For thousands of years humans have attempted to reduce complex phenomena—including thoughts, feelings, and behavior—to more simplistic, manageable explanations. Arguments have long existed about the relative importance of nature versus nurture—are a patient's difficulties primarily the result of the genes he or she was born with or the impact of his or her early experiences? It is clear, at this point, that the answer is usually both: a complicated interplay of genes and environment that develops over time.
The story of how and when environmental influences regulate complex biological processes in the brain has become one of the most exciting stories in neuroscience of the past several decades. Two key themes emerge from this work: first, that experience shapes the brain's development and functioning in clinically relevant ways, and second, that timing matters, with significant experiences at different phases of development producing distinct effects.
Epigenetics is the study of how the environment leads to stable changes in gene expression without altering the DNA sequence itself. In other words, in what ways can experience disrupt or enhance the transcription and translation of gene products? A central concept to this discussion is the idea that for genes to be expressed—that is, for proteins to be made—the DNA has to be accessible to transcription machinery. Two of the main mechanisms through which experience is known to affect this process are alteration to the structure of DNA itself (although the sequence remains unchanged) and alteration to the proteins around which DNA is looped (i.e., histones; Figure 1) (1).
Figure 1.
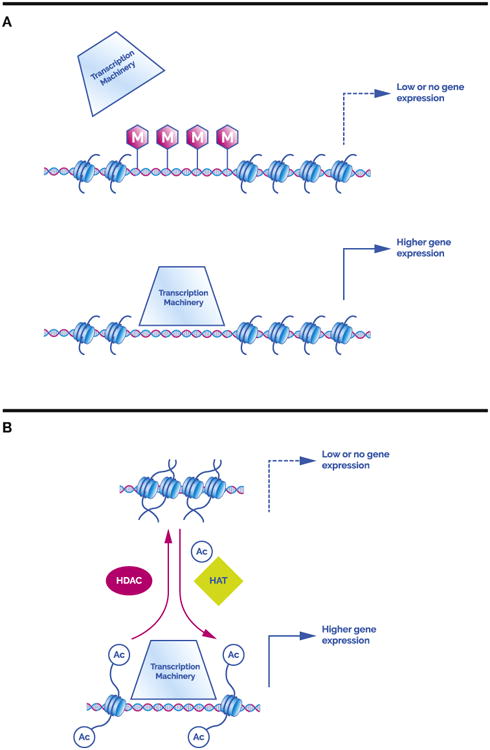
Epigenetic regulation of gene expression. (A) (Top) Methylated DNA prevents docking of the transcription machinery, resulting in low or no gene expression. (Bottom) Non-methylated DNA provides easier access for transcription machinery and facilitates gene expression. (B) (Top) Densely packed chromatin, with DNA wrapped tightly around histones, which are spaced close together; this configuration impedes access resulting in low gene expression. (Bottom) Acetylated (Ac) histones, which promote open, loose chromatin, which is more easily accessed and expressed. Histone deacetylases (HDAC) are enzymes that remove acetyl groups and histone acetyl transferases (HAT) are enzymes that add acetyl groups.
Epigenetic regulation at the DNA level is relatively straightforward. The primary mechanism is methylation, in which a sticky coating of methyl groups is added to the DNA, making it difficult for the transcription machinery to attach. This barrier leads to low gene expression or completely silenced genes. Though possible, it is relatively difficult to remove this layer of methyl groups. Thus, these changes are thought to result in relatively long-lasting inhibition of gene expression.
A second and more complicated type of regulation occurs at the level of the histone. Given the large amounts of DNA that must fit into tiny cells, DNA wraps around histones, or small protein complexes, and compacts into a dense, tight structure known as chromatin; for transcription to occur, chromatin must be unpacked. The histone code consists of a number of modification options for these protein complexes (e.g., methylation, acetylation, and several others), which act alone or in combination to regulate how loosely or tightly histones bind to DNA or how histones distance themselves from one another (1). Loose chromatin, in which DNA is relaxed and histones are spread apart, allows docking of the transcription machinery and promotes gene expression. Tightly coiled chromatin, with DNA and histones packed closely together, restricts DNA access and results in low gene expression. Acetylation is one of the best-studied modifications and usually is associated with open chromatin and enhanced gene expression (1). The enzymes that regulate acetylation (histone acetyl transferases to put on and histone deacetylases [HDACs] to take off) are constantly at work, making for a dynamic and fast-paced mode of gene access regulation.
Epigenetic mechanisms are sensitive to influences of both the cellular milieu and the life experience of the organism, as described below. They reflect fundamental processes through which experience alters gene expression and thereby influences the way that circuits, and ultimately people, behave. But not all experiences are equally impactful. Regulation of gene expression through epigenetics is highly dependent on both the specific type of experience and the developmental timing of those experiences.
The ability to respond effectively to a stressful event is a critical biological process. The hypothalamus plays a central role in both initiating and subsequently terminating this response, coordinating information from a variety of neural structures about internal states and external circumstances. Stressful experiences, ranging from stage fright to hand-to-hand combat, have a final common pathway of corticotropin-releasing hormone release from hypothalamic neurons. Corticotropin-releasing factor receptors on pituitary cells receive this signal and stimulate release of adrenocorticotropic hormone into the bloodstream. Adrenocorticotropic hormone stimulates cortisol release from the adrenal gland, which coordinates the actions of multiple organ systems to allow the person to contend with the stressor or flee.
Nearly as important as generating the proper stress response is efficiently terminating that response and returning the body to equilibrium after the acute stressor has resolved. Abnormalities in negative feedback to the hypothalamic-pituitary-adrenal (HPA) axis are linked to a variety of chronic health issues, including hypertension, obesity, metabolic syndrome, and a range of psychiatric illnesses (2). In general, input from structures like the prefrontal cortex and hippocampus provide negative regulation to the HPA axis, promoting termination of the stress response (3). The hypothalamus and these structures that regulate it are sensitive to early life experience, with stress during different developmental windows having selective effects.
Both human and animal studies suggest that severe stress to a pregnant mother is associated with HPA dysfunction in the offspring that persists into adulthood and carries an increased risk for psychiatric disorders such as depression; similar long-term effects are observed with direct stress to a neonate (4). Initially, the mechanism for these effects was not well understood. It is now clear that one prominent pathway is the epigenetic regulation of genes in prefrontal cortex and hippocampus, structures that negatively regulate the hypothalamus (5). Prenatally stressed animals have increased DNA methylation of important prefrontal developmental genes, such as brain-derived neurotrophic factor in excitatory neurons and glutamate decarboxylase 1 in inhibitory neurons. Consistent with an inhibitory effect of DNA methylation on gene expression, these animals show reduced brain-derived neurotrophic factor and glutamate decarboxylase 1 expression that persists into adulthood, which is linked with behavioral deficits (6).
The hippocampus is also sensitive to epigenetic programming by stress during the earliest developmental phases. Neglect during the first postpartum week in the rat, developmentally equivalent to the third trimester in humans, causes epigenetic changes in the hippocampus that drive significant HPA dysfunction in adulthood (7). A brief accessible review of this work can also be found online (8). This early stressful experience results in persistent DNA methylation of the glucocorticoid receptor (GR) gene in the hippocampus, resulting in long-lasting low GR expression. This low expression is linked with inefficient negative feedback in the HPA axis, prolonged stress hormone circulation, and a high stress reactivity phenotype. This epigenetic effect of early environment on gene expression is conserved across species, with increased hippocampal GR gene methylation and corresponding low GR expression observed in the postmortem brains of humans exposed to early life trauma (9).
In this issue of Biological Psychiatry, Morrison et al. (10) describe the impact that stress in the preadolescent period has on adult HPA regulation. They demonstrate that in both mice and human women, exposure to stress during the preadolescent period leads to dysregulated stress responses that emerged only during pregnancy and the peripartum period, suggesting that these long-lasting behavioral and endocrine effects emerge only within the hormonal milieu of pregnancy. Preadolescent stress and its downstream sequelae were linked to changes in adult hypothalamic gene expression, likely mediated by epigenetic mechanisms.
Taken together, these examples indicate that very early (prenatal or perinatal) stress epigenetically alters the prefrontal cortex and hippocampus, structures that negatively regulate the HPA axis, whereas preadolescent stress changes gene expression in the hypothalamus itself.
Epigenetics offers a biological framework for understanding the ways in which experience changes gene expression, thus providing a mechanism behind the clinical wisdom that most patients' difficulties are rooted in the interplay between genetic background and life experience. Early life experience appears particularly important, and different aspects of psychiatrically relevant circuits appear to be sensitive to epigenetic modification during different developmental windows.
The study of the epigenetic machinery paves the way for novel therapeutic approaches. For example, targeting epigenetic mechanisms in adulthood is able to reverse some of the stress-induced changes in gene expression described in animal models. One example is the use of histone deacetylase (HDAC) inhibitors, which promote histone acetylation (associated with loose, open chromatin and enhanced gene expression). HDAC inhibition in adulthood can reverse the impact of early life neglect on hippocampal GR gene expression in animals, overcoming the effects of persistent DNA methylation normally seen after early life neglect (7). While the search is on for selective compounds that act at specific epigenetic enzymes, some currently used psychiatric medicines have been shown to impact these systems. Both valproic acid and imipramine have HDAC inhibitory activity, and clozapine has been shown to reduce DNA methylation (6). Epigenetic mechanisms may also be at play in mediating the impact of reparative life experiences, such as psychotherapy. Epigenetics is a rapidly progressing field in psychiatric neuroscience, which emphasizes the power that experience has to change brain circuitry, with important implications for the etiology of mental illness and roads to recovery.
Acknowledgments
This work was supported by National Institutes of Health Grant Nos. R25 MH10107602S1 and R25 MH086466 07S1 (to DAR). This commentary was produced in collaboration with the National Neuroscience Curriculum Initiative (NNCI).
We thank Dr. Melissa Arbuckle for her contribution as NNCI editor and Amanda Wang for her role in developing the figure.
Footnotes
Disclosures: JBD reports no biomedical financial interests or potential conflicts of interest. DAR is co-chair of the NNCI and reports no other biomedical financial interests or potential conflicts of interest.
References
- 1.Szyf M. Epigenetics, a key for unlocking complex CNS disorders? Therapeutic implications. Eur Neuropsychopharmacol. 2015;25:682–702. doi: 10.1016/j.euroneuro.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laryea G, Muglia L, Arnett M, Muglia LJ. Dissection of glucocorticoid receptor-mediated inhibition of the hypothalamic-pituitary-adrenal axis by gene targeting in mice. Front Neuroendocri-nol. 2015;36:150–164. doi: 10.1016/j.yfrne.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jawahar MC, Murgatroyd C, Harrison EL, Baune BT. Epigenetic alterations following early postnatal stress: A review on novel aetiological mechanisms of common psychiatric disorders. Clin Epigenetics. 2015;7:122. doi: 10.1186/s13148-015-0156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Londono Tobon A, Diaz Stransky A, Ross DA, Stevens HE. Effects of maternal prenatal stress: Mechanisms, implications, and novel therapeutic interventions. Biol Psychiatry. 2016;80:e85–e87. doi: 10.1016/j.biopsych.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong E, Tueting P, Matrisciano F, Grayson DR, Guidotti A. Behavioral and molecular neuroepigenetic alterations in prenatally stressed mice: Relevance for the study of chromatin remodeling properties of antipsychotic drugs. Transl Psychiatry. 2016;6:e711. doi: 10.1038/tp.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: Clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- 8.Dwyer JB. Epigenetics and trauma. [Accessed February 17, 2017];2015 Available at: http://www.nncionline.org/course/epigenetics-borderline-personality-disorder/?course_type=content&course_page=3.
- 9.Labonte B, Yerko V, Gross J, Mechawar N, Meaney MJ, Szyf M, et al. Differential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse. Biol Psychiatry. 2012;72:41–48. doi: 10.1016/j.biopsych.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Morrison KE, Epperson CN, Sammel MD, Ewing G, Podcasy JS, Hantsoo L, et al. Preadolescent adversity programs a disrupted maternal stress reactivity in humans and mice. Biol Psychiatry. 2017;81:693–701. doi: 10.1016/j.biopsych.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]


