We demonstrate that intrinsic biases in numerical magnitude can directly predict the amount of money donated by an individual to an anonymous stranger during the dictator game. Furthermore, subliminally inducing perceptual biases in numerical-magnitude allocation can actively drive prosocial choices in the corresponding direction. Our findings provide evidence for numerical influences on decision making during performance of the dictator game. Accordingly, without the implementation of an adequate control for numerical influences, the dictator game and other tasks with an inherent numerical component (i.e., ultimatum game) should be employed with caution in the assessment of human behavior.
Keywords: decision making, dictator game, numerical magnitude allocation, vestibular cognition
Abstract
Over the past decade neuroscientific research has attempted to probe the neurobiological underpinnings of human prosocial decision making. Such research has almost ubiquitously employed tasks such as the dictator game or similar variations (i.e., ultimatum game). Considering the explicit numerical nature of such tasks, it is surprising that the influence of numerical cognition on decision making during task performance remains unknown. While performing these tasks, participants typically tend to anchor on a 50:50 split that necessitates an explicit numerical judgement (i.e., number-pair bisection). Accordingly, we hypothesize that the decision-making process during the dictator game recruits overlapping cognitive processes to those known to be engaged during number-pair bisection. We observed that biases in numerical magnitude allocation correlated with the formulation of decisions during the dictator game. That is, intrinsic biases toward smaller numerical magnitudes were associated with the formulation of less favorable decisions, whereas biases toward larger magnitudes were associated with more favorable choices. We proceeded to corroborate this relationship by subliminally and systematically inducing biases in numerical magnitude toward either higher or lower numbers using a visuo-vestibular stimulation paradigm. Such subliminal alterations in numerical magnitude allocation led to proportional and corresponding changes to an individual’s decision making during the dictator game. Critically, no relationship was observed between neither intrinsic nor induced biases in numerical magnitude on decision making when assessed using a nonnumerical-based prosocial questionnaire. Our findings demonstrate numerical influences on decisions formulated during the dictator game and highlight the necessity to control for confounds associated with numerical cognition in human decision-making paradigms.
NEW & NOTEWORTHY We demonstrate that intrinsic biases in numerical magnitude can directly predict the amount of money donated by an individual to an anonymous stranger during the dictator game. Furthermore, subliminally inducing perceptual biases in numerical-magnitude allocation can actively drive prosocial choices in the corresponding direction. Our findings provide evidence for numerical influences on decision making during performance of the dictator game. Accordingly, without the implementation of an adequate control for numerical influences, the dictator game and other tasks with an inherent numerical component (i.e., ultimatum game) should be employed with caution in the assessment of human behavior.
human prosocial behavior is suggested to be driven by an inherent desire to engage oneself for the greater societal good and by a concern for the outcomes of others (Crockett et al. 2014; Ruff et al. 2013). Across individuals, such tendencies are associated with a wide degree of heterogeneity and are poorly predicted by variables including, age, gender, income, wealth, taxation levels, and religious or cultural beliefs (Camerer et al. 2004).
To date, the neural basis of prosocial behavior has typically been assessed using the dictator game task, in which participants are required to decide how much, if any, of a monetary endowment to donate to an anonymous individual. During performance of this task or variants such as the ultimatum game (de Quervain et al. 2004; Fehr and Fischbacher 2003; Harbaugh et al. 2007; Hare et al. 2010; Kahneman et al. 1986; Morishima et al. 2012; Ruff et al. 2013; Tricomi et al. 2010), participants typically tend to anchor on a 50:50 split to either 1) ensure social norm compliance, as in the case of the dictator game, or 2) avoid any possible sanctions as found in the ultimatum game (Andreoni and Bernheim 2009). Accordingly, there is a tendency toward an equal split of the monetary endowment (Fehr and Fischbacher 2003). Considering that formulation of an equal split during the decision-making process necessitates an explicit numerical judgement (i.e., number pair bisection), we postulate that the decision-making process during the dictator game task recruits overlapping cognitive processes to those known to be engaged during number pair bisection (Zorzi et al. 2002).
Accordingly, we specifically predicted that intrinsic biases in numerical magnitude allocation would correlate with prosocial choices when assessed using the dictator game but not when assessed using a nonnumerical psychometric questionnaire (Rushton et al. 1981). More explicitly, there are six possible perspectives that one can adopt. If the dictator game does indeed measure prosocial behavior, an individual’s numerical bias may either be uncorrelated, positively, or negatively correlated with his or her prosocial attitudes. Conversely, if the dictator game does not measure prosocial behavior, the individual’s numerical bias may either be uncorrelated, positively correlated, or negatively correlated with their prosocial attitudes. We explicitly hypothesize that the dictator game does not measure prosocial behavior and an individual’s numerical biases as assessed by the number pair bisection task (Zorzi et al. 2002) are uncorrelated with their prosocial attitudes, such that an individual’s decision in the dictator game is partly misattributed. That is, they reflect an individual’s ability to perform mental arithmetic processes that is dependent on numerical cognition, and hence, magnitude biases may influence a participant’s response to an extent beyond that of pure noise. Moreover, if this is the case, then one would expect to observe 1) no comparative relationship between prosocial tendencies when evaluated with the dictator game (numerical dependent) and a prosocial questionnaire (nonnumerical dependent), and 2) that inducing subliminal and systematic biases in numerical magnitude perception toward either lower or higher numbers using a visuo-vestibular stimulation paradigm (Arshad et al. 2016b) should result in corresponding changes in prosocial choices during the dictator game but not when assessed using the nonnumerical prosocial questionnaire.
MATERIALS AND METHODS
Participants
Sixty right-handed participants [Handedness score >40 (Oldfield 1971)] were recruited in total (30 female, age range 18–28 yr, mean age 23.6 yr). No subjects had any history of otological, ophthalmological, psychiatric, or neurological disorders. All the participants were naïve to the purpose of the study. 40 of the 60 participants took part in experiment 1, and the remaining 20 subjects took part in experiment 2. All subjects provided written informed consent as approved by the local ethics research committee.
Experiment 1: Investigating the Relationship Between Intrinsic Numerical Biases and Prosocial Behavior
In experiment 1, we investigated whether intrinsic biases in numerical cognition assessed using the 1) number pair bisection task, or 2) random number generation task correlated with prosocial behavior assessed using either the dictator game or an altruism questionnaire. For each subject, the presentation of the four tasks was performed in a randomized order. We also assessed any potential relationship between decisions in the dictator game and the choices formulated during performance of the validated questionnaire both before and after correcting for numerical biasing.
Number pair bisection.
Participants were asked to complete 20 number pair bisection trials. Two numbers were presented through a radio speaker situated directly behind them. Participants were required to estimate the mid-point without calculation (3-s time limit; the average time to complete the task was 61.9 s). The following trials were completed in darkness in a randomized order: (33–87), (89–32), (37–91), (93–39), (41–66), (68–44), (47–90), (92–48), (52–91), (92–56), (57–89), (87–59), (61–99), (97–63), (67–95) (99–67), (58–124), (131–59), (55–131), and (132–58) (Arshad et al. 2016a, b). Note, as evident from the trials above, the number presented on the left of the pair varied between either the larger or the smaller value to avoid any effects associated with either spatial or temporal biasing. Bisection errors for each trial were calculated by subtracting the arithmetical midpoint from that reported by the participant. Percentage errors were calculated by dividing the errors by the number interval size (Zorzi et al. 2002). Positive mean percent bisection errors denote an overestimation, and negative mean percent bisection errors denote an underestimation from the actual midpoint.
Dictator game task.
A modified version of the dictator game task was implemented based on the design by Morishima et al. (2012). The specific modification made was that we relied on the monetary splits being presented verbally as opposed to visually. This modification ensured that we could employ the same task during visuo-vestibular stimulation (see experiment 2) in which visual fixation would suppress any vestibular activation.
During the dictator game task, participants were required to verbally state how they would like to readjust the presented monetary split. For example, a scenario could have been that the participant (dictator) has £7 and the stranger has £3. Subjects had one of three possible options: 1) keep the split the same, 2) donate £X amount to the stranger, or 3) take £X amount from the stranger. Note, subjects were instructed that they had full control of how they would like to adjust the monetary split with respect to the stranger, who was a fellow unknown participant in the experiment. To maintain consistency in the tactics employed, the participants were informed that their final payoff would be based on how they decided to donate to the stranger (fellow unknown participant in the experiment) in two trials selected at random. There was no deception. Twenty randomized trials were performed in total, with 10 trials setup such that the participant’s (i.e., the dictator) monetary split was given first (i.e., so that money donated would shift spatially from left to right): (£9-£1), (£8-£2), (£7-£4), (£6-£2),(£7-£3), (£8-£2), (£6-£2), (£7-£4), (£9-£2), and (£7-£2), and 10 trials where the participant’s (i.e., dictator) monetary split was given second (i.e., money donated would shift spatially from right to left): (£1-£9), (£2-£6), (£4-£7), (£2-£9) (£2-£8), (£3-£7), (£4-£6), (£2-£8), (£3-£7), and (£2-£7). Trials were performed in a randomized, counterbalanced order. Decisions in the dictator game were assessed by calculating the “mean amount donated” by the participant to the stranger across all 20 trials. No time limit was imposed for the task, and the average time taken to complete this task was 92.1 s.
Random number generation.
As previous work has 1) demonstrated the critical role of working memory and other nonspecific cognitive processes during a number pair bisection task (Aiello et al. 2012; Doricchi et al. 2005), and 2) demonstrated that working memory correlates with prosocial decision making (Brand et al. 2006; Hinson et al. 2003; Hsu et al. 2005; MacPherson et al. 2002), we employed a separate numerical task in 20 right-handed subjects (i.e., half of the original cohort) to control for the aforementioned variables, namely random number generation. The rationale for selecting this task is that it does not rely on an explicit magnitude adjustment and recruits distinct neural networks to those necessary for number bisection but critically does rely on neural mechanisms associated with 1) numerical cognition and 2) working memory (Arshad et al. 2016a; Ferrè et al. 2013; Priftis et al. 2006).
Participants were required to generate 20 random numbers between 1 and 9 in a random sequence. The number generations were paced by a series of tones at 2 Hz, which lasted ~10 s (Ferrè et al. 2013). Subjects heard a different tone to initiate the number generation. The data were analyzed with a previously adopted approach to assess the spatial component in random number generation task by calculating the ratio of large digits (6, 7, 8, and 9) indicating preferences for larger numerical magnitudes (right side of the mental number line) against small digits (Ferrè et al. 2013).
Altruism questionnaire.
To ensure that any potential findings between dictator game task performance and number-pair bisection were specifically related to numerical cognition and not prosocial tendencies per se, we replaced the dictator game with an altruism questionnaire that required no numerical judgements (Rushton et al. 1981). Ten questions were asked, to which one of three answers [yes (2 points), no (0 points), or possibly (1 point)] had to be given. The questions were as follows: 1) would you help push a stranger’s car in the snow; 2) would you provide change for a stranger; 3) would you give money to a charity; 4) would you give money to a stranger in the street who asked for it; 5) would you donate goods or clothes to charity; 6) would you perform voluntary work for a charity; 7) would you donate blood; 8) would you offer to help carry a stranger’s belongings; 9) would you delay an elevator for a stranger; and 10) would you offer your seat on public transport to a stranger? The mean score was calculated out of 20. No time limit was imposed for the task and the average time taken to complete this task was 164.8 s.
Experiment 2: Effects of Subliminal Bidirectional Modulation of Numerical Magnitude Perception on Prosocial Decision Making
In experiment 2, we proceeded to examine the effects of subliminally altering a participant’s perception of numerical magnitude on their formulation of prosocial choices during both the dictator game task and an altruism questionnaire.
Theoretical principles underpinning the visuo-vestibular stimulation paradigm.
We have recently demonstrated that certain combined visuo-vestibular stimuli can selectively induce interhemispheric conflict. For example, the combination of binocular rivalry that is predominantly processed by the right hemisphere (Knapen et al. 2011; Lumer et al. 1998) and a vestibular stimulus that is predominantly processed in the left hemisphere (either right sided cold (RIGHTCOLD) or left sided warm (LEFTWARM) water caloric irrigations) (Arshad 2014; Bronstein et al. 2015; Dieterich et al. 2003; Nigmatullina et al. 2016; Suzuki et al. 2001) will result in competition between the two hemispheres leading to unihemispheric inhibition.
The net result of interhemispheric conflict and subsequent unihemispheric inhibition is a systematic bias in numerical magnitude allocation. Namely, when combining binocular rivalry with a left-sided warm irrigation (LEFTWARM + RIV), there is a predominant left hemisphere response, subsequently biasing judgements toward larger numbers, whereas during the combination of rivalry with right-sided cold irrigations (RIGHTCOLD + RIV), there is left hemisphere inhibition and subsequent biasing of numerical judgements toward smaller numbers (Arshad et al. 2016b). It is important to note that no interhemispheric conflict occurs when rivalry is combined with caloric irrigations that are predominantly processed in the right hemisphere (i.e., LEFTCOLD or RIGHTWARM), providing an internal experimental control for any nonspecific effects associated with combined stimulation such as dizziness, visuo-vestibular mis-match, generalized arousal, attention, and eye movements (Arshad et al. 2016b).
Following combined stimulation, the numerical biasing observed is in line with previous findings from lesion studies that have demonstrated that right hemisphere front-parietal lesions induce a pathological bias during number pair bisection toward larger numerical magnitudes attributable to a left hemisphere predominant response (Aiello et al. 2012; Doricchi et al. 2005; Zorzi et al. 2002). One account for this biasing is that the lesion induces a rightward spatial attentional bias leading to a rightward shift on the mental number line toward larger numerical magnitudes (Dehaene et al. 1993; Zorzi et al. 2002). However, more recent research has challenged this account by demonstrating a double dissociation between numerical and spatial biases following right hemisphere lesions (Aiello et al. 2012; Doricchi et al. 2005). Moreover, our recent findings in healthy subjects to induce bidirectional numerical biases during combined visuo-vestibular stimulation do not simultaneously induce a spatial bias as assessed by straight ahead pointing (Arshad et al. 2016b). Thus it is proposed that there is context-dependent hemispheric allocation of numerical magnitude that is dissociated from spatial mechanisms. That is, the right hemisphere preferentially encodes and allocates smaller numerical magnitudes whereas the left hemisphere is preferentially involved in the encoding and allocation of larger numerical magnitudes (Arshad et al. 2016b). Thus RIGHTCOLD + RIV biases judgments toward smaller numbers, whereas LEFTWARM + RIV biases judgements toward larger magnitudes (Arshad et al. 2016b).
Experimental Paradigm
Binocular rivalry was induced using the afterimage technique. A point light source from two LEDs (illuminated at 80 cd for 60 s) positioned 42 cm in front of both eyes was passed through two striated lenses (ophthalmic Maddox rod) simultaneously, producing a streak of light. A vertically orientated light was projected to the right eye, and a horizontally orientated light was projected to the left. The perception of the retinal afterimages with eyes closed resulted in rivalry, with possible percepts including a vertical line (right eye), a horizontal line (left eye) or a mixed-cross percept (i.e., both eyes) (Arshad et al. 2012, 2016b).
For vestibular stimulation, participants lay supine on a reclining barber’s chair, with the head tilted up by 30° ensuring maximal activation of the horizontal semicircular canal. The external auditory meatus was irrigated with water either at 30°C (cold condition) or 44°C (warm condition) at a rate of 500 ml/min for 40 s (CHARTR VNG: ICS Medical). The onset of dizziness typically occurred 20 s after the start of the irrigation and reached a peak at ~60 s, lasting ~3 min in total, ensuring sufficient time to complete all the tasks (Seemungal et al. 2013). The experimental tasks employed were identical to those implemented in experiment 1; number-pair bisection, dictator game, and the altruism questionnaire. Tasks were performed immediately after the 40-s irrigation in a randomized order across participants. Note that this experiment utilized a within-subject design, and all conditions were performed in a randomized, counterbalanced order. Between conditions, subjects were provided with a 5-min rest period to avoid any vestibular carryover effects (Arshad et al. 2015).
RESULTS
Experiment 1: Investigating the Relationship Between Intrinsic Numerical Biases and Prosocial Behavior
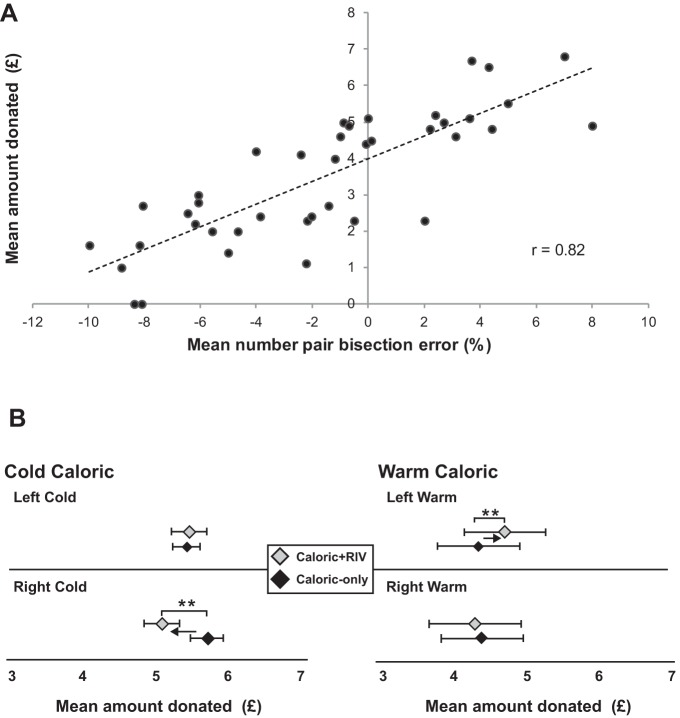
Figure 1A demonstrates that individual biases in number pair bisections were highly correlated with the degree of altruistic behavior (r = 0.82, P = 0.001; Pearson’s correlation). Subjects showing intrinsic numerical biases toward smaller numerical magnitudes formulated less favorable prosocial choices during the dictator game compared with those who had a bias toward larger numbers. When the dictator game task was substituted with a validated altruism questionnaire (Rushton et al. 1981) requiring no numerical judgement, intrinsic numerical magnitude biases did not predict prosocial choices (r = 0.023, P = 0.96; Pearson’s correlation).
Fig. 1.
Relationship between numerical magnitude and decision making during the dictator game. A: we observed a significant correlation between mean %bisection errors in the number pair bisection task (x-axis) with the amount of an individual’s donation during the dictator game task (y-axis). B; changing numerical magnitude perception using right-sided cold irrigation (RIGHTCOLD + RIV), which biases individuals toward smaller magnitudes results in subjects becoming less generous in their donation. Conversely, during left-sided warm irrigation (LEFTWARM + RIV) where subjects are biased toward larger numbers they correspondingly become more generous in their donation during the dictator game task. **P < 0.001, significant difference. Error bars indicate SE. Note; combined visuo-vestibular stimulation that did not alter numerical magnitude perception, namely left-sided cold irrigation (LEFTCOLD + RIV) and right-sided warm irrigation (RIGHTWARM + RIV), did not alter prosocial choices when compared with the corresponding caloric alone condition.
Note, given that the total amount available to donate was £148 over the 20 trials, this resulted in a maximum possible mean donation of £7.40, and if the participant (i.e., dictator) was to split this maximum donation equally [i.e., 50:50 (Andreoni and Bernheim 2009)], the mean average donation would be £3.70. In our sample, the lowest donation was £0, the highest was £6.80 [N.B. across each the 20 trials the minimum donated was £0 and the maximum was £9; our cohort (n = 40) was consistent in their decisions throughout, resulting in a similar distribution of the amount donated across each of the 20 trials] and the mean donation was £3.48.This mean value donated by our sample reflects just under a 50:50 split of each of the trials, which is what subjects typically tend to do when performing this task, and is further supported by the fact that our cohort had a mean bisection error of −1.64% (i.e., toward smaller magnitudes). Furthermore, we observed that when the midpoint in the monetary split was not a whole number, therefore more difficult to estimate accurately, participants tended to favor a split toward themselves (P < 0.05, paired t-test).
To control for generalized cognitive functions (i.e., working memory), as stated above, we substituted the bisection task with a random number generation task. We observed no relationship between numerical biases during random number generation (Ferrè et al. 2013) and decision making during the dictator game (r = 0.017; = 0.82, Pearson’s correlation). Furthermore, in our participants, we observed no relationship in the formulation of prosocial choices when comparing prosociality as assessed by the dictator game task and the validated questionnaire both when 1) not correcting for numerical biasing during the dictator game (r = 0.04, = 0.88; Pearson’s correlation) and 2) correcting for the numerical biases during the dictator game (r = −0.10, = 0.11).
Experiment 2: Effects of Subliminal Bidirectional Modulation of Numerical Magnitude Perception on Prosocial Decision Making
We proceeded to examine the effects of subliminal modulation of numerical magnitude allocation using a validated visuo-vestibular stimulation paradigm (see methods) on prosocial choice formulation.
We first assessed the degree to which combined visuo-vestibular stimulation could bias an individual’s numerical judgements selectively toward either higher (i.e., LEFTWARM + RIV) or lower (i.e., RIGHTCOLD + RIV) numerical magnitudes. A 2 × 2 × 2 repeated-measures ANOVA with factors (caloric side, 2 levels: right-left; rivalry, 2 levels: rivalry-no rivalry; and temperature: 2 levels: warm-cold irrigations) revealed a main effect for caloric side (F2,18 = 18.57, P < 0.001), a main effect for the presence of rivalry (F2,18 = 17.22, P < 0.001), and no main effect for temperature (F2,18 = 1.03, P > 0.05). There was a three-way interaction between caloric side × rivalry × temperature (F2,18 = 31.13, P < 0.001). Post hoc paired t-tests revealed that RIGHTCOLD + RIV biased numerical judgements toward smaller numerical magnitudes (P < 0.001, paired t-test). Conversely, we observed that during LEFTWARM + RIV participants were biased toward larger numerical magnitudes during the number-pair bisection task (P < 0.001, paired t-test), results directly in line with our previous observations (Arshad et al. 2016b). Moreover, as previously reported (Arshad et al. 2016b), no effects on magnitude allocation were observed in control conditions (LEFTCOLD + RIV and RIGHTWARM + RIV) (P > 0.05).
We proceeded to examine whether the combined stimulation paradigm used to induce subliminal biases in numerical magnitude allocation could affect prosocial choice formulation. A 2 × 2 × 2 repeated-measures ANOVA with factors (caloric side, 2 levels: right-left; rivalry, 2 levels: rivalry-no rivalry; and temperature: 2 levels: warm-cold irrigations) revealed a main effect for caloric side (F2,18 = 9.23, P < 0.001), a main effect for the presence of rivalry (F2,18 = 30.55, P < 0.001), and no main effect for temperature (F2,18 = 1.33, P > 0.05). There was a three-way interaction between caloric side × rivalry × temperature (F2,18 = 21.77, P < 0.001). Bonferroni corrected post hoc paired t-tests revealed that decision making in the dictator game during the CALORIC + RIV conditions was significantly different to caloric alone condition for both RIGHTCOLD + RIV and LEFTWARM + RIV (for right cold P < 0.001; for left warm P < 0.001).
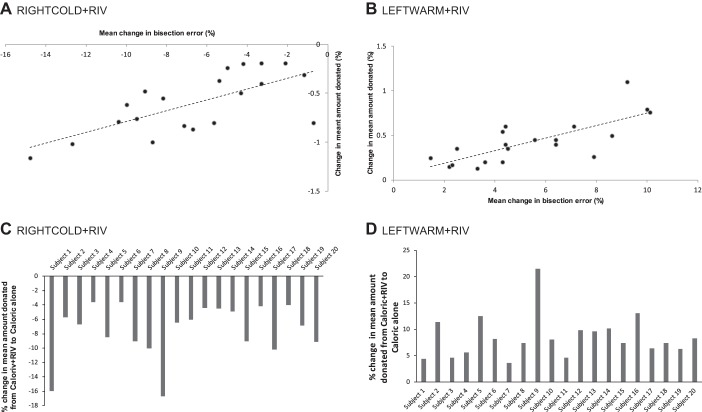
As illustrated in Figs. 1B and 2, RIGHTCOLD + RIV (which induces biases toward smaller numerical magnitudes) (Arshad et al. 2016b) resulted in a decrease in prosocial behavior during the dictator game. Contrastingly, LEFTWARM + RIV, which induces biases toward larger numbers (Arshad et al. 2016b), resulted in increased prosocial behavior. In the control conditions (i.e., LEFTCOLD + RIV or RIGHTWARM + RIV), no effects were observed on prosocial choices (P > 0.05), which as we show both here and in our previous work does not modulate numerical magnitude allocation. Furthermore, no effect of either caloric [vestibular stimulation vs. no stimulation (F2, 18 = 0.17 P > 0.05)] or binocular rivalry [rivalry vs. no rivalry (F2, 18 = 1.12, P > 0.05)] alone was observed. Additionally, we observed a significant correlation between the degree of the numerical bias induced and the subsequent impact on prosocial choices for both RIGHTCOLD + RIV (R2: 0.470; Fig. 2A and LEFTWARM + RIV (R2 0.576; Fig. 2).
Fig. 2.
A and B: relationship between mean percentage change in number-pair bisection error induced by the combined visuo-vestibular stimulation paradigm (x-axis) compared with the corresponding caloric alone condition and change in mean monetary amount donated during the dictator game task (y-axis). A: a negative correlation was observed during RIGHTCOLD + RIV stimulation, which biases participants toward smaller magnitudes. That is, individuals that manifested larger biases toward smaller number manifested a proportional change in decisions during the dictator game (i.e., became correspondingly less generous). B: conversely, during LEFTWARM + RIV which induces biases toward larger magnitudes resulted in increased monetary donations during the dictator game. C and D: %change in mean monetary amount donated for each individual, negative during the RIGHTCOLD + RIV condition (reflecting less generosity; C) and positive during the LEFTWARM + RIV condition (reflecting increased generosity; D).
To confirm that the above reported effects were attributable to the numerical nature of the task, we assessed whether biasing numerical magnitude could influence prosocial choice formulation on the altruism questionnaire. We observed no main effect for either the side of stimulation, rivalry nor temperature (Caloric vs Caloric + RIV) (P > 0.05, repeated-measures ANOVA).
DISCUSSION
Herewith, we demonstrate that intrinsic biases in numerical magnitude can directly predict the amount of money an individual donates to an anonymous stranger during the dictator game. Furthermore, experimentally inducing subliminal biases in numerical magnitude perception actively drives prosocial choices in the corresponding direction, in a directly proportional manner. Notably, the reported effects were only observed for prosocial choice formulation during the dictator game but not for the altruism questionnaire.
To date, prosocial tendencies have been associated with a large degree of unexplained heterogeneity, exemplified by the lack of traditional variables (i.e., age, gender, income, wealth, taxation levels, and religious or cultural beliefs) holding any predictive power (Harbaugh et al. 2007; Camerer et al. 2004). At first glance, our findings suggest that intrinsic biases in magnitude allocation play a role in choice formulation during the dictator game, in line with a previous report suggesting the importance of this variable (Furlong and Opfer 2009). However, if this was the case, then one would expect that prosocial tendencies assessed either with the dictator game or with the questionnaire would both correlate with biases in magnitude. However, we only observed the relationship for the dictator game, not the questionnaire, and furthermore, we observed no relationship between prosocial choices when comparing the two tasks. Therefore, we suggest that the relationship observed in our present study is attributable to the fact that as the dictator game task explicitly requires the act of readjusting the monetary spilt to a more abstract equitable position, and it recruits overlapping cognitive processes to those necessary for number pair bisection, hence explaining the observed relationship (Arshad et al. 2016b; Doricchi et al. 2005; Fehr and Camerer 2007; Knoch et al. 2008; Morishima et al. 2012; Ruff et al. 2013; Zorzi et al. 2002). Therefore, it seems to be the case that in economic and psychological experiments that implement tasks that require mental arithmetic processes are susceptible to numerical biasing, which subsequently results in influencing a participant’s response beyond that of pure noise.
We proceeded to corroborate the correlative relationship observed in experiment 1 by subliminally inducing biases in numerical magnitude perception systematically toward either higher or lower values using a visuo-vestibular stimulation paradigm (Arshad et al. 2016b). If the correlation we report was true, then we predicted that systematically inducing subliminal biases in numerical magnitude would actively drive prosocial choices during the dictator game in the corresponding direction. RIGHTCOLD + RIV stimulation, which biases numerical judgements toward smaller magnitudes, resulted in the formulation of less favorable prosocial choices, whereas LEFTWARM + RIV that biases numerical judgements toward larger magnitudes (Arshad et al. 2016b) resulted in subjects formulating more favorable choices. Moreover, the size of the induced subliminal biases was found to be correlated with the extent of the change in prosocial choices confirming the role of numerical magnitude on economic choice selection during the dictator game, as illustrated in Fig. 2.
It could be argued that the results we report following combined stimulation are attributable to nonspecific effects associated with our visuo-vestibular stimulation paradigm. However, we have previously and presently demonstrated that this technique does not induce biases in numerical magnitude due to nonspecific effects such as eye movements, visuo-vestibular mis-match, dizziness, or generalized arousal effects (Arshad et al. 2016b). Furthermore, we have previously shown that this stimulation technique does not modulate numerical magnitude allocation due to an induced spatial attention bias (Arshad et al. 2016b). That is, we have previously assessed for any potential spatial biases during combined stimulation implementing a straight-ahead pointing task and observed no effect (Arshad et al. 2016b). Moreover, by observing no relationship between decision making in the dictator game and the random number generation task, we can rule out more generalized, nonspecific cognitive effects such as numerical cognition per se and the role of working memory (Arshad et al. 2016a; Brand et al. 2006; Ferrè et al. 2013; Hinson et al. 2003; Hsu et al. 2005; MacPherson et al. 2002; Priftis et al. 2006). Finally, as expected we observed no effect of the combined stimulation paradigm on the formulation of prosocial choices during the altruism questionnaire (Rushton et al. 1981).
Taken together, our findings provide evidence for numerical influences on decision making in the dictator game, and hence have direct implications for neuro-economic, psychology, and social science research that have implemented the dictator game paradigm, or similar variants of the task that contain an inherent numerical component. This consideration is particularly important in those studies that also assess such behavior following asymmetric brain stimulation over frontal or parietal brain regions (Ruff et al. 2013; Arshad et al. 2016b).
Further supporting our viewpoint regarding the marked limitations of the dictator game is first provided by the contrasting findings of studies investigating the neural basis of human prosocial behavior that either implement a task with or without a numerical component. That is, assessing prosocial behavior using the dictator game has revealed parietal, prefrontal, and striatal involvement (Crockett et al. 2014; Fehr and Fischbacher 2003; Morishima et al. 2012; Ruff et al. 2013; Tricomi et al. 2010), regions that are either linked to or possess an enhanced sensitivity to numerical cognition (Cohen Kadosh et al. 2007; Hubbard et al. 2005; Rusconi et al. 2011). Contrastingly, the neural correlates of prosocial behavior using nonnumerical methodology by probing structural differences in the brains of kidney donors (i.e., altruistic individuals) vs. controls have revealed that prosocial individuals exhibit greater volume in the right amygdala (not implicated in numerical cognition) and an enhanced responsiveness to fearful faces (Marsh et al. 2014). These findings are in line with data from psychopathic individuals, who exhibit antisocial behavioral traits and have been shown to have both reduced amygdala volume and diminished responsiveness to fearful faces (Blair 2013).
To conclude, our results highlight the necessity to control for numerical influences during the dictator game and other tasks of a similar numerical nature. Without the implementation of an adequate control for these strong numerical influences, these tasks should be employed with caution in the assessment of human behavior.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.A. conceived and designed research; Q.A., S.S., M.F., S.M., S.R., and R.L. performed experiments; Q.A., Y.N., S.S., M.F., S.M., S.R., and R.E.R. analyzed data; Q.A., Y.N., M.F., R.L., P.M., R.E.R., and A.M.B. interpreted results of experiments; Q.A., S.S., S.M., R.L., P.M., and R.E.R. drafted manuscript; Q.A. and A.M.B. edited and revised manuscript; Q.A., Y.N., S.S., R.L., P.M., R.E.R., and A.M.B. approved final version of manuscript; Y.N., M.F., and S.M. prepared figures.
ACKNOWLEDGMENTS
We thank David Soto for useful comments. We also thank the four anonymous for insightful and helpful comments.
REFERENCES
- Aiello M, Jacquin-Courtois S, Merola S, Ottaviani T, Tomaiuolo F, Bueti D, Rossetti Y, Doricchi F. No inherent left and right side in human ‘mental number line’: evidence from right brain damage. Brain 135: 2492–2505, 2012. doi: 10.1093/brain/aws114. [DOI] [PubMed] [Google Scholar]
- Andreoni J, Bernheim BD. Social image and the 50–50 norm: a theoretical and experimental analysis of audience effects. Econometrica 77: 1607–1636, 2009. doi: 10.3982/ECTA7384. [DOI] [Google Scholar]
- Arshad Q. Behavioural Manipulations of Parietal Lobe Function (PhD thesis). London: Imperial College of London, 2014. [Google Scholar]
- Arshad Q, Kaski D, Buckwell D, Faldon ME, Gresty MA, Seemungal BM, Bronstein AM. A new device to quantify ocular counterroll using retinal afterimages. Audiol Neurootol 17: 20–24, 2012. doi: 10.1159/000324859. [DOI] [PubMed] [Google Scholar]
- Arshad Q, Nigmatullina Y, Bronstein AM. Handedness-related cortical modulation of the vestibular-ocular reflex. J Neurosci 33: 3221–3227, 2013. doi: 10.1523/JNEUROSCI.2054-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad Q, Nigmatullina Y, Nigmatullin R, Asavarut P, Goga U, Khan S, Sander K, Siddiqui S, Roberts RE, Cohen Kadosh R, Bronstein AM, Malhotra PA. Bidirectional Modulation of Numerical Magnitude. Cereb Cortex 26: 2311–2324, 2016b. doi: 10.1093/cercor/bhv344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad Q, Nigmatullina Y, Roberts RE, Bhrugubanda V, Asavarut P, Bronstein AM. Left cathodal trans-cranial direct current stimulation of the parietal cortex leads to an asymmetrical modulation of the vestibular-ocular reflex. Brain Stimul 7: 85–91, 2014. doi: 10.1016/j.brs.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad Q, Nigmatullina Y, Roberts RE, Goga U, Pikovsky M, Khan S, Lobo R, Flury AS, Pettorossi VE, Cohen-Kadosh R, Malhotra PA, Bronstein AM. Perceived state of self during motion can differentially modulate numerical magnitude allocation. Eur J Neurosci 44: 2369–2374, 2016a. doi: 10.1111/ejn.13335. [DOI] [PubMed] [Google Scholar]
- Arshad Q, Siddiqui S, Ramachandran S, Goga U, Bonsu A, Patel M, Roberts RE, Nigmatullina Y, Malhotra P, Bronstein AM. Right hemisphere dominance directly predicts both baseline V1 cortical excitability and the degree of top-down modulation exerted over low-level brain structures. Neuroscience 311: 484–489, 2015. doi: 10.1016/j.neuroscience.2015.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. The neurobiology of psychopathic traits in youths. 14: 786–799, 2013. doi: 10.1038/nrn3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Labudda K, Markowitsch HJ. Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Netw 19: 1266–1276, 2006. doi: 10.1016/j.neunet.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Patel M, Arshad Q. A brief review of the clinical anatomy of the vestibular-ocular connections—how much do we know? Eye (Lond) 29: 163–170, 2015. doi: 10.1038/eye.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer CF, Ho T and Chong JK. Behavioural game theory: thinking, learning and teaching. In: Advances in Understanding Strategic Behaviour. Berlin, Germany: Springer, 2004, p. 120–180. doi: 10.1057/9780230523371_8. [DOI] [Google Scholar]
- Cohen Kadosh R, Cohen Kadosh K, Kaas A, Henik A, Goebel R. Notation-dependent and -independent representations of numbers in the parietal lobes. Neuron 53: 307–314, 2007. doi: 10.1016/j.neuron.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Kurth-Nelson Z, Siegel JZ, Dayan P, Dolan RJ. Harm to others outweighs harm to self in moral decision making. Proc Natl Acad Sci USA 111: 17320–17325, 2014. [Erratum. Proc Natl Acad Sci USA 112: E381, 2015.] doi: 10.1073/pnas.1408988111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Fischbacher U, Treyer V, Schellhammer M, Schnyder U, Buck A, Fehr E. The neural basis of altruistic punishment. Science 305: 1254–1258, 2004. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Bossini S, Giraux P. The mental representation of parity and number magnitude. J Exp Psychol Gen 122: 371–396, 1993. doi: 10.1037/0096-3445.122.3.371. [DOI] [Google Scholar]
- Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, Brandt T. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex 13: 994–1007, 2003. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Guariglia P, Gasparini M, Tomaiuolo F. Dissociation between physical and mental number line bisection in right hemisphere brain damage. Nat Neurosci 8: 1663–1665, 2005. doi: 10.1038/nn1563. [DOI] [PubMed] [Google Scholar]
- Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends Cogn Sci 11: 419–427, 2007. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Fehr E, Fischbacher U. The nature of human altruism. Nature 425: 785–791, 2003. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- Ferrè ER, Vagnoni E, Haggard P. Galvanic vestibular stimulation influences randomness of number generation. 224: 233–241, 2013. doi: 10.1007/s00221-012-3302-6. [DOI] [PubMed] [Google Scholar]
- Furlong EE, Opfer JE. Cognitive constraints on how economic rewards affect cooperation. 20: 11–16, 2009. doi: 10.1111/j.1467-9280.2008.02244.x. [DOI] [PubMed] [Google Scholar]
- Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science 316: 1622–1625, 2007. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Knoepfle DT, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J Neurosci 30: 583–590, 2010. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL Whitney P. Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn 29: 298, 2003. [DOI] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science 310: 1680–1683, 2005. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Hubbard EM, Piazza M, Pinel P and Dehaene S. Interactions between number and space in parietal cortex. Nat Rev Neurosci 6: 435–448, 2005. doi: 10.1038/nrn1684. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Knetsch JL, Thaler R. Fairness as a constraint on profit seeking: entitlements in the market. Am Econ Rev 76: 728–741, 1986. [Google Scholar]
- Knapen T, Brascamp J, Pearson J, van Ee R, Blake R. The role of frontal and parietal brain areas in bistable perception. J Neurosci 31: 10293–10301, 2011. doi: 10.1523/JNEUROSCI.1727-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Nitsche MA, Fischbacher U, Eisenegger C, Pascual-Leone A, Fehr E. Studying the neurobiology of social interaction with transcranial direct current stimulation—the example of punishing unfairness. Cereb Cortex 18: 1987–1990, 2008. doi: 10.1093/cercor/bhm237. [DOI] [PubMed] [Google Scholar]
- Lumer ED, Friston KJ, Rees G. Neural correlates of perceptual rivalry in the human brain. Science 280: 1930–1934, 1998. doi: 10.1126/science.280.5371.1930. [DOI] [PubMed] [Google Scholar]
- MacPherson SE, Phillips LH, Della Sala S. Age, executive function, and social decision making: a dorsolateral prefrontal theory of cognitive aging. Psychol Aging 17: 598–609, 2002. doi: 10.1037/0882-7974.17.4.598. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Stoycos SA, Brethel-Haurwitz KM, Robinson P, VanMeter JW, Cardinale EM. Neural and cognitive characteristics of extraordinary altruists. Proc Natl Acad Sci USA 111: 15036–15041, 2014. doi: 10.1073/pnas.1408440111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, Schunk D, Bruhin A, Ruff CC, Fehr E. Linking brain structure and activation in temporoparietal junction to explain the neurobiology of human altruism. Neuron 75: 73–79, 2012. doi: 10.1016/j.neuron.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Nigmatullina Y, Siddiqui S, Khan S, Sander K, Lobo R, Bronstein AM, Arshad Q. Lateralisation of the vestibular cortex is more pronounced in left-handers. Brain Stimul 9: 942–944, 2016. doi: 10.1016/j.brs.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Priftis K, Zorzi M, Meneghello F, Marenzi R, Umiltà C. Explicit versus implicit processing of representational space in neglect: dissociations in accessing the mental number line. J Cogn Neurosci 18: 680–688, 2006. doi: 10.1162/jocn.2006.18.4.680. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Ugazio G, Fehr E. Changing social norm compliance with noninvasive brain stimulation. Science 342: 482–484, 2013. doi: 10.1126/science.1241399. [DOI] [PubMed] [Google Scholar]
- Rusconi E, Bueti D, Walsh V, Butterworth B. Contribution of frontal cortex to the spatial representation of number. Cortex 47: 2–13, 2011. doi: 10.1016/j.cortex.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Rushton JP, Chrisjohn RD, Fekken GC. The altruistic personality and the self-report altruism scale. Person Indiv Diff 2: 293–302, 1981. [Google Scholar]
- Seemungal BM, Guzman-Lopez J, Arshad Q, Schultz SR, Walsh V, Yousif N. Vestibular activation differentially modulates human early visual cortex and V5/MT excitability and response entropy. Cereb Cortex 23: 12–19, 2013. doi: 10.1093/cercor/bhr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Kitano H, Ito R, Kitanishi T, Yazawa Y, Ogawa T, Shiino A Kitajima K. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Brain Res Cogn Brain Res 12: 441–449, 2001. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Rangel A, Camerer CF, O’Doherty JP. Neural evidence for inequality-averse social preferences. Nature 463: 1089–1091, 2010. doi: 10.1038/nature08785. [DOI] [PubMed] [Google Scholar]
- Zorzi M, Priftis K, Umiltà C. Brain damage: neglect disrupts the mental number line. Nature 417: 138–139, 2002. doi: 10.1038/417138a. [DOI] [PubMed] [Google Scholar]