Abstract
The Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ)/5′-AMP–activated protein kinase (AMPK) phosphorylation cascade affects various Ca2+-dependent metabolic pathways and cancer growth. Unlike recombinant CaMKKβ that exhibits higher basal activity (autonomous activity), activation of the CaMKKβ/AMPK signaling pathway requires increased intracellular Ca2+ concentrations. Moreover, the Ca2+/CaM dependence of CaMKKβ appears to arise from multiple phosphorylation events, including autophosphorylation and activities furnished by other protein kinases. However, the effects of proximal downstream kinases on CaMKKβ activity have not yet been evaluated. Here, we demonstrate feedback phosphorylation of CaMKKβ at multiple residues by CaMKKβ-activated AMPK in addition to autophosphorylation in vitro, leading to reduced autonomous, but not Ca2+/CaM-activated, CaMKKβ activity. MS analysis and site-directed mutagenesis of AMPK phosphorylation sites in CaMKKβ indicated that Thr144 phosphorylation by activated AMPK converts CaMKKβ into a Ca2+/CaM-dependent enzyme as shown by completely Ca2+/CaM-dependent CaMKK activity of a phosphomimetic T144E CaMKKβ mutant. CaMKKβ mutant analysis indicated that the C-terminal domain (residues 471–587), including the autoinhibitory region, plays an important role in stabilizing an inactive conformation in a Thr144 phosphorylation–dependent manner. Furthermore, immunoblot analysis with anti-phospho-Thr144 antibody revealed phosphorylation of Thr144 in CaMKKβ in transfected COS-7 cells that was further enhanced by exogenous expression of AMPKα. These results indicate that AMPK-mediated feedback phosphorylation of CaMKKβ regulates the CaMKKβ/AMPK signaling cascade and may be physiologically important for intracellular maintenance of Ca2+-dependent AMPK activation by CaMKKβ.
Keywords: AMP-activated kinase (AMPK), Ca2+/calmodulin-dependent protein kinase (CaMK), calmodulin (CaM), phosphorylation, protein kinase
Introduction
The enzymatic activity of the Ca2+/calmodulin-dependent protein kinase (CaMK)2 family member CaMK kinase (CaMKK) is enhanced by binding with a Ca2+/CaM complex (1–3). CaMKK comprises α and β isoforms in mammals (4–6) and is conserved among higher and lower eukaryotes, including Caenorhabditis elegans and Aspergillus nidulans (7, 8). Unlike other CaMKs, CaMKK specifically phosphorylates downstream protein kinases, including CaMKI, CaMKIV, and AMPK, at specific Thr residues in activation loops (Thr177 in CaMKIα, Thr196 in CaMKIV, and Thr172 in AMPKα) to significantly enhance the enzymatic activity (9–15). CaMKK affects various physiological responses dependent on multiple downstream target kinases. For example, CaMKK/CaMKI cascades play important roles in neuronal development processes, including activity-dependent dendritic arborization, synaptogenesis, and axon outgrowth and specification (16–21). The CaMKK/CaMKIV cascade also affects transcriptional activation by phosphorylating transcription factors such as the cAMP-response element–binding protein and serum response factor (4, 22–25). Recent studies have indicated the involvement of CaMKKβ/AMPK signaling in metabolic regulation, including appetite control (26), adiponectin-induced PGC-1α expression in C2C12 myocytes (27), thyroid hormone triiodothyronine stimulation of mitochondrial fatty acid oxidation (28), amino acid starvation–induced autophagy (29), and cancer growth (30, 31).
Of the two CaMKK isoforms, CaMKKβ was shown to be responsible for phosphorylation/activation of AMPK in vivo and in vitro (13–15, 32). Previously, we demonstrated that a single residue in subdomain VIII of the CaMKK catalytic domain (Leu358 in CaMKKβ/Ile322 in CaMKKα) at least partly conferred the distinct recognition of AMPK (33). Extensive in vitro and in vivo studies using gene knockdown and the pharmacological inhibitor STO-609 (34) demonstrated that CaMKKβ-mediated phosphorylation cascade activation is Ca2+-dependent (13–15). Whereas CaMKK phosphorylates downstream protein kinases in multiple signaling cascades, cAMP-dependent protein kinase phosphorylates residues in the N-terminal domain (Thr108) and CaM-binding domain (Ser458) of CaMKKα, thus facilitating the recruitment of 14-3-3 protein and suppression of CaMKK activity in vivo and in vitro (35–38). In contrast to CaMKKα, recombinant CaMKKβ exhibits a higher basal activity (i.e. in the absence of Ca2+/CaM) (6, 39). This is partly attributed to intramolecular autophosphorylation at Thr482, resulting in partial disruption of the autoinhibitory mechanism (40). In addition, the N-terminal regulatory region (residues 129–151) was found to affect the autonomous activity of rat CaMKKβ as the deletion of this domain conferred Ca2+/CaM dependence on the kinase (39).
Beyond autophosphorylation, cyclin-dependent kinase 5 (CDK5) and glycogen synthase kinase 3 (GSK3) can phosphorylate multiple residues in the N-terminal regulatory domain (Ser129, Ser133, and Ser137 in human CaMKKβ), resulting in decreased autonomous activity (41). This observation is in agreement with the finding that CaMKKβ/AMPK pathway activation requires Ca2+/CaM signaling, whereas the CaMKKβ substrate AMPK is not Ca2+/CaM-dependent (13–15, 32). According to those studies, the maintenance of CaMKKβ as a Ca2+/CaM-dependent form appears to depend on multiple phosphorylation events, including autophosphorylation and the effects of other protein kinases. Because the effects of closely proximal downstream kinases on the activity of CaMKKβ have not yet been evaluated, we attempted to examine this activity during CaMKKβ-mediated AMPK activation. Here, we observed that, in vitro, phosphorylation by CaMKKβ-activated AMPK significantly decreased autonomous CaMKKβ activity. We also identified a single AMPK phosphorylation site in the N-terminal regulatory domain of CaMKKβ that acts as a switch for Ca2+/CaM dependence and suggests a unique enzymatic mechanism of CaMKKβ/AMPK signaling cascade regulation.
Results
Autonomous activity of recombinant CaMKKβ is suppressed during AMPK activation
Although recombinant CaMKKβ has been shown to exhibit a higher basal activity in the absence of Ca2+/CaM (autonomous activity) (6, 39), in intact cells CaMKKβ signaling activation requires an increased intracellular Ca2+ concentration (13–15, 32). Therefore, we first examined the activity of Escherichia coli-expressed CaMKKβ during a CaMKKβ-mediated AMPK activation reaction in which we incubated CaMKKβ with either wild-type or kinase-dead mutant (K45R) AMPK in the presence of Mg-ATP and EGTA for various time points (5–60 min) followed by the withdrawal of constant amounts of reaction mixture into buffer containing excess EDTA to stop the phosphorylation reaction. Subsequently, CaMKKβ activity levels in the samples were measured by a 10-min kinase reaction assay with glutathione S-transferase (GST)–tagged CaMKIα(1–293) K49E as the substrate in the absence of Ca2+/CaM (autonomous activity). When 100 μm [γ-32P]ATP was used (Fig. 1A), CaMKKβ autonomous activity decreased gradually in the presence of wild-type AMPK but was not affected by incubation with AMPK K45R mutant. To confirm that GST-CaMKIα(1–293) K49E phosphorylation was mediated by CaMKKβ at Thr177 (CaMKK phosphorylation site), we conducted a 5-min kinase reaction assay using non-radioisotopic ATP (Fig. 1B) followed by a dot-blotting assay and antibody-mediated detection of Thr177 phosphorylation in GST-CaMKIα(1–293) K49E (Fig. 1B, inset). The similar results obtained from these two different CaMKK activity assays confirmed the suppression of CaMKKβ autonomous activity by wild-type AMPK. We confirmed that AMPKα subunit was activated through phosphorylation at Thr172 by CaMKKβ during the incubation periods (see supplemental Fig. S1).
Figure 1.
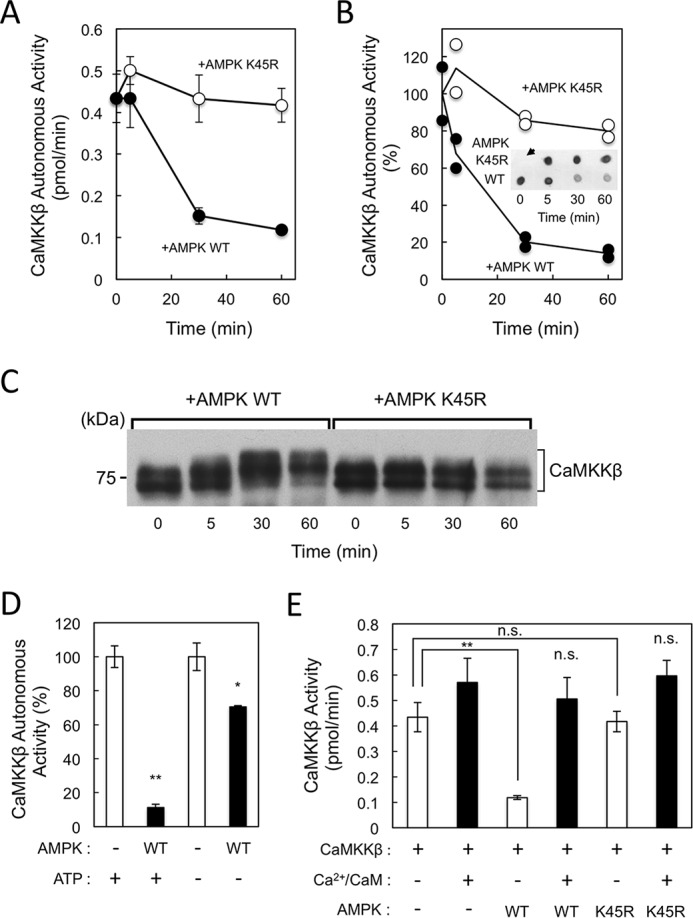
Suppression of the autonomous activity of CaMKKβ by AMPK phosphorylation. Recombinant CaMKKβ (1.2 μg) was incubated with either wild-type (WT) (closed circles) or K45R mutant (open circles) AMPK (1.2 μg) at 30 °C for the indicated time periods in a solution (20 μl) containing 50 mm HEPES, pH 7.5, 10 mm Mg(CH3COO)2, 1 mm DTT, and 1 mm ATP in the presence of 2 mm EGTA, and then the reaction was terminated followed by measuring CaMKKβ activity using GST-CaMKIα(1–293) K49E as a substrate in the presence of 2 mm EGTA and either 100 μm [γ-32P]ATP (A) or 100 μm ATP (B; dot-blot assay). B, inset, shows results of one set of dot-blot assays using anti-phospho-CaMKI (at Thr177) antibody. An arrow indicates the no-enzyme control. Autonomous activities of CaMKKβ in B are expressed as a percentage of the average value at 0 min, and the results represent two sets of dot-blot assays. C, reaction mixtures (50 ng of CaMKKβ) as shown in B were subjected to SDS-7.5% PAGE followed by immunoblot analysis using an anti-CaMKK antibody. The molecular mass in kilodaltons is indicated on the left. D, recombinant CaMKKβ (1.2 μg) was incubated without (−) or with WT AMPK (1.2 μg) at 30 °C for 60 min in a solution (20 μl) containing 50 mm HEPES, pH 7.5, 10 mm Mg(CH3COO)2, and 1 mm DTT in the presence of 2 mm EGTA either without (−) or with (+) 1 mm ATP, and then the reaction was terminated followed by measuring CaMKKβ activity using a dot-blot assay as described in B. Autonomous activities of CaMKKβ are expressed as a percentage of the average value in the absence of AMPK. E, recombinant CaMKKβ (1.2 μg) was incubated without (−) or with either WT or K45R mutant AMPK (1.2 μg) at 30 °C for 60 min in a solution (20 μl) containing 50 mm HEPES, pH 7.5, 10 mm Mg(CH3COO)2, and 1 mm DTT in the presence of 2 mm EGTA and 1 mm ATP, and then the reaction was terminated followed by measuring CaMKKβ activity using 100 μm [γ-32P]ATP in the presence of 2 mm EGTA (−) (white bars) or 2 mm CaCl2 and 6 μm CaM (+) (black bars). Results in A, D, and E are expressed as the mean ± S.D. of three experiments. Error bars represent S.D. Statistical differences are marked: *, p < 0.05, **, p < 0.01, n.s., not significant.
When samples collected at various time points (0–60 min) as shown in Fig. 1B were analyzed by immunoblotting using an anti-CaMKK antibody (Fig. 1C), the electrophoretic mobility of the CaMKKβ on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gradually shifted upward in the presence of wild-type AMPK but not K45R mutant enzyme, suggesting that this shift was mediated by AMPK-catalyzed phosphorylation. When we incubated wild-type AMPK and CaMKKβ without ATP, CaMKKβ autonomous activity decreased slightly (∼25%) relative to the 90% reduction observed in the presence of 1 mm ATP, indicating that the CaMKKβ-mediated, phosphorylation-dependent activation of AMPK is required for the subsequent inhibition of CaMKKβ activity by activated AMPK (Fig. 1D). The slight reduction in CaMKKβ activity in the absence of ATP was likely due to the phosphorylation of CaMKKβ by activated AMPK during the subsequent CaMKKβ activity assay. These results suggest that, following CaMKKβ-mediated phosphorylation, activated AMPK subsequently phosphorylates CaMKKβ to suppress its autonomous activity.
CaMKKβ catalytic activity is not affected by AMPK phosphorylation
To clarify the molecular mechanism by which activated AMPK suppresses CaMKKβ autonomous activity, we produced unphosphorylated (without AMPK or with AMPK K45R mutant) and phosphorylated CaMKKβ (with activated AMPK) and measured the Ca2+/CaM dependence of these enzymes (Fig. 1E). Consistent with Fig. 1, A and B, AMPK-mediated phosphorylation significantly suppressed the basal activity (autonomous activity) of recombinant CaMKKβ but did not affect the total activity in the presence of Ca2+/CaM. In other words, phosphorylation by AMPK was unable to suppress CaMKKβ catalytic activity but could convert the enzyme into a Ca2+/CaM-dependent kinase. We also performed a time-course experiment to confirm that the total activity of CaMKKβ was not altered by incubation with AMPK in the presence of Ca2+/CaM (supplemental Fig. S3).
AMPK phosphorylation of CaMKKβ Thr144 is involved in the suppression of autonomous activity
We next attempted to identify both autophosphorylation and AMPK phosphorylation sites in CaMKKβ via liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis to determine which were involved in the suppression of autonomous activity. In addition to seven autophosphorylation sites, we identified multiple AMPK phosphorylation sites, including Ser110, Ser126, Ser135, Thr144, Ser174, Thr446, Ser494, Ser510, and Ser552, in recombinant CaMKKβ incubated with K45R (autophosphorylation) or wild-type AMPK (autophosphorylation plus AMPK phosphorylation) in the presence of Mg-ATP at 30 °C for 60 min (Table 1, Fig. 2A, and supplemental Fig. S2). Because AMPK-catalyzed phosphorylation significantly reduced the Ca2+/CaM-independent activity (autonomous activity) of CaMKKβ without affecting its catalytic activity (Fig. 1), we first constructed three Ala-substituted mutants (S135A, T144A, and S494A) in which the phosphorylation sites were located either in the N-terminal regulatory region (residues 129–151) or the C-terminal autoinhibitory domain (AID) (residues 474–499). After expression and purification, these mutant CaMKKβs were evaluated to determine the effects of AMPK on autonomous activity (Fig. 2B). Whereas AMPK suppressed the autonomous activities of the S135A and S494A mutants in a manner similar to the suppression of wild-type CaMKKβ (Fig. 2B), the T144A mutant was unaffected, indicating that CaMKKβ autonomous activity is suppressed by AMPK through direct phosphorylation at Thr144. This conclusion was confirmed by the finding that a phosphomimetic mutant (T144E) of CaMKKβ was a completely Ca2+/CaM-dependent enzyme and exhibited no significant autonomous activity (Fig. 2C) compared with wild type (70–80% of total activity) and T144A CaMKKβ (∼50% of total activity). We measured specific activities in the presence of Ca2+/CaM of all the point mutants (S135A, 580 ± 31 nmol/min/mg; T144A, 390 ± 20 nmol/min/mg; S494A, 394 ± 18 nmol/min/mg; and T144E, 529 ± 16 nmol/min/mg) and wild-type CaMKKβ (515 ± 42 nmol/min/mg) using a 5-min standard CaMKK activity assay as described under “Experimental procedures,” indicating that the mutant CaMKKβs have total activities comparable with that of wild-type enzyme.
Table 1.
Identification of autophosphorylation and AMPK phosphorylation sites in rat CaMKKβ
Recombinant rat CaMKKβ (E. coli) was phosphorylated by either wild-type or kinase-dead mutant (K45R) AMPK for 60 min followed by LC-MS/MS analysis to identify the autophosphorylation (Autophos. site) and AMPK phosphorylation sites (AMPK site) as described under “Experimental procedures” (see supplemental Fig. S2).
| Phosphorylation | Residues | Peptide sequence | Phospho-amino acid residues |
|---|---|---|---|
| Autophos. site | 14–27 | AAPQDELG(p)SGGVSR | Ser22a |
| Autophos. site | 83–98 | DASEPESRSLL(p)SGGKM | Ser94 |
| AMPK site | 104–119 | SQGGPA(p)SSSSLDMNGR | Ser110 |
| AMPK site | 120–138 | CICPSL(p)SYSPASSPQSSPR | Ser126 |
| AMPK site | 128–138 | SPASSPQ(p)SSPR | Ser135 |
| AMPK site | 142–148 | RP(p)TVESH | Thr144 |
| Autophos. site | 165–175 | (p)TLKDEIGKGSY | Thr165 |
| AMPK site | 168–179 | DEIGKG(p)SYGVVK | Ser174 |
| Autophos. site | 214–226 | G(p)TRPAPGGCIQPR | Thr215a |
| AMPK site | 445–456 | V(p)TRHGAEPLPSE | Thr446 |
| Autophos. site | 473–485 | SVKHIPSLA(p)TVIL | Thr482a |
| AMPK site | 494–503 | (p)SFGNPFEGSR | Ser494 |
| AMPK site | 508–522c | SL(p)SAPGNLL(p)TKKPTR | Ser510 |
| Autophos. site | 508–522 | SLSAPGNLL(p)TKKPTR | Thr517b |
| AMPK site | 544–556 | ASPCGGGG(p)SALVK | Ser552 |
| Autophos. site | 557–574 | GGPCVE(p)SCGAPAPGSPPR | Ser563 |
a Autophosphorylation sites of rat CaMKKβ were identified previously (40).
b Autophosphorylation of Thr517 was identified in rat CaMKKβ incubated with AMPK K45R mutant.
Figure 2.
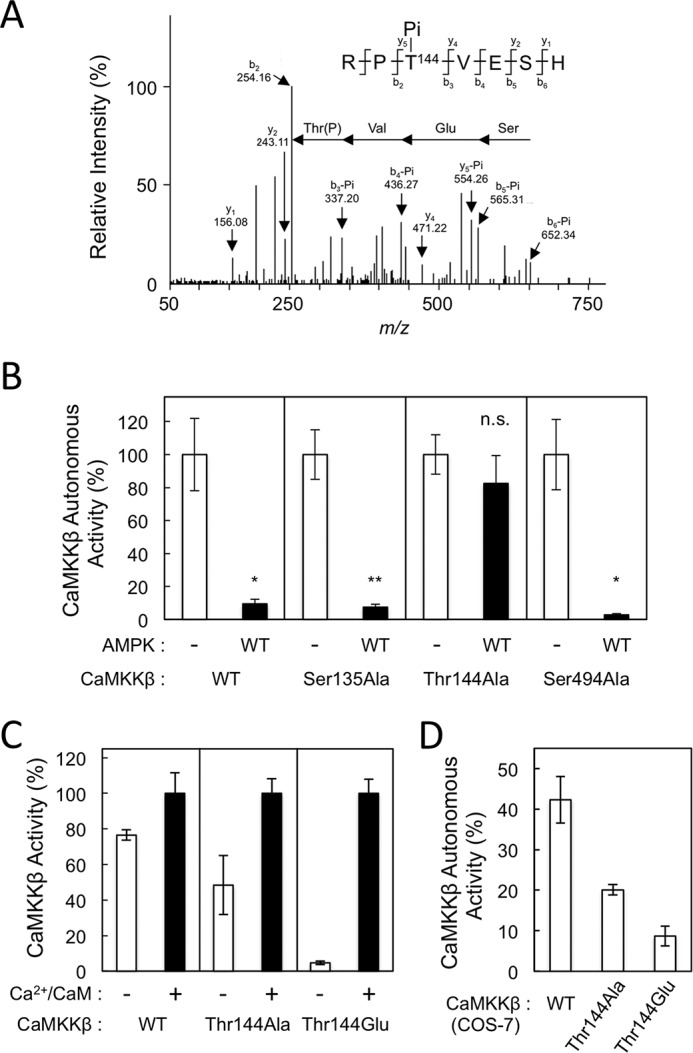
Identification of Thr144 in CaMKKβ as an AMPK phosphorylation site involved in reduction of the autonomous activity. A, recombinant CaMKKβ phosphorylated by AMPK for 60 min was subjected to SDS-PAGE, digested with a protease mixture, and analyzed by LC-MS/MS, resulting in identification of phospho-Thr144. The doubly charged ion of a peptide (residues 142–148) derived from CaMKKβ was subjected to MS/MS analysis as described under “Experimental procedures.” The observed b-ion and y-ion fragment series generated by collision-induced dissociation are indicated by arrows. The observed fragment ions are indicated above and below the peptide sequence. B, recombinant CaMKKβ mutants (S135A, T144A, and S494A) and WT enzyme were incubated without (−; white columns) or with 1.2 μg of AMPK WT (black columns) at 30 °C for 60 min in a solution (20 μl) containing 50 mm HEPES, pH 7.5, 10 mm Mg(CH3COO)2, and 1 mm DTT in the presence of 2 mm EGTA and 1 mm ATP, and then the reaction was terminated followed by measuring CaMKKβ autonomous activities using dot-blot assay in the presence of 2 mm EGTA. Autonomous activities of CaMKKβs in B are expressed as a percentage of the average value in the absence of AMPK. C, protein kinase activities of recombinant CaMKKβ mutants (T144A and T144E; 12 ng) and WT enzyme (12 ng) were measured in the presence of 2 mm EGTA (−; white columns) or 2 mm CaCl2 and 6 μm CaM (+; black columns) using a dot-blot assay as described under “Experimental procedures.” D, autonomous activities of COS-7 cells expressing CaMKKβ Thr144 mutants. CaMKKβs, including WT, T144A, and T144E, were expressed in COS-7 cells, and both autonomous (in the presence of EGTA) and total (in the presence of Ca2+/CaM) CaMKK activities of each cell lysate (1 μl) were measured as described in C except that 100 μm [γ-32P]ATP was used. CaMKKβ activities in C and D are expressed as a percentage of the average value in the presence of Ca2+/CaM. The results are expressed as the mean ± S.D. of three experiments. Error bars represent S.D. Statistical differences are marked: *, p < 0.05 versus the autonomous activities of non-treated enzymes; **, p < 0.01 versus the autonomous activity of non-treated enzyme; n.s., not significant.
In addition, we expressed CaMKKβ, including wild type and Thr144 mutants, in COS-7 cells and measured CaMKK activities using an equal volume of cell lysates (1 μl) (Fig. 2D). Whereas total activities (in the presence of Ca2+/CaM) of exogenously expressed CaMKKβ, including wild type (7.8 ± 1.9 pmol/min/μl of lysate), T144A mutant (7.8 ± 0.3 pmol/min/μl of lysate), and T144E mutant (7.9 ± 0.3 pmol/min/μl of lysate), are indistinguishable, we observed significant autonomous activity (∼40% of total activity) of wild-type enzyme expressed in COS-7 cells that was consistent with a previous report (39), and the Glu mutation significantly reduced the basal activity (∼10% of total activity). This is in good agreement with the results using E. coli-expressed CaMKKβ as shown in Fig. 2C.
Phosphorylation of CaMKKβ at Thr144 in vitro and in living cells
To confirm the physiologic significance of Thr144 phosphorylation of CaMKKβ, we generated a monoclonal antibody that specifically recognized a phosphorylated form of CaMKKβ at Thr144 as described under “Experimental procedures.” Immunoblot analysis (Fig. 3A, top panel) revealed that the antibody recognized CaMKKβ phosphorylated with wild-type AMPK but not an autophosphorylated CaMKKβ. In addition, CaMKKβ mutant T144A phosphorylated with wild-type AMPK was not recognized by the antibody, indicating that the antibody is capable of specifically recognizing the Thr144-phosphorylated form of CaMKKβ. Then we analyzed cell lysates of COS-7 cells transfected with rat CaMKKβ expression plasmid with or without HA-AMPKα expression plasmid by immunoblotting using the anti-phospho-Thr144 antibody. Fig. 3B (top and middle panels) clearly shows that the exogenously expressed CaMKKβ in COS-7 cells was phosphorylated at Thr144, and this phosphorylation was induced 3–7-fold by coexpression of HA-AMPKα, indicating the AMPK-mediated phosphorylation of CaMKKβ at Thr144 in living cells as well as in vitro. We confirmed the exogenous expression of HA-AMPKα in the cells (Fig. 3B, bottom panel) and that T144A mutant coexpressed with HA-AMPKα was not detected by the immunoblot analysis using the anti-phospho-Thr144 antibody (Fig. 3B, top panel) in good agreement with the result shown in Fig. 3A.
Figure 3.
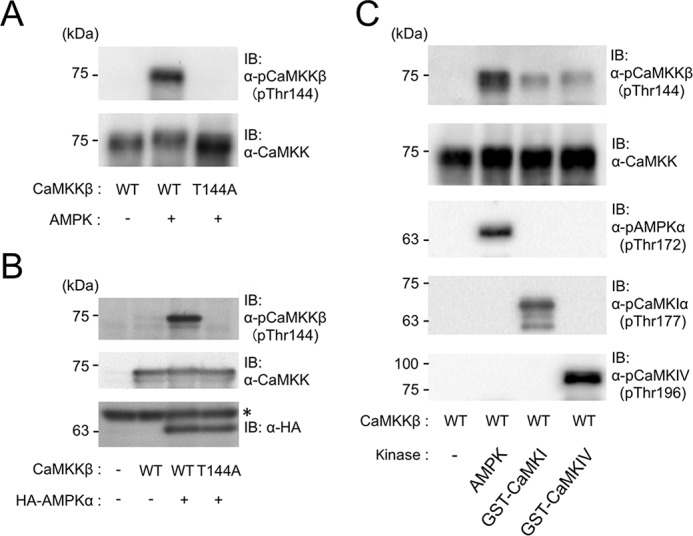
Phosphorylation of Thr144 in CaMKKβ in vitro and in living cells. A, specificity of anti-phospho-Thr144 CaMKKβ antibody. Recombinant CaMKKβ (1.2 μg) WT or T144A mutant was incubated without (−) or with (+) AMPK wild type (1.2 μg) in the presence of 1 mm ATP and 2 mm EGTA at 30 °C for 60 min as described in Fig. 1 legend. Then 100 ng of CaMKKβ was analyzed by immunoblotting with either anti-CaMKK antibody (lower panel) or anti-phospho-Thr144 CaMKKβ antibody (upper panel). B, COS-7 cells were transfected without (−) or with either WT or T144A CaMKKβ expression plasmid together with an empty vector (−) or HA-AMPKα expression plasmid (+) as described under “Experimental procedures,” and then cell lysates (10 μl) were analyzed by immunoblotting using anti-phospho-CaMKKβ (at Thr144) antibody (upper panel), anti-CaMKK antibody (middle panel), or anti-HA antibody (lower panel). The asterisk in the lower panel indicates endogenous proteins in COS-7 cells that nonspecifically bound to the primary antibody. C, recombinant CaMKKβ (1.2 μg) was incubated without (−) or with 1.2 μg of either wild-type AMPK (AMPK), GST-rat CaMKIα (GST-CaMKI), or GST-mouse CaMKIV (GST-CaMKIV) at 30 °C for 60 min in the presence of 1 mm ATP, 2 mm CaCl2, and 6 μm CaM as described in Fig. 1 legend. Then 180 ng of CaMKKβ was analyzed by immunoblotting with the indicated antibodies, including anti-phospho-CaMKKβ (at Thr144), anti-CaMKK, anti-phospho-AMPK (at Thr172), anti-phospho-CaMKI (at Thr177), and anti-phospho-CaMKIV (at Thr196) antibodies. The molecular masses in kilodaltons are indicated on the left. IB, immunoblot.
To test whether other CaMKK target kinases are capable of phosphorylating the Thr144 in CaMKKβ, we prepared CaMKKβ samples incubated with its downstream protein kinases, including GST-rat CaMKIα and GST-mouse CaMKIV, in the presence of Ca2+/CaM and Mg-ATP in vitro (Fig. 3C) followed by immunoblot analyses. Both downstream CaMKs were capable of phosphorylating CaMKKβ at Thr144 but less efficiently than AMPK (Fig. 3C, top panel). We confirmed that both downstream CaMKs were activated by phosphorylation of their activation-loop Thr residues (Thr177 in CaMKI and Thr196 in CaMKIV) with CaMKKβ during the incubation process in a similar manner to AMPK phosphorylation at Thr172 (Fig. 3C, middle panel), indicating that the feedback phosphorylation of CaMKKβ at Thr144 was catalyzed by activated CaMKK target kinases.
Phosphorylation-dependent reduction of CaMKKβ autonomous activity involves the C terminus
Our data, which suggest that Thr144 phosphorylation converts CaMKKβ to a Ca2+/CaM-dependent form, are in good agreement with many previous studies in which CaMKKβ activity was shown to require increases in intracellular Ca2+ concentration (13–15, 32). We next attempted to elucidate the molecular mechanism underlying the effect of Thr144 phosphorylation on the enzymatic regulation of CaMKKβ by using a C-terminal truncation mutant (Fig. 4A) that had been phosphorylated or not by AMPK. A CaMKKβ(2–470) molecule that lacked the C-terminal regulatory domain (Val474–Phe499), which contains an AID and CaM-binding region, was constitutively active (39). We confirmed that both the wild-type and CaMKKβ(2–470) mutant enzymes were phosphorylated at Thr144 by AMPK (Fig. 4C). In contrast to wild-type CaMKKβ whose basal activity was significantly reduced by AMPK-catalyzed phosphorylation, the C-terminal truncation mutant CaMKKβ(2–470) did not respond to the Thr144 phosphorylation by AMPK (Fig. 4B), indicating that the C-terminal region, including the AID (residues 474–499), plays an important Thr144 phosphorylation–dependent role in constructing an inactive conformation. This result also confirmed that the phosphorylation by AMPK was unable to suppress CaMKKβ total activity in the presence of Ca2+/CaM as shown in Fig. 1E and supplemental Fig. S3.
Figure 4.
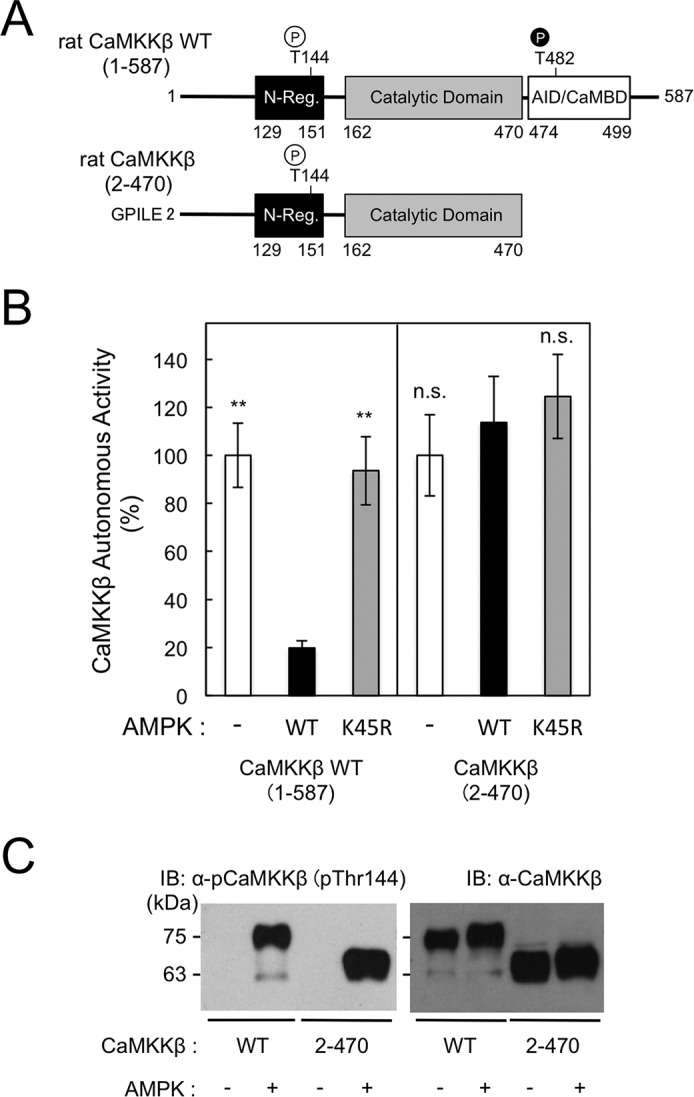
C-terminal region of CaMKKβ is involved in phosphorylation-dependent reduction of the autonomous activity. A, schematic representation of wild-type and a C-terminal truncation mutant of CaMKKβ. The amino acid sequence (Gly-Pro-Ile-Leu-Glu) at the digested N-terminal end of CaMKKβ mutant is indicated. Residue numbers in CaMKKβs are indicated. N-Reg., the N-terminal regulatory domain (39); CaMBD, CaM-binding domain (46). A site (Thr144) phosphorylated by AMPK and an autophosphorylation site (Thr482) (40) are indicated. B, CaMKKβ WT and C-terminal truncation mutant (CaMKKβ(2–470)) were incubated without (−; white columns) or with either WT (black columns) or K45R mutant (gray columns) AMPK (1.2 μg) at 30 °C for 60 min in a solution (20 μl) containing 50 mm HEPES, pH 7.5, 10 mm Mg(CH3COO)2, and 1 mm DTT in the presence of 2 mm EGTA and 1 mm ATP, and then the reaction was terminated followed by measuring CaMKKβ autonomous activity using a dot-blot assay in the presence of 2 mm EGTA. Autonomous activities of CaMKKβs in B are expressed as a percentage of the average value in the absence of AMPK. Results are expressed as the mean ± S.D. of three experiments. Error bars represent S.D. Statistical differences are marked: **, p < 0.01 versus the autonomous activities of the enzymes treated with AMPK wild type; n.s., not significant. C, recombinant rat CaMKKβ WT or CaMKKβ(2–470) mutant (2–470) was phosphorylated without (−) or with AMPK wild type in the presence of 1 mm ATP for 60 min as described in B followed by immunoblot analysis (120 ng of CaMKKβ) using either anti-phospho-Thr144 antibody (left panel) or anti-CaMKKβ antibody (right panel) as described under “Experimental procedures.” The molecular masses in kilodaltons are indicated on the left. IB, immunoblot.
Discussion
Our biochemical data demonstrate that the autonomous activity (in the absence of Ca2+/CaM) of CaMKKβ is reduced by an activated downstream kinase, AMPK, via feedback phosphorylation at Thr144. Thr144 phosphorylation converts CaMKKβ into a Ca2+/CaM-dependent enzyme, corroborating previous studies in which the activation of CaMKKβ-mediated signaling cascades, including AMPK, CaMKI, and CaMKIV pathways, was found to depend on increasing concentrations of intracellular Ca2+ (Fig. 5) (13–15, 32). This is a unique phosphorylation-dependent regulatory mechanism among CaMKs. CaMKII is known to undergo autophosphorylation at Thr286 (α-subunit within the AID) and thus induce autonomous activity (42–44). In the case of CaMKIV, Thr196 phosphorylation by CaMKK reduces the interaction of the catalytic core with the AID, resulting in generation of the autonomous activity (45). Unlike CaMKKα expressed in E. coli or COS-7 cells, which was found to be completely Ca2+/CaM-dependent kinase due to an autoinhibitory mechanism (46), CaMKKβ exhibits significant autonomous activity (70–80% of total activity), resulting from the impairment of a regulatory domain (residues 474–499)–mediated autoinhibitory mechanism (6, 39).
Figure 5.
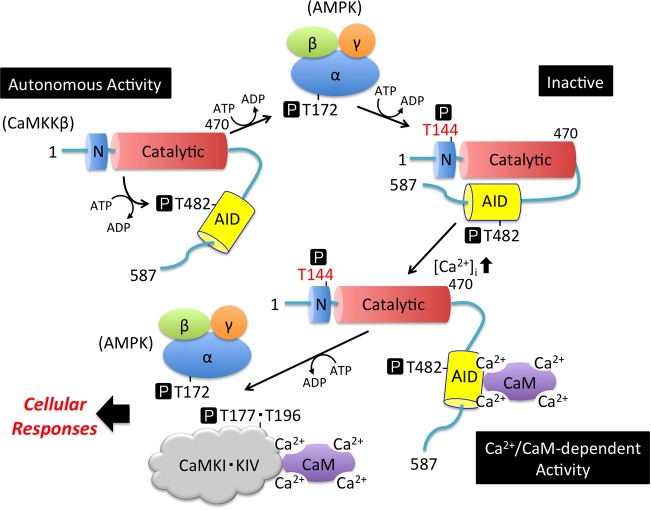
Schematic representation of the enzymatic regulation of CaMKKβ by AMPK through feedback phosphorylation at Thr144. CaMKKβ undergoes intramolecular autophosphorylation at Thr482 (Table 1), resulting in generation of the autonomous activity together with the function of the N-terminal regulatory domain (Autonomous Activity) (40). CaMKKβ phosphorylates the α-subunit of AMPK at Thr172 to activate its catalytic activity. Activated AMPK phosphorylates CaMKKβ at Thr144 to convert the enzyme to its inactive form in the absence of Ca2+/CaM (Inactive). Phosphorylated CaMKKβ is fully activated by Ca2+/CaM binding (Ca2+/CaM-dependent Activity) to phosphorylate and activate downstream protein kinases, including CaMKI, CaMKIV, and AMPK. N, N-terminal regulatory domain (residues 129–151) (39); Catalytic, catalytic domain (residues 162–470); AID, autoinhibitory domain containing the Ca2+/CaM-binding region (residues 474–499) (46); α/β/γ, α-subunit/β-subunit/γ-subunit of AMPK; CaMKI·KIV, Ca2+/CaM-dependent protein kinases I and IV; T, Thr residue. P in a black box indicates phosphorylation.
Previously, we have identified involvement of the N-terminal regulatory domain (residues 129–151) in rat CaMKKβ autonomous activity as deletion of this domain converts CaMKKβ into a Ca2+/CaM-dependent kinase (39). Rat CaMKKβ expressed in E. coli is highly autophosphorylated, and the Ca2+/CaM-independent, intramolecular phosphorylation of Thr482 in the AID (residues 474–499) was also detected in the present study (Table 1 and supplemental Fig. S2). Thr482 autophosphorylation, together with the N-terminal regulatory domain, results in Ca2+/CaM-independent, or basal, activity (Fig. 5, Autonomous Activity) (40). In this report, we found that the phosphorylation by AMPK at Thr144, within the N-terminal regulatory domain (residues 129–151), largely reduced the basal activity of CaMKKβ without significantly affecting the catalytic activity (Fig. 1E and Fig. 5, Inactive). This suggests that Thr144 phosphorylation disrupts the function of the N-terminal regulatory domain, which is necessary for autonomous activity (39), thus converting CaMKKβ into a Ca2+/CaM-dependent enzyme (Fig. 5, Ca2+/CaM-dependent Activity). This suggestion was confirmed by the finding that the enzymatic activity of a CaMKKβ mutant containing a single Thr144 phosphomimetic (Glu) mutation was entirely Ca2+/CaM-dependent (Fig. 2, C and D). Our observations agree well with an analogous mechanism demonstrated by Green et al. (41) in which the phosphorylation of multiple residues (Ser129, Ser133, and Ser137) in the N-terminal regulatory domain of human CaMKKβ by CDK5 and GSK3 led to decreased autonomous activity.
Notably, the CDK5 and GSK3 phosphorylation sites (Ser128, Ser132, and Ser136) in rat CaMKKβ were not phosphorylated by activated AMPK in vitro (Table 1). Our results suggest that the regulation of CaMKKβ by phosphorylation via activated AMPK might be physiologically relevant because AMPK is a direct downstream kinase in close proximity to CaMKKβ. This is confirmed by the fact that the exogenously expressed CaMKKβ in cultured cells has been shown to be phosphorylated at Thr144, and this phosphorylation was further enhanced by coexpression of AMPKα (Fig. 3B). We also observed significant Ca2+/CaM dependence of the T144E mutant CaMKKβ expressed in COS-7 cells (Fig. 2D). However, we cannot exclude the possibility that Thr144 phosphorylation is mediated by other downstream protein kinases, including CaMKI, CaMKIV, and another unidentified cellular protein kinase, because an equivalent Thr residue (Thr108) in CaMKKα has been shown to be phosphorylated by cAMP-dependent protein kinase (35, 36). Therefore, we tested the Thr144 phosphorylation in CaMKKβ by downstream kinases, including CaMKI and CaMKIV, in vitro (Fig. 3C). Immunoblot analysis revealed that both CaMKI and CaMKIV activated by CaMKKβ-mediated phosphorylation are capable of feedback-phosphorylating Thr144 in CaMKKβ but less efficiently than the activated AMPK. It would be interesting to determine whether Thr144 phosphorylation of CaMKKβ is regulated in a cellular context. Although the N-terminal regulatory domain containing Thr144 (in rat CaMKKβ) is conserved in various mammalian species (5, 6), a detailed mechanism underlying the release of the AID from the catalytic core by the N-terminal regulatory domain, thus resulting in the generation of autonomous activity, remains unclear (39). However, when we deleted the C-terminal region (residues 471–587), including the AID, of CaMKKβ, the autonomous activity of the mutant (CaMKKβ(2–470)) was no longer affected by AMPK-catalyzed Thr144 phosphorylation, even in the presence of the N-terminal regulatory domain, suggesting that the C-terminal region, including the AID, is involved in the Thr144 phosphorylation–dependent reduction of the autonomous activity (Fig. 4). We propose that the N-terminal regulatory region (residues 129–151) interacts with the C-terminal region (residues 471–587) in a Thr144 phosphorylation–dependent manner (Fig. 5, Inactive), thus promoting the interaction of the catalytic domain (residues 162–470) with the AID (residues 474–499). As intracellular Ca2+ levels increase in response to extracellular stimulation, Ca2+/CaM binds to the C terminus of AID and disrupts the interaction of the catalytic domain with AID to generate CaMKKβ kinase activity (Fig. 5, Ca2+/CaM-dependent Activity) (46), thus constituting a Ca2+-dependent signal transduction pathway. Further study is needed to verify this hypothesis and clarify how the phosphorylation of a single Thr144 would promote the autoinhibition of CaMKKβ and thus generate Ca2+/CaM dependence.
Experimental procedures
Materials
Recombinant rat CaMKKβ forms, including wild type and various site-directed mutants, were expressed in E. coli BL21 Star (DE3) cells and purified via chromatography with CaM-Sepharose and Q-Sepharose (39). GST-tagged rat CaMKKβ(2–470) was constructed using a pGEX-KG-PreS vector and expressed in E. coli BL21 Star (DE3) followed by purification via glutathione-Sepharose chromatography. GST tags were removed by treatment with PreScission protease (GE Healthcare) followed by purification on a glutathione-Sepharose column chromatography (39). GST-rat CaMKIα and GST-mouse CaMKIV were expressed and purified as described previously (45). GST-rat CaMKIα(1–293), K49E (GST-CaMKIα(1–293) K49E) was expressed in E. coli JM109 and purified as described previously (46). Recombinant wild-type AMPK and K45R mutant were expressed in E. coli BL21-CodonPlus (DE3) (Stratagene, La Jolla, CA) using a tricistronic pγ1β1His-α1 plasmid (kindly provided by Dr. Dietbert Neumann, Swiss Federal Institute of Technology, Zurich, Switzerland) and purified as described previously (47). Recombinant rat CaM was expressed in E. coli BL21 (DE3) using the plasmid pET-CaM (kindly provided by Dr. Nobuhiro Hayashi, Tokyo Institute of Technology, Yokohama, Japan) and purified as described previously (48). Anti-phospho-CaMKI (at Thr177) and anti-phospho-CaMKIV (at Thr196) monoclonal antibodies were generated as described previously (45). Anti-phospho-AMPKα (at Thr172) (clone 13E3) and anti-phospho-CaMKKβ (at Thr144) (clone A04) monoclonal antibodies were generated against synthetic phosphopeptides corresponding to residues 164–179 of rat AMPKα (MSDGEFLRpTSCGSPNYC where pT is phosphothreonine) and residues 137–151 of rat CaMKKβ (CPRMPRRPpTVESHHVS), respectively. Each peptide was conjugated with keyhole limpet hemocyanin via C-terminal or N-terminal cysteine and injected into BALB/c mice as described previously (49). Anti-AMPKα and anti-CaMKK antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA) and BD Transduction Laboratories, respectively. Anti-CaMKKβ antibody (sc-50341) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All other chemicals were obtained from standard commercial sources.
AMPK phosphorylation of CaMKKβ
Recombinant CaMKKβ (1.2 μg) was incubated without or with downstream protein kinases (1.2 μg), including wild-type AMPK, K45R mutant, GST-CaMKIα, and GST-CaMKIV, at 30 °C for the indicated time periods in a solution (20 μl) containing 50 mm HEPES, pH 7.5, 10 mm Mg(CH3COO)2, 1 mm DTT, and 1 mm ATP in the presence of 2 mm EGTA or 2 mm CaCl2 and 6 μm CaM. Reactions were terminated by a 25-fold dilution in ice-cold dilution buffer (50 mm HEPES, pH 7.5, 2 mg/ml bovine serum albumin, 10% ethylene glycol, and 2 mm EDTA) for the CaMKK activity assay or the addition of 5 or 20 μl of 2× SDS-PAGE sample buffer for either mass spectrometry or immunoblot analysis.
In vitro CaMKK activity assay
CaMKK activity was measured at 30 °C for 10 or 5 min (for dot-blot assay) in a solution (20 μl) containing 12 ng of CaMKKβ or cell lysates (1 μl), 50 mm HEPES, pH 7.5, 10 mm Mg(CH3COO)2, 1 mm DTT, 100 μm [γ-32P]ATP (2000–2500 cpm/pmol) or 100 μm ATP (for dot-blot assay), and 10 μg of GST-CaMKIα(1–293) K49E in the presence of 2 mm EGTA (autonomous activity) or 2 mm CaCl2 and 6 μm CaM (total activity). Each reaction was initiated by the addition of [γ-32P]ATP or ATP (for dot-blot assay). Reactions were terminated by spotting the samples onto P81 phosphocellulose paper (Whatman), which was extensively washed and subjected to scintillation counting to measure 32P incorporation into GST-CaMKIα(1–293) K49E. For the dot-blot kinase assay, reaction mixtures (4 μl) were spotted onto a nitrocellulose membrane, and Thr177-phosphorylated GST-CaMKIα(1–293) K49E was detected using an anti-phospho-CaMKI antibody. Antibody immunoreactivity was detected using a chemiluminescent reagent (PerkinElmer Life Sciences) and ChemiDoc XRS (Bio-Rad) followed by quantification of Thr177 phosphorylation with Quantity One® software (version 4.6.5; Bio-Rad).
Mass spectrometry analysis
Recombinant rat CaMKKβ (1.2 μg) was incubated with either wild-type or K45R AMPK (1.2 μg) in the presence of 1 mm ATP at 30 °C for 60 min as described above after which the reaction was terminated by adding 5 μl of 2× SDS-PAGE sample buffer. Phosphorylated CaMKKβ was separated by 7.5% SDS-PAGE and lightly stained with Coomassie Brilliant Blue followed by in-gel digestion (50) with a protease or protease mixture, including trypsin, chymotrypsin (Roche Diagnostics), trypsin with chymotrypsin, trypsin with Glu-C (Roche Diagnostics), trypsin with Asp-N (Roche Diagnostics), or chymotrypsin with Asp-N. The following protease concentrations were used: 10 μg/ml trypsin, 17 μg/ml chymotrypsin, 10 μg/ml Glu-C, and 4 μg/ml Asp-N. Trypsin and Asp-N were incubated at 37 °C, and chymotrypsin and Glu-C were incubated at 25 °C. The first and second digestions were incubated overnight and for 3 h, respectively. The digested peptides were eluted with 0.1% formic acid and subjected to LC-MS/MS analysis on an LC-MS-IT-TOF instrument (Shimadzu, Kyoto, Japan) interfaced with a nano reverse-phase LC system (Shimadzu) as described previously (40). MS/MS data were acquired in the datum-dependent mode using LC-MS solution software (Shimadzu) and converted to a single text file (containing the observed precursor peptide m/z, fragment ion m/z, and intensity values) using Mascot Distiller (Matrix Science, London, UK). MS/MS data were obtained independently and merged for the Mascot analysis. The following search parameters were used: database, rat CaMKKβ (578 amino acid residues); enzyme, all; variable modifications, carbamidomethyl (Cys), oxidation (Met), propionamide (Cys), and phospho (Ser/Thr).
Mutagenesis and construction of CaMKKβ expression plasmids
Site-directed mutagenesis was performed via inverse PCR using PrimeSTAR HS DNA polymerase (Takara, Japan), pET-rat CaMKKβ, and pME-rat CaMKKβ (39) as templates, and the following phosphorylated primers: CaMKKβ S135A, 5′-AGCCTGTGGGGAGCTGGCTGG-3′ and 5′-TCTCCCCGGATGCCCCGGCGG-3′; CaMKKβ T144A, 5′-AGCGGGCCGCCGGGGCATCCGGGG-3′ and 5′-GTGGAGTCGCACCACGTCTCC-3′; CaMKKβ T144E, 5′-CTCGGGCCGCCGGGGCATCCGGGG-3′ and 5′-GTGGAGTCGCACCACGTCTCC-3′; and CaMKKβ S494A, 5′-CCGTTTCCGAATCATGGT-3′ and 5′-AAACGGGCATTTGGGAACCCATTTGAA-3′. Sites of the mutation are indicated by underlines. The nucleotide sequences of all constructs were confirmed by sequencing on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Cell culture and transfection
COS-7 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum at 37 °C in 5% CO2. COS-7 cells in 6-well dishes were transfected with or without 1 μg of CaMKKβ expression plasmid (pME-CaMKKβ wild type or T144A mutant) together with 1 μg of HA-AMPKα expression plasmid (pME-HA-AMPKα) or the empty vector (pME-HA) using polyethylenimine MAX (Polysciences, Inc., Warrington, PA) according to the manufacturer's protocol. After 36-h culture, the cells were extracted with 1× SDS-PAGE sample buffer (100 μl) followed by immunoblot analyses. For the CaMKKβ activity assay, 2 μg of either an empty vector (pME18S) or CaMKKβ expression plasmid (pME-CaMKKβ wild type or mutants) were transfected into COS-7 cells as described above. The cells were extracted with 100 μl of lysis buffer (150 mm NaCl, 20 mm Tris-HCl, pH 7.5, 2 mm EDTA, 2 mm EGTA, 1% Nonidet P-40, 10% glycerol, 1:1000 protease inhibitor mixture (Nacalai Tesque, Kyoto, Japan), and 0.5 μm okadaic acid), and the cell extracts (1 μl) were subjected to an in vitro CaMKK activity assay (5-min reaction period) in the presence of 100 μm [γ-32P]ATP as described above.
Other methods
Immunoblot and dot-blot analyses were performed with the indicated primary antibodies and horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG (GE Healthcare) as the secondary antibody. A chemiluminescent reagent (PerkinElmer Life Sciences) was used for signal detection of immunoblots followed by quantification of the immunoreactivity with ImageJ software (51). Protein concentrations in samples were estimated by Coomassie Brilliant Blue (Bio-Rad) using bovine serum albumin as a standard. Student's t tests were used to evaluate the statistical significance of two-group comparisons. Probability (p) values <0.05 were considered statistically significant.
Author contributions
H. T. conceived and designed the study. A. N., Y. F., A. S., S. T., and H. A. performed the experiments. N. H. performed the mass spectrometry analysis. N. N. generated anti-phospho-AMPK (at Thr172) and anti-phospho-CaMKKβ (at Thr144) antibodies. All authors contributed to the analysis and interpretation of the data. M. M. and N. K. supervised the experiments. A. N. and Y. F. contributed to drafting the manuscript. H. T. wrote and prepared the final version of the manuscript.
Supplementary Material
This work was supported by a Grant-in-aid for Scientific Research 26440056 (to H. T.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Figs. S1–S3.
- CaMK
- Ca2+/calmodulin-dependent protein kinase
- CaMKK
- Ca2+/CaM-dependent protein kinase kinase
- AID
- autoinhibitory domain
- CaM
- calmodulin
- AMPK
- 5′-AMP–activated protein kinase
- CDK5
- cyclin-dependent kinase 5
- GSK3
- glycogen synthase kinase 3.
References
- 1. Soderling T. R., and Stull J. T. (2001) Structure and regulation of calcium/calmodulin-dependent protein kinases. Chem. Rev. 101, 2341–2352 [DOI] [PubMed] [Google Scholar]
- 2. Means A. R. (2008) The year in basic science: calmodulin kinase cascades. Mol. Endocrinol. 22, 2759–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wayman G. A., Lee Y. S., Tokumitsu H., Silva A. J., and Soderling T. R. (2008) Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron 59, 914–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tokumitsu H., Enslen H., and Soderling T. R. (1995) Characterization of a Ca2+/calmodulin-dependent protein kinase cascade. Molecular cloning and expression of calcium/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 270, 19320–19324 [DOI] [PubMed] [Google Scholar]
- 5. Kitani T., Okuno S., and Fujisawa H. (1997) Molecular cloning of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biochem. 122, 243–250 [DOI] [PubMed] [Google Scholar]
- 6. Anderson K. A., Means R. L., Huang Q. H., Kemp B. E., Goldstein E. G., Selbert M. A., Edelman A. M., Fremeau R. T., and Means A. R. (1998) Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization, and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biol. Chem. 273, 31880–31889 [DOI] [PubMed] [Google Scholar]
- 7. Kimura Y., Corcoran E. E., Eto K., Gengyo-Ando K., Muramatsu M. A., Kobayashi R., Freedman J. H., Mitani S., Hagiwara M., Means A. R., and Tokumitsu H. (2002) A CaMK cascade activates CRE-mediated transcription in neurons of Caenorhabditis elegans. EMBO Rep. 3, 962–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joseph J. D., and Means A. R. (2000) Identification and characterization of two Ca2+/CaM-dependent protein kinases required for normal nuclear division in Aspergillus nidulans. J. Biol. Chem. 275, 38230–38238 [DOI] [PubMed] [Google Scholar]
- 9. Tokumitsu H., and Soderling T. R. (1996) Requirements for calcium and calmodulin in the calmodulin kinase activation cascade. J. Biol. Chem. 271, 5617–5622 [DOI] [PubMed] [Google Scholar]
- 10. Selbert M. A., Anderson K. A., Huang Q. H., Goldstein E. G., Means A. R., and Edelman A. M. (1995) Phosphorylation and activation of Ca2+-calmodulin-dependent protein kinase IV by Ca2+-calmodulin-dependent protein kinase Ia kinase. Phosphorylation of threonine 196 is essential for activation. J. Biol. Chem. 270, 17616–17621 [DOI] [PubMed] [Google Scholar]
- 11. Lee J. C., and Edelman A. M. (1994) A protein activator of Ca2+-calmodulin-dependent protein kinase Ia. J. Biol. Chem. 269, 2158–2164 [PubMed] [Google Scholar]
- 12. Matsushita M., and Nairn A. C. (1998) Characterization of the mechanism of regulation of Ca2+/calmodulin-dependent protein kinase I by calmodulin and by Ca2+/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 273, 21473–21481 [DOI] [PubMed] [Google Scholar]
- 13. Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., and Carling D. (2005) Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2, 21–33 [DOI] [PubMed] [Google Scholar]
- 14. Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., and Hardie D. G. (2005) Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19 [DOI] [PubMed] [Google Scholar]
- 15. Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., and Witters L. A. (2005) The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280, 29060–29066 [DOI] [PubMed] [Google Scholar]
- 16. Saneyoshi T., Wayman G., Fortin D., Davare M., Hoshi N., Nozaki N., Natsume T., and Soderling T. R. (2008) Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/βPIX signaling complex. Neuron 57, 94–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wayman G. A., Impey S., Marks D., Saneyoshi T., Grant W. F., Derkach V., and Soderling T. R. (2006) Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 50, 897–909 [DOI] [PubMed] [Google Scholar]
- 18. Takemoto-Kimura S., Ageta-Ishihara N., Nonaka M., Adachi-Morishima A., Mano T., Okamura M., Fujii H., Fuse T., Hoshino M., Suzuki S., Kojima M., Mishina M., Okuno H., and Bito H. (2007) Regulation of dendritogenesis via a lipid-raft-associated Ca2+/calmodulin-dependent protein kinase CLICK-III/CaMKIγ. Neuron 54, 755–770 [DOI] [PubMed] [Google Scholar]
- 19. Wayman G. A., Kaech S., Grant W. F., Davare M., Impey S., Tokumitsu H., Nozaki N., Banker G., and Soderling T. R. (2004) Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I. J. Neurosci. 24, 3786–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horigane S., Ageta-Ishihara N., Kamijo S., Fujii H., Okamura M., Kinoshita M., Takemoto-Kimura S., and Bito H. (2016) Facilitation of axon outgrowth via a Wnt5a-CaMKK-CaMKIα pathway during neuronal polarization. Mol. Brain 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakamuta S., Funahashi Y., Namba T., Arimura N., Picciotto M. R., Tokumitsu H., Soderling T. R., Sakakibara A., Miyata T., Kamiguchi H., and Kaibuchi K. (2011) Local application of neurotrophins specifies axons through inositol 1,4,5-trisphosphate, calcium, and Ca2+/calmodulin-dependent protein kinases. Sci. Signal. 4, ra76. [DOI] [PubMed] [Google Scholar]
- 22. Enslen H., Sun P., Brickey D., Soderling S. H., Klamo E., and Soderling T. R. (1994) Characterization of Ca2+/calmodulin-dependent protein kinase IV. Role in transcriptional regulation. J. Biol. Chem. 269, 15520–15527 [PubMed] [Google Scholar]
- 23. Enslen H., Tokumitsu H., and Soderling T. R. (1995) Phosphorylation of CREB by CaM-kinase IV activated by CaM-kinase IV kinase. Biochem. Biophys. Res. Commun. 207, 1038–1043 [DOI] [PubMed] [Google Scholar]
- 24. Bito H., Deisseroth K., and Tsien R. W. (1996) CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87, 1203–1214 [DOI] [PubMed] [Google Scholar]
- 25. Miranti C. K., Ginty D. D., Huang G., Chatila T., and Greenberg M. E. (1995) Calcium activates serum response factor-dependent transcription by a Ras- and Elk-1-independent mechanism that involves a Ca2+/calmodulin-dependent kinase. Mol. Cell. Biol. 15, 3672–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson K. A., Ribar T. J., Lin F., Noeldner P. K., Green M. F., Muehlbauer M. J., Witters L. A., Kemp B. E., and Means A. R. (2008) Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 7, 377–388 [DOI] [PubMed] [Google Scholar]
- 27. Iwabu M., Yamauchi T., Okada-Iwabu M., Sato K., Nakagawa T., Funata M., Yamaguchi M., Namiki S., Nakayama R., Tabata M., Ogata H., Kubota N., Takamoto I., Hayashi Y. K., Yamauchi N., et al. (2010) Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 464, 1313–1319 [DOI] [PubMed] [Google Scholar]
- 28. Yamauchi M., Kambe F., Cao X., Lu X., Kozaki Y., Oiso Y., and Seo H. (2008) Thyroid hormone activates adenosine 5′-monophosphate-activated protein kinase via intracellular calcium mobilization and activation of calcium/calmodulin-dependent protein kinase kinase-β. Mol. Endocrinol. 22, 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghislat G., Patron M., Rizzuto R., and Knecht E. (2012) Withdrawal of essential amino acids increases autophagy by a pathway involving Ca2+/calmodulin-dependent kinase kinase-β (CaMKK-β). J. Biol. Chem. 287, 38625–38636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Massie C. E., Lynch A., Ramos-Montoya A., Boren J., Stark R., Fazli L., Warren A., Scott H., Madhu B., Sharma N., Bon H., Zecchini V., Smith D. M., Denicola G. M., Mathews N., et al. (2011) The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 30, 2719–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frigo D. E., Howe M. K., Wittmann B. M., Brunner A. M., Cushman I., Wang Q., Brown M., Means A. R., and McDonnell D. P. (2011) CaM kinase kinase β-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res. 71, 528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fujiwara Y., Hiraoka Y., Fujimoto T., Kanayama N., Magari M., and Tokumitsu H. (2015) Analysis of distinct roles of CaMKK isoforms using STO-609-resistant mutants in living cells. Biochemistry 54, 3969–3977 [DOI] [PubMed] [Google Scholar]
- 33. Fujiwara Y., Kawaguchi Y., Fujimoto T., Kanayama N., Magari M., and Tokumitsu H. (2016) Differential AMP-activated protein kinase (AMPK) recognition mechanism of Ca2+/calmodulin-dependent protein kinase kinase isoforms. J. Biol. Chem. 291, 13802–13808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tokumitsu H., Inuzuka H., Ishikawa Y., Ikeda M., Saji I., and Kobayashi R. (2002) STO-609, a specific inhibitor of the Ca2+/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 277, 15813–15818 [DOI] [PubMed] [Google Scholar]
- 35. Wayman G. A., Tokumitsu H., and Soderling T. R. (1997) Inhibitory cross-talk by cAMP kinase on the calmodulin-dependent protein kinase cascade. J. Biol. Chem. 272, 16073–16076 [DOI] [PubMed] [Google Scholar]
- 36. Matsushita M., and Nairn A. C. (1999) Inhibition of the Ca2+/calmodulin-dependent protein kinase I cascade by cAMP-dependent protein kinase. J. Biol. Chem. 274, 10086–10093 [DOI] [PubMed] [Google Scholar]
- 37. Davare M. A., Saneyoshi T., Guire E. S., Nygaard S. C., and Soderling T. R. (2004) Inhibition of calcium/calmodulin-dependent protein kinase kinase by protein 14-3-3. J. Biol. Chem. 279, 52191–52199 [DOI] [PubMed] [Google Scholar]
- 38. Ichimura T., Taoka M., Hozumi Y., Goto K., and Tokumitsu H. (2008) 14-3-3 Proteins directly regulate Ca2+/calmodulin-dependent protein kinase kinase α through phosphorylation-dependent multisite binding. FEBS Lett. 582, 661–665 [DOI] [PubMed] [Google Scholar]
- 39. Tokumitsu H., Iwabu M., Ishikawa Y., and Kobayashi R. (2001) Differential regulatory mechanism of Ca2+/calmodulin-dependent protein kinase kinase isoforms. Biochemistry 40, 13925–13932 [DOI] [PubMed] [Google Scholar]
- 40. Tokumitsu H., Hatano N., Fujimoto T., Yurimoto S., and Kobayashi R. (2011) Generation of autonomous activity of Ca2+/calmodulin-dependent protein kinase kinase β by autophosphorylation. Biochemistry 50, 8193–8201 [DOI] [PubMed] [Google Scholar]
- 41. Green M. F., Scott J. W., Steel R., Oakhill J. S., Kemp B. E., and Means A. R. (2011) Ca2+/calmodulin-dependent protein kinase kinase β is regulated by multisite phosphorylation. J. Biol. Chem. 286, 28066–28079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Colbran R. J. (1992) Regulation and role of brain calcium/calmodulin-dependent protein kinase II. Neurochem. Int. 21, 469–497 [DOI] [PubMed] [Google Scholar]
- 43. Hudmon A., and Schulman H. (2002) Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 71, 473–510 [DOI] [PubMed] [Google Scholar]
- 44. Fukunaga K., Rich D. P., and Soderling T. R. (1989) Generation of the Ca2+-independent form of Ca2+/calmodulin-dependent protein kinase II in cerebellar granule cells. J. Biol. Chem. 264, 21830–21836 [PubMed] [Google Scholar]
- 45. Tokumitsu H., Hatano N., Inuzuka H., Yokokura S., Nozaki N., and Kobayashi R. (2004) Mechanism of the generation of autonomous activity of Ca2+/calmodulin-dependent protein kinase IV. J. Biol. Chem. 279, 40296–40302 [DOI] [PubMed] [Google Scholar]
- 46. Tokumitsu H., Muramatsu M.-a., Ikura M., and Kobayashi R. (2000) Regulatory mechanism of Ca2+/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 275, 20090–20095 [DOI] [PubMed] [Google Scholar]
- 47. Neumann D., Woods A., Carling D., Wallimann T., and Schlattner U. (2003) Mammalian AMP-activated protein kinase: functional, heterotrimeric complexes by co-expression of subunits in Escherichia coli. Protein Expr. Purif. 30, 230–237 [DOI] [PubMed] [Google Scholar]
- 48. Hayashi N., Matsubara M., Takasaki A., Titani K., and Taniguchi H. (1998) An expression system of rat calmodulin using T7 phage promoter in Escherichia coli. Protein Expr. Purif. 12, 25–28 [DOI] [PubMed] [Google Scholar]
- 49. Kimura K., Nozaki N., Saijo M., Kikuchi A., Ui M., and Enomoto T. (1994) Identification of the nature of modification that causes the shift of DNA topoisomerase IIβ to apparent higher molecular weight forms in the M phase. J. Biol. Chem. 269, 24523–24526 [PubMed] [Google Scholar]
- 50. Hatano N., and Hamada T. (2008) Proteome analysis of pitcher fluid of the carnivorous plant Nepenthes alata. J. Proteome Res. 7, 809–816 [DOI] [PubMed] [Google Scholar]
- 51. Schneider C. A., Rasband W. S., and Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.