Abstract
An age-related decline in endogenous pain inhibitory processes likely places older adults at an increased risk for chronic pain. Limited research indicates that older adults may be characterized by deficient offset analgesia, an inhibitory temporal sharpening mechanism that increases the detectability of minor decreases in noxious stimulus intensity. The primary purpose of the study was to examine age differences in offset analgesia in community-dwelling younger, middle-aged, and older adults. An additional aim of the study was to determine whether the magnitude of offset analgesia predicted self-reported bodily pain. Eighty-seven younger adults, 42 middle-aged adults, and 60 older adults completed 4 offset analgesia trials and 3 constant temperature trials in which a noxious heat stimulus was applied to the volar forearm for 40-sec. The offset trials consisted of three continuous phases: an initial 10-sec painful stimulus (S1), either a 1.0°C or 0.4°C increase in temperature from S1 for 10-sec (S2), and either a 1.0°C or 0.4°C decrease back to the initial testing temperature for 20-sec (S3). During each trial, subjects rated pain intensity continuously using an electronic visual analogue scale (0–100). All subjects also completed the SF-36 Health Survey including the Bodily Pain subscale. The results indicated that older and middle-aged adults demonstrated reduced offset analgesia compared to younger adults in the 1.0°C and 0.4°C offset trials. Furthermore, the magnitude of offset analgesia predicted self-reported bodily pain, with those exhibiting reduced offset analgesia reporting greater bodily pain. Dysfunction of this endogenous inhibitory system could increase the risk of developing chronic pain for middle-aged and older adults.
Keywords: Pain inhibition, offset analgesia, aging, older adults
1.0 INTRODUCTION
Epidemiological studies indicate that the prevalence of chronic pain increases with age up to the seventh decade of life with rates reaching as high as 60–75% and then plateauing.8,12,37 While multiple mechanisms likely contribute, an age-related decline in endogenous pain modulatory processes is likely one mechanism placing older adults at an increased risk for the development of persistent pain compared to younger adults. Indeed, older adults exhibit diminished descending pain inhibitory capacity15,20,31 and increased pain facilitation likely caused by a sensitized central pain system.7,16,21 An imbalance of these systems likely underlies the increased transition from acute to chronic pain associated with aging.33
The majority of evidence indicating that older adults exhibit a diminished pain inhibitory capacity compared to younger adults has relied on assessment of pain inhibition with the condition pain modulation (CPM) paradigm.15,31 CPM is a dynamic quantitative sensory test that represents the behavioral correlate to diffuse noxious inhibitory controls, an inhibitory mechanism involving the spinal-bulbo-spinal loop.41 In this paradigm, a painful stimulus applied to a remote body site inhibits the experience of pain of another noxious stimulus applied to a different body site. A large body of research shows that reduced pain inhibition on the CPM test is a characteristic of many chronic pain conditions and represents a predisposition for chronic pain.18,40
Recently, another dynamic quantitative sensory test, termed offset analgesia, has gained attention as another assessment tool for endogenous pain inhibition in pain research.13 While CPM represents a spatial filtering mechanism, offset analgesia represents an inhibitory temporal sharpening mechanism that increases the detectability of small decreases in noxious stimulus intensity.10 Specifically, offset analgesia refers to a pronounced reduction in perceived pain intensity evoked by a small decrease in noxious temperature that is disproportionately large compared to the actual decrease in temperature.10 Functional imaging and pharmacological studies indicate that CPM and offset analgesia rely on different mechanisms, with offset analgesia likely reflecting more brain derived pain modulation compared to descending spinal modulatory effects of CPM.6,19,22,42 While less is known regarding the relevance of offset analgesia to the development of chronic pain, recent but limited evidence suggests that aging is also associated with reduced capacity to activate the temporal inhibitory mechanisms underlying this phenomenon.20
The primary purpose of this study was to further explore age-related differences in offset analgesia in a large sample of adults. We recently showed in a smaller study that older adults experience reduced offset analgesia compared to younger adults.20 The current study aimed to extend these findings in several ways. First, the age at which pain modulatory processes begin to decline is not well known, as most pain and aging studies have ignored the middle-age group. Importantly, a study by Lariviere and colleagues discovered that CPM begins to decline in middle age (40–55 years old).15 Thus, the primary aim of this study was to evaluate offset analgesia in young, middle-aged, and older adults. Secondly, some research indicates that age differences in pain may differ between sexes2,17,30; however, limited research has investigated or been sufficiently powered to detect sex by age interactions in pain inhibitory processes. Therefore, a second aim of this study was to evaluate whether age differences in offset analgesia differed as a function of sex. Third, previous offset studies have shown a disproportionate decrease in pain ratings following a 1, 2, or 3°C decrease in temperature.10 In addition to the most commonly used 1°C temperature decrease in offset trials, the current study assessed whether a temperature decrease of only 0.4°C could also evoke offset analgesia. Finally, while studies have shown that offset analgesia is reduced in neuropathic pain conditions and fibromyalgia23,24,27, whether the magnitude of offset is related to the experience of clinical pain in a community-based sample has not been determined. Thus, the current cross-sectional study examined whether the magnitude of offset analgesia predicted self-reported bodily pain in adults.
2.0 METHODS
2.1 Participants
Participants included 189 community-dwelling adults stratified into 3 age cohorts: 87 younger adults (Age: M=25.4, SD=5.7, age range: 18–39 years; 47 females and 40 males), 42 middle-aged adults (Age: M=50.8, SD=5.2, age range: 40–59 years; 23 females and 19 males), and 60 older adults (Age: M=68.1, SD=5.1, age range: 60–80 years; 29 females and 31 males). The overall sample consisted of 108 Caucasians (57%), 27 African Americans (14%), 24 Hispanics (13%), 16 Asians (8%) and 14 that endorsed mixed-race (7%) and were similarly distributed across the three age cohorts. The sample was recruited as part of a larger study examining age-related changes in pain inhibitory and facilitatory function (Study of The Effects of Aging on Experimental Models of Pain Inhibition and Facilitation). Recruitment and study procedures were approved by the University of Florida Institutional Review Board. Written informed consent was obtained from all participants, who were identified only by numbers.
Study exclusion criteria included a Mini Mental Status score below 23, current use of narcotics or chronic use of analgesics, uncontrolled hypertension, systemic disease that restricts normal daily activities, neurological problems with significant changes in somatosensory and pain perception at the intended stimulation sites, and serious psychiatric conditions (e.g., diagnosis of schizophrenia and bipolar disorder). Participants were asked to refrain from the use of any pain medication or coffee on the day of testing.
2.2 Apparatus
Testing was performed on a Thermo-Electric Stimulation System (Neuroanalytics, Gainesville FL). Thermal stimuli were administered by a thermode (Peltier thermoelectric device) housed in a free standing stimulation module that was adjustable from 20–28 inches in height. At rest, the 40×40 mm thermode is recessed behind a cutout in a thermally neutral plastic surface, out of contact with the skin, which rests on the plastic surface. The thermode is brought into reproducible light skin contact by a solenoid-powered mechanism with temperature and thermode position controlled by a computer.
Experimental pain was measured with an electronic visual analog scale (eVAS). The eVAS consisted of a low-friction sliding potentiometer of 100 mm travel. The left endpoint of the scale was identified as “no pain”, while the right endpoint was defined as “intolerable pain”. There were nine hash marks between these two anchors. The position of the slider was electronically converted into a pain rating between 0 and 100. The slider automatically returned to the left (“no pain”) position when so required by the protocol. The eVAS was mounted into the surface of a small inclined desk positioned to facilitate precise operation with minimal fatigue. The experimental set-up allows the participant to be separated from the investigator and facing away to minimize non-verbal communication and transmission of bias.
2.3 Study procedures
All subjects were tested in the Pain Clinical Research Unit of the University of Florida Pain Research and Intervention Center of Excellence.
2.3.1 Orientation and training session
Individuals who were interested in the study were provided information about the procedures, informed about privacy regulations and reviewed and signed an Informed Consent Form. Eligibility for the study was determined after participants completed a health history questionnaire, supplemented by interview and a blood pressure measurement. As part of this orientation participants watched a PowerPoint presentation with imbedded video and audio overlay that described using a 0–100 pain scale. Participants then received practice with the pain testing stimuli using several temperatures. Once oriented to the stimuli and rating pain, several series of ascending heat stimuli trials (+0.5°C per trial with starting temperature of 43°C) were administered at the palm with contact time of 5-sec and inter-stimulus intervals of 8-sec. Each series was terminated when ratings surpassed 50. The temperature at which participants rated closest to 40 was then administered to the forearm for 20-sec. If the 20-sec trial failed to reach a pain rating of 35, the next trial was administered at +0.5°C, if the pain rating surpassed 50 the next trial preceded at −0.5°C, if the pain rating peaked between 35–50, that temperature was repeated for the second trial. A third trial was performed with additional temperature adjustment if needed. The final (baseline) temperature was recorded and administered for a 40-sec trial at the forearm followed by an offset trial (10-sec baseline temperature, 10-sec at baseline +1.0°C, and returning to the baseline temperature for 20-sec. This allowed the use of a temperature during the subsequent testing session at which a participant would experience at least moderate pain during a 40-sec trial at a constant temperature, but not rate pain as intolerable during the offset trials. Additionally, participants completed the Pain Catastrophizing Questionnaire and the Short-Form Health Survey-36 (SF-36). A description of these questionnaires is provided below.
2.3.2 Testing Sessions
Participants were seated on a comfortable chair and relaxed for several minutes. Then, two blood pressure readings separated by 5 minutes were taken using a Datascope Accutorr Plus blood pressure monitor (Datascope Inc, Mahwah NJ). A third blood pressure measurement was taken if there was a change of greater than 5% in the first two readings. During the session, subjects completed an ascending/descending series, trials of offset analgesia, and temporal summation using thermal stimuli.
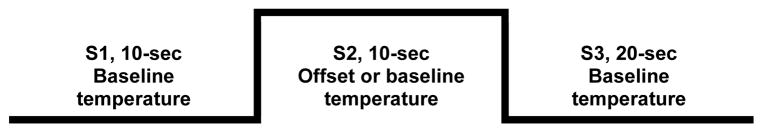
This paper presents data from a series of seven 40-sec trials. This includes a total of 4 offset trials with 2 trials increasing the baseline temperature +1.0°C and two trials increasing the baseline temperature +0.4°C, using a 10-sec baseline temperature (S1), 10-sec offset temperature (S2), and returning to the baseline temperature for the final 20-second paradigm (S3). Figure 1 shows the offset sequence. Three control trials were also administered in which the temperature remained constant for the entire 40-sec trial. All sets of trials began with a control trial. This was followed by series A: +0.4°C, control, +1.0°C and series B: +1.0°C, control, +0.4°C, or the reverse BA. The initial side of administration (R – L or L - R) and series order (A – B or B – A) were counterbalanced. All trials were administered to the volar forearm and separated by a 3-minute rest with the thermode position adjusted to minimize overlap.
Figure 1.
Offset trial sequence. At S1, a preheated thermode is applied the forearm at the participant’s individualized baseline testing temperature for 10 seconds. At S2, the thermode is heated an additional 1.0°C or 0.4°C for 10 seconds and then cooled back to the baseline temperature for 20 seconds during S3. For the control trials, the individualizred baseline temperature was maintained for the entire 40 second trial.
2.4 Questionnaires
Pain Catastrophizing Scale (PCS)
The Pain Catastrophizing Scale consists of 13 items rated on a 5-point likert scale.34 The PCS asks the respondents to reflect upon past painful experiences and to rate the degree to which they experienced negative thoughts or feelings about pain. The PCS measures three dimensions of catastrophizing: rumination, helplessness, and magnification that sum to a total score in which higher scores indicate greater pain catastrophizing.
Short-Form Health Survey-36 (SF-36)
The SF-36 is a health survey that yields 8-scale scores including: physical functioning, role limitations due to physical problems, bodily pain, vitality, general health perceptions, social functioning, role limitations due to emotional problems, and mental health.39 For the Bodily Pain Scale a higher score indicates less bodily pain.
2.5 Data Reduction and Analyses
SF-36 Bodily Pain score, individualized test temperatures for the offset analgesia trials, and the magnitude of offset analgesia for the control trials were analyzed with a two-way ANOVA with age and sex as between-subject factors. Thermode temperature was added as a covariate for the control trial analysis.
2.5.1. Did the magnitude of the offset temperature evoke a disproportionate increase or decrease in pain intensity?
We wanted to determine whether the temperature decrease from S2 to S3 produced greater changes in pain intensity ratings than the temperature increase from S1 to S2 and whether these changes differed by the magnitude in change on the offset temperature. Inspection of the data indicated 1) peak pain during S1 was experienced at 10-sec, 2) peak pain during S2 was experienced at 20 and 21-sec (at the end of S2) and 3) maximum offset analgesia was experienced during the interval 33–35-sec (S3). Thus, maximum S1 pain rating was taken from the ratings at 10-sec. Pain ratings at seconds 20–21 and 33–35 were averaged to create maximum S2 pain rating value and a minimum S3 pain rating value respectively. The absolute magnitude of change in pain intensity ratings was calculated from S1 to S2 and from S2 to S3 (offset). The dependent variables were collapsed across the two trials for each condition (+0.4°C, +1.0°C). They were analyzed with a 2-way repeated measures ANOVA, with temperature change (0.4°C, 1.0°C) and transition (S1S2, S2S3) as the within subject factors. Thermode temperature, age group and sex were included as a covariate.
2.5.2 Did the magnitude of the S1S2 increase of pain differ between age groups and sex?
Next, we tested for age and sex differences in the magnitude of the S1S2 increase in pain. This was important to determine whether differences in offset analgesia is not only due to a loss in temporal inhibition, but differences in the S1S2 increase in pain. To accomplish this, we performed a 3 (Age: young, middle, older) × 2 (Sex) × 2 (Condition: +0.4°C, +1.0°C) ANOVA with repeated measures on Condition. Thermode temperature was included as a covariate.
2.5.3 Did the magnitude of offset analgesia differ between age groups and sex?
To quantify the magnitude of offset analgesia, we calculated the relative magnitude of decrease in perceived pain following the temperature decrease. Thus, the magnitude of offset analgesia was calculated as the percent change from the S2 max pain rating with the following formula: the difference between maximum S2 and minimum S3 (ΔeVAS), corrected for the value of the peak eVAS during S2 [ΔeVASC= (ΔeVAS/maxS2)*100]. Consequently, this measures represents the percentage reduction in pain achieved from the offset sequence. The magnitude of offset analgesia was averaged across trials for each condition (control, +0.4°C +1.0°C). Offset analgesia magnitude for the experimental trials was analyzed using a 3 (Age: young, middle, older) × 2 (Sex) × 2 (Condition: +0.4°C, +1.0°C) ANOVA with repeated measures on Condition. This model also included the Bodily Pain Scale score, Pain Catastrophizing score, thermode temperature, and offset magnitude from the control trials as covariates. Post-hoc comparisons were made with Tukey’s HSD procedure.
2.5.4 Did magnitude of offset analgesia predict self-reported bodily pain?
Finally, linear regression was also conducted to determine whether magnitude of offset analgesia predicted Bodily Pain Scale score on the SF-36, after controlling for age, sex, and pain catastrophizing. Separate analyses were performed for offset of +0.4°C and +1.0°C because of multicollinearity. The control variables were entered in a first step with forced entry method followed by the offset variable in the second step. The F to change statistic for step two was used to test for statistical significance.
3.0 RESULTS
3.1 SF-36 Bodily Pain Score scale
The 2-way ANOVA revealed a significant effect of age group [F(2,183) = 8.677, p <.001], in which the younger adults (87.8±2.0) reported significantly less pain compared to the middle-aged adults (75.1±3.0) and older adults (76.4±2.6). No differences existed between the middle-aged and older adults.
3.2 Individualized Test Temperature
The 2-way ANOVA revealed a significant effect of sex [F(2,183) = 10.457, p =.001] with a higher thermode temperature was used for males (46.5±0.1°C) compared to females (45.9±0.1°C). No differences were observed between age groups (18–39, 46.1±0.1°C; 40–59, 46.0±0.2°C; 60–80, 46.4±0.3°C).
3.3 Magnitude of Offset Analgesia in the Control Trials
The two-way ANOVA conducted on the control trials did not show any significant effects of age, sex or an age by sex interaction on the magnitude of offset analgesia. Table 1 shows the magnitude of offset analgesia for the control trials for each age group.
Table 1.
Magnitude (Mean ± Standard Error) of Offset Analgesia for Control, +0.4C°, and +1.0C° Offset Trials for Each Age Group.
| Age group | Control Trials | +0.4C° Trials | +1.0C° Trials |
|---|---|---|---|
| Younger Adults | 9.2%±3.9 | 45.0%±2.9 | 62.6%±2.9 |
| Middle-age Adults | 6.9%±6.0 | 36.9%±4.1 | 50.0%±4.4 |
| Older Adults | 11.6%±4.1 | 31.0%±3.4 | 44.0%±3.7 |
| Total Sample | 9.4%±2.6 | 39.7%±2.2 | 53.6%±2.2 |
3.4. Did the magnitude of the offset temperature evoke a disproportionate increase or decrease in pain intensity?
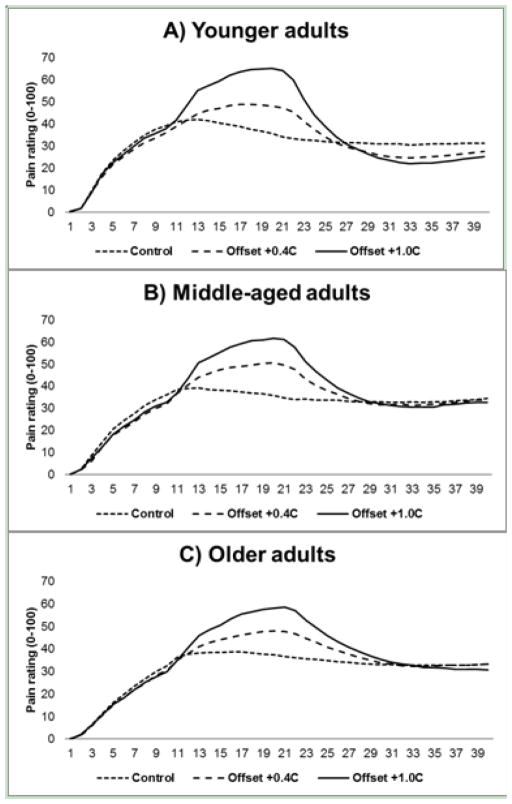
Figure 2 shows the average real-time eVAS pain ratings for each condition for younger (2A), middle-aged (2B), and older adults (2C). The 2-Way repeated measures ANOVA revealed a significant effect of transition change [F(1,185) = 6.210, p = .014] indicating that the change in pain ratings in the S2S3 offset sequence (27.2±1.3) were significantly greater than during the S1S2 sequence (21.3±0.9). The temperature by transition interaction was not significant indicating that the proportion of change in offset compared to the S1S2 increase was not different between 0.4°C.
Figure 2.
Average real-time eVAS rating during the +0.4°C offset trials, the 1.0°C offset trials, and the control trials for younger (A). middle-aged (B), and older adults (C).
3.5 Did the magnitude of S1S2 increase associated pain differ between age groups and sex?
The three-way ANOVA revealed the expected significant main effect of condition [F(1,182) = 150.019, p < .001] with a greater increase for the +1.0C° trials (28.1±1.4) compared to the +0.4C° trials (15.7±1.1). However, non-significant main effects were observed for age (p = .208) and sex (p = .269). The 2-way age x condition interaction approached significance [F(2,182) = 2.529, p = .083] and supports the use of relative magnitude of decrease in perceived pain as the primary offset variable as it adjusts for differences at S2.
3.6 Did the magnitude of offset analgesia differ between age groups and sex?
The three-way ANOVA revealed a main effect of condition [F(1,179) = 10.603, p=.001] and age group [F(2,179) = 6.338, p=.002]. As expected, a greater magnitude of offset analgesia was observed during the +1.0C° trials (53.6%±2.2) compared to the +0.4C° trials (39.7%±2.2). In regards to the age differences, younger adults (53.8%±2.8) exhibited significantly greater offset analgesia compared to the middle-aged (43.5%±3.9) and older adults (37.5%±3.3). The main effect of sex and the interaction effects were not significant.
3.7 Did magnitude of offset analgesia predict self-reported bodily pain?
Regression coefficients for both models testing for associations between offset analgesia and SF-36 Bodily Pain scores are presented in Table 2. In step one (the same for both offset +0.4°C and +1.0°C analyses), Age (p < .001) and PCS total scores (p = .012) were significantly associated greater SF-36 Bodily Pain scores. In the final step for offset at +0.4°C, Age (p < .001) and PCS total score (p = .012) remained significant but the variable representing offset analgesia at +0.4°C was not (p = .124). The F to change was 2.389, p = .124. In the final step for offset at +1.0°C, Age (p = .003), PCS total score (p = .007) and the variable representing offset analgesia at +1.0°C (p = .002) were significant and resulted in a significant F to change of 7.809, p = .002.
Table 2.
Regression coefficients for both models testing for associations between offset analgesia at +0.4°C and +0.4°C and SF-36 Bodily Pain Scale scores
| B (SE) | t | p. value | Δ R2 | |
|---|---|---|---|---|
| Step 1 (Model R2 = .11, F = (3, 184) = 6.417, p < .001) | ||||
| Age | −6.746 (1.806) | −3.735 | < .001 | |
| Sex | −3.416 (2.965) | −1.152 | .251 | |
| PCS total score | −.417 (.163) | −2.556 | .012 | |
| Step 2 for +0.4°C (Model R2 = .13, F (4, 183) = 5.454, p < .001) | .02 a | |||
| Age | −6.069 (1.850 | −3.280 | .001 | |
| Sex | −3.985 (2.974) | −1.340 | .182 | |
| PCS total score | −.411 (.162) | −2.534 | .012 | |
| Offset +0.4°C | −7.679 (4.969) | −1.545 | .124 | |
| Step 2 for +1.0°C (Model R2 = .17, F (4, 183) = 7.045, p < .001) | .06 b | |||
| Age | −5.466 (1.802) | −3.033 | .003 | |
| Sex | −3.514 (2.882) | −1.219 | .225 | |
| PCS total score | −.435 (.158) | −2.745 | .007 | |
| Offset +1.0°C | −11.852 (4.742) | −3.132 | .002 | |
F to change = 2.389, p = .124
F to change = 7.809, p = .002
Note: Step 1 with only covariates included is the same for both +0.4C° +1.0°C offse models. Higher scores on the SF-36 Bodily Pain Scale reflect less pain. Age was coded younger (18–39 years) = 1, middle-aged (40–59) = 2, and older (60–80) = 3, consequently the negative beta for age indicates that lower scores (greater pain) are associated higher coded age categories (older age). Thermode temperature was included as a covariate.
4.0 DISCUSSION
Several key findings emerged from this study. First, we showed for the first time that a temperature decrease as small as 0.4°C evokes a disproportionate decrease in pain intensity ratings (i.e., offset analgesia). Second, the magnitude of offset analgesia in 0.4°C and 1.0°C trials was reduced in middle-aged and older adults compared to younger adults. Third, sex differences in offset analgesia were not evident in any age group. Fourth, offset analgesia in the 1.0°C trials predicted self-reported bodily pain, suggesting that dysfunction of this inhibitory system may be related to the experience of clinical pain.
4.1 Age differences in offset analgesia
As hypothesized, older adults exhibited a reduced magnitude of offset analgesia compared to younger adults. Similar to our initial study examining age differences in offset, older adults did not show a disproportionate decrease in pain intensity ratings following the slight decrease in noxious temperatures.20 Importantly, we also showed for the first time that offset analgesia begins to decrease in middle-age. During the 1.0°C trials, the younger group’s pain intensity ratings descended approximately 63% from period S2 to S3, while the middle age group’s pain ratings fell on average 50% and the older adult group’s pain ratings decreased only 44%. Thus, the data suggest a gradual decline in offset analgesia across the lifespan. Most aging and pain modulation studies have focused on comparisons between the younger and older cohorts, providing little evidence for the functioning of the pain inhibitory systems in middle-age. In one of the few studies addressing the middle-age cohort, Lariviere and colleagues showed that pain inhibitory function measured with conditioned pain modulation also begins to decline in middle age.15 Therefore, the collective data suggests that the functioning of multiple endogenous inhibitory systems begin to deteriorate as early as middle-age, potentially placing middle-aged and older adults at a greater risk for developing persistent pain.
Studies examining the mechanisms underlying offset analgesia indicate that it involves more brain derived pain modulation compared to CPM, including a change in activation in the brain stem, cerebellum, and multiple cortical and subcortical regions.6,19,22,42 Indeed, several studies show that offset analgesia produces increased activity in the dorsolateral prefrontal cortex (DLPFC) and anterior insula, which mirrors the activity of brain regions activated in the modulation of pain by cognitive processes such as placebo, distraction, and meditation. Thus, Nahman-Averbuch and colleagues hypothesized that part of offset analgesia is mediated or amplified by cognitive processes associated to the prediction of the time course of pain.19 Additional research suggests an integral role of the cerebellum in offset analgesia.11 In particular, Ruscheweyh et al. discovered reduced offset analgesia in patients with cerebellar infarction compared to sex- and age- matched controls.32 Notably, normal aging is characterized by reduced integrity and white and grey matter volumes in the cerebellum3 and in cortical regions thought to play a role in the cognitive aspects of offset, including the insular and prefrontal cortex.1,35,36 Therefore, age-related brain atrophy could be one mechanism contributing to declining offset analgesia with age. However, it is also possible that pain-associated gray matter atrophy (versus age-associated atrophy) could account for these differences, given the association of offset with bodily pain in the present study.
Prior research also suggests that peripheral mechanisms may be involved in the initiation of offset analgesia. Naugle et al. discovered that offset analgesia occurs at the non-glabrous (“hairy”) skin of the forearm, but is absent in the glabrous skin of the palm.20 These data suggest that myelinated A-fiber mechano-heat Type II nociceptors (AMH-II), which are present in the non-glabrous skin35,36 but appear to be absent in the glabrous skin4,36, may be involved in the initiation of offset. Animal and human research indicate a selective loss of myelinated afferents25,26,38 and diminished function of the remaining A-fibers with age.14,38 Therefore, the diminished input of AMH-II fibers in older adults could be another mechanism underlying reduced offset analgesia in these individuals. Clearly, the proposed explanations are speculative and future research is needed to investigate the mechanisms causing an age-related decline in offset analgesia.
4.2. Sex differences in offset analgesia
Few aging studies examining endogenous pain modulation have examined or been sufficiently powered to detect sex differences. A recent meta-analysis on sex differences in pain inhibitory capacity on the CPM test revealed that men show greater pain inhibition compared to women.28 However, no evidence exists for sex differences in CPM in older adults. The evidence regarding sex differences in offset analgesia are mixed. Niesters and colleagues reported greater magnitude of offset analgesia in men compared to women in adults aged 20–80 years old.23 However, the sex differences were small and likely clinically insignificant. Naugle et al. did not show sex differences in offset analgesia among young or older adults4; however, that study was not powered to detect an age by sex interaction. Consistent with our earlier study,20 the current results suggest that sex differences in offset analgesia do not exist across the lifespan.
4.3. Prediction of clinical pain
Several studies have shown that offset analgesia is reduced in neuropathic pain patients23,24 and fibromyalgia patients.27 However, data demonstrating the relevance of offset analgesia for the experience of clinical pain is extremely limited. To the best of our knowledge, the current study provided the first evidence showing that the magnitude of offset analgesia predicts the experience of clinical pain in adults. Even after controlling for age and pain catastrophizing scores, adults who exhibited reduced offset analgesia reported greater bodily pain on the SF-36 Bodily Pain subscale. Notably, the association between magnitude of offset and bodily pain was only evidenced with the 1.0°C trials and not the 0.4°C trials. Bodily pain as measured is the aggregate of many acute and chronic pain experiences. Our demonstration of a link between reduced offset analgesia and pain in daily life supports the notion that pain modulatory deficits are associated with not just a chronic pain condition but with the experience of pain in general. The cross-sectional nature of this study renders it possible that greater bodily pain leads to reduced offset analgesia. Future research is warranted to verify the causal direction of the relationship between deficient offset analgesia and increased bodily pain in adults. Additionally, our sample was comprised of relatively healthy adults and these results may not generalize to adults with chronic pain. Nonetheless, accumulating evidence suggests that the capacity to activate this temporal pain inhibitory system plays a role in the experience of clinical pain.
4.4. Conclusions and Future Directions
In conclusion, the current study demonstrated that middle-aged and older adults are characterized by reduced offset analgesia. This study is limited by its cross-sectional nature and the inability to generalize to adults with a diagnosed chronic pain condition. In addition, using an individualized temperature to ensure participants experienced a moderate level of pain may have created a bias where highly sensitive participants received less intense stimuli thermal stimulation at higher intensities which induces greater rostral-caudal activation on spinal cord segments.5 The use of intolerable pain as a maximum anchor is a potential weakness, as well as the use of hash marks, which may have been used as anchors creating interval rather than ratio scale data. However, several strengths of this study exist. First, we have added to the extremely limited data showing that pain inhibitory processes begin to decline in middle age. Secondly, this study is one of the few pain modulation and aging studies that have been sufficiently powered to evaluate potential sex by age interactions. Third, we showed for the first time that a 0.4°C decrease in temperature can elicit a disproportionate drop in perceived pain intensity. Finally, the current study provides the first evidence of an association between bodily pain and magnitude of offset analgesia.
Several avenues of future research are warranted. Research is needed to determine whether deficits in offset analgesia increase the risk for the development of chronic pain in middle-aged and older adults. As deficient offset analgesia seems to be particularly characteristic of neuropathic pain patients, perhaps middle-aged and older adults with reduced pain inhibitory capacity on this dynamic quantitative sensory test are more susceptible to developing neuropathic pain conditions. Future studies should also investigate whether the magnitude of offset analgesia predicts the experience of clinical pain in chronic pain populations. Finally, additional research is also needed to determine the central and/or peripheral mechanisms that underlie the age-related decline in offset analgesia.
Perspective.
Older and middle-aged adults demonstrated reduced offset analgesia compared to younger adults. The significant association between reduced offset analgesia and pain in daily life supports the notion that pain modulatory deficits are associated with not just a chronic pain condition but with the experience of pain in general.
Highlights.
Older adults demonstrated reduced offset analgesia compared to younger adults
No sex differences in offset analgesia were evident in any age group
A reduction stimuli as small as 0.4°C resulted in significant offset analgesia
Reduced offset analgesia was associated with pain in daily life for all age groups
Footnotes
Disclosures
This research was supported by NIH-NIA Grant R01AG039659. Dr. Fillingim has received consulting fees, speaking fees, and/or honoraria from WebMD and Algynomics (less than $10,000 each) and owns stock or stock options in Algynomics. Other authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–60. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Bartley EJ, King CD, Sibille KT, Cruz-Almeida Y, Riley JL, III, Glover TL, Goodin BR, Sotolongo AS, Herbert MS, Bulls HW, Staud R, Fessler BJ, Redden DT, Bradley LA, Fillingim RB. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: Potential sex differences in central sensitization. Arthritis Care Res. 2016;68:472–80. doi: 10.1002/acr.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard JA, Seidler RD. Moving forward: age effects on the cerebellum underlie cognitive and motor declines. Neurosci Biobehav Rev. 2014;42:193–207. doi: 10.1016/j.neubiorev.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell JN, LaMotte RH. Latency to detection of first pain. Brain Res. 1983;266:203–8. doi: 10.1016/0006-8993(83)90650-9. [DOI] [PubMed] [Google Scholar]
- 5.Coghill RC, Mayer DJ, Price DD. The roles of spatial recruitment and discharge frequency in spinal cord coding of pain: a combined electrophysiological and imaging investigation. Pain. 1983;53:295–309. doi: 10.1016/0304-3959(93)90226-F. [DOI] [PubMed] [Google Scholar]
- 6.Derbyshire SWG, Osborn J. Offset analgesia is mediated by activation in the region of the periaqueductal grey and rostral ventromedial medulla. NeuroImage. 2009;47:1002–6. doi: 10.1016/j.neuroimage.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain. 2001;2:307–17. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 8.Ferrell BA, Ferrell BR, Osterweil D. Pain in the nursing home. J Am Geriatr Soc. 1990;38:409–14. doi: 10.1111/j.1532-5415.1990.tb03538.x. [DOI] [PubMed] [Google Scholar]
- 9.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 10.Grill JD, Coghill RC. Transient Analgesia Evoked by Noxious Stimulus Offset. J Neurophysiol. 2002;87:2205–8. doi: 10.1152/jn.00730.2001. [DOI] [PubMed] [Google Scholar]
- 11.Hamaguchi T, Kano M, Kanazawa M, Itoh M, Yanai K, Fukudo S. Effects of preceding stimulation on brain activation in response to colonic distention in humans. Psychosom Med. 2013;75:453–62. doi: 10.1097/PSY.0b013e3182926682. [DOI] [PubMed] [Google Scholar]
- 12.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17:417–31. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 13.Herman L, Calders P, Van Oosterwijck J, Vershelde A, Bertel E, Meeus M. An overview of offset analgesia and the comparison with conditioned pain modulation: A systematic Literature Review. Pain Physician. 2016;19:307–326. [PubMed] [Google Scholar]
- 14.Jacobs JM, Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain. 1985;108:897–24. doi: 10.1093/brain/108.4.897. [DOI] [PubMed] [Google Scholar]
- 15.Lariviere M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain. 2007;23:506–510. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- 16.Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115:410–418. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Leveille SG, Zhang Y, McMullen W, Kelly-Hayes M, Felson DT. Sex differences in musculoskeletal pain in older adults. Pain. 2005;116:332–8. doi: 10.1016/j.pain.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13:936–944. doi: 10.1016/j.jpain.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Nahman-Averbuch H, Martucci KT, Granovsky Y, Weissman-Fogel I, Yarnitsky D, Cognhill RC. Distinct brain mechanisms support spatial vs. temporal filtering of nociceptive information. Pain. 2014;155:2491–501. doi: 10.1016/j.pain.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naugle KM, Cruz-Almeida Y, Fillingim RB, Riley JL., 3rd Offset analgesia is reduced in older adults. Pain. 2013;154:2381–7. doi: 10.1016/j.pain.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naugle KM, Cruz-Almeida Y, Fillingim RB, Staud R, Riley JL., 3rd Novel method for assessing age-related differences in the temporal summation of pain. J Pain Res. 2016;9:195–205. doi: 10.2147/JPR.S102379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niesters M, Dahan A, Swartjes M, Noppers I, Fillingim RB, Aarts L, Sarton EY. Effect of ketamine on endogenous pain modulation in healthy volunteers. Pain. 2011;152:656–63. doi: 10.1016/j.pain.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Niesters M, Hoitsma E, Sarton E, Aarts L, Dahan A. Offset Analgesia in Neuropathic Pain Patients and Effect of Treatment with Morphine and Ketamine. Anesthesiology. 2011;115:1063–71. doi: 10.1097/ALN.0b013e31822fd03a. [DOI] [PubMed] [Google Scholar]
- 24.Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth. 2014;113:148–56. doi: 10.1093/bja/aeu056. [DOI] [PubMed] [Google Scholar]
- 25.Ochoa JL, Mair WG. The normal sural nerve in man. II. Changes in the axons and Schwann cells due to ageing. Acta Neuropathol. 1969;13:217–39. doi: 10.1007/BF00690643. [DOI] [PubMed] [Google Scholar]
- 26.O’Sullivan DJ, Swallow M. The fibre size and content of the radial and sural nerves. J Neurol Neurosurg Psychiatry. 1968;31:464–70. doi: 10.1136/jnnp.31.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oudejans LC, Smit JM, van Velzen M, Dahan A, Niesters M. The influence of offset analgesia on the onset and offset of pain in patients with fibromyalgia. Pain. 2015;156:2521–7. doi: 10.1097/j.pain.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 28.Popescu A, LeResche L, Truelove EL, Drangsholt MT. Gender differences in pain modulation by diffuse noxious inhibitory controls: a systematic review. Pain. 2010;150:309–18. doi: 10.1016/j.pain.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal Magnetic Resonance Imaging Studies of Older Adults: A Shrinking Brain. J Neurosci. 2013;23:3295–301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley JL, 3rd, Gilbert GH. Orofacial pain symptoms: an interaction between age and sex. Pain. 2001;90:245–56. doi: 10.1016/S0304-3959(00)00408-5. [DOI] [PubMed] [Google Scholar]
- 31.Riley JL, 3rd, King CD, Wong F, Fillingim RB, Mauderli AP. Lack of endogenous modulation and reduced decay of prolonged heat pain in older adults. Pain. 2010;150:153–160. doi: 10.1016/j.pain.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruscheweyh R, Kühnel M, Filippopulos F, Blum B, Eggert T, Straube A. Altered experimental pain perception after cerebellar infarction. Pain. 2014;155:1303–12. doi: 10.1016/j.pain.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother. 2012;12:577–585. doi: 10.1586/ern.12.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 35.Treede R-D, Meyer RA, Campbell JN. Myelinated Mechanically Insensitive Afferents From Monkey Hairy Skin: Heat-Response Properties. J Neurophysiol. 1998;80:1082–93. doi: 10.1152/jn.1998.80.3.1082. [DOI] [PubMed] [Google Scholar]
- 36.Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. The Journal of Physiol. 1995;483:747–58. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GL, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9:883–91. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. Journal of the Peripheral Nervous System. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 39.Ware J, Kosinski M, Dewey J. How to Score Version Two of the SF-36 Health Survey. Lincoln, RI: QualityMetric, Incorporated; 2000. [Google Scholar]
- 40.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 41.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23:611–615. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- 42.Yelle MD, Oshiro Y, Kraft RA, Coghill RC. Temporal Filtering of Nociceptive Information by Dynamic Activation of Endogenous Pain Modulatory Systems. The J Neurosci. 2009;29:10264–71. doi: 10.1523/JNEUROSCI.4648-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]