Abstract
Large-diameter myelinated phrenic afferents discharge in phase with diaphragm contraction, and smaller diameter fibers discharge across the respiratory cycle. In this article, we review the phrenic afferent literature and highlight areas in need of further study. We conclude that 1) activation of both myelinated and nonmyelinated phrenic sensory afferents can influence respiratory motor output on a breath-by-breath basis; 2) the relative impact of phrenic afferents substantially increases with diaphragm work and fatigue; 3) activation of phrenic afferents has a powerful impact on sympathetic motor outflow, and 4) phrenic afferents contribute to diaphragm somatosensation and the conscious perception of breathing. Much remains to be learned regarding the spinal and supraspinal distribution and synaptic contacts of myelinated and nonmyelinated phrenic afferents. Similarly, very little is known regarding the potential role of phrenic afferent neurons in triggering or modulating expression of respiratory neuroplasticity.
Keywords: afferent, diaphragm, phrenic, receptor, sensory
sensory afferent neurons comprise ~30–45% of phrenic nerve axons (Duron et al. 1978; Landau et al. 1962; Langford and Schmidt 1983). These fibers include large-diameter myelinated (group Ia, Ib, II), small-diameter myelinated (group III), and unmyelinated (group IV). Large-diameter myelinated phrenic afferents discharge in phase with diaphragm contraction, and smaller diameter fibers discharge across the respiratory cycle.
Activation of phrenic afferent neurons triggers a diverse range of physiological responses including increases in sympathetic neural outflow (Offner et al. 1992) and arterial blood pressure (Hussain et al. 1991), short-latency inhibition of phrenic motoneurons (Gill and Kuno 1963; Marlot et al. 1987), increases in ventilation (Marlot et al. 1987; Revelette et al. 1988), and decreases in intercostal motor output (De Troyer 1998). Phrenic afferents are also likely to play a role in the perception of breathing and responses to respiratory loading (Davenport et al. 2010; Davenport and Vovk 2009). In regard to the control of breathing, it is difficult to ascribe a precise functional role to phrenic afferents because of the diversity of techniques, species, and time domains in published studies of phrenic afferent activation. Nevertheless, it is very clear that activation of diaphragm sensory receptors has a powerful impact on respiratory motor output. Phrenic afferents have been reviewed previously, and the reader is referred to these prior publications for additional relevant citations of past work (Davenport 1995; Frazier and Revelette 1991; Hussain 1995; Road 1990). The goal of the current article is to provide a comprehensive and updated overview of the phrenic afferent literature, including a summary of recent studies and discussion of potential clinical relevance.
Diaphragm Receptors: Anatomical Distribution and Discharge Patterns
The diaphragm separates the thoracic and abdominal cavities and is composed of a central tendon and two distinct muscular portions termed “costal” and “crural” (for review of diaphragm anatomy and biomechanics, see Poole et al. 1997). Costal myofibers radiate from the central tendon to the xiphoid and costal arch cartilage of the lower ribs, and the crural myofibers project to the vertebral column. Unique features of the diaphragm include a high duty cycle, a high rate of blood flow per unit area, and susceptibility to atrophy (Powers et al. 2013).
The diaphragm is innervated by sensory afferents, and Table 1 provides a summary of the discharge patterns of diaphragm receptors based on neurophysiological recordings. Diaphragm muscle spindles (group Ia afferents) probably represent a low proportion of the total diaphragm receptor population. Histological studies report a total of 9 diaphragm muscle spindles in cats (Duron et al. 1978) and rabbits (Cuenod 1961) and 10 in humans (Winckler and Delaloye 1957). On the other hand, neurophysiological data suggest a total of 26 muscle spindles in the cat diaphragm (Bałkowiec et al. 1995). Across multiple experiments, Corda et al. (1965) recorded from a total of 750 filaments in the cat C5 dorsal root; of that total, 19 were identified as Ia afferents. The identified muscle spindles were found in the crural but not costal diaphragm. A subsequent study, however, found that receptors which were “unloaded” by contraction (presumably muscle spindles) were distributed in both the costal and crural feline diaphragm (Bałkowiec et al. 1995). Studies in rats have also reported spindles in the both the costal and crural regions (Barstad et al. 1965; Holt et al. 1991). Ex vivo studies of the rat diaphragm show that diaphragm muscle spindle afferents increase discharge with muscle lengthening (Holt et al. 1991), and this is consistent with reports of expiratory phase discharge in rat and cat (Corda et al. 1965; Hill 2001). Cat diaphragm muscle spindles have relatively high “resting” activity but are silenced during contraction of the diaphragm and can also be activated by low-threshold mechanical stimuli (Bałkowiec et al. 1995). Muscle spindle afferents also increase discharge rate during fatiguing contractions of the rat diaphragm (Hill 2001).
Table 1.
Discharge patterns of phrenic afferent neurons
| Group | Species | Comments | References |
|---|---|---|---|
| Ia | Cat, rat | Ia fibers discharge primarily in phase with muscle lengthening (i.e., expiration/diaphragm relaxation); activity increases during DIA fatigue | Bałkowiec et al. (1995); Barstad et al. (1965); Corda et al. (1965); Hill (2001); Holt et al. (1991) |
| Ib | Cat, rat | Ib fibers discharge in phase with inspiration; activity increases with respiratory effort; activity may decrease during fatigue | Bałkowiec et al. (1995); Balzamo et al. (1992); Corda et al. (1965); Duron and Caillol (1973); Holt et al. (1991); Jammes and Balzamo (1992); Jammes et al. (1986) |
| III–IV | Cat, rat | III–IV afferents discharge with irregular patterns during eupnea; afferents are activated during ischemia and lactic acid infusion; DIA fatigue increases discharge of group IV but not III | Bałkowiec et al. (1995); Graham et al. (1986); Hill (2000); Jammes and Balzamo (1992); Jammes et al. (1986); Revelette et al. (1988); Teitelbaum et al. (1993a) |
Listed are key publications providing descriptions of the discharge patterns of phrenic afferents. DIA, diaphragm.
Golgi tendon organs (GTOs; group Ib afferents) are found in the tendinous portions of the diaphragm and have a low mechanical threshold of activation (Corda et al. 1965). In rats, GTOs are found in the central tendon, myo-tendon borders, and the costal diaphragm margins. Increased GTO activity occurs in response to mechanical probing of the diaphragm (Holt et al. 1991). In the cat, Bałkowiec et al. (1995) observed receptors activated by diaphragm contraction (i.e., GTOs) throughout the central tendon. The activity of diaphragm GTOs increases during inspiratory-related contraction of the diaphragm followed by silence or reduced bursting during expiration. Diaphragm Ib afferents increase discharge rate as respiratory effort increases, and activity may decrease during diaphragm fatigue (Jammes and Balzamo 1992).
The diaphragm also contains pressure-sensitive mechanoreceptors that are distinct from spindle and GTOs (Corda et al. 1965). These receptors respond to diaphragm mechanical probing but are not activated by succinylcholine (which activates spindles) or spinal ventral root stimulation (to induce diaphragm contraction). Bałkowiec et al. (1995) described diaphragm receptors that had negligible resting activity and did not respond to diaphragm contraction but that could be activated by focal pressure application. Holt et al. (1991) also found similar pressure-sensitive mechanoreceptors near tendinous borders of diaphragm. Goshgarian and Roubal (1986) suggested that rapidly adapting receptors in the diaphragm were Pacinian corpuscles.
Unmyelinated sensory axons are more prevalent than myelinated sensory fibers in the phrenic nerve (Duron and Marlot 1980; Frazier and Revelette 1991). The most abundant receptors in the diaphragm are small-diameter, myelinated free nerve endings (group III) and unmyelinated C fibers (group IV). Group III and IV afferent endings are evenly distributed across the cat diaphragm (Duron et al. 1978) and have been functionally classified as metabotropic and ergotropic receptors (Bałkowiec et al. 1995; Graham et al. 1986; Jammes et al. 1986). The nerve fibers innervating these receptors show irregular resting activity but are strongly activated by bradykinin, capsaicin, and lactic acid (Bałkowiec et al. 1995; Teitelbaum et al. 1993a). Diaphragm fatigue in rat activates group IV, but not III, afferents (Hill 2000; Jammes and Balzamo 1992; Revelette et al. 1988).
The Phrenic Nerve
The phrenic nerve provides innervation of the entire diaphragm, and the associated efferent projections exit the spinal cord from the mid-cervical ventral roots. There is species and even interspecies variability in regard to the cervical segments that give rise to the phrenic nerve. For example, a study in cadavers found multiple phrenic nerve rootlet patterns in the mid-cervical spinal cord (Mendelsohn et al. 2011). The most common pattern (26% of observations) was for the phrenic nerve to emerge from the C3 and C4 ventral roots, with no observed contribution from C5. In 22% of cases, the phrenic nerve emerged from the C3, C4, and C5 rootlets (Mendelsohn et al. 2011). In rats, the phrenic nerve is typically described as arising from C3–C6 (Goshgarian and Rafols 1981; Gottschall 1981), and in dogs it arises from C5–C7 (Brichant and De Troyer 1997). There is an accessory phrenic nerve with rare and variable incidence in humans (Mendelsohn et al. 2011). It arises from the fifth cervical ventral ramus and joins the main phrenic nerve inside the thoracic cavity (Paraskevas et al. 2016). In the rat, the accessory phrenic nerve contains ~10% of all efferent phrenic motor axons (DeVries and Goshgarian 1989).
Of the total axons in the phrenic nerve (i.e., including both sensory afferent and motor efferent fibers), ~60% of axons are myelinated and 40% are unmyelinated (Duron and Marlot 1980; Langford and Schmidt 1983). Hinsey et al. (1939) established that the phrenic nerve in cat has both myelinated and unmyelinated sensory afferents. Landau et al. (1962) subsequently reported that 35% of myelinated phrenic axons are afferent projections; a similar estimate was provided by Langford and Schmidt (1983). As reviewed above (Diaphragm Receptors: Anatomical Distribution and Discharge Patterns), these myelinated phrenic axons include group Ia-Ib, II, and III fibers. Most unmyelinated afferent axons in the phrenic nerve arise from the mid-cervical dorsal root ganglia, but unmyelinated afferents can also come from the cervical sympathetic chain and even the ventral root (Bałkowiec and Szulczyk 1992; Langford and Schmidt 1983). The ventral root fibers have been suggested to be preganglionic efferent fibers (Langford and Schmidt 1983). In addition to postganglionic sympathetic axons, the phrenic nerve contains pericardial mechanosensitive afferents that discharge in phase with the cardiac rhythm (Kostreva and Pontus 1993b; Ruckebusch 1961). Some phrenic afferents also respond to light pressure applied to the hepatic veins, hepatic parenchyma, and the inferior vena cava (Kostreva and Pontus 1993a). These afferents merge with the phrenic nerve above the level of heart in rats (Razook et al. 1995).
The left and the right phrenic nerves are not identical in composition. Song et al. (1999) reported that the right phrenic nerve of the adult rat has ~20% more axons than the left nerve. This asymmetry was ascribed to a possible larger overall innervation area of the diaphragm by the right phrenic nerve (see also Hebb et al. 1964; Laskowski et al. 1991), increases in the number of mechanoreceptor afferents in the right phrenic nerve (Kostreva and Pontus 1993a), or differences in postganglionic visceral efferent fibers.
Spinal Cord Projections of Phrenic Afferent Neurons
Anatomical studies.
There are relatively few published reports of the anatomical projections of phrenic afferents, and these are summarized in Table 2. Gottschall (1981) applied horseradish peroxidase (HRP) to the rat phrenic nerve and observed HRP-labeled dorsal root ganglia cell bodies from C3–C6, with 68% of the labeled soma at C4. Another study applied fast blue to the feline phrenic nerve while another tracer (nuclear yellow) was injected into the cervical dorsal column. This dual tracing approach colabeled dorsal root ganglia cell bodies, and the authors speculated that these were group Ia and Ib afferents (Larnicol et al. 1984). Goshgarian and Roubal (1986) applied HRP to the phrenic nerve in the adult rat. HRP-positive soma were observed in the ipsilateral C3–C7 dorsal root ganglia, with the majority (88%) of labeling present at C4–C5. HRP-labeled afferent fibers were prominent in the C4–C5 fasciculus cuneatus as well as gray matter laminae I–IV. Collateral axons from fasciculus cuneatus fibers were also noted to enter lamina I–IV. HRP-labeled fibers were not observed, however, in the C3 or C6–C7 segments. The authors suggested that a majority of the HRP-labeled afferent fibers arose from diaphragmatic Pacinian corpuscles (Goshgarian and Roubal 1986). Song et al. (1999) comprehensively evaluated the spinal distribution of phrenic afferents in rats at embryonic stages E13–E21 and postnatal days P0, P4, and P7 by using prolonged application of carbocyanine dye (DiI) to the phrenic nerve. Phrenic afferents could be found in cervical gray matter by E13, and dorsal root ganglia soma were primarily labeled at C5. By E17, phrenic afferents could be clearly observed in three regions: 1) dorsal horn laminae I–V, 2) medial laminae V–VII or near the central canal (laminae X), and 3) laminae VIII and IX in the immediate vicinity of phrenic motoneurons. The authors suggested that visceral afferents carried in the phrenic nerve were more likely to terminate in lamina V or X and that the ventral horn projections reflected Ia or Ib afferents.
Table 2.
Histological and neurophysiological data regarding spinal projections of phrenic afferent neurons
| Group | Location | Method/Species | Comments | References |
|---|---|---|---|---|
| Ia, Ib, II | Mid-cervical laminae I–IV, V–VII, and X | Histology, neonatal and adult rat | Labeled afferents in dorsomedial horn and intermediate gray | Goshgarian and Roubal (1986); Nair et al. (2017); Song et al. (1999) |
| Ia or Ib (likely) | Mid-cervical laminae VIII and IX | Histology, neonatal and adult rat | Labeled afferents reach immediate vicinity of Phr nucleus | Nair et al. (2017); Song et al. (1999) |
| II, III | Cervical cord | Neurophysiology, adult nonhuman primates, rats, cats, and guinea pigs | Phr E-stim alters cervical IN discharge; diverse responses; most recordings are from dorsal horn | Biscoe and Sampson (1970); Bolser et al. (1991a); Cleland and Getting (1993); Iscoe and Duffin (1996); Razook et al. (1995) |
| III–IV | Mid-cervical laminae I–II | Histology, adult rat | CGRP and IB4 immunostaining in dorsal laminae | Nair et al. (2017) |
| III–IV | Mid-cervical laminae I–II | Histology, adult rat | Phr E-stim results in C-Fos expression in dorsal horn | Malakhova and Davenport (2001) |
| III–IV | Cervical cord | Neurophysiology; adult dogs | Activation of III–IV afferents excites PhrMN; response preserved after C2 transection |
Hussain et al. (1990b); Revelette et al. (1988) |
| III–IV | Cervical and thoracic cord | Neurophysiology, dog | Phr E-stim inhibits rostral IC | De Troyer (1998) |
| III–IV (for longer latency inhibition); Ia, Ib (for shorter latency inhibition) | Cervical cord | Neurophysiology, adult cats | Phr E-stim inhibits ipsilateral and contralateral Phr output; abolished by ipsilateral dorsal rhizotomy | Gill and Kuno (1963); Marlot et al. (1988); Speck (1987); Speck and Revelette (1987a) |
The group is estimated on the basis of latency data in neurophysiology experiments and transport properties of neuroanatomical tracers and/or anatomical location of labeling. E-stim, electrical stimulation; Phr, phrenic nerve; PhrMN, phrenic motoneurons; IN, interneurons. Note: studies describing projection fibers (e.g., dorsal column fibers) are included in Table 3.
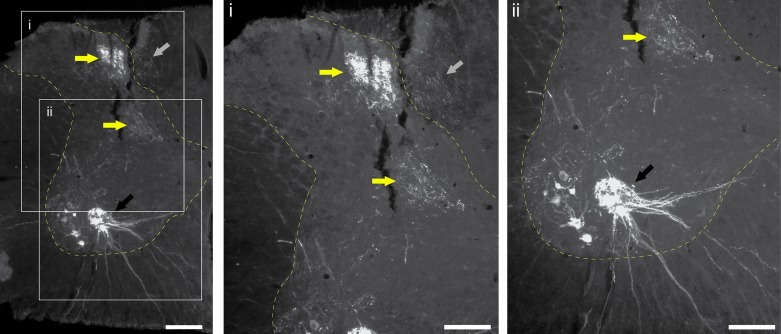
We recently reported a method of phrenic nerve “soak” with either cholera toxin or cascade blue to label phrenic afferents in adult rats (Nair et al. 2017). The results may reconcile some of the apparent differences between the results of the aforementioned studies. Phrenic afferent labeling was observed in the same locations as observed by Goshgarian and Roubal (1986) (e.g., prominent in fasciculus cuneatus, laminae I–III), but phrenic afferent projections were also present in mid-cervical intermediate gray matter (laminae IV and VII) as well as laminae X. Last, phrenic afferent projections were identified in the immediate vicinity of phrenic motoneurons, and this was verified by staining motoneurons with choline acetyltransferase (ChAT) to differentiate between phrenic dendrites and afferent fibers. Figure 1 provides a representative histological section from the mid-cervical spinal cord of an adult rat following labeling of phrenic afferent neurons using cholera toxin β-subunit per our published method (Nair et al. 2017). Note the robust labeling in the dorsal horn with projections extending to laminae X near the central canal.
Fig. 1.
Histological example of phrenic afferent labeling in the C4 spinal cord. Cholera toxin β-subunit (CT-β) was applied to the phrenic nerve in an adult Sprague-Dawley rat using the method published by Nair et al. (2017). Tissues were harvest after 96 h and immunochemically processed to enable visualization of CT-β labeling. Phrenic motoneuron labeling is clearly observed (black arrows). A dense pocket of afferent projections is observed in the dorsal horn laminae II and III and projecting into the deeper lamina (yellow arrows). Afferent fibers are also labeled in the dorsal columns (gray arrows). Scale bars: 200 µm (left) and 50 µm (enlarged insets i and ii).
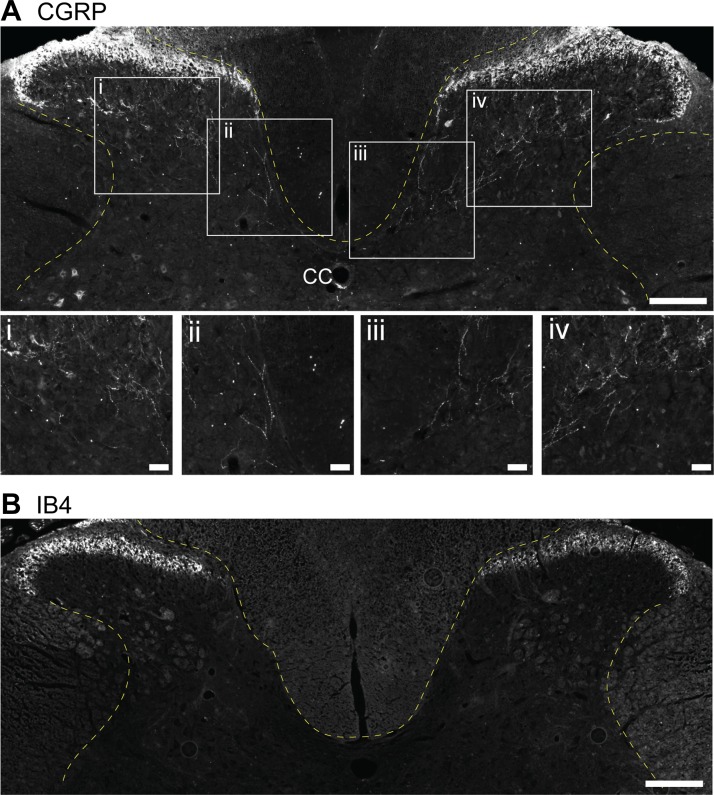
To our knowledge, the spinal cord projections of small myelinated or unmyelinated phrenic afferent neurons have not been specifically traced. However, calcitonin gene-related peptide (CGRP) and isolectin-B4 (IB4) immunochemical staining in the mid-cervical spinal cord provides some insight. CGRP labels peptidergic C-fibers (Gibson et al. 1984), and IB4 labels nonpeptidergic C-fiber afferents (Basbaum et al. 2009; Snider and McMahon 1998). Figure 2 provides representative histological sections from the mid-cervical (C4) spinal cord of an adult rat that were processed with antibodies against IB4 and CGRP using our published method (Nair et al. 2017). Note that CGRP immunoreactivity is prominent in lamina I and can also be seen in deeper laminae. In contrast, IB4 immunoreactivity is concentrated in lamina II but is absent from the deeper laminae. In our recent study, CGRP- and IB4-positive cell bodies were present in dorsal root ganglia soma at C4. When cascade blue was applied to the phrenic nerve, dual labeling in the dorsal root ganglia soma was noted for CGRP but not IB4, suggesting that cascade blue is capable of labeling peptidergic C-fibers. However, small-diameter afferents in dorsal horn lamina I and II were not observed to be either IB4 or CGRP positive (Nair et al. 2017). Additional studies are needed to determine the spinal cord projections of group III and IV phrenic afferents.
Fig. 2.
Histological example of calcitonin gene-related peptide (CGRP) and isolectin-B4 (IB4) immunochemical staining in the C4 spinal cord. Tissue sections from an adult Sprague-Dawley rat were immunochemically stained using the method published by Nair et al. (2017). A: CGRP staining is prominent in dorsal horn laminae I and II, and CGRP-positive fibers can be seen extending to deeper laminae. B: IB4 immunoreactivity is concentrated in lamina II but is absent from the deeper laminae. Scale bars: 200 µm (A and B) and 50 µm (enlarged insets i–iv).
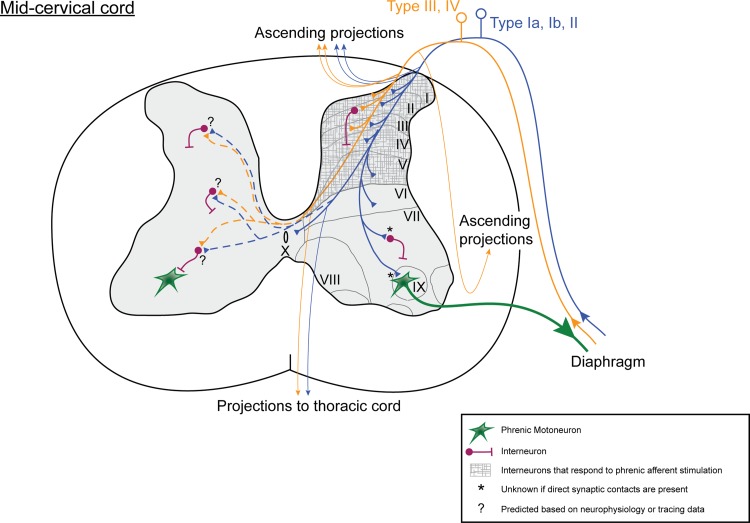
The schematic diagram shown in Fig. 3 provides a summary of known and predicted (e.g., based on neurophysiological data) anatomical projections of phrenic afferent fibers in the mid-cervical spinal cord.
Fig. 3.
Diagram illustrating known and hypothesized phrenic afferent projections to the mid-cervical spinal cord. The drawing is based on neurophysiological and anatomical studies as outlined in Table 2.
Do spinal projections of phrenic afferents reach phrenic motoneurons via monosynaptic or polysynaptic projections?
Short-latency inhibitory responses to phrenic nerve stimulation suggest a spinal synaptic connection to phrenic motoneurons (Gill and Kuno 1963; Speck and Revelette 1987a). To our knowledge, there are two reports that phrenic afferent neurons project to the immediate vicinity of the phrenic motoneuron pool (i.e., laminae VII, VIII, and IX). Song et al. (1999) showed very clear DiI-labeled afferent projections in the mid-cervical spinal cord that reached the ventral horn in neonatal rats. Light microscopy revealed no evidence for a direct contact between these afferents and motoneurons, but as the authors point out, electron microscopy would be needed to verify this hypothesis. We recently observed that phrenic afferent projections in the adult rat reach lamina IX and the immediate vicinity of retrogradely identified phrenic motoneurons. Whether or not these afferents make a direct (monosynaptic) connection with phrenic motoneurons remains unknown, but synapses with cervical interneurons are more likely. There is one anatomical study that suggests phrenic afferents terminate in the vicinity of cervical interneurons identified as being synaptically coupled to the phrenic motor pool (Nair et al. 2017), and multiple reports show that electrical stimulation of the phrenic nerve can activate cervical interneurons (see Spinal cord neuronal activation following phrenic nerve stimulation).
Mead (1979) suggested that the diaphragm has a phrenic afferent-mediated stretch reflex that optimizes myofiber length in response loading in humans. Consistent with this suggestion, Frazier et al. (1985) evoked a diaphragm stretch reflex in anesthetized cats. Rapid diaphragm stretch produced a triphasic diaphragm electromyographic (EMG) response beginning 60 ms after the stretch. The authors concluded, however, that the reflex was a polysynaptic response involving supraspinal connections (i.e., not a “spinal reflex loop”). In many motor pools, activation of muscle spindle (Ia) afferents can evoke a monosynaptic excitation of motoneurons via a spinal loop (i.e., the “H-reflex”). Stimulation of cervical dorsal roots in an in situ neonatal rat can evoke a phrenic H-reflex (Formenti and Zocchi 2014). A caveat, however, is that stimulation of the dorsal root does not selectively activate phrenic afferents, and thus it cannot be certain that the H-reflex was induced via phrenic or other (e.g., forelimb) afferent fibers. In adult mammals, including rat, lamb, and cat, attempts to evoke a phrenic H-reflex have been unsuccessful (Davenport P, unpublished observations).
Spinal cord neuronal activation following phrenic nerve stimulation.
Cervical interneurons can be synaptically coupled to respiratory motoneurons (Sanlioglu et al. 2000) or sympathetic preganglionic neurons (Deuchars et al. 2005). In addition, cervical interneurons with both inspiratory or expiratory discharge patterns are common (Bellingham and Lipski 1990; Iscoe and Duffin 1996; Palisses et al. 1989; Streeter et al. 2016). Neurophysiological studies indicate that phrenic afferents are synaptically coupled to this propriospinal network, although the prevalence of mono- vs. polysynaptic connections is not known. Bellingham and Lipski (1990) tested the response of 24 cervical (C5) interneurons to stimulation of the ipsilateral phrenic nerve in cats, and 3 cells responded with increased discharge (latency 2–7 ms). Phrenic nerve stimulation in adult guinea pigs causes a diverse cervical interneuron response that includes increases and decreases in discharge rates (Cleland and Getting 1993). In a similar study, cervical interneurons in both the ipsilateral and contralateral C1–C2 dorsal horn were activated at a latency of ~9 ms following phrenic nerve stimulation (Razook et al. 1995). Based on an estimated conduction velocity of 3.0 m/s, it was inferred that group III–IV afferents were responsible. In the study by Razook et al. (1995), C1–C2 interneurons were not activated if the phrenic nerve was stimulated “downstream” from the heart, thus suggesting a pericardial origin of the relevant afferents (also see Kostreva and Pontus 1993b). Iscoe and Duffin (1996) observed that phrenic nerve stimulation induced a graded suppression of ipsi- but not contralateral phrenic nerve activity, and this was associated with both excitation and inhibition of cervical interneurons. Histological data also confirm that phrenic afferent stimulation activates cervical interneurons following 30-min of phrenic nerve stimulation in anesthetized rats. Following this procedure, neurons positive for c-Fos (an indirect marker of neuronal activation) can be observed in laminae I and II of the ipsilateral C3–C5 spinal cord (Malakhova and Davenport 2001). Activation of spinal interneurons via phrenic afferent stimulation is also inferred from studies of phrenic-to-phrenic or phrenic-to-intercostal reflexes (see Phrenic afferent projections to somatosensory cortex and their role in perception of breathing and Tables 2 and 4).
Table 4.
Functional impact of phrenic afferent neuron activation
| Fiber Type | Species | Comment | References |
|---|---|---|---|
| Large | Cat | Blocking Ia and Ib afferents prolongs PhrMN discharge | Dolivo (1952); Jammes et al. (1986) |
| Large | Cat | Stimulating GTOs inhibits PhrMN activity | Shannon et al. (1985) |
| Large | Cat | GTOs are activated by diaphragm tension; may attenuate DIA EMG | Revelette et al. (1992) |
| Large | Cat | ↓DIA EMG when DIA is lengthened; response is abolished after cervical rhizotomy | Cheeseman and Revelette (1990) |
| Small diameter, myelinated | Cat | Phr nerve E-stim initially causes ↓Vt and ↑Te; then induces a sustained ↑Vt and ↑V̇e | Marlot et al. (1987) |
| Small | Cat | Procaine block of III–IV fibers does not impact eupneic Phr output, E-stim of III–IV fibers can ↓PhrMN discharge | Jammes et al. (1986) |
| Small | Dog | Capsaicin or K+ stimulation of III–IV afferents leads to ↑tonic EMG DIA | Frazier et al. (1985); Hussain et al. (1990b, 1991); Revelette et al. (1988); Supinski et al. (1993) |
| Small | Dog | DIA ischemia activates III–IV afferents leading to ↑DIA EMG | Teitelbaum et al. (1993b) |
| Small | Dog | E-stim of III–IV afferents produce ↑DIA EMG, ↑MAP, and ↑V̇e | Road et al. (1987) |
| All; authors speculate III–IV are driving responses | Dog | Phr E-stim causes ↑V̇e; ↑genioglossus EMG, variable impact on abdominal muscles; larger ↑V̇e when limb and Phr afferents are both stimulated | Ward et al. (1992a, 1992b) |
| Large (ipsilateral), group III (contralateral) | Cat | Phr E-stim causes ↓ipsilateral and/or contralateral PhrMN output, latency ~5–15 ms | Gill and Kuno (1963); Marlot et al. (1988); Speck and Revelette (1987a) |
| Small group III | Cat | Phr E-stim causes ↑PhrMN discharge; response is blunted by E-stim of contralateral Phr | Macron et al. (1988) |
| Mechanosensitive group II–III | Monkey | Implications for referred pain from the diaphragm and heart | Bolser et al. (1991a) |
| Mechanosensitive | Cat | Respiratory timing alterations after lower body negative pressure are mediated by Phr afferents | Fryman and Frazier (1987) |
Listed are key publications that provide evidence regarding the functional role of phrenic afferents. EMG, electromyogram; Vt, tidal volume; Te, expiratory time; V̇e, minute ventilation; MAP, mean arterial pressure.
In summary, activation of phrenic afferents can alter propriospinal discharge, but the functional significance of the diverse responses that have been reported is not clear. It is likely that cervical interneurons can relay information arising from the activation of diaphragm receptors to propriospinal neurons and/or spinal motoneurons, and also to supraspinal centers involved in autonomic and/or respiratory control.
Supraspinal Projections of Phrenic Afferents
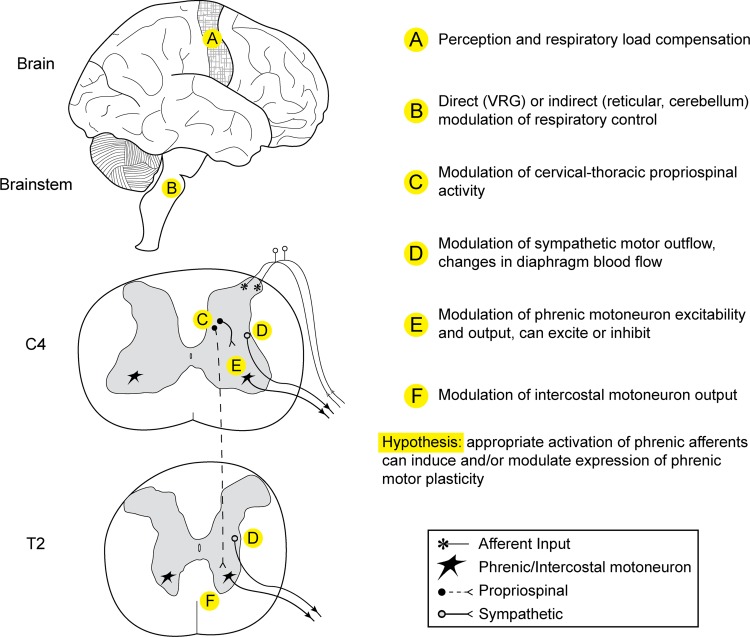
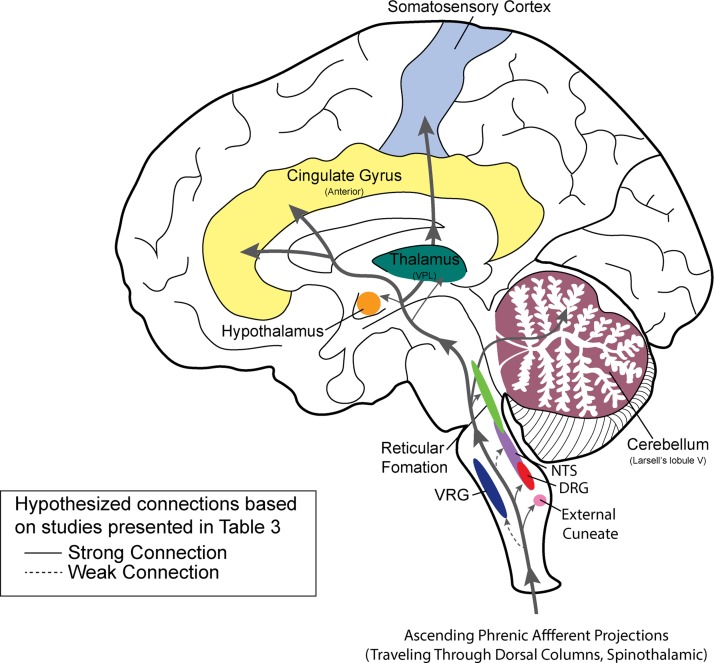
Histological and neurophysiological studies confirm that phrenic afferent projections reach the brain stem and higher brain centers (Fig. 4). As summarized in Table 2, ascending (spinobulbar) projections from phrenic afferents travel in the cervical dorsal columns (Chou and Davenport 2005; Goshgarian and Roubal 1986; Larnicol et al. 1984; Nair et al. 2017) as well as the lateral portion of the spinal cord (Speck and Revelette 1987b), including the spinothalamic tract (Bolser et al. 1991a; Bolser et al. 1991b).
Fig. 4.
Diagram illustrating known spinal and supraspinal projections of phrenic afferents neurons. The drawing is based on neurophysiological and anatomical studies as outlined in Table 3.
Brain stem, cerebellar, and thalamic projections.
Brain stem projections have been confirmed in multiple investigations (Table 3). Phrenic nerve stimulation experiments indicate direct (monosynaptic) inputs to the external cuneate nucleus in the medulla (Larnicol et al. 1985; Marlot et al. 1985), which is part of the dorsal column-medial lemniscus pathway. Phrenic nerve stimulation can also activate neurons in the reticular formation (Macron et al. 1985; Malakhova and Davenport 2001), the dorsal and ventral respiratory groups (Malakhova and Davenport 2001; Shannon et al. 1985; Speck and Revelette 1987b), cerebellum (Marlot et al. 1985; Marlot et al. 1984), hypothalamus (Malakhova and Davenport 2001), and thalamus (Bolser et al. 1991a; Bolser et al. 1991b; Zhang and Davenport 2003). Group I and II phrenic afferents activate thalamic ventroposterolateral (VPL) neurons (Zhang and Davenport 2003), and the VPL nucleus has projections to the diaphragm somatosensory cortex (Yates et al. 1994). Thus ascending projections from phrenic afferent neurons reach areas with both direct and indirect impact on respiratory motor control (Fig. 4), and this is consistent with the body of literature showing that phrenic afferent activation can modulate respiratory motor drive via supraspinal pathways (e.g., Speck 1987; see also Table 3).
Table 3.
Supraspinal projections of phrenic afferent neurons
| Location | Method/Species | Comment | Citations |
|---|---|---|---|
| Dorsal columns | Tract tracing and histology, rat; neurophysiology, cat | Phr afferent fibers clearly observed in dorsal columns; Phr E-stim evokes cord dorsum potentials | Chou and Davenport (2005); Goshgarian and Roubal (1986); Larnicol et al. (1984); Nair et al. (2017) |
| Outside of dorsal column | Neurophysiology, cat | Dorsal column lesion does not abolish VRG responses following Phr E-stim | Speck and Revelette (1987b) |
| Cervical spinothalamic tract | Neurophysiology, monkey | Phr E-stim or punctate mechanical stimulation of the diaphragm activates or inhibits STT neurons | Bolser et al. (1991a, 1991b) |
| External cuneate | Neurophysiology and tracing, cat | Monosynaptic connections from group I–II afferents | Larnicol et al. (1985); Marlot et al. (1985) |
| Nucleus of solitary tract | c-Fos in rat | Direct connections are unknown? | Malakhova and Davenport (2001) |
| Ventrolateral reticular formation | Neurophysiology, cats | Nucleus ambiguous discharge pattern was strongly affected by Phr E-stim; concluded that Phr afferents relay in VLRN before projecting to the cerebellar cortex | Macron et al. (1985); Malakhova and Davenport (2001) |
| Dorsal respiratory group | Neurophysiology, cat | Strong connections, hypothesized to be from group III | Speck and Revelette (1987b) |
| Ventral respiratory group | Neurophysiology in cat; c-Fos in rat | Weak connection | Malakhova and Davenport (2001); Speck and Revelette (1987b) |
| Hypothalamus | c-Fos in rat | Malakhova and Davenport (2001) | |
| Thalamus | Neurophysiology, cat, rat; histology, rats | Phr E-stim and diaphragm mechanical stim activates contralateral VPL neurons; |
Malakhova and Davenport (2001); Zhang and Davenport (2003) |
| paraventricular thalamic nucleus has c-fos expression following Phr stimulation | |||
| Cerebellar cortex | Neurophysiology, cat | E-stim of C5–C6 Phr branches evokes cerebellar cortex potentials; ascending fibers are in contralateral ventrolateral spinal cord | Marlot et al. (1984, 1985) |
| Cingulate gyrus | Neurophysiology, human | Evoked potentials in anterior cingulate gyrus with transcutaneous Phr E-stim | Straus et al. (1997) |
| Amygdala | Histology, rat | Central nucleus of amygdala has c-fos expression following Phr E-stim | |
| Somatosensory cortex | Neurophysiology, human, cat, and rat | Cortical evoked potentials from Phr E-stim in cats and rats. Brief inspiratory occlusion in humans evokes somatosensory cortex EEG response | Davenport et al. 1985, 1986, 2006, 2010); Frankstein et al. (1979); Revelette and Davenport (1990); Yates et al. (1994) |
Listed are key publications that provide evidence for the location of ascending pathways containing phrenic afferent information, as well as the potential destination of pathways from diaphragm receptors. VRG, ventral respiratory group; STT, spinothalamic tract; VLRN, ventrolateral reticular formation; VPL, ventral posterolateral nucleus.
Phrenic afferent projections to somatosensory cortex and their role in perception of breathing.
Diaphragm loading, ischemia, and fatigue can all trigger increases in phrenic afferent discharge (Table 1). Thus phrenic afferents respond to stimuli that are related to the overall work of breathing and/or respiratory effort. Accordingly, Davenport and others have hypothesized that diaphragm receptors are part the neurophysiological substrate enabling perception of respiratory effort (Campbell et al. 1961; Davenport et al. 1985). In this article, we briefly review the relevant literature including studies of cortical activation in animals and humans; for further discussion of this topic see Davenport and Vovk (2009).
The first study to establish a neurophysiological link between phrenic afferents and the somatosensory cortex was completed by Frankstein et al. (1979). They demonstrated that electrically stimulating the phrenic nerve in anesthetized cats produced an evoked potential in the contralateral somatosensory cortex. Davenport et al. (1985) then showed that electrical stimulation of phrenic nerve afferents in the cat activated sensorimotor cortex neurons located medial to forelimb and lateral to hindlimb representation. A related study by the Jammes laboratory (Balzamo et al. 1992) verified somatosensory cortical evoked potentials following phrenic nerve stimulation in anesthetized rats and showed that the evoked potentials were altered by diaphragm fatigue. They concluded that diaphragm fatigue alters the “cortical integration” of phrenic sensory afferent information. The latency of phrenic afferent-evoked cortical potentials suggests that it is elicited by myelinated group I and II fibers (Davenport et al. 1985; Frankstein et al. 1979; Yates et al. 1994). This was confirmed in another study by the Davenport laboratory showing bilateral somatosensory cortical activation when large phrenic fibers were electrically stimulated in anesthetized cats (Davenport et al. 2010). The activated cortical neurons were located in the “trunk region” of the cat homunculus, medial to forelimb and intercostal representation (Davenport et al. 1993). The authors concluded that phrenic afferents are cortically represented on the basis of diaphragm “body position” rather than spinal segment. Another observation was that ipsilateral (i.e., relative to nerve stimulation) cortical activation occurred after the contralateral cortex had been activated, potentially indicating a cortico-cortical pathway via the corpus callosum.
The confirmed activation of somatosensory cortical neurons in animal studies indicates that phrenic afferents provide information regarding diaphragm activation to the cerebral cortex, and this information is likely to contribute to diaphragmatic proprioception and somatosensation. Validating this hypothesis is difficult, but a review of the human literature is instructive. Brief inspiratory occlusion in humans evokes a strong diaphragm contraction and clear electroencephalographic (EEG) potentials in the contralateral sensory motor cortex (Davenport et al. 1986). Cortical potentials evoked by occlusion can be detected bilaterally (larger in the right vs. left cortex; Revelette and Davenport 1990) are larger when occlusion occurs during mid inspiration (Revelette and Davenport 1990) and can be evoked in double-lung transplant, tracheotomized patients (Davenport et al. 2006). This latter observation shows that cortical evoked potentials in response to respiratory loading can occur without upper airway or lung (vagal) sensory inputs, and this implicates phrenic afferents as a logical candidate for mediating the responses.
Recordings from the anterior cingulate gyrus during phrenic nerve stimulation indicate that phrenic afferents also project to the limbic system in humans (Straus et al. 1997). This suggests a possible link between diaphragm sensory afferents and emotional state. Consistent with this possibility, cortical evoked responses to brief inspiratory occlusions are strongly modulated by the affective state in humans (Von Leupoldt et al. 2010). Phrenic afferents may also be involved in shoulder or neck pain. Mechanical and/or noxious stimulation of the central diaphragm in humans produces referred angina-like pain in these regions (initially reported by Capps 1911, cited in Chandler et al. 1999). This response likely reflects activation of group III and IV phrenic afferents, which converge with the spinothalamic tract in high cervical spinal cord (Chandler et al. 1998, 1999; Razook et al. 1995).
In summary, animal data confirm that diaphragm sensory afferents activate neurons in the somatosensory cortex, and human data are fully consistent with these observations. These pathways likely play an important role in the perception (and modification) of respiratory motor output.
Phrenic Afferent Activation: Impact on Respiratory Motor Output
A cervical dorsal rhizotomy study from the early 1960s led to the conclusion that phrenic afferents have little to no effect on respiratory motor output (Sant’Ambrogio et al. 1962); however, the literature unequivocally confirms that phrenic afferents play a strong role in modulating respiratory motor output. Reported discrepancies are likely due to the diversity of phrenic afferent fibers, the experimental challenges associated with selectively activating specific types of afferents, and the difficulty in quantifying complex respiratory responses that may involve both excitation and inhibition of different groups of neurons (Tables 2–4). Below we review several of the seminal studies that have contributed to our understanding of the functional consequences of phrenic afferent activation with regard to breathing; see Table 4 for a more comprehensive list.
Ventilation.
Early studies indicated that activation of phrenic afferents via electrical stimulation of the phrenic nerve can trigger an increase in breathing (Kohrman et al. 1947), and many subsequent studies have validated this observation (Table 4). One strong conclusion that emerges from the literature is that activation of group III and IV phrenic afferents results in increases in breathing (Supinski et al. 1993; Yu and Younes 1999). A study by Yu and Younes (1999) is particularly informative in this regard. The respiratory impact of phrenic nerve electrical stimulation was quantified at different levels of anesthesia in dogs. Animals were allowed to recover naturally from pentobarbital anesthesia; as the anesthetic plane became lighter, phrenic afferent activation produced increased in ventilation of up to 400% of baseline values. Based on the stimulus currents applied to the phrenic nerve, the authors concluded that group III and IV afferents were primarily responsible for the robust increase in ventilation. The data also suggest that many studies may have underestimated the ability of phrenic afferents to stimulate ventilation secondary to the impact of anesthesia.
Activation of large-diameter phrenic afferents also impacts respiratory motor output, and most studies suggest an overall inhibitory impact (Table 4). However, group I and II afferents may produce both inhibition and excitation of breathing, but on a different timescale. For example, Marlot et al. (1987) reported a biphasic respiratory response to phrenic nerve stimulation in anesthetized dogs. Initially, there was a decrease in tidal volume and minute ventilation lasting a few respiratory cycles. However, this was followed by an increase in both of these variables. The authors concluded that the increases in breathing were due to activation of myelinated phrenic afferents, but they acknowledged that the phrenic nerve stimulation paradigm may have activated both myelinated and unmyelinated afferent fibers.
The impact of phrenic afferent neurons on ventilation is likely to involve activation of spinal, brain stem, and cortical neurons (Figs. 3 and 4), and the complexity of the respiratory response to phrenic afferent stimulation has been highlighted by previous authors (Davenport 1995; Frazier and Revelette 1991; Hussain 1995; Road 1990). This complexity can be appreciated by considering that during each respiratory cycle, diaphragm contraction, relaxation and associated changes in blood flow result in a wide range of sensory fiber activation patterns (see Table 1). These sensory afferent signals will interact to produce the overall respiratory response. Moreover, the prevailing metabolic and/or experimental conditions influence how activation of these afferents changes ventilation. The latter point was illustrated by Ward et al. (1992b), who showed that activation of limb muscle afferents interacts with phrenic afferent stimulation to synergistically increase ventilation. It should also be emphasized that anesthesia has a robust impact on the ventilatory response to afferent nerve stimulation (Yu and Younes 1999). Based on the studies summarized in Table 4, we conclude that activating small-diameter (III, IV) diaphragm afferents will robustly stimulate ventilation and that activating larger diameter myelinated (Ia, Ib) afferents has a more variable impact, but with short-term inhibition being likely.
Phrenic afferent reflexes.
The initial response to electrical stimulation of the phrenic nerve is a short-latency inhibition of phrenic discharge commonly referred to as the phrenic-to-phrenic reflex (Gill and Kuno 1963). Many of the key findings in this area are summarized in Table 2. Gill and Kuno showed that phrenic motoneurons were hyperpolarized by electrical stimulation of the contralateral phrenic nerve, an effect abolished by sectioning the contralateral cervical dorsal roots. Subsequently, Speck and Revelette (1987b) provided a comprehensive study of this reflex in anesthetized cats. Electrical stimulation of the C5 phrenic nerve root evoked bilateral inhibition of phrenic motor output; this response was abolished by ipsilateral C4–C6 rhizotomy. Based on observed latency of 8–12 ms, the authors suggested that group III afferents were primarily responsible for the observed phrenic inhibition. An even shorter latency (5 ms) ipsilateral inhibition was evoked by stimulation of the phrenic nerve in the thoracic region, and this likely involves large diameter afferents (Speck and Revelette 1987b).
The phrenic-to-phrenic reflex is not altered by sectioning the dorsal columns at C2 (Speck and Revelette 1987a), consistent with a spinal mechanism. On the other hand, the inhibition of intercostal reflexes via phrenic afferents does have a supraspinal component (Speck 1987). The phrenic-to-phrenic inhibitory reflex also has been demonstrated in humans (Butler et al. 2003). When the phrenic nerve was stimulated in the cervical region, inhibition of the contralateral diaphragm was readily observed in EMG recordings as well as single motor unit discharge patterns. The authors suggested that the inhibition was driven by activation of large-diameter afferents and that the latency of the response suggested involvement of brain stem circuits (Butler et al. 2003).
Activation of phrenic afferents can also modulate intercostal muscle output. Diaphragm paralysis immediately increases external intercostal muscle activity, and the relative increase is considerably greater than would be expected on the basis of changes in arterial blood gases. Thus paralysis-induced inhibition of diaphragm mechanoreceptors appears to produce disinhibition of the inspiratory intercostal muscles (Brichant and De Troyer 1997). Consistent with this hypothesis, stimulating the C5 ventral phrenic roots causes inhibition of inspiratory intercostal EMG activity, and this effect is abolished when the cervical dorsal roots are cut (De Troyer 1998). A role for diaphragm mechanoreceptors in this reflex is further supported by studies showing that direct pressure to the diaphragm central tendon (activating GTOs) results in decreased activation of the external intercostal muscles (De Troyer et al. 1999). The inhibition of intercostal muscles was blunted after cutting of the ipsilateral cervical dorsal roots, and this confirms a role of phrenic afferents. DeTroyer et al. suggested that the inhibitory phrenic-to-intercostal reflex is supraspinally mediated.
Phrenic Afferents and Autonomic Function
As illustrated in Fig. 4, phrenic afferents project to brain stem regions associated with autonomic control. In addition, the dense spinal projections of phrenic afferents (e.g., Figs. 1–3) and the robust cervical interneuron response to phrenic afferent stimulation (see Spinal cord neuronal activation following phrenic nerve stimulation) suggest possible links to sympathetic preganglionic neurons. Consistent with this suggestion, activation of group III and IV phrenic afferents evokes increase in mean arterial pressure (Hussain et al. 1990a; Road et al. 1987; Supinski et al. 1993). For example, delivering capsaicin to the phrenic artery increases both systemic arterial pressure and heart rate in dogs (Hussain et al. 1990a). Supinski et al. (1993) activated group III and IV diaphragm afferents with potassium injections to the canine inferior phrenic artery (all other sources of blood flow the diaphragm were ligated). Potassium infusion triggered an increase in mean arterial pressure of ~20 mmHg as well as robust increases in diaphragm EMG activity (Supinski et al. 1993). The aforementioned studies indicate that group III and IV phrenic afferent stimulation increases sympathetic outflow, and this was confirmed in a study of anaesthetized cats (Offner et al. 1992). Responses in both cardiac and renal sympathetic nerves could be evoked by electrical stimulation of the ipsilateral phrenic nerve. Both spinal and supraspinal pathways were suggested to be involved in this reflex activation of sympathetic outflow (Offner et al. 1992).
Because phrenic afferents can stimulate sympathetic motor outflow, it follows that diaphragm workload could impact vascular conductance in the diaphragm and/or limb muscles (Harms et al. 1997). Strong support for this hypothesis comes from a study in humans showing that peroneal nerve sympathetic activity increases during diaphragm fatigue (St Croix et al. 2000). A resistive breathing paradigm sufficient to fatigue the diaphragm also produced an increase arterial blood pressure and sympathetic drive to resting limb muscle. This response required diaphragm fatigue, because breathing paradigms that increased central “respiratory drive” without diaphragm fatigue did not alter muscle sympathetic outflow or arterial blood pressure. St. Croix et al. (2000) therefore concluded that diaphragm fatigue likely triggered a phrenic afferent-mediated increase in sympathetic outflow to resting limb muscles.
Finally, McCallister et al. (1986) showed that reflex bronchodilation can be evoked when the phrenic nerve is stimulated in dogs. This effect was only induced at high stimulus currents, indicating that group III and IV fibers are responsible. The dilation requires cholinergic input to pulmonary airways because it can be blocked by atropine.
Phrenic Afferents and Pain
Phrenic afferents may also contribute to the sensation of referred pain. Clinical presentations associated with inflammation of the superior or inferior surfaces of the diaphragm, such as pleural effusions or subdiaphragmatic infections, can include sharply localized referred pain to the trapezius ridge (Capps 1911). These areas of referred pain include mid- to lower cervical dermatomes that overlap with the segmental projections of phrenic afferents. In humans with disorders involving the diaphragm that manifest as referred pain, mechanical stimulation of the central portions of this muscle elicited pain referred to the trapezius ridge (Capps 1911; Hinsey and Phillips 1940).
The mechanisms responsible for referred pain of visceral origin were first proposed by Ruch in 1961. This hypothesis holds that convergence of somatic and visceral sensory information onto spinothalamic tract neurons accounts for the sensations of pain distant from the organ of origin (Ruch 1961). This hypothesis has been supported by a robust literature including both basic and clinical observations (Foreman et al. 2015; Rosen 2012). For example, in the anesthetized primate, electrical or mechanical stimulation of the phrenic nerve excites spinothalamic tract neurons in the mid- to lower cervical spinal cord that have somatic receptive fields that include the shoulder and thorax (Bolser et al. 1991a). However, the activity patterns of other spinothalamic tract neurons with somatic receptive fields restricted to the distal forelimb are not altered by phrenic afferent stimulation (Bolser et al. 1991a). These observations provide support for the suggestion that phrenic afferents contribute to referred noxious sensations originating from the diaphragm. They also support the concept that pain from the central regions of the diaphragm is referred to axial regions of the body that are innervated by cervical dermatomes associated with the spinal segmental entry of phrenic afferents.
Phrenic Afferents and Neuroplasticity
The neurons and neuronal networks that regulate breathing in adult mammals are capable of changing based on experience (reviewed in Fuller and Mitchell 2016). Such “respiratory neuroplasticity” can be expressed in multiple time domains (e.g., seconds, hours, days) leading to increased or decreased respiratory-related motor output. In nonrespiratory motor circuits, activation of sensory afferent neurons can evoke neuroplastic changes, especially in the injured spinal cord (Courtine et al. 2009; Minassian et al. 2016). Much less is known, however, in regard to phrenic afferent neurons and expression of neuroplasticity in respiratory-related neurons or networks. Phrenic afferent activation can evoke increases or decreases in respiratory motor output (Tables 1 and 2), but whether these responses can also lead to plasticity associated with the changes in synaptic activity is not known. In the most well-studied form of spinal respiratory neuroplasticity, persistent changes in phrenic motor output do not appear to be initiated via classical “Hebbian” mechanisms that depend on increased synaptic activity; rather, release of neuromodulators (e.g., serotonin) can trigger synaptic plasticity leading to sustained increases in motor output. Phrenic afferents may project to brain stem networks that can trigger release of serotonin or other neuromodulators in the vicinity of respiratory motor neuron pools. It is unknown, however, if phrenic afferent activation can lead to sustained changes in respiratory motor output via this type of mechanism. Another consideration is that reductions in synaptic activity can be a powerful stimulus leading to persistent changes in respiratory motor output (Strey et al. 2013). Thus phrenic afferent-mediated inhibition of respiratory motor output could also lead to neuroplasticity. Below we review evidence suggesting that phrenic afferents may play a role in triggering persistent changes in respiratory motor output.
Persistent changes in breathing after phrenic afferent stimulation.
Very few studies have formally evaluated prolonged (e.g., longer than several minutes) changes in breathing after a period of phrenic afferent stimulation. The vast majority of published reports focus on the response during phrenic afferent activation and a short poststimulus recovery phase (typically millisecond time domain). Road et al. (1993) observed that 1 min of phrenic nerve stimulation induced a transient increase of inspiratory phrenic nerve burst amplitude, but this was followed by a prolonged (30 min) decrease in inspiratory phrenic nerve burst amplitude in anesthetized dogs. The effect was attributed to poststimulus inhibition associated with activation of thin fiber (III, IV) afferents. In a recent study utilizing a brain stem-spinal cord preparation, C4 dorsal roots were stimulated while phrenic output was recorded from C4 ventral roots. Five consecutive stimulus trains were delivered at 3-min intervals, and this paradigm produced an increase in phrenic inspiratory burst frequency that lasted 10–15 min (Formenti and Zocchi 2014). The authors speculated that activation of low-threshold, large-diameter fibers led to this response. Additional studies in anesthetized animals have also reported increases in diaphragm EMG bursting and/or ventilation that persist following activation of phrenic afferents, but, as mentioned above, the persistent impact has rarely been formally evaluated (see Table 4). Overall, the available literature on this topic, which is quite limited, indicates that increases (Formenti and Zocchi 2014; Hussain et al. 1990b; Supinski et al. 1993) or decreases (Road et al. 1993) in respiratory motor output can occur after a period of phrenic afferent stimulation. To our knowledge, there are no available data regarding the potential physiological mechanisms underlying sustained changes in respiratory output after phrenic afferent stimulation. Thus there is a need for further study in this area, and the impact of phrenic afferent activation on respiratory neuroplasticity remains an open question.
Impact of phrenic afferent denervation on respiratory neuroplasticity.
In addition to playing a role in the induction of neuroplasticity, phrenic afferent input (or lack thereof) may modulate the expression of respiratory plasticity induced by other stimuli. One way this could happen is via removal of an inhibitory influence of phrenic afferents on local spinal circuits and/or supraspinal respiratory centers (i.e., “disinhibition”). For example, a study from our laboratory showed that sectioning the phrenic nerve distal to the location of neurophysiological recordings enhances the subsequent capacity for phrenic motor plasticity (Sandhu et al. 2010). Specifically, when phrenicotomy preceded exposure to acute intermittent hypoxia (AIH) in anesthetized rats, the magnitude of AIH-induced long-term facilitation of phrenic burst amplitude was enhanced. Although potential effects of phrenic motoneuron axotomy cannot be excluded, this observation is consistent with a role for phrenic afferents in modulating expression of plasticity (Sandhu et al. 2010). Phrenic afferents can also have a robust impact on the neuroplastic response of the phrenic motor system to high cervical spinal cord injury (SCI). Goshgarian (1981) demonstrated that cutting the mid-cervical dorsal roots (contralateral to injury and diaphragm EMG recordings) resulted in immediate restoration of inspiratory EMG busting in rats with high cervical SCI. Thus phrenic (or other mid-cervical) afferents can inhibit respiratory motor output after SCI. On the other hand, there is also evidence that phrenic afferents can facilitate motor output after SCI. For instance, phrenicotomy can abolish phrenic inspiratory activity recorded proximal to the nerve cut after acute (Vinit et al. 2007) or chronic cervical SCI in rats (Golder et al. 2003). Thus both ipsilateral (Goshgarian 1981) and contralateral (Golder et al. 2003; Vinit et al. 2007) phrenic and/or mid-cervical afferents can have powerful modulatory effects on respiratory motor output after SCI. Nothing is known, however, regarding the mechanisms by which phrenic afferents impact respiratory motor output and/or spontaneous recovery after SCI. A final consideration is that chronic deafferentation of the mid-cervical spinal cord produces profound neurochemical changes in the mid-cervical spinal cord. Chronic cervical dorsal rhizotomy results in enhanced serotonergic innervation of the cervical dorsal horn (Marlier et al. 1991; Zhang et al. 1993) as well as the ventral horn in the immediate vicinity of the phrenic motor nucleus (Kinkead et al. 1998). Chronic cervical dorsal rhizotomy also leads to strengthening of spinal synaptic pathways to phrenic motoneurons (Fuller et al. 2002) and enhanced expression of respiratory neuroplasticity (Kinkead et al. 1998). However, cervical dorsal rhizotomy is not specific to diaphragm afferents, and it is unknown if phrenic afferent denervation is an important component of the aforementioned responses.
Phrenic afferents and respiratory rehabilitation.
In this final section, we speculate about a possible role for phrenic afferent neurons in clinical strategies for maintaining or rehabilitating breathing in certain neuromuscular disorders. It is well established that musculoskeletal afferents are important for neurorehabilitation in nonrespiratory motor systems, but, to our knowledge, the role of respiratory afferents in rehabilitation is unknown. In the lower limbs, activation of musculoskeletal afferents can help to restore standing and stepping ability following incomplete SCI (Courtine et al. 2009). Appropriate musculoskeletal afferent feedback can also engage spinal interneurons to produce locomotor output after SCI (Roy et al. 2012), and this is generally considered to be an important component of the neurorehabilitation process.
Does respiratory rehabilitation activate diaphragm sensory afferents? Certainly, it can be expected that respiratory muscle training approaches such as inspiratory threshold loading (Martin et al. 2011; Sapienza et al. 2011) will activate phrenic afferents as patients breathe against inspiratory loads. Increases in GTO and muscle spindle discharge would be expected, and as diaphragm work and blood flow increases, discharge of group III and IV afferents will also likely increase. As discussed throughout this review, activation of these sensory afferents can activate (or inhibit) spinal motoneurons and interneurons and can also modulate supraspinal neuronal discharge. Furthermore, there is evidence that phrenic afferent-driven activation of respiratory neurons and/or neuronal networks may contribute to neuroplastic changes in the control of breathing (see Persistent changes in breathing after phrenic afferent stimulation and Impact of phrenic afferent denervation on respiratory neuroplasticity); whether this is true in the context of respiratory neurorehabilitation is a hypothesis that should be tested in future experiments.
Another clinical approach that will robustly activate diaphragm sensory receptors and phrenic afferent neurons is intramuscular stimulation of the diaphragm muscle via the phrenic nerve motor point (termed “diaphragm pacing”). This method was developed to replace long-term mechanical ventilation in cases where the phrenic nerve and diaphragm are intact (Onders et al. 2010). Diaphragm pacing is now used acutely following cervical SCI to promote ventilator weaning and avoid ventilator-associated complications (Posluszny et al. 2014). Compared with mechanical ventilation, patients using diaphragm pacing experience fewer respiratory infections and improved speech and olfaction, and they report overall better quality of life (Romero et al. 2012). Evidence from both animals and humans indicates that intramuscular diaphragm stimulation can mitigate the diaphragm atrophy that can be induced by mechanical ventilation (Ayas et al. 1999; Yang et al. 2013). Thus one likely benefit of diaphragm pacing is a healthier (i.e., less atrophied) diaphragm compared with what would occur during mechanical ventilation. However, if and how intramuscular diaphragm stimulation impacts diaphragm receptor activation (e.g., Table 1) and phrenic afferent input to the spinal cord and/or supraspinal centers is unknown. Certainly, it can be expected that the vigorous diaphragm contractions induced by pacing will activate both large-fiber mechanoreceptors and small-fiber metabo- and ergoreceptors (Table 1).
Could diaphragm pacing (and the associated activation of phrenic afferents) also have a neurorehabilitative impact in clinical cases of respiratory insufficiency associated with neuromuscular disorders? In other words, can diaphragm pacing do more than preserve muscle function? The answer is unknown, but anecdotal reports indicate that some patients with cervical SCI and severe paresis of the diaphragm demonstrate increasing activation of the diaphragm and recovery of independent breathing such that pacing is discontinued (Onders et al. 2011). In an ongoing study of diaphragm pacing following acute SCI, our research team has observed similar findings (Fox EJ, unpublished observations). Many explanations could be offered, but a potential role of phrenic afferent activation is intriguing. In this regard, there is indirect evidence for phrenic afferents evoking diaphragm activity in humans with SCI. Banzett et al. (1981) studied adults with tetraplegia and showed that diaphragm electrical activity reflexively increased when negative pressure was applied to the lower torso. Because the patients had complete C6–C7 lesions, the authors suggested that phrenic afferents might be mediating the response (Banzett et al. 1981).
A recent report provides support for the notion that diaphragm pacing can provide a neurorehabilitative impact (Smith et al. 2016). Smith and colleagues studied patients with a rare neuromuscular disorder known as Pompe disease. This condition results in neural and muscular pathology in the phrenic-diaphragm motor system and often leads to severe respiratory insufficiency. Direct diaphragm muscle EMG recordings via intramuscular wires in Pompe patients indicated that the ability to voluntarily activate the diaphragm improved over weeks and months following initiation of the diaphragm pacing procedures. This apparent increase in phrenic neuromotor drive was associated with an increased ability to breathe for longer durations and with larger tidal volumes during periods with no external respiratory support (Smith et al. 2016). It remains possible that the pacing-induced diaphragm contraction (and associated activation of phrenic afferent pathways) is a stimulus for triggering plasticity and rehabilitation of the respiratory networks that control phrenic output.
Conclusion
In this article we have systematically reviewed the evidence that is summarized in Fig. 5 and have emphasized the impact of phrenic afferents on respiratory-related outcome measures. We conclude that activation of both myelinated and nonmyelinated phrenic sensory afferents is likely to influence respiratory motor output on a breath-by-breath basis, even during resting or “eupneic” conditions. However, the relative impact of phrenic afferents on respiratory motor output will substantially increase with diaphragm work and fatigue. Activation of group III and IV phrenic afferents also has a powerful impact on sympathetic motor outflow. Last, the information carried via phrenic afferents contributes to diaphragm somatosensation and the conscious perception of breathing.
Fig. 5.
Diagram providing an overall summary of the anatomical projections and functional impact of phrenic afferent neurons. See text for further descriptions.
This article has also highlighted areas in need of further study. For example, much remains to be learned regarding the spinal and supraspinal distribution and synaptic contacts of both myelinated and nonmyelinated phrenic afferents. Similarly, very little is known regarding the potential role of phrenic afferent neurons in triggering or modulating expression of respiratory neuroplasticity, particularly in the context of rehabilitation following neurological injury and/or neuromuscular disease.
GRANTS
Funding was provided by National Institutes of Health Grants 1OT2OD001983-01 (D. C. Bolser, D. D. Fuller, and P. W. Davenport), 1R01NS080180-01A1 (D. D. Fuller), Craig H. Neilsen Foundation 313369 (S. M. Turner) 1F32NS095620-01 (K. A. Streeter), and T32ND043730 (M. D. Sunshine), the Craig H. Neilsen Foundation (E. J. Fox), and the State of Florida Brain and Spinal Cord Injury Research Trust Fund (D. D. Fuller), awarded through the McKnight Brain Institute at the University of Florida.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.N. and D.D.F. conceived and designed research; J.N., S.M.T., and D.D.F. performed experiments; J.N. and D.D.F. analyzed data; J.N. and D.D.F. interpreted results of experiments; J.N. and K.A.S. prepared figures; J.N., K.A.S., and D.D.F. drafted manuscript; J.N., K.A.S., S.M.T., M.D.S., D.C.B., E.J.F., P.W.D., and D.D.F. edited and revised manuscript; J.N., K.A.S., S.M.T., M.D.S., D.C.B., E.J.F., P.W.D., and D.D.F. approved final version of manuscript.
REFERENCES
- Ayas NT, McCool FD, Gore R, Lieberman SL, Brown R. Prevention of human diaphragm atrophy with short periods of electrical stimulation. Am J Respir Crit Care Med 159: 2018–2020, 1999. doi: 10.1164/ajrccm.159.6.9806147. [DOI] [PubMed] [Google Scholar]
- Bałkowiec A, Kukuła K, Szulczyk P. Functional classification of afferent phrenic nerve fibres and diaphragmatic receptors in cats. J Physiol 483: 759–768, 1995. doi: 10.1113/jphysiol.1995.sp020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bałkowiec A, Szulczyk P. Properties of postganglionic sympathetic neurons with axons in phrenic nerve. Respir Physiol 88: 323–331, 1992. doi: 10.1016/0034-5687(92)90006-I. [DOI] [PubMed] [Google Scholar]
- Balzamo E, Lagier-Tessonnier F, Jammes Y. Fatigue-induced changes in diaphragmatic afferents and cortical activity in the cat. Respir Physiol 90: 213–226, 1992. doi: 10.1016/0034-5687(92)90082-8. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Inbar GF, Brown R, Goldman M, Rossier A, Mead J. Diaphragm electrical activity during negative lower torso pressure in quadriplegic men. J Appl Physiol Respir Environ Exerc Physiol 51: 654–659, 1981. [DOI] [PubMed] [Google Scholar]
- Barstad JA, Kristoffersen A, Lillheil G, Staaland H. Muscle spindles in the rat diaphragm. Experientia 21: 533–534, 1965. doi: 10.1007/BF02138980. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 139: 267–284, 2009. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res 533: 141–146, 1990. doi: 10.1016/0006-8993(90)91807-S. [DOI] [PubMed] [Google Scholar]
- Biscoe TJ, Sampson SR. An analysis of the inhibition of phrenic motoneurones which occurs on stimulation of some cranial nerve afferents. J Physiol 209: 375–393, 1970. doi: 10.1113/jphysiol.1970.sp009170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, Hobbs SF, Chandler MJ, Ammons WS, Brennan TJ, Foreman RD. Convergence of phrenic and cardiopulmonary spinal afferent information on cervical and thoracic spinothalamic tract neurons in the monkey: implications for referred pain from the diaphragm and heart. J Neurophysiol 65: 1042–1054, 1991a. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Hobbs SF, Chandler MJ, Foreman RD. Inhibitory effects of phrenic afferent fibers on primate lumbosacral spinothalamic tract neurons. Brain Res 557: 162–166, 1991b. doi: 10.1016/0006-8993(91)90130-N. [DOI] [PubMed] [Google Scholar]
- Brichant JF, De Troyer A. On the intercostal muscle compensation for diaphragmatic paralysis in the dog. J Physiol 500: 245–253, 1997. doi: 10.1113/jphysiol.1997.sp022014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Gandevia SC. Reflex inhibition of human inspiratory muscles in response to contralateral phrenic nerve stimulation. Respir Physiol Neurobiol 138: 87–96, 2003. doi: 10.1016/S1569-9048(03)00161-7. [DOI] [PubMed] [Google Scholar]
- Campbell EJ, Freedman S, Smith PS, Taylor ME. The ability of man to detect added elastic loads to breathing. Clin Sci 20: 223–231, 1961. [PubMed] [Google Scholar]
- Capps JA. An experimental study of the pain sense in the pleural membranes. Arch Int Med 8: 717–733, 1911. [Google Scholar]
- Chandler MJ, Qin C, Yuan Y, Foreman RD. Convergence of trigeminal input with visceral and phrenic inputs on primate C1–C2 spinothalamic tract neurons. Brain Res 829: 204–208, 1999. doi: 10.1016/S0006-8993(99)01348-7. [DOI] [PubMed] [Google Scholar]
- Chandler MJ, Zhang J, Foreman RD. Phrenic nerve inputs to upper cervical (C1–C3) spinothalamic tract neurons in monkeys. Brain Res 798: 93–100, 1998. doi: 10.1016/S0006-8993(98)00412-0. [DOI] [PubMed] [Google Scholar]
- Cheeseman M, Revelette WR. Phrenic afferent contribution to reflexes elicited by changes in diaphragm length. J Appl Physiol (1985) 69: 640–647, 1990. [DOI] [PubMed] [Google Scholar]
- Chou YL, Davenport PW. Phrenic nerve afferents elicited cord dorsum potential in the cat cervical spinal cord. BMC Physiol 5: 7, 2005. doi: 10.1186/1472-6793-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland CL, Getting PA. Respiratory-modulated and phrenic afferent-driven neurons in the cervical spinal cord (C4–C6) of the fluorocarbon-perfused guinea pig. Exp Brain Res 93: 307–311, 1993. doi: 10.1007/BF00228399. [DOI] [PubMed] [Google Scholar]
- Corda M, Voneuler C, Lennerstrand G. Proprioceptive innervation of the diaphragm. J Physiol 178: 161–177, 1965. doi: 10.1113/jphysiol.1965.sp007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12: 1333–1342, 2009. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenod M. [Proprioceptive reflexes of the diaphragm in the rabbit]. Helv Physiol Pharmacol Acta 19: 360–372, 1961. [PubMed] [Google Scholar]
- Davenport PW. Intercostal and diaphragm myelinated afferents. In: The Thorax–Part A: Physiology (In Three Parts) (2nd ed), edited by Roussos C. New York: Marcel Dekker, 1995. [Google Scholar]
- Davenport PW, Friedman WA, Thompson FJ, Franzén O. Respiratory-related cortical potentials evoked by inspiratory occlusion in humans. J Appl Physiol (1985) 60: 1843–1848, 1986. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Martin AD, Chou YL, Alexander-Miller S. Respiratory-related evoked potential elicited in tracheostomised lung transplant patients. Eur Respir J 28: 391–396, 2006. doi: 10.1183/09031936.06.00095005. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Reep RL, Thompson FJ. Phrenic nerve afferent activation of neurons in the cat SI cerebral cortex. J Physiol 588: 873–886, 2010. doi: 10.1113/jphysiol.2009.181735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport PW, Shannon R, Mercak A, Reep RL, Lindsey BG. Cerebral cortical evoked potentials elicited by cat intercostal muscle mechanoreceptors. J Appl Physiol (1985) 74: 799–804, 1993. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Thompson FJ, Reep RL, Freed AN. Projection of phrenic nerve afferents to the cat sensorimotor cortex. Brain Res 328: 150–153, 1985. doi: 10.1016/0006-8993(85)91334-4. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respir Physiol Neurobiol 167: 72–86, 2009. doi: 10.1016/j.resp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Brunko E, Leduc D, Jammes Y. Reflex inhibition of canine inspiratory intercostals by diaphragmatic tension receptors. J Physiol 514: 255–263, 1999. doi: 10.1111/j.1469-7793.1999.255af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer AD. The canine phrenic-to-intercostal reflex. J Physiol 508: 919–927, 1998. doi: 10.1111/j.1469-7793.1998.919bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars SA, Milligan CJ, Stornetta RL, Deuchars J. GABAergic neurons in the central region of the spinal cord: a novel substrate for sympathetic inhibition. J Neurosci 25: 1063–1070, 2005. doi: 10.1523/JNEUROSCI.3740-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries KL, Goshgarian HG. Spinal cord localization and characterization of the neurons which give rise to the accessory phrenic nerve in the adult rat. Exp Neurol 104: 88–90, 1989. doi: 10.1016/0014-4886(89)90013-7. [DOI] [PubMed] [Google Scholar]
- Dolivo M. [Effects of interruption of nerve conduction in the phrenic nerve on the respiratory frequency]. Helv Physiol Pharmacol Acta 10: 366–371, 1952. [PubMed] [Google Scholar]
- Duron B, Caillol MC. Investigation of afferent activity in the intact phrenic nerve with bipolar electrodes. Acta Neurobiol Exp (Wars) 33: 428–432, 1973. [PubMed] [Google Scholar]
- Duron B, Jung-Caillol MC, Marlot D. Myelinated nerve fiber supply and muscle spindles in the respiratory muscles of cat: quantitative study. Anat Embryol (Berl) 152: 171–192, 1978. doi: 10.1007/BF00315923. [DOI] [PubMed] [Google Scholar]
- Duron B, Marlot D. The non-myelinated fibers of the phrenic and the intercostal nerves in the cat. Z Mikrosk Anat Forsch 94: 257–268, 1980. [PubMed] [Google Scholar]
- Foreman RD, Garrett KM, Blair RW. Mechanisms of cardiac pain. Compr Physiol 5: 929–960, 2015. doi: 10.1002/cphy.c140032. [DOI] [PubMed] [Google Scholar]
- Formenti A, Zocchi L. Error signals as powerful stimuli for the operant conditioning-like process of the fictive respiratory output in a brainstem-spinal cord preparation from rats. Behav Brain Res 272: 8–15, 2014. doi: 10.1016/j.bbr.2014.06.038. [DOI] [PubMed] [Google Scholar]
- Frankstein SI, Smolin LN, Sergeeva ZN, Sergeeva TI. Cortical representation of the phrenic nerve. Exp Neurol 63: 447–449, 1979. doi: 10.1016/0014-4886(79)90139-0. [DOI] [PubMed] [Google Scholar]
- Frazier DT, Revelette WR. Role of phrenic nerve afferents in the control of breathing. J Appl Physiol (1985) 70: 491–496, 1991. [DOI] [PubMed] [Google Scholar]
- Frazier DT, Revelette WR, Fryman D, Jewell L. A role for phrenic afferents? In: Neurogenesis of Central Respiratory Rhythm: Electrophysiological, Pharmacological & Clinical Aspects, edited by Bianchi AL and Denavit-Saubie M. Lancaster, UK: MTP, 1985, p. 243–246. [Google Scholar]
- Fryman DL, Frazier DT. Diaphragm afferent modulation of phrenic motor drive. J Appl Physiol (1985) 62: 2436–2441, 1987. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Johnson RA, Mitchell GS. Chronic cervical spinal sensory denervation reveals ineffective spinal pathways to phrenic motoneurons in the rat. Neurosci Lett 323: 25–28, 2002. doi: 10.1016/S0304-3940(02)00121-0. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Mitchell GS. Respiratory neuroplasticity – Overview, significance and future directions. Exp Neurol 27: 144–152, 2016. doi: 10.1016/j.expneurol.2016.05.022. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Polak JM, Bloom SR, Sabate IM, Mulderry PM, Ghatei MA, McGregor GP, Morrison JF, Kelly JS, Evans RM. Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. J Neurosci 4: 3101–3111, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill PK, Kuno M. Excitatory and inhibitory actions on phrenic motoneurones. J Physiol 168: 274–289, 1963. doi: 10.1113/jphysiol.1963.sp007192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci 23: 2494–2501, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol 72: 211–225, 1981. doi: 10.1016/0014-4886(81)90139-4. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The phrenic nucleus of the albino rat: a correlative HRP and Golgi study. J Comp Neurol 201: 441–456, 1981. doi: 10.1002/cne.902010309. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Roubal PJ. Origin and distribution of phrenic primary afferent nerve fibers in the spinal cord of the adult rat. Exp Neurol 92: 624–638, 1986. doi: 10.1016/0014-4886(86)90304-3. [DOI] [PubMed] [Google Scholar]
- Gottschall J. The diaphragm of the rat and its innervation. Muscle fiber composition; perikarya and axons of efferent and afferent neurons. Anat Embryol (Berl) 161: 405–417, 1981. doi: 10.1007/BF00316051. [DOI] [PubMed] [Google Scholar]
- Graham R, Jammes Y, Delpierre S, Grimaud C, Roussos C. The effects of ischemia, lactic acid and hypertonic sodium chloride on phrenic afferent discharge during spontaneous diaphragmatic contraction. Neurosci Lett 67: 257–262, 1986. doi: 10.1016/0304-3940(86)90318-6. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol (1985) 82: 1573–1583, 1997. [DOI] [PubMed] [Google Scholar]
- Hebb CO, Krnjevic K, Silver A. Acetylcholine and choline acetyltransferase in the diaphragm of the rat. J Physiol 171: 504–513, 1964. doi: 10.1113/jphysiol.1964.sp007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM. Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res 856: 240–244, 2000. doi: 10.1016/S0006-8993(99)02366-5. [DOI] [PubMed] [Google Scholar]
- Hill JM. Increase in the discharge of muscle spindles during diaphragm fatigue. Brain Res 918: 166–170, 2001. doi: 10.1016/S0006-8993(01)02988-2. [DOI] [PubMed] [Google Scholar]
- Hinsey JC, Hare K, Phillips RA. Sensory components of the phrenic nerve of the cat. Proc Soc Exp Biol Med 41: 411–414, 1939. doi: 10.3181/00379727-41-10693. [DOI] [Google Scholar]
- Hinsey JC, Phillips RA. Observations upon diaphragmatic sensation. J Neurophysiol 3: 175–181, 1940. [Google Scholar]
- Holt GA, Dalziel DJ, Davenport PW. The transduction properties of diaphragmatic mechanoreceptors. Neurosci Lett 122: 117–121, 1991. doi: 10.1016/0304-3940(91)90207-A. [DOI] [PubMed] [Google Scholar]
- Hussain H. Small-fiber phrenic afferents in breathing. In: The Thorax–Part A: Physiology (In Three Parts) (2nd ed), edited by Roussos C. New York: Dekker, 1995. [Google Scholar]
- Hussain S, Chatillon A, Magder S, Roussos C. The respiratory and vascular responses evoked by chemical activation of small fiber phrenic nerve afferents in dogs. Chest 97, Suppl: 44S–45S, 1990a. doi: 10.1378/chest.97.3_Supplement.44S-a. [DOI] [PubMed] [Google Scholar]
- Hussain SN, Chatillon A, Comtois A, Roussos C, Magder S. Chemical activation of thin-fiber phrenic afferents. 2. Cardiovascular responses. J Appl Physiol (1985) 70: 77–86, 1991. [DOI] [PubMed] [Google Scholar]
- Hussain SN, Magder S, Chatillon A, Roussos C. Chemical activation of thin-fiber phrenic afferents: respiratory responses. J Appl Physiol (1985) 69: 1002–1011, 1990b. [DOI] [PubMed] [Google Scholar]
- Iscoe S, Duffin J. Effects of stimulation of phrenic afferents on cervical respiratory interneurones and phrenic motoneurones in cats. J Physiol 497: 803–812, 1996. doi: 10.1113/jphysiol.1996.sp021811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes Y, Balzamo E. Changes in afferent and efferent phrenic activities with electrically induced diaphragmatic fatigue. J Appl Physiol (1985) 73: 894–902, 1992. [DOI] [PubMed] [Google Scholar]
- Jammes Y, Buchler B, Delpierre S, Rasidakis A, Grimaud C, Roussos C. Phrenic afferents and their role in inspiratory control. J Appl Physiol (1985) 60: 854–860, 1986. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci 18: 8436–8443, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrman RM, Nolasco JB, Wiggers CJ. Types of afferent fibers in the phrenic nerve. Am J Physiol 151: 547–553, 1947. [DOI] [PubMed] [Google Scholar]
- Kostreva DR, Pontus SP. Hepatic vein, hepatic parenchymal, and inferior vena caval mechanoreceptors with phrenic afferents. Am J Physiol Gastrointest Liver Physiol 265: G15–G20, 1993a. [DOI] [PubMed] [Google Scholar]
- Kostreva DR, Pontus SP. Pericardial mechanoreceptors with phrenic afferents. Am J Physiol Heart Circ Physiol 264: H1836–H1846, 1993b. [DOI] [PubMed] [Google Scholar]
- Landau BR, Akert K, Roberts TS. Studies on the innervation of the diaphragm. J Comp Neurol 119: 1–10, 1962. doi: 10.1002/cne.901190102. [DOI] [Google Scholar]
- Langford LA, Schmidt RF. An electron microscopic analysis of the left phrenic nerve in the rat. Anat Rec 205: 207–213, 1983. doi: 10.1002/ar.1092050211. [DOI] [PubMed] [Google Scholar]
- Larnicol N, Rose D, Duron B. Identification of phrenic afferents in the dorsal columns: a fluorescent double-labeling study in the cat. Neurosci Lett 52: 49–53, 1984. doi: 10.1016/0304-3940(84)90349-5. [DOI] [PubMed] [Google Scholar]
- Larnicol N, Rose D, Duron B. Identification of phrenic afferents to the external cuneate nucleus: a fluorescent double-labeling study in the cat. Neurosci Lett 62: 163–167, 1985. doi: 10.1016/0304-3940(85)90349-0. [DOI] [PubMed] [Google Scholar]
- Laskowski MB, Norton AS, Berger PK. Branching patterns of the rat phrenic nerve during development and reinnervation. Exp Neurol 113: 212–220, 1991. doi: 10.1016/0014-4886(91)90177-E. [DOI] [PubMed] [Google Scholar]
- Macron JM, Marlot D, Duron B. Phrenic afferent input to the lateral medullary reticular formation of the cat. Respir Physiol 59: 155–167, 1985. doi: 10.1016/0034-5687(85)90004-0. [DOI] [PubMed] [Google Scholar]
- Macron JM, Marlot D, Wallois F, Duron B. Phrenic-to-phrenic inhibition and excitation in spinal cats. Neurosci Lett 91: 24–29, 1988. doi: 10.1016/0304-3940(88)90243-1. [DOI] [PubMed] [Google Scholar]
- Malakhova OE, Davenport PW. c-Fos expression in the central nervous system elicited by phrenic nerve stimulation. J Appl Physiol (1985) 90: 1291–1298, 2001. [DOI] [PubMed] [Google Scholar]
- Marlier L, Poulat P, Rajaofetra N, Privat A. Modification of serotonergic immunoreactive pattern in the dorsal horn of the rat spinal cord following dorsal root rhizotomy. Neurosci Lett 128: 9–12, 1991. doi: 10.1016/0304-3940(91)90748-I. [DOI] [PubMed] [Google Scholar]
- Marlot D, Macron JM, Duron B. Projections of phrenic afferences to the cat cerebellar cortex. Neurosci Lett 44: 95–98, 1984. doi: 10.1016/0304-3940(84)90227-1. [DOI] [PubMed] [Google Scholar]
- Marlot D, Macron JM, Duron B. Projection of phrenic afferents to the external cuneate nucleus in the cat. Brain Res 327: 328–330, 1985. doi: 10.1016/0006-8993(85)91529-X. [DOI] [PubMed] [Google Scholar]
- Marlot D, Macron JM, Duron B. Inhibitory and excitatory effects on respiration by phrenic nerve afferent stimulation in cats. Respir Physiol 69: 321–333, 1987. doi: 10.1016/0034-5687(87)90086-7. [DOI] [PubMed] [Google Scholar]
- Marlot D, Macron JM, Duron B. Effects of ipsilateral and contralateral cervical phrenic afferents stimulation on phrenic motor unit activity in the cat. Brain Res 450: 373–377, 1988. doi: 10.1016/0006-8993(88)91578-8. [DOI] [PubMed] [Google Scholar]
- Martin AD, Smith BK, Davenport PD, Harman E, Gonzalez-Rothi RJ, Baz M, Layon AJ, Banner MJ, Caruso LJ, Deoghare H, Huang TT, Gabrielli A. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care 15: R84, 2011. doi: 10.1186/cc10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallister LW, McCoy KW, Connelly JC, Kaufman MP. Stimulation of groups III and IV phrenic afferents reflexly decreases total lung resistance in dogs. J Appl Physiol (1985) 61: 1346–1351, 1986. [DOI] [PubMed] [Google Scholar]
- Mead J. Responses to loaded breathing. A critique and a synthesis. Bull Eur Physiopathol Respir 15, Suppl: 61–73, 1979. [PubMed] [Google Scholar]
- Mendelsohn AH, Deconde A, Lambert HW, Dodson SC, Daney BT, Stark ME, Berke GS, Wisco JJ. Cervical variations of the phrenic nerve. Laryngoscope 121: 1920–1923, 2011. doi: 10.1002/lary.21894. [DOI] [PubMed] [Google Scholar]
- Minassian K, Hofstoetter US, Danner SM, Mayr W, Bruce JA, McKay WB, Tansey KE. Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord-injured individuals. Neurorehabil Neural Repair 30: 233–243, 2016. doi: 10.1177/1545968315591706. [DOI] [PubMed] [Google Scholar]
- Nair J, Bezdudnaya T, Zholudeva LV, Detloff MR, Reier PJ, Lane MA, Fuller DD. Histological identification of phrenic afferent projections to the spinal cord. Respir Physiol Neurobiol 236: 57–68, 2017. doi: 10.1016/j.resp.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner B, Dembowsky K, Czachurski J. Characteristics of sympathetic reflexes evoked by electrical stimulation of phrenic nerve afferents. J Auton Nerv Syst 41: 103–111, 1992. doi: 10.1016/0165-1838(92)90132-Z. [DOI] [PubMed] [Google Scholar]
- Onders RP, Khansarinia S, Weiser T, Chin C, Hungness E, Soper N, Dehoyos A, Cole T, Ducko C. Multicenter analysis of diaphragm pacing in tetraplegics with cardiac pacemakers: positive implications for ventilator weaning in intensive care units. Surgery 148: 893–897, 2010. doi: 10.1016/j.surg.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Onders RP, Ponsky TA, Elmo M, Lidsky K, Barksdale E. First reported experience with intramuscular diaphragm pacing in replacing positive pressure mechanical ventilators in children. J Pediatr Surg 46: 72–76, 2011. doi: 10.1016/j.jpedsurg.2010.09.071. [DOI] [PubMed] [Google Scholar]
- Palisses R, Perségol L, Viala D. Evidence for respiratory interneurones in the C3–C5 cervical spinal cord in the decorticate rabbit. Exp Brain Res 78: 624–632, 1989. doi: 10.1007/BF00230250. [DOI] [PubMed] [Google Scholar]
- Paraskevas G, Koutsouflianiotis K, Kitsoulis P, Spyridakis I. Abnormal origin and course of the accessory phrenic nerve: case report. Acta Med (Hradec Kralove) 59: 70–71, 2016. doi: 10.14712/18059694.2016.55. [DOI] [PubMed] [Google Scholar]
- Poole DC, Sexton WL, Farkas GA, Powers SK, Reid MB. Diaphragm structure and function in health and disease. Med Sci Sports Exerc 29: 738–754, 1997. doi: 10.1097/00005768-199706000-00003. [DOI] [PubMed] [Google Scholar]
- Posluszny JA Jr, Onders R, Kerwin AJ, Weinstein MS, Stein DM, Knight J, Lottenberg L, Cheatham ML, Khansarinia S, Dayal S, Byers PM, Diebel L. Multicenter review of diaphragm pacing in spinal cord injury: successful not only in weaning from ventilators but also in bridging to independent respiration. J Trauma Acute Care Surg 76: 303–309, 2014. doi: 10.1097/TA.0000000000000112. [DOI] [PubMed] [Google Scholar]
- Powers SK, Smuder AJ, Fuller D, Levine S. CrossTalk proposal: mechanical ventilation-induced diaphragm atrophy is primarily due to inactivity. J Physiol 591: 5255–5257, 2013. doi: 10.1113/jphysiol.2013.254680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razook JC, Chandler MJ, Foreman RD. Phrenic afferent input excites C1–C2 spinal neurons in rats. Pain 63: 117–125, 1995. doi: 10.1016/0304-3959(95)00026-O. [DOI] [PubMed] [Google Scholar]
- Revelette R, Reynolds S, Brown D, Taylor R. Effect of abdominal compression on diaphragmatic tendon organ activity. J Appl Physiol (1985) 72: 288–292, 1992. [DOI] [PubMed] [Google Scholar]
- Revelette WR, Davenport PW. Effects of timing of inspiratory occlusion on cerebral evoked potentials in humans. J Appl Physiol (1985) 68: 282–288, 1990. [DOI] [PubMed] [Google Scholar]
- Revelette WR, Jewell LA, Frazier DT. Effect of diaphragm small-fiber afferent stimulation on ventilation in dogs. J Appl Physiol (1985) 65: 2097–2106, 1988. [DOI] [PubMed] [Google Scholar]
- Road JD. Phrenic afferents and ventilatory control. Lung 168: 137–149, 1990. doi: 10.1007/BF02719685. [DOI] [PubMed] [Google Scholar]
- Road JD, Osborne S, Wakai Y. Delayed poststimulus decrease of phrenic motoneuron output produced by phrenic nerve afferent stimulation. J Appl Physiol (1985) 74: 68–72, 1993. [DOI] [PubMed] [Google Scholar]
- Road JD, West NH, Van Vliet BN. Ventilatory effects of stimulation of phrenic afferents. J Appl Physiol (1985) 63: 1063–1069, 1987. [DOI] [PubMed] [Google Scholar]
- Romero FJ, Gambarrutta C, Garcia-Forcada A, Marín MA, Diaz de la Lastra E, Paz F, Fernandez-Dorado MT, Mazaira J. Long-term evaluation of phrenic nerve pacing for respiratory failure due to high cervical spinal cord injury. Spinal Cord 50: 895–898, 2012. doi: 10.1038/sc.2012.74. [DOI] [PubMed] [Google Scholar]
- Rosen SD. From heart to brain: the genesis and processing of cardiac pain. Can J Cardiol 28, Suppl: S7–S19, 2012. doi: 10.1016/j.cjca.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Roy RR, Harkema SJ, Edgerton VR. Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Arch Phys Med Rehabil 93: 1487–1497, 2012. doi: 10.1016/j.apmr.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Ruch TC. Pathophysiology of pain. In: Neurophysiology, edited by Ruch TC, Patton HD, Woodbury JW, and Towe AL. Philadelphia, PA: Saunders, 1961, p. 350–368. [Google Scholar]
- Ruckebusch Y. [Afferent impulses of pericardial origin in the phrenic nerves]. C R Seances Soc Biol Fil 155: 524–527, 1961. [PubMed] [Google Scholar]
- Sandhu MS, Lee KZ, Fregosi RF, Fuller DD. Phrenicotomy alters phrenic long-term facilitation following intermittent hypoxia in anesthetized rats. J Appl Physiol (1985) 109: 279–287, 2010. doi: 10.1152/japplphysiol.01422.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlioglu S, Benson PK, Yang J, Atkinson EM, Reynolds T, Engelhardt JF. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation. J Virol 74: 9184–9196, 2000. doi: 10.1128/JVI.74.19.9184-9196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant’Ambrogio G, Wilson MF, Frazier DT. Somatic afferent activity in reflex regulation of diaphragmatic function in the cat. J Appl Physiol 17: 829–832, 1962. [Google Scholar]
- Sapienza C, Troche M, Pitts T, Davenport P. Respiratory strength training: concept and intervention outcomes. Semin Speech Lang 32: 21–30, 2011. doi: 10.1055/s-0031-1271972. [DOI] [PubMed] [Google Scholar]
- Shannon R, Shear WT, Mercak AR, Bolser DC, Lindsey BG. Non-vagal reflex effects on medullary inspiratory neurons during inspiratory loading. Respir Physiol 60: 193–204, 1985. doi: 10.1016/0034-5687(85)90103-3. [DOI] [PubMed] [Google Scholar]
- Smith BK, Fuller DD, Martin AD, Lottenberg L, Islam S, Lawson LA, Onders RP, Byrne BJ. Diaphragm pacing as a rehabilitative tool for patients with Pompe disease who are ventilator-dependent: case series. Phys Ther 96: 696–703, 2016. doi: 10.2522/ptj.20150122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron 20: 629–632, 1998. doi: 10.1016/S0896-6273(00)81003-X. [DOI] [PubMed] [Google Scholar]
- Song A, Tracey DJ, Ashwell KW. Development of the rat phrenic nerve and the terminal distribution of phrenic afferents in the cervical cord. Anat Embryol (Berl) 200: 625–643, 1999. doi: 10.1007/s004290050310. [DOI] [PubMed] [Google Scholar]
- Speck DF. Supraspinal involvement in the phrenic-to-phrenic inhibitory reflex. Brain Res 414: 169–172, 1987. doi: 10.1016/0006-8993(87)91341-2. [DOI] [PubMed] [Google Scholar]
- Speck DF, Revelette WR. Attenuation of phrenic motor discharge by phrenic nerve afferents. J Appl Physiol (1985) 62: 941–945, 1987a. [DOI] [PubMed] [Google Scholar]
- Speck DF, Revelette WR. Excitation of dorsal and ventral respiratory group neurons by phrenic nerve afferents. J Appl Physiol (1985) 62: 946–951, 1987b. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol 529: 493–504, 2000. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus C, Zelter M, Derenne J-P, Pidoux B, Willer JC, Similowski T. Putative projection of phrenic afferents to the limbic cortex in humans studied with cerebral-evoked potentials. J Appl Physiol (1985) 82: 480–490, 1997. [DOI] [PubMed] [Google Scholar]
- Streeter KA, Sunshine MD, Patel SR, Liddell SS, Denholtz LE, Reier PJ, Fuller DD, Baekey DM. Coupling multi-electrode array recordings with silver labeling of recording sites to study cervical spinal network connectivity. J Neurophysiol 117: 1014–1029, 2016. doi: 10.1152/jn.00638.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strey KA, Baertsch NA, Baker-Herman TL. Inactivity-induced respiratory plasticity: protecting the drive to breathe in disorders that reduce respiratory neural activity. Respir Physiol Neurobiol 189: 384–394, 2013. doi: 10.1016/j.resp.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Dick T, Stofan D, DiMarco AF. Effects of intraphrenic injection of potassium on diaphragm activation. J Appl Physiol (1985) 74: 1186–1194, 1993. [DOI] [PubMed] [Google Scholar]
- Teitelbaum J, Borel CO, Magder S, Traystman RJ, Hussain SN. Effect of selective diaphragmatic paralysis on the inspiratory motor drive. J Appl Physiol (1985) 74: 2261–2268, 1993a. [DOI] [PubMed] [Google Scholar]
- Teitelbaum J, Vanelli G, Hussain SN. Thin-fiber phrenic afferents mediate the ventilatory response to diaphragmatic ischemia. Respir Physiol 91: 195–206, 1993b. doi: 10.1016/0034-5687(93)90099-V. [DOI] [PubMed] [Google Scholar]
- Vinit S, Stamegna JC, Boulenguez P, Gauthier P, Kastner A. Restorative respiratory pathways after partial cervical spinal cord injury: role of ipsilateral phrenic afferents. Eur J Neurosci 25: 3551–3560, 2007. doi: 10.1111/j.1460-9568.2007.05619.x. [DOI] [PubMed] [Google Scholar]
- Von Leupoldt A, Vovk A, Bradley MM, Keil A, Lang PJ, Davenport PW. The impact of emotion on respiratory-related evoked potentials. Psychophysiology 47: 579–586, 2010. doi: 10.1111/j.1469-8986.2009.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward ME, Deschamps A, Roussos C, Hussain SN. Effect of phrenic afferent stimulation on pattern of respiratory muscle activation. J Appl Physiol (1985) 73: 563–570, 1992a. [DOI] [PubMed] [Google Scholar]
- Ward ME, Vanelli G, Hashefi M, Hussain SN. Ventilatory effects of the interaction between phrenic and limb muscle afferents. Respir Physiol 88: 63–76, 1992b. doi: 10.1016/0034-5687(92)90029-V. [DOI] [PubMed] [Google Scholar]
- Winckler G, Delaloye B. [Presence of neuromuscular spindles in the human diaphragm]. Acta Anat (Basel) 29: 114–116, 1957. doi: 10.1159/000141163. [DOI] [PubMed] [Google Scholar]
- Yang M, Wang H, Han G, Chen L, Huang L, Jiang J, Li S. Phrenic nerve stimulation protects against mechanical ventilation-induced diaphragm dysfunction in rats. Muscle Nerve 48: 958–962, 2013. doi: 10.1002/mus.23850. [DOI] [PubMed] [Google Scholar]
- Yates JS, Davenport PW, Reep RL. Thalamocortical projections activated by phrenic nerve afferents in the cat. Neurosci Lett 180: 114–118, 1994. doi: 10.1016/0304-3940(94)90500-2. [DOI] [PubMed] [Google Scholar]
- Yu J, Younes M. Powerful respiratory stimulation by thin muscle afferents. Respir Physiol 117: 1–12, 1999. doi: 10.1016/S0034-5687(99)00056-0. [DOI] [PubMed] [Google Scholar]
- Zhang B, Goldberger ME, Murray M. Proliferation of SP- and 5HT-containing terminals in lamina II of rat spinal cord following dorsal rhizotomy: quantitative EM-immunocytochemical studies. Exp Neurol 123: 51–63, 1993. doi: 10.1006/exnr.1993.1139. [DOI] [PubMed] [Google Scholar]
- Zhang W, Davenport PW. Activation of thalamic ventroposteriolateral neurons by phrenic nerve afferents in cats and rats. J Appl Physiol (1985) 94: 220–226, 2003. doi: 10.1152/japplphysiol.00334.2002. [DOI] [PubMed] [Google Scholar]