Abstract
Recent studies on molecular carcinogenesis suggest that the chemo-resistance of some cancers is largely due to presence of cancer stem cells (CSCs), which affect the chemotherapy outcome for hepatocellular carcinoma (HCC). However, currently no consensus on a CSC phenotype in HCC has been obtained. Here, we examined Sox12 as a novel CSC marker in HCC. Sox12+ versus Sox12− cells were purified from HCC cell lines. The Sox12+ cells were compared with Sox12− HCC cells for tumor sphere formation, chemo-resistance, tumor formation after serial adoptive transplantations in nude mice, and the frequency of developing distal metastasis. We found that compared to Sox12− HCC cells, Sox12+ HCC cells generated significantly more tumor spheres in culture, were more chemo-resistant to cisplatin, were detected in circulation more frequently, and formed distal tumor more frequently. Moreover, Sox12 appeared to functionally contribute to the stemness of HCC cells. Thus, we conclude that Sox12 may be a novel marker for enriching CSCs in HCC.
Keywords: cancer stem cells (CSCs), hepatocellular carcinoma (HCC), Sox12
INTRODUCTION
Hepatocellular carcinoma (HCC) is among the top 5 most common solid tumor worldwide and the third major cause of cancer-related death (Verslype et al., 2009). Most HCC patients are diagnosed at intermediate or advanced stages of the disease, in which the outgrowth and distal metastasis of the primary tumor have occurred (Llovet and Bruix, 2008). Sorafenib and Cisplatin are the first line anti-HCC drugs used in clinic, but overall, the chemotherapy for advanced HCC is not very effective with undetermined mechanisms (Brower, 2016; Ikeda et al., 2016).
Recent studies on molecular carcinogenesis suggest that the chemo-resistance of some cancers is largely due to presence a group of cancer cells that have characteristics of stem cells, and are tumorigenic, highly proliferative and highly invasive (Sun et al., 2017). These cells are called cancer stem cells (CSCs), and treatments targeting CSCs are expected to improve current cancer therapies with experimental evidence (Perez-Losada and Balmain, 2003; Petersson and Niemann, 2012; Singh et al., 2003; Singh, 2012).
Although some cell surface markers [like CD133 (Zhang and Li, 2010) and CD44 (Yan et al., 2015)] are generally used for isolation of CSCs, none of these cell surface markers are satisfactorily robust and specific. Moreover, some non-surface markers [like Lgr5 in colorectal carcinoma (Nakata et al., 2014)] have also been applied for identification of CSCs in certain cancers. In addition, tumor cells with increased activity of aldehyde dehydrogenase (ALDH) appeared to be Aldefluor-positive in flow cytometry analysis (Duester, 2000; Magni et al., 1996). Increased activity of ALDH has been detected in some stem/progenitor cells (Armstrong et al., 2004; Hess et al., 2004; 2006; 2008). Recent evidence suggests that enhanced ALDH activity may be a characteristic for cancer stem cells (CSC) (Ma and Allan, 2011; Silva et al., 2011). Since all CSC-markers only enrich CSCs from a certain tumor, most “purified” CSCs are actually not CSCs, but are CSC-like cells (Fang et al., 2010; Ma et al., 2008; Nagata et al., 2011; Ottaiano, 2010; Shi et al., 2010). In HCC, although there is still no consensus on a CSC phenotype in HCC, single or combined use of CSC markers has been used to identify such a small population with CSC properties including self-renewing and the ability to form original tumor characteristics (Chiba et al., 2016). So far, the gold standard for defining a CSC or CSC-like cell is combined application of tumor sphere formation in vitro and tumor formation in receipts after serial adoptive transplantations (Huang and Rofstad, 2017; Iqbal et al., 2016).
SRY-related HMG-box gene 12 (Sox12) is a member of transcription factor superfamily homologous with sex-determining gene SRY, and has been shown to play critical roles in embryonic development and cell fate determination (Dy et al., 2008; Hoser et al., 2008). A recent study showed that Sox12 was elevated in HCC tissues, which correlated with loss of tumor encapsulation and higher cancer invasion and metastasis, seemingly through enhanced expression of Twist1 and fibroblast growth factor binding protein 1 (FGFBP1) (Huang et al., 2015). However, the association of Sox12 positivity and CSCs in HCC has not been reported.
Here, we examined Sox12 as a novel CSC marker in HCC. Sox12+ versus Sox12− cells were purified from HCC cell lines. The Sox12+ cells were compared with Sox12− HCC cells for tumor sphere formation, chemo-resistance, tumor formation after serial adoptive transplantations in nude mice, and the frequency of developing distal metastasis. Our results suggest that Sox12 may be a novel marker for enriching CSCs in HCC.
MATERIALS AND METHODS
Protocol approval
All the experimental methods including animal experiments have been approved by the research committee and the Institutional Animal Care and Use Committee at the People’s Hospital of Ganzhou. The research work was done following these guidelines and in accordance with the instruction from institutional Ethics Committee.
Cell culture and treatment
Two human HCC cell lines SNU-182 and HepG2 were both purchased from ATCC (American Type Culture Collection, USA). These cells were cultured in Dulbecco’s Modified Eagle’s Medium suppled with 10% Fetal Bovine Serum (FBS, Invitrogen, USA). The cells were incubated in a 37°C incubator with 5% CO2. Cisplatin (Sigma-Aldrich, USA) was given to HCC cells at the concentration of 20 μmol/l in culture for 24 h before analysis.
Cell transduction
The HCC cells were co-transduced with 2 lentiviruses. The first lentivirus carries a red fluorescent protein (RFP) reporter under the control of a Sox12 promoter. The second lentivirus carries a luciferase (LUC) reporter and a green fluorescent protein (GFP) reporter under a cytomegalovirus (CMV) promoter. LUC and GFP reporters are connected by a 2A sequence to allow co-expression of 2 genes by one promoter. The plasmids for generating these 2 viruses were prepared from a pcDNA3.1-CAG-GFP plasmid, a pcDNA3.1-CAG-RFP plasmid, and a pcDNA3.1-CMV-luciferase plasmid as backbones (Clontech, USA), using molecular cloning. Sox12 (Gene ID: 6666) promoter was cloned by PCR using human genomic DNA as a template. For lentiviral production, seeded HEK293T cells (ATCC) were co-transfected with 5 μg of pSox12-luciferase-2A-GFP and 5 μg each of packaging plasmids (REV, pMDL and VSV-G) by Lipofectamine-3000 assay (Invitrogen). The supernatant was removed 48 hours after transfection and filtered through the 0.45 μm syringe filter, after which the lentivirus in supernatant was isolated for titration. A multiplicity of infection (MOI) of 100 was used for both lentivirus to transduce HCCs in vitro, which resulted in more than 90% transduction efficiency for both lines.
Animal work
For examining tumor growth in vivo, male nude mice of 12 weeks of age (SLAC Laboratory Animal Co. Ltd, China) were subcutaneously transplanted with 106 transduced/labelled HCC cells. The bioluminescence was monitored 4 weeks after tumor implantation. For serial adoptive transplantation of tumor cells, male nude mice of 12 weeks of age were subcutaneously transplanted with 50 Sox12+ or Sox12− HCC cells. The presence of circulated blood cells was examined 2 weeks after tumor implantation. The tumor formation was evaluated by bioluminescence 8 weeks after tumor implantation, in a 3-round serial adoptive transplantation. Fifty cancer cells were isolated from implanted tumor and grafted to the new mice with the same protocol for the next round. Formation of tumor was monitored according to the overall luciferase activity of in vivo formed tumor. After anesthetized with 3% isoflurane (Sigma-Aldrich), mice that have received tumor cell transplantation got i.v. infusion of 50 mg/kg body weight luciferin (Sigma-Aldrich). Ten minutes later, the bioluminescence detection was performed and the images were recorded with IVIS imaging system (Xenogen Corp., USA), with an acquisition time of 60-second and binning of 10.
Flow cytometry
For flow cytometry analysis and cell sorting, the cell preparations were either labeled with PEcy7-conjugated anti-human CD133 antibody (Becton-Dickinson Biosciences, USA), or labeled with an Aldefluor Kit (StemCell Technologies, China), according to the manufacturer’s instructions. GFP and RFP were detected by direct fluorescence. Flow cytometry was performed using a FACSAria (Becton-Dickinson Biosciences) flow cytometer, and the data were analyzed and presented using Flowjo software (Flowjo LLC, USA).
Primary tumor sphere culture
The single cell preparation of the specific tumor cell fractions were re-suspended in tumor sphere media (TSM, DMEM suppled with 20 ng/ml human recombinant epidermal growth factor, 20 ng/ml basic fibroblast growth factor, 10 ng/ml leukemia inhibitory factor and 60 μg/ml N-acetylcysteine. After smeared in 60mm petri dish at a density of 2 × 104 cells/plate, the formation of tumor sphere was evaluated.
Cell viability assay
The cell viability was determined with an CCK-8 detection kit (Sigma-Aldrich), as instructed by the manufacturer. The absorbance of wells in microplate was read at 450 nm with microplate reader. The cell viability was calculated as: the percentage of absorbance value in detected well with reference to control well.
Statistical analysis
The statistical analysis was performed with the GraphPad Prism 6 (GraphPad Software, USA), using one-way analysis of variance (ANOVA) test followed by a Turkey multiple comparison post-hoc analysis. The values were shown as mean ± standard deviation (SD). A value of p<0.05 was considered as significant after Bonferroni correction.
RESULTS
Genetic labeling of Sox12+ vs Sox12− HCC cells with different fluorescence and luciferase
Two human HCC cell lines SNU-182 and HepG2 were used in the current study. Both lines were generated from HCC tissue, and SNU-182 was more aggressive than HepG2. These 2 HCC cell lines were co-transduced with 2 lentiviruses. The first lentivirus carries a RFP reporter under the control of a Sox12 promoter (pSox12-RFP). The second lentivirus carries a luciferase (LUC) reporter and a GFP reporter under a CMV promoter (pCMV-LUC-GFP) (Fig. 1A). Using a MOI of 100, both lines were transduced with 2 lentiviruses efficiently. Flow cytometry analysis showed that many HCC cells were GFP+, and these GFP+ cells were transduced cells. Among GFP+ cells, there were a few cells appeared to be RFP+ and thus yellow fluorescent (positive for both RFP and GFP), and these cells (GFP+RFP+) were Sox12+ HCC cells. On the other hand, the GFP+RFP− cells (green fluorescent due to absence of RFP) were Sox12− HCC cells (Fig. 1B). To confirm that RFP+ cells were indeed enriched for Sox12-expressing cells, we examined the mRNA level of SOX12 in GFP+RFP+ and GFP+RFP− cells, compared to total GFP+ cells. Our data showed that the mRNA level of SOX12 in GFP+RFP+ cells was increased by more than 9 times, compared to total GFP+ cells in both lines, while the mRNA level of SOX12 in GFP+RFP− cells was decreased by more than 80%, compared to total GFP+ cells in both lines (Fig. 1C). These data suggest that RFP+ cells were indeed enriched for Sox12-expressing cells. When all the fluorescent cells (GFP+RFP+ and GFP+RFP−) were sorted and put in culture, we found that all the cells were GFP+, from which a few are RFP−, confirmed the flow cytometry-based cell sorting (Fig. 1D).
Fig. 1. Genetic labeling of Sox12+ vs Sox12− HCC cells with different fluorescence and luciferase.
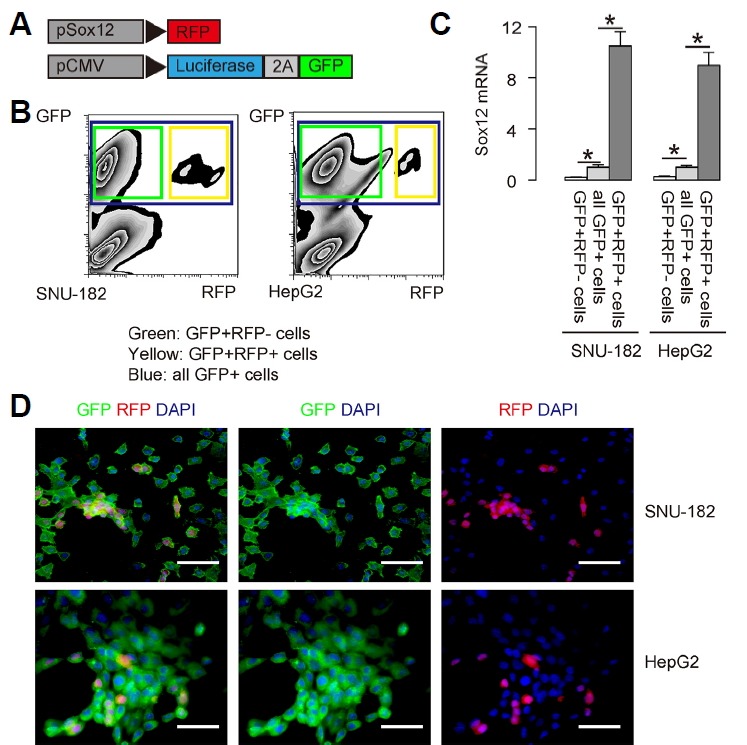
(A) Two human HCC cell lines SNU-182 and HepG2 were co-transduced with 2 lentiviruses. The first lentivirus carries a RFP reporter under the control of a Sox12 promoter (pSox12-RFP). The second lentivirus carries a luciferase (LUC) reporter and a GFP reporter under a CMV promoter (pCMV-LUC-GFP). The viral structure is shown. (B) Representative flow cytometry for GFP and RFP in the HCC cells infected with both lentiviruses. (C) RT-qPCR for the purified GFP+RFP− and GFP+RFP+ cells, compared to total GFP+ cells. (D) The purified GFP+ (RFP+ or FRP−) HCC cells in culture. DAPI: nuclei staining. Scale bars are 50 μm.
CSC marker expression in Sox12+ versus Sox12− HCC cells
The fluorescent HCC cells (GFP+RFP+ cells that represent Sox12+ cells and GFP+RFP− cells that represent Sox12− cells) were subjected to flow cytometry analysis for Aldefluor and CD133, two CSC markers, respectively. The ratio of Aldeflu−or+ versus Aldefluor− cells were quantified in both GFP+RFP+ HCC cell fraction and GFP+RFP− HCC cell fraction (Fig. 2A). We found that the percentage of Aldefluor+ cells was significantly higher in GFP+RFP+ HCC cell fraction, than in GFP+RFP− HCC cell fraction, shown by representative flow chart (Fig. 2A), and by quantification (Fig. 2B). Moreover, the ratio of CD133+ versus CD133− cells were quantified in both GFP+RFP+ HCC cell fraction and GFP+RFP− HCC cell fraction (Fig. 2C). We found that the percentage of CD133+ cells was significantly higher in GFP+RFP+ HCC cell fraction, than in GFP+RFP− HCC cell fraction, shown by representative flow chart (Fig. 2C), and by quantification (Fig. 2D). On the top of these interesting findings, the presence of a few Aldefluor+ or CD133+ cells in both lines suggests that both HCC cell lines here are heterogeneous and contain cancer stem cells. Thus, CSC marker expression in Sox12+ cells was significantly higher than in Sox12− HCC cells, suggesting that the levels of Sox12 may be higher in CSC cells in HCC.
Fig. 2. CSC marker expression in Sox12+ versus Sox12-HCC cells.
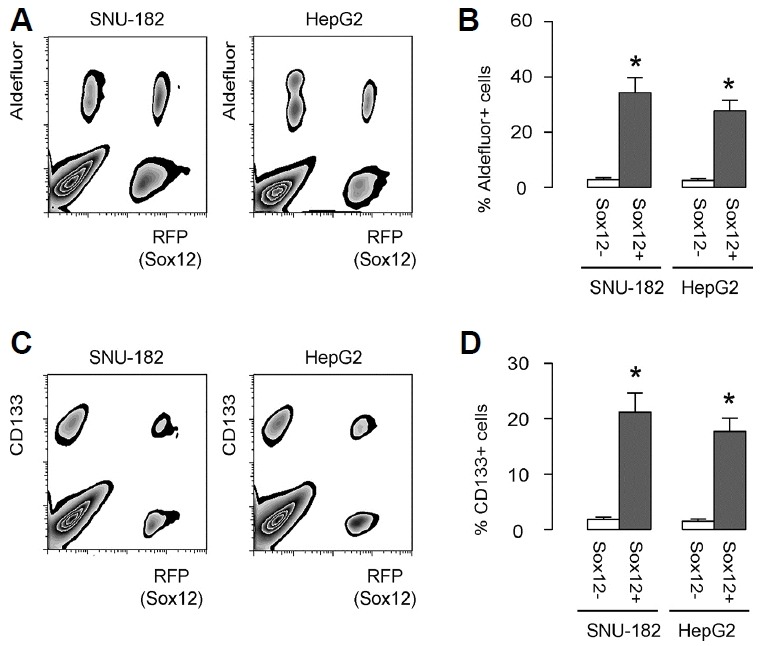
(A, B) The different fractions of HCC cells (GFP+RFP+ cells that represent Sox12+ cells and GFP+RFP− cells that represent Sox12− cells) were subjected to flow cytometry analysis for Aldefluor. The ratio of Aldefluor+ versus Aldefluor− cells were quantified in both GFP+RFP+ HCC cell fraction and GFP+RFP− HCC cell fraction, shown by representative flow chart (A), and by quantification (B). (C–D) The fluorescent HCC cells (GFP+RFP+ HCC cells and GFP+RFP− HCC cells) were subjected to flow cytometry analysis for CD133. The ratio of CD133+ versus CD133− cells were quantified in both GFP+RFP+ HCC cell fraction and GFP+RFP− HCC cell fraction, shown by representative flow chart (C), and by quantification (D). *p < 0.05. N = 5.
Sox12+ HCC cells generate more tumor spheres and are more chemo-resistant in vitro
In order to determine whether Sox12 indeed plays a role in the stemness of HCC cells or is just a bystander, we added one gain-of-function control (GFP+RFP− cells transfected with a Sox12-expressing plasmid; GFP+RFP−/Sox12) and one loss-of-function control [GFP+RFP+ cells transfected with a short hairpin interfering RNA (shSox12)-expressing plasmid; GFP+RFP+/shSox12]. First, the Sox12 mRNA levels in these cells were determined by RT-qPCR (Fig. 3A). The GFP+RFP−/Sox12, GFP+RFP−, GFP+RFP+ and GFP+RFP+/shSox12 HCC cells from both lines were then examined for their CSC properties in vitro. First, in a tumor sphere formation assay, we found that compared to GFP+RFP− HCC cells, GFP+RFP+ HCC cells in both lines generated significantly more tumor spheres, shown by representative images (Fig. 3B), and by quantification (Fig. 3C). Expression of Sox12 in GFP+RFP− cells significantly increased tumor sphere formation, while depletion of Sox12 in GFP+RFP+ cells decreased tumor sphere formation (Figs. 3B and 3C). Next, these HCC cells were treated with cisplatin, a first line chemotherapeutic drug for HCC. We found that compared to GFP+RFP− HCC cells, GFP+RFP+ HCC cells had better survival against cisplatin, in an CCK-8 assay (Fig. 3D). Expression of Sox12 in GFP+RFP− cells significantly increased HCC cell survival, while depletion of Sox12 in GFP+RFP+ cells significantly cell survival against cisplatin (Fig. 3D). Hence, Sox12+ HCC cells generate more tumor spheres and are more chemo-resistant in vitro, likely through Sox12-controlled stemness of HCC.
Fig. 3. Sox12+ HCC cells generate more tumor spheres and are more chemo-resistant in vitro.
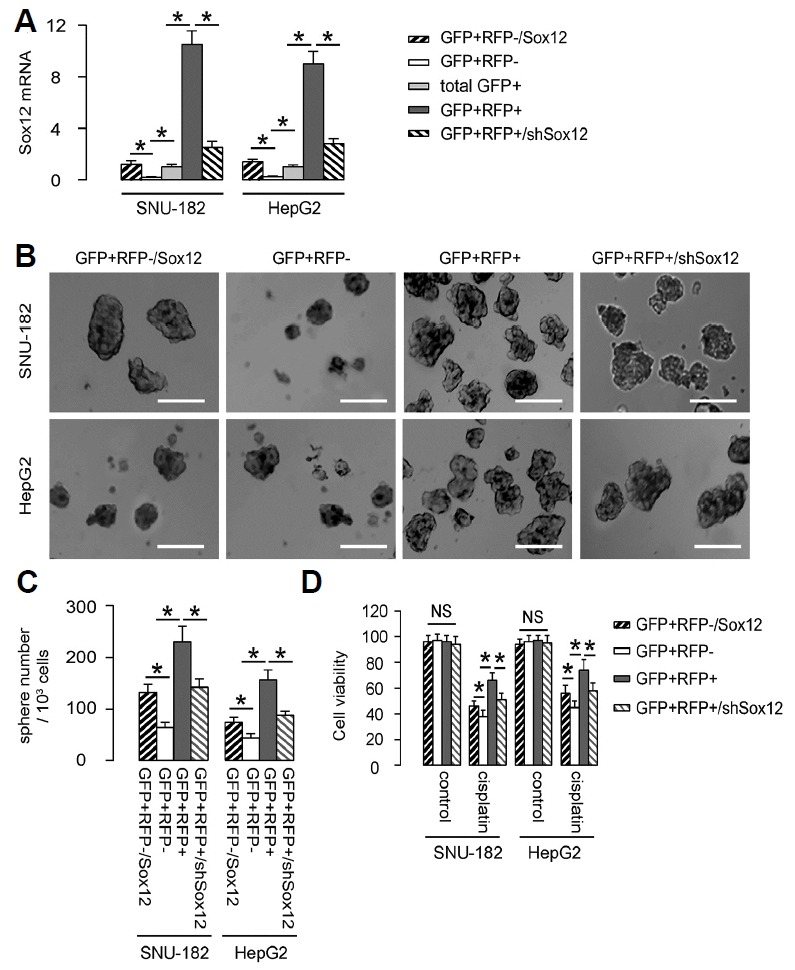
(A) GFP+RFP− cells transfected with a Sox12-expressing plasmid (GFP+RFP−/Sox12) were used as gain-of-function control. GFP+RFP+ cells transfected with a short hairpin interfering RNA (shSox12)-expressing plasmid (GFP+RFP+/shSox12) were used loss-of-function control. The Sox12 mRNA levels in these cells were determined by RT-qPCR. (B) The GFP+RFP−/Sox12, GFP+RFP−, GFP+RFP+ and GFP+RFP+/shSox12 HCC cells from both lines were then examined in a tumor sphere formation assay, shown by representative images (B), and by quantification (C). (D) The GFP+RFP−/Sox12, GFP+RFP−, GFP+RFP+ and GFP+RFP+/shSox12 HCC cells from both lines were treated with cisplatin in vitro, after which cell viability was determined in an CCK-8 assay. *p < 0.05. N = 5.
Transplanted Sox12+ HepG2 cells generate bigger tumor in vivo
GFP+RFP+/Sox12+ HCC cells and GFP+RFP−/Sox12− HCC cells of 106 in number were subcutaneously transplanted into nude mice, and the formed tumor was examined by bioluminescence after 4 weeks. We found that compared to GFP+RFP−/Sox12− HCC cells, GFP+RFP+/Sox12+ HCC cells generated significantly bigger tumor, shown by quantification (Fig. 4A) and by representative images (Fig. 4B).
Fig. 4. Transplanted Sox12+ HepG2 cells generate bigger tumor in vivo.
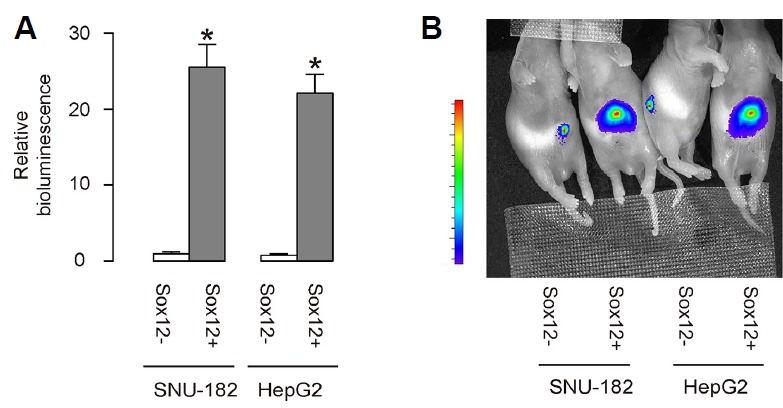
GFP+RFP+/Sox12+ HCC cells and GFP+RFP−/Sox12− HCC cells of 106 in number were subcutaneously transplanted into nude mice, and the formed tumor was examined by bioluminescence after 4 weeks. (A, B) The luciferase activity was determined in mice, shown by quantification (A) and by representative images (B). *p < 0.05. N = 10 mice that were used in each experimental group.
More circulated tumor cells and more tumor formation are detected after serial adoptive transplantation of Sox12+ HCC cells
In order to check the difference in cell invasion and metastasis, circulated tumor cells were examined 2 weeks after tumor cell implantation, by flow cytometry, based on GFP (regardless of presence of RFP). We found that GFP+ tumor cells were more frequently detected in the circulation of the mice transplanted with GFP+RFP+/Sox12+ HCC cells, compared to those transplanted with GFP+RFP−/Sox12− HCC cells (Fig. 5A). In a 3-round serial adoptive transplantation approach, tumor formation was more frequently detected in the mice transplanted with GFP+RFP+/Sox12+ HCC cells, compared to those transplanted with GFP+RFP−/Sox12− HCC cells (Figs. 5B and 5C). Together, our data suggest that Sox12 may be a novel marker for enriching CSCs in HCC.
Fig. 5. More circulated tumor cells and more tumor formation are detected after serial adoptive transplantation of Sox12+ HCC cells.
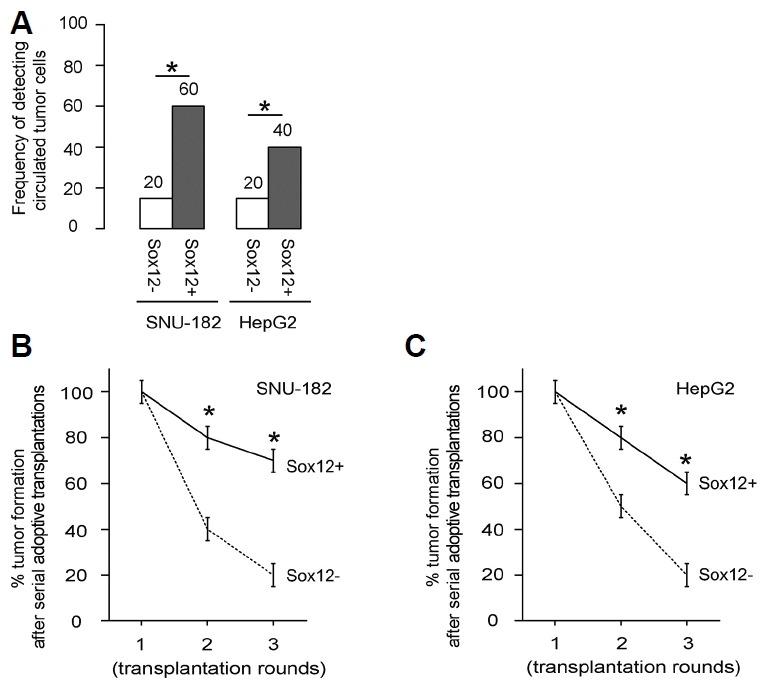
(A) In order to check the difference in cell invasion and metastasis, circulated tumor cells were examined 2 weeks after tumor cell implantation, by flow cytometry, based on GFP (regardless of presence of RFP). A total of 100 μl of mouse blood was taken for detection of GFP+ cells by flow cytometry. The frequency was shown. (B, C) The tumor formation was evaluated by bioluminescence 8 weeks after tumor implantation, in a 3-round serial adoptive transplantation. Fifty cancer cells were isolated from implanted tumor and grafted to the new mice with the same protocol for the next round. Formation of tumor was monitored according to the overall luciferase activity of in vivo formed tumor. Frequency of developing tumor by SNU-182 cells (B) and by HepG2 cells (C), after serial adoptive transplantation. *p < 0.05. N = 20.
DISCUSSION
In the current study, we analyzed Sox12 as a novel CSC marker for HCC. Our approach was theoretically supported by 2 recent studies. In the first study, Huang et al. showed that Sox12 upregulation was significantly correlated with loss of tumor encapsulation, microvascular invasion, and an advanced cancer stage in human HCC patients (Huang et al., 2015). Mechanistically, they showed evidence to demonstrate that forkhead box Q1 directly binds to the Sox12 promoter and then trans-activates its expression, to induce epithelial-mesenchymal transition (EMT) through direct targets for Sox12, Twist1 and FGFBP1 (Huang et al., 2015). Since Twist (Matsuo et al., 2009; Yang et al., 2009; Zhang et al., 2012; 2015) and FGFBP1 (Ray et al., 2014; Yang et al., 2014; Zhu et al., 2016) are important regulators for tumor invasion, angiogenesis and metastasis, Sox12 may be expected to contribute to the invasive manner for CSC cells in HCC. In another study, Jiang et al. showed that a tumor suppressive microRNA, miR-874, was downregulated in HCC tissue, resulting in the augmentation of Sox12 levels through loss of a direct binding-mediated translational control (Jiang et al., 2017). In a previous study, Sox12 was found to be a direct promoter for HCC cell migration, invasion, and EMT (Jiang et al., 2017). Thus, the contribution of Sox12 to the HCC cell stemness may be primarily on cell invasive manner, suggesting that combination of another CSC marker, which functions through cell cycle control on self-renewal, with Sox12, may be further improve the purification of CSC-like cells in HCC. This hypothesis may be tested in future study.
Here, we used 2 lentiviruses to co-transduce the HCC cells. Although one cell may be only infected by one virus but not the other, we think that this possibility should be low, since the 2 viruses are of same type and similar structure (Cockrell and Kafri, 2007; Houghton et al., 2015; McCarron et al., 2016). A MOI of 100 further rendered this possibility even lower. Moreover, the absence of RFP+GFP− cells after viral infection did not support this possibility. Furthermore, our isolation of GFP+ cells, regardless of RFP positivity, made the influence of this possibility to the interpretation of the data very limited. Together, the technique used in the current study should be validated.
We chose two human HCC lines in this study, since they were commonly used HCC lines, but processed different malignancy. Analysis on both lines increased the reliability of the study and the results may be more applicable to primary HCC. Indeed, previous studies have shown the association of Sox12 upregulation was an independent and significant risk factor for recurrence and reduced survival after curative resection (Huang et al., 2015). Studies on more clinical HCC specimens may increase our confidence of Sox12 as a clinic-relevant CSC marker.
REFERENCES
- Armstrong L., Stojkovic M., Dimmick I., Ahmad S., Stojkovic P., Hole N., Lako M. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–1151. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- Brower V. Sorafenib plus cisplatin for hepatocellular carcinoma. Lancet Oncol. 2016;17:e424. doi: 10.1016/S1470-2045(16)30447-8. [DOI] [PubMed] [Google Scholar]
- Chiba T., Iwama A., Yokosuka O. Cancer stem cells in hepatocellular carcinoma: Therapeutic implications based on stem cell biology. Hepatol Res. 2016;46:50–57. doi: 10.1111/hepr.12548. [DOI] [PubMed] [Google Scholar]
- Cockrell A.S., Kafri T. Gene delivery by lentivirus vectors. Mol Biotechnol. 2007;36:184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem. 2000;267:4315–4324. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- Dy P., Penzo-Mendez A., Wang H., Pedraza C.E., Macklin W.B., Lefebvre V. The three SoxC proteins--Sox4, Sox11 and Sox12--exhibit overlapping expression patterns and molecular properties. Nucleic Acids Res. 2008;36:3101–3117. doi: 10.1093/nar/gkn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D.D., Kim Y.J., Lee C.N., Aggarwal S., McKinnon K., Mesmer D., Norton J., Birse C.E., He T., Ruben S.M., et al. Expansion of CD133(+) colon cancer cultures retaining stem cell properties to enable cancer stem cell target discovery. Br J Cancer. 2010;102:1265–1275. doi: 10.1038/sj.bjc.6605610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D.A., Meyerrose T.E., Wirthlin L., Craft T.P., Herrbrich P.E., Creer M.H., Nolta J.A. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- Hess D.A., Wirthlin L., Craft T.P., Herrbrich P.E., Hohm S.A., Lahey R., Eades W.C., Creer M.H., Nolta J.A. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D.A., Craft T.P., Wirthlin L., Hohm S., Zhou P., Eades W.C., Creer M.H., Sands M.S., Nolta J.A. Widespread nonhematopoietic tissue distribution by transplanted human progenitor cells with high aldehyde dehydrogenase activity. Stem Cells. 2008;26:611–620. doi: 10.1634/stemcells.2007-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoser M., Potzner M.R., Koch J.M., Bosl M.R., Wegner M., Sock E. Sox12 deletion in the mouse reveals nonreciprocal redundancy with the related Sox4 and Sox11 transcription factors. Mol Cell Biol. 2008;28:4675–4687. doi: 10.1128/MCB.00338-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton B.C., Booth C., Thrasher A.J. Lentivirus technologies for modulation of the immune system. Curr Opin Pharmacol. 2015;24:119–127. doi: 10.1016/j.coph.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Huang R., Rofstad E.K. Cancer stem cells (CSCs), cervical CSCs and targeted therapies. Oncotarget. 2017;8:35351–35367. doi: 10.18632/oncotarget.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Chen Z., Shang X., Tian D., Wang D., Wu K., Fan D., Xia L. Sox12, a direct target of FoxQ1, promotes hepatocellular carcinoma metastasis through up-regulating Twist1 and FGFBP1. Hepatology. 2015;61:1920–1933. doi: 10.1002/hep.27756. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Shimizu S., Sato T., Morimoto M., Kojima Y., Inaba Y., Hagihara A., Kudo M., Nakamori S., Kaneko S., et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol. 2016;27:2090–2096. doi: 10.1093/annonc/mdw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal W., Alkarim S., AlHejin A., Mukhtar H., Saini K.S. Targeting signal transduction pathways of cancer stem cells for therapeutic opportunities of metastasis. Oncotarget. 2016;7:76337–76353. doi: 10.18632/oncotarget.10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Guan L.Y., Ye Y.S., Liu H.Y., Li R. MiR-874 inhibits metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma by targeting SOX12. Am J Cancer Res. 2017;7:1310–1321. [PMC free article] [PubMed] [Google Scholar]
- Llovet J.M., Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl 1):S20–37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Ma I., Allan A.L. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev. 2011;7:292–306. doi: 10.1007/s12015-010-9208-4. [DOI] [PubMed] [Google Scholar]
- Ma S., Chan K.W., Lee T.K., Tang K.H., Wo J.Y., Zheng B.J., Guan X.Y. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- Magni M., Shammah S., Schiro R., Mellado W., Dalla-Favera R., Gianni A.M. Induction of cyclophosphamide-resistance by aldehyde-dehydrogenase gene transfer. Blood. 1996;87:1097–1103. [PubMed] [Google Scholar]
- Matsuo N., Shiraha H., Fujikawa T., Takaoka N., Ueda N., Tanaka S., Nishina S., Nakanishi Y., Uemura M., Takaki A., et al. Twist expression promotes migration and invasion in hepatocellular carcinoma. BMC Cancer. 2009;9:240. doi: 10.1186/1471-2407-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron A., Donnelley M., McIntyre C., Parsons D. Challenges of up-scaling lentivirus production and processing. J Biotechnol. 2016;240:23–30. doi: 10.1016/j.jbiotec.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Nagata T., Sakakura C., Komiyama S., Miyashita A., Nishio M., Murayama Y., Komatsu S., Shiozaki A., Kuriu Y., Ikoma H., et al. Expression of cancer stem cell markers CD133 and CD44 in locoregional recurrence of rectal cancer. Anticancer Res. 2011;31:495–500. [PubMed] [Google Scholar]
- Nakata S., Phillips E., Goidts V. Emerging role for leucine-rich repeat-containing G-protein-coupled receptors LGR5 and LGR4 in cancer stem cells. Cancer Manag Res. 2014;6:171–180. doi: 10.2147/CMAR.S57846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaiano A. Finding markers for cancer stem cells in renal cell carcinoma: looking beyond CD133. Cell Cycle. 2010;9:4431. doi: 10.4161/cc.9.22.13823. [DOI] [PubMed] [Google Scholar]
- Perez-Losada J., Balmain A. Stem-cell hierarchy in skin cancer. Nat Rev Cancer. 2003;3:434–443. doi: 10.1038/nrc1095. [DOI] [PubMed] [Google Scholar]
- Petersson M., Niemann C. Stem cell dynamics and heterogeneity: implications for epidermal regeneration and skin cancer. Curr Med Chem. 2012;19:5984–5992. [PubMed] [Google Scholar]
- Ray P.E., Al-Attar A., Liu X.H., Das J.R., Tassi E., Wellstein A. Expression of a Secreted Fibroblast Growth Factor Binding Protein-1 (FGFBP1) in Angioproliferative Kaposi Sarcoma. J AIDS Clin Res. 2014;5 doi: 10.4172/2155-6113.1000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Tian R., Wang M., Wang X., Jiang J., Zhang Z., Li X., He Z., Gong W., Qin R. CD44+ CD133+ population exhibits cancer stem cell-like characteristics in human gallbladder carcinoma. Cancer Biol Ther. 2010;10:1182–1190. doi: 10.4161/cbt.10.11.13664. [DOI] [PubMed] [Google Scholar]
- Silva I.A., Bai S., McLean K., Yang K., Griffith K., Thomas D., Ginestier C., Johnston C., Kueck A., Reynolds R.K., et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.R. Stem cell niche in tissue homeostasis, aging and cancer. Curr Med Chem. 2012;19:5965–5974. doi: 10.2174/092986712804485917. [DOI] [PubMed] [Google Scholar]
- Singh S.K., Clarke I.D., Terasaki M., Bonn V.E., Hawkins C., Squire J., Dirks P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Sun B., Zhang D., Zhao N., Zhao X. Epithelial-to-endothelial transition and cancer stem cells: two cornerstones of vasculogenic mimicry in malignant tumors. Oncotarget. 2017;8:30502–30510. doi: 10.18632/oncotarget.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslype C., Van Cutsem E., Dicato M., Arber N., Berlin J.D., Cunningham D., De Gramont A., Diaz-Rubio E., Ducreux M., Gruenberger T., et al. The management of hepatocellular carcinoma. Ann Oncol; Current expert opinion and recommendations derived from the 10th World Congress on Gastrointestinal Cancer; Barcelona. 2008; 2009. pp. vii1–vii6. [DOI] [PubMed] [Google Scholar]
- Yan Y., Zuo X., Wei D. Concise review: emerging Role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl Med. 2015;4:1033–1043. doi: 10.5966/sctm.2015-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.H., Chen C.L., Chau G.Y., Chiou S.H., Su C.W., Chou T.Y., Peng W.L., Wu J.C. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- Yang Z., Yang Z., Zou Q., Yuan Y., Li J., Li D., Liang L., Zeng G., Chen S. A comparative study of clinicopathological significance, FGFBP1, and WISP-2 expression between squamous cell/adenosquamous carcinomas and adenocarcinoma of the gallbladder. Int J Clin Oncol. 2014;19:325–335. doi: 10.1007/s10147-013-0550-9. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li S.Y. Research progression of CD133 as a marker of cancer stem cells. Chinese J Cancer. 2010;29:243–247. doi: 10.5732/cjc.009.10587. [DOI] [PubMed] [Google Scholar]
- Zhang C.H., Xu G.L., Jia W.D., Li J.S., Ma J.L., Ren W.H., Ge Y.S., Yu J.H., Liu W.B., Wang W. Activation of STAT3 signal pathway correlates with twist and E-cadherin expression in hepatocellular carcinoma and their clinical significance. J Surg Res. 2012;174:120–129. doi: 10.1016/j.jss.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Zhang C., Guo F., Xu G., Ma J., Shao F. STAT3 cooperates with Twist to mediate epithelial-mesenchymal transition in human hepatocellular carcinoma cells. Oncol Rep. 2015;33:1872–1882. doi: 10.3892/or.2015.3783. [DOI] [PubMed] [Google Scholar]
- Zhu H.Y., Bai W.D., Liu J.Q., Zheng Z., Guan H., Zhou Q., Su L.L., Xie S.T., Wang Y.C., Li J., et al. Up-regulation of FGFBP1 signaling contributes to miR-146a-induced angiogenesis in human umbilical vein endothelial cells. Sci Rep. 2016;6:25272. doi: 10.1038/srep25272. [DOI] [PMC free article] [PubMed] [Google Scholar]


