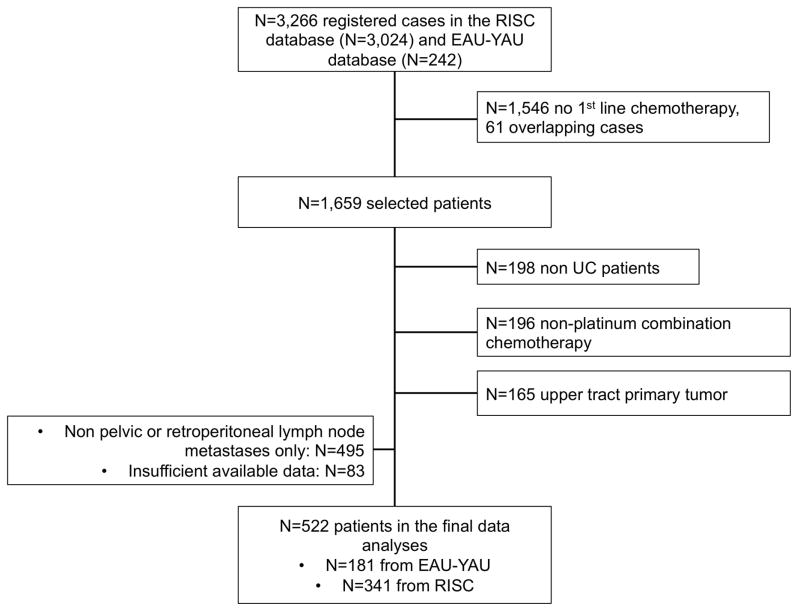
The study flow chart is presented in Figure 1. Of 3,266 registered cases, 522 patients, from 34 contributing centers, treated between January 2000 and June 2015, were suitable for analyses. Of these, 242 (46.4%) patients received PC-LND and 280 (53.6%) did not. Cystectomy was performed in all patients who were included in the PC-LND group. PC-LND consisted of pelvic LND in 193 (79.8%) and retroperitoneal LND in 40 (16.5%) patients (9 patients received LND and were included in the analyses, but extent is unknown).
Figure 1.
Study flow chart, with counts and reasons for patient selection.
Abbreviations: EAU-YAU: European Association of Urology-Young Academic Urologists; RISC: Retrospective International Study of Invasive/Advanced Cancer of the Urothelium; UC: urothelial carcinoma.
The median follow-up was 32.6 months (IQR: 17.2–55.9 months) and the median number of cycles was the same between operated and non operated patients (n=4, IQR: 4–4). Two hundred and eighty deaths were recorded, of which 116 and 164 occurred among treated and untreated patients, respectively.
Table 1 shows the distribution of main patient and disease characteristics together with SD. For this statistic a value >50% is generally considered an index of severe imbalance, while values <20% (or a more restrictive 10%) are reassuring. It is possible to observe that the 50% threshold was frequently exceeded, delineating the main differences between the groups in the clinical practice. The majority of the few patients who started chemotherapy due to a relapse after local treatment did not receive postchemotherapy surgery subsequently (SD=187.58%). Poor response to chemotherapy (SD=95.95%) as well as poor performance status at the end of chemotherapy (SD=26.51%) were other important drivers for treatment selection, as it was expected. Finally, there were 177 (33.9%) patients with retroperitoneal metastases, while 345 (66.1%) patients had pelvic lymph nodes only, delineating significant difference in the distribution between the two groups (SD=37.36%). The GBM model used to build the propensity score yielded as a by-product relative influence measures that may be regarded as a multivariate summary of the degree of imbalance in each covariate between the two cohorts (Supplementary Figure 1). In addition, Supplementary Figure 2 shows the flow of patients’ treatments.
Table 1.
Characteristics of patients in the study population and according to subgroup, and comparison of the standardized difference values for the main characteristics, according to the type of analysis.
| Characteristic | Overall: N (%) | No-PC-LND: N (%) | PC-LND: N (%) | Unweighted standardized difference (%) | Standardized difference after IPTW (%) | Standardized difference after 1:1 matching (%) |
|---|---|---|---|---|---|---|
|
| ||||||
| Total number of patients | 522 | 280 | 242 | 522 | 522 | 84 |
|
| ||||||
| Age at diagnosis: median years (IQR) | 64 (57–70) | 67 (60–73) | 61 (53–67) | 59.26 | 9.87 | 9.87 |
|
| ||||||
| Smoking history: | ||||||
| Never smoker | 122 (24.7) | 68 (24.3) | 54 (25.2) | 4.74 | ||
| Former smoker | 229 (46.4) | 143 (51.1) | 86 (40.2) | 32.46 | NA* | NA* |
| Current smoker | 143 (28.9) | 69 (24.6) | 74 (34.6) | 12.88 | ||
| Missing | 28 | - | 28 | 36.17 | ||
|
| ||||||
| ECOG-PS:** | ||||||
| 0 | 235 (53.9) | 104 (44.4) | 131 (64.8) | 34.10 | 15.78 | −4.76 |
| 1 | 159 (36.5) | 100 (42.7) | 59 (29.2) | 26.51 | 26.15 | −2.52 |
| ≥2 | 42 ( 9.6) | 30 (12.9) | 12 (6.0) | 26.51 | 11.50 | −17.19 |
| Missing | 86 | 46 | 40 | 0.27 | 15.78 | 24.27 |
|
| ||||||
| Clinical T stage: | ||||||
| T1–2 | 268 (52.3) | 172 (61.4) | 96 (41.4) | 44.48 | 33.17 | −4.78 |
| T3–4 | 244 (47.7) | 108 (38.6) | 136 (58.6) | 35.53 | 24.38 | −4.78 |
| Missing | 10 | - | 10 | 20.76 | - | - |
|
| ||||||
| Lymph node extent: | ||||||
| Pelvic LN | 345 (66.1) | 164 (58.6) | 181 (74.8) | 37.36 | 27.16 | −9.89 |
| RP LN | 177 (33.9) | 116 (41.4) | 61 (25.2) | 37.36 | 27.16 | −9.89 |
|
| ||||||
| Prior local treatment: | ||||||
| No | 283 (64.0) | 136 (48.6) | 147 (90.7) | 24.93 | 40.94 | −20.39 |
| Yes | 159 (36.0) | 144 (51.4) | 15 (9.3) | 187.58 | 54.17 | 20.39 |
| Missing | 80 | - | 80 | 70.27 | 70.27 | - |
|
| ||||||
| Prior perioperative CT: | ||||||
| No | 467 (89.5) | 232 (82.9) | 235 (97.1) | 85.03 | 36.18 | 15.81 |
| Yes | 55 (10.5) | 48 (17.1) | 7 (2.9) | 85.03 | 36.18 | −15.81 |
|
| ||||||
| Type of CT: | ||||||
| Cisplatin-based | 386 (75.7) | 194 (69.3) | 192 (83.5) | 24.83 | 0.41 | −11.80 |
| Carboplatin-based | 124 (24.3) | 86 (30.7) | 38 (16.5) | 41.26 | 14.08 | 11.80 |
| Missing | 12 | - | 12 | 22.84 | 22.84 | - |
|
| ||||||
| Best response to CT: | ||||||
| CR | 79 (19.9) | 35 (16.0) | 44 (24.7) | 14.73 | 2.96 | 0.00 |
| PR | 188 (47.4) | 78 (35.6) | 110 (61.8) | 35.34 | 5.54 | 16.62 |
| SD | 81 (20.4) | 62 (28.3) | 19 (10.7) | 53.13 | 28.45 | −12.07 |
| PD | 49 (12.3) | 44 (20.1) | 5 (2.8) | 95.95 | 52.04 | 0.00 |
| Missing | 125 | 61 | 64 | 10.57 | 37.80 | −13.31 |
Abbreviations: CR: complete response; CT: chemotherapy; ECOG-PS: Eastern Cooperative Oncology Group Performance Status: IPTW: inverse probability of treatment weighting; IQR: interquartile range; LN: lymph node; NA: not available; PC-LND: postchemotherapy lymphadenectomy; PD: progressive disease; PR: partial response; RP: retroperitoneal; SD: stable disease.
Not considered for propensity score weighting and matching analyses.
Assessed after chemotherapy completion (i.e., at the time of surgery in operated patients).
Results of the PS-matched Cox models
Cox models results are shown in Table 2. The crude comparison between treated and untreated patients yielded a highly significant result (p<0.0001) and an estimated HR (HR=0.60, 95%CI, 0.47–0.76) denoting an unrealistic 40% relative hazard reduction. Paired 1:1 matching was clearly effective in controlling covariate imbalance. As shown in Table 1, SD obtained for the matched sample were generally shrunk below 20%. A drawback, however, was the exclusion of many observations from the analysis, as far as only 84 treated patients and as many untreated patients could be matched. The treatment effect estimated with the Cox model in the matched sample was much less strong (HR=0.91, 95%CI, 0.60–1.36) and no longer statistically significant (p=0.628).
Table 2.
Results of the Cox analyses
| 2A) Unadjusted comparison: | ||||
|---|---|---|---|---|
|
| ||||
| Covariate | HR | Lower 0.95 | Upper 0.95 | p-value |
|
| ||||
| Group: | <0.001 | |||
| • LND vs. No LND | 0.60 | 0.47 | 0.76 | |
|
| ||||
| 2B) Matched analysis (N=84): | ||||
|
| ||||
| Group: | 0.628 | |||
| • LND vs. No LND | 0.91 | 0.60 | 1.36 | |
|
| ||||
| 2C) Propensity score-adjusted comparison (ATT approach): | ||||
|
| ||||
| Group: | 0.628 | |||
| • LND vs. No LND | 0.78 | 0.52 | 1.16 | |
|
| ||||
| 2D) Doubly-robust procedure (ATT approach): | ||||
|
| ||||
| Group: | 0.479 | |||
| • LND vs. No LND | 0.86 | 0.56 | 1.31 | |
|
| ||||
| Age: | 0.634 | |||
| • 70 vs. 57 | 0.93 | 0.71 | 1.24 | |
|
| ||||
| ECOG-PS: | 0.268 | |||
| • 1 vs. 0 | 1.08 | 0.68 | 1.73 | |
| • ≥2 vs. 0 | 1.82 | 0.76 | 4.38 | |
|
| ||||
| Clinical T stage: | 0.483 | |||
| • T3–4 vs. T1–2 | 1.16 | 0.76 | 1.76 | |
|
| ||||
| Nodal extent: | 0.083 | |||
| • RP vs. Pelvic | 1.47 | 0.95 | 2.26 | |
|
| ||||
| Prior local treatment: | 0.849 | |||
| • Yes vs. No | 1.06 | 0.56 | 2.03 | |
|
| ||||
| Prior PO-CT: | 0.881 | |||
| • Yes vs. No | 1.07 | 0.434 | 2.64 | |
|
| ||||
| CT regimen: | 0.433 | |||
| • CBDCA vs. CDDP | 1.26 | 0.70 | 2.28 | |
|
| ||||
| Best response to CT: | 0.080 | |||
| • PR vs. CR | 1.39 | 0.79 | 2.42 | |
| • SD vs. CR | 1.94 | 0.92 | 4.09 | |
| • PD vs. CR | 2.92 | 1.16 | 7.37 | |
Abbreviations: ATT: average treatment effect among the treated; CBDCA: carboplatin; CDDP: cisplatin; CR: complete response; CT: chemotherapy; ECOG-PS: Eastern Cooperative Oncology Group Performance Status; HR: hazard ratio; LND: lymphadenectomy; PD: progressive disease; PO: perioperative; PR: partial response; RP: retroperitoneal; SD: stable disease.
Results of the PS-weighted Cox models
Using the IPTW approach, the estimated treatment effect (HR=0.78, 95%CI 0.52–1.16, p=0.216) was toned down compared to the raw estimate of the unadjusted model, but not so much as in paired matching. At the same time, compared to the latter, the IPTW approach was slightly less effective in controlling baseline imbalance, as revealed by Table 1 and the plot of SD (Supplementary Figure 3), with the figures many times in the range between 20–50% rather than below the more satisfactory 20%. For this reason we also performed “doubly robust” estimation, with which we achieved a further slight correction in the estimated treatment effect (HR=0.86, 95%CI, 0.56–1.31, p=0.479). The 3-month landmark analysis demonstrated little impact of immortal time bias of treatment effect (HR: 0.89, 95%CI: 0.58–1.36, p=0.592). None of the covariates was statistically significant, but low p values were obtained for lymph node extent (p=0.083) and for response to chemotherapy (p=0.080). Namely, increased hazard of death was observed for patients with retroperitoneal metastases compared to those with pelvic involvement (HR=1.47, 95%CI, 0.95–2.26), while a trend toward increased mortality was observed over the distinct response categories from CR to PD.
Additional analyses
Forest plots of the treatment effect across the subgroups are shown in Supplementary Figure 4. As for the interaction between surgical treatment and extent of lymph node involvement, stratum-specific estimates of treatment effect were HR=0.75 (95%CI, 0.52–1.08) for the pelvic group and HR=1.12 (95%CI, 0.68–1.84) for the pelvic + retroperitoneal group, respectively. The corresponding interaction test failed to reach statistical significance (Pinteraction=0.182). Interaction tests, however, notoriously suffer from low power. Confirmation of this finding was sought also considering the matched set, and the results we obtained thereof were HR=0.71 (95%CI 0.42–1.20) for the pelvic group and HR=1.37 (95%CI: 0.71–2.66) for the pelvic + retroperitoneal group, respectively. Interestingly, PC-LND seemed to be beneficial for patients who had failed prior local treatment.
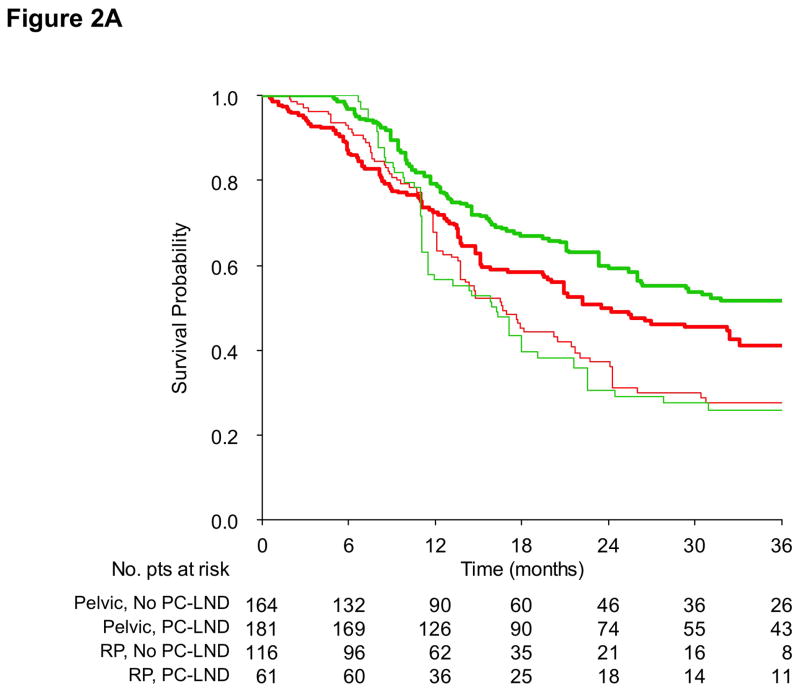
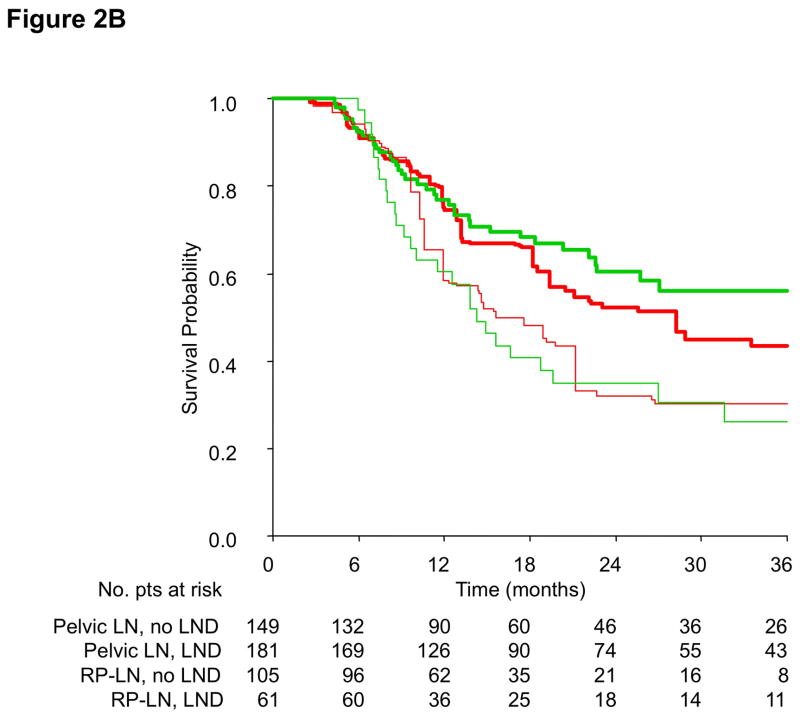
Finally, PS-adjusted OS curves according to the treatment group and site of lymph node metastases are shown in Figure 2, either with (2A) or without (2B) landmark analysis. The treatment effect was confined in the pelvic group of patients, coherently with Cox model results. In this case, 36-month OS for operated patients was 51.7% vs. 41.1%, corresponding to a survival benefit of 10.6%. PS-adjusted OS curves according to the treatment group and the clinical history of prior local treatment for muscle-invasive disease are provided in Supplementary Figure 5.
Figure 2.
Propensity score-adjusted overall survival curves according to the study group (PC-LND: green line; No PC-LND: red line) and extent of lymph node metastases (pelvic lymph nodes: thick line; retroperitoneal lymph nodes: thin line); without (2A) and with (2B) a 3-month landmark.
Abbreviations: LN: lymph nodes; PC-LND: postchemotherapy lymph node dissection; RP: retroperitoneal.
Discussion
This retrospective study provides one of the largest comparative data analyses, at the individual patient-level, on the role of surgical clearance of residual disease after chemotherapy for advanced UBC. The optimal management of patients with clinically-evident lymph node involvement from UBC is still debated. Indeed, elucidating the role of surgical clearance of residual disease (i.e., radical cystectomy with pelvic lymph node dissection) after chemotherapy is of noteworthy importance, as such patients are usually recommended to receive upfront chemotherapy instead of surgery. At present, one large population-based Netherlands Cancer Registry (NCR) study, another analysis from the U.S. National Cancer Database (NCDB) and one U.S.-European multicenter study, added to a few smaller studies, attempted to analyze the net benefit from administering multimodal therapy in such patients.[13–15] As important differences from our study, the above studies focused on patients presenting with enlarged pelvic lymph nodes as the only metastatic site, and focused on de novo metastatic patients. According to their analyses, performing PC-LND was beneficial for OS, and a proportion of patients became long-term survivors.
Conversely, very limited information is available regarding those patients who present with more extensive disease and received first-line chemotherapy followed by surgery. In particular, patients who presented with enlarged lymph nodes in the retroperitoneum, or those with lymph node-only relapse after prior surgery, radiation or chemoradiation for muscle-invasive UBC were included in our study, and comparative outcomes of surgery versus observation were provided for the first time to our knowledge. Additionally, data of patients who did not benefit from partial or complete response to chemotherapy, who were usually excluded from prior studies, were included in our analysis. Of note, PC-LND did not provide statistically significant OS improvement regardless of response to chemotherapy, although the bias of small numbers may be acknowledged in the CR subgroup. However, a numerical improvement in OS should be acknowledged in the cohort of pelvic lymph node-involved patients, and it might be important in clinical decision-making. Most noteworthy, performing PC-LND in patients with metastatic disease beyond the pelvic boundaries is not indicated.
Of course there are a few biases in interpreting the present findings. First, and most importantly, our study mirrors the real world practice by showing that there is substantial difference in patient and disease characteristics between patients who are considered for multimodal approach and those who are not. The most relevant parameters are age, prior local therapy, and Eastern Cooperative Oncology Group (ECOG) performance status, as resulted from the GBM analysis. These confounders may be partly unaccounted for even with the use of advanced statistics, and explain why our study was negative. Secondly, as in the majority of retrospective studies aimed at analyzing real world data, the assessment of clinical lymph node involvement may be difficult in some cases unless a biopsy for histological confirmation is performed. Unfortunately, we were unable to get this information in our database, as well as we did not capture the results of any additional staging procedure, such as fluorodeoxyglucose positron emission tomography (FDG-PET) scans. Third, as in our prior analysis from part of the same database,[1] the response to chemotherapy parameter relied on the investigators’ judgment rather than the result of a formal assessment applying standardized criteria. The heterogeneity of lymphadenectomy templates is another important, unrecognized bias.
Some important strengths should be recognized in our study as well. Advanced statistical approaches were used to minimize the confounding effect of several covariates, so that we can compare present results with those from the available studies. The analyses mainly relied on the use of the PS. In particular, the IPTW approach gets rid of the above limitation by allowing analysis of the full sample, while case-mix adjustment is achieved with the use of suitable weights to individual observations. Doubly robust procedure was further applied, allowing us to obtain the prognostic role of multiple covariates added to the effect of PC-LND. Finally, to corroborate our findings, a matched-paired analysis was performed. Provided that even findings from the latter analysis should be taken with caution, considering that also “incomplete matching” is likely to generate bias,[16] two groups of patients that were comparable according to their baseline characteristics were obtained.
Furthermore, in our study we were able to access detailed information about the type of administered chemotherapy, and get patients who had received platinum-based chemotherapy included. The possible inclusion of patients who had received single-agent or non-platinum chemotherapy did represent a bias in the NCDB analysis. Finally, the doubly robust procedure allowed us to weight the effect of surgical treatment in the two cohorts of patients with pelvic only and retroperitoneal lymph nodes, and any trend to survival benefit disappeared in the latter group. Owing to the fact that it will be unlikely to ascertain randomized, prospective trial data of postchemotherapy surgery, large retrospective appraisals like the present one may provide useful information to enhance clinical practice and patient counseling.
Conclusions
In summary, in this large retrospective, multicenter study, we provided original data on the role of surgery after chemotherapy for clinically metastatic patients with pelvic or retroperitoneal lymph nodes. Results showed a non-statistically significant improvement in OS with surgery in patients with pelvic lymph node extent. In the remaining patients, no role for more extended lymphadenectomy, like retroperitoneal lymph node dissection, was found. In future clinical trials, more active treatments may provide a viable alternative to active surveillance for these patients.
Supplementary Material
Supplementary Figure 1. Generalized boosted regression model for propensity score estimation: relative influence measured for each variable.
Abbreviations: ECOG PS: Eastern Cooperative Oncology Group performance status.
Supplementary Figure 2. Flow of the selected patients’ treatment.D
Abbreviations: UC: urothelial carcinoma.
Supplementary Figure 3. Radar plot showing the magnitude of standardized differences (SD) for each patient and treatment characteristic in PC-LND versus no PC-LND group. Legend: blue area: unweighted SD; red area: SD after inverse probability of treatment weighting adjustment; green area: SD after 1:1 matched-paired analysis.
Abbreviations: CBDCA: carboplatin; CDDP: cisplatin; CR: complete response; CT: chemotherapy; ECOG PS: Eastern Cooperative Oncology Group performance status; LN: lymph node; PD: disease progression; PR: partial response; SD: stable disease.
Supplementary Figure 4. Forest plot showing the hazard ratios (HR) and p-values of interaction tests of postchemotherapy lymphadenectomy vs. no lymphadenectomy according to age, ECOG performance status, lymph node extent, prior local treatment, prior perioperative chemotherapy, chemotherapy regimen, and response to chemotherapy.
Abbreviations: CI: confidence interval; CT: chemotherapy; CBDCA: carboplatin; CDDP: cisplatin; CR: complete response; ECOG-PS: Eastern Cooperative Oncology Group performance status; HR: hazard ratio; PD: disease progression; PO-CT: perioperative chemotherapy; PR: partial response; SD: stable disease.
Supplementary Figure 5. Propensity score-adjusted overall survival curves according to postchemotherapy lymphadenectomy (no lymphadenectomy: red line; lymphadenectomy: green line) and prior administration of local treatment (no: thick line; yes: thin line).
Abbreviations: LND: lymph node dissection; LT: local treatment.
Take Home Message.
In contemporary cohorts of patients with metastatic pelvic or retroperitoneal lymph nodes from bladder cancer, we found no survival benefit from postchemotherapy surgery vs. observation in a retrospective study. Performing postchemotherapy lymphadenectomy remains investigational in patients with metastatic bladder cancer.
Acknowledgments
Funding: This work was not supported by any grant funding.
Footnotes
Presented at the 2016 Annual Meeting of the Society of Urologic Oncology (SUO), November 29–December 1, 2016, San Antonio, TX, USA.
Conflict of interest statement: None of the authors have a relevant conflict of interest to disclose.
References
- 1.Necchi A, Sonpavde G, Lo Vullo S, et al. Nomogram-based Prediction of Overall Survival in Patients with Metastatic Urothelial Carcinoma Receiving First-line Platinum-based Chemotherapy: Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC) Eur Urol. 2017;71:281–289. doi: 10.1016/j.eururo.2016.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Necchi A, Giannatempo P, Lo Vullo S, et al. Postchemotherapy lymphadenectomy in patients with metastatic urothelial carcinoma: long-term efficacy and implications for trial design. Clin Genitourin Cancer. 2015;13:80–86. doi: 10.1016/j.clgc.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Dodd PM, McCaffrey JA, Herr H, et al. Outcome of postchemotherapy surgery after treatment with methotrexate, vinblastine, doxorubicin, and cisplatin in patients with unresectable or metastatic transitional cell carcinoma. J Clin Oncol. 1999;17:2546–2552. doi: 10.1200/JCO.1999.17.8.2546. [DOI] [PubMed] [Google Scholar]
- 4.Herr HW, Donat SM, Bajorin DF. Post-chemotherapy surgery in patients with unresectable or regionally metastatic bladder cancer. J Urol. 2001;165:811–814. [PubMed] [Google Scholar]
- 5.Abe T, Shinohara N, Harabayashi T, et al. Impact of multimodal treatment on survival in patients with metastatic urothelial cancer. Eur Urol. 2007;52:1106–1113. doi: 10.1016/j.eururo.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney P, Millikan R, Donat M, et al. Is there a therapeutic role for post-chemotherapy retroperitoneal lymph node dissection in metastatic transitional cell carcinoma of the bladder? J Urol. 2003;169:2113–2117. doi: 10.1097/01.ju.0000067601.29966.4a. [DOI] [PubMed] [Google Scholar]
- 7.de Vries RR, Nieuwenhuijzen JA, Meinhardt W, Bais EM, Horenblas S. Long-term survival after combined modality treatment in metastatic bladder cancer patients presenting with supra-regional tumor positive lymph nodes only. Eur J Surg Oncol. 2009;35:352–355. doi: 10.1016/j.ejso.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Siefker-Radtke AO, Walsh GL, Pisters LL, et al. Is there a role for surgery in the management of metastatic urothelial cancer? The M.D. Anderson experience. J Urol. 2004;171:145–148. doi: 10.1097/01.ju.0000099823.60465.e6. [DOI] [PubMed] [Google Scholar]
- 9.Otto T, Krege S, Suhr J, Rübben H. Impact of surgical resection of bladder cancer metastases refractory to systemic therapy on performance score: a phase II trial. Urology. 2001;57:55–59. doi: 10.1016/s0090-4295(00)00867-0. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann J, Suttmann H, Albers P, et al. Surgery for Metastatic Urothelial Carcinoma with Curative Intent: The German Experience (AUO AB 30/05) Eur Urol. 2009;55:1293–1299. doi: 10.1016/j.eururo.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Abe T, Kitamura H, Obara W, et al. Outcome of metastasectomy for urothelial carcinoma: A multiinstitutional retrospective study in Japan. J Urol. 2014;191:932–936. doi: 10.1016/j.juro.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Matsuguma H, Yoshino I, Ito H, et al. Is there a role for pulmonary metastasectomy with a curative intent in patients with metastatic urinary transitional cell carcinoma? Ann Thorac Surg. 2011;92:449–454. doi: 10.1016/j.athoracsur.2011.03.097. [DOI] [PubMed] [Google Scholar]
- 13.Hermans TJ, Fransen van de Putte EE, Horenblas S, et al. Pathological downstaging and survival after induction chemotherapy and radical cystectomy for clinically node-positive bladder cancer-Results of a nationwide population-based study. Eur J Cancer. 2016;69:1–8. doi: 10.1016/j.ejca.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Zargar-Shoshtari K, Zargar H, Lotan Y, et al. A multi-institutional analysis of outcomes of patients with clinically node positive urothelial bladder cancer treated with induction chemotherapy and radical cystectomy. J Urol. 2016;195:53–59. doi: 10.1016/j.juro.2015.07.085. [DOI] [PubMed] [Google Scholar]
- 15.Galsky MD, Stensland K, Sfakianos JP, et al. Comparative effectiveness of treatment strategies for bladder cancer with clinical evidence of regional lymph node involvement. J Clin Oncol. 2016;34:2627–2635. doi: 10.1200/JCO.2016.67.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCaffrey DF, Griffin BA, Almirall D, et al. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388–3414. doi: 10.1002/sim.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32:2837–2849. doi: 10.1002/sim.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colea SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Generalized boosted regression model for propensity score estimation: relative influence measured for each variable.
Abbreviations: ECOG PS: Eastern Cooperative Oncology Group performance status.
Supplementary Figure 2. Flow of the selected patients’ treatment.D
Abbreviations: UC: urothelial carcinoma.
Supplementary Figure 3. Radar plot showing the magnitude of standardized differences (SD) for each patient and treatment characteristic in PC-LND versus no PC-LND group. Legend: blue area: unweighted SD; red area: SD after inverse probability of treatment weighting adjustment; green area: SD after 1:1 matched-paired analysis.
Abbreviations: CBDCA: carboplatin; CDDP: cisplatin; CR: complete response; CT: chemotherapy; ECOG PS: Eastern Cooperative Oncology Group performance status; LN: lymph node; PD: disease progression; PR: partial response; SD: stable disease.
Supplementary Figure 4. Forest plot showing the hazard ratios (HR) and p-values of interaction tests of postchemotherapy lymphadenectomy vs. no lymphadenectomy according to age, ECOG performance status, lymph node extent, prior local treatment, prior perioperative chemotherapy, chemotherapy regimen, and response to chemotherapy.
Abbreviations: CI: confidence interval; CT: chemotherapy; CBDCA: carboplatin; CDDP: cisplatin; CR: complete response; ECOG-PS: Eastern Cooperative Oncology Group performance status; HR: hazard ratio; PD: disease progression; PO-CT: perioperative chemotherapy; PR: partial response; SD: stable disease.
Supplementary Figure 5. Propensity score-adjusted overall survival curves according to postchemotherapy lymphadenectomy (no lymphadenectomy: red line; lymphadenectomy: green line) and prior administration of local treatment (no: thick line; yes: thin line).
Abbreviations: LND: lymph node dissection; LT: local treatment.