Abstract
Optical aberrations lead to defects in image-forming, the image obtained being imperfect and thereby decreasing the quality of vision. When an optic system is not perfect, as happens with the eye, the rays of light that pass through the system produce optical aberrations.
The purpose of this review is to describe optical aberrations and their impact on vision and how refractive surgery outcomes are influenced by them.
The main optical aberrations of the eye are as follows: spherical aberration, chromatic aberration, oblique astigmatism and high order aberrations. When the patient undergoes various types of surgeries (cataract surgery, corneal refractive surgery) the properties of the eye change and the eye doctor must take into account the correction of optical aberrations to improve vision quality.
Abbreviations: LASIK (laser in situ keratomileusis), PRK (photorefractive keratectomy), UDVA (uncorrected distance visual acuity), SA (spherical aberrations), HOA (higher-order aberrations), RMS (root mean square)
Keywords: optical aberrations, refractive surgery, LASIK, wavefront-guided ablation
Introduction
Laser refractive surgery represents one of the most remarkable inventions in eye surgery. Since 1990 when the first laser in-situ keratomileusis (LASIK) procedure was described by Pallikaris [1], people worldwide have turned to refractive surgery and gave up glasses or contact lenses. Nowadays the most used refractive procedures are lamellar (LASIK) and surface (like photorefractive keratectomy (PRK)) ablations of the cornea who aim to achieve an uncorrected distance visual acuity (UDVA) of 20/20 Snellen. Despite the good UDVA results there are patients unsatisfied with their surgery outcome and one of the reasons blamed might be the increase in high -order optical aberrations. Some of the most frequent complications after refractive surgery are glare and halo, especially if the surgeon deals with large pupils or uses a small ablation diameter [2]. Many studies regarding changes in corneal [3] and wavefront aberrations [4,5] show that best corrected image quality decreases after refractive surgery.
The purpose of this review is to describe optical aberrations and their impact on vision and how refractive surgery outcomes are influenced by them. The review is divided in three parts: one regarding optical aberrations, the second regarding laser refractive surgery and the last concerning the impact refractive surgery has on eye’s optical aberrations and quality of vision.
Optical aberrations:
According to Dr J. Holladay [6] good vision is more than 20/20 on a Snellen visual chart. The modern ophthalmologist should understand that contrast sensitivity, near and distance vision, performance under light and dark conditions, and the brain’s interpretation of input from the sensory apparatus, are all important elements in patients’ quality of vision [6]. Quality of vision is influenced by the presence of aberrations in the eye’s optical system.
An optical aberration is defined as an optical phenomenon resulting from the failure of an optical system to produce a good image; the image of an object is distorted due to the presence of optical aberrations while the rays of light do not obey the laws describing perfect optical systems [7]. The human eye is not a perfect optical system, especially for large pupil diameters:
1) First of all, the optic and visual axis do not coincide as they should for a perfect vision in order that the image with the highest resolution to project on the retina with the highest resolution (fovea centralis) [8]. The angle between the optic and visual axes is called angle alpha. It measures about 5 degrees for humans and it was considered the most reliable reference in refractive surgery because it had the lowest variability between patients [6]. However there is a high debate on where to best center refractive surgery procedures and devices according to the visual axis or to use the line of sight [8,9,10].
Also the lens and cornea are slightly tilted and decentered relative to each other [11]. As a consequence the eye functions only at about 40% of the performance it could theoretically achieve [6].
2) Secondly, the human eye is not a fixed optical system; the pupil center is not static due to modifying its refractive state by accommodation and light [12].
Only hyperopia, myopia and regular astigmatism are correctable by spectacles or contact lenses, so in the past they were the only aberrations of clinical interest [13]. However the eye suffers from other optical imperfections (called high-order aberrations) which cannot be corrected by conventional means. Like defocus, optical aberrations blur the retinal image, reducing image contrast and limiting the range of spatial frequencies available to further stages of the visual processing. The contribution of aberrations to optical degradation is typically smaller than is that of defocus or astigmatism; the blurring effect of aberrations becomes more noticeable for large pupils [14].
The main aberrations of the eye could be classified as low-order (defocus, regular astigmatism) and high-order aberrations (spherical aberration, distortion, coma, astigmatism of oblique incidence, other higher-order aberrations), as monochromatic (measured at a single wavelength) and chromatic aberrations [15]. Since the 19th century scientists know about the presence of high-order aberrations, but only recently in the 1990s wavefront sensors were developed to allow routine estimation of these ocular aberrations [16].
In order to measure optical aberrations we must understand the concept of wavefront aberration. The aberrations of an optical system, such as the eye, prevent a spherical wavefront from remaining spherical as it passes through the system (Fig. 1). This aberrated wavefront can be compared with an ideal spherical wavefront, whose centre of curvature on the image side of the system is at the ideal image position. The difference between the actual wavefront and the ideal wavefront is the wave aberration [13]; the most convenient position for comparing the two wavefronts is at the exit pupil of the system [15]. If the actual wavefront is ahead of the ideal one, the wave aberration is considered positive, otherwise is negative. Wave aberrations are small quantities and are usually expressed in micrometres or wavelengths. At a wavelength of 500 nm, one micrometre (pm) is equivalent to two wavelengths [15]. Wavefront aberrations can be mathematically represented as the sum of a series of polynomial functions of different orders to show the departure from perfection and classify the shape of aberration maps. The most used polynomial functions are the Taylor and Zernicke polyn omial series [17] (Fig. 2). With their use we can compare and standardize different models of aberration and reproduce them in order to correct them by laser surgery.
Fig. 1.
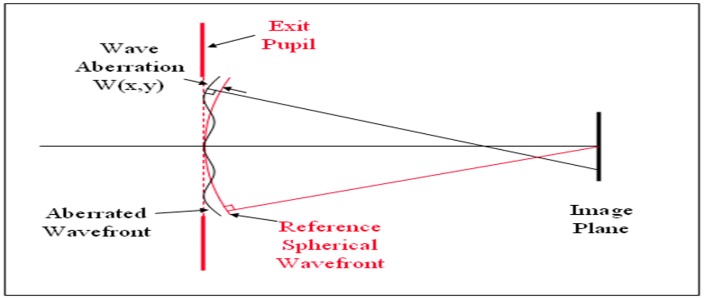
The aberrations of an optical system, such as the eye, prevent a spherical wavefront from remaining spherical as it passes through the system. This aberration of wavefront can be compared with an ideal spherical wavefront, whose center of curvature on the image side of the system is at the ideal image position. The difference between the actual wavefront and the ideal wavefront is the wave aberration [13]. (Image reproduced with approval of Jie Shen [31])
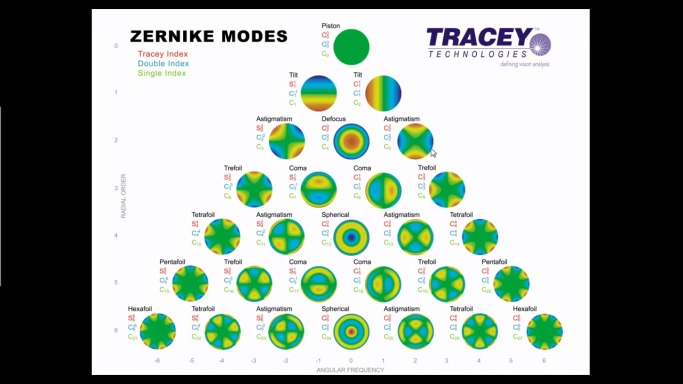
Fig. 2.
This figure shows the most common shapes of aberrations when a wavefront of light passes through an eye with imperfect vision. A theoretically perfect eye is represented by an aberration-free flat plane, named piston (top). Wavefront aberrations can be mathematically represented as the sum of a series of polynomial functions of different orders to show the departure from perfection and classify the shape of aberration maps. The most used are Zernike polynomials, each polynomial represent a particular mode of optical aberration, reconstructed here as a wavefront map. The 2nd order terms represent sphere and cylinder. The 3rd order terms and higher represent higher order aberrations. Here are shown the Zernike aberrations till the 6th order (Image reproduced with courtesy of Tracey Technologies, Inc.).
Here are the most frequent types of aberrations:
Defocus refers to both myopia and hyperopia. The wave aberration has a paraboidal or bowl shape. For myopia the corresponding wave aberration is called positive defocus (even though myopia is corrected by minus lenses), while for hyperopia is called negative defocus (even though hypeopia is corrected by positive lenses).
Regular astigmatism refers to a change in refraction from one principle meridian of the eye to the other, the two meridians always being at right angles [13]. We refer to “with the rule” astigmatism when the steepest axe (meridian with the greatest refractive power) tends to be vertical (around 90º), and “against the rule” astigmatism when the steepest axe tends to be horizontal (around 180º). The wave aberration associated with regular astigmatism has a cylindrical shape.
Myopia, hyperopia and regular astigmatism represent low-order aberrations, but in terms of Zernike polynomials they are classified as second-order aberrations. Lower order aberrations make up about 85 per cent of all aberrations in the eye [18].
In Spherical aberration (SA) rays of light entering the eye near the pupil edge are focused in front of the retina (positive SA), while rays near the pupil center are focused further behind (negative SA). The distance between these focal points is known as the axial spherical aberration. Point objects form a retinal blur circle. The SA is about 2 D and is maximum at 2-4 mm from the visual axis [19]. SA is the reason for night myopia. In low light conditions the pupil enlarges, more peripheral rays enter the eye and the focus shifts anteriorly, making the patient more myopic. The effect of spherical aberration increases as the fourth power of the pupil diameter. Doubling pupil diameter increases spherical aberration 16 times [20]. The human eye has innate adaptations that minimize SA:
1) The cornea is not spherical and flattens towards the periphery (it has a prolate shape); therefore there is less refractive power at the periphery resulting in a reduction in refraction of peripheral rays of light [7].
2) The crystalline lens has a varying refractive index and curvature; the central nucleus has a higher refractive index than the cortex so that the central rays are refracted more, and SA is reduced overall [7].
3) Foveal cones are excited more strongly when the light incident upon them enters through the center of the pupil rather than through the periphery [21]. The Stiles Crawford’s effect and its consequence is that peripheral light rays that are refracted more due to SA will be perceived less [7].
SA is represented by fourth-order Zernike polynomials.
A study about pupil sizes and visual acuity has shown that a normal daytime pupil size (this mean 3-3.2 mm) is the optimum pupil size for achieving best UCVA in a normal emetropic eye, balancing the diffraction effect that appears at small pupil sizes (especially below 2mm), against the aberrations let in by a large pupil [22].
Coma makes the image rays to “flare out” from the image point in a fashion reminiscent of a comet’s tail. The wave aberration has the shape of a lounge chair [13]. Coma is pupil independent and is increased when multiple optical elements do not share the same optical axes [23]. Vertical and horizontal come are described by third-order Zernike polynomials.
Astigmatism of oblique incidence is described by a fourth-order Zernike polynomial; it shouldn’t be confused with regular astigmatism.
Other higher-order aberrations (HOA) as trefoil, trefoil and other aberrations that don’t have a name, but are described only in mathematic terms by Zernike polynomials, have a lower impact on visual quality.
Laser eye surgery for refractive errors:
The most used methods in laser surgery are lamellar and surface ablations:
1) The laser in situ keratomilieusis (LASIK) uses a lamellar procedure in which the excimer laser ablation is done under a partial thickness lamellar corneal flap [2]. The flap could be done by a microkeratome or by a femtosecond laser and is repositioned at the end of the surgery.
2) Photorefractive keratectomy is the most used of the surface ablations nowadays. In this procedure the excimer laser ablates the most anterior portion of the corneal stroma after the epithelium was removed. The corneal healing occurs from the surrounding epithelial cells which migrate and divide to correct the epithelial defect. Compared to LASIK the wound-healing might associate greater stromal haze and scarring [2].
Until recently the surgeon could only decide where to do the laser ablation, choosing either LASIK or a surface ablation to correct the sphere and cylinder error. With the help of wavefront aberrometry which measures the more subtle, high order aberrations of the eye [24], the surgeon has options on the method he uses: standard ablations versus wavefront ablations.
There are two main methods of using wavefront measurements in laser eye surgery [13]:
1) Wavefront-optimized ablations – try to preserve the eye’s pre-existing optical aberration (the adjustments are done on average population data and the ablation profile is based on an ideal model, without evaluating the patient’s own aberrometry). Its aim is to optimize the asphericity of the cornea, to precompensate for the expected 4th-order spherical aberration and higher-order astigmatism in the average eye [13,25].
2) Wavefront –customized ablations (also known as wavefront guided ablations) – take into consideration the patient’s own aberration profile aiming to correct not only the spherocylindrical error but also the pre-existing HOA or those HOA that might be induced by conventional laser corrections [4].
Refractive surgery outcomes:
Both LASIK and PRK increase wavefront aberrations of the cornea, particularly increasing coma and spherical aberrations [5,26]. Oshika T. et al. [5] showed that conventional LASIK induced more SA than PRK when dealing with large pupil, but considered that this might be the consequence of a smaller transition zone of the laser ablation in LASIK.
Skiamoto et al. [2] reviewed the results for wavefront-guided versus conventional laser ablation for myopia. The review revealed that in wavefront guided LASIK 89% of patients achieved 20/20 or better uncorrected distance visual acuity, whereas only 72% of patients with conventional LASIK did. The same review also approached the FDA studies for patients with hyperopia – their findings suggest that although wavefront-guided treatments might prevent some worse outcomes, they do not improve the chances of obtaining the best outcomes; wavefront-guided ablation do not solve the problems of unpredictable wound healing and biomechanics, which are important determinants in the outcome of hyperopic LASIK.
A prospective study published in 2013 sustains better visual performance after wavefront guided LASIK compared to conventional LASIK for myopia, especially for eyes with high-magnitude root mean square [27].
Feng et al. [27,28] reviewed the outcomes for wavefront-guided and wavefront-optimized LASIK for myopia. He included 930 eyes in the meta-analysis, but found no statistically significant differences in the eyes achieving uncorrected distance visual acuity of 20/20, nor did the HOA differ between the two groups unless the preoperative root mean square of higher order aberrations (RMS) was higher than 0.3 μm. This meta-analysis suggested that both wavefront-guided and wavefront-optimized LASIK have excellent efficacy, safety, and predictability.
In 2014 Am J Ophthalmology published a study [29] comparing also wavefront-guided and wavefront-optimized LASIK for myopia where wavefront-guided treatment platforms appeared to offer significant advantages in terms of residual refractive error, uncorrected distance acuity and contrast sensitivity. The study found no differences in levels of residual astigmatism or in HOA.
A recent study [30] comparing the refractive outcome of wavefront-guided LASIK and wavefront-guided PRK in patients with high preoperative HOA (root mean square more than >0.35 μm) showed similar efficacy, safety and predictability, though wavefront guided - PRK induced less HOA.
Conclusions
These new surgical procedures have proved their benefit and efficacy. Some of the techniques are very new and need to be further tested and observed in time. However, at a close look we see that the need and demand for laser surgery is increasing every day, patients expect very good results and is our duty to offer them the best that we can. Taking into consideration the studies presented here, the benefit of using wavefront-guided ablations seems to matter for myopic eyes that have preoperative HOA with a RMS more than 0.3 μm. The results seem to be better when comparing wavefront-guided ablations with wavefront-optimized ablations, so for good outcomes the surgeon should take into account the presence of HOA before laser eye surgery. Further studies should also focus on hyperopic patients and the results of wavefront ablations on such eyes. For the moment conventional laser surgery offers good and stable results, but advancements in technology push forward the development in laser eye surgery, the hopes and expectations of both surgeons and patients.
Acknowledgement: This paper is partly supported by the Sectorial Operational Programme Human Resources Development (SOPHRD), financed by the European Social Fund and the Romanian Government under the contract number POSDRU 141531.
References
- 1.Pallikaris IG, Papatzanaki ME, Stathi EZ, Frenschock O, Georgiadis A. Laser in situ keratomileusis. Lasers Surg.Med. 1990;10:463–468. doi: 10.1002/lsm.1900100511. [DOI] [PubMed] [Google Scholar]
- 2.Sakimoto T, Rosenblatt MI, Azar DT. Laser eye surgery for refractive errors. Lancet . 2006;367:1432–1447. doi: 10.1016/S0140-6736(06)68275-5. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez JR, Villa C, Anera RG, Gutierrez R, del Barco LJ. Binocular visual performance after LASIK. J.Refract.Surg. 2006;22:679–688. doi: 10.3928/1081-597X-20060901-09. [DOI] [PubMed] [Google Scholar]
- 4.Marcos S. Aberrations and visual performance following standard laser vision correction. J.Refract.Surg. 2001;17:S596–S601. doi: 10.3928/1081-597X-20010901-19. [DOI] [PubMed] [Google Scholar]
- 5.Oshika T, Klyce SD, Applegate RA, Howland HC, El Danasoury MA. Comparison of corneal wavefront aberrations after photorefractive keratectomy and laser in situ keratomileusis. Am.J.Ophthalmol. 1999;127:1–7. doi: 10.1016/s0002-9394(98)00288-8. [DOI] [PubMed] [Google Scholar]
- 6.Holladay J. T. Quality of Vision: Essential Optics for the Cataract and Refractive Surgeon. Slack Incorporated; 2006. pp. 2–5. [Google Scholar]
- 7.Janet Voke. Understanding the Basics of Ocular Aberrations. http://www.optometry.co.uk/ 2010 [Google Scholar]
- 8.Uozato H, Guyton DL. Centering corneal surgical procedures. Am.J.Ophthalmol. 1987;103:264–275. [PubMed] [Google Scholar]
- 9.Pande M, Hillman JS. Optical zone centration in keratorefractive surgery. Entrance pupil center, visual axis, coaxially sighted corneal reflex, or geometric corneal center? Ophthalmology. 1993;100:1230–1237. [PubMed] [Google Scholar]
- 10.Chang DH, Waring GO. The subject-fixated coaxially sighted corneal light reflex: a clinical marker for centration of refractive treatments and devices. Am.J.Ophthalmol. 2014;158:863–874. doi: 10.1016/j.ajo.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Mester U, Sauer T, Kaymak H. Decentration and tilt of a single-piece aspheric intraocular lens compared with the lens position in young phakic eyes. J.Cataract Refract.Surg. 2009;35:485–490. doi: 10.1016/j.jcrs.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Wilson MA, Campbell MC, Simonet P, The Julius F. Neumueller Award in Optics, 1989: change of pupil centration with change of illumination and pupil size. Optom.Vis.Sci. 1992;69:129–136. doi: 10.1097/00006324-199202000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Yanoff M, Ducker J. S. Ophthalmology. Fourth edition. Elsevier Saunders; 2014. pp. 76–90. [Google Scholar]
- 14.Marcos S. Image quality of the human eye. Int.Ophthalmol.Clin. 2003;43:43–62. doi: 10.1097/00004397-200343020-00007. [DOI] [PubMed] [Google Scholar]
- 15.Atchison DA. Recent advances in representation of monochromatic aberrations of human eyes. Clin.Exp.Optom. 2004;87:138–148. doi: 10.1111/j.1444-0938.2004.tb03166.x. [DOI] [PubMed] [Google Scholar]
- 16.Atchison DA. Recent advances in measurement of monochromatic aberrations of human eyes. Clin.Exp.Optom. 2005;88:5–27. doi: 10.1111/j.1444-0938.2005.tb06659.x. [DOI] [PubMed] [Google Scholar]
- 17.Dimitri T.Azar, Damien Gatinel, Thang Hoang-Xuan. Refractive surgery. Second Edition. Philadelphia: Mosby Elsevier; 2007. pp. 10–150. [Google Scholar]
- 18.Mirko Resan, Miroslav Vukosavljeviæ , Milorad Milivojeviæ. Wavefront Aberrations. Advances in Ophthalmology. 2012:191. [Google Scholar]
- 19.Charman WN. The retinal image in the human eye. Osborne NN et al Ed. Progress in Retinal Research 2. Oxford: Pergaman; 1983. pp. 1–50. [Google Scholar]
- 20.Basic and Clinical Science Course, Section 3: Clinical Optics . American Academy of Ophthalmology. 2011-2012. 2012 [Google Scholar]
- 21.Coble JR, Rushton WA. Stiles-Crawford effect and the bleaching of cone pigments. J.Physiol. 1971;217:231–242. doi: 10.1113/jphysiol.1971.sp009568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holladay JT, Lynn MJ, Waring GO, III, Gemmill M, Keehn GC, Fielding B. The relationship of visual acuity, refractive error, and pupil size after radial keratotomy. Arch.Ophthalmol. 1991;109:70–76. doi: 10.1001/archopht.1991.01080010072036. [DOI] [PubMed] [Google Scholar]
- 23.Applegate RA, Hilmantel G, Howland HC, Tu EY, Starck T, Zayac EJ. Corneal first surface optical aberrations and visual performance. J.Refract.Surg. 2000;16:507–514. doi: 10.3928/1081-597X-20000901-04. [DOI] [PubMed] [Google Scholar]
- 24.Schwiegerling J. Theoretical limits to visual performance. Surv.Ophthalmol. 2000;45:139–146. doi: 10.1016/s0039-6257(00)00145-4. [DOI] [PubMed] [Google Scholar]
- 25.Mrochen M, Donitzky C, Wullner C, Loffler J. Wavefront-optimized ablation profiles: theoretical background. J.Cataract Refract.Surg. 2004;30:775–785. doi: 10.1016/j.jcrs.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Oliver KM, Hemenger RP, Corbett MC, O'Brart DP, Verma S, Marshall J, et al. Corneal optical aberrations induced by photorefractive keratectomy. J.Refract.Surg. 1997;13:246–254. doi: 10.3928/1081-597X-19970501-10. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Zhou YH, Li R, Tian L. Visual performance after conventional LASIK and wavefront-guided LASIK with iris-registration: results at 1 year. Int.J.Ophthalmol. 2013;6:498–504. doi: 10.3980/j.issn.2222-3959.2013.04.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng Y, Yu J, Wang Q. Meta-analysis of wavefront-guided vs. wavefront-optimized LASIK for myopia. Optom.Vis.Sci. 2011;88:1463–1469. doi: 10.1097/OPX.0b013e3182333a50. [DOI] [PubMed] [Google Scholar]
- 29.He L, Liu A, Manche EE. Wavefront-guided versus wavefront-optimized laser in situ keratomileusis for patients with myopia: a prospective randomized contralateral eye study. Am.J.Ophthalmol. 2014;157:1170–1178. doi: 10.1016/j.ajo.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 30.Arora R, Goel Y, Goyal JL, Goyal G, Garg A, Jain P. Refractive outcome of wavefront guided laser in situ keratomileusis and wavefront guided photorefractive keratectomy in high pre-existing higher order aberration. Cont.Lens Anterior.Eye . 2015;38:127–133. doi: 10.1016/j.clae.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Jie Shen. Ocular Aberrations and Image Quality, Contact Lens and MYOPIA Progression, Ophthalmology - Current Clinical and Research Updates, Associate Prof. Pinakin Davey (Ed.), ISBN: 978-953-51-1721-6, InTech, DOI: 10.5772/58456. 2014. Available from: http://www.intechopen.com/books/ophthalmology-current-clinical-and-research-updates/ocular-aberrations-and-image-quality-contact-lens-and-myopia-progression. [Google Scholar]