Abstract
Angle-closure glaucoma includes a number of entities with closed angle, elevated intraocular pressure, in association with optic nerve damage and visual field defects as common markers. These entities are characterized by irido-trabecular apposition, irido-trabecular synechiae or both. The angle configuration must be systematically checked at least one time in patients presenting with raised intraocular pressure or glaucoma.
Gonioscopy represented for a long time the gold standard for clinically assessing anterior chamber angle structures and their configuration. However, the interpretation of gonio-scopic findings is subjective and only semiquantitative.
With the development of new imaging techniques of the anterior segment, new analysis methods have also emerged. Ultrabiomicroscopy was the first method of analyzing the anterior segment and is still the only imaging technique for all anterior segment structures (especially the ciliary body). Another method is optical coherence tomography, a non-contact technique by which angle configuration can be assessed in a more rapid and less invasive manner. Recently developed Pentacam technology could represent in the near future a more quantitative, rapid and non-invasive screening tool which could allow early detection of angle closure glaucoma and narrow angle configurations by measuring a set of anterior chamber parameters.
List of abbreviations:
ACG –angle closure glaucoma, ASOCT-anterior segment optical coherence tomography
UMB- ultrasound biomicroscopy (ultrabiomicroscopy), PAS-posterior angle synechiae
ACD-anterior chamber depth, ACV-anterior chamber volume, PLI-periphery laser iridotomy
Keywords: anterior chamber angle, gonioscopy, ultrabiomicroscopy, Pentacam
Introduction
Angle-closure glaucoma represents the second most common type of glaucoma, but its impact is more critical due to a greater likelihood of blindness than in patients with open angle glaucoma. A timely and accurate diagnosis is essential in order to start the appropriate and specific treatment that may prevent progression to greater and irreversible damage [1].
Angle closure entities can be classified into primary and secondary forms. Primary angle-closure glaucoma occurs in an anatomically and functionally predisposed eye, not as a consequence of other ocular or systemic abnormalities. Secondary forms of angle-closure glaucoma are caused by other ocular or systemic abnormalities (uveitis, neovascular glaucoma, Marfan´s Syindrome), medications, such as topiramate [2].
Depending on the characteristics of the anterior chamber angle, intraocular pressure and optic nerve findings, we distinguish four distinct categories of primary angle closure: angle closure suspect (occludable angle), acute angle closure, intermittent angle closure and chronic angle-closure glaucoma [1].
In primary angle-closure suspects, the trabecular meshwork can only be seen in 180º or less by gonioscopy, the intraocular pressure remains within normal limits and no structural damage to the optic nerve is present. Eyes with intermittent angle closure have similar gonioscopic findings but the intraocular pressure becomes occasionally elevated and drops to normal values shortly, with still no damage to the optic nerve. Additionally, chronic angle-closure glaucoma eyes have evident signs of optic nerve damage. If the intraocular pressure remains at a very high level due to circumferential iris apposition to the trabecular meshwork, an acute angle closure must be considered [1,3].
Ocular anatomic characteristics
Primary angle-closure glaucoma eyes have an average axial length about 1 mm shorter than normal eyes, making hyperopes more predisposed than emmetropes or myopes to angle closure. Other anatomical risk factors that should also be sought in the fellow eye of a patient with acute angle-closure glaucoma, are the following:
-occludable angles
-short eyes
-shallower anterior chamber depth
-thicker lenses
-a closer relationship between the lens and the posterior iris surface [4, 5].
Due to the fact that eyes with occludable angles are characterized by shorter axial lengths, women tend to more at risk by having shorter eyes than men (22.07 mm vs. 22.58 mm respectively). In a similar way the proportion of lens thickness to axial length is significantly greater in this group of patients. About 22% of occludable angles progress towards closed-angle glaucoma [6].
Angle anatomy
In normal angles the following structures should be seen in gonioscopy: Schwalbe´s line, trabecular meshwork, scleral spur and the ciliary body band. Some other findings may be present in normal or abnormal angles. Schwalbe's line is the most anterior structure seen on gonioscopy - a collagen condensation of Descemet´s membrane, which lies between the corneal endothelium and the trabecular meshwork. It is normally seen as a thin, translucent line that protrudes into the anterior chamber. This prominence is quite variable and may have heavy pigmentation over it.
Continuing posterior to Schwalbe´s line is the trabecular meshwork. It extends to the scleral spur, has a dull gray appearance and is somewhat translucent, except for a tenuous pigmentation of the lower half of the trabecular meshwork. Schlemm’s canal can be seen through it sometimes, when blood refluxes during gonioscopy.
The next posterior structure is the scleral spur; a short extension of sclera forming the inferior wall of a scleral pocket where Schlemm's canal rests and the longitudinal ciliary muscle normally inserts. It appears white and opaque and is seen as a thin white line below the trabecular meshwork.
The ciliary body band is seen on gonioscopy below the scleral spur as a pale gray to dull brown band. The width of visible ciliary band will depend on the iris insertion, and this fact makes it variable [2,4].
Other findings
- Pigmentation
Chronic episodes of angle closure may leave patches of pigment at the level of contact, mostly at the trabecular meshwork, but depending on the degree of apposition, the pigment clumps may be seen above Schwalbe’s line. Heavy, but diffuse, pigmentation of the trabecular meshwork is more typical in pigment dispersion syndrome and pseudoexfoliation. The presence of more diffuse and somewhat grey pigmentation above Schwalbe’s line is called Sampaolesi’s line, and is highly suggestive of pseudoexfoliation syndrome. It can be concurrent with ACG, especially when the zonules begin to become affected and the lens tends to move forward. Previous trauma is another cause of angle pigmentation, but it is usually accompanied by other more prominent signs, such as pupillary sphincter ruptures, angle recession or a cyclodialysis cleft, but it can also be confirmed by the subtle finding of disinserted or ruptured iris processes.
- Iris processes
Normal iris processes are fine strands of iris tissue that can reach the scleral spur, or even the posterior third of the trabecular meshwork. Long iris processes are more anterior and reach anterior portions of the trabecular meshwork.
- Blood vessels
Blood vessels may be a normal finding or a sign of disease. Normal blood vessels are usually found circumferential and close to the scleral spur, but never above it. Abnormal vessels on the other hand, are usually due to retinal hypoxia or some forms of uveitis. They cross over the scleral spur and cover the trabecular meshwork, initially in segments. Neovessels eventually interfere with aqueous outflow, and cause secondary angle closure due to peripheral anterior synechiae (PAS). When associated with Fuch’s heterochomic iridocyclitis, neovessels tend to be finer, more fragile, and almost never reach beyond the trabecular meshwork or cause PAS [2,5].
Angle exploration methods
VAN HERRICK’S GRADING
Van Herick´s method is an integral part of eye examination and is used to describe the peripheral anterior chamber depth by using an oblique beam of light at the slit-lamp. The angle is considered as non-occludable when there is an space between the endothelium and the anterior iris surface that measures at least one half of the peripheral corneal thickness [7].
Van Herick´s method is easy to perform and correlates well with gonioscopy, but cannot replace it (Table 1). If one uses the van Herrick´s method like the only form of angle evaluation, important information will be missed, such as peripheral iris shape, relationship between the anterior surface of the lens and the posterior surface of the iris, number of angle structures seen with or without indentation, presence or absence of PAS and its extension or changes in angle opening in dark/light conditions [7, 8].
Table 1.
Van Herrick´s grading system versus Schaeffer’s gonioscopic classification
| Grade | Risc of angle closure | Schaeffer | Van Herrick | |
|---|---|---|---|---|
| 0 | 0° | yes | Irido-corneal contact | Irido-corneal contact |
| I | 10° | yes | Schwalbe’s line | < 1/4 of corneal thickness |
| II | 20° | possible | Trabecular meshwork | > 1/4 < 1/2 of corneal thickness |
| III | 25-35° | no | Scleral spur | > ½ corneal thickness |
| IV | 35-45° | no | Ciliary band | = corneal thickness |
GONIOSCOPY
Gonioscopy is the oldest method used to determine the anterior chamber angle characteristics such as the level of iris insertion, the shape of the peripheral iris, the width of the angle, the degree of trabecular pigmentation and areas of PAS or apposition. The anterior chamber angle can be evaluated by direct or indirect gonioscopy [9].
- Direct gonioscopy
In direct gonioscopy, light from the anterior chamber passes through the cornea and through a contact goniolens, permitting a direct and adequately magnified view of angle structures, and making simultaneous comparison of both eyes possible. One of the most common goniolenses used for this technique is Koeppe’s contact goniolens [ 9,10].
- Indirect gonioscopy
In this technique light from the anterior chamber is reflected on a mirror, allowing an inverted view of the anterior chamber angle. Indirect gonioscopy must be performed in all glaucoma patients and suspects at least once a year. It represents the gold standard technique for categorizing glaucoma suspects into open or closed-angle categories.
The three-mirror Goldmann’s lens facilitates application of laser (trabeculoplasty), but requires rotating the lens in order to view all quadrants at the same time and cannot be used for performing indentation gonioscopy. Indentation gonioscopy must be done when van Herick’s grading is suggestive of angle-closure or the patient is being evaluated as an angle-closure glaucoma suspect [10, 11].
- Common classifications of the anterior chamber angle
Gonioscopy grading systems are useful to record findings using a systematic approach.
They help classify patients into open, occludable or closed angle varieties and allow comparisons between repeated gonioscopic observations in the same eye. There are two types of classification: simpler systems that only evaluate the degree of angle opening (Shaffer and Scheie systems) and more comprehensive ones, such as Spaeth’s system, that also evaluates the level of iris insertion, iris configuration and extent of angle opening. The latter is a bit more time consuming because of its sophistication, and might be difficult to perform on a demanding setting [2, 10].
For routine clinical evaluation we prefer Shaffer´s system, which evaluates the number of visible angle structures while maintaining the surface of the gonioscopic lens perpendicular to the observation axis, taking care to avoid inadvertently changing angle structures during examination. Dynamic indentation and dark/light gonioscopy should be performed in all cases being evaluated for narrow angles or when van Herrick´s method is suggestive of angle-closure, in order to verify the presence of PAS, or apposition between the iris and trabecular structures (Table 1). Scheie’s system is designed to describe closure, also based on the number of visible angle structures, so grade 0 corresponds to a wide open angle and 4 to a closed angle [11, 12].
Ultrabiomicroscopy
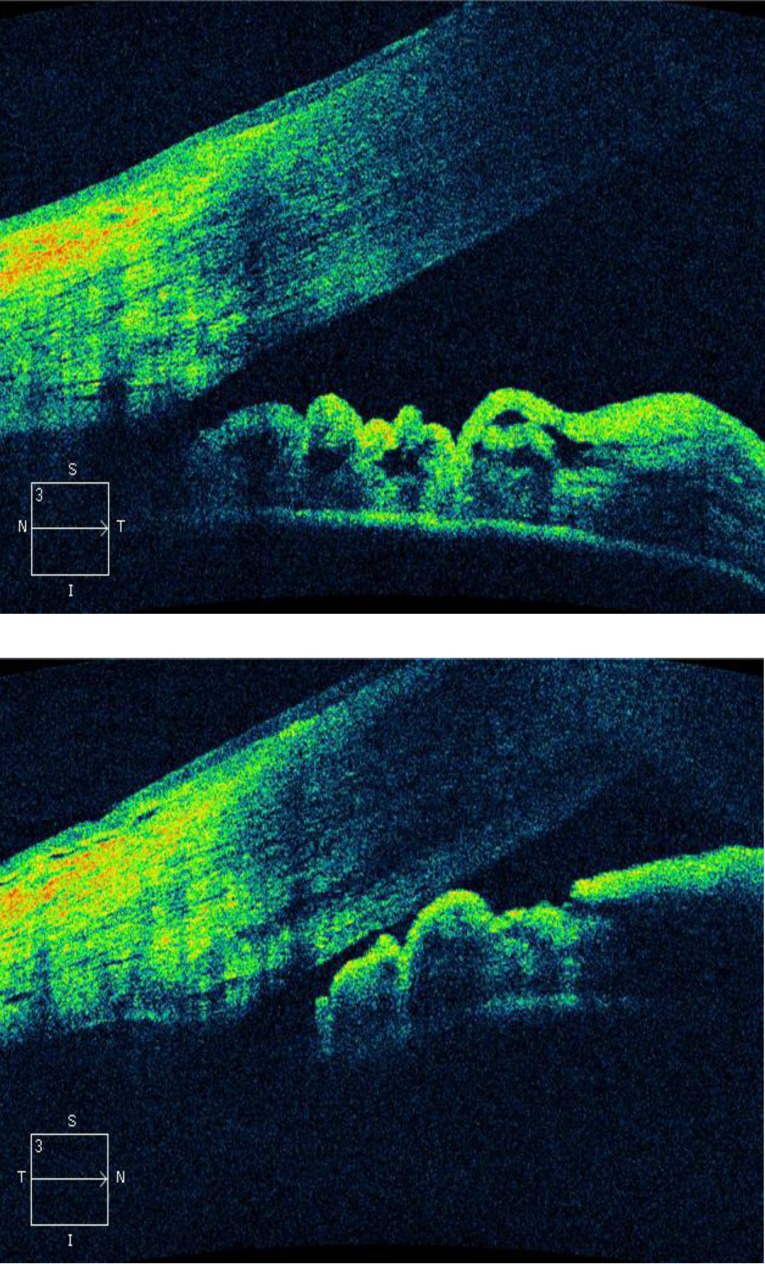
Modern ultrasound techniques which are now available contribute to the understanding of anatomical mechanisms participating in angle-closure. UBM is a relatively new technique developed in 1990 by Pavlin and Foster. It is a very high frequency ultrasound (50-80 MHz) that allows visualization of anterior segment structures with a lateral resolution of 50 microns and an axial resolution of 20 microns. It can obtain images of the ciliary body, zonule, lens, iris, angles, anterior chamber and cornea (Fig. 1). Higher frequencies (100 MHz) have been developed enabling the visualization of Schlemm’s canal [13,14].
Fig. 1.
The UBM appearance of the anterior segment of a normal eye. The cornea (C), anterior chamber (AC), iris (I), lens capsule (LC), posterior chamber (PC), angle (white arrow), scleral spur (thin black arrow), Schwalbe's line (thick blackarrow) sclera (S), and ciliary body (CB) are visible [14]
It is possible to analyze in vivo mechanisms of interaction among anterior segment structures. Ritch et al. have used UBM to identify four possible anatomic sites of origin of angle-closure: the iris (papillary block), ciliary body (plateau iris), lens (phacomorphic glaucoma) and space behind the lens (malignant glaucoma) [15].
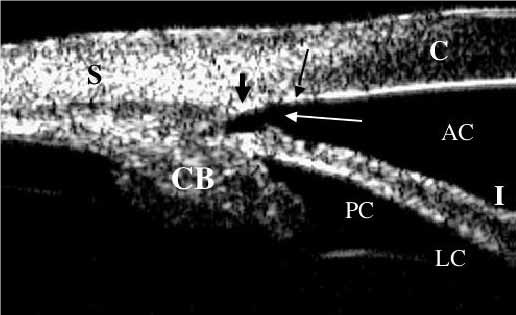
UBM is extremely useful in establishing the pathophysiological changes involving the anterior segment architecture (Fig. 2). It has traditionally been used as the standard for anterior segment imaging but, while rendering excellent quality images, it can be difficult to use as it requires a scleral shell (water bath) in order to obtain echographic coupling [16,17].
Fig. 2.
Pupillary block angle-closure - the iris has a convex configuration (white arrows) due to the relative pressure difference between the posterior chamber and the anterior chamber [14]
However, new generation linear probes have made UBM more practical. In that they no longer require a water bath, and need less user expertise. Lateral distortion is also minimized by the linear scan. UBM can be also useful in evaluating secondary angle closure glaucoma, such as those caused by iridociliary cysts, lens subluxation or microspherophakia [18,19].
Anterior segment optical coherence tomography
ASOCT is a non-contact, optical instrument that uses a wavelength of 1310 nm and permits acquisition of images of anterior segment with a transverse resolution of 60 microns and an axial resolution of 10-20 microns. It has the disadvantage of light absorption by the sclera and iris, so structures such as the ciliary body and the iris-anterior capsule interaction are not visible. Even though the scleral spur is harder to detect by anterior segment optical coherence tomography in open and closed angles, a quantitative analysis of the angle is still possible [20,21].
The use of the infrared laser and the non-contact technique during examination allows capturing of the angle morphology in the dark. Moreover, ASOCT has the potential to provide valuable quantitative and spatial information regarding dynamic changes of the angle configuration not provided by standard gonioscopy. Compared to other diagnostic techniques that analyze the anterior segment, such as UBM, ASOCT has more advantages for the patient because it is a non-invasive, reproducible, fast and well-tolerated method [22-24].
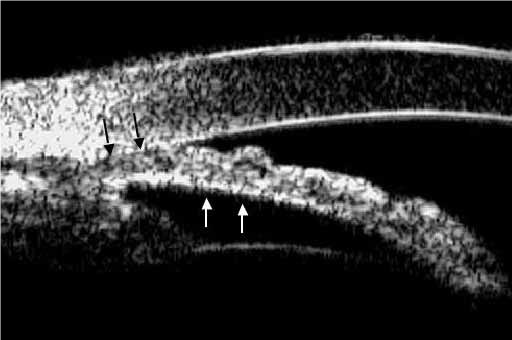
ASOCT has become a useful tool in the evaluation and biometric analysis of the anterior segment in angle closure. Iris apposition to the trabecular meshwork is the final common pathway of angle closure/angle closure glaucoma, caused by one or more abnormalities in the relative or absolute sizes or positions of the anterior segment structures or by abnormal forces in the posterior segment that alter the anatomy of the anterior segment (Fig. 3).
Fig. 3.
ASOCT comparison between a normal open anterior chamber angle (left) versus a narrow angle at risc of iminent angle closure (right)
Even if dynamic indentation gonioscopy with a gonioprism is the current reference standard for clinically assessing anterior chamber angle structures and their configuration, ASOCT is a technique for completing the clinical examination that could provide more than simple qualitative infor-mation and, when associated with optic nerve and retinal thickness assessment, allows a broader assessment of a clinical state [25,26].
This method seems to offer a more convenient and rapid method of assessing the anterior chamber configuration and may help during routine clinical assessment and treatment of patients with narrow or closed angles, particularly when gonioscopy is difficult to interpret, as in highly pigmented angle [26-28].
With the development of a new generation of OCT and the emergence of three-dimensional acquisition, ASOCT currently allows imaging of the entire circumference of the angle rather than one meridian, making this technique clinically more accomplished [29-31].
The pentacam glaucoma module
Rotating Scheimpflug imaging technology is used by instruments such as Pentacam (Oculus) for measuring the anterior and posterior corneal surfaces, as well as other anterior segment structures. Initially, Scheimpflug photography was used to obtain images of lens opacities for objective evaluation, but Pentacam - Scheimpflug camera is much wider in its clinical applications than the Scheimpflug photography alone, as a diagnostic tool not only for corneal diseases like keratoconus, cataract and refractive surgery planning, but glaucoma specialists as well [32,33].
The Pentacam represents the latest development among ophthalmic camera systems based on Scheimpflug’s principle, the first multipurpose instrument providing five different measurement options for the anterior eye segment. These are: pachymetry, corneal topography, anterior and posterior corneal curvature, astigmatism determination and Scheimpflug photography of the lens [34].
UBM has a higher resolution and is used as a diagnostic tool for the anterior segment of the eye providing images that delineate anterior segment structures in vivo; however, it requires an immersion technique, limiting its usefulness for patient with corneal epithelial problems, infectious disorders and postoperative patients. Noncontact methods, such as the Pentacam, proved to be more advantageous for corneal conditions associated with epithelial disorders, almost as a rule [35,36].
- Glaucoma Screening using the Pentacam
The Pentacam-Sheimpflug camera is a non-contact high resolution imaging system that constructs a 3 dimensional image of anterior segment. As far as its applications in glaucoma, the parameters like anterior chamber depth (ACD), central as well as peripheral; anterior chamber volume (ACV), corneal thickness (apical) and anterior chamber angle, as well as inbuilt IOP correction formulae, have been used in patients with narrow angles [36,37].
Following are some of the clinical applications in glaucoma:
1. The Pentacam glaucoma module is able to directly measure the effect of pilocarpine on ACD and ACV in eyes with narrow angle and open angles. Pilocarpine 2% solution decreased central ACD, ACV but had angle opening effect by causing relatively less shallowing of peripheral ACD. Studies prove that pilocarpine causes shallowing of anterior chamber—central ACD decreased by 97 microns (p = 0.48). ACV decreased by 5.7 mm3, which was statistically insignificant (p = 0.70) compared to the degree of angle widening [38].
2. ACV has been found to be a good screening tool for diagnosing eyes with narrow angles. Several studies confirm a good sensitivity and specificity for ACV in eyes with narrow angles. The software provides a colored map of the anterior chamber depth-both central and peripheral [39]. With ACV of 110 mm3 as cut off to define narrow angle, the Pentacam had a sensitivity of 88.37% and specificity = 90.62% with a positive predictive value of 92.7 and negative predictive value of 85.3. Any patient having ACV of <110 mm3 has 9,42 times chance of having a narrow angle on gonioscopy [39,40]
3. The dynamics of the anterior chamber including the ACV can be studied following procedures like laser peripheral iridotomy (PLI). Studies show that after PLI there was a significant increase in ACV by 28.36 mm3, which was significantly persistent at 1-week and 4-week post PLI. The percentage change in peripheral ACD was maximum and the effect of PLI increased with increasing distance from the optical axis. The central ACD did not change significantly immediately after PLI [40].
4. Oka et al reported that the ACV for the narrow angle group (74.5 +/– 21.1) was significantly smaller than for the other groups (post PLI group: 96.4 +/– 21.4; open angle group: 144.2 +/–31.6, p < 0.001). The most significant association was detected between ACV and the peripheral ACD. Only two parameters, ACV and peripheral ACD, increased significantly after PLI and concluded that the measurement of the ACV and the peripheral ACD using Pentacam is useful for evaluating the anterior segment topography in eyes with narrow angles [41].
Various IOP correction formulae are incorporated in the Pentacam software based on the central corneal thickness. However it is limited in its evaluation of the anterior chamber angle as compared to ASOCT. This is due to the reflectance from the scleral surface and hence, inability to visualize the scleral spur. Direct angle visualization of the scleral spur, ciliary body, and ciliary sulcus is possible only with the ASOCT and UBM. Pentacam measurements in closed angles have a limited correlation with gonioscopy and ultrabiomicroscopy [42].
Fig. 4.
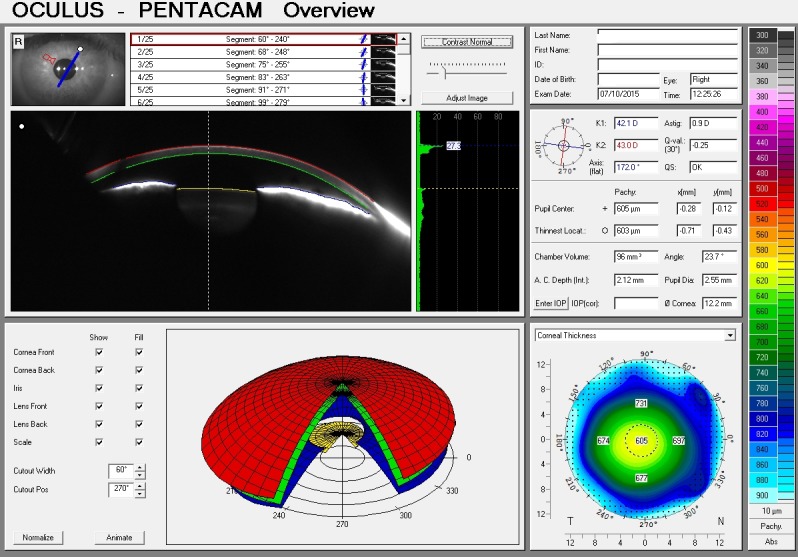
Pentacam glaucoma module showing low ACD and ACV measurements, suggesting a positive diagnosis of angle closure glaucoma.
Conclusions
Presently, we possess a wide range of instruments which allow us to accurately explore the configuration of the anterior chamber angle, enabling us to confirm or infirm the diagnosis of angle closure glaucoma and to further guide our patient towards the appropriate therapeutic approach.
Although gonioscopy still represents a redoubtable method for detailed visualization of the irido-corneal angle architecture, it becomes sometimes time-consuming and rather uncomfortable for the patient, not to say difficult to use in cases of damaged corneal epithelium or infections disorders.
The same limitations must be taken into consideration when exploring the anterior segment using UBM because, although its resolution and ability to identify different structures is clearly superior to gonioscopy, it requires a scleral bath for coupling and a well-trained operator in order to correctly perform the examination.
By contrast, ASOCT is a rapid and non-invasive method for quickly assessing the anterior chamber angle. It renders simple qualitative information capable of guiding us through routine clinical assessment and treatment of patients with narrow or closed angles, particularly when goni¬oscopy is difficult to apply or interpret.
The Pentacam is also a non-contact instrument which proved to be useful in measuring the irido-corneal angle, although difficult in complete 360° because of eyelid interference. It offers a more quantitative approach to the anterior chamber than ASOCT, by measuring peripheral ACD and ACV. The pachymetric measurements are useful when time is of the essence and a quick evaluation must be made. Moreover, the examination using the Pentacam is helpful in educating the patient about his disease, and making evident the effects of the treatment. Therefore, it has the potential to become a future screening tool for diagnosing angle closure glaucoma.
References
- 1.European Glaucoma Society Terminology and guidelines for glaucoma. EUGS Publicomm. 2014:100–119. [Google Scholar]
- 2.Yanoff M, Duker JS. Ophtalmology. Mosby; 2004. pp. 367–390. [Google Scholar]
- 3.Congdon N, Friedman D. Angle-closure glaucoma: impact, etiology, diagnosis, and treatment. Curr Opin Ophthalmol. 2003;14(2):70–73. doi: 10.1097/00055735-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Singh P, Tyagi M, Kumar Y, Kuldeep K, Das Sharma P. Gonioscopy: A Review. Open Journal of Ophthalmology. 2013;3:118–121. [Google Scholar]
- 5.Priya L, Dabasia B, Edgar DF, Lawrenson JG. Methods of measurement of the anterior chamber angle Part 1: Angle closure glaucoma and gonioscopy. Optometry in Practice. 2013;14(3):107–117. [Google Scholar]
- 6.Aung T, Lim MC, Chan YH, Rojanapongpun P, Chew PT. Configuration of the drainage angle, intraocular pressure, and optic disc cupping in subjects with chronic angle-closure glaucoma. Ophthalmology. 2005;112(1):28–32. doi: 10.1016/j.ophtha.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Källmark FP, Sakhi M. Evaluation of Nasal and Temporal Anterior Chamber Angle with Four Different Techniques. International Journal of Clinical Medicine. 2013;4:548–555. [Google Scholar]
- 8.Pettersson L, Källmark F. Difference in the anterior chamber angle of the four meridians. Journal of Applied Medical Sciences. 2012;1(1):1–13. [Google Scholar]
- 9.Dabasia PL, Edgar DF, Murdoch IE, Lawrenson JG. Noncontact Screening Methods for the Detection of Narrow Anterior Chamber Angles. Invest Ophthalmol Vis Sci. 2015;56:3929–3935. doi: 10.1167/iovs.15-16727. DOI:10.1167/ iovs.15-16727. [DOI] [PubMed] [Google Scholar]
- 10.Foster PJ, Devereux JG, Alsbirk PH, Lee PS, Uranchimeg D, Machin D, Johnson GJ, Baasanhu J. Detection of gonioscopically occludable angles and primary angle closure glaucoma by estimation of limbal chamber depth in Asians: modified grading scheme. Br J Ophthalmol . 2000;84(2):186–192. doi: 10.1136/bjo.84.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman DS. Anterior chamber angle assessment techniques. Surv Ophthalmol . 2008;53(3):250–273. doi: 10.1016/j.survophthal.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Marsh BC, Cantor LB. The Spaeth Gonioscopic Grading System. Glaucoma Today. 2005;102(2):15–23. [Google Scholar]
- 13.Gohdo T, Tsumura T, Iijima H, Kashiwagi K, Tsukahara S. Ultrasound biomicroscopic study of ciliary body thickness in eyes with narrow angles. Am J Ophthalmol . 2000;129(3):342–346. doi: 10.1016/s0002-9394(99)00353-0. [DOI] [PubMed] [Google Scholar]
- 14.Silverman RH. High-resolution ultrasound imaging of the eye – a review. Clin Experiment Ophthalmol. 2009 Jan;37(1):54–67. doi: 10.1111/j.1442-9071.2008.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritch R, Schlötzer-Schrehardt U. Exfoliation Syndrome. Surv Ophthalmol. 2001;45(4):265–315. doi: 10.1016/s0039-6257(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 16.Yoo C, Oh JH, Kim YY, Jung HR. Peripheral anterior synechiae and ultrasound biomicroscopic parameters in angle-closure glaucoma suspects. Korean J Ophthalmol. 2007;21(2):106–110. doi: 10.3341/kjo.2007.21.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mingguang H, Friedman DS, Ge J, Huang W, Jin C, Cai X, Khaw PT, Foster PJ. Laser Peripheral Iridotomy in Eyes with Narrow Drainage Angles: Ultrasound Biomicroscopy Outcomes. The Liwan Eye Study Ophthalmology. 2007;114(8):56–62. doi: 10.1016/j.ophtha.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Puech M. UBM and glaucoma: diagnosis and follow-up of plateau iris. Réalités Ophtalmologiques. 2013;204(1):145–150. [Google Scholar]
- 19.Ishikawa H, Schuman JS. Anterior segment Imaging: ultrasound biomicroscopy. Ophthalmol Clin North Am. 2004;17(1):7–20. doi: 10.1016/j.ohc.2003.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L. Anatomical Changes of the Anterior Chamber Angle With Anterior-Segment Optical Coherence Tomography. Arch Ophthalmol. 2008;126(12):1682–1686. doi: 10.1001/archopht.126.12.1682. doi:10.1001/archopht.126.12.1682. [DOI] [PubMed] [Google Scholar]
- 21.Wong HT, Lim MC, Sakata LM, Aung HT, Amerasinghe N, Friedman DS, Aung T. High-definition optical coherence tomography imaging of the iridocorneal angle of the eye. Arch Ophthalmol. 2009;127(3):256–260. doi: 10.1001/archophthalmol.2009.22. [DOI] [PubMed] [Google Scholar]
- 22.Dada T, Sihota R, Gadia R, Aggarwal A, Mandal S, Gupta V. Comparison of anterior segment optical coherence tomography and ultrasound biomicroscopy for assessment of the anterior segment. J Cataract Refract Surg . 2007;33(5):837–840. doi: 10.1016/j.jcrs.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Sakata LM, Lavanya R, Friedman DS, Aung HT, Seah SK, Foster PJ, Aung T. Assessment of the scleral spur in anterior segment optical coherence tomography images. Arch Ophthalmol. 2008;126(2):181–185. doi: 10.1001/archophthalmol.2007.46. [DOI] [PubMed] [Google Scholar]
- 24.See JL, Chew PT, Smith SD, Nolan WP, Chan YH, Huang D, Zheng C, Foster PJ, Aung T, Friedman DS. Changes in anterior segment morphology in response to illumination and after laser iridotomy in Asian eyes: an anterior segment OCT study. Br J Ophthalmol. 2007;91(11):1485–1489. doi: 10.1136/bjo.2006.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakata LM, Lavanya R, Friedman DS, Aung HT, Gao H, Kumar RS, Foster PJ, Aung T. Comparison of gonioscopy and anterior segment ocular coherence tomography in detecting angle closure in different quadrants of the anterior chamber angle. Ophthalmology. 2008;115(5):769–774. doi: 10.1016/j.ophtha.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Leung CK, Cheung CY, Wong L, Pang CP, Weinreb RN, Lam DS. Repeatability and reproducibility of anterior chamber angle measurement with anterior segment optical coherence tomography. Br J Ophthalmol. 2007;91(11):1490–1492. doi: 10.1136/bjo.2007.118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radhakrishnan S, Goldsmith J, Huang D, Westphal V, Dueker DK, Rollins AM, Izatt JA, Smith SD. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol. 2005;123 (8):1053–1059. doi: 10.1001/archopht.123.8.1053. [DOI] [PubMed] [Google Scholar]
- 28.Mansouri K, Sommerhalder J, Shaarawy T. Prospective comparison of ultrasound biomicroscopy and anterior segment optical coherence tomography for evaluation of anterior chamber dimensions in European eyes with primary angle closure. Eye (Lond) . 2010;24 (2):1476–5454. doi: 10.1038/eye.2009.103. [DOI] [PubMed] [Google Scholar]
- 29.Muller M, Dahmen G, Porksen E, Geerling G, Laqua H, Ziegler A, Hoerauf H. Anterior chamber angle measurement with optical coherence tomography: intraobserver and interobserver variability. J Cataract Refract Surg . 2006;32 (11):1803–1808. doi: 10.1016/j.jcrs.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Pekmezci M, Porco TC, Lin SC. Anterior segment optical coherence tomography as a screening tool for the assessment of the anterior segment angle. Ophthalmic Surg Lasers Imaging. 2009;40(4):389–398. doi: 10.3928/15428877-20096030-07. [DOI] [PubMed] [Google Scholar]
- 31.Nolan WP, See JL, Chew PT, Friedman DS, Smith SD, Radhakrishnan S, Zheng C, Foster PJ, Aung T. Detection of primary angle closure using anterior segment optical coherence tomography in Asian eyes. Ophthalmology. 2007;114 (1):1549–4713. doi: 10.1016/j.ophtha.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 32.Alonsa RS, Ambrosio R, Parahnos A, Sakata LM, Ventura MP. Glaucoma anterior chamber morphometry based on optical Scheimpflug images. Arq Bras Oftalmol. 2010;73(6):497–500. doi: 10.1590/s0004-27492010000600005. [DOI] [PubMed] [Google Scholar]
- 33.Holladay JT, Michelson MA, Woodhams JT, Ahmed IK. The Pentacam: Precision, Confidence, Results, and Accurate “Ks!”. Insert to Cataract&Refractive Surgery Today . 2007 [Google Scholar]
- 34.Mou D, Fu J, Li S, Wang L, Wang X, Wu G, Qing G, Peng Y, Wang N. Narrow and open-angle measurements with anterior-segment optical coherence tomography and Pentacam. Ophthalmic Surg Lasers Imaging. 2010;41(6):1938–2375. doi: 10.3928/15428877-20100929-06. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Wang Z, Cao X, Hu L, Tian F, Dai H. Pentacam could be a useful tool for evaluating and qualifying the anterior chamber morphology. Int J Clin Exp Med. 2014;7(7):1878–1882. [PMC free article] [PubMed] [Google Scholar]
- 36.Rabsilder TM, Koramnia R, Auffarth GU. Anterior chamber measurements using Pentacam rotating Scheimpflug camera. Journal of Cataract and Refractive Surgery. 2006;32(3):456–459. doi: 10.1016/j.jcrs.2005.12.103. DOI: 10.1016/j.jcrs.2005.12.103. [DOI] [PubMed] [Google Scholar]
- 37.Esmaeili A, Barazandeh B, Ahmadi S, Haghi A, Abolbashari F. Assessment of the anterior chamber parameters after laser iridotomy in primary angle close suspect using Pentacam and gonioscopy. Int J Ophatlmol. 2013;6(5):680–684. doi: 10.3980/j.issn.2222-3959.2013.05.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain R, Grewal D, Grewal SPS. Pentacam evaluation of changes in Anterior Chamber Depth and Volume in cases with Primary Angle Closure after instillation of Pilocarpine. Asian J Ophtalmol. 2006;8(6):310. [Google Scholar]
- 39.Jain R, Grewal D, Grewal SPS. Predictive value of Anterior Chamber Volume on Scheimpflug Imaging in Eyes with Narrow Angle. Asian J Ophtalmol. 2007;9(1):58. [Google Scholar]
- 40.Jain R, Grewal D, Grewal SPS. Changes in anterior chamber depth and Volume following laser peripheral laser iridotomy in eyes with Primary Angle closure using Pentacam. Asian J Ophtalmol. 2006;8(6):64. [Google Scholar]
- 41.Oka N, Otori Y, Okada M. Clinical study of anterior ocular segment topography in angle-closure glaucoma using the three-dimensional anterior segment analyzer Pentacam. Nippon Ganka Gakkai Zasshi. 2006;110(5):398–403. [PubMed] [Google Scholar]
- 42.Yi J, Lee H, Hong S. Anterior Chamber Measurements by Pentacam and AS-OCT in Eyes With Normal Open Angles. Korean J Ophthalmol. 2008;22:242–245. doi: 10.3341/kjo.2008.22.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]