Abstract
In this work, we developed a facile fluorescence method for quantitative detection of human serum albumin (HSA) based on the inhibition of poly(thymine) (poly T)-templated copper nanoparticles (CuNPs) in the presence of HSA. Under normal circumstances, poly T-templated CuNPs can display strong fluorescence with excitation/emission peaks at 340/610 nm. However, in the presence of HSA, it will absorb cupric ion, which will prevent the formation of CuNPs. As a result, the fluorescence intensity will become obviously lower in the presence of HSA. The analyte HSA concentration had a proportional linear relationship with the fluorescence intensity of CuNPs. The detection limit for HSA was 8.2 × 10−8 mol·L−1. Furthermore, it was also successfully employed to determine HSA in biological samples. Thus, this method has potential applications in point-of-care medical diagnosis and biomedical research.
Keywords: label-free, human serum albumin, copper nanoparticles
1. Introduction
Quantitative detection of proteins is of great importance in a number of areas, such as chemical and biochemical analyses, immunodiagnostics, and biotechnology [1]. As one of the most abundant blood proteins in plasma, human serum albumin (HSA) plays a very important role in maintaining the oncotic pressure of blood and serves as a carrier for many neutral and weak acidic metabolites and drugs [2,3]. The concentration of HSA in body fluids is almost considered a reliable sign of health [4]. For example, research has shown that there is an intimate connection between cardiovascular disease and kidney damage, and the critical indictor for kidney damage is the concentration of HSA in human urine [5]. Several methods to determine the concentration of HSA have been developed, including visible absorption spectroscopy [6,7,8], electrochemical [9,10], Immunoassays [11,12,13] and High-performance liquid chromatography (HPLC) [14]; however, there are some problems for detecting the concentration of HSA with these methods. For example, Tu and co-workers reported a low-cost method with Immunoassays to detect the HSA [15]. However, this method requires special testing instruments, and a lot of time. Yu Ermolenko et al., reported a methodology for the determination of HSA by electrophoresis, which is sensitive [16]. But the operative cost impedes its application. Therefore, a sensitive, rapid and inexpensive method for the quantitative analysis of HSA is still highly desirable.
As a type of fluorescent probe, DNA-templated copper nanoparticles (CuNPs) have attracted a lot of attention due to some outstanding advantages, such as being cost-effective, environmentally friendly, and highly sensitivity in many areas [17,18,19]. In particular, it can be synthesized more rapidly and at lower cost than AgNCs or AuNPS as a signaling nanomaterial [20]. Thus, CuNPs have been utilized as a label-free, low-cost and facile analysis method in various bio-assays. It has been reported that two kinds of DNA-templated CuNPs, the poly(thymine)-templated CuNPs and the dsDNA-templated CuNPs, have been synthesized [21,22,23]. It is known to us that DNA-templated CuNPs have been used for the detection of many materials, including DNA [24,25], proteins [26,27,28], enzymes [29,30,31], microRNA [32,33], and small molecules [34,35,36].
Here, a fluorescence-based strategy is prepared and applied in detecting the concentration of HSA based on the ploy T-templated CuNPs. HSA will absorb Cu2+ because of the strong binding ability between HSA and Cu2+, which will prevent Cu2+ from being reduced to Cu0 by Vitamin c (Vc). As a result, CuNPs can’t be formatted normally. That’s to say, the fluorescence intensity will become lower obviously in the presence of HSA. Thus, the levels of HSA can be determined by monitoring the variation in fluorescence intensity. Furthermore, our method can be used well in detecting HSA in human serum.
2. Materials and Methods
2.1. Materials and Reagents
HSA was purchased from Sigma Aldrich (St. Louis, MO, USA). Standard solutions of HSA were prepared daily by further dilution of its stock solution (0.03325 g HSA was dissolved in 1 mL ultrapure water and the concentration of HSA is 0.5 mM), which was stored at −20 °C. 3′-(N-morpholino) propanesulfonic acid (MOPS), Vitamin c (Vc) and copper sulfate (CuSO4) were purchased from Sinopharm Chemical Reagent (Shanghai, China). The oligonucleotide poly-T, T40, was purchased from Sangon Biological Engineering Technology & Services (Shanghai, China). The DNA sequence is as follows. T40: 5′-TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTT-3′. Ultrapure water (18.2 MΩ·cm) from a Milli-Q water purification system (Millipore Corp, Bedford, MA, USA) was used in all the experiments. The buffer is as follows: MOPS buffer (10 mM MOPS, 150 mM NaCl, and pH 7.5).
2.2. Apparatus
All fluorescence measurements were performed on an F-2700 spectrophotometer (Hitachi, Tokyo, Japan) with excitation at 340 nm and emission at 530–650 nm for the poly T-templated CuNPs. The excitation slits and emission slits were set for 10.0 and 10.0 nm, respectively. Each experiment was carried out in a final volume of 100 µL.
2.3. Detection of HSA
For assaying the concentration of HSA, 100 μM Cu2+ and different concentrations of HSA were mixed in reaction buffer (10 mM MOPS, 150 mM NaCl, pH 7.5) and incubated for 15 min at room temperature. Then 500 nM T40 and 1 mM Vc were subsequently added to the reaction solution and incubated for 10 min at room temperature before measurement. The total volume of the reaction system is 100 μL.
In order to test the application of the proposed assay in biological systems, spiked samples were prepared by addition of different concentrations of HSA to human serum.
3. Results and Discussion
3.1. Verification of the Feasibility of HSA Detection Using Poly T-Templated CuNPs
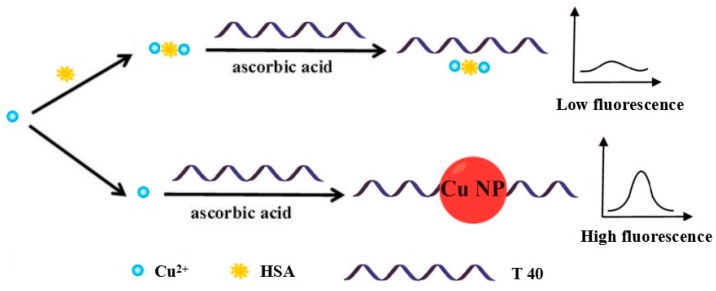
The principle of the detection method is illustrated in Scheme 1. Our experimental method selected Poly T strand (T40) as the template for CuNPs formation [37]. Under normal circumstances, Cu2+ could be reduced to Cu0 by Vc, which reacts with T40 to form the poly T-templated CuNPs and generates a strongly fluorescent signal. However, in the presence of HSA, it will absorb the Cu2+ to prevent the reduction reaction by ascorbic acid. As the result, poly T-templated CuNPs could not be formed after adding T40 to the reaction solution, which meant that the mixture could not generate nearly the same fluorescence intensity. Therefore, we were able to detect the concentration of HSA by monitoring the variation in fluorescence intensity.
Scheme 1.
Rapid and sensitive monitoring of HSA based on poly(thymine)-templated copper nanoparticles.
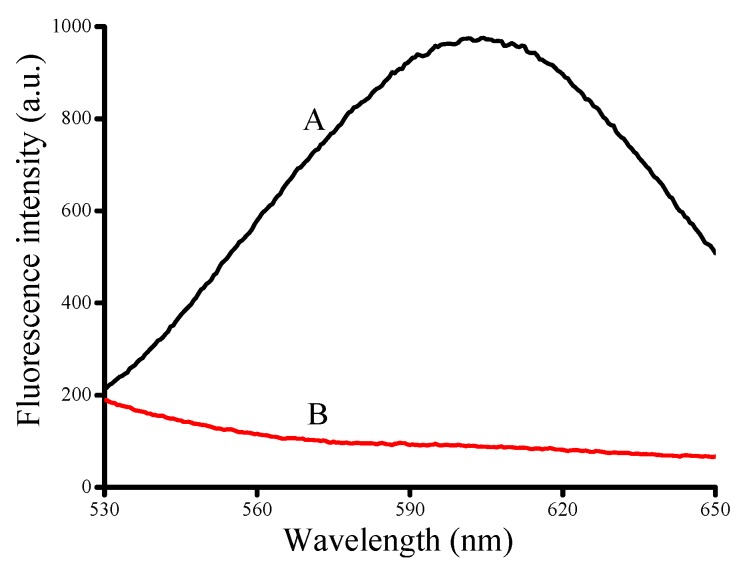
The feasibility of the proposed method was verified. It could apparently observe strong levels of fluorescence at 340 nm excitation when T40, Cu2+ and Vc were added to the buffer (Figure 1A). In contrast, we could see that it didn’t form any fluorescence after adding HSA (Figure 1B). In summary, the results of this experiment demonstrated the feasibility of the proposed strategy for detection of HSA.
Figure 1.
The feasibility of the proposed method. (A) Synthesis of CuNPs with 500 nM T40, 100 μM CuSO4 and 1 mM Vc; (B) 500 nM T40, 100 μM CuSO4 and 1 mM Vc were incubated for 10 min at room temperature after prep-incubation of the CuSO4 with 5 μM HSA for 15 min at room temperature.
3.2. Optimization of Experimental Conditions
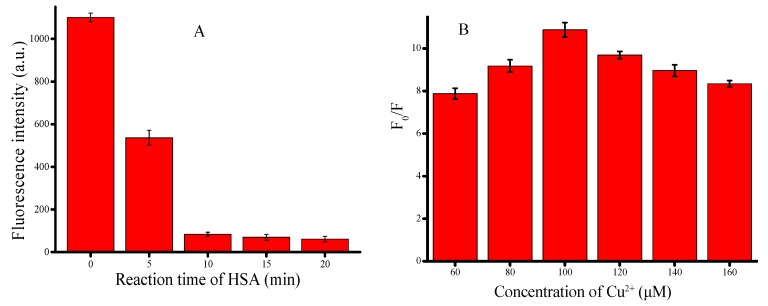
After verifying the feasibility of the proposed method, we investigated the effects of different concentrations of Cu2+ and the reaction time of HSA with Cu2+ on the sensitivity and fluorescence response characteristics of the proposed method. Firstly, we conducted an experiment about the reaction time of HSA with the Cu2+. After a series of fluorescence measurements, it could be found that the fluorescence intensity reduced with the reaction time and reached a steady value after 15 min (Figure 2A). In order to get more stable experimental results, 15 min was considered as the optimal reaction time between HSA and Cu2+.
Figure 2.
Optimization of experimental conditions. (A) Changes in fluorescence intensity with reaction time of HSA (0, 5, 10, 15, 20 min); (B) Changes in fluorescence intensity with different concentrations of CuSO4 (60, 80, 100, 120, 140, 160 μM). Error bars were estimated from three replicate measurements.
Then, the effect of different concentrations of Cu2+ was investigated. As can be seen in Figure 2B, the optimum rate of increase in fluorescence intensity (F0/F, where F0 represented the fluorescence intensity without HSA and F for the fluorescence intensity with HSA) occurred when the concentration of Cu2+ was 100 μM. Thus, 100 μM Cu2+ was used for subsequent experiments.
3.3. Quantitative Measurement of HSA
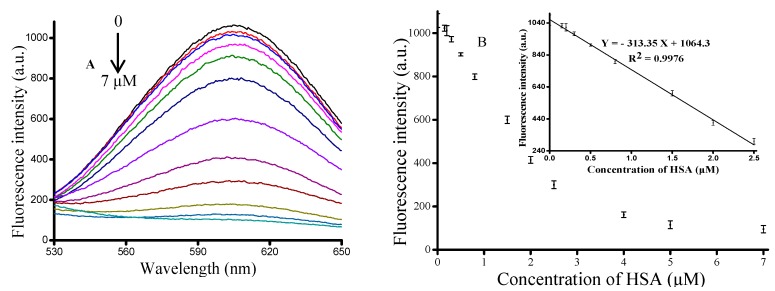
In order to evaluate the sensitivity of the proposed method, we chose a series concentration of HSA ranging from 0 to 7 μM (0, 0.15, 0.2, 0.3, 0.5, 0.8, 1.5, 2, 2.5, 4, 5, 7 μM). As seen in Figure 3A, we found that the fluorescence intensity at 610 nm dynamically decreased with an increase in the concentration of HSA. Figure 3A shows the relationship between fluorescence intensity and the concentration of HSA. The inset of Figure 3B showed that the fluorescence intensity had a linear relationship (R2 = 0.9976) with HSA concentration in the concentration range of 0.15–2.5 μM, and the regression equation was Y = −313.35 X + 1064.3, where Y was the fluorescence intensity at 610 nm and X was the HA concentration. The limit of detection (LOD) of the proposed strategy was estimated to be 0.082 μM, which was lower and more rapid than the other methods [38,39,40]. Therefore, a simple, rapid, effective and sensitive method of HA determination was established.
Figure 3.
(A) Fluorescence emission spectra of the concentration of HSA (0, 0.15, 0.2, 0.3, 0.5, 0.8, 1.5, 2, 2.5, 4, 7 μM). Optimized assay conditions were used; (B) Graph depicting the changes in fluorescence output at 610 nm as a function of HSA concentration. Inset: Linear relationship between fluorescence intensity and low HSA concentrations. Error bars were estimated from three replicate measurements.
3.4. Study of Interferences
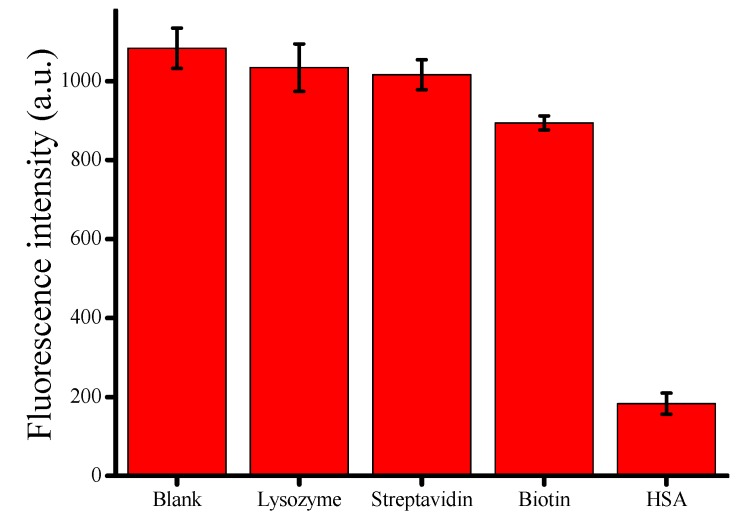
We tested several proteins, such as lysozyme, streptavidin and biotin, by the proposed assay under the optimized concentrations to investigate the selectivity. As seen in Figure 4, we found that none of the proteins influenced the synthesis of CuNPs except HSA. That’s to say, the proposed assay was specific for the detection of HSA, showing a great potential in biological samples.
Figure 4.
Fluorescence intensity of CuNPs in the presence of lysozyme, streptavidin, biotin and HSA under optimized concentrations. Error bars were estimated from three replicate measurements.
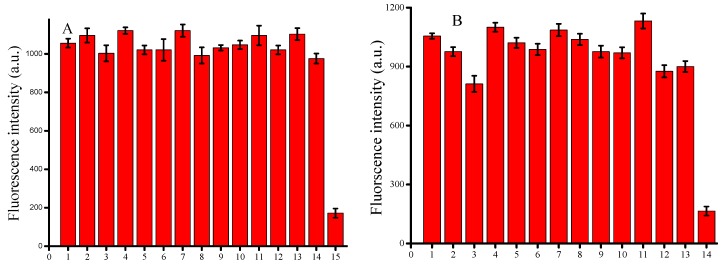
Organic and inorganic compounds typically occurring in human serum were studied as potential interferents. It was considered not to interfere when various kinds of ions and amino acids caused minimal changes in the fluorescence intensity. The results are shown in Figure 5A,B. Therefore, our method has a wider linear range for detecting the HSA in human serum.
Figure 5.
(A) Fluorescence emission spectra of the reaction system with HSA (7 μM) and various ions (7 μM) under the optimized concentrations. 1, Blank; 2, K+; 3, Mn2+; 4, Ca2+; 5, Na+; 6, Mg2+; 7, Li+; 8, Al3+; 9, Zn2+; 10, Hg2+; 11, Cl−; 12, NO3−; 13, SO42−; 14, CO32−; 15, HAS; (B) Fluorescence emission spectra of the reaction system with HSA (7 μM) and various organics (7 μM) under the optimized concentrations. 1, Blank; 2, arginine; 3, glutamic; 4, glycine; 5, isoleucine; 6, leucine; 7, lyscine; 8, valine; 9, proline; 10, alanine; 11, glucose; 12, sucrose; 13, carbamide; 14, HSA.
3.5. Application of the Proposed Assay in Biological Systems
In order to evaluate the practical application of the proposed sensing platform, we attempted to detect HSA in 1% human serum. We added different concentrations of HSA into the diluted blood samples and detected it again with the proposed method. The results are shown in Table 1: the recovery rates of different concentrations of HSA in human serum were 97.0% for 0.86 μM, 101.0% for 1.36 μM, and 99.7% for 1.86 μM. These results indicated that the method might have practical applications for HSA detection in biological systems.
Table 1.
Recovery of HSA in diluted human serum using the proposed method.
| Sample | HSA in Plasma (μM) | Added (μM) | Detected (μM) | Recovery (%) |
|---|---|---|---|---|
| 1 | 0.36 | 0.5 | 0.834 ± 0.036 | 97.0 |
| 2 | 0.36 | 1 | 1.374 ± 0.064 | 101.0 |
| 3 | 0.36 | 1.5 | 1.854 ± 0.094 | 99.7 |
4. Conclusions
In summary, we have successfully demonstrated a fluorescence method for HSA detection based on the poly T-templated copper nanoparticles. The proposed method exhibits high sensitivity to HSA, with a detection limit of 8.2 × 10−8 mol·L−1 under the optimized conditions. Besides, this method is simple and cost-effective, without any labels or complicated operations. The proposed strategy was also successfully applied in detection of HSA in serum samples, and satisfactory results were obtained. We envision that our strategy based on poly T-templated copper nanoparticles for detection of HSA may be applied in in point-of-care medical diagnosis.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 21205142, 31370104), The Research Innovation Program for Graduates of Central South University (2016zzts580, 2017zzts347).
Author Contributions
C.M. conceived and designed the experiments; M.C. and X.X. performed the experiments; K.W. and H.H. analyzed the data; X.X. and H.C. contributed reagents/materials/analysis tools; M.C. and C.M. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zhang Y., Guo Y.M., Xian Y.L., Chen W.W., Zhao Y.Y., Jiang X.Y. Nanomaterials for Ultrasensitive Protein Detection. Adv. Mater. 2013;25:3802–3819. doi: 10.1002/adma.201301334. [DOI] [PubMed] [Google Scholar]
- 2.Huang S.S., Li F.F., Liao C.Y., Zheng B.Z., Du J., Xiao D. A selective and sensitive fluorescent probe for the determination of HSA and trypsin. Talanta. 2017;170:562–568. doi: 10.1016/j.talanta.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 3.McCallum M.M., Pawlak A.J., Shadrick W.R., Simeonov A., Jadhav A., Yasgar A., Maloney D.J., Arnold L.A. A fluorescence-based high throughput assay for the determination of small molecule-human serum albumin protein binding. Anal. Bioanal. Chem. 2014;406:1867–1875. doi: 10.1007/s00216-013-7560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gui W.Y., Chen X.Q., Ma Q. A novel detection method of human serum albumin based on CuInZnS quantum dots—Co2+ sensing system. Anal. Bioanal. Chem. 2017;409:3871–3876. doi: 10.1007/s00216-017-0332-8. [DOI] [PubMed] [Google Scholar]
- 5.Fanali G., Masi A.D., Trezza V., Marino M., Fasano M., Ascenzi P. Human Serum Albumin: From Bench to Bedside. Mol. Aspects Med. 2012;33:209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Huang Z.Z., Wang H.N., Yang W.S. Gold Nanoparticle-Based Facile Detection of Human Serum Albumin and Its Application as an INHIBIT Logic Gate. ACS Appl. Mater. Interfaces. 2015;7:8990–8999. doi: 10.1021/acsami.5b01552. [DOI] [PubMed] [Google Scholar]
- 7.Sun H.X., Xiang J.F., Zhang X.F., Chen H.B., Yang Q.F., Li Q., Guan A.J., Shang Q., Tang Y.L., Xu G.Z. A Colorimetric and Fluorometric Dual-Modal Supramolecular Chemosensor and Its Application for HSA Detection. Analyst. 2014;139:581–584. doi: 10.1039/C3AN01929B. [DOI] [PubMed] [Google Scholar]
- 8.Zeng X.D., Ma M.S., Zhu B.C., Zhu L. A near infrared fluorescence probe for sensitive determination of human serum albumin. Anal. Sci. 2016;32:1291–1294. doi: 10.2116/analsci.32.1291. [DOI] [PubMed] [Google Scholar]
- 9.Stojanovic Z., Erdossy J., Keltai K., Scheller F.W., Gyurcsanyi R.E. Electrosynthesized moleculary imprinted polyscopoletin nanofilms for human serum albumin detection. Anal. Chim. Acta. 2017;977:1–9. doi: 10.1016/j.aca.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 10.Dabrowski M., Cieplak M., Sharma P.S., Borowicz P., Noworyta K., Lisowski W., Souza F.D., Kuhn A. Hierarchical templating in deposition of semi-covalently imprinted inverse opal polythiophene film for femtomolar determination of human serum albumin. Biosens. Bioelectron. 2017;94:155–161. doi: 10.1016/j.bios.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 11.Caballero D., Martinez E., Bausells J., Errachid A., Samitier J. Impedimetric Immunosensor for Human Serum Albumin Detection on a Direct Aldehyde-Functionalized Silicon Nitride Surface. Anal. Chim. Acta. 2012;720:43–48. doi: 10.1016/j.aca.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Jovanovic V.B., Pavicevic I.D., Takic M.M., Penezic-Romanjuk A.Z., Acimovic J.M., Mandic L.M. The influence of fatty acids on determination of human serum albumin thiol group. Anal. Biochem. 2014;448:50–57. doi: 10.1016/j.ab.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Tu M.C., Chang Y.Z., Kang Y.T., Chang H.Y., Chang P., Yew T.R. A quantum dot-based optical immunosensor for human serum albumin detection. Biosens. Bioelectron. 2012;34:286–290. doi: 10.1016/j.bios.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 14.Contois J.H., Hartigan C., Rao L.V., Snyder L.M., Thompson M.J. Analytical Validation of an HPLC Assay for Urinary Albumin. Clin. Chim. Acta. 2006;367:150–155. doi: 10.1016/j.cca.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Ermolenko Y., Anshakova A., Osipova N., Kamentsev M., Maksimenko O., Balabanyan V., Gelperina S. Simultaneous determination of rifabutin and human serum albumin in pharmaceutical formulations by capillary electrophoresis. J. Pharmacol. Toxicol. Methods. 2017;85:55–60. doi: 10.1016/j.vascn.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Singh R., Crow F.W., Babic N., Lutz W.H., Lieske J.C., Larson T.S., Kumar R. A Liquid Chromatography−Mass Spectrometry Method for the Quantification of Urinary Albumin Using a Novel 15N-Isotopically Labeled Albumin Internal Standard. Clin. Chem. 2007;53:540–542. doi: 10.1373/clinchem.2006.078832. [DOI] [PubMed] [Google Scholar]
- 17.Qing Z., Zhu L., Yang S., Cao Z., He X., Wang K., Yang R. In situ formation of fluorescent copper nanoparticles for ultrafast zero-background Cu2+, detection and its toxicides screening. Biosens. Bioelectron. 2016;78:471–476. doi: 10.1016/j.bios.2015.11.057. [DOI] [PubMed] [Google Scholar]
- 18.Wu C., Zhou C., Wang E., Dong S. A label-free and enzyme-free system for operating various logic devices using poly(thymine)-templated CuNPs and SYBR Green I as signal transducers. Nanoscale. 2016;8:14243–14249. doi: 10.1039/C6NR04069A. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y.M., Cao E.P., Lei X.L., Mang L.H., Cheng S.J., Song J.T. Fluorescent copper nanoparticles: Recent advances in synthesis and applications for sensing metal ions. Nanoscale. 2016;8:4852–4863. doi: 10.1039/C6NR00145A. [DOI] [PubMed] [Google Scholar]
- 20.Chen J., Ji X., Tinnefeld P., He Z. Multifunctional Dumbbell-Shaped DNA-Templated Selective Formation of Fluorescent Silver Nanoclusters or Copper Nanoparticles for Sensitive Detection of Biomolecules. ACS Appl. Mater. Interfaces. 2016;8:1786–1794. doi: 10.1021/acsami.5b09678. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S.S., Zhang L., Cai Q.Y., Dong Z.Z., Geng X., Ge J., Li Z.H. A facile label-free aptasensor for detecting ATP based on fluorescence enhancement of poly(thymine)-templated copper nanoparticles. Anal. Bioanal. Chem. 2016;408:6711–6717. doi: 10.1007/s00216-016-9788-1. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Si L., Bao J., Wang Z., Dai Z. Fluorescence Regulation of Poly(thymine)-templated Copper Nanoparticles via an Enzyme-triggered Reaction towards Sensitive and Selective Detection of Alkaline Phosphatase. Anal. Chem. 2017;89:3681–3686. doi: 10.1021/acs.analchem.6b05112. [DOI] [PubMed] [Google Scholar]
- 23.Sun J., Hu T., Xu X.L., Wang L., Yang X. A fluorescent ELISA based on the enzyme-triggered synthesis of poly(thymine)-templated copper nanoparticles. Nanoscale. 2016;8:16846–16850. doi: 10.1039/C6NR06446A. [DOI] [PubMed] [Google Scholar]
- 24.Song C., Yang X., Wang K., Wang Q., Huang J., Liu J., Liu W., Liu P. Label-free and non-enzymatic detection of DNA based on hybridization chain reaction amplification and dsDNA-templated copper nanoparticles. Anal. Chim. Acta. 2014;827:74–79. doi: 10.1016/j.aca.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Hu W., Ning Y., Kong J., Zhang X. Formation of copper nanoparticles on poly(thymine) through surface-initiated enzymatic polymerization and its application for DNA detection. Analyst. 2015;140:5678–5684. doi: 10.1039/C5AN01109D. [DOI] [PubMed] [Google Scholar]
- 26.Yang L., Wang Y., Li B., Jin Y. High-throughput identification of telomere-binding ligands based on the fluorescence regulation of DNA-copper nanoparticles. Biosens. Bioelectron. 2017;87:915–917. doi: 10.1016/j.bios.2016.09.055. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J., Hu S., Cao Y., Zhang B., Li G. Electrochemical detection of protein based on hybridization chain reaction-assisted formation of copper nanoparticles. Biosens. Bioelectron. 2015;66:327–331. doi: 10.1016/j.bios.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 28.Wang H.B., Zhang H.D., Chen Y., Liu Y.M. A fluorescent biosensor for protein detection based on poly(thymine)-templated copper nanoparticles and terminal protection of small molecule-linked DNA. Biosens. Bioelectron. 2015;74:581–586. doi: 10.1016/j.bios.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Qing T.B., He X.X., He D.G., He X., Shangguan J., Liu J., Yuan B., Wang K. Dumbbell DNA-templated CuNPs as a nano-fluorescent probe for detection of enzymes involved in ligase-mediated DNA repair. Biosens. Bioelectron. 2017;94:456–463. doi: 10.1016/j.bios.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 30.Zhao H.Z., Dong J.J., Zhou F.L., Li B.X. One facile fluorescence strategy for Sensitive detection of endonuclease activity using DNA-templated copper nanoclusters as signal indicators. Sens. Actuators B Chem. 2017;238:828–833. doi: 10.1016/j.snb.2016.07.083. [DOI] [Google Scholar]
- 31.Cai Q.Y., Ge J., Xu H.H., Zhang L., Hu Y.L., Huang Z.M., Li Z.H. A label-free aptasensor for highly sensitive ATP detection by using exonuclease I and oligonucleotide-templated fluorescent copper nanoparticles. Anal. Methods. 2017;9:2710–2714. doi: 10.1039/C7AY00424A. [DOI] [Google Scholar]
- 32.Chi B.Z., Liang R.P., Qiu W.B., Yuan Y.H., Qiu J.D. Direct fluorescence detection of microRNA based on enzymatically engineered primer extension poly-thymine (EPEPT) reaction using copper nanoparticles as nano-dye. Biosens. Bioelectron. 2016;87:216–221. doi: 10.1016/j.bios.2016.08.042. [DOI] [PubMed] [Google Scholar]
- 33.Park K.W., Batule B.S., Kang K.S., Park K.S., Park H.G. Rapid and ultrasensitive detection of microRNA by target-assisted isothermal exponential amplification coupled with poly (thymine)-templated fluorescent copper nanoparticles. Nanotechnology. 2016;27:425–502. doi: 10.1088/0957-4484/27/42/425502. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L., Cai Q., Li J., Ge J., Wang J., Dong Z., Li Z.H. A label-free method for detecting biothiols based on poly(thymine)-templated copper nanoparticles. Biosens. Bioelectron. 2015;69:77–82. doi: 10.1016/j.bios.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Cui H., Cao Z., Lau C., Lu J. Additive and enhanced fluorescence effects of hairpin DNA template-based copper nanoparticles and their application for the detection of NAD. Talanta. 2016;154:574–580. doi: 10.1016/j.talanta.2015.12.067. [DOI] [PubMed] [Google Scholar]
- 36.Lai Q.Q., Liu M.D., Gu C.C., Nie H.G., Xu X.J., Li Z., Yang Z., Huang S.M. A novel label-free fluorescence strategy for methyltransferase activity assay based on dsDNAtemplated copper nanoparticles coupled with an endonuclease-assisted signal transduction system. Analyst. 2016;141:1383–1389. doi: 10.1039/C5AN02123E. [DOI] [PubMed] [Google Scholar]
- 37.Qing Z., He X., He D., Wang K., Xu F., Qing T., Yang X. Poly(thymine)-Templated Selective Formation of Fluorescent Copper Nanoparticles. Angew. Chem. Int. Ed. 2013;52:9719–9722. doi: 10.1002/anie.201304631. [DOI] [PubMed] [Google Scholar]
- 38.Eertmans F., Bogaert V., Puype B. Development and validation of a high-performance liquid chromatography (HPLC) method for the determination of human serum albumin (HSA) in medical devices. Anal. Methods. 2011;3:1296–1302. doi: 10.1039/c1ay05148b. [DOI] [Google Scholar]
- 39.Ramezani A.M., Manzoori J., Amjadi M., Jouyban A. Spectrofluorimetric Determination of Human Serum Albumin Using Terbium-Danofloxacin Probe. Sci. World. J. 2012;1:940541–940550. doi: 10.1100/2012/940541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boiero C., Allemandi D., Longhi M., Llabot J.M. RP-HPLC method development for simultaneous determination of timolol maleate and human serum albumin in albumin nanoparticles. J. Pharm. Biomed. Anal. 2015;111:186–189. doi: 10.1016/j.jpba.2015.03.031. [DOI] [PubMed] [Google Scholar]