Abstract
Background
Children with sickle cell disease (SCD) are at increased risk of complications from influenza. However, despite widespread recommendations that these patients receive an annual influenza immunization, reported vaccination rates remain very low at under 50%.
Procedure
Our aim was to increase the influenza vaccination rate among our pediatric patients with SCD aged 6 months to 21 years over two influenza seasons, 2012–2013 and 2013–2014, to 80%, consistent with the Health People 2020 goal. We used multiple quality improvement methods, based on the literature and our previous experience in other aspects of SCD care, including parent and provider education, enhancement of our EHR, use of a SCD patient registry and reminder and recall done by a patient navigator.
Results
We vaccinated 80% of our pediatric patients with SCD for influenza during the 2012–2013 season and 90% of patients in 2013–2014. Our early season vaccination rates were nearly double that of those for the general population.
Conclusions
Use of quality improvement methods can increase rates of influenza vaccination for this high-risk population, suggesting that less health care utilization and lower cost might result.
Keywords: quality improvement, sickle cell disease, vaccination
INTRODUCTION
Influenza infections are associated with >200,000 hospitalizations and >30,000 deaths annually in the US [1]. Since 2008, the American Academy of Pediatrics has recommended that all children ages 6 months to 18 years receive an annual influenza vaccine, which has been shown to reduce clinic visits and hospitalizations due to influenza [2,3]. People with sickle cell disease (SCD) are a high-risk population for complications of influenza. Although there are not direct data available, reasons postulated include increased susceptibility to bacterial infections with encapsulated organisms due to decreased splenic function, increased airway hyperactivity, and risk of acute chest syndrome following respiratory infections [4–6]. A 2010 study showed that children with SCD are hospitalized for influenza at a rate 56 times that of children without SCD and have double the rate of hospitalization for influenza compared to children with cystic fibrosis [6].
Given this high risk of complications, annual influenza vaccination for individuals with SCD has been recommended by the Centers for Disease Control and Prevention (CDC) since 1978, is endorsed in multiple national guidelines, and has been identified as an important quality indicator in pediatric sickle cell care [7–10]. However, despite evidence of safety and efficacy, influenza vaccination rates are low in children and adults with SCD [11,12]. A study of Wisconsin Medicaid patients reported only 22% adherence to influenza vaccination among children and adults with SCD [13]. In addition, a survey by the CDC reported that only 50% of parents of a child with SCD said their child “had received or had an appointment scheduled to receive influenza vaccine” during the 2011–2012 influenza season [14].
A variety of quality improvement efforts, such as using a database to target high risk patients for reminder and recall letters, placement of an alert in the EHR, and use of non-physician personal and direct outreach, have been shown to increase vaccination rates in other high risk populations [15–17]. We used similar quality improvement methods to increase our rate of annual influenza vaccination among pediatric patients with SCD over two seasons, 2012–2013 and 2013–2014 compared with our baseline rates in 2011–2012. Our goal was to vaccinate over 80% of our patients as per the Health People 2020 objectives [18].
METHODS
We performed this study in a pediatric hematology practice based in an urban, safety net hospital that cared for approximately 180 pediatric patients with SCD aged ≤21 years. Our goal was to provide influenza vaccinations to ≥80% of patients with SCD aged 6 months to 21 years in the 2012–2013 and 2013–2014 influenza seasons. Therefore, our primary outcome was the proportion of children with SCD who received the influenza vaccine. In addition, the CDC recommends receipt of the influenza vaccine “as early as possible,” defining “early season” influenza vaccination as that given before mid-November [19]. We calculated the proportion of children who received the influenza vaccine by November 15th as a secondary outcome measure. Finally, we calculated the number of “protected days,” which we defined as the number of days between receipt of influenza vaccine and the end of the influenza season (March 31st), with an aim of increasing the total number of protected days for the population and for each individual.
Documentation was required for all vaccinations. We used internal hospital data for patients who received the vaccine at our institution. For patients who reported receiving the vaccine elsewhere our patient navigator (or in some cases the clinic nurse) obtained written confirmation of the vaccination and the date it was given. This was then entered into our EHR and was available in our database.
We employed four tactics to improve influenza vaccination rates: (i) education of families and providers; (ii) use of an enhanced electronic health record (EHR); (iii) creation of a patient registry; and (iv) use of a patient navigator. First, starting in 2012 we provided education to families and providers about the importance of influenza vaccination for children with SCD. Using existing materials from the CDC about SCD and influenza, one of the authors (PS) created educational materials explaining the importance of influenza vaccination in our patient population. We distributed these materials to patients and families during influenza season during pediatric hematology clinic visits, by direct mail and email, and via our practice’s Facebook page. We also shared them with primary care providers at our institution to increase recognition of the need for influenza vaccination for all patients with SCD.
Second, we used the EHR to help drive our improvement efforts. Although data on receipt of influenza vaccine had been available in the EHR, it was not easily accessible in a clinic note. From 2011 to 2012, we refined the sickle cell encounter form to integrate immunization dates directly into the clinic note, making it easier for the provider to recognize the child’s eligibility for influenza vaccine at the point of care.
Third, we created a patient registry for children with SCD in April 2012 to assist with all of our improvement efforts, including providing influenza vaccine [20]. After the first season we determined that there were still many patients without documented influenza vaccinations, but we could not be certain if these patients had received the vaccine elsewhere and wanted to be more proactive about bringing patients in to be vaccinated if they had not. Therefore, beginning in September 2013, monthly reports were generated from our institution’s data warehouse which contained patient names, phone numbers and date of vaccination. Our patient navigator reviewed the registry reports monthly and identified which patients did not have a documented influenza vaccination. She contacted each family to ask if the patient had been vaccinated elsewhere. If they had she obtained the outside documentation to confirm this, including the date the vaccine was given. This data were then entered into our EHR. If a patient had not yet been vaccinated, she helped the family identify the most convenient place to obtain the influenza vaccine (e.g., primary care or pediatric hematology clinic, local pharmacies, etc.) and schedule a visit.
RESULTS
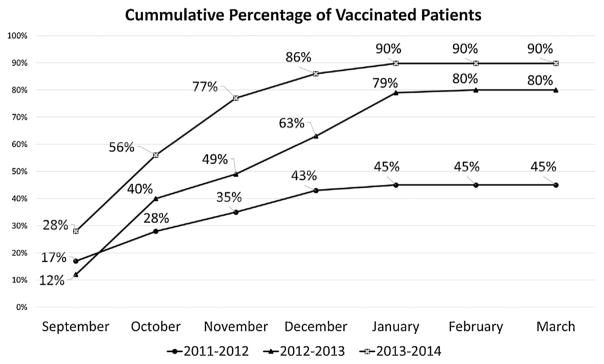
During the 2012–2013 influenza season, 80% (132/164) of patients with SCD ages 6 months to 21 years followed in our clinic received the influenza vaccine (Fig. 1). With the added efforts of the patient navigator in the 2013–2014 season, we vaccinated 90% (151/167) of this patient population (P =0.0014). This was a substantial increase from the season before our quality improvement efforts started when only 45% (75/167) of patients were vaccinated.
Fig. 1.
Influenza vaccination rates for pediatric patients with sickle cell disease.
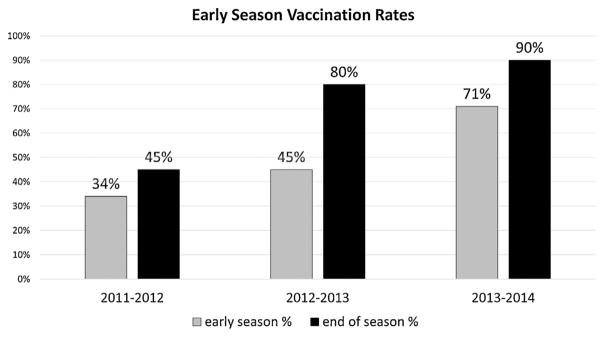
Our “early season” vaccination rates rose from 45% (78/172) in 2012–2013 to 71% (125/176) in 2013–2014 (P <0.0001), compared with 34% in 2011–2012 (Fig. 2). Total protected days for the pediatric SCD population were 18,810 and 24,831 days for the 2012–2013 and 2013–2014 seasons, respectively (P <0.0001), an increase from 11,963 for the 2011–2012 season.
Fig. 2.
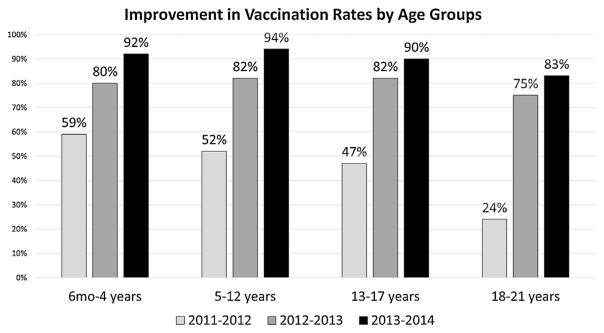
Vaccination rates by age group.
Our vaccination rates were somewhat higher in younger age groups compared with patients ages 18–21 (Fig. 3); however, all groups achieved over 80% vaccination coverage by the end of the project.
Fig. 3.
Early season vaccination rates.
DISCUSSION
In this study, we showed that it is possible to exceed the Healthy People 2020 goal for influenza vaccination rates for a medically and socially high risk population through educational materials, use of an enhanced EHR and patient registry, and efforts of a patient navigator to conduct outreach with patients and families. The rates of influenza vaccination achieved in our study appear to exceed those reported in the literature for patients with SCD or other chronic illnesses [13,14,21]. In addition, our early season influenza vaccination rate in 2013–2014 was nearly double (71% vs. 39.5%) that reported by the CDC for the general population [19]. By vaccinating earlier in the 2013–2014 influenza season, we also provided 32% more protected days compared to the 2012–2013 season. We improved influenza vaccination rates in the first year of our project by providing specific educational materials to patients and providers, increasing provider awareness, and making vaccine data more readily available in the electronic clinic note. We achieved greater success in the second year of the project by involving the patient navigator to proactively reach out to patients without a documented influenza vaccination in our EHR, determine if they had received one elsewhere, and if not bring them in to be vaccinated.
Identifying patients at high risk who should be targeted to receive vaccination is a barrier to improving vaccination rates [15]. Use of a database to target reminders to high risk patients followed by a recall letter for those remaining unvaccinated, was shown to increase influenza vaccination rates in a group of pediatric practices [15]. Establishing our database of patients with SCD allowed up to target those individuals by mail, email, and phone and maximize our vaccination coverage. In another study, leveraging the EHR through use of an alert resulted in an increase in influenza vaccination rates among immunocompromised rheumatology patients from 47% to 65% [16]. Similarly, embedding our registry in our EHR allowed us to target unvaccinated patients throughout the influenza season. A meta-analysis of improvement initiatives for community-dwelling adults showed success with the use of non-physician personnel, activating patients through direct outreach, and audit and feedback aimed at physicians [17]. These steps were successful for us as well, using a non-clinician patient navigator for direct outreach, and providing updates to the clinical team on our overall vaccination rates throughout the project.
There are several limitations to this study. These interventions were done at a specialty clinic in a single urban academic center, and may not be generalizable to different practice settings, although similar methods have been shown to achieve success in other studies. We were not able to link increased receipt of influenza immunization to outcomes, such as emergency department visits or hospitalizations, due to the variation in efficacy of the vaccine year to year, our relatively small sample size, and the large number of medical centers in our region making it difficult to determine where a patient may have presented for care. However, prior studies have shown that the influenza vaccine is effective in reducing hospitalizations among pediatric patients, and is recommended for good clinical care [22]. Our effort was possible because of our institutions advanced EHR and patient registry which allowed the generation of reports detailing unvaccinated patients. However, as more institutions adopt EHRs and are compliant with meaningful use requirements, these tools may become more widely available. While we cannot prove that our increased in vaccination rates were linked to our efforts, during the time of our project rates for influenza vaccination of children in Massachusetts changed only slightly from 63% in 2011–2012 to 72% in 2013–2014, adding evidence that our more substantial increase was due to our quality improvement efforts [23].
Finally, the use of a grant-supported patient navigator played a key role in meeting our goal. However, the work of the patient navigator in this project could be performed by existing staff members. For example, information on the importance of influenza vaccination in SCD could be handed to patients by check-in staff, or sent via mail, email, or text by the institution or practice. A clinic nurse could contact families to determine if they had received the flu vaccine elsewhere; in states with an electronic vaccine registry this step would be even less time consuming as information could be obtained online. As more training programs include quality improvement education, a similar project, including reminder calls and obtaining outside records, could be done by a motivated medical student or resident [24]. Our patient navigator estimated that she spent several hours at the beginning of the project setting up her own database and tracking appointments, and then approximately 30 min per day during flu season updating records, which is an achievable amount of time to dedicate to a project that results in improved outcomes and likely reduces costs. Depending on local circumstances, these are examples of potential ways to replicate our quality improvement efforts without the need for a grant-funded position.
Vaccinating a majority of patients at high risk for complications may decrease health care utilization and costs associated with influenza in this population. In an analysis of influenza related hospitalizations across four states, Bundy et al. calculated a cost saving of $1.9 million in direct hospital charges if the children with SCD in their study had been admitted at the same rate as children with without SCD [6]. If we extrapolate this to children across the US, and include indirect costs such as lost work productivity for parents, cost savings could be even higher [25]. Our methods could also be used in other high risk patient populations, thereby reducing cost even further and making the role of patient navigator cost effective.
In conclusion, using a combination of quality improvement initiatives, our pediatric hematology clinic vaccinated 80%–90% of our patients with SCD against influenza during the two years of the project, exceeding the Healthy People 2020 goal for vaccination rates for healthy children. This demonstrates that close to universal vaccination is possible for children and adolescents with SCD and should serve as a benchmark for other practices. More work needs to be done to demonstrate the improvement in health status and cost savings of influenza vaccination, and extend this work into adult populations.
Acknowledgments
Grant sponsor: Health Resources and Service Administration Sickle Cell Disease; Grant sponsor: Newborn Screening Program; Grant number: U38 MC22215-01-00.
This work was funded through a Health Resources and Service Administration Sickle Cell Disease and Newborn Screening Program grant, #U38 MC22215-01-00. Thank you to Ning Chen for statistical support.
Footnotes
Conflict of interest: Nothing to declare.
References
- 1.Thompson WW, Comanor L, Shay DK. Epidemiology of seasonal influenza: Use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis. 2006;194:S82–S91. doi: 10.1086/507558. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Infectious Diseases. Prevention of influenza: Recommendations for influenza immunization of children, 2008–2009. Pediatrics. 2008;122:1135–1141. doi: 10.1542/peds.2008-2449. [DOI] [PubMed] [Google Scholar]
- 3.Katayose M, Hosoya M, Haneda T, Yamaguchi H, Kawasaki Y, Sato M, Wright PF. The effectiveness of trivalent inactivated influenza vaccine in children over six consecutive influenza seasons. Vaccine. 2011;29:1844–1849. doi: 10.1016/j.vaccine.2010.12.049. [DOI] [PubMed] [Google Scholar]
- 4.Caboot JB, Allen JL. Pulmonary complications of sickle cell disease in children. Curr Opin Pediatr. 2008;20:279–287. doi: 10.1097/MOP.0b013e3282ff62c4. [DOI] [PubMed] [Google Scholar]
- 5.van Tuijn CF, Nur E, van Beers EJ, Zaaijer HL, Biemond BJ. Acute chest syndrome in sickle cell disease due to the new influenza A (H1N1) virus infection. Am J Hematol. 2010;85:303–304. doi: 10.1002/ajh.21638. [DOI] [PubMed] [Google Scholar]
- 6.Bundy DG, Strouse JJ, Casella JF, Miller MR. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics. 2010;125:234–243. doi: 10.1542/peds.2009-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Center for Disease Control (CDC) Recommendation of the public health service advisory committee on immunization practices: Influenza vaccine. MMWR Morb Mortal Wkly Rep. 1978;27:285–292. [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) [Accessed May 29, 2014];Sickle cell disease (SCD) 5 tips to help prevent infection. http://www.cdc.gov/ncbddd/sicklecell/healthyliving-prevent-infection.html Updated January 16, 2014.
- 9.National Heart Lung and Blood Institute. [Accessed February 27, 2014];The management of sickle cell disease. http://www.nhlbi.nih.gov/health/prof/blood/sickle/sc_mngt.pdf. Updated 2002.
- 10.Wang CJ, Kavanagh PL, Little AA, Holliman JB, Sprinz PG. Quality-of-care indicators for children with sickle cell disease. Pediatrics. 2011;128:484–493. doi: 10.1542/peds.2010-1791. [DOI] [PubMed] [Google Scholar]
- 11.Howard-Jones M, Randall L, Bailey-Squire B, Clayton J, Jackson N. An audit of immunisation status of sickle cell patients in coventry. UK J Clin Pathol. 2009;62:42–45. doi: 10.1136/jcp.2008.058982. [DOI] [PubMed] [Google Scholar]
- 12.Souza AR, Braga JA, de Paiva TM, Loggetto SR, Azevedo RS, Weckx LY. Immunogenicity and tolerability of a virosome influenza vaccine compared to split influenza vaccine in patients with sickle cell anemia. Vaccine. 2010;28:1117–1120. doi: 10.1016/j.vaccine.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 13.Beverung LM, Brousseau D, Hoffmann RG, Yan K, Panepinto JA. Ambulatory quality indicators to prevent infection in sickle cell disease. Am J Hematol. 2014;89:256–260. doi: 10.1002/ajh.23627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Influenza vaccination practices of physicians and caregivers of children with neurologic and neurodevelopmental conditions—united states, 2011–12 influenza season. MMWR Morb Mortal Wkly Rep. 2013;62:744–746. [PMC free article] [PubMed] [Google Scholar]
- 15.Daley MF, Barrow J, Pearson K, Crane LA, Gao D, Stevenson JM, Berman S, Kempe A. Identification and recall of children with chronic medical conditions for influenza vaccination. Pediatrics. 2004;113:e26–e33. doi: 10.1542/peds.113.1.e26. [DOI] [PubMed] [Google Scholar]
- 16.Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61:1505–1510. doi: 10.1002/art.24873. [DOI] [PubMed] [Google Scholar]
- 17.Lau D, Hu J, Majumdar SR, Storie DA, Rees SE, Johnson JA. Interventions to improve influenza and pneumococcal vaccination rates among community-dwelling adults: A systematic review and meta-analysis. Ann Fam Med. 2012;10:538–546. doi: 10.1370/afm.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services. [Accessed April 2, 2014];Healthy people 2020 objectives. http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=23. Published August 28, 2013. Updated 2013.
- 19.Centers for Disease Control and Prevention (CDC) [Accessed July 8, 2014];National early season flu vaccination coverage, united states. 2013 Nov; http://www.cdc.gov/flu/fluvaxview/nifs-estimates-nov2013.htm. Updated 2013.
- 20.Kavanagh PL, Sobota AE, McClure ES, Sprinz PG, Adams WG. Using an electronic health record-based registry to improve pediatric sickle cell care. JCOM. 2014;21:159. [Google Scholar]
- 21.Centers for Disease Control Prevention (CDC) Vaccination coverage among persons with asthma—united states, 2010–2011 influenza season. MMWR Morb Mortal Wkly Rep. 2013;62:973–978. [PMC free article] [PubMed] [Google Scholar]
- 22.Committee on Infectious Diseases. American Academy of Pediatrics. Reduction of the influenza burden in children. Pediatrics. 2002;110:1246–1252. doi: 10.1542/peds.110.6.1246. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) [Accessed September 24, 2014];2010–11 through 2013–14 state, regional, and national vaccination trend report. http://www.cdc.gov/flu/fluvaxview/reports/reporti1314/trends/index.htm Updated 2014.
- 24.Mann KJ, Craig MS, Moses JM. Quality improvement educational practices in pediatric residency programs: Survey of pediatric program directors. Acad Pediatr. 2014;14:23–28. doi: 10.1016/j.acap.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Ortega-Sanchez IR, Molinari NA, Fairbrother G, Szilagyi PG, Edwards KM, Griffin MR, Cassedy A, Poehling KA, Bridges C, Staat MA. Indirect, out-of-pocket and medical costs from influenza-related illness in young children. Vaccine. 2012;30:4175–4181. doi: 10.1016/j.vaccine.2012.04.057. [DOI] [PubMed] [Google Scholar]