Abstract
Mapping the expression of transcription factors in the mouse spinal cord has identified ten progenitor domains, four of which are cardinal classes of molecularly defined, ventrally located interneurons that are integrated in the locomotor circuitry. This review focuses on the properties of these interneuronal populations and their contribution to hindlimb locomotor central pattern generation. Interneuronal populations are categorized based on their excitatory or inhibitory functions and their axonal projections as predictors of their role in locomotor rhythm generation and coordination. The synaptic connectivity and functions of these interneurons in the locomotor central pattern generators (CPGs) have been assessed by correlating their activity patterns with motor output responses to rhythmogenic neurochemicals and sensory and descending fibers stimulations as well as analyzing kinematic gait patterns in adult mice. The observed complex organization of interneurons in the locomotor CPG circuitry, some with seemingly similar physiological functions, reflects the intricate repertoire associated with mammalian motor control and is consistent with high transcriptional heterogeneity arising from cardinal interneuronal classes. This review discusses insights derived from recent studies to describe innovative approaches and limitations in experimental model systems and to identify missing links in current investigational enterprise.
Keywords: locomotor circuitry, molecularly defined spinal interneurons, rhythm generation, rhythm coordination, mouse spinal cord
terrestrial movements are executed by coordinated contractions of multiple joint-specific flexor and extensor muscles on both sides of the body (Engberg and Lundberg 1969). Ultimately, motor coordination is achieved via the spatiotemporally integrated synaptic connectome of numerous populations of interposed spinal interneurons onto motoneurons (MNs). Locomotor-related interneurons are recruited to generate behaviorally relevant responses to stimuli from sensory afferents and supraspinal projections. They can be flexibly driven and reflexively integrate environmental interactions to generate rhythmic activity patterns that underlie repetitive motor behavior, the most fundamental of which is locomotion. Muscle contractions are controlled by circuits of interconnected spinal interneurons (INs), the central pattern generators (CPGs). CPGs constitute rhythm-generating and rhythm-coordinating networks whose composition may not be mutually exclusive. Rhythm-generating networks regulate the timing and frequency of rhythmic locomotor activity. Rhythm-coordinating networks control the strength and coordination of ipsilateral flexor-extensor alternating motor activities, and bilateral, left-right alternating or synchronous movements (Brown 1911; Butt and Kiehn 2003; Grillner 2003; Guertin 2009; Kiehn 2006; Kullander 2005; McCrea and Rybak 2008; Pearson 1993).
The neuronal circuitry responsible for generating locomotor activity is contained entirely within the spinal cord, and it has a high degree of autonomy as reflected by its ability to trigger rhythmic motor bouts in the absence of descending inputs from supraspinal areas or peripheral sensory inputs (Brown 1911; Grillner and Wallén 1985; Kiehn and Butt 2003; Rossignol et al. 2006). Locomotor CPG circuits can be recruited by descending projections from several areas in the brain (Dubuc et al. 2008; Grillner and Robertson 2015; Jordan et al. 2008), as well as afferent activity at various segments of the cord (Bonnot et al. 2002; Lev-Tov et al. 2010; Whelan et al. 2000). The bidirectional communication between the brain and spinal locomotor circuits (Grillner et al. 2005; Hantman and Jessell 2010; Lemon 2008; Oscarsson 1965) as well as feedback from proprioceptive and cutaneous signals adjust the activity pattern of these circuits in response to changes in the external environment (Akay et al. 2014; Büschges et al. 2011; Grillner 1985; for review, see McCrea 2001; Pearson 2004; Rossignol et al. 2006; Windhorst 2007; Zehr and Stein 1999).
Early studies have demonstrated that groups of spinal interneurons that are likely to play fundamental roles in the locomotor circuitry are spatially dispersed in the adult cat spinal cord (Jankowska 2008). The task of categorizing them as identified populations with specific functions in the CPG has been challenging, partly because of their wide distribution in various laminae of the spinal cord and the inability to verify their functions with certainty in repeated experiments (Johnson and Sears 1988; McDonagh et al. 1999; for review, see Jankowska 2001; Rossignol et al. 2006).
The fundamental neural circuit organization that generates complex patterns of motor coordination necessary for locomotion is already present at birth, and it has been studied in the isolated neonatal rodent spinal cord (Hayes et al. 2009; Jiang et al. 1999; Kiehn and Kjaerulff 1996; Kudo and Yamada 1987; Smith and Feldman 1987). The in vitro approach revolutionized experimental procedures for dissecting the basic organization of mammalian CPGs associated with rhythmic limb movements. It provides recording stability and accessibility to neurons whose activity could be correlated with locomotor-like motor outputs generated by stimulation of descending systems and afferent pathways and/or pharmacological substances (Cazalets et al. 1995; Cowley and Schmidt 1997; Kjaerulff and Kiehn 1996; Kremer and Lev-Tov 1997; Kudo and Yamada 1987; Smith and Feldman 1987). Several experimental approaches have been utilized to activate the lumbar CPG in the isolated spinal cord (Hochman et al. 2012), and the contribution of an identified interneuronal population to locomotor-related motor activity has been assessed by correlating the activity pattern of interneurons with rhythmic motor outputs (e.g., Gordon and Whelan 2006; Gosgnach et al. 2006; Hinckley et al. 2005b; Wilson et al. 2005).
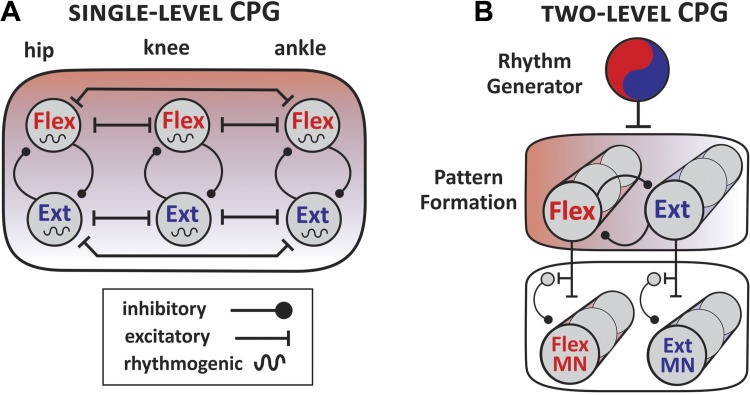
Several computational models have been constructed to provide a framework for the functional integration and organization of neurons that are essential for executing a wide spectrum of tetrapod locomotor activities. The models are based on intrinsic properties of oscillatory, presumably rhythm-generating interneurons, the strength of their synaptic connections with excitatory/inhibitory interneurons, and network modulation by supraspinal and sensory inputs. Currently, two conceptual and not mutually exclusive models describe the fundamental organization of the locomotor CPG: the single-level CPG, a unit burst generator (UBG), and the two-level model of rhythm generator with multiple pattern formation modules (Fig. 1). Both models broadly embrace modular organization, but they differ in their hierarchical architecture (Grillner 2011; Jordan 1991; Kiehn 2016; Stein and Daniels-McQueen 2002). The single-level CPG model includes multiple independent half-centers of interconnected oscillators across joints that coordinate locomotor rhythms and drive the activity of functional groups of MNs (Grillner 2011) (Fig. 1A). The single-level modular organization comprises first-order interneurons (drive other interneurons) that also function as last-order interneurons (drive MNs). The oscillators provide independent excitatory drive to MN pools controlling movements such as the axial bending in lamprey (Grillner et al. 1988; Tråvén et al. 1993) and driving flexor-extensor muscle activities at each joint in limbed vertebrates, including rodents (Kiehn and Kjaerulff 1998; Sherwood et al. 2011) and cats (Deliagina et al. 1983; Grillner and Zangger 1979).
Fig. 1.
Schematic models of single- and two-level central pattern generators (CPGs). A: in a single-level CPG, multiple, loosely interconnected flexor (Flex)/extensor (Ext)-related oscillators at each joint control the activity of motoneuron (MN) pools in limbed vertebrates. B: in a two-level CPG, the rhythm generator and the pattern formation units are separated. A distributed single rhythm generator controls the activity of multiple pattern formation modules that regulate the activity of flexor/extensor MNs at each joint.
The proposed two-level CPG circuitry has a different architecture in which a single rhythm generator (RG) controls the activity of multiple pattern formation (PF) modules that drive specific groups of MNs. Similar to the single-level UBG model, the RG comprises intrinsically rhythmic network of first-order interneurons that dictate the frequency and duration of the bursting phases (Fig. 1B). The second level of PF circuitry comprises both first- and last-order interneurons that regulate the strength and coordination of the alternating rhythmic oscillations (McCrea and Rybak 2008; Rybak et al. 2006). This model has been proposed to explain the independent expression of spontaneous perturbations of alternating locomotor patterns in synergistic and antagonistic MNs. During such perturbations, oscillatory motor bouts are altered/deleted in a group of synergistic MNs but remain unchanged in the group of antagonistic MNs. Spontaneous deletions in recruitment of agonist and antagonist muscles were observed during fictive locomotion in cats (Lafreniere-Roula and McCrea 2005), rats (Hochman et al. 2012), and mice (Zhong et al. 2012) and during rhythmical rostral scratching in turtle (Stein 2008; Stein and Daniels-McQueen 2004). Observed deletions either have no consequences on the timing of the ongoing locomotor cycle (nonresetting deletions) or lead to a fundamental shift in onset of the subsequent burst phase, resetting the timing of the locomotor cycle (resetting deletions). The influence of synaptic inputs on the timing of oscillatory activities could be interpreted as acting on the RG or the PF. Stimulation of proprioceptive afferents that reset fictive locomotion by triggering a permanent shift, delaying or advancing the onset of subsequent rhythmic episodes is regarded as evidence of actions of the RG (Hultborn et al. 1998; McCrea 2001; Pearson 2000). Therefore, whether a specific population of interneurons is part of the rhythm- or pattern-generating networks could be determined based on the characterization of spontaneous or afferent evoked deletions during fictive locomotion (Hinckley et al. 2010). This approach has been used to determine the role of interneuronal population in the locomotor CPG, and it is discussed in more detail in subsequent sections of this review.
Functional identification of spinal interneuronal populations and their role in motor behavior is based on their intrinsic properties, neurotransmitter phenotype, patterns of axonal projections and synaptic inputs onto their target cells (for review, see Jankowska 2001, 2008). Numerous reviews describe and interrelate the organization of spinal networks responsible for generating locomotion in other vertebrate classes and their common genetic underpinnings (Büschges et al. 2011; Goulding 2009; Grillner and El Manira 2015; Grillner and Jessell 2009; Kiehn 2016; McLean and Dougherty 2015). Much of our early understanding on the identification and segmental circuit organization of locomotor-related interneurons in the vertebrate locomotor CPG arose from studies on swimming in adult lamprey and Xenopus embryos employing primarily electrophysiological and morphological techniques (Sillar et al. 2008). Optical and genetic approaches have more recently been carried out in zebrafish to examine the principles governing the functions of molecularly defined interneurons in swimming motor behavior, including important cellular and network mechanisms underlying interneuronal recruitments at various locomotor speeds (Ampatzis et al. 2014; Björnfors and El Manira 2016; Fetcho and McLean 2010; McLean and Fetcho 2008).
Axial bending in swimming is controlled by numerically smaller neural networks than those regulating limb movements (Buchanan 1982; Buchanan and Grillner 1987; Cohen and Harris-Warrick 1984; Roberts et al. 1998). Tetrapod evolution entailed the emergence of additional neuronal circuits to accommodate for the more complex motor coordination across multiarticular limbs. This review focuses on tetrapod locomotion with an emphasis on mammals, and more specifically on genetic and physiological studies in the de facto mouse animal model. The discovery that spinal interneurons are derived from defined progenitor domains governed by the expression of a distinct combination of transcription factors has been a transformative advance to studies on locomotor mechanisms (for review, see Goulding and Pfaff 2005; Jessell 2000). The emergent view was of a genetically guided circuit construction based on a platform of molecularly distinct populations of interneurons with specified synaptic connections. Electrophysiological studies of locomotor networks in the in vitro neonatal mouse model leveraged molecular interneuron identity with genetic approaches that allow their selective ablation, visualization, and activity control. Analyses examined the effects of such manipulations on the timing, robustness, and left-right/flexor-extensor coordination (for review, see Brownstone and Bui 2010; Goulding 2009; Grillner and Jessell 2009; Kiehn 2006; Kiehn and Kullander 2004; Stein 2013; Stepien and Arber 2008). With experimental designs to determine the role of specific interneuronal groups in the locomotor circuity, a complete reconstruction of the mammalian hindlimb locomotor CPG seemed at hand.
It is important to emphasize that studies designed to examine the effects of genetically driven removal of interneuronal groups or silencing their transmission on locomotion have been performed predominantly in the developmentally immature neonate in vitro. Moreover, initial transgenic approaches designed to determine the role of a specific group of interneurons in generating/coordinating fictive locomotion or hindlimb movements involved lethal germline deletions of that group, resulting in compensatory circuit reconstruction that complicate data analysis in mutant mice (Vallstedt et al. 2001). In recent years, important complementary studies have examined the behavioral consequences of such manipulations in adult mice with mature synaptic network organization, using inducible genetic manipulations, including widely available optogenetic and chemogenetic approaches that enable acute circuit activation or inhibition.
Principal Premotor Interneurons with Identified Functions in the Mouse Locomotor Circuitry
The identification of functionally discrete groups of spinal interneurons intrinsic to the locomotor CPG forms the requisite foundation toward understanding the cellular and synaptic basis of their recruitment, the flexibility of their operation via extrinsic synaptic sources (e.g., brain and periphery), and the contribution of intrinsic and extrinsic neuromodulation to their function. This review focuses primarily on genetically distinct interneuronal populations that have been studied as putative components of the locomotor CPG in mice, but it also includes assessment of other differentiable populations of interneurons of uncertain progenitor origin with probable functions in the locomotor circuitry. Sections below are organized into populations based on their excitatory and inhibitory functions and their ipsilateral, contralateral, and bilateral axonal projections.
To date, ten cardinal classes of spinal interneurons have been recognized based on their origin from genetically defined progenitor domains governed by the expression of transcription factors. Four of these fundamental classes, V0–V3, develop from basal plate progenitors (Jessell 2000). Postmitotically, they are distributed in ventral laminae VII, VIII, and X, the regions of the mammalian spinal cord that house the locomotor CPG (Kiehn 2006). Six classes of interneurons, dI1–dI6, develop from the dorsal plate. Most settle in the dorsal horn and function as sensory interneurons, some with probable functions in spinal reflex pathways (Gross et al. 2002; Grossmann et al. 2010; Müller et al. 2002; Wildner et al. 2006). For example, intermediate laminae dI3 INs include premotor interneurons that relay cutaneous signals from low-threshold mechanoreceptors to locomotor networks and MNs (Bui et al. 2013; Panek et al. 2014; for review, see Garcia-Campmany et al. 2010). The dI6 class of premotor interneurons migrate ventrally to laminae VII/VIII and are divided into subgroups performing various functions in the locomotor circuitry (Andersson et al. 2012; Dyck et al. 2012).
In intact-limbed animals, activation of the CPG circuitry is initiated and regulated by synaptic drive from primary sensory afferents and/or descending pathways. Multisensory input originating from muscle, skin, and joint afferents converge onto common spinal interneurons interposed in motor behaviors (Lundberg 1979), including interneurons that are integral components of the CPG. Such an example is the feedback from skeletal muscle proprioceptors that can trigger or modulate left-right and flexor-extensor coordinated locomotor activity (Gossard et al. 1994; for review see Hultborn et al. 1998; McCrea 2001; Pearson 2004; Rossignol et al. 2006; Rybak et al. 2006; Windhorst 2007). Similarly, in the isolated neonatal rodent spinal cord, afferent stimulation can initiate locomotor-like rhythms or reset the oscillatory activity induced by neurotransmitter agonists (Iizuka et al. 1997; Kiehn et al. 1992; Lev-Tov and Delvolvé 2000; Marchetti et al. 2001; Smith et al. 1988; Whelan et al. 2000). Low-threshold afferent stimulation has been employed to determine the role of molecularly defined interneurons in triggering or regulating locomotor-related motor outputs (e.g., Gordon and Whelan 2006; Hinckley et al. 2010; Zhong et al. 2012). However, because of the incomplete myelination of afferent projections during the first few weeks after birth (Foran and Peterson 1992; Vejsada et al. 1985; Ziskind-Conhaim 1988), threshold-based identification of afferents as undertaken in the adult cat (McCrea 2001) cannot reliably differentiate somatosensory afferents in neonatal rodents (Fitzgerald 1987; García-Ramirez et al. 2014; Hochman et al. 2012; Mandadi et al. 2009). Stimulating descending bulbospinal pathways reliably activates the locomotor CPG in the in vitro brain stem-spinal cord preparation (Gordon and Whelan 2008; Zaporozhets et al. 2004; for review, see Jordan et al. 2008). Therefore, the ability to activate specific descending pathways (Hägglund et al. 2010; Liu and Jordan 2005; Szokol et al. 2011) can provide important evidence for the assumed role of a defined interneuronal population in the locomotor circuitry.
Ipsilateral Excitatory Interneurons
Oscillatory drive in locomotor networks of all vertebrates is initiated by ipsilaterally projecting excitatory interneurons that innervate neurons in the CPG circuitry (Brownstone and Wilson 2008; Grillner 2003; Jordan et al. 2008; Kiehn 2006; McLean and Dougherty 2015). Functional identification of rhythm-generating interneurons that control the frequency and robustness of locomotor output has been based primarily on three criteria: 1) changes in their oscillatory frequency or resetting their activity are correlated with changes in rhythmic motor outputs or modulate the frequency of existing oscillatory motor bouts, 2) specific disruption of their activity by altering their intrinsic properties or synaptic transmission significantly reduces the amplitude and frequency of drug-induced or electrically evoked rhythmic motor bouts, and 3) the interneurons can be reconfigured to express intrinsic membrane voltage oscillations that characterize pacemaker neurons. Molecular markers have identified several populations of ipsilaterally projecting excitatory interneurons that fit the criteria of rhythmogenic interneurons, but to date genetically driven removal or silencing any of the putative rhythm-generating interneurons described in this section has not blocked the onset of rhythmic motor outputs.
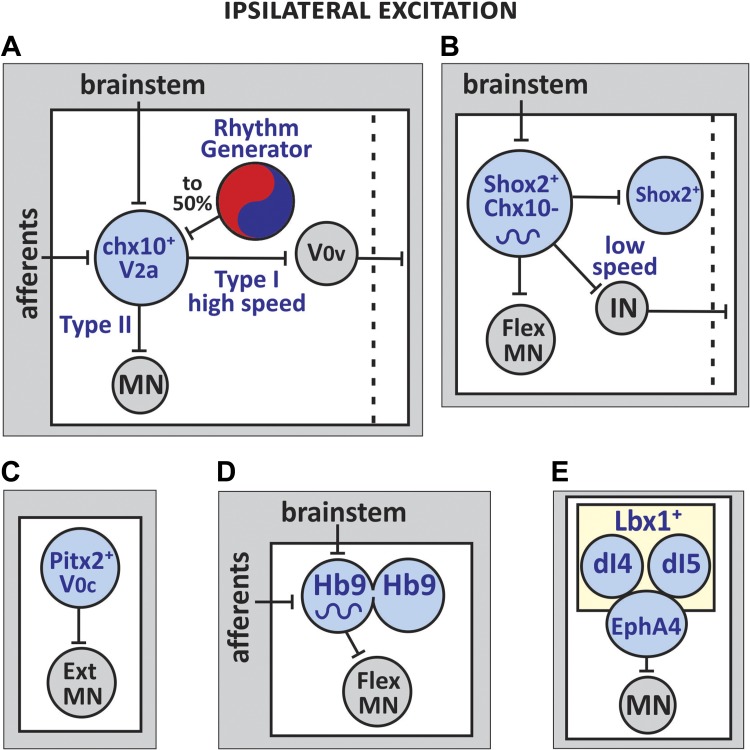
Multiple ipsilaterally projecting excitatory interneurons from several progenitor domains that have been identified as putative rhythm generators are distributed in different laminae, suggesting that they serve different functions in locomotor microcircuits. The interneuronal populations are derived primarily from domains pV0, pV2, and pV3 and their postmitotic development gives rise to four populations of excitatory ipsilateral interneurons: 1) glutamatergic Chx10-expressing V2a INs, 2) glutamatergic Shox2-expressing INs, 3) cholinergic Pitx2-expressing V0c INs, and 4) glutamatergic Sim1-derived small (<15%) subset of ipsilateral V3 INs. The majority of V3 INs project contralaterally, therefore, their role in the locomotor circuitry is discussed in relation to their function as commissural excitatory interneurons. Classes of locomotor-related interneurons of unknown progenitor origin include: 1) glutamatergic Hb9-expressing INs and 2) glutamatergic EphA4-positive INs (Table 1, Fig. 2).
Table 1.
Interneuronal populations engaged in the mouse locomotor central pattern generation
| Neuronal Population | Neurotransmitter | Target Neurons | Proposed Role in Locomotor CPG |
|---|---|---|---|
| Ipsilateral excitatory interneurons | |||
| Chx10-expressing V2a INs | Glutamate | Dbx1-Evx1-expressing V0v INs | High-speed left-right coordination* |
| Shox2-expressing, nonV2a INs | Glutamate | MNs | Rhythm generators |
| Shox2 INs | Low-speed left-right coordination | ||
| Flexor-related MNs | |||
| Commissural INs | |||
| Pitx2-expressing V0c INs | Acetylcholine | Extensor-related MNs | Task-dependent modulation of motor outputs* |
| Hb9-expressing INs | Glutamate | Hb9 INs | Rhythm generators |
| Flexor-related MNs | |||
| unidentified INs | |||
| EphA4 INs (dI4, dI5) | Glutamate | MNs | Non-weight-bearing left-right coordination (dI4 and dI5) |
| Ipsilateral inhibitory interneurons | |||
| En-1-expressing V1 INs | GABA/glycine | Locomotion speed* | |
| Numerous (>19) subpopulations | MNs to specific muscles | ||
| 20% comprise: | |||
| RCs | Reciprocal MNs | ||
| Ia INs | |||
| Ia INs (V1) | Flexor-related MNs | Flexor-extensor alternation (V1+V2b)* | |
| GATA3/4-expressing V2b INs | GABA/glycine | Extensor-related MNs | Flexor-extensor coordination (V1+V2b)* |
| GAD65-expressing dorsal INs | GABA/glycine | MNs | Termination of rhythmic motor output |
| Commissural excitatory interneurons | |||
| Dbx1-Evx1-expressing V0v INs | Glutamate | Contralateral inhibitory INs | High-speed left-right coordination* |
| Contralateral RCs | |||
| Contralateral Ia INs | |||
| Sim1-expressing V3 INs | Glutamate | <15% Ipsilateral MNs | |
| Sim1-expressing V3 INs | Glutamate | Contralateral MNs | Left-right coordination* |
| Contralateral RCs | |||
| Contralateral Ia INs | |||
| Comprised of: | |||
| Ventral V3 (V3v) | Contralateral MNs | Left-right coordination* | |
| Dorsal V3 (V3d) | Contralateral INs | Weight bearing | |
| Left-right coordination* | |||
| Commissural inhibitory interneurons | |||
| Dbx1-Pax7-expressing V0d INs | GABA/glycine | Contralateral MNs | Low-speed left-right coordination |
| WT1-expressing dI6 INs | GABA/glycine | Left-right coordination? | |
| Subpopulation dI6 | ? | Ipsilateral INs? | Rhythm generation? |
| Subpopulation dI6 | ? | Ipsilateral MNs? | Pattern formation? |
| GAD67-expressing ventral INs | GABA/glycine | Contralateral MNs | Left-right coordination |
| Contralateral RCs | |||
| Neurons in numerous laminae | |||
| Bilateral inhibitory interneurons | |||
| Dmrt3-expressing INs | GABA/glycine | Ipsilateral + contralateral MNs | High-speed movements |
| Flexor-extensor, left-right coordination* | |||
CPG, central pattern generation; INs, interneurons; MNs, motoneurons; RCs, Renshaw cells.
Locomotor activity examined in juvenile/adult mice.
Fig. 2.
Schematics of the known synaptic inputs and output connections of five ipsilaterally projecting excitatory interneurons (INs) in the locomotor circuitry. A: approximately half the population of V2a INs receive inputs from rhythmogenic interneurons. Type II V2a INs synapse onto ipsilateral MNs and type I INs innervate the commissural excitatory V0v INs and are involved in high-speed left-right locomotion. B: Shox2+ INs possess rhythmogenic properties are thought to be part of rhythm-generating networks. They innervate other Shox2+ INs, flexor-related ipsilateral MNs as well as commissural INs. The interneurons play a role in generating low-speed left-right alternating motor bouts. C: V0c INs modulate the firing frequency of ipsilateral extensor-related MNs. D: Hb9 INs display rhythmogenic properties and are thought to be a functional component of the rhythm-generating circuitry. The interneurons are electrically coupled and they innervate primarily flexor-related ipsilateral MNs. E: EphA4 INs project to ipsilateral MNs. EphA4-expressing interneurons derived from dI4 and dI5 progenitor domains extend ectopic contralateral projections to MNs in EphA4 knockout mice (not shown).
Ipsilateral V2a glutamatergic interneurons.
LIM/homeobox protein Lhx3 defines ventral interneurons that arise from the domain pV2. Postmitotically the interneurons develop into populations of both excitatory and inhibitory interneurons with ipsilateral target neurons (Goulding et al. 2002; Peng et al. 2007). V2a INs are excitatory glutamatergic neurons delineated by the expression of the transcription factor Chx10 (Lundfald et al. 2007), and V2b INs are inhibitory GABAergic/glycinergic interneurons marked by the transcription factors GATA2/3 (Al-Mosawie et al. 2007). The V2a population is probably the best characterized class of ipsilateral excitatory interneurons in the rodent locomotor circuitry. Based on the expression of the vesicular glutamate transporter 2 (vGlut2) in spinal neurons, the V2a INs comprise ~30% of glutamatergic interneurons in the ventral horn (Crone et al. 2008). Morphological analyses demonstrated that the interneurons form synaptic contacts onto 1) Dbx1-Evx1-expressing V0v commissural excitatory interneurons (Crone et al. 2008) that play an important role in high-speed locomotion and 2) ipsilateral MNs (Dougherty and Kiehn 2010b; Lundfald et al. 2007; Stepien et al. 2010) (Fig. 2A).
Locomotor-like rhythmic firing in V2a INs is phase locked with bursts of ipsilateral motor outputs initiated by neuroactive substances, electrical stimulation of peripheral afferents, or brain stem projections in the isolated brain stem-spinal cord preparations (Crone et al. 2008; Dougherty and Kiehn 2010b; Zhong et al. 2010). Genetic ablation of V2a INs during embryonic development does not change the frequency of drug-induced fictive locomotion or the ipsilateral flexion-extension alternations, but the pattern of left-right alternation is disrupted at high-frequency locomotion (Crone et al. 2008). Kinematic analyses of treadmill stepping of adult transgenic mouse in which V2a INs have been ablated show alternating gaits (trotting) at low speed that transition to synchronous gaits (galloping) at high-speed locomotion (Crone et al. 2008; Crone et al. 2009; Zhong et al. 2010; Zhong et al. 2011a). Synaptic drive from sensory afferents and brain stem stimuli fail to activate the locomotor circuitry in mutant mice, suggesting that V2a INs receive excitatory drive from multiple sources including intra/intersegmental rhythm-generating neurons, sensory afferents and brain stem projections. Computational models constructed to include interactions between spinal interneurons involved in speed-dependent left-right coordination have proposed that at high-speed locomotion V2a INs regulate the activity patterns of commissural excitatory V0v INs (Shevtsova et al. 2015).
The contribution of rhythmically firing V2a INs to the coordination of left-right alternating motor outputs was further examined by analyzing episodes of spontaneous MNs deletions and rhythm perturbations during drug- and neural-induced fictive locomotion (Zhong et al. 2012). The majority of the deletions do not change the phase onset of subsequent cycles, categorizing them as nonresetting deletions. The rhythmic V2a INs comprise two groups that function differently during the deletions. Type II V2a INs, similar to MNs, cease firing during the ipsilateral deletions. This group of interneurons synapse onto MNs (Harris-Warrick RM, personal communication) and presumably regulate MN activity patterns. Type I rhythmic V2a INs (60% of the population) exhibit unperturbed oscillatory activity during ipsilateral deletions and they may drive commissural interneurons such as V0v INs (Fig. 2A) that are involved in high-speed left-right alternations (Talpalar et al. 2013).
The V2a population comprises heterogeneous subgroups with diverse electrical and morphological properties as well as synaptic connectivity (Crone et al. 2008; Dougherty and Kiehn 2010a). The subgroups differ in their recruitment during low- and high-speed locomotor activity. Half the population of V2a INs is rhythmically driven by excitatory synaptic inputs, and the percent of rhythmically firing V2a INs increases at higher motor cycle frequency (Zhong et al. 2010). It has been proposed that their higher firing frequencies results from increased excitatory drive from rhythm-generating neurons that recruit V2a INs with subthreshold oscillations (Zhong et al. 2011a). The other half of the V2a population does not receive rhythmic synaptic drive during drug-induced fictive locomotion and their function in the locomotor circuitry is yet to be determined.
The recruitment of different groups of interneurons at increasing speeds has been studied extensively in zebrafish. In the larval zebrafish, the activity of interneurons controlling swimming at higher speeds is correlated with the silencing of interneurons that are recruited during swimming at lower speeds (McLean et al. 2008). The V2a INs constitute a phylogenetically conserved interneuronal population, and in zebrafish V2a INs provide excitatory locomotor drive to MNs (Kimura et al. 2006) and their genetically driven ablation significantly impairs the generation of rhythmic locomotion (Eklöf-Ljunggren et al. 2012). Furthermore, selective optogenetic activation of this population initiates coordinated rhythmic movements (Ljunggren et al. 2014), providing additional evidence that V2a INs are essential for generating locomotor rhythms in the zebrafish spinal cord. Three subpopulations of V2a INs have been identified in the adult zebrafish as part of microcircuits architecture that control the recruitment of slow, intermediate, and fast MNs (Ampatzis et al. 2014). Whether a similar subspecialization of projections to MN subtypes exists in other vertebrate classes, including mammals, is currently unknown. Unlike the mouse locomotor circuitry, a subpopulation that coordinates high-speed left-right alternating movements has not been identified in zebrafish. The concept of a network organization that encodes locomotor speed by adding microcircuits at increasing locomotor frequency highlights the complexity of the organization of neural circuits controlling basic movements in vertebrates.
Ipsilateral Shox2-expressing non-V2a glutamatergic interneurons.
Ipsilaterally projecting excitatory interneurons that express the homeodomain transcription factor Shox2 partially overlap with V2a INs (Dougherty et al. 2013). Based on the expression of Shox2 and/or Chx10, the interneurons are divided into three subpopulations: Chx10-expressing V2a INs (V2a INs), Shox2-Chx10-expressing V2a INs (Shox2 V2a INs), and Shox2-expressing, non-Chx10-expressing INs (Shox2 INs). Shox2 INs are distributed in the lumbar intermediate zone along the mediolateral axis. Morphological analyses and electrophysiological recordings demonstrate that Shox2 INs synapse onto other Shox2 INs and commissural interneurons (Fig. 2B). Analyses of their rostral-caudal distribution reveal that a significant percent of them make direct contacts onto MNs located in flexor-related L1–L3 segments (Dougherty et al. 2013), the presumed dominant segments generating drug-induced fictive locomotion (Kiehn and Kjaerulff 1998). The majority of Shox2 INs fire rhythmically and in phase with ipsilateral motor bouts during induced locomotor-like activity.
Ablation of the subpopulation Shox2 V2a INs increases the variability of the amplitude and duration of locomotor cycles, but it neither affects the frequency nor the coordinated pattern of left-right/flexor-extensor alternations (Dougherty et al. 2013), suggesting that the interneurons are not involved in rhythm-generating or rhythm-coordinating circuits. In contrast, blocking the output of Shox2 INs by a specific Vglut2 inactivation or optogenetic silencing, significantly reduces the frequency of drug-induced or brain stem-evoked locomotor-like activity without changing the left-right/flexor-extensor alternating patterns. These findings suggest that Shox2 INs are functionally integrated into rhythm-generating circuits in the mouse spinal cord. It has been speculated that, through their synaptic connectivity with commissural interneurons, Shox2 INs together with yet undetermined population of ipsilateral excitatory interneurons control left-right alternations at low-speed locomotion (Dougherty et al. 2013).
Ipsilateral V0c cholinergic interneurons.
V0 INs develop from pV0 progenitor cells at the dorsal-ventral border of the developing neural tube (Pierani et al. 1999), and postmitotically they give rise to three subpopulations: ipsilaterally projecting V0c INs, commissural excitatory V0v INs, and commissural inhibitory V0d INs. V0c INs are marked by the homeodomain protein Pitx2 and most of them are cholinergic in nature (Zagoraiou et al. 2009). The interneurons are positioned in small clusters close to the central canal, and their axons project ipsilaterally to synapse onto MNs (Fig. 2C). V0c INs have been identified as the single source of cholinergic C-boutons, (Zagoraiou et al. 2009), solving a long-standing query regarding the identity of the neuronal population that establishes these unique synaptic terminals on somata and proximal dendrites of MNs (Li et al. 1995; Nagy et al. 1993). C-boutons are associated with muscarinic receptor type 2 (m2) and Kv2.1 type potassium channels (Hellström et al. 2003; Muennich and Fyffe 2004) that reduce the amplitude of action potential hyperpolarization, resulting in MN firing at higher frequency (Miles et al. 2007).
Drug-induced fictive locomotion triggers episodes of rhythmic firing in V0c INs that are phase locked with ipsilateral rhythmic motor outputs, raising the possibility that the interneurons are functional components of locomotor circuits (Zagoraiou et al. 2009). To determine their role in regulating motor behavior, right-left and flexor-extensor alternations were analyzed using EMG recordings from the gastrocnemius (Gs) and tibialis anterior (TA) muscles in adult mice. Selective ablation of choline acetyltransferase (ChAT) in Pitx2-expressing V0c INs silences their synaptic output, but it does not affect the alternating gait pattern. However, it limits the normally twofold increase in Gs EMG activity seen during swimming compared with overground locomotion (Zagoraiou et al. 2009). Presumably, the reduced Gs EMG amplitude is due to loss at C-synapses of m2 receptor-mediated increases in MN firing frequency. Thus V0c INs represent a distinct class of neuromodulatory cholinergic interneurons that increase the gain in extensor motor output for effective swimming locomotor behavior. It is yet to be determined whether these interneurons are similarly recruited in other locomotor activities required to facilitate extensor drive [e.g., upslope locomotion (Carlson-Kuhta et al. 1998)].
Ipsilateral Hb9-expressing glutamatergic interneurons of unknown progenitor origin.
The progenitor origin of the interneurons that continue to express the homeobox gene Hb9 postnatally (Hb9 INs) is unknown, but the embryonic expression of HB9 occurs in multiple interneuronal classes (Caldeira et al. 2017). The Hb9 gene is a selective marker for spinal MNs (Arber et al. 1999); therefore it is conceivable that Hb9 INs develop from the same neural progenitors as MNs. Hb9 INs are clustered in medial lamina VIII of the lower thoracic and upper lumbar segments of the spinal cord, regions with robust rhythmogenic activity along the rostrocaudal and ventrodorsal axes (Kjaerulff and Kiehn 1996; Kremer and Lev-Tov 1997). Morphological and immunohistochemical observations suggest that they are premotor interneurons with axonal projections that terminate on MNs (Ziskind-Conhaim et al. 2010). Electrical coupling can synchronize the activity between clustered Hb9 INs (Fig. 2D) (Hinckley and Ziskind-Conhaim 2006; but see Wilson et al. 2007) in newborn and juvenile mice. Spontaneous, synchronous bursts of excitatory postsynaptic currents in coupled Hb9 INs suggest that their activity is coordinated by common synaptic inputs (Hinckley and Ziskind-Conhaim 2006). Drug-induced locomotor-like oscillations in Hb9 INs are phased-locked with ipsilateral motor bouts (Hinckley et al. 2005b; Wilson et al. 2005), and their rhythmic activity is independent of fast excitatory and inhibitory synaptic transmission. The induced rhythmic activity is generated by persistent sodium current (Tazerart et al. 2008; Ziskind-Conhaim et al. 2008), a prevalent current in various rhythmogenic CNS neurons.
Low-intensity stimulation of dorsal roots, presumably via projections from muscle spindle afferents, generates relatively short-latency excitatory postsynaptic currents in Hb9 INs (Hinckley et al. 2010). Moreover, axon terminals of dorsal root afferents immunoreactive for VGluT1, a marker for primary afferent projections (Alvarez et al. 2004; Oliveira et al. 2003), are in close apposition to dendrites and somata of Hb9 INs, suggesting that neonatal Hb9 INs are innervated by proprioceptive inputs. Stimulation of flexor-related afferents during the flexor but not the extensor phase resets the oscillatory activity to flexion phase onset (Hinckley et al. 2010). These findings are consistent with the observations that proprioceptive afferents reset locomotor activities in the cat spinal cord during limb flexion but not extension (Rybak et al. 2006). In comparison, higher threshold flexor reflex afferents can reset L-dopa fictive locomotion in spinal cat to flexor onset regardless of locomotor phase [e.g., prolonged flexion during submit flexion and resetting to flexion during extensor phase (Schomburg et al. 1998)]. Together, the findings support the concept that Hb9 INs are selective and possibly integral to flexor rhythm-generation in the neonatal mouse spinal cord.
To confirm that Hb9 INs are functional components of the rhythm-generating circuits, it is essential to determine whether their removal or silencing their synaptic output perturbs the induced motor outputs and gait patterns. Selective Vglut2 inactivation and silencing glutamate release from Hb9 INs results in a significant attenuation of the frequency of drug-induced and descending fiber-evoked locomotor rhythms in newborn mice (Caldeira et al. 2017). The disruption of glutamatergic synaptic release does not affect right-left or flexor-extensor alternating patterns, and a specific silencing of glutamatergic transmission from MNs does not affect locomotor frequencies (Caldeira et al. 2017). These findings conclusively support the model that Hb9 INs are involved in rhythm generation in the mouse locomotor CPG.
That the onset of rhythmic episodes of MN spiking in L1–L3 ventral roots can occur before spiking in Hb9 INs calls into question the role of Hb9 INs in rhythm-generating networks (Kwan et al. 2009). However, it should be noted that axons emanating from L1–L3 ventral roots include axonal projections from preganglionic sympathetic neurons as well as lateral and medial motor column MNs that innervate the limb and axial musculature, respectively (Vanderhorst and Holstege 1997). Therefore, it is not surprising that there are small differences in spiking onset. To relate Hb9 IN spike timing to motor activity in specific limb muscles, it is essential to perform simultaneous recordings from Hb9 INs and identified motor pools.
Ipsilateral EphA4-positive glutamatergic interneurons of unknown progenitor origin.
EphA4, a tyrosine kinase axon guidance receptor, is expressed in growing axons of excitatory spinal neurons (Restrepo et al. 2011), and its effect on axonal pathfinding influences the organization of spinal cord networks. The interaction between EphA4 and the midline-expressed chemorepellent EphrinB3 prevents EphA4-expressing axonal projections from crossing the midline (Beg et al. 2007; Kullander et al. 2003; Wegmeyer et al. 2007). EphA4-positive INs synapse onto MNs (Fig. 2E) and their induced locomotor-like rhythmic firing is in phase with ipsilateral motor activity (Butt et al. 2005). Genetic manipulations that perturb the EphhA4 signaling pathways lead to aberrant midline crossing of ipsilateral excitatory interneurons, resulting in a rabbitlike hopping locomotor phenotype (Butt et al. 2005; Kullander et al. 2003). Gait transition from alternating left-right hindlimb movements to synchronized, hopping behavior persisted in adult knockout mice (Akay et al. 2006).
The change in motor behavior can be attributed to midline crossing of deviant axonal projections of excitatory neurons either in the spinal cord and/or in the brain. Selective deletion of EphA4 signaling in the forebrain that includes corticospinal tract neurons or in spinal neurons demonstrates that EphA4 expression in spinal glutamatergic interneurons not cortical neurons is essential for the assembly of functional locomotor circuitry and the alternating gait patterns (Borgius et al. 2014). EphA4 is expressed in a subset of V2a INs, raising the possibility that ipsilaterally projecting excitatory V2a INs comprise a major component of the aberrant crossover projections in EphA4 knockout mice. However, midline crossing in the mutant mice does not originate from Chx10-expressing V2a INs (Lundfald et al. 2007), rather EphA4 mutation targeting Lbx1-marked neurons leads to aberrant crossover in the dorsal horn (Paixão et al. 2013). Many of the Lbx1-expressing neurons derived from dI4–dI6 progenitor domains are premotor interneurons (Tripodi et al. 2011) with wide distribution in the spinal cord. dI4–dI5 INs are positioned in the dorsal horn whereas dI6 INs are distributed in lamina VIII. Transsynaptic virus-based methods have been used to identify dI4 and dI5 subpopulations as the primary source of ectopic contralateral projections to bilateral MNs in EphA4-null mice (Fig. 2E) (Satoh et al. 2016). These mice have alternating gait walking on ground, but synchronous gait during decreased weight-bearing movements such as swimming. It is yet to be determined what subpopulation of interneurons is responsible for overground synchronized hindlimb movements.
Rhythm generation in the absence of glutamatergic synaptic transmission.
To date, multiple subclasses of ipsilateral excitatory INs have been identified as probable components of the locomotor rhythm-generating circuits in the mouse spinal cord, but none is capable of completely abolishing motor rhythmogenesis when silenced. The general consensus is that neuroactive substances activate ipsilateral excitatory interneurons that trigger the onset of persistent locomotor-related rhythmic activity in the in vitro mouse preparation (Talpalar and Kiehn 2010; Whelan et al. 2000; for review, see Grillner 2006; Kiehn 2006). Both NMDA and non-NMDA receptors contribute to the induction of fictive locomotion in the isolated spinal cord of newborn rats (Cazalets et al. 1992), and in the neonatal mouse spinal cord the various ionotropic glutamate receptors independently regulate the speeds of locomotor-like activity. AMPA and kainate receptors mediate high-speed locomotor activity whereas NMDA receptors are involved in persistent, steady locomotion (Talpalar and Kiehn 2010). However, the concept that glutamatergic transmission is essential for rhythmic activity has been challenged by the generation of drug-induced fictive locomotion in embryonic Vglut2 mutant mice, in which left-right alternating motor bouts persist in the absence of glutamatergic transmission (Wallén-Mackenzie et al. 2006, Talpalar et al. 2011). The persistence of flexor-extensor and left-right alternating rhythmic activity in Vglut2 mutant mice implies that reciprocal inhibitory interactions between Ia INs projecting to antagonist MNs and between left-right commissural inhibitory INs can support motor coordination consistent with hindlimb locomotion (Talpalar et al. 2011). However, it is important to note that inactivation of glutamate release does not affect the expression of NMDA receptors, and rhythm generation in these studies required bath-applied NMDA as a source of excitatory glutamatergic drive. Therefore, it seems more likely that excitatory circuits normally generate and drive the rhythms that are further coordinated by reciprocal interactions. Reciprocal inhibitory synaptic actions between neurons expressing postinhibitory rebound spiking is fundamental to sustain stable antiphasic oscillations in many neural circuits (Friesen 1994; Miller and Selverston 1982a, 1982b; Perkel and Mulloney 1974), including the vertebrate locomotor CPG in Xenopus tadpole (Moult et al. 2013). Additional form of synaptic compensation that might underlie the generation of rhythmic activity in the absence of glutamatergic transmission is the increased efficacy of excitatory cholinergic transmission from spinal neurons that corelease glutamate and acetylcholine (Li et al. 2004; Liu et al. 2009; Mentis et al. 2005).
Ipsilateral Inhibitory Interneurons
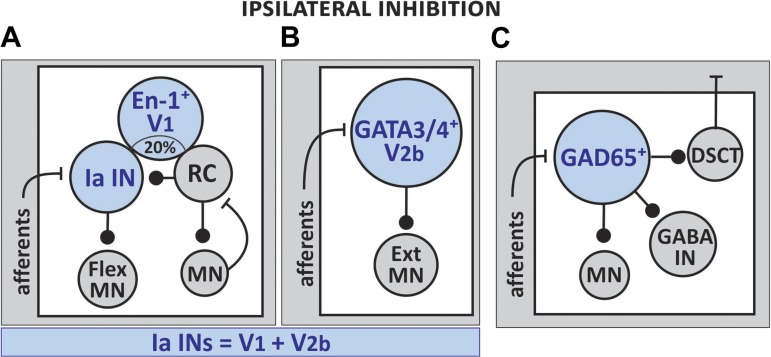
Premotor ipsilaterally projecting inhibitory interneurons are the core components of spinal microcircuits that: 1) coordinate alternating flexor-extensor muscle contractions during leg movements (Fu et al. 1975; Kjaerulff and Kiehn 1996; Lanuza et al. 2004) and 2) modulate the speed of locomotor activity (Gosgnach et al. 2006). To date, two groups of molecularly defined last-order interneurons have been shown to affect the pattern of flexion-extension movements, and together they encompass all the reciprocal inhibitory interneurons in the neonatal mouse spinal cord (Zhang et al. 2014). The interneurons are derived primarily from progenitor domains pV1 and pV2, and give rise to two postmitotic populations of inhibitory ipsilateral interneurons: 1) GABAergic/glycinergic, engrailed 1-expressing V1 INs, and 2) GABAergic/glycinergic, GATA3/4-expressing V2b INs. A dorsally located population of glutamic acid decarboxylase (GAD)-65-expressing INs of unknown progenitor domain has also been identified as a component of the locomotor circuitry (Table 1, Fig. 3).
Fig. 3.
Schematics of the known input-output connections of three ipsilaterally projecting inhibitory INs. The three populations project directly to ipsilateral MNs. A: a fraction (~20%) of V1 INs includes Renshaw cells (RC) and reciprocal inhibitory interneurons Ia (Ia INs). Ia INs synapse primarily onto flexor-related MNs. B: a fraction (~20%) of V2b INs includes Ia INs. The interneurons synapse primarily onto extensor-related MNs. Together, V1 and V2b INs account for the entire population of Ia INs and control flexor-extensor alternating locomotor activity. C: GAD65-expressing INs project to ipsilateral MNs, other GABAergic INs, and dorsal spinocerebellar tract (DSCT) neurons.
Ipsilateral V1 interneurons.
The ventral interneurons derived from the progenitor domain pV1 express the homeobox proteins Dbx2 and Nkx6–2, and postmitotically they express the transcription factor engrailed-1 (En-1). V1 INs differentiate into heterogeneous populations of ipsilaterally projecting premotor inhibitory interneurons located primarily in close proximity to MNs (Saueressig et al. 1999). The V1 INs include Renshaw cells (RCs) and the reciprocal inhibitory interneurons Ia (Ia INs) that in the adult spinal cord account for only 9% and 13–29% of the V1 neural population, respectively (Alvarez et al. 2005; Zhang et al. 2014).
renshaw cells.
RCs are innervated by axon collaterals of alpha MNs innervating distal musculature, and they form reciprocal synaptic contacts on MNs that excite them (Fig. 3A), thus establishing a negative feedback mechanism that regulates the firing rate of MNs (for review, see Hultborn 2006). The neurons also innervate Ia INs that receive monosynaptic excitatory inputs from muscle spindle afferents, and they synapse onto MNs that innervate antagonistic muscles (Eccles et al. 1957). Given this synaptic organization, RCs cannot be fundamental to the CPG network operation.
reciprocal inhibitory interneurons.
V1-derived En-1-expressing Ia INs are integral components of the local circuitry that plays an important role in reciprocal inhibition between flexor-extensor MNs. They are part of a segmental network in which active Ia afferents excite MNs of agonist muscles and through Ia INs they prevent the firing of MNs of antagonist muscles, thereby establishing reciprocal inhibition between flexor-extensor MNs (Eccles et al. 1956; Feldman and Orlovsky 1975). In the adult mouse, 80% of these interneurons are glycinergic and about a third are GABAergic, raising the possibility of glycine/GABA corelease at target neurons (Benito-Gonzalez and Alvarez 2012; Gao and Ziskind-Conhaim 1995). The loss of Ia INs might be expected to initiate flexor-extensor coactivation, but drug-induced flexor-extensor and left-right alternating patterns remain unperturbed in neonatal mice following their substantial loss or acute silencing (Gosgnach et al. 2006). Instead, burst durations are lengthened causing a three- to fourfold decrease in fictive locomotion frequency. Important behavioral support of the role of V1 INs in the generation of faster locomotor speeds has been demonstrated in adult En-1 knockout mice that fail to execute rapid stepping at higher rotarod speeds (Gosgnach et al. 2006).
Ia INs comprise a fraction (13–29%) of the V1 population (Alvarez et al. 2005; Zhang et al. 2014), and most of V1-derived neurons remained uncharacterized until recently when microarray analysis revealed a surprisingly broad genetic heterogeneity within V1 population. The interneurons can be divided into numerous molecularly defined subpopulations based on the expression of 32 transcription factors (Bikoff et al. 2016). Antibodies were generated for 19/32 of the transcription factors, and based on their molecular identity, the 19 marked V1 subpopulations are spatially segregated along the dorsoventral and mediolateral axes. Electrophysiological recordings show that the 19 diverse subpopulations express different biophysical properties. Bayesian sparse regression analysis on the same population of neurons predicts that if a full transcription factor analysis were done there would be ~50 functionally distinct V1 subpopulations distributed in defined spatial domains (Gabitto et al. 2016). It is possible that ensembles of numerous discrete microcircuits are designed to have distinct fractionated control regulating complex motor activity. The spatial segregation of V1 subpopulations relative to specific MN pools demonstrates particular distribution patterns close to MN pools innervating proximal and distal muscles. The most ventrally positioned V1R INs are located close to gluteus MNs that innervate the hip extensor, and V1Sp8 INs are distributed most dorsally in lamina VII, close to tibialis anterior and intrinsic foot MNs innervating the ankle flexor and foot plantar-flexor muscles, respectively (Bikoff et al. 2016). The organization of interneuronal subgroups in specific spatial positions is suitable for receiving selective inputs from specific proprioceptive afferents, and the establishment of diverse inhibitory microcircuits that modulate the activity of motor outputs to specific classes of limb muscles. It is conceivable that these subpopulations are constituents of modular, loosely coupled UBGs, so that V1Sp8 INs, for example, are associated functionally with modular UBGs at the ankle joint, and they regulate alternating activity around the ankle.
Ipsilateral V2b interneurons.
Ventral interneurons derived from the progenitor domain pV2 give rise to at least two subpopulations: 1) Chx10-expressing V2a excitatory interneurons, and 2) GATA3/4-expressing V2b inhibitory interneurons (Karunaratne et al. 2002). Both subpopulations settle in lamina VII, form synaptic contacts with ipsilateral MNs, and receive synaptic inputs from primary afferents in the adult mouse spinal cord (Fig. 3B) (Al-Mosawie et al. 2007). Similar to V1 INs, a fraction (~20%) of V2b INs fit the morphological properties of Ia INs, and it has been shown that, collectively, V1 and V2b account for the entire population of Ia INs (Zhang et al. 2014). Inactivation of V2b synaptic input increases the phase variability of flexor-extensor alternating fictive locomotion, but the phase coordination remains alternating, not synchronous as expected if V2b INs are solely responsible for flexor-extensor alternating activity. Drug-induced synchronous flexor-extensor rhythmic activity in the isolated mouse spinal cord occurs only when synaptic transmission is inactivated in both V1 and V2b INs (Zhang et al. 2014).
The specific functions of V2b and V1 INs in regulating flexor-extensor alternation was assessed in juvenile and adult mice in which diphtheria toxin receptor was expressed selectively in V2b and/or V1 INs and ablating them by exposure to diphtheria toxin (Britz et al. 2015). Using this approach, it has been demonstrated that the two populations have opposite actions on limb movements. Ablation of V1 INs results in limb hyperflexion whereas ablation of V2b leads to hyperextension, suggesting that V1 INs are essential for inhibiting flexor-related MNs whereas V2b are required for inhibiting extensor-related MNs. Their different actions on flexion-extension movement has been attributed to their preferential innervation of MN pools: V1 INs innervate the ankle flexor MNs, and V2b INs innervate the ankle extensor MNs (Britz et al. 2015).
Ipsilateral GAD65-expressing dorsal horn interneurons of unknown progenitor origin.
Glutamic acid decarboxylase-65 (GAD65)-expressing interneurons in medial laminae V/VI have been identified as ipsilateral inhibitory interneurons with direct synaptic inputs onto MNs, other GABAergic interneurons in the same area, and neurons in Clarke’s column (Fig. 3C) (Wilson et al. 2010). The dorsomedial GAD65::GFP interneurons are innervated by low-threshold afferents, presumably Ia muscle spindle afferents. Based on c-Fos expression, an indirect measure of activity, there is a ninefold increase in the number of active interneurons during overground locomotion. Based on calcium transients recorded during drug-induced fictive locomotion in the isolated hemisected spinal cord, the interneurons are rhythmically active during the interburst phase of ipsilateral bouts of motor activity. GAD65::GFP INs provide direct rhythmic inhibitory inputs to MNs and contribute to the termination of ipsilateral oscillatory motor activity. The interneurons are positioned in the same region as group I nonreciprocal inhibitory interneurons described in the cat spinal cord (Brink et al. 1983; Jankowska et al. 1981), and they share similar physiological/synaptic properties.
Commissural Excitatory Interneurons
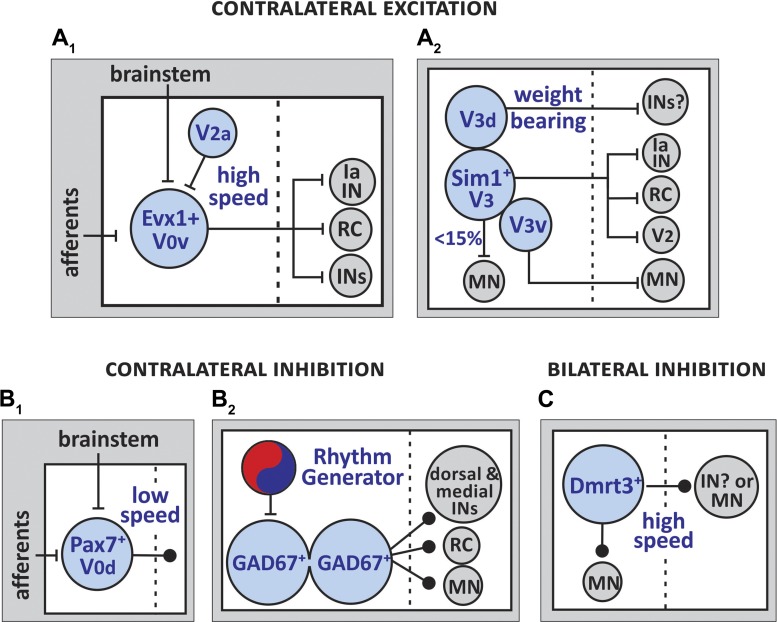
Commissural interneurons are key constituents of the pattern-generating circuits that regulate left-right limb coordination. Contralaterally projecting excitatory interneurons that act directly on MNs and interneurons are responsible for 1) crossed excitation and the transition from alternating to synchronous gaits and 2) crossed inhibition by acting on premotor inhibitory interneurons in the contralateral side of the cord (Bannatyne et al. 2003; Björnfors and El Manira 2016; Quinlan and Kiehn 2007). In the mouse spinal cord, commissural excitatory interneurons originate primarily from pV0 and pV3 progenitor domains and postmitotically give rise to: 1) glutamatergic, Evx1-expressing V0v INs and 2) glutamatergic Sim-1-expressing V3 INs (Table 1, Fig. 4A).
Fig. 4.
Schematics of known synaptic inputs and output connections of contralaterally projecting excitatory INs (A), contralaterally projecting inhibitory INs (B), and bilaterally projecting INs (C). A1: V0v INs project contralaterally to innervate Ia INs, RCs, and other INs. The interneurons modulate high-speed left-right alternating motor bouts. A2: the majority of V3 INs project contralaterally to innervate Ia INs and RCs. The subpopulation V3v innervates contralateral MNs and V3d INs synapse onto undefined INs. As a population, V3 INs coordinate left-right alternating motor outputs. B1: V0d inhibitory INs project contralaterally and play a role in low-speed left-right alternating locomotion. B2: GAD67-expressing INs are electrically coupled. They receive synaptic inputs from rhythmogenic neurons and they innervate contralateral MNs, RCs, and INs in the medial and dorsal areas of the spinal cord. C: Dmrt3+ inhibitory INs project to ipsilateral MNs and contralateral INs and/or MNs. The interneurons contribute to the generation of high-speed left-right alternating limb movements.
Commissural V0v interneurons.
V0 INs develop from pV0 progenitor cells, the dorsal region of the ventral neural tube, and postmitotically they give rise to three subpopulations with different neurotransmitter phenotypes: 1) ventrally located commissural glutamatergic V0v INs that constitute the majority (58%) of V0 INs (Griener et al. 2015), 2) dorsally positioned commissural GABAergic/glycinergic inhibitory V0d INs (Lanuza et al. 2004), and 3) medially located ipsilaterally projecting Pitx2-expressing cholinergic V0c INs described earlier (Zagoraiou et al. 2009). Both V0v and V0d subpopulations receive synaptic contacts from serotonergic fibers and primary afferents. V0v and V0d arise from Dbx1-expressing progenitors (Pierani et al. 2001), but postmitotically V0v express the homeodomain protein Evx1 and V0d INs express the transcription factor Pax7 (Moran-Rivard et al. 2001). V0v INs constitute part of the disynaptic inhibitory pathways via excitation of contralateral inhibitory interneurons, including RCs and Ia INs (Fig. 4A). Genetic ablation of V0 INs in Dbx1 mutant mice results in synchronous left-right fictive motor pattern (Moran-Rivard et al. 2001), demonstrating that the interneurons contribute to left-right locomotor activity (Lanuza et al. 2004; Quinlan and Kiehn 2007). The quadrupedal rabbitlike hopping with synchronized leg movements in V0-ablated adult mice provides additional support for the concept that V0 INs are involved in the coordination of left-right alternating limb movements (Talpalar et al. 2013).
Taking advantage of the different molecular markers of V0v and V0d, a recent study conducted both in vivo and in vitro mouse models examined the contribution of these transmitter-defined subpopulation to left-right locomotion at different speeds (Talpalar et al. 2013). It appears that V0v and V0d subpopulations are organized as part of a dual-mode, speed-dependent operation of networks responsible for left-right alternating activities (Fig. 4, A1–B1). Selective ablation of the inhibitory V0d INs results in left-right alternating limbs at high frequency, but the alternations are not present at low frequency. In contrast, V0v-deficient mice are capable of performing left-right limb movements at low but not high frequency. Thus it is apparent that V0d INs are recruited to the circuitry coordinating left-right alternations during low-speed movements, whereas V0v INs are active during high-speed movements.
Computational models have been constructed to better understand the functional integration of identified interneurons controlling speed-dependent left-right alternations in the locomotor CPG (Shevtsova et al. 2015). The models can reproduce experimental data of left-right coordinated motor outputs at various speeds in wild-type mice and in mice with a specific ablation of neuronal population(s). They include left-right rhythm-generating circuitry with three parallel networks that incorporate V2a–V0v, V0d, and V3 interneurons that regulate left-right limb movements at different speeds (Shevtsova et al. 2015).
Commissural V3 interneurons.
V3 INs are derived from Nkx2.2-expressing p3 progenitors and are postmitotically marked by the transcription factor Sim1 (Briscoe et al. 1999; Goulding et al. 2002; Sugimori et al. 2007). Early in embryonic development (embryonic day 12.5), 81% of V3 INs have ipsilaterally projecting axons, but by birth only a subset of them (<15%) project ipsilaterally, making synaptic contacts onto MNs (Fig. 4A2) (Zhang et al. 2008). It is unlikely that the ipsilaterally projecting V3 INs are integral component of the rhythm generating networks, because only a small fraction of them exhibit intrinsic membrane properties characteristic of rhythmogenic interneurons. It is unknown whether the same population of V3 INs or yet to be identified molecularly distinct subpopulations project axons to both sides of the spinal cord. Nevertheless, the interneurons serve a unique role in the locomotor circuitry by influencing motor outputs in the two sides of the cord.
V3 INs play a significant role in contralateral excitation of MNs and inhibitory interneurons. It has been shown that they provide ~20% of the VGlut2+ contacts onto MNs and ~25% of the VGlut2+ contacts onto RCs and Ia INs (Zhang et al. 2008). Selective suppression of V3 synaptic output using tetanus toxin significantly reduces the robustness and regularity of induced fictive locomotion. Similarly, acute decrease of V3 INs excitability by ligand-induced selective activation of the hyperpolarizing allatostatin insect receptor in the neonatal mouse in vitro preparation disrupts the pattern of rhythmic motor outputs with a significant increase in the variability of flexor-related burst duration between the left-right sides of the cord (Zhang et al. 2008). To determine the role of V3 INs in walking behavior of adult mice, the interneurons were silenced using allatostatin applied to lumbar segments. Similar to the in vitro data, kinematic analyses point to uncoordinated gait with variable timing and phasing of stance/swing stepping (Zhang et al. 2008). Taken together, the findings suggest that V3 INs are required for normal walking gait by coordinating motor outputs from the left-right sides of the spinal cord.
Additional support for the role of V3 INs in the locomotor circuitry comes from experiments that disrupt other axonal projections that cross the midline but leave V3 crossing projections intact. Netrins are axon guidance molecules that bind to the netrin receptor DCC and provide chemotrophic cues for commissural axons (Kennedy et al. 1994). The majority of commissural interneurons fail to cross the midline in netrin-1 knockout mice, but the mutation does not affect V3 axonal projections (Serafini et al. 1996). Synchronous left-right motor activities are preserved in netrin-1 mutant mice (Rabe Bernhardt et al. 2012), supporting the role of V3 INs in stabilizing gaits and synchronizing the activities across the midline by controlling the excitatory and inhibitory drives between the two sides of the spinal cord.
V3 INs are divided into two subpopulations: V3v INs, ventral neurons distributed in both the medial area of laminae VII–VIII and X, and V3d INs that migrate dorsally during embryonic development and settle in deep dorsal horn neurons (Fig. 4A2) (Grossman et al. 2012). V3v and V3d INs exhibit heterogeneous morphological and electrophysiological properties that are indicative of their different functions in the locomotor circuitry (Borowska et al. 2013). V3v INs have relatively simple, small dendritic arbors and their axonal projections establish synaptic contacts on contralateral MNs. This subset of interneurons is capable of producing rapid tonic firing. V3d INs have larger and more complex dendritic arborizations and they do not contact contralateral MNs. They display diverse firing patterns and their overall firing frequency is significantly slower than that of V3v INs. Based on c-Fos expression, V3v INs are recruited during swimming and running whereas V3d INs are active only during running. These findings suggest that V3v INs contribute to limb coordination associated with general locomotor activities and the V3d INs serve as relay neurons that receive intense sensory inputs from cutaneous afferents and are likely to be recruited primarily during weight bearing gaits (Borowska et al. 2013).
Commissural Inhibitory Interneurons
Commissural inhibitory interneurons play a fundamental role in controlling left-right alternating locomotor activity (Jankowska and Noga 1990). In the mouse spinal cord, the interneurons are derived from neural progenitors pV0 and pd6 and develop postmitotically to include 1) Dbx1-expressing GABAergic/glycinergic V0d INs, and 2) Wt1-expressing dI6 INs. This group of commissural inhibitory interneurons also includes ventrally located glutamic acid decarboxylase-67-expressing interneurons of unknown progenitor domain (Table 1, Fig. 4B). The probable role of Pax7-expressing commissural inhibitory V0d INs in the locomotor circuitry was discussed above. A role for Dmrt3-expressing bilateral inhibitory dl6 INs is described below.
Commissural V0d interneurons.
V0d INs contribute to networks regulating low-speed left-right limb movements (Fig. 4B1) (Talpalar et al. 2013), but not flexor-extensor alternations that are controlled by ipsilateral inhibitory interneurons (V2b and V1 INs). It has been demonstrated that a cluster of V0d INs located lateral to the central canal receive primary afferent synaptic inputs and are active during drug-induced fictive locomotion. Interneurons in the same location are part of oligosynaptic pathway that project toward contralateral motoneurons (Griener et al. 2015), implying that this subset of V0 INs are involved in coordinating left-right alternation during fictive locomotion.
Commissural dI6 interneurons.
dI6 INs arise from progenitor domain pd6, just dorsal to the pV0 domain, and they migrate ventrally during embryogenesis to settle in medial laminae VII/VIII (Müller et al. 2002). Similar to their neighboring V0d INs, they express the transcription factors Pax7, but not Dbx1 (Gross et al. 2002; Pierani et al. 2001). It has been proposed that postmitotically these commissural inhibitory interneurons (Pillai et al. 2007) express the homeodomain factor Lbx1 (Gross et al. 2002; Rabe et al. 2009) and the transcription factor WT1 (Goulding 2009). In the absence of Dbx1 function, the number of Lbx1-expressing dI6-like INs increases significantly, suggesting that, postmitotically, potential V0d INs obtain the molecular characteristics of dI6 INs, supporting the theory that dI6 INs have similar role to V0d INs in the locomotor circuitry (Lanuza et al. 2004). In the absence of a specific molecular marker for dI6 INs it has been difficult to examine their function in the locomotor CPG.
To explore the role of dI6 in the locomotor circuitry, a transgenic mouse line has been generated in which dI6 INs located close to the central canal could be visualized for electrophysiological recordings (Dyck et al. 2012). Drug-induced fictive locomotion triggers rhythmic firing in most dI6 INs, but based on their intrinsic properties and firing patterns the interneurons belong to two subsets of interneurons (Dyck et al. 2012). One subset expresses nonlinear membrane properties characteristic of rhythm-generating neurons, and their firing patterns are loosely correlated with ipsilateral rhythmic motor bouts. The interneurons oscillate intrinsically when chemical synaptic transmission is blocked, a hallmark of neurons involved in rhythm generation. Dyck and colleagues (2012) have proposed that this is a group of excitatory interneurons that is part of the rhythm-generating networks that might innervate the Hb9 INs. The interneurons also contribute to rhythm coordination via their innervation of contralaterally projecting inhibitory dI6 INs. The second subset of excitatory dI6 INs does not exhibit intrinsic oscillatory properties and their rhythmic firing during fictive locomotion appears tightly correlated with rhythmic motor outputs. Based on the proposed model, these ipsilaterally projecting interneurons synapse onto MNs (Dyck et al. 2012). To validate the proposed model that the dI6 INs located close to the central canal are ipsilaterally projecting excitatory interneurons with roles in rhythm-generating and rhythm-coordinating networks, it is essential identify their molecular marker(s) and then characterize their neurotransmitter phenotype, axonal projections, and the effects of their acute activation or inactivation on the patterns of locomotor outputs.
Commissural GAD67-expressing ventral interneurons of unknown progenitor origin.
GABAergic interneurons play a major role in generating drug-induced left-right alternating fictive locomotion in the postnatal mouse spinal cord (Hinckley et al. 2005a; Ziskind-Conhaim 2013). It has been suggested that a group of GABAergic interneurons located in medial lamina VIII of GAD67::GFP transgenic mice is a functional component of the rhythm-coordinating networks in the locomotor circuitry. The interneurons are electrically coupled and they have distinct morphological properties with widespread multipolar dendritic arbors, suggesting that they receive synaptic inputs from various neuronal populations (Wu et al. 2011). The axonal projections of GAD67 INs branch into contralateral ventral, medial, and dorsal laminae to synapse onto diverse neuronal types including MNs and RCs (Fig. 4B2) (Wu et al. 2011). These findings imply that the interneurons integrate a large number of synaptic inputs that are transmitted to numerous neuronal targets in the contralateral side. Based on their similar electrophysiological and morphological properties, it is likely that they constitute a functionally homogenous population.
Drug-induced fictive locomotion triggers rhythmic excitatory drive to GAD67 INs that is correlated with membrane voltage oscillations, raising the possibility that the interneurons are innervated by rhythmogenic interneurons. Their oscillatory firing is out of phase with episodes of fictive contralateral motor outputs and phase locked with ipsilateral bursts of motor activity. GAD67::GFP INs express three voltage-gated channels often associated with currents generated in rhythmically active neurons: T-type calcium current, persistent sodium current, and hyperpolarizing-activated inward current (Wu et al. 2011). Taken together, it is reasonable to assume that the interneurons are integral part of networks of commissural inhibitory interneurons regulating contralateral local circuits responsible for alternating left-right motor activity. The genetic identity of this group of interneurons remains unknown, making it difficult to confirm their function in the locomotor CPG using genetic deletion or silencing.
Bilateral Inhibitory Interneurons
Interneurons that project both ipsilaterally and contralaterally might play a unique role in regulating flexor-extensor and left-right alternating locomotor activity. To date, only Dmrt3-expressing interneurons have been identified with certainty as bilateral inhibitory interneurons (Table 1, Fig. 4C).
Bilateral Dmrt3-expressing interneurons.
The transcription factor Dmrt3 overlaps with Pax2 and is expressed in dI4, dI6, and V0d INs. It has been shown that a mutation in the DMRT3 gene has a great impact on the pattern of locomotion in horses (Andersson et al. 2012). The effects on alternate gaits varies depending on the breed, but all are manifested at speeds higher than walking, in a variable manner that depends on the breed. Typically, a transition of three gaits is evident with increasing speed: walk, trot, and gallop. Horses with the mutation are able to perform alternate gait of trotting instead of galloping at high speed. To determine the role of Dmrt3-expressing interneurons in spinal circuits controlling stride, their probable functions have been examined in mice. The interneurons are positive for the vesicular inhibitory amino acid transporter (VIAAT) and negative for the transporter Vglut2, indicating that they are inhibitory neurons. Retrograde labeling and pseudorabies virus tracing demonstrate direct ipsi- and contralateral axonal projections from Dmrt3-expressing INs onto MNs (Fig. 4C) (for review, see Vallstedt and Kullander 2013). Therefore, it is conceivable that Dmrt3-expressing INs are capable of executing multiple functions in the locomotor circuitry.
Drug-induced fictive locomotion in the isolated neonatal spinal cords of Dmrt3 knockout mice are characterized by increased burst and interburst durations as well as uncoordinated left-right and flexion-extension outputs. As a consequence, left-right motor coordination in mutant mice is disrupted at high speed (Andersson et al. 2012). Gait analysis in the adult demonstrates a substantial increase in both swing time and stride length in all limbs. The size of the dI6 population as well as the neighboring dI5 and V0 populations does not change in Dmrt3 knockout mice, but the number of Wt1-expressing dI6 INs interneurons increases and the number of commissural interneurons decreases (Andersson et al. 2012), pointing to a change in the fate of these interneurons. It is apparent that Dmrt3-expressing interneurons are constituents of multiple subpopulations that arise from various progenitor cells and they have an important role in left-right and flexor-extensor motor coordination (Vallstedt and Kullander 2013). Future studies in the mouse model may benefit from genetic approaches that allow for inducible activation or inactivation that exclude possible compensatory processes during development.
Contemporary Issues Concerning the Functional Integration of Spinal Interneurons in the Mammalian Locomotor Circuitry
It is now clear that the construction and operation of mammalian locomotor circuits is determined by a large number of molecularly distinct subpopulations of interneurons assembled to participate in modules of rhythm- and pattern-generating networks. Further subdivision of interneurons into differentiable microcircuits may be necessary to account for the diverse range of behaviors that underlie mammalian locomotion. A deeper understanding of interneuron diversity in locomotor circuit operation will require additional refinement of experimental approaches and data analyses. In the following segments we briefly describe the challenges associated with the complexity of the mammalian locomotor circuitry and the limitations of current experimental approaches.
If the early unique expression of transcription factors forms the basis for identifying distinct functional classes, recent work suggests that their total number may be in the hundreds. For example, V1 INs are likely subdivided by the expression of various transcription factors into ~50 subpopulations that altogether account for ~90% of V1 INs (Bikoff et al. 2016; Gabitto et al. 2016). The large increase in neuron numbers and the expansion of subpopulations in the mammalian spinal cord compared with other vertebrate locomotor model systems is associated with an evolutionary increase in behavioral complexity (Harrigan and Commons 2014). For example, there are ~8,000 neurons in the adult mudpuppy C2 spinal segment (Cheng et al. 1998) compared with ~600,000 neurons in the neonatal rat L2 spinal segment (Cina and Hochman 2000) with similar estimated numbers in various primates (Burish et al. 2010). Similarly, the total numbers of CNS neurons in the larval zebrafish (100,000) and adult zebrafish (~10 million) are significantly lower than those reported in the mouse CNS (71 million) and rat (200 million) (World Heritage Encyclopedia).
To determine the role of a specific group of interneurons in the locomotor circuitry, genetically driven chronic ablation/silencing of the group is often used to examine the effect on locomotor output and movement. However, the task of confirming neuronal function can be challenging because of homeostatic mechanisms that could compensate for the disruption of a specific neuronal pathway. Homeostatic processes that stabilize and restore networks activity might include 1) recruitment of another group of neurons (interneurons and/or motoneurons) with similar function and synaptic connectivity (Chub and O’Donovan 1998), and 2) changes in the number of either excitatory or inhibitory interneurons that form new synaptic connections in response to the perturbation (Borodinsky et al. 2004; Moult et al. 2013; Spitzer 2015; Vallstedt A. et al. 2001). Therefore, to reduce the probability of cellular/network compensation, acute rather than chronic disruption of neuronal function would be a more desirable approach in future studies.
Advancing our understanding of the complex assembly and operation of the mammalian locomotor spinal circuits requires appreciation of limitations and differences in experimental approaches. Inclusion or exclusion of neuronal populations as components of the CPG may also depend on behavioral state and the recruitment of diverse neuromodulatory systems. In the neonate, various neuromodulators act on signal transduction pathways with ability to activate or modulate locomotor-like activity (Barrière et al. 2004, 2005; Kiehn and Kjaerulff 1996; Kiehn et al. 1999; Sqalli-Houssaini and Cazalets 2000). In vitro studies are undertaken in the neonate and rely on provision of neuromodulators or electrical stimulation of descending/sensory systems. It is apparent that cellular and synaptic properties change considerably with circuit maturation (e.g., Husch et al. 2015). Therefore, to increase our comprehension of the role of various interneurons in locomotion, it is advantageous to link proposed functions of interneuronal groups in the neonates with their contributions to movement performances in adults. Studies that use optogenetic or chemogenetic approaches to selectively and reversibly perturb or activate molecularly defined spinal neurons during ongoing unrestrained locomotor behavior in adult mouse may provide the most reliable indicator of functional role in locomotor behavior (e.g., Akay et al. 2008; Zhang et al. 2008).
The dramatic pace of genetic approaches that identify, monitor, and manipulate activity in molecularly defined interneurons has likely advanced beyond technologies capable of distinguishing subtle behavioral changes in circuit behavior (Anderson and Perona 2014). Methodological limitations in performing behavioral analyses may also prevent attributing a specific role of interneuronal populations in locomotion. A case in mind is the frequency-dependent recruitment of V2a INs and their importance in the transition from trotting to galloping as an example of the type of effect on locomotor structure that could have been overlooked if not examined under the right experimental condition (Crone et al. 2009; Zhong et al. 2011b). Mammalian locomotor behavior is undertaken under varied environmental interactions whose spinal interneuron recruitment remains untested. This includes up/downslope and weight-bearing locomotion, turning/veering, and backward/side-to-side locomotion, to mention a few. Newer approaches that combine high-resolution 3D imaging of mouse behavior with computational models hold promise for detection of distinct modules of locomotor behavior, in which manipulation of locomotor-related interneuronal subpopulations leads to identifiable changes in movement patterns (Wiltschko et al. 2015).
Conclusions
Postmitotic neurons express unique transcription factors that further divide the cardinal classes into subpopulations with distinct properties that include neurotransmitter phenotype, spatial distribution in the spinal cord, synaptic connections, projections to target neurons, and unique function in motor behavior (e.g., Arber 2012; Bikoff et al. 2016). Experiments undertaken in immature and adult mouse spinal cord support various predominantly ventrally located interneuronal groups as constituents of the locomotor circuitry. Progress has been made determining the synaptic interactions between some of these populations, and further elucidation of their synaptic inputs and output profiles is crucial for a complete construction of locomotor circuit operation. Their convergent synaptic integration and response to activation of identified descending and afferent systems will refine our understanding of their role in locomotion. Computational models will undoubtedly continue to provide insights into the interactive organization of the locomotor circuitry.
None of the cardinal class-derived group of interneurons discussed in this review is by itself necessary and sufficient for rhythm generation, but that should be expected based on neural circuit diversity associated with mammalian evolutionary expansion of behavioral complexity. The number of genetically identified subpopulations of spinal neurons implicated in the operation of the mammalian locomotor CPG is likely much larger than in other vertebrate locomotor models. The complex composition of patterned outputs that constitute a locomotor behavior necessitate flexible recruitments of microcircuits. The superposition of environmental variability, existence of distinct locomotor phenotypes, and the influence of behavioral state would benefit from multiple locomotor pattern-generating circuits. Powerful genetic tools enable exacting reversible control of distinct subclasses of spinal locomotor-related neurons, but the current lack of sophisticated behavioral analyses that permit detection of micromodular deficits in locomotor function may be the greater challenge to our understanding of functional integration of spinal neurons in mammalian locomotor microcircuits.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.Z.-C. and S.H. prepared figures; L.Z.-C. and S.H. drafted manuscript; L.Z.-C. and S.H. edited and revised manuscript; L.Z.-C. and S.H. approved final version of manuscript.
GRANTS
We sincerely appreciate the support of NIH grants that funded our locomotor research throughout the years: NS23808 (L. Ziskind-Conhaim) and NS40893, NS40440, and NSF IOS-0745164 (S. Hochman).
REFERENCES
- Akay T, Acharya HJ, Fouad K, Pearson KG. Behavioral and electromyographic characterization of mice lacking EphA4 receptors. J Neurophysiol 96: 642–651, 2006. doi: 10.1152/jn.00174.2006. [DOI] [PubMed] [Google Scholar]
- Akay T, Fouad K, Pearson KG. New technique for drug application to the spinal cord of walking mice. J Neurosci Methods 171: 39–47, 2008. doi: 10.1016/j.jneumeth.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Akay T, Tourtellotte WG, Arber S, Jessell TM. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc Natl Acad Sci USA 111: 16877–16882, 2014. doi: 10.1073/pnas.1419045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mosawie A, Wilson JM, Brownstone RM. Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur J Neurosci 26: 3003–3015, 2007. doi: 10.1111/j.1460-9568.2007.05907.x. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Jonas PC, Sapir T, Hartley R, Berrocal MC, Geiman EJ, Todd AJ, Goulding M. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J Comp Neurol 493: 177–192, 2005. doi: 10.1002/cne.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol 472: 257–280, 2004. doi: 10.1002/cne.20012. [DOI] [PubMed] [Google Scholar]
- Ampatzis K, Song J, Ausborn J, El Manira A. Separate microcircuit modules of distinct v2a interneurons and motoneurons control the speed of locomotion. Neuron 83: 934–943, 2014. doi: 10.1016/j.neuron.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Perona P. Toward a science of computational ethology. Neuron 84: 18–31, 2014. doi: 10.1016/j.neuron.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Andersson LS, Larhammar M, Memic F, Wootz H, Schwochow D, Rubin CJ, Patra K, Arnason T, Wellbring L, Hjälm G, Imsland F, Petersen JL, McCue ME, Mickelson JR, Cothran G, Ahituv N, Roepstorff L, Mikko S, Vallstedt A, Lindgren G, Andersson L, Kullander K. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature 488: 642–646, 2012. doi: 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S. Motor circuits in action: specification, connectivity, and function. Neuron 74: 975–989, 2012. doi: 10.1016/j.neuron.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 23: 659–674, 1999. doi: 10.1016/S0896-6273(01)80026-X. [DOI] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci 18: 2273–2284, 2003. doi: 10.1046/j.1460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrière G, Bertrand S, Cazalets JR. Peptidergic neuromodulation of the lumbar locomotor network in the neonatal rat spinal cord. Peptides 26: 277–286, 2005. doi: 10.1016/j.peptides.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Barrière G, Mellen N, Cazalets JR. Neuromodulation of the locomotor network by dopamine in the isolated spinal cord of newborn rat. Eur J Neurosci 19: 1325–1335, 2004. doi: 10.1111/j.1460-9568.2004.03210.x. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sommer JE, Martin JH, Scheiffele P. alpha2-Chimaerin is an essential EphA4 effector in the assembly of neuronal locomotor circuits. Neuron 55: 768–778, 2007. doi: 10.1016/j.neuron.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Benito-Gonzalez A, Alvarez FJ. Renshaw cells and Ia inhibitory interneurons are generated at different times from p1 progenitors and differentiate shortly after exiting the cell cycle. J Neurosci 32: 1156–1170, 2012. doi: 10.1523/JNEUROSCI.3630-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikoff JB, Gabitto MI, Rivard AF, Drobac E, Machado TA, Miri A, Brenner-Morton S, Famojure E, Diaz C, Alvarez FJ, Mentis GZ, Jessell TM. Spinal inhibitory interneuron diversity delineates variant motor microcircuits. Cell 165: 207–219, 2016. doi: 10.1016/j.cell.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnfors ER, El Manira A. Functional diversity of excitatory commissural interneurons in adult zebrafish. eLife 5: 5, 2016. doi: 10.7554/eLife.18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnot A, Whelan PJ, Mentis GZ, O’Donovan MJ. Spatiotemporal pattern of motoneuron activation in the rostral lumbar and the sacral segments during locomotor-like activity in the neonatal mouse spinal cord. J Neurosci 22: RC203, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgius L, Nishimaru H, Caldeira V, Kunugise Y, Löw P, Reig R, Itohara S, Iwasato T, Kiehn O. Spinal glutamatergic neurons defined by EphA4 signaling are essential components of normal locomotor circuits. J Neurosci 34: 3841–3853, 2014. doi: 10.1523/JNEUROSCI.4992-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 429: 523–530, 2004. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Borowska J, Jones CT, Zhang H, Blacklaws J, Goulding M, Zhang Y. Functional subpopulations of V3 interneurons in the mature mouse spinal cord. J Neurosci 33: 18553–18565, 2013. doi: 10.1523/JNEUROSCI.2005-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E, Jankowska E, McCrea DA, Skoog B. Inhibitory interactions between interneurones in reflex pathways from group Ia and group Ib afferents in the cat. J Physiol 343: 361–373, 1983. doi: 10.1113/jphysiol.1983.sp014897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O’Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature 398: 622–627, 1999. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Britz O, Zhang J, Grossmann KS, Dyck J, Kim JC, Dymecki S, Gosgnach S, Goulding M. A genetically defined asymmetry underlies the inhibitory control of flexor-extensor locomotor movements. eLife 4: 04718, 2015. doi: 10.7554/eLife.04718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B 84: 308–319, 1911. doi: 10.1098/rspb.1911.0077. [DOI] [Google Scholar]
- Brownstone RM, Bui TV. Spinal interneurons providing input to the final common path during locomotion. Prog Brain Res 187: 81–95, 2010. doi: 10.1016/B978-0-444-53613-6.00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM, Wilson JM. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res Brain Res Rev 57: 64–76, 2008. doi: 10.1016/j.brainresrev.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JT. Identification of interneurons with contralateral, caudal axons in the lamprey spinal cord: synaptic interactions and morphology. J Neurophysiol 47: 961–975, 1982. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Grillner S. Newly identified ‘glutamate interneurons’ and their role in locomotion in the lamprey spinal cord. Science 236: 312–314, 1987. doi: 10.1126/science.3563512. [DOI] [PubMed] [Google Scholar]
- Bui TV, Akay T, Loubani O, Hnasko TS, Jessell TM, Brownstone RM. Circuits for grasping: spinal dI3 interneurons mediate cutaneous control of motor behavior. Neuron 78: 191–204, 2013. doi: 10.1016/j.neuron.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burish MJ, Peebles JK, Baldwin MK, Tavares L, Kaas JH, Herculano-Houzel S. Cellular scaling rules for primate spinal cords. Brain Behav Evol 76: 45–59, 2010. doi: 10.1159/000319019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büschges A, Scholz H, El Manira A. New moves in motor control. Curr Biol 21: R513–R524, 2011. doi: 10.1016/j.cub.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Kiehn O. Functional identification of interneurons responsible for left-right coordination of hindlimbs in mammals. Neuron 38: 953–963, 2003. doi: 10.1016/S0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Lundfald L, Kiehn O. EphA4 defines a class of excitatory locomotor-related interneurons. Proc Natl Acad Sci USA 102: 14098–14103, 2005. doi: 10.1073/pnas.0503317102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira V, Dougherty KJ, Borgius L, Kiehn O. Spinal Hb9:Cre-derived excitatory interneurons contribute to rhythm generation in the mouse. Sci Rep 7: 41369, 2017. doi: 10.1038/srep41369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson-Kuhta P, Trank TV, Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol 79: 1687–1701, 1998. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci 15: 4943–4951, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol 455: 187–204, 1992. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Stein RB, Jovanović K, Yoshida K, Bennett DJ, Han Y. Identification, localization, and modulation of neural networks for walking in the mudpuppy (Necturus maculatus) spinal cord. J Neurosci 18: 4295–4304, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chub N, O’Donovan MJ. Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. J Neurosci 18: 294–306, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cina C, Hochman S. Diffuse distribution of sulforhodamine-labeled neurons during serotonin-evoked locomotion in the neonatal rat thoracolumbar spinal cord. J Comp Neurol 423: 590–602, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Harris-Warrick RM. Strychnine eliminates alternating motor output during fictive locomotion in the lamprey. Brain Res 293: 164–167, 1984. doi: 10.1016/0006-8993(84)91464-1. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol 77: 247–259, 1997. [DOI] [PubMed] [Google Scholar]
- Crone SA, Quinlan KA, Zagoraiou L, Droho S, Restrepo CE, Lundfald L, Endo T, Setlak J, Jessell TM, Kiehn O, Sharma K. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron 60: 70–83, 2008. doi: 10.1016/j.neuron.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci 29: 7098–7109, 2009. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Pavlova GA. The capacity for generation of rhythmic oscillations is distributed in the lumbosacral spinal cord of the cat. Exp Brain Res 53: 81–90, 1983. doi: 10.1007/BF00239400. [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Kiehn O. Firing and cellular properties of V2a interneurons in the rodent spinal cord. J Neurosci 30: 24–37, 2010a. doi: 10.1523/JNEUROSCI.4821-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KJ, Kiehn O. Functional organization of V2a-related locomotor circuits in the rodent spinal cord. Ann N Y Acad Sci 1198: 85–93, 2010b. doi: 10.1111/j.1749-6632.2010.05502.x. [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Zagoraiou L, Satoh D, Rozani I, Doobar S, Arber S, Jessell TM, Kiehn O. Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron 80: 920–933, 2013. doi: 10.1016/j.neuron.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Brocard F, Antri M, Fénelon K, Gariépy JF, Smetana R, Ménard A, Le Ray D, Viana Di Prisco G, Pearlstein E, Sirota MG, Derjean D, St-Pierre M, Zielinski B, Auclair F, Veilleux D. Initiation of locomotion in lampreys. Brain Res Brain Res Rev 57: 172–182, 2008. doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Dyck J, Lanuza GM, Gosgnach S. Functional characterization of dI6 interneurons in the neonatal mouse spinal cord. J Neurophysiol 107: 3256–3266, 2012. doi: 10.1152/jn.01132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. Synaptic actions on motoneurones in relation to the two components of the group I muscle afferent volley. J Physiol 136: 527–546, 1957. doi: 10.1113/jphysiol.1957.sp005778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Fatt P, Landgren S. The inhibitory pathway to motoneurones. Prog Neurobiol 2: 72–82, 1956. [PubMed] [Google Scholar]
- Eklöf-Ljunggren E, Haupt S, Ausborn J, Dehnisch I, Uhlén P, Higashijima S, El Manira A. Origin of excitation underlying locomotion in the spinal circuit of zebrafish. Proc Natl Acad Sci USA 109: 5511–5516, 2012. doi: 10.1073/pnas.1115377109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I, Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand 75: 614–630, 1969. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- Feldman AG, Orlovsky GN. Activity of interneurons mediating reciprocal 1a inhibition during locomotion. Brain Res 84: 181–194, 1975. doi: 10.1016/0006-8993(75)90974-9. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, McLean DL. Some principles of organization of spinal neurons underlying locomotion in zebrafish and their implications. Ann N Y Acad Sci 1198: 94–104, 2010. doi: 10.1111/j.1749-6632.2010.05539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. Prenatal growth of fine-diameter primary afferents into the rat spinal cord: a transganglionic tracer study. J Comp Neurol 261: 98–104, 1987. doi: 10.1002/cne.902610108. [DOI] [PubMed] [Google Scholar]
- Foran DR, Peterson AC. Myelin acquisition in the central nervous system of the mouse revealed by an MBP-Lac Z transgene. J Neurosci 12: 4890–4897, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen WO. Reciprocal inhibition: a mechanism underlying oscillatory animal movements. Neurosci Biobehav Rev 18: 547–553, 1994. doi: 10.1016/0149-7634(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Gabitto MI, Pakman A, Bikoff JB, Abbott LF, Jessell TM, Paninski L. Bayesian sparse regression analysis documents the diversity of spinal inhibitory interneurons. Cell 165: 220–233, 2016. doi: 10.1016/j.cell.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao BX, Ziskind-Conhaim L. Development of glycine- and GABA-gated currents in rat spinal motoneurons. J Neurophysiol 74: 113–121, 1995. [DOI] [PubMed] [Google Scholar]
- Garcia-Campmany L, Stam FJ, Goulding M. From circuits to behaviour: motor networks in vertebrates. Curr Opin Neurobiol 20: 116–125, 2010. doi: 10.1016/j.conb.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ramírez DL, Calvo JR, Hochman S, Quevedo JN. Serotonin, dopamine and noradrenaline adjust actions of myelinated afferents via modulation of presynaptic inhibition in the mouse spinal cord. PLoS One 9: e89999, 2014. doi: 10.1371/journal.pone.0089999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon IT, Whelan PJ. Deciphering the organization and modulation of spinal locomotor central pattern generators. J Exp Biol 209: 2007–2014, 2006. doi: 10.1242/jeb.02213. [DOI] [PubMed] [Google Scholar]
- Gordon IT, Whelan PJ. Brainstem modulation of locomotion in the neonatal mouse spinal cord. J Physiol 586: 2487–2497, 2008. doi: 10.1113/jphysiol.2007.148320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature 440: 215–219, 2006. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res 98: 213–228, 1994. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci 10: 507–518, 2009. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M, Lanuza G, Sapir T, Narayan S. The formation of sensorimotor circuits. Curr Opin Neurobiol 12: 508–515, 2002. doi: 10.1016/S0959-4388(02)00371-9. [DOI] [PubMed] [Google Scholar]
- Goulding M, Pfaff SL. Development of circuits that generate simple rhythmic behaviors in vertebrates. Curr Opin Neurobiol 15: 14–20, 2005. doi: 10.1016/j.conb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Griener A, Zhang W, Kao H, Wagner C, Gosgnach S. Probing diversity within subpopulations of locomotor-related V0 interneurons. Dev Neurobiol 75: 1189–1203, 2015. doi: 10.1002/dneu.22277. [DOI] [PubMed] [Google Scholar]
- Grillner S. Neurobiological bases of rhythmic motor acts in vertebrates. Science 228: 143–149, 1985. doi: 10.1126/science.3975635. [DOI] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci 4: 573–586, 2003. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52: 751–766, 2006. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods, and fish. Compr Physiol 1179–1236, 2011. doi: 10.1002/cphy.cp010226. [DOI] [Google Scholar]
- Grillner S, Buchanan JT, Lansner A. Simulation of the segmental burst generating network for locomotion in lamprey. Neurosci Lett 89: 31–35, 1988. doi: 10.1016/0304-3940(88)90476-4. [DOI] [PubMed] [Google Scholar]
- Grillner S, El Manira A. The intrinsic operation of the networks that make us locomote. Curr Opin Neurobiol 31: 244–249, 2015. doi: 10.1016/j.conb.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Ménard A, Saitoh K, Wikström MA. Mechanisms for selection of basic motor programs—roles for the striatum and pallidum. Trends Neurosci 28: 364–370, 2005. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol 19: 572–586, 2009. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Robertson B. The basal ganglia downstream control of brainstem motor centers—an evolutionarily conserved strategy. Curr Opin Neurobiol 33: 47–52, 2015. doi: 10.1016/j.conb.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallén P. Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci 8: 233–261, 1985. doi: 10.1146/annurev.ne.08.030185.001313. [DOI] [PubMed] [Google Scholar]
- Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res 34: 241–261, 1979. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Gross MK, Dottori M, Goulding M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron 34: 535–549, 2002. doi: 10.1016/S0896-6273(02)00690-6. [DOI] [PubMed] [Google Scholar]
- Grossman RG, Frankowski RF, Burau KD, Toups EG, Crommett JW, Johnson MM, Fehlings MG, Tator CH, Shaffrey CI, Harkema SJ, Hodes JE, Aarabi B, Rosner MK, Guest JD, Harrop JS. Incidence and severity of acute complications after spinal cord injury. J Neurosurg Spine 17, Suppl 1: 119–128, 2012. doi: 10.3171/2012.5.AOSPINE12127. [DOI] [PubMed] [Google Scholar]
- Grossmann KS, Giraudin A, Britz O, Zhang J, Goulding M. Genetic dissection of rhythmic motor networks in mice. Prog Brain Res 187: 19–37, 2010. doi: 10.1016/B978-0-444-53613-6.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin PA. The mammalian central pattern generator for locomotion. Brain Res Brain Res Rev 62: 45–56, 2009. doi: 10.1016/j.brainresrev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Hägglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat Neurosci 13: 246–252, 2010. doi: 10.1038/nn.2482. [DOI] [PubMed] [Google Scholar]
- Hantman AW, Jessell TM. Clarke’s column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci 13: 1233–1239, 2010. doi: 10.1038/nn.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan WJ, Commons ML. The stage of development of a species predicts the number of neurons. Behav Dev Bull 19: 12–21, 2014. doi: 10.1037/h0101077. [DOI] [Google Scholar]
- Hayes HB, Chang YH, Hochman S. An in vitro spinal cord-hindlimb preparation for studying behaviorally relevant rat locomotor function. J Neurophysiol 101: 1114–1122, 2009. doi: 10.1152/jn.90523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström J, Oliveira AL, Meister B, Cullheim S. Large cholinergic nerve terminals on subsets of motoneurons and their relation to muscarinic receptor type 2. J Comp Neurol 460: 476–486, 2003. doi: 10.1002/cne.10648. [DOI] [PubMed] [Google Scholar]
- Hinckley C, Seebach B, Ziskind-Conhaim L. Distinct roles of glycinergic and GABAergic inhibition in coordinating locomotor-like rhythms in the neonatal mouse spinal cord. Neuroscience 131: 745–758, 2005a. doi: 10.1016/j.neuroscience.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L. Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J Neurophysiol 93: 1439–1449, 2005b. doi: 10.1152/jn.00647.2004. [DOI] [PubMed] [Google Scholar]
- Hinckley CA, Wiesner EP, Mentis GZ, Titus DJ, Ziskind-Conhaim L. Sensory modulation of locomotor-like membrane oscillations in Hb9-expressing interneurons. J Neurophysiol 103: 3407–3423, 2010. doi: 10.1152/jn.00996.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley CA, Ziskind-Conhaim L. Electrical coupling between locomotor-related excitatory interneurons in the mammalian spinal cord. J Neurosci 26: 8477–8483, 2006. doi: 10.1523/JNEUROSCI.0395-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman S, Gozal EA, Hayes HB, Anderson JT, DeWeerth SP, Chang YH. Enabling techniques for in vitro studies on mammalian spinal locomotor mechanisms. Front Biosci (Landmark Ed) 17: 2158–2180, 2012. doi: 10.2741/4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H. Spinal reflexes, mechanisms and concepts: from Eccles to Lundberg and beyond. Prog Neurobiol 78: 215–232, 2006. doi: 10.1016/j.pneurobio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Conway BA, Gossard JP, Brownstone R, Fedirchuk B, Schomburg ED, Enríquez-Denton M, Perreault MC. How do we approach the locomotor network in the mammalian spinal cord? Ann N Y Acad Sci 860: 70–82, 1998. doi: 10.1111/j.1749-6632.1998.tb09039.x. [DOI] [PubMed] [Google Scholar]
- Husch A, Dietz SB, Hong DN, Harris-Warrick RM. Adult spinal V2a interneurons show increased excitability and serotonin-dependent bistability. J Neurophysiol 113: 1124–1134, 2015. doi: 10.1152/jn.00741.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M, Kiehn O, Kudo N. Development in neonatal rats of the sensory resetting of the locomotor rhythm induced by NMDA and 5-HT. Exp Brain Res 114: 193–204, 1997. doi: 10.1007/PL00005628. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol 533: 31–40, 2001. doi: 10.1111/j.1469-7793.2001.0031b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Brain Res Rev 57: 46–55, 2008. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Fu TC, Lundberg A. Reciprocal Ia inhibition during the late reflexes evoked from the flexor reflex afferents after DOPA. Brain Res 85: 99–102, 1975. doi: 10.1016/0006-8993(75)91012-4. [DOI] [PubMed] [Google Scholar]
- Jankowska E, McCrea D, Mackel R. Pattern of ‘non-reciprocal’ inhibition of motoneurones by impulses in group Ia muscle spindle afferents in the cat. J Physiol 316: 393–409, 1981. doi: 10.1113/jphysiol.1981.sp013796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Noga BR. Contralaterally projecting lamina VIII interneurones in middle lumbar segments in the cat. Brain Res 535: 327–330, 1990. doi: 10.1016/0006-8993(90)91618-Q. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 1: 20–29, 2000. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res 816: 493–499, 1999. doi: 10.1016/S0006-8993(98)01199-8. [DOI] [PubMed] [Google Scholar]
- Johnson IP, Sears TA. Ultrastructure of interneurons within motor nuclei of the thoracic region of the spinal cord of the adult cat. J Anat 161: 171–185, 1988. [PMC free article] [PubMed] [Google Scholar]
- Jordan LM. Brainstem and spinal cord mechanisms for the initiation of locomotion. In: Neurobiological Basis of Human Locomotion, edited by Shimamura M, Grillner S, Edgerton VR. Tokyo: Japan Scientific Societies, 1991, p. 3–20. [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Brain Res Rev 57: 183–191, 2008. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Karunaratne A, Hargrave M, Poh A, Yamada T. GATA proteins identify a novel ventral interneuron subclass in the developing chick spinal cord. Dev Biol 249: 30–43, 2002. doi: 10.1006/dbio.2002.0754. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78: 425–435, 1994. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29: 279–306, 2006. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 17: 224–238, 2016. doi: 10.1038/nrn.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Butt SJ. Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog Neurobiol 70: 347–361, 2003. doi: 10.1016/S0301-0082(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Iizuka M, Kudo N. Resetting from low threshold afferents of N-methyl-D-aspartate-induced locomotor rhythm in the isolated spinal cord-hindlimb preparation from newborn rats. Neurosci Lett 148: 43–46, 1992. doi: 10.1016/0304-3940(92)90800-M. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol 75: 1472–1482, 1996. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Ann N Y Acad Sci 860: 110–129, 1998. doi: 10.1111/j.1749-6632.1998.tb09043.x. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kullander K. Central pattern generators deciphered by molecular genetics. Neuron 41: 317–321, 2004. doi: 10.1016/S0896-6273(04)00042-X. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Sillar KT, Kjaerulff O, McDearmid JR. Effects of noradrenaline on locomotor rhythm-generating networks in the isolated neonatal rat spinal cord. J Neurophysiol 82: 741–746, 1999. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci 26: 5684–5697, 2006. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J Neurophysiol 77: 1155–1170, 1997. [DOI] [PubMed] [Google Scholar]
- Kudo N, Yamada T. N-methyl-D,L-aspartate-induced locomotor activity in a spinal cord-hindlimb muscles preparation of the newborn rat studied in vitro. Neurosci Lett 75: 43–48, 1987. doi: 10.1016/0304-3940(87)90072-3. [DOI] [PubMed] [Google Scholar]
- Kullander K. Genetics moving to neuronal networks. Trends Neurosci 28: 239–247, 2005. doi: 10.1016/j.tins.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydström A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science 299: 1889–1892, 2003. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- Kwan AC, Dietz SB, Webb WW, Harris-Warrick RM. Activity of Hb9 interneurons during fictive locomotion in mouse spinal cord. J Neurosci 29: 11601–11613, 2009. doi: 10.1523/JNEUROSCI.1612-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol 94: 1120–1132, 2005. doi: 10.1152/jn.00216.2005. [DOI] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron 42: 375–386, 2004. doi: 10.1016/S0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A, Delvolvé I. Pattern generation in non-limb moving segments of the mammalian spinal cord. Brain Res Bull 53: 671–675, 2000. doi: 10.1016/S0361-9230(00)00400-7. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A, Etlin A, Blivis D. Sensory-induced activation of pattern generators in the absence of supraspinal control. Ann N Y Acad Sci 1198: 54–62, 2010. doi: 10.1111/j.1749-6632.2009.05424.x. [DOI] [PubMed] [Google Scholar]
- Li W, Ochalski PA, Brimijoin S, Jordan LM, Nagy JI. C-terminals on motoneurons: electron microscope localization of cholinergic markers in adult rats and antibody-induced depletion in neonates. Neuroscience 65: 879–891, 1995. doi: 10.1016/0306-4522(94)00511-3. [DOI] [PubMed] [Google Scholar]
- Li WC, Soffe SR, Roberts A. Glutamate and acetylcholine corelease at developing synapses. Proc Natl Acad Sci USA 101: 15488–15493, 2004. doi: 10.1073/pnas.0404864101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J Neurophysiol 94: 1392–1404, 2005. doi: 10.1152/jn.00136.2005. [DOI] [PubMed] [Google Scholar]
- Liu TT, Bannatyne BA, Jankowska E, Maxwell DJ. Cholinergic terminals in the ventral horn of adult rat and cat: evidence that glutamate is a cotransmitter at putative interneuron synapses but not at central synapses of motoneurons. Neuroscience 161: 111–122, 2009. doi: 10.1016/j.neuroscience.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren EE, Haupt S, Ausborn J, Ampatzis K, El Manira A. Optogenetic activation of excitatory premotor interneurons is sufficient to generate coordinated locomotor activity in larval zebrafish. J Neurosci 34: 134–139, 2014. doi: 10.1523/JNEUROSCI.4087-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. Prog Brain Res 50: 11–28, 1979. doi: 10.1016/S0079-6123(08)60803-1. [DOI] [PubMed] [Google Scholar]
- Lundfald L, Restrepo CE, Butt SJ, Peng CY, Droho S, Endo T, Zeilhofer HU, Sharma K, Kiehn O. Phenotype of V2-derived interneurons and their relationship to the axon guidance molecule EphA4 in the developing mouse spinal cord. Eur J Neurosci 26: 2989–3002, 2007. doi: 10.1111/j.1460-9568.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- Mandadi S, Nakanishi ST, Takashima Y, Dhaka A, Patapoutian A, McKemy DD, Whelan PJ. Locomotor networks are targets of modulation by sensory transient receptor potential vanilloid 1 and transient receptor potential melastatin 8 channels. Neuroscience 162: 1377–1397, 2009. doi: 10.1016/j.neuroscience.2009.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Beato M, Nistri A. Alternating rhythmic activity induced by dorsal root stimulation in the neonatal rat spinal cord in vitro. J Physiol 530: 105–112, 2001. doi: 10.1111/j.1469-7793.2001.0105m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol 533: 41–50, 2001. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Brain Res Rev 57: 134–146, 2008. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh JC, Gorman RB, Gilliam EE, Hornby TG, Reinking RM, Stuart DG. Electrophysiological and morphological properties of neurons in the ventral horn of the turtle spinal cord. J Physiol Paris 93: 3–16, 1999. doi: 10.1016/S0928-4257(99)80131-4. [DOI] [PubMed] [Google Scholar]
- McLean DL, Dougherty KJ. Peeling back the layers of locomotor control in the spinal cord. Curr Opin Neurobiol 33: 63–70, 2015. doi: 10.1016/j.conb.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Using imaging and genetics in zebrafish to study developing spinal circuits in vivo. Dev Neurobiol 68: 817–834, 2008. doi: 10.1002/dneu.20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DL, Masino MA, Koh IY, Lindquist WB, Fetcho JR. Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci 11: 1419–1429, 2008. doi: 10.1038/nn.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Alvarez FJ, Bonnot A, Richards DS, Gonzalez-Forero D, Zerda R, O’Donovan MJ. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci USA 102: 7344–7349, 2005. doi: 10.1073/pnas.0502788102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci USA 104: 2448–2453, 2007. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JP, Selverston AI. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. II. Oscillatory properties of pyloric neurons. J Neurophysiol 48: 1378–1391, 1982a. [DOI] [PubMed] [Google Scholar]
- Miller JP, Selverston AI. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. IV. Network properties of pyloric system. J Neurophysiol 48: 1416–1432, 1982b. [DOI] [PubMed] [Google Scholar]
- Moran-Rivard L, Kagawa T, Saueressig H, Gross MK, Burrill J, Goulding M. Evx1 is a postmitotic determinant of v0 interneuron identity in the spinal cord. Neuron 29: 385–399, 2001. doi: 10.1016/S0896-6273(01)00213-6. [DOI] [PubMed] [Google Scholar]
- Moult PR, Cottrell GA, Li WC. Fast silencing reveals a lost role for reciprocal inhibition in locomotion. Neuron 77: 129–140, 2013. doi: 10.1016/j.neuron.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muennich EA, Fyffe RE. Focal aggregation of voltage-gated, Kv2.1 subunit-containing, potassium channels at synaptic sites in rat spinal motoneurones. J Physiol 554: 673–685, 2004. doi: 10.1113/jphysiol.2003.056192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Brohmann H, Pierani A, Heppenstall PA, Lewin GR, Jessell TM, Birchmeier C. The homeodomain factor lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron 34: 551–562, 2002. doi: 10.1016/S0896-6273(02)00689-X. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Yamamoto T, Jordan LM. Evidence for the cholinergic nature of C-terminals associated with subsurface cisterns in alpha-motoneurons of rat. Synapse 15: 17–32, 1993. doi: 10.1002/syn.890150103. [DOI] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hökfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse 50: 117–129, 2003. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Functional organization of the spino- and cuneocerebellar tracts. Physiol Rev 45: 495–522, 1965. [DOI] [PubMed] [Google Scholar]
- Paixão S, Balijepalli A, Serradj N, Niu J, Luo W, Martin JH, Klein R. EphrinB3/EphA4-mediated guidance of ascending and descending spinal tracts. Neuron 80: 1407–1420, 2013. doi: 10.1016/j.neuron.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek I, Bui T, Wright AT, Brownstone RM. Cutaneous afferent regulation of motor function. Acta Neurobiol Exp (Wars) 74: 158–171, 2014. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Common principles of motor control in vertebrates and invertebrates. Annu Rev Neurosci 16: 265–297, 1993. doi: 10.1146/annurev.ne.16.030193.001405. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Neural adaptation in the generation of rhythmic behavior. Annu Rev Physiol 62: 723–753, 2000. doi: 10.1146/annurev.physiol.62.1.723. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res 143: 123–129, 2004. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Peng CY, Yajima H, Burns CE, Zon LI, Sisodia SS, Pfaff SL, Sharma K. Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord. Neuron 53: 813–827, 2007. doi: 10.1016/j.neuron.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel DH, Mulloney B. Motor pattern production in reciprocally inhibitory neurons exhibiting postinhibitory rebound. Science 185: 181–183, 1974. doi: 10.1126/science.185.4146.181. [DOI] [PubMed] [Google Scholar]
- Pierani A, Brenner-Morton S, Chiang C, Jessell TM. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell 97: 903–915, 1999. doi: 10.1016/S0092-8674(00)80802-8. [DOI] [PubMed] [Google Scholar]
- Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron 29: 367–384, 2001. doi: 10.1016/S0896-6273(01)00212-4. [DOI] [PubMed] [Google Scholar]
- Pillai A, Mansouri A, Behringer R, Westphal H, Goulding M. Lhx1 and Lhx5 maintain the inhibitory-neurotransmitter status of interneurons in the dorsal spinal cord. Development 134: 357–366, 2007. doi: 10.1242/dev.02717. [DOI] [PubMed] [Google Scholar]
- Quinlan KA, Kiehn O. Segmental, synaptic actions of commissural interneurons in the mouse spinal cord. J Neurosci 27: 6521–6530, 2007. doi: 10.1523/JNEUROSCI.1618-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe N, Gezelius H, Vallstedt A, Memic F, Kullander K. Netrin-1-dependent spinal interneuron subtypes are required for the formation of left-right alternating locomotor circuitry. J Neurosci 29: 15642–15649, 2009. doi: 10.1523/JNEUROSCI.5096-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe Bernhardt N, Memic F, Gezelius H, Thiebes AL, Vallstedt A, Kullander K. DCC mediated axon guidance of spinal interneurons is essential for normal locomotor central pattern generator function. Dev Biol 366: 279–289, 2012. doi: 10.1016/j.ydbio.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Restrepo CE, Margaryan G, Borgius L, Lundfald L, Sargsyan D, Kiehn O. Change in the balance of excitatory and inhibitory midline fiber crossing as an explanation for the hopping phenotype in EphA4 knockout mice. Eur J Neurosci 34: 1102–1112, 2011. doi: 10.1111/j.1460-9568.2011.07838.x. [DOI] [PubMed] [Google Scholar]
- Roberts A, Soffe SR, Wolf ES, Yoshida M, Zhao FY. Central circuits controlling locomotion in young frog tadpoles. Ann N Y Acad Sci 860: 19–34, 1998. doi: 10.1111/j.1749-6632.1998.tb09036.x. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol 577: 617–639, 2006. doi: 10.1113/jphysiol.2006.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh D, Pudenz C, Arber S. Context-dependent gait choice elicited by Epha4 mutation in Lbx1 spinal interneurons. Neuron 89: 1046–1058, 2016. doi: 10.1016/j.neuron.2016.01.033. [DOI] [PubMed] [Google Scholar]
- Saueressig H, Burrill J, Goulding M. Engrailed-1 and netrin-1 regulate axon pathfinding by association interneurons that project to motor neurons. Development 126: 4201–4212, 1999. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Exp Brain Res 122: 339–350, 1998. doi: 10.1007/s002210050522. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87: 1001–1014, 1996. doi: 10.1016/S0092-8674(00)81795-X. [DOI] [PubMed] [Google Scholar]
- Sherwood WE, Harris-Warrick R, Guckenheimer J. Synaptic patterning of left-right alternation in a computational model of the rodent hindlimb central pattern generator. J Comput Neurosci 30: 323–360, 2011. doi: 10.1007/s10827-010-0259-y. [DOI] [PubMed] [Google Scholar]
- Shevtsova NA, Talpalar AE, Markin SN, Harris-Warrick RM, Kiehn O, Rybak IA. Organization of left-right coordination of neuronal activity in the mammalian spinal cord: insights from computational modelling. J Physiol 593: 2403–2426, 2015. doi: 10.1113/JP270121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillar KT, Combes D, Ramanathan S, Molinari M, Simmers J. Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis. Brain Res Brain Res Rev 57: 94–102, 2008. doi: 10.1016/j.brainresrev.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods 21: 321–333, 1987. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Smith JC, Feldman JL, Schmidt BJ. Neural mechanisms generating locomotion studied in mammalian brain stem-spinal cord in vitro. FASEB J 2: 2283–2288, 1988. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Neurotransmitter switching? No surprise. Neuron 86: 1131–1144, 2015. doi: 10.1016/j.neuron.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sqalli-Houssaini Y, Cazalets JR. Noradrenergic control of locomotor networks in the in vitro spinal cord of the neonatal rat. Brain Res 852: 100–109, 2000. doi: 10.1016/S0006-8993(99)02219-2. [DOI] [PubMed] [Google Scholar]
- Stein PS. Motor pattern deletions and modular organization of turtle spinal cord. Brain Res Brain Res Rev 57: 118–124, 2008. doi: 10.1016/j.brainresrev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS. Molecular, genetic, cellular, and network functions in the spinal cord and brainstem. Ann N Y Acad Sci 1279: 1–12, 2013. doi: 10.1111/nyas.12083. [DOI] [PubMed] [Google Scholar]
- Stein PS, Daniels-McQueen S. Modular organization of turtle spinal interneurons during normal and deletion fictive rostral scratching. J Neurosci 22: 6800–6809, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS, Daniels-McQueen S. Variations in motor patterns during fictive rostral scratching in the turtle: knee-related deletions. J Neurophysiol 91: 2380–2384, 2004. doi: 10.1152/jn.01184.2003. [DOI] [PubMed] [Google Scholar]
- Stepien AE, Arber S. Probing the locomotor conundrum: descending the ‘V’ interneuron ladder. Neuron 60: 1–4, 2008. doi: 10.1016/j.neuron.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Stepien AE, Tripodi M, Arber S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron 68: 456–472, 2010. doi: 10.1016/j.neuron.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Sugimori M, Nagao M, Bertrand N, Parras CM, Guillemot F, Nakafuku M. Combinatorial actions of patterning and HLH transcription factors in the spatiotemporal control of neurogenesis and gliogenesis in the developing spinal cord. Development 134: 1617–1629, 2007. doi: 10.1242/dev.001255. [DOI] [PubMed] [Google Scholar]
- Szokol K, Glover JC, Perreault MC. Organization of functional synaptic connections between medullary reticulospinal neurons and lumbar descending commissural interneurons in the neonatal mouse. J Neurosci 31: 4731–4742, 2011. doi: 10.1523/JNEUROSCI.5486-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A, Kiehn O. Dual-mode operation of neuronal networks involved in left-right alternation. Nature 500: 85–88, 2013. doi: 10.1038/nature12286. [DOI] [PubMed] [Google Scholar]
- Talpalar AE, Endo T, Löw P, Borgius L, Hägglund M, Dougherty KJ, Ryge J, Hnasko TS, Kiehn O. Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron 71: 1071–1084, 2011. doi: 10.1016/j.neuron.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Talpalar AE, Kiehn O. Glutamatergic mechanisms for speed control and network operation in the rodent locomotor CpG. Front Neural Circuits 4: 4, 2010. doi: 10.3389/fncir.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazerart S, Vinay L, Brocard F. The persistent sodium current generates pacemaker activities in the central pattern generator for locomotion and regulates the locomotor rhythm. J Neurosci 28: 8577–8589, 2008. doi: 10.1523/JNEUROSCI.1437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tråvén HG, Brodin L, Lansner A, Ekeberg O, Wallén P, Grillner S. Computer simulations of NMDA and non-NMDA receptor-mediated synaptic drive: sensory and supraspinal modulation of neurons and small networks. J Neurophysiol 70: 695–709, 1993. [DOI] [PubMed] [Google Scholar]
- Tripodi M, Stepien AE, Arber S. Motor antagonism exposed by spatial segregation and timing of neurogenesis. Nature 479: 61–66, 2011. doi: 10.1038/nature10538. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Kullander K. Dorsally derived spinal interneurons in locomotor circuits. Ann N Y Acad Sci 1279: 32–42, 2013. doi: 10.1111/j.1749-6632.2012.06801.x. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Muhr J, Pattyn A, Pierani A, Mendelsohn M, Sander M, Jessell TM, Ericson J. Different levels of repressor activity assign redundant and specific roles to Nkx6 genes in motor neuron and interneuron specification. Neuron 31: 743–755, 2001. doi: 10.1016/S0896-6273(01)00412-3. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol 382: 46–76, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Vejsada R, Paleček J, Hník P, Soukup T. Postnatal development of conduction velocity and fibre size in the rat tibial nerve. Int J Dev Neurosci 3: 583–595, 1985. doi: 10.1016/0736-5748(85)90048-6. [DOI] [PubMed] [Google Scholar]
- Wallén-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygård A, Enjin A, Fujiyama F, Fortin G, Kullander K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci 26: 12294–12307, 2006. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmeyer H, Egea J, Rabe N, Gezelius H, Filosa A, Enjin A, Varoqueaux F, Deininger K, Schnütgen F, Brose N, Klein R, Kullander K, Betz A. EphA4-dependent axon guidance is mediated by the RacGAP alpha2-chimaerin. Neuron 55: 756–767, 2007. doi: 10.1016/j.neuron.2007.07.038. [DOI] [PubMed] [Google Scholar]
- Whelan P, Bonnot A, O’Donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J Neurophysiol 84: 2821–2833, 2000. [DOI] [PubMed] [Google Scholar]
- Wildner H, Müller T, Cho SH, Bröhl D, Cepko CL, Guillemot F, Birchmeier C. dILA neurons in the dorsal spinal cord are the product of terminal and non-terminal asymmetric progenitor cell divisions, and require Mash1 for their development. Development 133: 2105–2113, 2006. doi: 10.1242/dev.02345. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Blagovechtchenski E, Brownstone RM. Genetically defined inhibitory neurons in the mouse spinal cord dorsal horn: a possible source of rhythmic inhibition of motoneurons during fictive locomotion. J Neurosci 30: 1137–1148, 2010. doi: 10.1523/JNEUROSCI.1401-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Cowan AI, Brownstone RM. Heterogeneous electrotonic coupling and synchronization of rhythmic bursting activity in mouse Hb9 interneurons. J Neurophysiol 98: 2370–2381, 2007. doi: 10.1152/jn.00338.2007. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci 25: 5710–5719, 2005. doi: 10.1523/JNEUROSCI.0274-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, Abraira VE, Adams RP, Datta SR. Mapping sub-second structure in mouse behavior. Neuron 88: 1121–1135, 2015. doi: 10.1016/j.neuron.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull 73: 155–202, 2007. doi: 10.1016/j.brainresbull.2007.03.010. [DOI] [PubMed] [Google Scholar]
- World Heritage Encyclopedia List of Animals by Number of Neurons. http://www.worldlibrary.org/articles/list_of_animals_by_number_of_neurons [29 April 2017].
- Wu L, Sonner PM, Titus DJ, Wiesner EP, Alvarez FJ, Ziskind-Conhaim L. Properties of a distinct subpopulation of GABAergic commissural interneurons that are part of the locomotor circuitry in the neonatal spinal cord. J Neurosci 31: 4821–4833, 2011. doi: 10.1523/JNEUROSCI.4764-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron 64: 645–662, 2009. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaporozhets E, Cowley KC, Schmidt BJ. A reliable technique for the induction of locomotor-like activity in the in vitro neonatal rat spinal cord using brainstem electrical stimulation. J Neurosci Methods 139: 33–41, 2004. doi: 10.1016/j.jneumeth.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol 58: 185–205, 1999. doi: 10.1016/S0301-0082(98)00081-1. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lanuza GM, Britz O, Wang Z, Siembab VC, Zhang Y, Velasquez T, Alvarez FJ, Frank E, Goulding M. V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron 82: 138–150, 2014. doi: 10.1016/j.neuron.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, Fan CM, Goulding M. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 60: 84–96, 2008. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Droho S, Crone SA, Dietz S, Kwan AC, Webb WW, Sharma K, Harris-Warrick RM. Electrophysiological characterization of V2a interneurons and their locomotor-related activity in the neonatal mouse spinal cord. J Neurosci 30: 170–182, 2010. doi: 10.1523/JNEUROSCI.4849-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Sharma K, Harris-Warrick RM. Frequency-dependent recruitment of V2a interneurons during fictive locomotion in the mouse spinal cord. Nat Commun 2: 274, 2011a. doi: 10.1038/ncomms1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Shevtsova NA, Rybak IA, Harris-Warrick RM. Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization. J Physiol 590: 4735–4759, 2012. doi: 10.1113/jphysiol.2012.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong GZH, Husch AAH, Harris-Warrick RRMH. The frequency dependent recruitment of interneurons in the rodent spinal cord. Integr Comp Biol 51: E156, 2011b. [Google Scholar]
- Ziskind-Conhaim L. Physiological and morphological changes in developing peripheral nerves of rat embryos. Brain Res 42: 15–28, 1988. doi: 10.1016/0165-3806(88)90198-8. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. Neuronal correlates of the dominant role of GABAergic transmission in the developing mouse locomotor circuitry. Ann N Y Acad Sci 1279: 43–53, 2013. doi: 10.1111/nyas.12064. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Mentis GZ, Wiesner EP, Titus DJ. Synaptic integration of rhythmogenic neurons in the locomotor circuitry: the case of Hb9 interneurons. Ann N Y Acad Sci 1198: 72–84, 2010. doi: 10.1111/j.1749-6632.2010.05533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Wu L, Wiesner EP. Persistent sodium current contributes to induced voltage oscillations in locomotor-related hb9 interneurons in the mouse spinal cord. J Neurophysiol 100: 2254–2264, 2008. doi: 10.1152/jn.90437.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]