Abstract
Oncogenic signaling in cancer cells alters glucose uptake and utilization to supply sufficient energy and biosynthetic intermediates for survival and sustained proliferation. Oncogenic signaling also prevents oxidative stress and cell death caused by increased production of reactive oxygen species. However, elevated glucose metabolism in cancer cells, especially in glioblastoma, results in the cells becoming sensitive to glucose deprivation (i.e. in high glucose dependence), which rapidly induces cell death. However, the precise mechanism of this type of cell death remains unknown. Here, we report that glucose deprivation alone does not trigger glioblastoma cell death. We found that, for cell death to occur in glucose-deprived glioblastoma cells, cystine and glutamine also need to be present in culture media. We observed that cystine uptake through the cystine/glutamate antiporter xCT under glucose deprivation rapidly induces NADPH depletion, reactive oxygen species accumulation, and cell death. We conclude that although cystine uptake is crucial for production of antioxidant glutathione in cancer cells its transport through xCT also induces oxidative stress and cell death in glucose-deprived glioblastoma cells. Combining inhibitors targeting cancer-specific glucose metabolism with cystine and glutamine treatment may offer a therapeutic approach for glioblastoma tumors exhibiting high xCT expression.
Keywords: amino acid transport, cell death, glioblastoma, glucose, reactive oxygen species (ROS), cystine
Introduction
Cancer cells increase glucose uptake and metabolism to support survival and sustained proliferation. Thus, transporters or enzymes involved in altered glucose metabolism have been considered as potential therapeutic targets for many cancers (1, 2). One consequence of elevated glucose metabolism in cancer cells, especially in glioblastoma cells, is that they become sensitive to glucose deprivation (glucose addiction). Glioblastoma is the most aggressive malignant brain tumor in humans with poor survival (3), and previous studies have demonstrated that withdrawal of glucose from culture medium rapidly induces cell death in multiple glioblastoma cell lines (4–6). Because normal astrocytes are not sensitive to glucose deprivation, the difference in glucose sensitivity may provide an effective therapeutic approach for glioblastoma. Glucose deprivation-induced rapid cell death requires increased reactive oxygen species (ROS)2 levels in cells (5–8). However, the precise mechanism of this type of cell death remains unknown.
ROS are produced in all cells as a by-product of normal cellular metabolism, and at low levels, ROS increase cell proliferation and survival. However, when ROS levels become excessively high, they cause oxidative stress, leading to cell death (9, 10). In cancer cells, ROS are produced at high levels and contribute to accelerating the accumulation of additional mutations and amplifying the tumorigenic phenotype. Therefore, cancer cells up-regulate antioxidant systems and reduce ROS to moderate levels to avoid harmful oxidative stress and cell death (9, 10). Thus, elucidation of molecular mechanisms underlying how ROS levels are tightly regulated in cancer cells provides opportunities for novel therapies against cancer.
Cancer cells also exhibit altered amino acid uptake and utilization, and therefore they often express high levels of cell surface amino acid transporters. Among them, xCT (SLC7A11), the light chain of the cystine/glutamate antiporter system xc−, is up-regulated in brains of patients with glioblastoma and in glioblastoma cell lines, and its expression correlates with tumor invasion and poor survival (11–17). The system xc− is composed of xCT and the heavy chain subunit 4F2 (4F2hc, SLC3A2) that exchanges extracellular cystine for intracellular glutamate at the plasma membrane. The substrate specificity and activity of system xc− depend on xCT, and increased expression of xCT leads to enhanced activity of cystine/glutamate exchange. In contrast, 4F2hc is known to form heterodimers with several light chains of specific amino acid transporters including xCT, leading to the surface expression of amino acid transporters (18–20). Within the cells, the imported cystine is reduced to cysteine, which is the rate-limiting amino acid for reduced glutathione synthesis. Glutathione is one of the most important antioxidants in many types of cancer including glioblastoma. Conversely, treatment with pharmacological inhibitors of xCT or depletion of glutathione in several types of cancer cells triggers iron-dependent nonapoptotic cell death termed ferroptosis (21–23). Thus, intracellular transport of cystine is important to reduce elevated ROS levels and to avoid oxidative stress-induced cell death in cancer cells. However, xCT has recently been reported to have an opposite effect on cell viability during glucose deprivation (24, 25). In this study, we show that, in glioblastoma cells expressing high levels of xCT, intracellular transport of cystine through xCT rapidly induces NADPH depletion, ROS accumulation, and cell death.
Results
Cystine and glutamine are required for glucose deprivation-induced cell death in glioblastoma cells
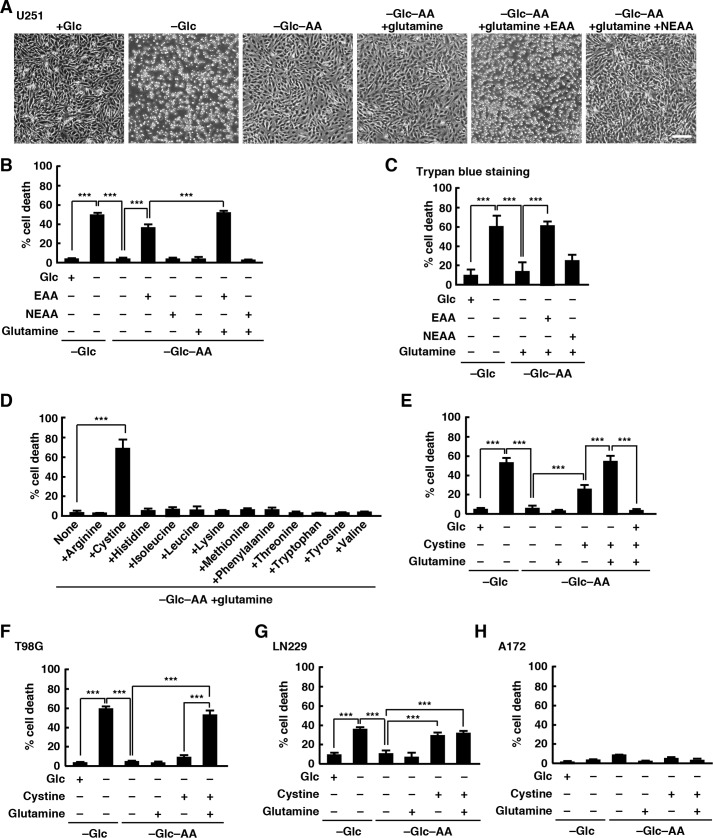
As reported previously (5), we used U251 glioblastoma cell line to show that glucose deprivation from the medium rapidly induced cell death: cells were detached from the substrate within 24 h. Unexpectedly, when U251 cells were deprived of both glucose and all amino acids in the medium, few cells were detached from the substrate over 24 h (Fig. 1A). To quantify U251 cell death, we measured lactate dehydrogenase (LDH) release in the culture medium at 24 h after deprivation of glucose or glucose and amino acids. Glucose deprivation significantly increased cell death, whereas deprivation of both glucose and amino acids did not (Fig. 1B). We next used essential amino acid (EAA) solution (containing arginine, cystine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, tyrosine, and valine) and nonessential amino acid (NEAA) solution (containing alanine, asparagine, aspartic acid, glutamic acid, glycine, proline, and serine) to examine whether addition of amino acids restored glucose deprivation-induced cell death. Addition of EAA solution, but not NEAA solution or glutamine (glutamine is not included in NEAA solution used here), in the glucose- and amino acid-free medium restored cell death but not completely, and the combination of glutamine and EAA solution was sufficient to restore glucose deprivation-induced cell death (Fig. 1, A and B), suggesting that an amino acid(s) in EAAs and glutamine are required for glucose deprivation-induced cell death in U251 cells. Similar results were obtained using a trypan blue exclusion assay (Fig. 1C). To determine which amino acid(s) in EAA solution was responsible for the glucose deprivation-induced rapid cell death, glutamine and each amino acid in the EAA solution were added in the glucose- and amino acid-free medium, and cell death was analyzed by LDH release assay. We found that addition of cystine with glutamine in the glucose- and amino acid-free medium triggered cell death (Fig. 1D). Addition of cystine alone also induced cell death, but the combination of cystine and glutamine was required to induce cell death at levels comparable with those induced by deprivation of glucose alone (Fig. 1E). Addition of glucose in the glucose- and amino acid-free medium completely inhibited cell death induced by cystine and glutamine, suggesting that cystine and glutamine do not affect cell viability in the presence of glucose. We used other glioblastoma cell lines to show that this is not limited to U251 cells. Similar results were obtained in T98G and LN229 cells, but they showed different responses to glutamine. In T98G cells, cell death induced by cystine alone was very weak, and addition of glutamine greatly promoted cystine-induced cell death in the glucose- and amino acid-free medium, although glutamine alone had little effect on cell viability (Fig. 1F). Conversely, the level of glucose deprivation-induced cell death in LN229 cells was low compared with those in U251 and T98G cells, and addition of glutamine had little effect on cystine-induced cell death (Fig. 1G). In contrast, A172 cells did not die without glucose, and neither addition of cystine alone nor addition of a combination of cystine and glutamine under glucose and amino acid deprivation conditions induced cell death in A172 cells within 24 h (Fig. 1H). These results suggest that cystine and glutamine are required for glucose deprivation-induced cell death in glioblastoma cells.
Figure 1.
Cystine and glutamine induce cell death during glucose deprivation. A and B, U251 cells were placed in glucose-free or glucose- and amino acid-free medium with or without glucose (5 mm), EAA, or NEAA solutions for 24 h. Scale bar, 200 μm (A). Quantification of cell death was performed using an LDH release assay. Cells treated with 0.1% Tween 20 were used for calculating 100% cell death (B). C, quantification of cell death was performed using a trypan blue exclusion assay. D–H, cells were placed in glucose-free or glucose- and amino acid-free medium with or without glucose, glutamine (2 mm), or cystine (0.2 mm) for 24 h (D, E, G, and H) or 12 h (F). Quantification of cell death was performed using an LDH release assay. Error bars represent S.D. (n = 3). ***, p < 0.001, calculated by one-way ANOVA with Tukey's post hoc test. Glc, glucose; AA, amino acids.
The cystine/glutamate antiporter xCT is involved in glucose deprivation-induced cell death
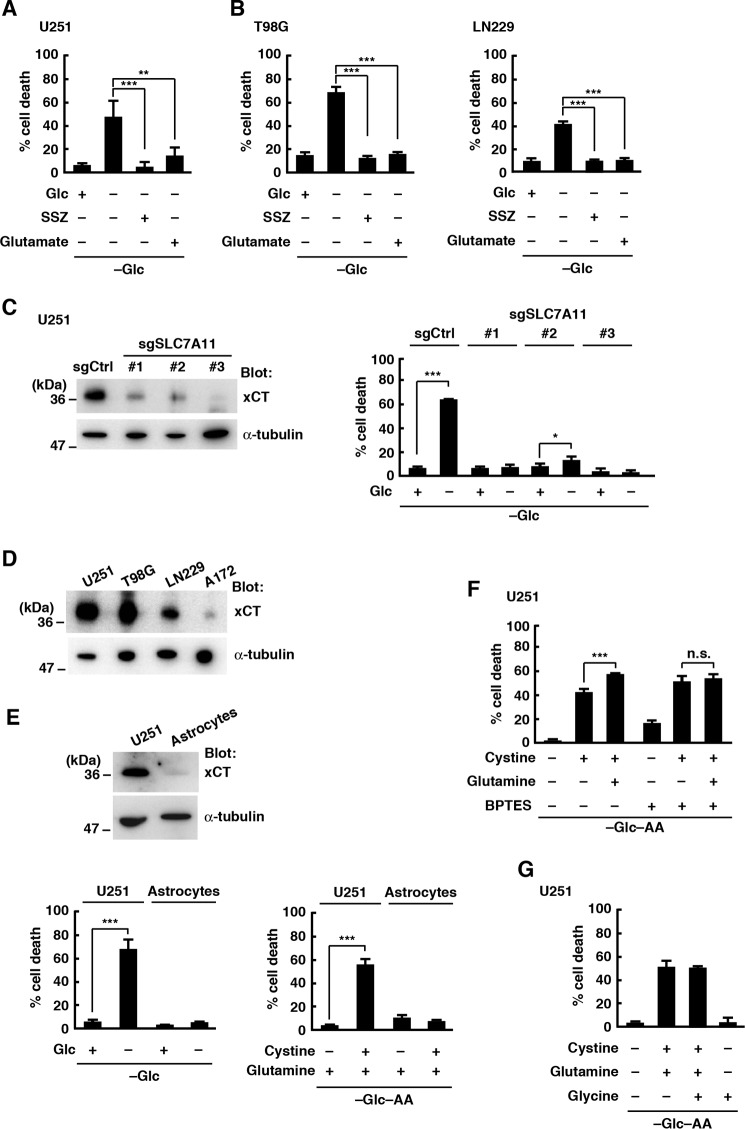
Because xCT, a subunit of the cysteine/glutamate antiporter system xc−, is up-regulated in glioblastoma cell lines (12, 16, 17), we speculated that cystine uptake through xCT triggered cell death during glucose deprivation. In U251 cells, the xCT inhibitor sulfasalazine (SSZ) or a high concentration (10 mm) of glutamate, both of which inhibit xCT-mediated cystine uptake (20, 26), blocked glucose deprivation-induced cell death (Fig. 2A). Similar results were obtained in T98G and LN229 cells (Fig. 2B). To confirm the involvement of xCT in glucose deprivation-induced cell death, xCT activity was genetically suppressed in U251 cells using single-guide RNAs (sgRNAs). We could not obtain complete xCT knock-out cells: this might be due to the fact that cells derived from xCT knock-out mice fail to proliferate and finally die under normal culture conditions (20, 27–29). However, glucose deprivation-induced cell death was greatly inhibited in cells with reduced expression of xCT using sgRNAs targeting different regions of SLC7A11 gene (sgSLC7A11-1, -2, and -3; Fig. 2C). Like parental cells, control sgRNA-expressing cells died in the glucose-free medium. We also found that A172 cells, which were much less sensitive to glucose depletion, expressed very low levels of xCT compared with other glioblastoma cell lines (Fig. 2D). These results suggest that xCT mediates glucose deprivation-induced cell death in glioblastoma cells. It has been reported that normal astrocytes express less xCT than glioblastoma cells and are not sensitive to glucose deprivation (5, 17). Consistent with these findings, the expression level of xCT in normal astrocytes prepared from rat cerebral cortex was very low compared with that of U251 cells, and glucose deprivation from the medium or addition of cystine and glutamine in the glucose- and amino acid-free medium had little effect on the viability of normal astrocytes within 24 h (Fig. 2E). Glutamine is transported into cells and metabolized to glutamate, and cystine uptake by xCT is coupled to the export of glutamate. Our results show that glutamine promoted, rather than inhibited, cystine-induced cell death (Fig. 1), suggesting that the uptake of cystine, rather than depletion of intracellular glutamate, contributes to xCT-mediated cell death under glucose deprivation conditions in glioblastoma cells. In addition, glutamine did not promote the cystine-induced cell death when U251 cells were treated with bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES), a glutaminase inhibitor, although BPTES alone had a slight cytotoxic effect (Fig. 2F). This result also supports the idea that the conversion of glutamine to glutamate drives xCT-mediated cystine uptake and cell death. One of the important roles of xCT is to supply cysteine necessary for glutathione synthesis (18–20). Glutathione is synthesized from three amino acids, cysteine, glutamate, and glycine, but addition of glycine had little effect on cystine- and glutamine-induced cell death (Fig. 2G).
Figure 2.
Glucose deprivation-induced cell death involves xCT. A and B, cells were placed in glucose-free medium with or without glucose (5 mm), SSZ (250 μm), or glutamic acid (10 mm) for 24 h. Quantification of cell death was performed using an LDH release assay. Error bars represent S.D. (n = 3). **, p < 0.01; ***, p < 0.001, calculated by one-way ANOVA with Tukey's post hoc test. C, immunoblot analysis of U251 cells after CRISPR-based gene editing with SLC7A11 sgRNAs (sgSLC7A11) or sgCtrl. Quantification of cell death was performed 24 h after glucose deprivation using an LDH release assay. Error bars represent S.D. (n = 3). *, p < 0.05; ***, p < 0.001, calculated by one-way ANOVA with Tukey's post hoc test. D, immunoblot analysis of glioblastoma cell lines. E, immunoblot analysis of U251 cells and rat astrocytes. Quantification of cell death was performed 24 h after glucose deprivation using an LDH release assay. Error bars represent S.D. (n = 3). *, p < 0.05; ***, p < 0.001, calculated by one-way ANOVA with Tukey's post hoc test. F and G, cells were placed in glucose- and amino acid-free medium with or without cystine (0.2 mm), glutamine (2 mm), glycine (0.4 mm), or BPTES (10 μm) for 24 h. Quantification of cell death was performed using an LDH release assay. Error bars represent S.D. (n = 3). **, p < 0.01; ***, p < 0.001, calculated by one-way ANOVA with Tukey's post hoc test. n.s., not significant; Glc, glucose; AA, amino acids.
Cystine uptake through xCT induces ROS accumulation and cell death during glucose deprivation
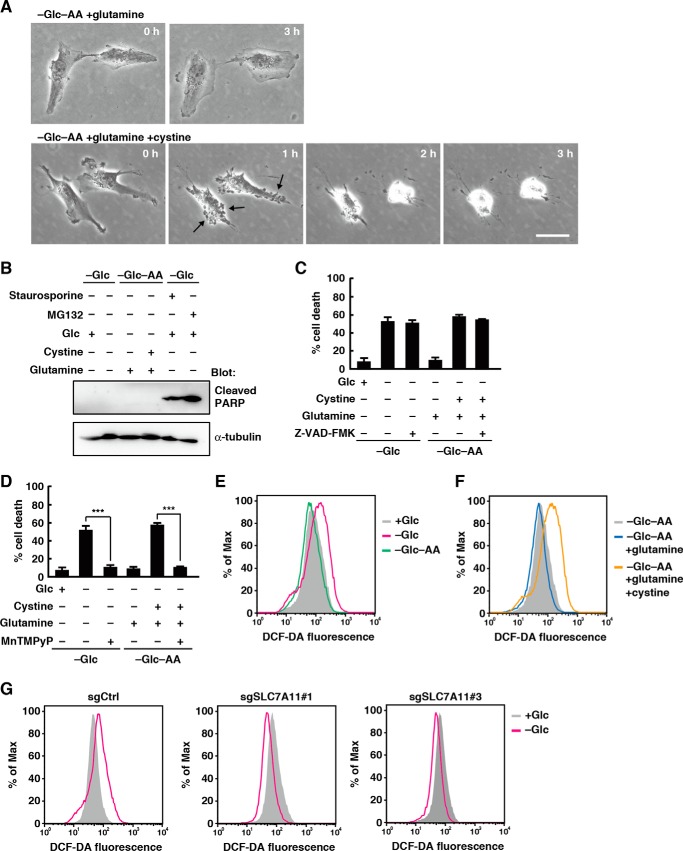
We next examined the morphological changes of U251 cells after addition of cystine and glutamine in the glucose- and amino acid-free medium. Addition of cystine and glutamine rapidly triggered the formation of blebbing-like structures on the plasma membrane and shrinking of the cell size (Fig. 3A, arrows indicate typical blebbing-like structures). These morphological changes were not observed in the absence of cystine. The level of cleaved poly(ADP-ribose) polymerase, an apoptosis marker, did not change after glucose deprivation from the medium or addition of cystine and glutamine in the glucose- and amino acid-free medium (Fig. 3B; staurosporine and MG132, both of which induce apoptosis in glioblastoma cells (30, 31), were used as positive controls). In addition, the caspase inhibitor Z-VAD-fmk had no effect on glucose deprivation- or cystine-induced cell death (Fig. 3C). In contrast, treatment with a cell-permeable ROS scavenger, MnTMPyP, completely suppressed glucose deprivation- or cystine-induced cell death (Fig. 3D). These results suggest that cystine induces ROS-dependent nonapoptotic cell death during glucose deprivation. Flow cytometric analysis of U251 cells loaded with the cell-permeable fluorescent ROS probe 2′,7′-dichlorofluorescein diacetate revealed that although glucose deprivation increased intracellular ROS levels combined depletion of glucose and amino acids did not (Fig. 3E). However, addition of cystine and glutamine in the glucose- and amino acid-free medium caused an increase in intracellular ROS levels (Fig. 3F). Addition of glutamine alone had little effect on ROS levels. We next used xCT-deficient cells to investigate whether xCT is involved in the ROS accumulation under glucose deprivation. Although glucose withdrawal increased intracellular ROS levels in control sgRNA-expressing cells, it caused a decrease, rather than an increase, in intracellular ROS levels in xCT-deficient cells (Fig. 3G). Thus, xCT promotes ROS accumulation under glucose deprivation conditions in glioblastoma cells. Because ROS are produced by mitochondrial oxidative phosphorylation in most cancer cells (2, 32), glucose deprivation may decrease mitochondrial oxidative metabolism and therefore reduce intracellular ROS generation, although the exact reason for reduced ROS levels in glucose-deprived xCT-deficient cells is unknown. In contrast, recent studies have revealed nonapoptotic iron-dependent cell death, termed ferroptosis, which is driven by accumulation of lipid-based ROS (21–23). However, the iron chelator deferoxamine or the lipophilic antioxidant Trolox had little effect on the cystine-induced cell death (data not shown).
Figure 3.
Cystine uptake through xCT causes accumulation of ROS under glucose deprivation. A, after the medium was replaced with glucose- and amino acid-free medium containing glutamine (2 mm) or glutamine and cystine (0.2 mm), phase-contrast images of U251 cells were captured at the indicated times. Scale bar, 20 μm. B, immunoblot analysis of U251 cells after the medium was replaced with glucose-free or glucose- and amino acid-free medium with or without glucose (5 mm), glutamine (2 mm), cystine (0.2 mm), staurosporine (0.1 μm), or MG132 (10 μm) for 24 h. C and D, U251 cells were placed in glucose-free or glucose- and amino acid-free medium with or without glucose (5 mm), glutamine (2 mm), cystine (0.2 mm), Z-VAD-fmk (10 μm), or MnTMPyP (10 μm) for 24 h. Quantification of cell death was performed using an LDH release assay. Error bars represent S.D. (n = 3). ***, p < 0.001, calculated by one-way ANOVA with Tukey's post hoc test. E–G, FACS analysis of ROS generation in U251 cells. Cells were placed in glucose-free or glucose- and amino acid-free medium with or without glucose or cystine for 3 h. Dark traces, cells placed in the medium with glucose (E and G) or in the medium without glucose and amino acids (F). Similar results were obtained in three independent experiments. Glc, glucose; AA, amino acids; DCF-DA, 2′,7′-dichlorofluorescein diacetate.
Cystine uptake through xCT promotes NADPH depletion during glucose deprivation
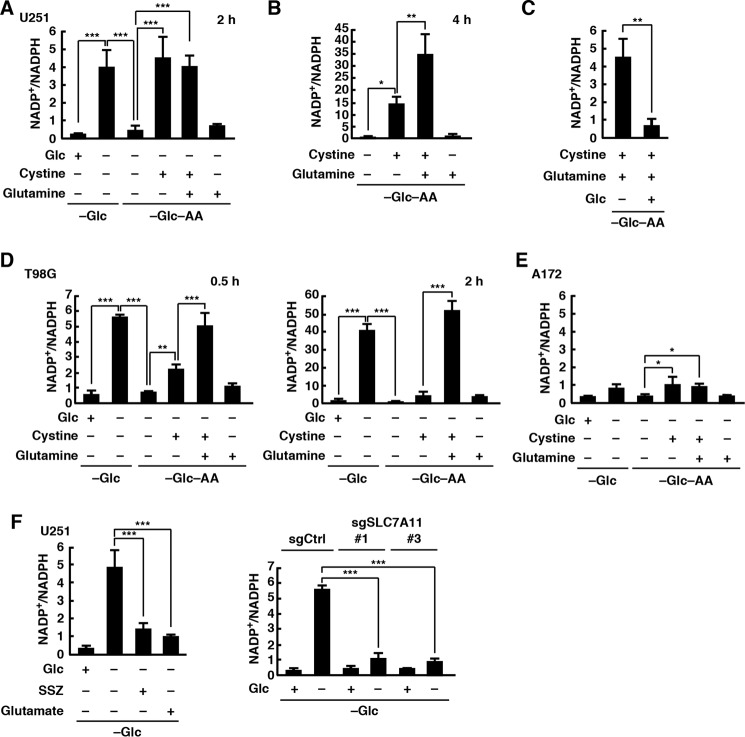
It is known that glucose metabolism generates NADPH, which is important for protection against oxidative stress in cancer cells (32, 33). We found that although glucose deprivation in U251 cells rapidly increased the intracellular NADP+/NADPH ratio combined depletion of glucose and amino acids did not. However, addition of cystine in the glucose- and amino acid-free medium increased the NADP+/NADPH ratio at levels comparable with those induced by deprivation of glucose alone (Fig. 4A). Although glutamine neither promoted nor inhibited the cystine-induced increase in the NADP+/NADPH ratio 2 h after changing the medium, it significantly promoted NADPH depletion by 4 h (Fig. 4B). The cystine-induced NADPH depletion was completely suppressed by addition of glucose (Fig. 4C). Thus, although glucose is essential for the maintenance of NADPH levels, glucose deprivation alone does not induce NADPH depletion. Similar results were obtained in T98G cells, but they responded more rapidly than U251 cells (Fig. 4D). In addition, the increase in the NADP+/NADPH ratio induced by cystine alone was very weak, and it was greatly promoted by addition of glutamine. These results are consistent with the results of cell death assays. In contrast, the intracellular NADP+/NADPH ratio in A172 cells was low even when they were placed in glucose-free medium containing cystine and glutamine (Fig. 4E). To investigate whether xCT is involved in the glucose deprivation-induced NADPH depletion, we used xCT inhibitors and xCT-deficient cells. We found that xCT inhibitors SSZ and high concentration of glutamate or loss of xCT expression significantly blocked glucose deprivation-induced NADPH depletion (Fig. 4F). These results suggest that cystine uptake through xCT triggers NADPH depletion under glucose deprivation conditions.
Figure 4.
Cystine uptake through xCT induces NADPH depletion during glucose deprivation. After the medium was replaced with glucose-free or glucose- and amino acid-free medium containing glucose (5 mm), glutamine (2 mm), cystine (0.2 mm), SSZ (250 μm), or glutamic acid (10 mm) for 2 (A, C, E, and F), 0.5 or 2 (D), or 4 h (B), the NADP+/NADPH ratio was measured. Error bars represent S.D. (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001, calculated by one-way ANOVA with Tukey's post hoc test. Glc, glucose; AA, amino acids.
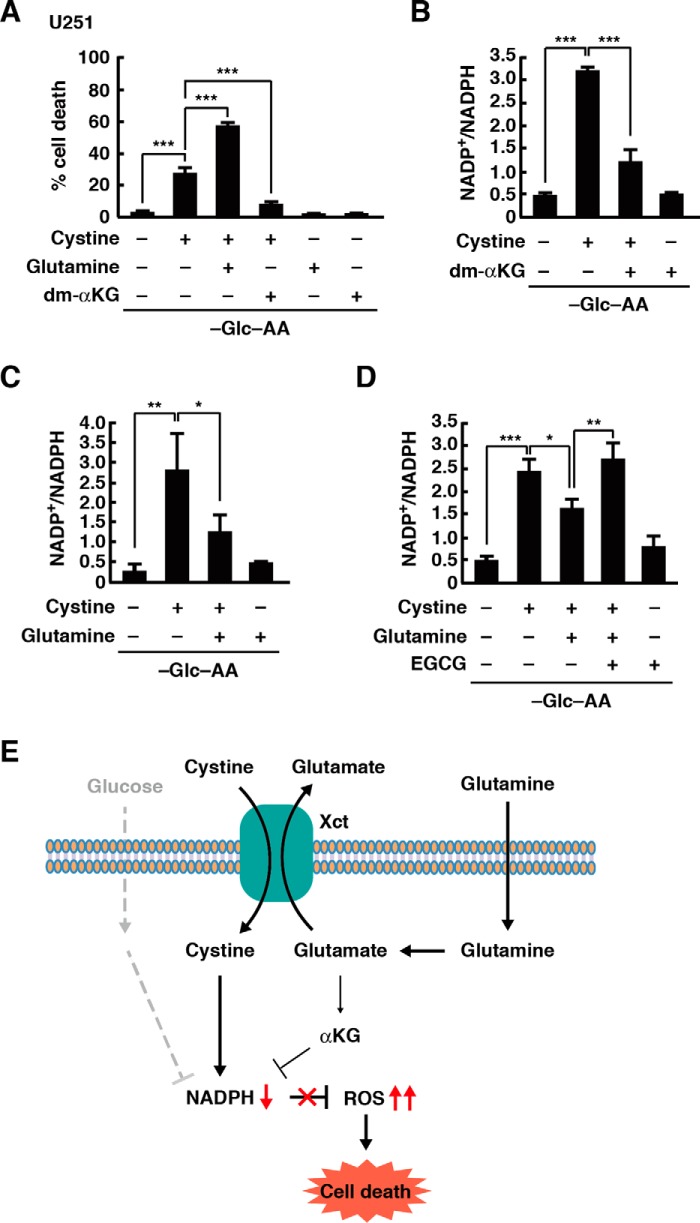
Glutamine has opposite effects on xCT-mediated cell death
In glioblastoma cells, glutamine metabolism is important for cell survival when glucose metabolism is impaired (34). Glutamine is transported into cells and metabolized to glutamate, which is then converted into α-ketoglutarate, and a recent study reported that dimethyl α-ketoglutarate (dm-αKG), a cell-permeable form of α-ketoglutarate, greatly improved cell viability during glucose deprivation in cancer cells (24, 25). Consistent with this, treatment with dm-αKG inhibited cystine-induced cell death in U251 cells, although glutamine had the opposite effect on cell viability (Fig. 5A). Addition of dm-αKG also inhibited the cystine-induced NADPH depletion during glucose deprivation (Fig. 5B). Because the promoting effect of glutamine on the cystine-induced NADPH depletion was observed at 4 h, but not 2 h, after glucose deprivation, we investigated the effect of glutamine on the NADP+/NADPH ratio for a short period of time. At 1 h after glucose deprivation, glutamine significantly inhibited the cystine-induced increase in the NADP+/NADPH ratio (Fig. 5C). This inhibitory effect of glutamine was suppressed by epigallocatechin gallate (EGCG), an inhibitor of glutamate dehydrogenase that catalyzes the conversion of glutamate to α-ketoglutarate (Fig. 5D). These results suggest that glutamine is metabolized into α-ketoglutarate to inhibit the NADPH depletion and cell death under glucose deprivation conditions.
Figure 5.
Glutamine and α-ketoglutarate inhibit cystine-induced NADPH depletion. A, cells were placed in glucose- and amino acid-free medium with or without glutamine (2 mm), cystine (0.2 mm), or dm-αKG (4 mm) for 24 h. Quantification of cell death was performed using an LDH release assay. B–D, after the medium was replaced with glucose- and amino acid-free medium containing glutamine (2 mm), cystine (0.2 mm), dm-αKG (4 mm), or EGCG (20 μm) for 2 (B) or 1 h (C and D), the NADP+/NADPH ratio was measured. Error bars represent S.D. (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001, calculated by one-way ANOVA with Tukey's post hoc test. E, model of xCT-mediated cell death under glucose deprivation conditions. Glc, glucose; AA, amino acids.
Discussion
The reprogrammed glucose metabolism in cancer cells is required for their growth and survival. In particular, the production of NADPH from glucose is important to reduce the intracellular ROS levels, and therefore glucose deprivation can lead to cell death in cancer cells compared with normal cells. NADPH is utilized for H2O2 detoxification by the glutathione system (glutathione, glutathione reductase, and glutathione peroxidase) and the thioredoxin system (thioredoxin, thioredoxin reductase, and peroxiredoxins). However, the precise mechanism by which glucose deprivation in cancer cells rapidly induces cell death has remained elusive. In this study, we identified amino acids cystine and glutamine as key mediators for glucose deprivation-induced cell death in glioblastoma cells, and we show that glucose deprivation alone does not trigger cell death. In the absence of glucose, cystine uptake through the cysteine/glutamate antiporter xCT triggers depletion of NADPH and elevation of ROS levels, which eventually cause cell death. Thus, in glioblastoma cells with high xCT activity, glucose metabolism protects against oxidative stress and cell death induced by cystine uptake through xCT. We also found that glutamine has opposite effects on glioblastoma cell viability under glucose deprivation conditions. Glutamine via glutamate is converted to α-ketoglutarate and produces NADPH, which results in the suppression of ROS accumulation and cell death during glucose deprivation. The generation of NADPH by glutamine may involve the malic enzymes (35, 36). Conversely, glutamine finally promotes xCT-mediated NADPH depletion and cell death. Because xCT transports cystine into the cell in exchange for glutamate, glutamine appears to be required to provide glutamate for xCT-mediated cystine uptake within the cells (Fig. 5E).
Recent studies have reported that inhibition of xCT in cancer cells improves cell viability after glucose deprivation by using glutamine as a carbon source (24, 25). α-Ketoglutarate is a tricarboxylic acid (TCA) cycle component, and the conversion of glutamine to α-ketoglutarate maintains the TCA cycle and cell survival. Expression of high levels of xCT suppresses glutamate-dependent processes by exporting glutamate and thus renders cancer cells highly dependent on glucose. In addition, xCT also contributes to glutamine anaplerosis (37). Our results suggest that cystine uptake through xCT induces ROS accumulation in glioblastoma cells. Because glutamate is utilized for synthesis of the antioxidant glutathione, xCT-mediated ROS accumulation may drive glutamine metabolism to glutathione synthesis, which results in dysfunction of the TCA cycle. However, it remains unclear how cystine causes NADPH depletion under glucose deprivation. Intracellularly, cystine is reduced cysteine, and it has been assumed that this reaction is mediated by glutathione and/or thioredoxin reductase 1 (20, 38, 39), both of which require NADPH for their activities. Because glucose is a major source of NADPH, high NADPH consumption due to reduction of imported cystine may rapidly cause NADPH depletion under glucose deprivation. Another possible mechanism is that glutamate export by xCT impairs the conversion of glutamine to α-ketoglutarate and α-ketoglutarate-mediated NADPH production, leading to NADPH depletion in the absence of glucose. Further studies are required to elucidate how cystine uptake induces ROS accumulation and influences glutamine metabolism in cancer cells with high levels of xCT.
Because cancer cells exhibit higher levels of ROS than normal cells to facilitate their growth, they are more susceptible to oxidative stress (40, 41). Cystine is mainly imported via system xc− in glioblastoma (15), and it is well known that under normal glucose conditions cystine is immediately reduced to cysteine within the cell and used for glutathione synthesis. Glutathione is one of the most important antioxidants in cancer cells, and therefore xCT is up-regulated under oxidative stress in various cancer cell types including glioblastoma (11, 19, 20). Recent studies have shown that treatment with pharmacological inhibitors of xCT or depletion of glutathione in several types of cancer cells results in iron-dependent accumulation of lipid ROS and cell death, termed ferroptosis. Thus, it has been accepted that xCT has a positive effect on cancer cell survival. Indeed, glioblastoma cells often up-regulate xCT, and the expression level of xCT is highly associated with tumor growth (11–17). However, cystine uptake by xCT promotes oxidative stress and eventually cell death under glucose deprivation conditions. Glucose transporters or enzymes involved in reprogrammed glucose metabolism have been considered as potential therapeutic targets for cancer (32). Therefore, the combination of inhibitors targeting cancer-specific glucose metabolism and amino acids cystine and glutamine could be an attractive therapeutic approach for glioblastoma with high xCT expression.
Experimental procedures
Reagents and antibodies
Minimum essential medium essential amino acids solution (50×) was purchased from Wako Pure Chemical Industries. Nonessential amino acids solution (100×) and all amino acids were purchased from Nacalai Tesque. The medium without glucose and amino acids was prepared by dissolving 0.2 g of CaCl2, 0.1 mg of Fe(NO3)3·9H2O, 97.67 mg of MgSO4, 0.4 g of KCl, 3.7 g of NaHCO3, 6.4 g of NaCl, 0.109 g of NaH2PO4, and 10 ml of minimum essential medium vitamin solution (100×) liquid (Thermo Fisher Scientific) in 1 liter of water. Inhibitors were used at the following concentrations: SSZ (Sigma-Aldrich), 250 μm, glutamic acid (Sigma-Aldrich), 10 mm, MnTMPyP (Merck), 10 μm, staurosporine (Sigma-Aldrich), 0.1 μm; MG132 (Wako Pure Chemical Industries), 10 μm; Z-VAD-fmk (R&D Systems), 10 μm; Trolox (Santa Cruz Biotechnology), 100 μm; deferoxamine mesylate salt (Santa Cruz Biotechnology), 100 μm; dm-αKG (Sigma-Aldrich), 4 mm; EGCG (Sigma-Aldrich), 20 μm; and BPTES (Sigma-Aldrich), 10 μm. Antibodies against xCT/SLC7A11 (D2M7A) and poly(ADP-ribose) polymerase (Asp-214) (D64E10) were purchased from Cell Signaling Technology. The α-tubulin antibody was purchased from Sigma-Aldrich. Secondary antibodies conjugated to horseradish peroxidase were purchased from Dako.
Cell culture and transfection
U251 cells were obtained from European Collection of Authenticated Cell Cultures (catalog number EC09063001). A172 and T98G cells were provided by the RIKEN BioResource Center through the National Bio-Resource Project of the Ministry of Education, Culture, Sports, Science and Technology, Japan (A172, catalog number RCB2530; T98G, catalog number RCB1954). LN229 cells were obtained from ATCC (catalog number CRL-2611). Cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 4 mm glutamine, 100 units/ml penicillin, and 0.1 mg/ml streptomycin under humidified air containing 5% CO2 at 37 °C. Cells were transfected with the indicated expression vectors using Lipofectamine 2000 (Life Technologies). All cell lines were tested and found to be negative for Mycoplasma contamination using the EZ-PCR Mycoplasma Test kit (Biological Industries).
Primary rat astrocytes were prepared from postnatal day 2 rat cerebral cortex. The cerebral cortex was dissected in ice-cold Hanks' balanced salt solution and incubated in Hanks' balanced salt solution with 0.25% trypsin and 0.1% DNase for 15 min at 37 °C. After washing in DMEM, the astrocytes were grown in DMEM containing 10% fetal bovine serum, 4 mm glutamine, 100 units/ml penicillin, and 0.1 mg/ml streptomycin under humidified air containing 5% CO2 at 37 °C.
Generation of xCT-deficient U251 cells
To generate xCT knock-out U251 cells, we used the CRISPR/Cas9-mediated homology-independent knock-in system (42). sgRNAs targeting SLC7A11 sequences were cloned into the tandem sgRNA expression vector peSpCAS9(1.1)-2xsgRNA (Addgene plasmid 80768), which has a Cas9 with enhanced specificity (eSpCas9) and tandem expression cassettes of sgRNAs. The first sgRNA targets SLC7A11, and the second sgRNA targets the donor vector pDonor-tBFP-NLS-Neo (Addgene plasmid 80766). The cleavage site of pDonor-tBFP-NLS-Neo is located upstream of the cytomegalovirus promoter to enable insertion of the sequence encoding blue fluorescent protein (tBFP) fused with a triplicated nuclear localization signal (NLS). U251 cells were seeded in two 6-cm dishes (250,000 cells/dish). Twenty-four hours later, the cells were cotransfected with peSpCAS9(1.1)-2xsgRNA containing sgRNA targeting SLC7A11 and pDonor-tBFP-NLS-Neo. Two days after transfection, the cells were collected and seeded in two 10-cm dishes in medium containing 250 μg/ml G418 (Wako) to eliminate untransfected cells. Ten days after selection, colonies grown from single cells with nuclear tBFP fluorescence were isolated. These clones were expanded and screened by immunoblotting with anti-xCT antibody.
The following primers were used to clone sgRNA into peSpCAS9(1.1)-2xsgRNA: sgSLC7A11-1F, caccaccatagtagggacacacgg; sgSLC7A11-1R, aaacccgtgtgtccctactatggt; sgSLC7A11-2F, cacctgcagggaaatgttaacggg; sgSLC7A11-2R, aaaccccgttaacatttccctgca; sgSLC7A11-3F, caccccccgtgtgtccctacta; sgSLC7A11-3R, aaactagtagggacacacgggg; sgCtrl-F, cacctgagcgacaacgagatccag; and sgCtrl-R, aaacctggatctcgttgtcgctca. The control sgRNA (sgCtrl) vector used in this study contains sgRNA targeting the human scribble sequence we tried to use for another study, but this sgRNA had no effect on scribble protein expression, although cells with nuclear tBFP fluorescence were isolated. sgSLC7A11-1 and -2 were designed using the online tool CRISPOR (http://crispor.tefor.net/crispor.py)3 and Fusi/Doench scores (43). sgSLC7A11-3 was designed based on a previous report (19).
Glucose and amino acid deprivation conditions and cell death experiments
On the day before the experiment, cells (20,000 cells/well) were seeded in a 48-well plate (Greiner Bio-One, catalog number 677180). On the day of the experiment, cells were rinsed twice with PBS, and the medium was replaced with glucose-free or glucose- and amino acid-free medium containing 10% dialyzed FBS (HyClone) for 24 h. Cell death was measured by LDH release assay or trypan blue exclusion assay. The LDH release assay was performed using an MTX LDH kit (Kyokuto Pharmaceutical Industrial) according to the manufacturer's instruction. The optical density was measured at 595 nm using a microplate reader (Tecan, GENious). The value of LDH release after treatment with 0.1% Tween 20 was defined as 100% cell death. Glucose and amino acids were used in the medium at the following concentrations: glucose, 5 mm; arginine-HCl, 0.4 mm; cystine-2HCl, 0.2 mm; histidine-HCl-H2O, 0.2 mm; isoleucine, 0.8 mm; leucine, 0.8 mm; lysine-HCl, 0.8 mm; methionine, 0.2 mm; phenylalanine, 0.4 mm; threonine, 0.8 mm; tryptophan, 0.08 mm; tyrosine-2Na-2H2O, 0.4 mm; valine, 0.8 mm; glutamine, 2 mm; and glycine, 0.4 mm.
Measurement of intracellular ROS
On the day before the experiment, U251 cells (50,000 cells/well) were seeded in a 24-well plate (Greiner Bio-One, catalog number 662160). On the day of the experiment, cells were rinsed twice with PBS, and the medium was replaced with glucose-free or glucose- and amino acid-free medium with or without 5 mm glucose or 0.2 mm cystine for 3 h. 2′,7′-Dichlorofluorescein diacetate (10 μm; Sigma-Aldrich) was added for the last 30 min. The cells were washed with PBS and harvested by trypsinization. Then the cells were resuspended in 50 μl of ice-cold PBS and analyzed using a flow cytometer (FACSCalibur, BD Biosciences). Data were collected from the FL1 channel. At least 30,000 cells were analyzed per condition.
Immunoblotting
Cell lysates were separated by SDS-PAGE and electrophoretically transferred onto a polyvinylidene difluoride membrane (Millipore Corp.). The membrane was blocked with 3% low-fat milk in Tris-buffered saline and then incubated with primary antibodies diluted with 3% low-fat milk or Can Get Signal (Toyobo). The primary antibodies were detected with horseradish peroxidase-conjugated secondary antibodies and a chemiluminescence detection kit (ECL Prime, GE Healthcare or Chemi-Lumi One L, Nacalai Tesque). The signals were captured with the LAS-3000 image analysis system (Fujifilm).
NADPH assay
On the day before the experiment, cells (50,000 cells/well) were seeded in a 24-well plate. On the day of the experiment, cells were rinsed twice with PBS, and the medium was replaced with glucose-free or glucose- and amino acid-free medium with or without 5 mm glucose or 0.2 mm cystine and 2 mm glutamine for the indicated times. The intracellular levels of NADPH and total NADP (NADPH + NADP+) were measured using enzymatic cycling methods as described previously (32) with modifications. Briefly, cells were lysed in 100 μl of extraction buffer (20 mm nicotinamide, 20 mm NaHCO3, and 100 mm Na2CO3) and centrifuged. For NADPH extraction, 40 μl of the supernatant was incubated at 60 °C for 30 min. The cell extract (5 μl) was added to a 96-well plate (Greiner Bio-One, catalog number 655180) containing 40 μl of NADP cycling buffer (100 mm Tris-HCl, pH 8.0, 0.5 mm thiazolyl blue, 2 mm phenazine ethosulfate, and 5 mm EDTA) containing 0.325 unit of glucose-6-phosphate dehydrogenase. After a 1-min incubation, 5 μl of 10 mm glucose 6-phosphate was added to the mixture, and the change in absorbance at 595 nm was measured every 5 min for 30 min at room temperature using a microplate reader. The NADP+/NADPH ratio was calculated as ([total NADP] − [NADPH])/[NADPH].
Data analysis
Data were analyzed using analysis of variance (ANOVA) with Tukey honest significant difference post hoc test. p < 0.05 was considered significant. Statistical analyses were performed using KaleidaGraph (Synergy Software).
Author contributions
H. K. and M. N. conceived and designed the study. H. K., T. G., and K. T. performed the experiments. H. K. wrote the manuscript.
Acknowledgments
We thank Dr. Yohei Katoh and Prof. Kazuhisa Nakayama for providing peSpCAS9(1.1)-2xsgRNA and pDonor-tBFP-NLS-Neo.
This work was supported by Grant-in-aid for Scientific Research 15K07043 from the Ministry of Education, Science, Sports, and Culture of Japan and a grant from Takeda Science Foundation. The authors declare that they have no conflicts of interest with the contents of this article.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party-hosted site.
- ROS
- reactive oxygen species
- LDH
- lactate dehydrogenase
- EAA
- essential amino acid
- NEAA
- nonessential amino acid
- SSZ
- sulfasalazine
- sgRNA
- single-guide RNA
- BPTES
- bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide
- dm-αKG
- dimethyl α-ketoglutarate
- EGCG
- epigallocatechin gallate
- BFP
- blue fluorescent protein
- NLS
- nuclear localization signal
- Z
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone
- MnTMPyP
- Mn(III) tetrakis(1-methyl-4-pyridyl)porphyrin
- TCA
- tricarboxylic acid
- CRISPR
- clustered regularly interspaced short palindromic repeats
- ANOVA
- analysis of variance
- sgCtrl
- control sgRNA.
References
- 1. Hanahan D., and Weinberg R. A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 2. Ward P. S., and Thompson C. B. (2012) Metabolic reprogramming: a cancer hallmarks even Warburg did not anticipate. Cancer Cell 21, 297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Furnari F. B., Fenton T., Bachoo R. M., Mukasa A., Stommel J. M., Stegh A., Hahn W. C., Ligon K. L., Louis D. N., Brennan C., Chin L., DePinho R. A., and Cavenee W. K. (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 21, 2683–2710 [DOI] [PubMed] [Google Scholar]
- 4. Elstrom R. L., Bauer D. E., Buzzai M., Karnauskas R., Harris M. H., Plas D. R., Zhuang H., Cinalli R. M., Alavi A., Rudin C. M., and Thompson C. B. (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 64, 3892–3899 [DOI] [PubMed] [Google Scholar]
- 5. Jelluma N., Yang X., Stokoe D., Evan G. I., Dansen T. B., and Haas-Kogan D. A. (2006) Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal astrocytes. Mol. Cancer Res. 4, 319–330 [DOI] [PubMed] [Google Scholar]
- 6. Graham N. A., Tahmasian M., Kohli B., Komisopoulou E., Zhu M., Vivanco I., Teitell M. A., Wu H., Ribas A., Lo R. S., Mellinghoff I. K., Mischel P. S., and Graeber T. G. (2012) Glucose deprivation activates a metabolic and signaling amplification loop to cell death. Mol. Syst. Biol. 8, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee Y. J., Galoforo S. S., Berns C. M., Chen J. C., Davis B. H., Sim J. E., Corry P. M., and Spitz D. R. (1998) Glucose deprivation-induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J. Biol. Chem. 273, 5294–5299 [DOI] [PubMed] [Google Scholar]
- 8. Ahmad I. M., Aykin-Burns N., Sim J. E., Walsh S. A., Higashikubo R., Buettner G. R., Venkataraman S., Mackey M. A., Flanagan S. W., Oberley L. W., and Spitz D. R. (2005) Mitochondrial O2˙̄ and H2O2 mediate glucose deprivation-induced cytotoxicity and oxidative stress in human cancer cells. J. Biol. Chem. 280, 4254–4263 [DOI] [PubMed] [Google Scholar]
- 9. Cairns R. A., Harris I. S., and Mak T. W. (2011) Regulation of cancer cell metabolism. Nat. Rev. Cancer 11, 85–95 [DOI] [PubMed] [Google Scholar]
- 10. Sabharwal S. S., and Schumacker P. T. (2014) Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat. Rev. Cancer 14, 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim J. Y., Kanai Y., Chairoungdua A., Cha S. H., Matsuo H., Kim D. K., Inatomi J., Sawa H., Ida Y., and Endou H. (2001) Human cysteine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochim. Biophys. Acta 1512, 335–344 [DOI] [PubMed] [Google Scholar]
- 12. Chung W. J., Lyons S. A., Nelson G. M., Hamza H., Gladson C. L., Gillespie G. Y., and Sontheimer H. (2005) Inhibition of cystine uptake disrupts the growth of primary brain tumors. J. Neurosci. 25, 7101–7110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Savaskan N. E., Heckel A., Hahnen E., Engelhorn T., Doerfler A., Ganslandt O., Nimsky C., Buchfelder M., and Eyüpoglu I. Y. (2008) Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat. Med. 14, 629–632 [DOI] [PubMed] [Google Scholar]
- 14. Takeuchi S., Wada K., Toyooka T., Shinomiya N., Shimazaki H., Nakanishi K., Nagatani K., Otani N., Osada H., Uozumi Y., Matsuo H., and Nawashiro H. (2013) Increased xCT expression correlates with tumor invasion and outcome in patients with glioblastoma. Neurosurgery 72, 33–41 [DOI] [PubMed] [Google Scholar]
- 15. Sleire L., Skeie B. S., Netland I. A., Førde H. E., Dodoo E., Selheim F., Leiss L., Heggdal J. I., Pedersen P. H., Wang J., and Enger P. Ø. (2015) Drug repurposing: sulfasalazine sensitizes glioma to gamma knife radiosurgery by blocking cystine uptake through system Xc−, leading to glutathione depletion. Oncogene 34, 5951–5959 [DOI] [PubMed] [Google Scholar]
- 16. Tsuchihashi K., Okazaki S., Ohmura M., Ishikawa M., Sampetrean O., Onishi N., Wakimoto H., Yoshikawa M., Seishima R., Iwasaki Y., Morikawa T., Abe S., Takao A., Shimizu M., Masuko T., et al. (2016) The EGF receptor promotes the malignant potential of glioma by regulating amino acid transport system xc(−). Cancer Res. 76, 2954–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polewski M. D., Reveron-Thornton R. F., Cherryholmes G. A., Marinov G. K., Cassady K., and Aboody K. S. (2016) Increased expression of system xc− in glioblastoma confers an altered metabolic state and temozolomide resistance. Mol. Cancer Res. 14, 1229–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verrey F., Closs E. I., Wagner C. A., Palacin M., Endou H., and Kanai Y. (2004) CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 447, 532–542 [DOI] [PubMed] [Google Scholar]
- 19. Conrad M., and Sato H. (2012) The oxidative stress-inducible cystine/glutamate antiporter, system xc−: cystine supplier and beyond. Amino Acids 42, 231–246 [DOI] [PubMed] [Google Scholar]
- 20. Lewerenz J., Hewett S. J., Huang Y., Lambros M., Gout P. W., Kalivas P. W., Massie A., Smolders I., Methner A., Pergande M., Smith S. B., Ganapathy V., and Maher P. (2013) The cystine/glutamate antiporter system xc− in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 18, 522–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dixon S. J., Lemberg K. M., Lamprecht M. R., Skouta R., Zaitsev E. M., Gleason C. E., Patel D. N., Bauer A. J., Cantley A. M., Yang W. S., Morrison B. 3rd, Stockwell B. R. (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang W. S., SriRamaratnam R., Welsch M. E., Shimada K., Skouta R., Viswanathan V. S., Cheah J. H., Clemons P. A., Shamji A. F., Clish C. B., Brown L. M., Girotti A. W., Cornish V. W., Schreiber S. L., and Stockwell B. R. (2014) Regulation of ferroptotic cancer death by GPX4. Cell 156, 317–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang W. S., and Stockwell B. R. (2016) Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 26, 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shin C. S., Mishra P., Watrous J. D., Carelli V., D'Aurelio M., Jain M., and Chan D. C. (2017) The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nat. Commun. 8, 15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koppula P., Zhang Y., Shi J., Li W., and Gan B. (2017) The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J. Biol. Chem. 292, 14240–14249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Briggs K. J., Koivunen P., Cao S., Backus K. M., Olenchock B. A., Patel H., Zhang Q., Signoretti S., Gerfen G. J., Richardson A. L., Witkiewicz A. K., Cravatt B. F., Clardy J., and Kaelin W. G. Jr. (2016) Paracrine induction of HIF by glutamate in breast cancer: EgIN1 senses cysteine. Cell 166, 126–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sato H., Shiiya A., Kimata M., Maebara K., Tamba M., Sakakura Y., Makino N., Sugiyama F., Yagami K., Moriguchi T., Takahashi S., and Bannai S. (2005) Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem. 280, 37423–37429 [DOI] [PubMed] [Google Scholar]
- 28. Shih A. Y., Erb H., Sun X., Toda S., Kalivas P. W., and Murphy T. H. (2006) Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J. Neurosci. 26, 10514–10523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nabeyama A., Kurita A., Asano K., Miyake Y., Yasuda T., Miura I., Nishitai G., Arakawa S., Shimizu S., Wakana S., Yoshida H., and Tanaka M. (2010) xCT deficiency accelerates chemically induced tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 6436–6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamasaki F., Hama S., Yoshioka H., Kajiwara Y., Yahara K., Sugiyama K., Heike Y., Arita K., and Kurisu K. (2003) Staurosporine-induced apoptosis is independent of p16 and p21 and achieved via arrest at G2/M and at G1 in U251MG human glioma cell line. Cancer Chemother. Pharmacol. 51, 271–283 [DOI] [PubMed] [Google Scholar]
- 31. Zanotto-Filho A., Braganhol E., Battastini A. M., and Moreira J. C. (2012) Proteasome inhibitor MG132 induces selective apoptosis in glioblastoma cells through inhibition of PI3K/Akt and NFκB pathways, mitochondrial dysfunction. And activation of p38-JNK1/2 signaling. Invest. New Drugs 30, 2252–2262 [DOI] [PubMed] [Google Scholar]
- 32. Hay N. (2016) Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat. Rev. Cancer 16, 635–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jeon S. M., Chandel N. S., and Hay N. (2012) AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485, 661–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang C., Sudderth J., Dang T., Bachoo R. M., Bachoo R. G., McDonald J. G., and DeBerardinis R. J. (2009) Glioblastoma cells require glutamine dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 69, 7986–7993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Son J., Lyssiotis C. A., Ying H., Wang X., Hua S., Ligorio M., Perera R. M., Ferrone C. R., Mullarky E., Shyh-Chang N., Kang Y., Fleming J. B., Bardeesy N., Asara J. M., Haigis M. C., et al. (2013) Glutamine supports pancreatic cancer cell growth through KRAS-regulated metabolic pathway. Nature 496, 101–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Altman B. J., Stine Z. E., and Dang C. V. (2016) From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 619–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muir A., Danai L. V., Gui D. Y., Waingarten C. Y., Lewis C. A., and Vander Heiden M. G. (2017) Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. eLife 6, e27713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mandal P. K., Seiler A., Perisic T., Kölle P., Banjac Canak A., Förster H., Weiss N., Kremmer E., Lieberman M. W., Bannai S., Kuhlencordt P., Sato H., Bornkamm G. W., and Conrad M. (2010) System xc− and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J. Biol. Chem. 285, 22244–22253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pader I., Sengupta R., Cebula M., Xu J., Lundberg J. O., Holmgren A., Johansson K., and Arnér E. S. (2014) Thioredoxin-related protein of 14 kDa is an efficient L-cystine reductase and S-denitrosylase. Proc. Natl. Acad. Sci. U.S.A. 111, 6964–6969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nogueira V., and Hay N. (2013) Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 19, 4309–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Panieri E., and Santoro M. M. (2016) ROS homeostasis and metabolism: a dangerous liaison in cancer cells. Cell Death Dis. 7, e2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Katoh Y., Michisaka S., Nozaki S., Funabashi T., Hirano T., Takei R., and Nakayama K. (2017) Practical method for targeted disruption of cilia-related genes by using CRISPR/Cas9-mediated homology-independent knock-in system. Mol. Biol. Cell 28, 898–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Doench J. G., Fusi N., Sullender M., Hegde M., Vaimberg E. W., Donovan K. F., Smith I., Tothova Z., Wilen C., Orchard R., Virgin H. W., Listgarten J., and Root D. E. (2016) Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]