Abstract
Background
Orthostatic tremor (OT), a rare and complex movement disorder, is characterized by rapid tremor of both legs and the trunk while standing. These disappear while the patient is either lying down or walking. OT may be idiopathic/primary or it may coexist with several neurological conditions (secondary OT/OT plus). Primary OT remains an enigmatic movement disorder and its pathogenesis and neural correlates are not fully understood.
Methods
A PubMed search was conducted in July 2017 to identify articles for this review.
Results
Structural and functional neuroimaging studies of OT suggest possible alterations in the cerebello-thalamo-cortical network. As with essential tremor, the presence of a central oscillator has been postulated for OT; however, the location of the oscillator within the tremor network remains elusive. Studies have speculated a possible dopaminergic deficit in the pathogenesis of primary OT; however, the evidence in favor of this concept is not particularly robust. There is also limited evidence favoring the concept that primary OT is a neurodegenerative disorder, as a magnetic resonance spectroscopic imaging study revealed significant reduction in cerebral and cerebellar N-acetyl aspartate (NAA) levels, a marker of neuronal compromise or loss.
Discussion
Based on the above, it is clear that the pathogenesis of primary OT still remains unclear. However, the available evidence most strongly favors the existence of a central oscillatory network, and involvement of the cerebellum and its connections.
Keywords: Orthostatic tremor, pathogenesis, cerebellum, neurodegeneration
Introduction
Orthostatic tremor (OT) is a rare and enigmatic neurological disorder. The term “orthostatic tremor” itself is a relatively recent one, having been coined in 1984 by Heilman.1 OT is defined as a rapid tremor that appears only while standing, and chiefly affecting the lower limbs and the trunk with a characteristic frequency of 13–18 Hz.2 The tremor is associated with a subjective sensation of unsteadiness, which improves markedly on leaning or touching sturdy objects such as walls and tables, and on starting to walk; the sensation disappears on sitting or lying down, along with resolution of the tremor. In fact, the sensation of unsteadiness may be the predominant presenting symptom in the majority of patients with OT. The first description of this phenomenon dates back to 1970, when Pazzaglia and colleagues reported an “unusual disorder of erect standing position” in three patients.3 The majority of studies have reported the onset of OT in the sixth decade of life, with a preponderance for female gender.4 On visual inspection, OT is characterized by rapid tremor of the legs, and palpation and auscultation reveal the presence of a thrill and a thumping sound (helicopter sign), respectively.4,5 However, the aforementioned signs and symptoms may not be sensitive and it can be challenging to distinguish OT from orthostatic myoclonus (OM) by clinical evaluation alone.6 Thus, confirmation of the diagnosis by surface electromyography (EMG) is of additional importance. OT may be idiopathic/primary or it may be associated with several neurologic and medical conditions (secondary OT/OT plus).7 Initial reports presumed OT to be a variant of essential tremor (ET), as there are reports of the presence of postural and kinetic tremor of the upper limbs in patients with OT, and a family history of ET in patients with OT has been reported in a number of published case series.8,9 However, subsequent clinical and neurophysiological studies have suggested that OT may have a distinct pathomechanistic substrate.10–12
Longitudinal observations have indicated that OT is a progressive disorder,13 which may be associated with a number of non-motor symptoms such as depression and cognitive impairment.14,15 Moreover, patients with OT have a markedly reduced quality of life.16 Hence, the pathological underpinnings of this complex disorder need to be understood so that specific therapeutic avenues may be explored and better treatments implemented. So far, however, even three decades after its first description, the pathophysiology of OT is not completely understood and remains controversial. A number of electrophysiological and advanced neuroimaging studies have explored the neural correlates of this rare movement disorder. Similar to ET, the theories regarding the pathogenesis of OT have revolved around the concept of the existence of central oscillators and alterations in the cerebello-thalamo-cortical network. In addition, studies also speculate that OT may be a neurodegenerative disorder. This article reviews studies of the pathogenesis of primary OT and comprehensively discusses the concepts and controversies regarding the pathogenesis of this enigmatic movement disorder. As literature on secondary OT is largely in the form of case reports, and all of the original research aimed at exploring the pathogenesis of OT has been conducted on patients with primary OT, in this article, we limit our discussion to primary OT.
Methodology
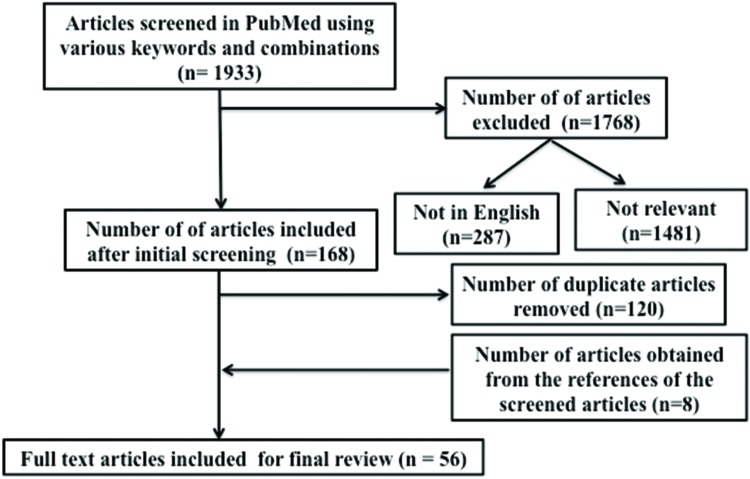
In July 2017, the authors utilized PubMed to search the relevant literature using the term “orthostatic tremor” with additional search terms being “pathogenesis”, “pathology”, “pathophysiology”, “biology”, “neurodegeneration”, “cerebellum”, “thalamus”, “red nucleus”, “neuroimaging”, “MRI”, “PET”, “SPECT”, and “spectroscopy”. This yielded 1,933 articles (Table 1, Figure 1). During the initial screening of the abstracts/full texts, the publications that were not relevant to this review (e.g., papers on secondary OT), duplicates, and those that were published in languages other than English were removed, leaving 48 remaining articles. The references from these articles were also thoroughly searched for any additional articles, yielding nine more articles. In total, 57 articles pertinent to this topic were included for this review (Table 1, Figure 1).
Table 1. Results of Search for Articles from PubMed Using Various Key Words and their Combinations.
| Key Words and Combinations | Number of Publications | ||
|---|---|---|---|
| Total | Included | Excluded | |
| Orthostatic tremor AND pathogenesis | 545 | 42 | 503 (not in English: 95, not relevant: 408) |
| Orthostatic tremor AND pathology | 173 | 7 | 166 (not in English: 35, not relevant: 131) |
| Orthostatic tremor AND physiology | 801 | 53 | 738 (not in English: 93, not relevant: 645) |
| Orthostatic tremor AND biology | 12 | 0 | 12 (not in English: 0, not relevant: 12) |
| Orthostatic tremor AND neurodegeneration | 29 | 0 | 29 (not in English: 8, not relevant: 21) |
| Orthostatic tremor AND cerebellum | 96 | 10 | 85 (not in English: 14, not relevant: 71) |
| Orthostatic tremor AND thalamus | 62 | 9 | 52 (not in English: 6, not relevant: 46) |
| Orthostatic tremor AND red nucleus | 10 | 0 | 10 (not in English: 2, not relevant: 8) |
| Orthostatic tremor AND inferior olive | 7 | 0 | 7 (not in English: 2, not relevant: 5) |
| Orthostatic tremor AND neuroimaging | 41 | 5 | 33 (not in English: 1, not relevant: 32) |
| Orthostatic tremor AND MRI | 91 | 9 | 79 (not in English: 20, not relevant: 59) |
| Orthostatic tremor AND PET | 16 | 3 | 13 (not in English: 3, not relevant: 10) |
| Orthostatic tremor AND SPECT | 24 | 6 | 18 (not in English: 6, not relevant: 12) |
| Orthostatic tremor AND spectroscopy | 26 | 2 | 23 (not in English: 2, not relevant: 21) |
| Total number of articles included for review after removing the duplicates | 48 | ||
| Total number of articles included from the reference sections of the shortlisted articles | 8 | ||
| Final number of articles included for review | 56 | ||
Abbreviations: MRI, Magnetic Resonance Imaging; PET, Positron Emission Tomography; SPECT, Single-Photon Emission Computed Tomography.
Figure 1. Flow Diagram Summarizing our Literature Search.
Results
The role of the cerebellum and its connections in primary OT: insights from the neuroimaging studies
Certain clinical features of OT such as subjective unsteadiness17 and ataxia,18 and the similarities of OT to ET as described above have prompted researchers to explore the role of the cerebellum and its connections in the pathogenesis of primary OT. Neuroimaging studies using several modalities, including positron emission tomography (PET),19,20 magnetic resonance spectroscopy (MRS),21 resting state functional magnetic resonance imaging (rs-fMRI),22,23 and voxel-based morphometry (VBM),23 have explored the neuroanatomical and neurophysiological correlates of primary OT, and all these studies have reported structural and/or functional alterations in the cerebellum and/or its connections.
Wills et al.19 conducted a PET study to explore the relationship between the postural tremor in patients with OT and cerebellar blood flow. The study focused on four patients with primary OT who also had a 14–16 Hz postural tremor of the arms. The authors demonstrated significantly greater activation of both cerebellar hemispheres when the right arm was in an outstretched position (Table 2).19 Similarly, when the arms were at rest, there was greater activation of both cerebellar hemispheres and the vermis in the patients with primary OT than in age- and gender-matched controls. As the authors had previously reported nearly similar results in patients with ET,24 they speculated that irrespective of the etiology, increased cerebellar activity is a feature of tremor disorders. Although this study was primarily designed to explore the relationship between the postural tremor in OT and cerebellar blood flow, it provided a potential clue regarding the role of cerebellum in primary OT. Later, Schöberl et al.20 quantified cerebral glucose metabolism in 10 patients with primary OT and 10 age- and gender-matched controls, both in the supine and the standing positions (Table 2). In that study, patients with primary OT had a significant increase in glucose metabolism compared with controls, both in the supine and the standing positions, in the posterior cerebellum (including the dentate nucleus), ventral intermediate (VIM), and ventral posterolateral nuclei of the thalamus, pontine tegmentum, and bilateral primary motor cortex. In addition, a decrease in glucose metabolism was noted in the mesiofrontal cortical areas and bilateral anterior insula. Overall, the results suggested that there is altered ponto-cerebellar-thalamic-cortical activation in patients with OT at rest that is further activated when the patients stand. Other than the pontine tegmentum, brain regions with higher glucose metabolism in this study are components of the cerebello-thalamo-cortical loop, which is presumably altered in patients with ET.25,26 Further evidence of involvement of the cerebellum and its connections was revealed in a rs-fMRI study (Table 2).23 In that study, the connectivity between the cerebellar hemispheres and supplementary motor cortex was significantly increased in patients with primary OT compared with controls, and it correlated with the disease duration and tremor severity. The notion of cerebellar involvement was further reinforced by the observation that repetitive transcranial magnetic stimulation of the cerebellum resulted in a decrease in tremor severity as well as a decrease in the functional connectivity of the cerebellar hemispheres and supplementary motor cortex. In the same study, patients with primary OT had decreased gray matter volume in the cerebellar hemispheres, and increased grey matter volume in the vermis and the supplementary motor cortex. In a recently published rs-fMRI study (Table 2), patients with primary OT had decreased connectivity compared with controls in the cerebellum and sensorimotor networks.22 In addition, the OT group had increased connectivity within several resting state networks, which are presumed to be associated with cognition. Despite the large amount of evidence favoring a role of the cerebellum and its connections in OT, one wonders why there is a lack of association between OT and spinocerebellar ataxia. However, it may be difficult for patients with such ataxias to recognize a separate source of unsteadiness in the legs.
Table 2. Summary of the Neuroimaging Studies on Patients with Primary Orthostatic Tremor.
| Authors | Imaging Modality | Subjects | Principal Results |
|---|---|---|---|
| Wills et al.19 1996 | PET (H215O) | OT: 4 Controls: 4 |
Increased activation in bilateral cerebellar hemisphere and vermis in OT both at rest and with outstretched arms. NO activation of medulla and inferior olive |
| Katzenschlager et al.51 2003 | SPECT | OT: 11 PD: 12 Controls: 12 |
Striatal tracer binding in OT was significantly lower than that in controls and higher than that in PD, suggesting deficits in the dopaminergic system in OT |
| Wegner et al.56 2008 | SPECT | OT: 2 | Normal dopaminergic and serotonergic innervation in OT |
| Trocello et al.54 2008 | SPECT | OT: 12 Controls: 12 |
Normal presynaptic dopaminergic uptake in OT |
| Raudino et al.55 2009 | SPECT | OT: 1 | Normal presynaptic dopaminergic uptake in OT |
| Benito-León et al.22 2016 | Resting state fMRI | OT: 13 Controls: 13 |
Increased connectivity in the RSN involved in cognitive processes (DMN, FPN) in OT Decreased connectivity within the cerebellar and sensorimotor network in OT |
| Gallea et al.23 2016 | Resting state fMRI and VBM hemispheres | OT: 17 Controls: 17 |
VBM: Increased volume of vermis and supplementary motor area, and decreased volume of cerebellar hemispheres in patients with OT. fMRI: Reduced connectivity between cerebellar hemispheres and supplementary motor area (connectivity was restored with rTMS over cerebellum) |
| Schöberl et al.20 2016 | PET (FDG) | OT: 10 Controls: 10 |
Increased glucose metabolism in pontine tegmentum, posterior cerebellum, bilateral primary motor cortex, and ventral intermediate and ventroposterolateral nuclei of thalamus. Decreased glucose metabolism in mesiofrontal cortical areas, and bilateral anterior insula |
| Benito-León et al.21 2016 | MRS | OT: 14 Controls: 14 |
Reduced NAA in cerebellar vermis, cerebellar white matter, and mid-parietal gray matter |
Abbreviations: DMN, Default Mode Network; FDG, Flourodeoxy Glucose; fMRI, Functional Magnetic Resonance Imaging; FPN, Frontoparietal Network; MRS, Magnetic Resonance Spectroscopy; NAA, N-Acetyl Aspartate; OT, Orthostatic Tremor; PD, Parkinson’s Disease; PET, Positron Emission Tomography; rTMS, Repetitive Transcranial Magnetic Stimulation; RSN, Resting State Network; SPECT, Single Photon Emission Computerized Tomography; VBM, Voxel-based Morphometry.
Clues from thalamic deep brain stimulation: further support for the cerebello-thalamo-cortical network hypothesis
Based on the evidence for involvement of the cerebello-thalamo-cortical network in OT, as suggested by the neuroimaging studies discussed above, some clinicians have been motivated to assess the possible beneficial effects of deep brain stimulation (DBS) of the VIM nucleus of thalamus in patients with medically refractory primary OT.27–30 In a recently published international, multicenter study aimed at evaluating the efficacy of thalamic DBS in 17 medically refractory OT patients, Merola et al.31 reported that DBS of the VIM nucleus of the thalamus is safe, well tolerated, and yields sustained benefit in a majority of the patients so treated. Although the benefit with DBS in these cases does not provide direct information about the pathogenesis of OT, these observations further support the cerebello-thalamo-cortical network hypothesis. However, lack of as robust benefit as ET suggests a different pathological substrate within the network.
Where is the central oscillator in primary OT?
Akin to ET, it has been postulated that a central oscillator is perhaps the prime generator of tremor in primary OT. This idea has largely been based on observations from electrophysiological studies. Köster et al.32 performed spectral analysis of EMG recordings from all of the affected muscles in six patients with primary OT, and revealed a high level of coherence. This high intermuscular coherence between all muscles suggests the existence of a unique central oscillator, which generates tremor. In other words, muscles on both sides innervated by different segments of the neuraxis probably receive tremor signals from a common central source. Later, Marsden et al. performed a detailed phase analysis of tremor in five patients with primary OT and two controls, which showed that although phase relation in different muscles remains constant in a muscle, it varies among subjects and changes based on posture. They interpreted this to suggest that OT is an unmasking of central postural control mechanisms.33 Further studies have predicted that the central oscillators in OT are probably located in the posterior fossa.34 Thus, Wu et al.34 in a study of six patients with primary OT demonstrated that very low-intensity galvanic vestibular stimulation (GVS) with electrodes applied to both mastoids was able to reset the tremor. On the other hand, GVS of healthy controls can elicit EMG bursts of 14–18 Hz, with persistent frequency within a subject with a delay of 700 ms and persistence of 1–2 seconds after cessation of stimulus. A study using the acoustic startle response (ASR) suggested that the brainstem may be one of the anatomical substrates in the pathogenesis of primary OT.35 In that study, the response rate of ASR was suppressed in seven patients with primary OT compared with 13 controls.35 ASR is a brainstem reflex, which represents a defense mechanism that follows sudden, unexpected loud stimuli. Interestingly, the tremor frequency of primary OT overlaps with the frequency of synchronization seen in the ASR,36 thus suggesting possible functional involvement of the brainstem in primary OT.
The presence of intermittent 16 Hz spasms in the legs (with intermuscular coherence) in a paraplegic patient raised the possibility of the existence of an OT oscillator in the spinal cord.37 This prediction was further corroborated by a study that reported significant improvement in unsteadiness in two medically refractory primary OT patients who had undergone chronic spinal cord stimulation.38 However, other than these few observations, there is no robust evidence to support the notion that the central oscillator of OT is located in the spinal cord. Later, a study by Muthuraman et al.39 characterized the central oscillatory network in OT through coherent source analysis of simultaneous electroencephalography (EEG) and EMG recordings. In that study, dynamic imaging of coherent source analysis was used to find the sources in the brain that are coherent with the peripheral tremor signal. The approach revealed that the network for the tremor frequency is represented by unilateral activation of the primary motor area corresponding to the legs, primary sensory cortex, supplementary motor area, premotor area, prefrontal cortex, cerebellum, and thalamus.39 The study further reinforced the concept that tremor in OT is driven by a central oscillator and it is perhaps a network, rather than an isolated brain region.
In summary, the bulk of the evidence suggests the existence of a central oscillating mechanism that generates the tremor in OT. The central oscillator appears to be a network, rather than a single structure and is perhaps located in the posterior fossa.
Is primary OT neurodegenerative?
The natural course of primary OT is similar to that of most of the neurodegenerative disorders, i.e. its onset is insidious, and symptoms are progressive. Moreover, longitudinal studies have reported progressive disability in patients with primary OT, and some of the patients may develop additional neurological signs in the long run.13,40 An MRS study provided additional insights into the possible neurodegenerative mechanisms of OT.21 Benito-León et al.21 performed a proton MRS study on 14 patients with OT (nine primary OT, five OT patients with mild parkinsonism), and reported a significant reduction in the absolute concentration of N-acetyl aspartate (NAA) in the cerebellar vermis, cerebellar white matter, and in the mid-parietal gray matter. A reduction in NAA is highly suggestive of greater neuronal dysfunction or increased neuronal loss, and suggests an underlying neurodegenerative process in primary OT. Similar findings were also seen in MRS-based studies of ET,41,42 which has been increasingly recognized as a neurodegenerative disease.43,44 Results of the Benito-León et al.21 study raise the possibility of a neurodegenerative basis for OT; however, additional studies are warranted.
Is there a dopaminergic deficit in primary OT?
Several clinical observations have led to speculation that there may be a dopaminergic deficit in primary OT. These include a case series describing eight patients with primary OT, which documented significant improvement with levodopa in five of these.45 Another case report documented significant improvement of OT symptoms with pramipexole46, further suggesting a possible alteration in dopaminergic transmission in primary OT. Moreover, some patients with OT may develop incident Parkinson’s disease (PD)13,45,47 or progressive supranuclear palsy48 and OT may even appear in long-standing PD.49,50
These clinical observations gain additional support from a molecular imaging study of 11 patients with primary OT.51 In this single-photon emission computed tomography (SPECT)-based study, the authors used 123I-FP-CIT ([123I]-2β-carbomethoxy-3β-(-4-iodophenyl)-N-(3-fluoropropyl)-nortropane) as a dopamine transporter tracer and reported significant reduction in the striatal tracer binding in primary OT patients compared with controls. The study suggests a presynaptic dopaminergic deficit in primary OT. Further evidence in favor of a dopaminergic deficit in primary OT comes from a transcranial sonographic (TCS) study of the substantia nigra (SN).52 Hyperechogenicity of the SN may be observed with TCS in more than 90% of patients with PD.53 The pathological correlates of hyperechogenicity of the SN remain elusive and it is not clear whether this finding represents structural changes in the tissue or accumulation of metals such as iron. Similar to the TCS findings in PD, Spiegel et al. reported hyperechogenic SN in all the four subjects with OT in their study,52 thus, reinforcing the conjecture that altered dopaminergic transmission could be involved in the pathogenesis of OT.
While there is some evidence of a dopaminergic deficit in OT, subsequent studies based on molecular imaging did not find any evidence of a presynaptic dopaminergic deficit in patients with primary OT (reviewed below).54–56 Furthermore, although the study by Katzenschlage et al.51 demonstrated a significant reduction in overall tracer uptake in OT (41.9% less compared with controls), levodopa challenge did not result in a significant improvement in tremor. Moreover, there was no correlation of tracer uptake with disease duration. Another study using the same tracer in 12 OT patients found no difference when compared with age- and gender-matched controls, despite the fact that eight of 12 patients had a disease duration that was greater than 10 years.54 A normal FP-CIT SPECT scan was reported in two other studies.55,56 Although these additional studies do not entirely rule out the possibility of a dopaminergic deficit in OT, they lead to a conjecture that there may be several subtypes of OT, some associated with dopaminergic deficits and some that are not. Based on this concept, it has been suggested that primary OT may be categorized into three different subtypes:54 1) type A, corresponding to primary OT without evidence of dopaminergic deficit; 2) type B, corresponding to primary OT with dopaminergic deficits but without signs of parkinsonism; and 3) type C, corresponding to OT associated with PD.
Conclusion
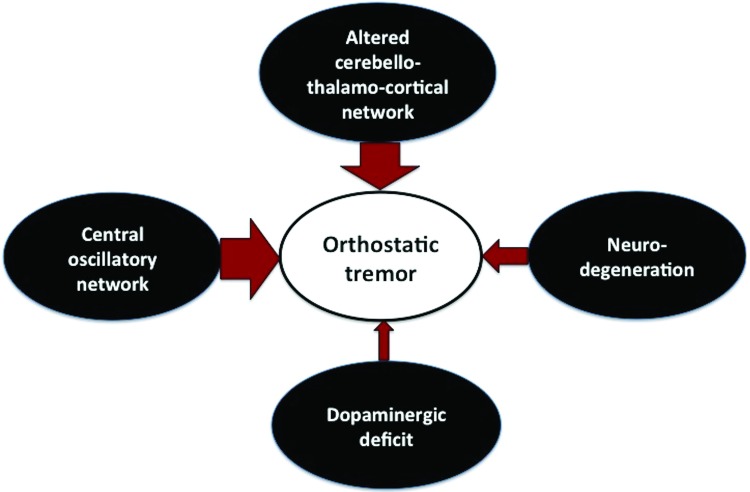
Primary OT is a rare and complex movement disorder, which is associated with a poor quality of life. Several neuroimaging studies using a variety of modalities have examined the neural correlates of primary OT; however, the exact pathogenesis of this disease remains elusive. The majority of studies have implicated the role of cerebellum and/or its connections in the genesis of tremor in primary OT and have posited the presence of a central oscillatory network. The exact location of the central oscillator is not fully clear; however, the posterior fossa appears to be a candidate in many studies. So far, only one study using MRS has provided evidence that OT could be a cerebellar neurodegenerative disorder and this notion needs to be confirmed by further studies. In addition, a dopaminergic deficit may be one of the neurobiological underpinnings in a subset of patients with OT. To summarize, three mechanisms related to the pathogenesis of OT, i.e. an aberrant central oscillator involving the cerebello-thalamo-cortical network, cerebellar neurodegeneration, and a dopaminergic deficit, have been suggested by previous studies (Figure 2, Table 3). It is difficult to ascertain whether these putative mechanisms overlap or they are mutually exclusive in primary OT and this may be an interesting question for future research on primary OT. Considering the rarity of this disorder, additional studies, and especially those based on neuropathology (of which there are currently none), are warranted in order to gain further insights into the pathogenesis of primary OT.
Figure 2. Summary of the Observations from the Studies Exploring the Pathogenesis of Primary Orthostatic Tremor. Major theories regarding the pathogenesis of primary orthostatic tremor (The thicker the arrow, the stronger the evidence).
Table 3. Take-home Messages.
| Primary orthostatic tremor (OT) is an enigmatic movement disorder, the pathogenesis of which is not fully understood |
| Neuroimaging studies indicate structural and functional abnormalities in the cerebellum and/or its connections |
| Electrophysiological studies suggest the presence of an aberrant central oscillator involving the cerebello-thalamo-cortical network, with a central oscillator possibly in the posterior fossa |
| Speculation that primary OT is associated with cerebellar neurodegeneration comes from a single magnetic resonance spectroscopy study |
| Although controversial, a dopaminergic deficit may occur in a subset of patients with primary OT |
| The aforementioned mechanisms may not be mutually exclusive and they may overlap in some of the patients |
| Further studies, especially those based on neuropathological examination, are needed to gain better insights into the pathogenesis of primary OT |
Footnotes
Funding: None.
Financial Disclosures: Dr. Lenka is sponsored by the Indian Council of Medical Research (ICMR, New Delhi) for his MD-PhD (Clinical Neurosciences) fellowship at the National Institute of Mental Health and Neurosciences, Bangalore, India. Dr. Pal has received grants from the Indian Council of Medical Research (ICMR, New Delhi), Department of Science and Technology (DST) and Department of Biotechnology of the Government of India. Dr. Bhatti is in the speaker panel for Teva Neurosciences and Accadia Pharmaceuticals, in the advisory board of Abbvie and Merz Pharmaceuticals. He is the phase IV study site PI for Abbvie funded trial. He has internal funding grants from University of Nebraska Medical Center for Driving in PD research; Co-PI in OT balance and coordination study that is funded by Nebraska OT Research Fund and grants from Nebraska Medicine and University of Nebraska Medical Center. Dr. Louis has received research support from the National Institutes of Health: NINDS #R01 NS094607 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #R01 NS046436 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator) and NINDS #R01 NS088257 (principal investigator). He has also received support from the Claire O’Neil Essential Tremor Research Fund (Yale University).
Conflicts of Interest: The authors report no conflict of interest.
Ethics Statement: Not applicable for this category of article.
References
- 1.Heilman KM. Orthostatic tremor. Arch Neurol. 1984;41:880–881. doi: 10.1001/archneur.1984.04050190086020. doi: 10.1001/archneur.1984.04050190086020. [DOI] [PubMed] [Google Scholar]
- 2.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13((Suppl. 3)):2–23. doi: 10.1002/mds.870131303. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 3.Erro R, Antelmi E, Bhatia KP. “A disorder which occurs on standing”: the earliest account of orthostatic tremor by Pazzaglia. Mov Disord Clin Pract. 2015;2:39–40. doi: 10.1002/mdc3.12127. doi: 10.1002/mdc3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerschlager W, Brown P. Orthostatic tremor – a review. Handb Clin Neurol. 2011;100:457–462. doi: 10.1016/B978-0-444-52014-2.00035-5. doi: 10.1016/B978-0-444-52014-2.00035-5. [DOI] [PubMed] [Google Scholar]
- 5.DeOrchis VS, Geyer HL, Herskovitz S. Teaching video neuroimages: orthostatic tremor: the helicopter sign. Neurology. 2013;80:e161. doi: 10.1212/WNL.0b013e31828ab301. doi: 10.1212/WNL.0b013e31828ab301. [DOI] [PubMed] [Google Scholar]
- 6.van Gerpen JA. Shaky legs: more than meets the eyes (or ears) Parkinsonism Relat Disord. 2015;21:162. doi: 10.1016/j.parkreldis.2014.10.030. doi: 10.1016/j.parkreldis.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Benito-Leon J, Domingo-Santos A. Orthostatic tremor: an update on a rare entity. Tremor Other Hyperkinet Mov. 2016:6. doi: 10.7916/D81N81BT. doi: 10.7916/d81n81bt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papa SM, Gershanik OS. Orthostatic tremor: an essential tremor variant? Mov Disord. 1988;3:97–108. doi: 10.1002/mds.870030201. doi: 10.1002/mds.870030201. [DOI] [PubMed] [Google Scholar]
- 9.FitzGerald PM, Jankovic J. Orthostatic tremor: an association with essential tremor. Mov Disord. 1991;6:60–64. doi: 10.1002/mds.870060111. doi: 10.1002/mds.870060111. [DOI] [PubMed] [Google Scholar]
- 10.Britton TC, Thompson PD, van der Kamp W, Rothwell JC, Day BL, Findley LJ, et al. Primary orthostatic tremor: further observations in six cases. J Neurol. 1992;239:209–217. doi: 10.1007/BF00839142. doi: 10.1007/BF00839142. [DOI] [PubMed] [Google Scholar]
- 11.Piboolnurak P, Yu QP, Pullman SL. Clinical and neurophysiologic spectrum of orthostatic tremor: case series of 26 subjects. Mov Disord. 2005;20:1455–1461. doi: 10.1002/mds.20588. doi: 10.1002/mds.20588. [DOI] [PubMed] [Google Scholar]
- 12.McAuley JH, Britton TC, Rothwell JC, Findley LJ, Marsden CD. The timing of primary orthostatic tremor bursts has a task-specific plasticity. Brain. 2000;123((Pt 2)):254–266. doi: 10.1093/brain/123.2.254. doi: 10.1093/brain/123.2.254. [DOI] [PubMed] [Google Scholar]
- 13.Ganos C, Maugest L, Apartis E, Gasca-Salas C, Cáceres-Redondo MT, Erro R, et al. The long-term outcome of orthostatic tremor. J Neurol Neurosurg Psychiatry. 2016;87:167–172. doi: 10.1136/jnnp-2014-309942. doi: 10.1136/jnnp-2014-309942. [DOI] [PubMed] [Google Scholar]
- 14.Benito-León J, Louis ED, Puertas-Martín V, Romero JP, Matarazzo M, Molina-Arjona JA, et al. Cognitive and neuropsychiatric features of orthostatic tremor: a case-control comparison. J Neurol Sci. 2016;361:137–143. doi: 10.1016/j.jns.2015.12.031. doi: 10.1016/j.jns.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidailhet M, Roze E, Maugest L, Gallea C. Lessons I have learned from my patients: everyday life with primary orthostatic tremor. J Clin Mov Disord. 2017;4:1. doi: 10.1186/s40734-016-0048-5. doi: 10.1186/s40734-016-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerschlager W, Katzenschlager R, Schrag A, Lees AJ, Brown P, Quinn N, et al. Quality of life in patients with orthostatic tremor. J Neurol. 2003;250:212–215. doi: 10.1007/s00415-003-0980-9. doi: 10.1007/s00415-003-0980-9. [DOI] [PubMed] [Google Scholar]
- 17.Yaltho TC, Ondo WG. Orthostatic tremor: a review of 45 cases. Park Relat Disord. 2014;20:723–725. doi: 10.1016/j.parkreldis.2014.03.013. doi: 10.1016/j.parkreldis.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Setta F, Jacquy J, Hildebrand J, Manto MU. Orthostatic tremor associated with cerebellar ataxia. J Neurol. 1998;245:299–302. doi: 10.1007/s004150050222. doi: 10.1007/s004150050222. [DOI] [PubMed] [Google Scholar]
- 19.Wills AJ, Thompson PD, Findley LJ, Brooks DJ. A positron emission tomography study of primary orthostatic tremor. Neurology. 1996;46:747–752. doi: 10.1212/wnl.46.3.747. doi: 10.1212/WNL.46.3.747. [DOI] [PubMed] [Google Scholar]
- 20.Schöberl F, Feil K, Xiong G, Bartenstein P, la Fougére C, Jahn K, et al. Pathological ponto-cerebello-thalamo-cortical activations in primary orthostatic tremor during lying and stance. Brain. 2017;140((Pt 1):83–97. doi: 10.1093/brain/aww268. doi: 10.1093/brain/aww268. [DOI] [PubMed] [Google Scholar]
- 21.Benito-León J, Louis ED, Mato-Abad V, Dydak U, Álvarez-Linera J, Hernández-Tamames JA, et al. In vivo neurometabolic profiling in orthostatic tremor. Medicine (Baltimore) 2016;95:e4848. doi: 10.1097/MD.0000000000004848. doi: 10.1097/MD.0000000000004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benito-León J, Louis ED, Manzanedo E, Hernández-Tamames JA, Álvarez-Linera J, Molina-Arjona JA, et al. Resting state functional MRI reveals abnormal network connectivity in orthostatic tremor. Medicine (Baltimore) 2016;95:e4310. doi: 10.1097/MD.0000000000004310. doi: 10.1097/MD.0000000000004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallea C, Popa T, García-Lorenzo D, Valabregue R, Legrand A, Apartis E, et al. Orthostatic tremor: a cerebellar pathology? Brain. 2016;139:2182–2197. doi: 10.1093/brain/aww140. doi: 10.1093/brain/aww140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins IH, Bain PG, Colebatch JG, Thompson PD, Findley LJ, Frackowi RSJ, et al. A positron emission tomography study of essential tremor: evidence for overactivity of cerebellar connections. Ann Neurol. 1993;34:82–90. doi: 10.1002/ana.410340115. doi: 10.1002/ana.410340115. [DOI] [PubMed] [Google Scholar]
- 25.Lenka A, Bhalsing KS, Panda R, Jhunjhunwala K, Naduthota RM, Saini J, et al. Role of altered cerebello-thalamo-cortical network in the neurobiology of essential tremor. Neuroradiology. 2017;59:157–168. doi: 10.1007/s00234-016-1771-1. doi: 10.1007/s00234-016-1771-1. [DOI] [PubMed] [Google Scholar]
- 26.Benito-León J, Louis ED, Romero JP, Hernández-Tamames JA, Manzanedo E, Álvarez-Linera J, et al. Altered functional connectivity in essential tremor: a resting-state fMRI study. Medicine (Baltimore) 2015;94:e1936. doi: 10.1097/MD.0000000000001936. doi: 10.1097/MD.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espay AJ, Duker AP, Chen R, Okun MS, Barrett ET, Devoto J, et al. Deep brain stimulation of the ventral intermediate nucleus of the thalamus in medically refractory orthostatic tremor: preliminary observations. Mov Disord. 2008;23:2357–2362. doi: 10.1002/mds.22271. doi: 10.1002/mds.22271. [DOI] [PubMed] [Google Scholar]
- 28.Lehn AC, Olson S, Salari M, Hospital PA, Coast G, Hospital PA. Case reports thalamic ventral intermediate nucleus deep brain stimulation for orthostatic tremor. Tremor Other Hyperkinet Mov. 2017;7 doi: 10.7916/D8280JHR. doi: 10.7916/D8280JHR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyons MK, Behbahani M, Boucher OK, Caviness JN, Evidente VGH. Orthostatic tremor responds to bilateral thalamic deep brain stimulation. Tremor Other Hyperkinet Mov. 2012;2 doi: 10.7916/D8TQ608K. doi: 10.7916/D8TQ608K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaltho TC, Ondo WG. Thalamic deep brain stimulation for orthostatic tremor. Tremor Other Hyperkinet Mov. 2011:1. doi: 10.7916/D8NZ86C1. doi: 10.7916/D8NZ86C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merola A, Fasano A, Hassan A, Ostrem JL, Contarino MF, Lyons M, et al. Thalamic deep brain stimulation for orthostatic tremor: a multicenter international registry. Mov Disord. 2017;32:1240–1244. doi: 10.1002/mds.27082. doi: 10.1002/mds.27082. [DOI] [PubMed] [Google Scholar]
- 32.Köster B, Lauk M, Timmer J, Poersch M, Guschlbauer B, Deuschl G, et al. Involvement of cranial muscles and high intermuscular coherence in orthostatic tremor. Ann Neurol. 1999;45:384–388. doi: 10.1002/1531-8249(199903)45:3<384::aid-ana15>3.0.co;2-j. doi: 10.1002/1531-8249(199903)45:3<384::AID-ANA15>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 33.McAuley JH, Britton TC, Rothwell JC, Findley LJ, Marsden CD. The timing of primary orthostatic tremor bursts has a task-specific plasticity. Brain. 2000;123:254–266. doi: 10.1093/brain/123.2.254. doi: 10.1093/brain/123.2.254. [DOI] [PubMed] [Google Scholar]
- 34.Wu YR, Ashby P, Lang AE. Orthostatic tremor arises from an oscillator in the posterior fossa. Mov Disord. 2001;16:272–279. doi: 10.1002/mds.1045. doi: 10.1002/mds.1045. [DOI] [PubMed] [Google Scholar]
- 35.Kiziltan ME, Gündüz A, Kiziltan G, Sayilir T, Benbir G, Uyanik Ö. Acoustic startle response in patients with orthostatic tremor. Neurosci Lett. 2012:525. doi: 10.1016/j.neulet.2012.07.060. doi: 10.1016/j.neulet.2012.07.060. [DOI] [PubMed] [Google Scholar]
- 36.Grosse P, Brown P. Acoustic startle evokes bilaterally synchronous oscillatory EMG activity in the healthy human. J Neurophysiol. 2003;90:1654–1661. doi: 10.1152/jn.00125.2003. doi: 10.1152/jn.00125.2003. [DOI] [PubMed] [Google Scholar]
- 37.Norton JA, Wood DE, Day BL. Is the spinal cord the generator of 16-Hz orthostatic tremor? Neurology. 2004;62:632–634. doi: 10.1212/wnl.62.4.632. doi: 10.1212/WNL.62.4.632. [DOI] [PubMed] [Google Scholar]
- 38.Krauss JK, Weigel R, Blahak C, Bäzner H, Capelle H-H, Grips E, et al. Chronic spinal cord stimulation in medically intractable orthostatic tremor. J Neurol Neurosurg Psychiatry. 2006;77:1013–6. doi: 10.1136/jnnp.2005.086132. doi: 10.1136/jnnp.2005.086132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muthuraman M, Hellriegel H, Paschen S, Hofschulte F, Reese R, Volkmann J, et al. The central oscillatory network of orthostatic tremor. Mov Disord. 2013;28:1424–1430. doi: 10.1002/mds.25616. doi: 10.1002/mds.25616. [DOI] [PubMed] [Google Scholar]
- 40.Feil K, Böttcher N, Guri F, Krafczyk S, Schöberl F, Zwergal A, et al. Long-term course of orthostatic tremor in serial posturographic measurement. Park Relat Disord. 2015;21:905–910. doi: 10.1016/j.parkreldis.2015.05.021. doi: 10.1016/j.parkreldis.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 41.Pagan FL, Butman JA, Dambrosia JM, Hallett M. Evaluation of essential tremor with multi-voxel magnetic resonance spectroscopy. Neurology. 2003;60:1344–1347. doi: 10.1212/01.wnl.0000065885.15875.0d. doi: 10.1212/01.WNL.0000065885.15875.0D. [DOI] [PubMed] [Google Scholar]
- 42.Louis ED, Shungu DC, Chan S, Mao X, Jurewicz EC, Watner D. Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neurosci Lett. 2002;333:17–20. doi: 10.1016/s0304-3940(02)00966-7. doi: 10.1016/S0304-3940(02)00966-7. [DOI] [PubMed] [Google Scholar]
- 43.Louis ED. Essential tremors a family of neurodegenerative disorders? Arch Neurol. 2009;66:1202–1208. doi: 10.1001/archneurol.2009.217. doi: 10.1001/archneurol.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benito-León J. Essential tremor: a neurodegenerative disease? Tremor Other Hyperkinet Mov. 2014;4 doi: 10.7916/D8765CG0. doi: 10.7916/D8765CG0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wills AJ, Brusa L, Wang HC, Brown P, Marsden CD. Levodopa may improve orthostatic tremor: case report and trial of treatment. J Neurol Neurosurg Psychiatry. 1999;66:681–684. doi: 10.1136/jnnp.66.5.681. doi: 10.1136/jnnp.66.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finkel MF. Pramipexole is a possible effective treatment for primary orthostatic tremor (shaky leg syndrome) Arch Neurol. 2000;57:1519–20. doi: 10.1001/archneur.57.10.1519. doi: 10.1001/archneur.57.10.1519. [DOI] [PubMed] [Google Scholar]
- 47.Gerschlager W, Münchau A, Katzenschlager R, Brown P, Rothwell JC, Quinn N, et al. Natural history and syndromic associations of orthostatic tremor: a review of 41 patients. Mov Disord. 2004;19:788–795. doi: 10.1002/mds.20132. doi: 10.1002/mds.20132. [DOI] [PubMed] [Google Scholar]
- 48.De Bie RMA, Chen R, Lang AE. Orthostatic tremor in progressive supranuclear palsy. Mov Disord. 2007;22:1192–1194. doi: 10.1002/mds.21434. doi: 10.1002/mds.21434. [DOI] [PubMed] [Google Scholar]
- 49.Apartis E, Tison F, Arné P, Jedynak CP, Vidailhet M. Fast orthostatic tremor in Parkinson’s disease mimicking primary orthostatic tremor. Mov Disord. 2001;16:1133–1136. doi: 10.1002/mds.1218. doi: 10.1002/mds.1218. [DOI] [PubMed] [Google Scholar]
- 50.Leu-Semenescu S, Roze E, Vidailhet M, Legrand A, Trocello J, Cochen V, et al. Myoclonus or tremor in orthostatism: an under-recognized cause of unsteadiness in Parkinson’s disease. Mov Disord. 2007;22:2063–2069. doi: 10.1002/mds.21651. doi: 10.1002/mds.21651. [DOI] [PubMed] [Google Scholar]
- 51.Katzenschlager R, Costa D, Gerschlager W, O’Sullivan J, Zijlmans J, Gacinovic S, et al. [123I]-FP-CIT-SPECT demonstrates dopaminergic deficit in orthostatic tremor. Ann Neurol. 2003;53:489–496. doi: 10.1002/ana.10475. doi: 10.1002/ana.10475. [DOI] [PubMed] [Google Scholar]
- 52.Spiegel J, Behnke S, Fuss G, Becker G, Dillmann U. Echogenic substantia nigra in patients with orthostatic tremor. J Neural Transm. 2005;112:915–920. doi: 10.1007/s00702-004-0236-6. doi: 10.1007/s00702-004-0236-6. [DOI] [PubMed] [Google Scholar]
- 53.Berg D, Siefker C, Becker G. Echogenicity of the substantia nigra in Parkinson’s disease and its relation to clinical findings. J Neurol. 2001;248:684–689. doi: 10.1007/s004150170114. doi: 10.1007/s004150170114. [DOI] [PubMed] [Google Scholar]
- 54.Trocello JM, Zanotti-Fregonara P, Roze E, Apartis E, Legrand AP, Habert MO, et al. Dopaminergic deficit is not the rule in orthostatic tremor. Mov Disord. 2008;23:1733–1738. doi: 10.1002/mds.22224. doi: 10.1002/mds.22224. [DOI] [PubMed] [Google Scholar]
- 55.Raudino F, Muscia F, Osio M. Orthostatic tremor and I123-FP-CIT-SPECT: report of a case. Neurol Sci. 2009;30:365–366. doi: 10.1007/s10072-009-0105-z. doi: 10.1007/s10072-009-0105-z. [DOI] [PubMed] [Google Scholar]
- 56.Wegner F, Strecker K, Boeckler D, Wagner A, Preul C, Lobsien D, et al. Intact serotonergic and dopaminergic systems in two cases of orthostatic tremor. J Neurol. 2008;255:1840–1842. doi: 10.1007/s00415-008-0023-7. doi: 10.1007/s00415-008-0023-7. [DOI] [PubMed] [Google Scholar]