Abstract
Hamman’s syndrome (spontaneous subcutaneous emphysema and pneumomediastinum) is a rare complication of diabetic ketoacidosis (DKA), with a multifactorial etiology. Awareness of this syndrome is important: it is likely underdiagnosed as the main symptom of shortness of breath is often attributed to Kussmaul’s breathing and the findings on chest radiograph can be subtle and easily missed. It is also important to be aware of and consider Boerhaave’s syndrome as a differential diagnosis, a more serious condition with a 40% mortality rate when diagnosis is delayed. We present a case of pneumomediastinum, pneumopericardium, epidural emphysema and subcutaneous emphysema complicating DKA in an eighteen-year-old patient. We hope that increasing awareness of Hamman’s syndrome, and how to distinguish it from Boerhaave’s syndrome, will lead to better recognition and management of these syndromes in patients with diabetic ketoacidosis.
Learning points:
Hamman’s syndrome (spontaneous subcutaneous emphysema and pneumomediastinum) is a rare complication of DKA.
Presentation may be with chest or neck pain and shortness of breath, and signs are subcutaneous emphysema and Hamman’s sign – a precordial crunching or popping sound during systole.
Boerhaave’s syndrome should be considered as a differential diagnosis, especially in cases with severe vomiting.
The diagnosis of pneumomediastinum is made on chest radiograph, but a CT thorax with water-soluble oral contrast looking for contrast leak may be required if there is high clinical suspicion of Boerrhave’s syndrome.
Hamman’s syndrome has an excellent prognosis, self-resolving with the correction of the ketoacidosis in all published cases in the literature.
Background
Hamman’s syndrome (spontaneous subcutaneous emphysema and pneumomediastinum) is a rare complication of diabetic ketoacidosis (DKA). It is important to consider Boerhaave’s syndrome as a differential diagnosis, particularly in patients with DKA and severe vomiting. Patients have similar symptoms, but Boerhaave’s syndrome is a more serious condition with a 40% mortality rate when diagnosis is delayed.
Case presentation
An eighteen-year-old man attended the Accidents & Emergency (A&E) department following six hours of vomiting. He was diagnosed with type 1 diabetes mellitus aged 8 years, controlled with an insulin pump. He had a recent HbA1c of 7.4% and no microvascular or macrovascular complications. He described coryzal symptoms, myalgia and headaches for one week prior to admission. He had no other significant past medical history, particularly no respiratory disease, and did not take any other regular medications. He was a non-smoker and did not take any illicit drugs. Prior to presenting to A&E, his blood glucose was 24 mmol/L, with urine ketones ++++.
Investigation
When reviewed in A&E, he was alert and orientated, with dry mucous membranes. On clinical examination, he had Kussmaul’s breathing, was tachycardic, had enlarged non-exudative tonsils and anterior cervical non-tender lymphadenopathy. The rest of the clinical examination was unremarkable. His venous blood gas was as follows: pH: 7.39, Base Excess:18.7, pCO2: 3.6 kPa, HCO3: 16.3 mmol/L, lactate: 2.6 mmol/L and glucose: 27.5 mmol/L. Laboratory bloods showed WCC: 33.7 × 109/L, CRP: 3.2 mg/L, K: 5.1 mmol/L, Na: 129 mmol/L, creatinine: 159 μmol/L, amylase: 34 U/L and capillary ketones: 5.1 mmol/L. Urine dip showed glucose +++, ketones ++, blood +. A diagnosis of diabetic ketoacidosis (DKA), secondary to viral illness, was made and he was managed, as per hospital protocol, with intravenous fluids and a fixed rate insulin infusion.
Treatment
A diagnosis of diabetic ketoacidosis (DKA), secondary to viral illness, was made and he was managed, as per hospital protocol, with intravenous fluids and a fixed rate insulin infusion.
The next morning, he complained of neck pain, and on examination, he had mild surgical emphysema bilaterally in the supraclavicular fossae. A chest radiograph showed supraclavicular subcutaneous emphysema and pneumomediastinum (Fig. 1). A CT thorax with water-soluble oral contrast was performed and pneumomediastinum, pneumopericardium, epidural emphysema and surgical emphysema in the soft tissues of the neck and supraclavicular fossae, extending deep into the fascial planes below the pectoralis minor (Fig. 2). There was no contrast leak from the esophagus.
Figure 1.
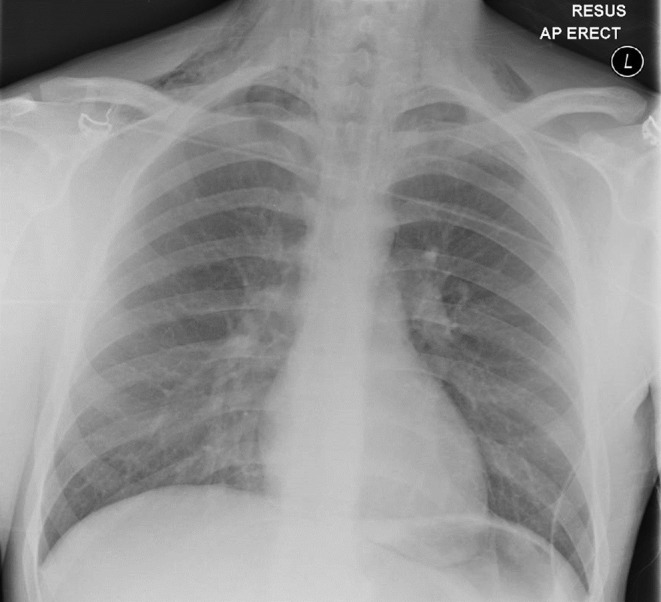
Chest radiograph showing pneumomediastinum and bilateral subcutaneous cervical surgical emphysema.
Figure 2.
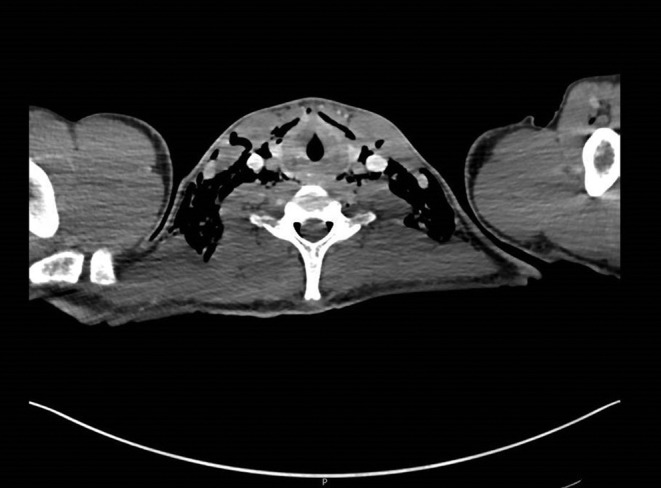
CT showing epidural emphysema and surgical emphysema in the soft tissues of the neck.
Outcome and follow-up
Following resolution of the DKA and review by a diabetologist to ensure correct pump function, he was discharged with an appointment for a repeat chest radiograph in six weeks and diabetic follow-up. He had no complications or lasting effects at 8 months.
Discussion
What is pneumomediastinum?
Pneumomediastinum is a rare complication of DKA. It is an accumulation of air within the mediastinum and can be primary/spontanenous or secondary to respiratory disease and trauma. High intrathoracic pressures lead to overdistension and rupture of alveoli, allowing air to leak and track along the bronchovascular bundles to the mediastinum (1). Air can then track to the subcutaneous tissues of the neck, the pericardium, retroperitoneum or through the posterior mediastinum and intervertebral foramen to the epidural space. Rupture of the mediastinal pleura can also lead to pneumothorax (2).
Pathogenesis of pneumomediastinum in DKA
The pathogenesis of pneumomediastinum in DKA is multifactorial. Kussmaul’s breathing, the respiratory compensation mechanism in metabolic acidosis, increases the alveolar pressure 20–30 mmHg above normal inspiratory pressures, predisposing to alveolar rupture. Vomiting in DKA also increases intrathoracic pressures, secondary to the Valsalva maneuver. DKA is often associated with severe vomiting, especially in the first 24 h, caused by acidosis. Gastroparesis is also worsened by hyperglycaemia and exacerbates vomiting. Possibly contributing are the recently described fibrotic changes in the lungs of people with poorly controlled diabetes, predisposing to alveolar rupture at lower intrathoracic pressures (3).
Symptoms and signs
Symptoms include chest or neck pain and shortness of breath. Signs are subcutaneous emphysema, and Hamman’s sign – a precordial crunching or popping sound during systole, associated with 50% of cases (2).
Diagnosis
A diagnosis of pneumomediastinum can be made on chest radiograph. A CT thorax is necessary to look for esophageal rupture or mediastinitis. Boerhaave’s syndrome, the syndrome of esophageal perforation due to raised intraluminal pressures, can complicate violent vomiting and is occasionally seen in DKA (4, 5). Other sequelae of raised venous pressures secondary to violent vomiting, such as subconjunctival hemorrhage and facial petechiae, may be present and raise clinical suspicion of Boerhaave’s syndrome. Clinical judgment must be exercised as Boerhaave’s is a rare syndrome and CT gives high radiation doses to often young patients. A CT thorax with water-soluble oral contrast shows contrast leak, though care should be taken interpreting a negative result, since the high pressure during possible perforation will no longer be present.
Management
The management of Hamman’s syndrome in DKA involves reversal of the underlying cause. DKA guidelines advocate high dose fixed rate insulin to reverse the ketoacidosis quickly, thereby reducing the compensatory hyperventilation and antiemetics to manage vomiting. It is worth noting that gastroparesis may co-exist in patients with multiple diabetes complications, and these patients often respond better to prokinetic antiemetics such as metoclopramide and domperidone. If vomiting continues despite pharmacological interventions, consideration can be given to Ryles’ tube insertion. If Boerhaave’s syndrome is suspected, CT thorax, prompt intravenous antibiotics and liaison with the surgical team are essential.
Although a rare complication of DKA, Hamman’s syndrome is not currently mentioned as a complication in the Joint British Diabetes Societies (JBDS) DKA 2013 guidelines. Including it when the guidelines are next updated may lead to raised awareness and improved management.
Prognosis
Hamman’s syndrome associated with DKA has an excellent prognosis, self-resolving with the correction of the ketoacidosis in all published cases in the literature. When diagnosis and surgical intervention is delayed, Boerrhave’s syndrome has a high mortality of 40% (6).
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written, informed consent was obtained from the patient for the publication of this article.
Author contribution statement
All authors contributed equally to the design, writing and editing of the article.
References
- 1.Hamman L. 1939. Spontaneous mediastinal emphysema. Bulletin of the Johns Hopkins Hospital 64 1–21. [Google Scholar]
- 2.Panacek EA, Singer AJ, Sherman BW, Prescott A, Rutherford WF. 1992. Spontaneous pneumomediastinum: clinical and natural history. Annals of Emergency Medicine 21 1222–1227. ( 10.1016/S0196-0644(05)81750-0) [DOI] [PubMed] [Google Scholar]
- 3.Hu Y, Ma Z, Guo Z, Zhao F, Wang Y, Cai L, Yang J. 2014. Type 1 diabetes mellitus is an independent risk factor for pulmonary fibrosis. Cell Biochemistry and Biophysics 70 1385–1391. ( 10.1007/s12013-014-0068-4) [DOI] [PubMed] [Google Scholar]
- 4.Alkhuja S, Gazizov N, Charles G. 2013. Pneumomediastinum complicating diabetic ketoacidosis and Boerhaave’s syndrome. Case Reports in Medicine 2013 598720 ( 10.1155/2013/598720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauw RG, van der Werf TS, van Dullemen HM, Dullaart RP. 2007. Mediastinal emphysema complicating diabetic ketoacidosis: plea for conservative diagnostic approach. Netherlands Journal of Medicine 65 368–371. [PubMed] [Google Scholar]
- 6.Shaker H, Elsayed H, Whittle I, Hussein S, Shackcloth M. 2010. The influence of the ‘golden 24-h rule’ on the prognosis of oesophageal perforation in the modern era. European Journal of Cardio-Thoracic Surgery 38 216–222. ( 10.1016/j.ejcts.2010.01.030) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a