Introduction
Chronic rhinosinusitis (CRS) is a common but heterogeneous disease process defined by local inflammation of sinuses and upper airway with persistent symptoms for 12 weeks.1 CRS affects 10.9% of populations in Europe and 12.5% in the United States.2 Symptoms include, but are not limited to, nasal blockage, congestion, rhinorrhea, smell dysfunction, and facial pain or pressure. CRS is clinically categorized into two major phenotypes based on endoscopic findings: CRS with nasal polyposis (CRSwNP) and CRS without nasal polyposis (CRSsNP). Different T cell-dependent mechanisms are proposed to underlie CRSsNP (predominantly Th1 inflammation with high levels of IFNγ and TGFβ and CRSwNP (Th2 inflammation with high levels of IL5 and IgE).3 In addition to the putative role of IgE in some CRS subtypes, the contributions of other antibody isotypes, particularly IgD, are less well characterized.
Humans express five classes of immunoglobulins (IgM, IgD, IgG, IgA and IgE). Unlike the other isotypes, the anatomic distribution and function of IgD and the plasma cells that produce this isotype are incompletely understood. The evolutionary origins of IgD extend back to teleost fishes.5 IgD is expressed in primates, dog, mouse, and rat, but not in several other species6,7 and was initially identified in humans in 1965.4 Recognized primarily for its expression on the surface membrane of naive and several low-prevalence B cell subsets, the functions of the secreted protein are not well defined.8
Clues to the specificity and activity of IgD derive from its relatively selective expression in plasma cells in the upper respiratory mucosa (e.g., tonsil and nasal mucosa)8, and its increased levels with chronic tonsillitis.9 Moreover, the binding of surface IgD on naïve B cells by proteins from respiratory pathogens, e.g. Haemophilus influenzae and Moraxella catarrhalis, drives T cell-independent polyclonal B cell activation.10,11 Such non-specific activation may serve as a bacterial strategy to subvert antigen-specific antibody responses that might otherwise help control these organisms to nonspecific polyclonal responses.10 Secreted IgD binds to viruses (rubella and measles), bacteria (Escherichia coli, specific surface proteins on H. influenzae and M. catarrhalis), and selected bacterial virulence factors (e.g., streptolysin O from S. pyogenes, diphtheria toxin).12, 13 Allergic inflammation can also promote binding of IgD to granulocytes such as neutrophils and eosinophils in the skin.14, 15
The IgD expressed as a transmembrane receptor on mature naïve B cells is generated by alternative splicing of pre-mRNA containing both IgM and IgD heavy chain constant regions. IgD is also produced by plasma cells and secreted into mucosal fluids or serum.7 Most class switching from IgM+IgD+ naïve cells to IgG, IgM, IgA or IgE occurs in the germinal centers of lymphoid follicles. However, whether naïve B cells differentiate into IgD-producing plasma cells without typical class switch is less clear and may involve somewhat different mechanisms.16 Whether germinal centers are present in the sinus mucosa to support classical class switch mediated by activation-induced cytidine deaminase (AID) and other related mediators is also controversial.8,33
In summary, IgD is increased in tonsillar tissue of subjects with chronic tonsillitis9 but whether these findings extend to effector sites, such as the sinuses, during chronic disease is unknown. Upper respiratory pathogens can selectively activate IgD+ B cells in vitro,10 and IgD may induce antimicrobial and proinflammatory responses in basophils.8 In addition, the two forms of chronic rhinosinusitis likely result from varying inflammatory milieu in the mucosa3. Therefore, we studied the differential expression of IgD plasma cells in sinus tissues and protein in secretions of adults with CRSsNP, CRSwNP, and control subjects to evaluate the expression of IgD during CRS using human sinus tissue and secretions.
Methods
Human Subjects
Written informed consent was obtained from patients as approved by the Colorado Multi-Institution Review Board (COMIRB 11-1442). Ethmoid sinus tissue and middle meatal swabs were obtained from patients diagnosed with CRSwNP, CRSsNP subjects, and control subjects (n= 6 each) during endoscopic sinus surgery performed for standard clinical indications (Tables 1a and 1b).17 Control subjects were undergoing endoscopic approaches for skull base surgery or dacrocystorhinostomy (DCR) for epiphora, without endoscopic or radiographic evidence of inflammation. CT scans and nasal endoscopy were scored according to the Lund-McKay and Lund-Kennedy scoring criteria, respectively.18,19 Nasal swabs (BD CultureSwabs, Franklin Lakes, NJ) of the middle meatus were tested for total immunoglobulins by ELISA from nine control subjects, eleven patients with CRSsNP, and thirteen patients with CRSwNP.
Table 1.
Clinical Characteristics of Two Groups of Control Subjects and Patients with Chronic Rhinosinusitis with (CRSwNP) and without Polyps (CRSsNP)
| Table 1A: Subjects tested for plasma cells in sinus tissue by immunohistochemistry. | |||
|---|---|---|---|
| Control | CRSsNP | CRSwNP | |
| No. of Subjects | 6 | 6 | 6 |
| Age Mean +/− SD | 51.2 +/− 14.9 | 51 +/− 13.8 | 42.8 +/− 20.0 |
| Gender M/F | 2/4 | 3/3 | 1/5 |
| Aspirin Sensitivity (%) | 0(0) | 1(16.7) | 2(33.3) |
| Allergic Rhinitis (%) | 1(16.7) | 3(50) | 4(66.7) |
| Asthma (%) | 0(0) | 3(50) | 5(83.3) |
| CT Score# Mean +/− SD | 0 +/− 0 | 9.67 +/− 6.8 | 18.33 +/− 4.6 |
| Endoscopy Score*Mean +/− SD | 0 +/− 0 | 3.67 +/− 3.3 | 7.8 +/− 2.8 |
| Table 1B. Subjects tested for immunoglobulin levels in sinus secretion by ELISA. | |||
|---|---|---|---|
| B: ELISA | Control | CRSsNP | CRSwNP |
| No. of Subjects | 9 | 11 | 13 |
| Age Mean +/− SD | 40.7 +/− 16.1 | 49 +/− 11.2 | |
| Gender M/F | 4/5 | 6/5 | 7/6 |
| Aspirin Sensitivity (%) | 0(0) | 0(0) | 5(38.4) |
| Allergic Rhinitis (%) | 4(44.4) | 2(18.2) | 9(69.2) |
| Asthma (%) | 3(33.3) | 3(27.3) | 10(76.9) |
| CT Score# Mean +/− SD | 1.1 +/− 3.2 | 3.7 +/− 2.7 | 9.6 +/− 6.7 |
| Endoscopy Score* Mean +/− SD | 0.3 +/− 0.7 | 4.6 +/− 3.5 | 13.9 +/− 6.4 |
CT score: The Lund-Mackay score is a widely used method for radiologic staging of CRS. A score of 0 (no abnormality), 1 (partial opacification), or 2 (complete opacification) is assigned to each of the sinuses, with a maximum score of 24. The ostiomeatal complex is assigned a score of either 0 (not obstructed) or 2 (obstructed). See reference 18.
Endoscopy score: The Lund-Kennedy endoscopic scoring system quantifies the pathologic states of the nose and paranasal sinuses, focusing on the presence of polyps, discharge, edema, scarring, or adhesions and crusting. Scores range from 0 to 20. Polyps are graded as absent (0), present in the middle meatus (1), or present beyond the middle meatus (3). Discharge is graded as not present (0), thin (1), or thick and purulent (2). Edema, scarring, and crusting are each graded as absent (0), mild (1), or severe (2). See reference 19.
Immunohistochemistry
Immunohistochemistry was performed as recently described.20 Briefly, tissue specimens were paraffin-embedded and fixed onto glass slides in 4 micrometer sections. Deparaffinization was performed using mixed xylenes, followed by rehydration using decreasing concentration of ethanol (EtOH). High temperature antigen retrieval was then performed using a citric acid buffer at pH of 6.5. Slides were blocked with 10% normal goat serum x 1 hour, stained with a mixture of directly-labeled F(ab’)2 Goat Anti-Human IgD (Alexa Fluor (R) 647- conjugated; 1:100), IgA (Alexa Fluor (R) 488; 1:100), and IgM (Alexa Fluor(R) 594; 1:100) (Jackson Immunology, West Grove, PA, USA). After DAPI nuclear staining, mounting media was applied with cover-slips. Separate slides were co-stained for IgD and CD138 (Alexa Fluor(R) 594- conjugated AffiniPure F(ab’)2 Fragment Goat Anti-Human CD138) (Jackson Immunology, West Grove, PA, USA) to verify presence of plasma cells. For H&E staining to identify the presence of epithelium, WBC, cilia, and lymphoid aggregates or follicles, cryostat sections were prepared and mounted on frosted glass slides in 4 micrometer sections and stored at −40C until staining.
Image processing and cell counting
Slides were scanned using an Olympus VS120 scanner outfitted with an OrcaR2 camera (Olympus; Center Valley, PA). Image processing was performed using the Olympus VS-ASW FL2.7 software ((c) Olympus Corporation 2004–2013). We identified 10 random regions of interest (ROIs) containing epithelium and lamina propria in each slide, each 0.1735mm2 in area. Image channels were separated into DAPI, Cy5, FITC and TRITC only images and uploaded into the Cellprofiler cell image analysis software v 2.1.1 (Broad Imaging Institute, Cambridge MA). The frequencies of DAPI+, IgD+, IgM+, and IgA+ cells and the proportions of each cell types were calculated per mm2 by dividing cell count by the area of the ROI, and isotype by total DAPI+ cells, respectively. Manual confirmation of these counts was then performed using ImageJ, a freely available java-based public-domain image processing and analysis program developed at the National Institutes of Health (NIH).
Statistical Analysis
Values for the 3 groups were compared by one-way ANOVA of counts per mm2. Those with significant differences were then compared by 2-group comparisons with unpaired t-tests were performed using GraphPad Prism version 6.00 (GraphPad Software; La Jolla, CA). Data are presented as scatter plots of average counts per mm2 per subject with bars representing mean values. Statistical significance was set at α= 0.01.
Total Antibody in secretions by Enzyme Linked Immunosorbent Assay (ELISA)
We coated Nunc Maxisorp (R) microtiter plates (eBioscience; San Diego USA) with goat anti-human IgA, G, M (Jackson Immunology, West Grove, PA, USA), and D (Southern Biotech; Birmingham, AL, USA) in carbonate buffer pH 9.6 overnight at four degrees. Nasal swabs were diluted in 400uL of PBS and serially diluted with standards in duplicates for two hours at room temperature. We detected levels of antibodies with HRPO-labeled affinity purified goat anti-human IgA, G, M and D at one hour at room temperature. 3,3′,5,5′-Tetramethylbenzidine (TMB) was used as the developer and plates where read at 450 nm. Total protein levels were determined by Pierce BCA protein assay (ThermoFisher Scientific; Waltham, MA USA). We then coated the microtiter plates with rabbit anti-human albumin (Dako; Santa Clara, CA, USA) in carbonate buffer pH 9.6 overnight at four degrees. Plates were blocked with 10% rabbit serum for two hours. Nasal swabs and standards were diluted in duplicates and incubated for two hours at room temperature. We detected levels of antibody with rabbit anti-human HRPO (Dako; Santa Clara, CA, USA) at one hour, at room temperature. TMB was used as the developer and plates were read at 450 nm.
Histologic Lymphoid Structure Analysis
6 control, 8 CRSsNP, and 13 CRSwNP slides stained with hematoxylin and eosin (H&E) were independently evaluated by a board certified hematopathologist (QN). Surfaces lined by ciliated pseudostratified, columnar epithelium including identifiable mucocytes (goblet cells) were referered to as the Schneiderian membrane with normal histology. CRSsNP was defined by submucosal edema with mixed inflammatory cells, including mature lymphocytes with variable plasma cells, eosinophils, histiocytes, and neutrophils. CRSwNP was characterized by respiratory epithelium with variable squamous metaplasia, basement membrane thickening, edematous stroma with mixed chronic inflammatory infiltrate, predominantly composed of eosinophils, plasma cells and lymphocytes, and characteristically, an absence of submucosal seromucous glands. Lymphoid aggregates were considered present when there was a dense and sizable (>50 lymphoid cells) aggregate with predominantly lymphoid cells (i.e. chronic inflammatory cells including plasma cells, eosinophils, histiocytes, where neutrophils constitute only a minor subset within the aggregate). All other lymphoid patterns are considered a part of the chronic inflammatory infiltrate. Germinal centers were considered present when conspicuous presence of centroblasts, centrocytes, small lymphocytes, tangible body macrophages, and follicular dendritic cells were identified within a lymphoid nodule. In order to examine whether there is a significant correlation between IgD+ plasma cells and degree of inflammation, the slides were graded on a 4 point scale of 0 to 3, with 0 being no inflammation, and 3 being highly inflamed.38 The amount of inflammation is judged based on the observed density of inflammatory cells, which include variable proportion of plasma cells, eosinophils, histiocytes, and neutrophils. The grade of inflammation was then correlated with the density of IgD+ plasma cell using Pearson correlation analysis.
Results
Patient Characteristics
Disease-related variables (presence of allergies, polyps, CT and endoscopy scores), but not age or gender, differed between the 6 control subjects, 6 patients with CRSsNP and 6 with CRSwNP from whom sinus tissue samples were obtained (Table 1A). Similarly, the demographics of the 3 groups from whom secreted antibodies were studied were comparable (Table 1B).
Localization of Plasma Cells in Sinus Tissues
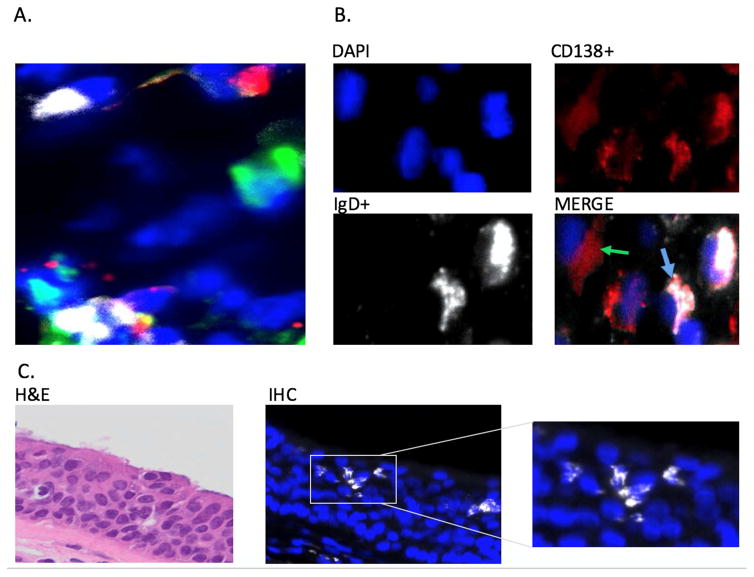
We identified discrete individual cells staining for cytoplasmic IgD, IgA, and IgM dispersed throughout the tissues (Figure 1A). No cells co-expressed multiple intracytoplasmic isotypes (e.g., cIgD and cIgM). The cytoplasmic localization of IgD and co-expression of IgD in the cytoplasm with CD138 (syndecan-1) on the cell surface confirmed that these IgD+ cells were consistent with plasma cells, rather than naïve B cells expressing surface IgD (Figure 1B). By manual counts, CD138+ cells in the lamina propria (non-epithelial cells) comprised a median of 29.4% (range 21.5–40.6%) of over 2500 DAPI+ cells from four random samples from three subjects with CRSwNP samples (609–742 cells/sample). Among these CD138+ lamina propria cells, the majority (median 70.0% [range 61.9–81.7%]) expressed cytoplasmic IgD, and virtually all cells with cytoplasmic IgD co-expressed CD138.
Figure 1.
A. Immunofluorescent triple staining for cells expressing cytoplasmic IgD (white), IgM (red), and IgA (green) in sinus tissue. Nuclei are stained with DAPI (blue). Results are shown from a representative patient with CRSsNP. Original magnification: 400x. B. Immunoflourescent staining showing co-expression of cytoplasmic IgD (white), plasma cell marker CD 138 (red) and nuclear staining (blue). Blue Arrow: IgD/CD138 Costaining. Green Arrow: CD138 without IgD staining. Results shown are representative of 18 samples. Original magnification: 400x. C. Localization of IgD plasma cells in the sinus epithelium. IgD+ plasma cells: White, DAPI nuclear stain: Blue. Inset: higher magnification showing morphology of IgD+ plasma cells Original magnification: 400x.
We excluded the possibility that IgD+ cells expressed only surface IgD rather than cytoplasmic IgD. Simultaneous staining of control tonsil tissue from adults showed a clear discrimination of surface IgD-staining cells surrounding germinal centers, whereas cells with cytoplasmic IgD were identified in the extrafollicular areas (Supplemental Figure 1). The surface IgD+ cells also expressed IgM, consistent with naïve B cells, whereas, as noted, no cell with cytoplasmic IgD expressed another isotype.
A unique feature of plasma cells with cytoplasmic IgD was their selective presence in the epithelium, whereas those with cytoplasmic IgA+ and IgM+ cells were absent in this venue (Figure 1C). We observed intraepithelial IgD+ cells consistent with plasma cells in 3/6 control subjects, 4/6 subjects with CRSsNP, and 3/6 subjects with CRSwNP. Intraepithelial IgD+ plasma cells could not be stained with antibody to CD138 due to co-expression of syndecan-1, the CD138 ligand for the antibody, on plasma cells and epithelial cells.20
Plasma cell frequencies
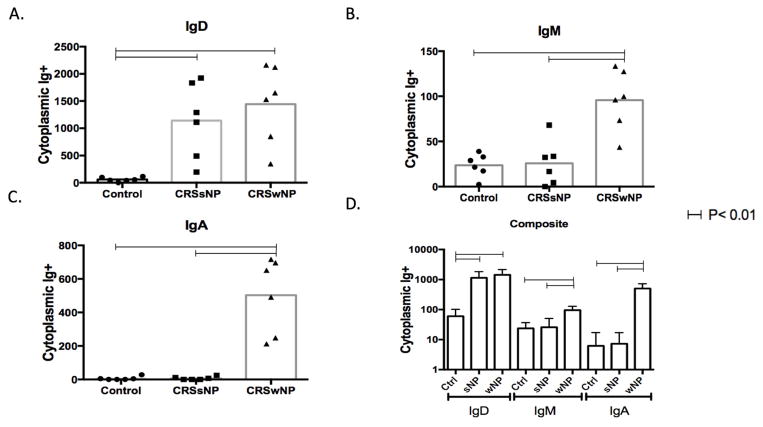
The densities of cells expressing cytoplasmic IgD in the sinus lamina propria exceeded those with IgM and IgA in each of the 3 patient groups (Figure 2C). The frequency of IgD+ plasma cells per mm2 was higher in both CRS groups compared with controls (p< 0.01) (Figure 2A). Unlike results with IgD, both IgM+ and IgA+ plasma cells were higher in those with CRSwNP compared with controls and with CRSsNP (p< 0.01). Values for the latter two groups were similar (Figure 2B and 2C). Thus, IgD+ plasma cells most consistently distinguished patients with CRS from controls.
Figure 2.
Frequencies of cytoplasmic Ig+ cells in sinus tissues. Cells expressing cytoplasmic IgD (A), IgA (B) and IgM (C) were detected by 4-color immunofluorescence in sinus tissues from healthy control subjects (circle), patients with CRSsNP (squares) and CRSwNP (triangles). Dots represent mean cell counts from 10 regions of interest per tissue sample and the box represents the median values. Horizontal bars represent statistical significance between groups by ANOVA. (p<0.01) Figure 2D represents a composite graph of IgD, IgM, IgA in a logarithm10 scale showing relative counts of the antibodies in each disease state. Horizontal bars represent statistical significance between two points. Error bars represent standard deviation. Statistical significance calculated by ANOVA.
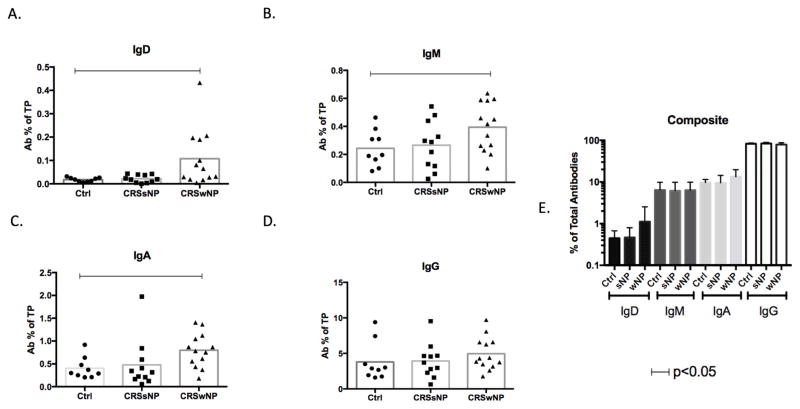
Mucus antibody levels
Secreted IgD was detected in the mucus of all subjects (Figure 3A). Unlike with plasma cells, IgG and IgA comprised the majority of locally secreted antibodies, rather than IgD, which was present in the lowest amounts of any isotype (Figure 3E). Consistent with results with plasma cells, levels of IgD, IgM and IgA were all significantly higher in CRSwNP than controls. In contrast to the relative distribution of plasma cells, levels of IgD protein were not increased, but were very similar in secretions from subjects with CRSsNP and controls, as were IgM and IgA. Secreted IgM and IgA were not significantly higher in CRSwNP compared with CRSsNP (Figure 3B, C). We found no differences in IgG, which comprised the majority of secreted antibodies, between the 3 groups (Figure 3D and 3E).
Figure 3.
Levels of antibodies in sinus tissue secretions eluted from swabs and meaured by ELISA. Values for IgD (A), IgA (B), IgM (C), and IgG (D) from each subject (one dot per subject) are normalized and expressed as antibody (Ab) percent of total protein (TP). Horizontal bars represent significance differences between two points calculated by ANOVA. (p< 0.05) Figure 3E represents a composite graph showing relative antibody values as % of total antibodies on a log10 scale.
Lymphoid structure by histology
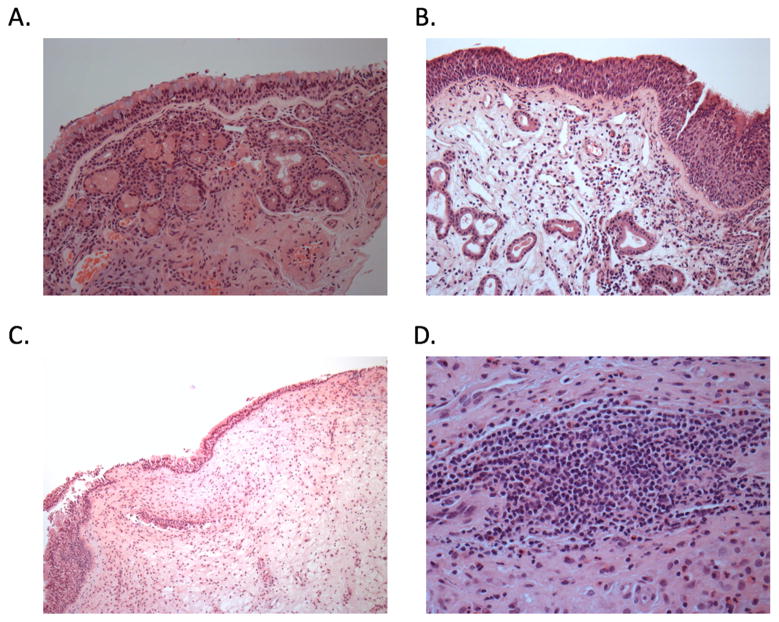
We surveyed sinus tissues for histologic evidence of lymphoid follicles and active germinal centers. Among 27 tissues reviewed by a hematopathologist (QN), fully organized germinal centers were not histologically identified in any sinus tissue, regardless of clinical diagnosis. However, non-organized lymphoid aggregates, which are typically collections of B cells without follicular structure, were identified in sinus tissues from a minority of subjects (1 of 6 control subjects (16.7%), 1 of 8 with CRSsNP (12.5%), and 6 of 13 with CRSwNP (46.2%)) (Fig 4D); p=0.19 (chi-square test). We observed a significant correlation between the frequency of cells with cytoplasmic IgD+ and the degree of inflammation (grades 0 to 3) (R=0.531, p=0.023) using Pearson correlation analysis.
Figure 4.
H&E staining of sinus tissue. A. Normal control: Normal sinonasal mucosa lined by ciliated pseudotratified, columnar epithelium with identifiable mucocytes (goblet cells) with underlying seromucous glands scattered throughout the submucosa. Magnification 200x B. CRSsNP: Submucosal edema with mixed inflammatory cells, including mature lymphocytes with variable plasma cells, eosinophils, histiocytes, and neutrophils. Magnification 200x. C. CRSwNP: The surface epithelium is composed of intact respiratory epithelium. The underlying stroma is markedly edematous and is noteworthy for the absence of seromucous glands. A mixed chronic inflammatory infiltrate is present and is predominantly composed of eosinophils, plasma cells, and lymphocytes. Magnification 100x D. Lymphoid follicle: Dense and sizable (>50 lymphoid cells) aggregate with predominantly lymphoid cells (i.e. other chronic inflammatory including plasma cells, eosinophils, histiocytes, and neutrophils cells constitute only a minor subset within the aggregate). All other lymphoid patterns are considered a part of the chronic inflammatory infiltrate. Magnification 400x.
Discussion
We have shown that cells expressing intracellular IgD represent a dominant isotype of antibody-secreting cells in the sinus tissue from healthy control subjects, as well as patients with two forms of CRS, compared with IgA or IgM. Although IgD can also be present on the surface of naive and other B cell subsets, such surface IgD+ cells were not identified in sinus tissue. The identification of IgD+ cells as being consistent with plasma cells in sinus tissues was confirmed by absence of IgD/IgM co-staining, cytoplasmic localization of the antibody and co-expression of CD138 (Syndecan-1) (Fig 1B). This cytoplasmic localization is distinct from the surface staining of IgD+ naïve cells in tonsil controls (Supplemental Fig 1). In the context of sinus pathology, increased frequencies of IgD+ plasma cells in the sinus tissue most consistently distinguished patients with CRS (with or without polyps) from control subjects.
In addition to their presence in the sinus lamina propria, only cells with cytoplasmic IgD+ were identified in the epithelial layer. However, we cannot exclude the presence of cells expressing cytoplasmic IgG and IgE that were not examined. Despite the high frequency of cells with cytoplasmic IgD in the lamina propria and epithelium, levels of IgD protein were quite low in secretions.
Consistent with the results of Hulse et.al.,24 IgA and IgM are both present at increased concentrations in patients with CRSwNP. These polymeric antibodies engage the polymeric Ig receptor (pIgR) on the basolateral surface of mucosal epithelial cells and undergo active receptor-mediated transport from lamina propria to lumen as secretory IgA (S-IgA) or S-IgM. pIgR expression and transport is upregulated in the presence of inflammation.27,28 IgG also may be actively transported across the epithelium by the neonatal Fc receptor (FcRN),29 which is prominently expressed in in the upper respiratory mucosa. In contrast, no specific or active mechanisms by which IgD may be transported to the lumen have been identified, which may explain its presence within the epithelial layer in the absence of other antibodies. Thus, the function of IgD may be most active within the sinus tissue rather than sinus secretions.
We identified high frequencies of IgD+ plasma cells in the lamina propria of sinus tissues from healthy adults. Increased frequencies of these cells in CRS suggests that pathologic stimulatory or regulatory mechanisms may enhance and drive their development. In contrast to our results, Cerruti and colleagues demonstrated that virtually none of the IgD+IgM- cells isolated in tonsils were positive for CD138.8 Unlike in the paper by Chen et al, cells that we localized were consistent with mature plasma cells, capable of secreting IgD antibodies into the lamina propria. One likely explanation for this is that IgD+ CD138- plasmablasts identified by Chen et.al in the tonsil could represent an earlier stage of differentiation. Given that most of the IgD+ cells that we identified were also CD138+, it is a possibility that IgD- switched cells differentiate elsewhere (e.g tonsils, local lymphoid tissue) and enter the sinus as a terminally differentiated population. Subjects with CRSwNP but not CRSsNP also showed greater numbers of IgA+ and IgM+ plasma cells, indicating that the pathogenesis of these two syndromes differ to some extent in the same target tissue.
Pathogen-specific functions of IgD are poorly characterized, but may depend largely on the antigen-binding Fab variable region of the molecule. Binding of bacterial surface antigens of upper respiratory pathogens by the exposed Fab fragments of IgD or IgM on naïve B cells may initiate B cell activation and drive antigen-specific antibody responses which could support agglutination or, potentially, phagocytosis. In organized germinal centers in secondary lymphoid tissues, such as tonsils (Supplemental Figure 1), T cell-dependent mechanisms may contribute prominently to this evolution. However, selective cross-linking of the constant region Fc of IgD by selective IgD-binding surface proteins on respiratory mucosal bacteria such as H. influenzae and M. catarrhalis10,11 may activate and initiate non-antigen-specific differentiation of these IgD+ cells into IgD+ plasma cells in the local environment. In the sinus, T cell-independent mechanisms may be sufficient to drive this process to generate IgD+ plasma cells in the sinus lamina propria. Extrafollicular T cell-independent mechanisms of B cell differentiation into IgD plasma cells may include internalization of H. influenzae into B cells to stimulate TLR9 as well as soluble BAFF and APRIL derived from IgD-activated basophils, each of which can also facilitate B cell activation alone or in combination.10 The presence of lymphoid aggregates, rather than follicles, in sinus tissue serves as evidence for local lymphocyte proliferation in the sinus tissue. We found such lymphoid aggregates in 46.3% of patients with CRSwNP (Fig 4D), consistent with a recent publication highlighting the presence of tertiary lymphoid organ-like structures in eosinophilic nasal polyps.32 A prior study by Tsurumaru et. al.33 identified immunologically activated lymphoid follicles in sinus tissue that produce plasma cells, as evidenced by the patterns of T and B lymphocyte subpopulations and follicular dendritic cells (FDCs).
Another isotype, IgE, has also been shown to be more prevalent in the mucosa of subjects with CRSwNP, and has been implicated in its pathogenesis.34 Baba et.al., demonstrated local increase in total IgE, as well as class switch recombination to IgE in nasal polyps in chronic rhinosinusitis. The Th2 inflammatory pathway, and increased concentrations of IL-5 and total IgE, are proposed to play a role in the pathogenesis of CRSwNP 35 That increased IgE levels are reported in CRSwNP, coupled with the current findings that cells with cytoplasmic IgG are also elevated in this population, suggests mature B cells may migrate and localize to polyp tissue. Factors that govern class-switch recombination and further tissue specific differentiation of plasma cells warrant further investigation. From a clinical context, further work is required to compare the relative prevalence of IgE+ and IgD+ plasma cells in normal controls, and subjects with different forms of CRS.
Potential limitations of this study include the inclusion of different study cohorts for immunohistochemistry and sinus fluid analyses. However, the study design limits potential bias by performing a second test (ELISA) on a different cohort in order to build on the findings in the initial immunostaining. Although automated cell counting software for enumeration of isotype-specific plasma cells in IHC (Cellprofiler) may generate inaccuracies, we verified the cell counts using a manual cell counting software (ImageJ), which showed 98% correlation of image counting. Despite the descriptive nature of this study with a somewhat small sample size (n=6–13 per group), we have shown consistent, statistically significant and biologically relevant differences between CRS and control subjects. Finally, we did not stain for IgG or IgE, as earlier studies have already demonstrated that IgG plasma cells are also prominent in sinus tissues.25
In summary, chronic rhinosinusitis is associated with increased expression of IgD plasma cells in tissue and secreted IgD. Current work in our laboratory is directed to determine whether sinus tissue-derived IgD+ plasma cells do or do not show evidence of antigen specificity. Both secreted antibodies and those that on the B cell surface may bind to local microbiota and/or mucosal pathogens based on specific binding of the Fab fragments to discrete bacterial antigens. Antibodies may also bind to IgD-binding proteins on the surface of e.g., H. influenzae or M. catarrhalis via the constant regions. Such binding by secreted IgD may serve as a “decoy” to sterically block more functional bacteria-specific antibodies.36,37 Binding by the constant regions on the surface of B cells could also activate B cells to secrete irrelevant antibodies, thereby diverting more effective specific antibody production. Determining the specificity and functions of IgD in sinus tissue is an intriguing and relevant target of our ongoing work to consider the mechanisms of IgD induction and the activity of these antibodies in health and disease.
Supplementary Material
Acknowledgments
Funding Sources: Research reported in this publication was supported by the National Institute On Deafness and Other Communication Disorders and the National Institute of Allergy and Infectious Diseases, of the National Institutes of Health (K23DC014747 (VRR) and R01AI108479 (ENJ)), by Flight Attendants Medical Research Institute grant CIA13006 (DNF) and the Veteran Affairs Research Service (Merit Review I01CX001464 to ENJ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor the Department of Veterans Affairs.
Footnotes
Level of Evidence: N/A (Basic Science Research)
Conflicts of interest: None of the authors have any conflicts of interests relevant to this study.
Contributions: MS conceived the study, performed the experiments, analyzed the data, and contributed to the manuscript; VRR conceived the study, analyzed the data, and contributed to the manuscript; JR and JMK designed and performed the experiments, and contributed to the manuscript; DNF performed the experiments and analyzed the data; AG and TTK recruited subjects for the study and contributed to the manuscript; QN performed histopathology experiments and contributed to the manuscript; ENJ conceived the study, supervised the performed experiments, analyzed the data, and contributed to the manuscript. All authors discussed the results and implications and contributed to the final manuscript.
Trial Registration: Not applicable
IRB-approved study (COMIRB protocol number 11-1442)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;(23):1–298. 3 p preceding table of contents. [PubMed] [Google Scholar]
- 2.Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011;128:693–707. doi: 10.1016/j.jaci.2011.08.004. quiz 708–699. [DOI] [PubMed] [Google Scholar]
- 3.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 4.ROWE DS, FAHEY JL. A NEW CLASS OF HUMAN IMMUNOGLOBULINS. I. A UNIQUE MYELOMA PROTEIN. J Exp Med. 1965;121:171–184. doi: 10.1084/jem.121.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson M, Bengtén E, Miller NW, Clem LW, Du Pasquier L, Warr GW. A novel chimeric Ig heavy chain from a teleost fish shares similarities to IgD. Proc Natl Acad Sci U S A. 1997;94:4593–4597. doi: 10.1073/pnas.94.9.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CL, Lehmeyer JE, Cooper MD. Evidence for an IgD homologue on chicken lymphocytes. J Immunol. 1982;129:2580–2585. [PubMed] [Google Scholar]
- 7.Martin LN, Leslie GA. In vivo effects of antiserum to IgD on surface immunoglobulins, serum immunoglobulins and lymphocyte blastogenesis in rhesus monkeys. Immunology. 1979;37:253–262. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen K, Xu W, Wilson M, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandtzaeg P, Surjan L, Berdal P. Immunoglobulin systems of human tonsils. I. Control subjects of various ages: quantification of Ig-producing cells, tonsillar morphometry and serum Ig concentrations. Clin Exp Immunol. 1978;31:367–381. [PMC free article] [PubMed] [Google Scholar]
- 10.Singh K, Nordström T, Mörgelin M, Brant M, Cardell LO, Riesbeck K. Haemophilus influenzae resides in tonsils and uses immunoglobulin D binding as an evasion strategy. J Infect Dis. 2014;209:1418–1428. doi: 10.1093/infdis/jit593. [DOI] [PubMed] [Google Scholar]
- 11.Möllenkvist A, Nordström T, Halldén C, Christensen JJ, Forsgren A, Riesbeck K. The Moraxella catarrhalis immunoglobulin D-binding protein MID has conserved sequences and is regulated by a mechanism corresponding to phase variation. J Bacteriol. 2003;185:2285–2295. doi: 10.1128/JB.185.7.2285-2295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salonen EM, Hovi T, Meurman O, Vesikari T, Vaheri A. Kinetics of specific IgA, IgD, IgE, IgG, and IgM antibody responses in rubella. J Med Virol. 1985;16:1–9. doi: 10.1002/jmv.1890160102. [DOI] [PubMed] [Google Scholar]
- 13.Luster MI, Armen RC, Hallum JV, Leslie GA. Measles virus-specific IgD antibodies in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1976;73:1297–1299. doi: 10.1073/pnas.73.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunyadi J, Hamerlinck F, Cormane RH. Immunoglobulin and complement bearing polymorphonuclear leukocytes in allergic contact dermatitis and psoriasis vulgaris. Br J Dermatol. 1976;94:417–422. doi: 10.1111/j.1365-2133.1976.tb06119.x. [DOI] [PubMed] [Google Scholar]
- 15.Dikeacou TC, van Joost T, Cormane RH. The recruitment of inflammatory cells using the skin-window technique. Arch Dermatol Res. 1979;265:1–7. doi: 10.1007/BF00412695. [DOI] [PubMed] [Google Scholar]
- 16.Rouaud P, Saintamand A, Saad F, et al. Elucidation of the enigmatic IgD class-switch recombination via germline deletion of the IgH 3′ regulatory region. J Exp Med. 2014;211:975–985. doi: 10.1084/jem.20131385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152:S1–S39. doi: 10.1177/0194599815572097. [DOI] [PubMed] [Google Scholar]
- 18.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31:183–184. [PubMed] [Google Scholar]
- 19.Lund VJ, Kennedy DW. Quantification for staging sinusitis. The Staging and Therapy Group. Ann Otol Rhinol Laryngol Suppl. 1995;167:17–21. [PubMed] [Google Scholar]
- 20.Gustafson CE, Higbee D, Yeckes AR, et al. Limited expression of APRIL and its receptors prior to intestinal IgA plasma cell development during human infancy. Mucosal Immunol. 2014;7:467–477. doi: 10.1038/mi.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pleil JD. QQ-plots for assessing distributions of biomarker measurements and generating defensible summary statistics. J Breath Res. 2016;10:035001. doi: 10.1088/1752-7155/10/3/035001. [DOI] [PubMed] [Google Scholar]
- 22.Rowe DS, Crabbé PA, Turner MW. Immunoglobulin D in serum, body fluids and lymphoid tissues. Clin Exp Immunol. 1968;3:477–490. [PMC free article] [PubMed] [Google Scholar]
- 23.Swierczynska Z, Wozniczko-Orlowska G, Maldyk H. An IgD myeloma protein with anti-streptolysin O activity. Immunochemistry. 1976;13:379–382. doi: 10.1016/0019-2791(76)90371-2. [DOI] [PubMed] [Google Scholar]
- 24.Hulse KE, Norton JE, Suh L, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131:1075–1083. 1083.e1071–1077. doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lü J, Liu HG. Expression and significance of IgG4 in inflammatory disease of nasal cavity and paranasal sinuses. Zhonghua Bing Li Xue Za Zhi. 2013;42:386–391. doi: 10.3760/cma.j.issn.0529-5807.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Gray T, Coakley R, Hirsh A, et al. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1beta in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L320–330. doi: 10.1152/ajplung.00440.2002. [DOI] [PubMed] [Google Scholar]
- 27.Richardson JM, Kaushic C, Wira CR. Polymeric immunoglobin (Ig) receptor production and IgA transcytosis in polarized primary cultures of mature rat uterine epithelial cells. Biol Reprod. 1995;53:488–498. doi: 10.1095/biolreprod53.3.488. [DOI] [PubMed] [Google Scholar]
- 28.Natvig IB, Johansen FE, Nordeng TW, Haraldsen G, Brandtzaeg P. Mechanism for enhanced external transfer of dimeric IgA over pentameric IgM: studies of diffusion, binding to the human polymeric Ig receptor, and epithelial transcytosis. J Immunol. 1997;159:4330–4340. [PubMed] [Google Scholar]
- 29.Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS. FcRn: The Architect Behind the Immune and Nonimmune Functions of IgG and Albumin. J Immunol. 2015;194:4595–4603. doi: 10.4049/jimmunol.1403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche AM, Richard AL, Rahkola JT, Janoff EN, Weiser JN. Antibody blocks acquisition of bacterial colonization through agglutination. Mucosal Immunol. 2015;8:176–185. doi: 10.1038/mi.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Übelhart R, Hug E, Bach MP, et al. Responsiveness of B cells is regulated by the hinge region of IgD. Nat Immunol. 2015;16:534–543. doi: 10.1038/ni.3141. [DOI] [PubMed] [Google Scholar]
- 32.Lau A, Lester S, Moraitis S, et al. Tertiary lymphoid organs in recalcitrant chronic rhinosinusitis. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.08.052. [DOI] [PubMed] [Google Scholar]
- 33.Tsurumaru H. Lymphoid follicle formation in sinus mucosa of chronic sinusitis. Nihon Jibiinkoka Gakkai Kaiho. 1996;99:1662–1675. doi: 10.3950/jibiinkoka.99.1662. [DOI] [PubMed] [Google Scholar]
- 34.Van Zele T, Holtappels G, Gevaert P, Bachert C. Differences in initial immunoprofiles between recurrent and nonrecurrent chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2014;28:192–198. doi: 10.2500/ajra.2014.28.4033. [DOI] [PubMed] [Google Scholar]
- 35.Baba S, Kondo K, Toma-Hirano M, et al. Local increase in IgE and class switch recombination to IgE in nasal polyps in chronic rhinosinusitis. Clin Exp Allergy. 2014;44:701–712. doi: 10.1111/cea.12287. [DOI] [PubMed] [Google Scholar]
- 36.Forsgren A, Brant M, Möllenkvist A, et al. Isolation and characterization of a novel IgD-binding protein from Moraxella catarrhalis. J Immunol. 2001;167:2112–2120. doi: 10.4049/jimmunol.167.4.2112. [DOI] [PubMed] [Google Scholar]
- 37.Jendholm J, Samuelsson M, Cardell LO, Forsgren A, Riesbeck K. Moraxella catarrhalis-dependent tonsillar B cell activation does not lead to apoptosis but to vigorous proliferation resulting in nonspecific IgM production. J Leukoc Biol. 2008;83:1370–1378. doi: 10.1189/jlb.1107788. [DOI] [PubMed] [Google Scholar]
- 38.Watelet JB, Demetter P, Claeys C, Cauwenberge P, Cuvelier C, Bachert C. Wound healing after paranasal sinus surgery: neutrophilic inflammation influences the outcome. Histopathology. 2006;48:174–181. doi: 10.1111/j.1365-2559.2005.02310.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.