Abstract
Retinal vein occlusion (RVO) is the second most common retinal vein disease with significant visual loss via thrombus or compression of vein wall.
Thrombophilia is the predisposition to vascular thrombosis with the existence of genetic defect that leads to blood hypercoagulability.
This report describes the case of a 55 year old male patient, with an active life who presented himself at the emergency room with acute visual lose, insidious and progressive visual field constriction, without any known history of neurological or vascular diseases.
The examinations revealed unilateral optic nerve head edema, the fluorescein angiography was specific for nonischemic central retinal vein occlusion CRVO complicated with macular edema. Blood examinations has emphasized the presence of the heterozygous mutation A1298C in the methylenetetrahydrofolate reductase gene (MTHFR), the only one presented from the thrombophilia screen panel and a slightly elevated cholesterol level.
During the follow-up period, the patient received anti-VEGF treatment (Bevacizumab, 3x 0.1 ml intravitreal injections) with improved visual acuity and amendment of macular edema.
The complex etiology calls for interdisciplinary approach to determine better the cause of this ophthalmological disease.
Although studies have found a correlation between some thrombophilia mutations and retinal vein occlusion, more studies that contain a larger number of patients are necessary in order to determine the final role of these gene variants.
Keywords: hyperviscosity, hypercoagulable state, (methylenetetrahydrofolate reductase gene) MTHFR, retinal vein occlusion
Introduction
Retinal vein occlusion (RVO) is one of the most common retinal vein disease, second to diabetic retinopathy.
Central retinal vein thrombosis is typically a disease of older patients (90% are over 50 years old), rarely occurring in young adults. [1, 2, 3]
RVO is divided into three types according to Hayreh: BRVO (Branch retinal vein occlusion) is divided further into major BRVO and macular BRVO; CRVO is divided into ischemic and nonischemic types; and hemi-CRVO with involvement of only one half of the retina surface. [1]
Worldwide, approximately 16.4 million adults are affected by RVO and 2.5 million adults suffer of central retinal vein occlusion (CRVO). [4]
Visual acuity is decreased due to one of two conditions macular edema and/or retinal ischemia. Although it was first documented over a century ago, the exact pathogenesis still remains unclear, despite the various systemic and ophthalmic risk factors that have been identified. [1,5]
The condition is due to a combination of three systemic changes known as Virchow's triad:
• hemodynamic changes,
• degenerative changes of the vessel wall, and
• blood hypercoagulability. [1]
Hypertension, diabetes, vasculitis and high cholesterol levels are the most frequent factors associated with vascular diseases, therefore retinal occlusions. [6,3]
The common hematological tests performed are: complete blood count, glucose tolerance test, lipid profile, serum protein electrophoresis, chemistry profile and syphilis serology. If the history suggests systemic clotting diathesis, further hematological tests such as lupus anticoagulant level, anticardiolipin antibody, and protein S and protein C levels should be performed. [3,7]
Completely normal-medical laboratory evaluation results and history are found in 25% of patients. [8]
Coagulopathy and thrombophilia should also be considered where the RVO etiology isn't obvious or if the patient is young. [1]
A large number of studies have reported that increased blood viscosity, factor V Leiden mutation, hyperhomocysteinemia, and protein C or S deficiency may play an important role in the etiology of CRVO. [1,9,10]
The role of thrombophilic risk factors in RVO is still controversial. Various studies have conflicting results, and results should be interpreted with caution. [2,10]
Thrombophilia is the predisposition to vascular thrombosis with the existence of genetic defect that leads to blood hypercoagulability.
Methylene tetrahydrofolate reductase (MTHFR) catalyzes a substrate for homocysteine to methionine. Genetic variation in this gene make individuals susceptibility to occlusive vascular disease, neural tube defects, Alzheimer's disease, colon cancer, and acute leukemia. The enzyme is coded on chromosome 1 location p36.3 in humans. Two of the most investigated nucleotides are C677T and A1298C [11,10]
Homocysteine levels are determined by mutations in the thermolabile gene MTHFR, but also depend on diet, by levels of folic acid, vitamin B6 and vitamin B12. So, for elderly patients there is the theory that the dietary factor is more important than the genetic one. [10]
Case report
A 55-year-old male, former athlete, presented to the emergency room with acutely reduced visual acuity and a central scotoma in the left eye.
The patient's history revealed unknown vascular or neurological diseases. The patient history underlines the absence of vascular comorbidities: a non-smoker patient, with an active life, former athlete.
The current pathology occurred approximately 4 days prior to hospital admission, with the appearance of a central scotoma in the left eye that persisted till the admission day.
From the ophthalmological examination we emphasize on:
Best-corrected visual acuity: RE (right eye) 1.0 with + 1.00 DSph correction
LE (left eye) 0.2
Refraction: RE = +1.50 DSh - -0. 50Dcyl ax 42º
LE = + 1.50 DSh
Intraocular pressure (Goldman tonometry): 17-17 mmHg
The anterior segment evaluation was within the normal range, without pupillary defects.
Ophthalmoscopy (Fundus examination) of the - RE showed: normal optic nerve head and stage II angiopathy, (Fig. 1)
Fig. 1.
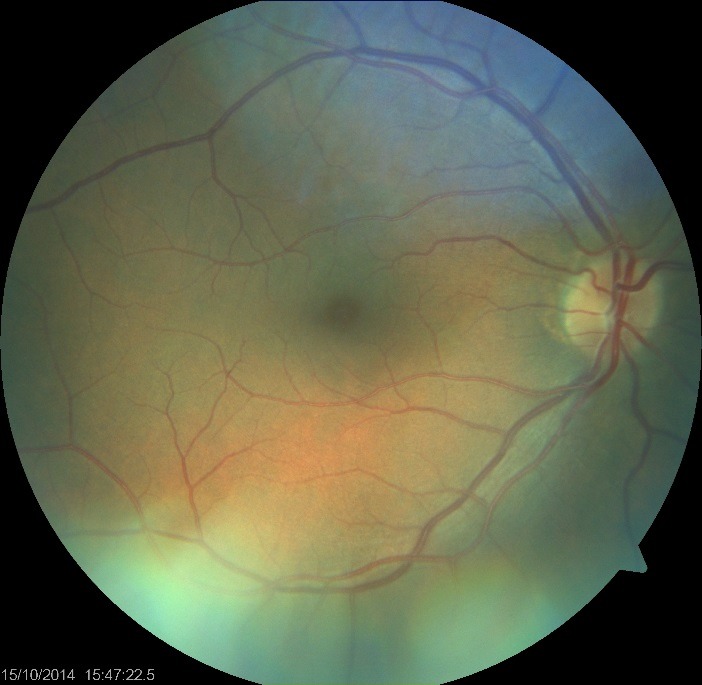
Fundus photography RE
- LE revealed dot and blot hemorrhages in all quadrants of the retina and blurring of the optic disc, the retinal venules appeared tortuous (Fig. 2);
Fig. 2.
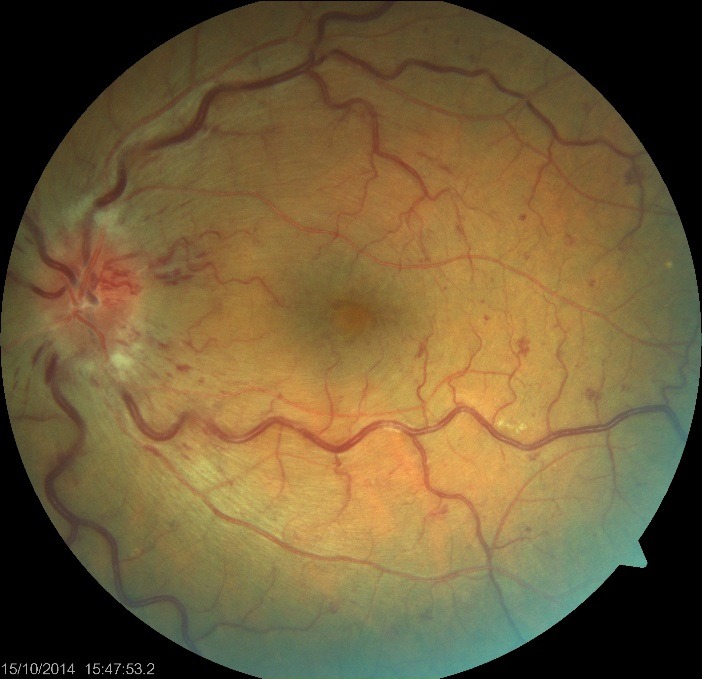
Fundus photography LE
The patient's history, general and ophthalmic examination point to a vascular retinal pathology, suggested by the means of debut and the fundus imagine.
For the certainty of the diagnosis ancillary tests where performed: (we present only the relevant issues)
1. Computerized perimetry: RE normal aspect, LE multiple central and paracentral scotoma (Fig. 3)
Fig. 3.
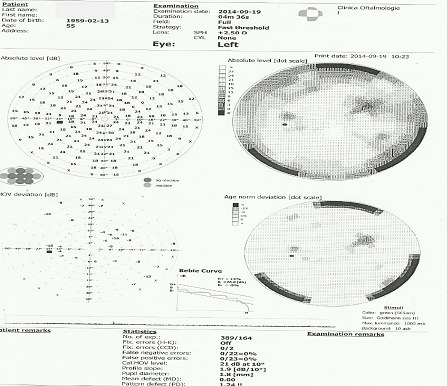
Computerized perimetry LE
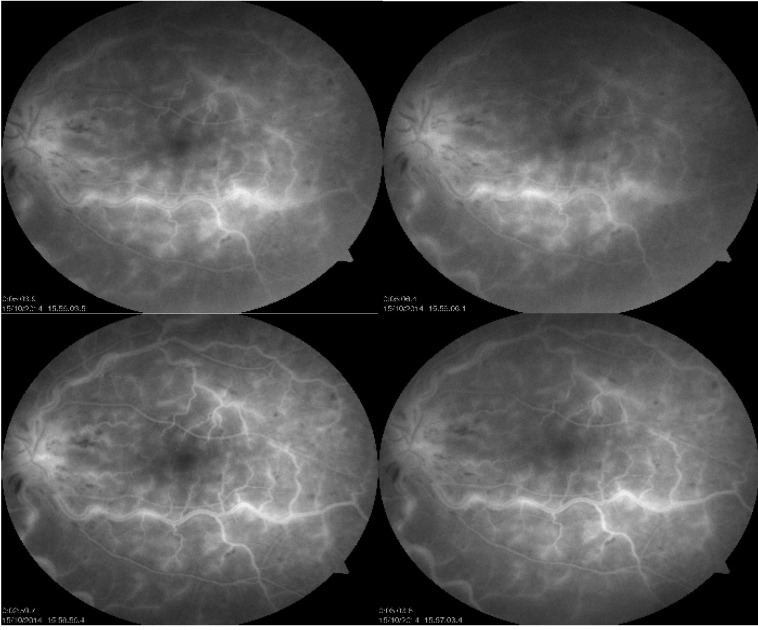
2.Angiofluorgraphy: in the LE revealed normal arterial filling but delayed arteriovenous filling, and there was late disc hyperfluorescence, staining along retinal veins, microaneurysms and dilated optic nerve head capillaries. (Fig. 4)
Fig. 4.
Fluorescein angiography LE
3.Paraclinical investigations
o cholesterol 240 mg/dl,
o creatinine 1.19 mg/dl,
o glucose 87.5mg/dl,
o anti-dsDNA antibodies 3.5 U/ml
anti-cardiolipin antibodies IgG 1.73.5 U/ml
o uric acid 4.4mg/dl,
o ASAT(GOT) 87.5 U/l, ALAT(GPT) 24 U/l
Blood examinations emphasized the presence of the heterozygous mutation A1298C in the methylenetetrahydrofolate reductase gene (MTHFR), the only one present from the thrombophilia screen panel (factor V Leiden nutation, factor II mutation, MTHFR C677T gene mutation and antibodies from ANA profile were all absent).
Other investigations:
• Cardiologic consultation: Sinus Bradycardia,
• Dental consultation: without infections pathology
• ENT consultation: Deviated septum, Cronic rhinosinusitis, operated basal cell cancer
• Pulmonology consultation: without acute pathology
• Urology consultation: without acute pathology
• Neurology consultation: normal Doppler ultrasound (carotid artery)
• MRI and angio MRI: without alterations in the signal sequence T2 and flair to plead for demyelination, without vascular caliber changes of vertebrobasilar and internal carotid.
From the patient's history, ancillary tests and general examination we established the following: Nonischemic central retinal vein occlusion associated with heterozygosity for the MTHFR A1298C genotype LE, Stage II angiopathie RE, Hypermetropia BE, Presbyopia BE, Sinus Bradicardia.
During follow-up period the patient developed one of CRVO complication, macular edema, therefore he received anti-VEGF treatment (Bevacizumab, 3x 0.1 ml intravitreal injections) with improvement of visual acuity and amendment of macular edema.
Fig. 5.
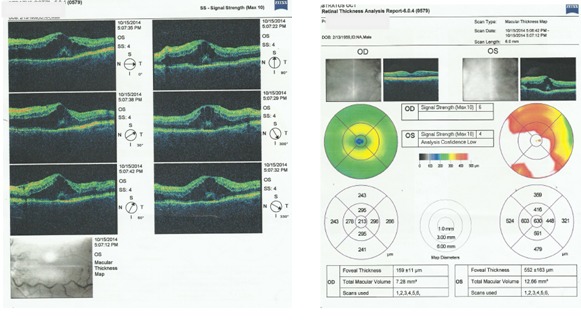
OCT examination LE (macular edema)
Differential Diagnosis:
• Ischemic versus nonischemic CRVO- Ischemic CRVO is usually associated with more extensive 4 quadrant hemorrhage and retinal edema, variable numbers of cotton-wool spots.
• Retinopathy due to carotid occlusive disease (ocular ischemic syndrome) - blurred vision in a darker room suggestive of carotid artery disease, not tortoise veins. Hemorrhages are commonly splotchy, located in the midperipheral retina.
• Acute hypertensive retinopathy (bilateral) - grade 3/4 with retinal hemorrhages and/or exudate, disc swelling
• Papillophlebitis or optic disc vasculitis - an inflammatory optic neuritis or vasculitis
• Hyperviscosity syndromes (bilateral retinopathy) - polycytemia vera, leukemia,
• Sever anemia with thrombocytopenia - excluded by blood examinations
Discussions
The Central Vein Occlusion Study Group noted that 34% of nonischemic CVRO progressed to ischemic aspect within 3 years. [5]
The prognosis of visual recovery is highly dependent upon the subtype of the occlusion:
1. Less than 10% of the patients who have the nonischemic type may experience a complete recovery of the vision.
2. 7% of patients with CVO develop venous occlusion of the fellow eye within 2 years.
3. The risk of any vascular occlusion in the fellow eye is estimated to be 0.9% per year[5]
Neovascularization of the anterior segment is rare under 2% of cases [12]
In the present case the prognosis is dismal knowing that thrombophilia induces a higher risk for vascular thrombosis.
The particularities of the case are: occurrence of a vascular pathology in a patient without knows diseases, and the discovery of genetic mutation at an older age.
Conclusions
When dealing with a retinal vein occlusion, the patient should be investigated for hypertension, glaucoma and diabetes mellitus. The estimation of plasma viscosity, a full blood count and lipid levels are cheap investigations and should be performed in every case.
It is mandatory to establish a hypercoagulable state, meaning thrombophilia screen in young patients or to those without other known pathologies.
Thrombophilia screening of these patients poses a particularly difficult diagnostic challenge; it seems likely that several risk factors, both genetic and acquired, need to be present for thrombosis to occur.
The complex pathogenesis and increase in the incidence of occlusion syndrome call for interdisciplinary approach to better determine the etiology.
Although studies have revealed a correlation between some thrombophilia mutations and retinal vein occlusion, more studies that contain a larger number of patients are necessary in order to determine the final role of these gene variants.
Disclosures
None
References
- 1.Kolar P. Risk factors of central and branch vein occlusion A meta-analysis of published clinical data. J Ophthalmol. 2014;1155(10):724–780. doi: 10.1155/2014/724780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilleul J, Glacet-Bernard A. Underlying conditions associated with the occurrence of retinal vein occlusions. J Fr Ophtalmol. 2011;34(5):318–324. doi: 10.1016/j.jfo.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Salaun N, Delyfer MN, Rougier JMBH, Korobelnik JF. Assessment of risk factors for retinal vein occlusions in patients under 60 years of age. J Fr Ophtalmol. 2007;30(9):918–923. doi: 10.1016/s0181-5512(07)74029-9. [DOI] [PubMed] [Google Scholar]
- 4.Stem MS, Talwar N, Comer GM. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology. 2013;120(2):362–370. doi: 10.1016/j.ophtha.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanoff M, Duker J.S. Venous obstructive disease of the retina. Ophthalmology. Mosby. 2004;115:862–869. [Google Scholar]
- 6.Pierre-Filho Pde T, Pierre AM, Nascimento MA, Marcondes AM. Central retinal vein prethrombosisas an initial manifestation of protein S deficiency. Sao Paulo Med J. 2004;122(3):134–135. doi: 10.1590/S1516-31802004000300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz Negrete FJ, Casas-Lleras P, Pérez-López M, Rebolleda G. Hypercoagulable workup in ophtalmology. When and What. Arch Soc Esp Oftalmol. 2009;84(7):325–332. doi: 10.4321/s0365-66912009000700003. [DOI] [PubMed] [Google Scholar]
- 8.Popa A, Voinea L, Pop M. Sindrom antifosfolipidic primar. Oftalmologia. 2008;52:13–17. [PubMed] [Google Scholar]
- 9.Fegan CD. Central retinal vein occlusion and thrombophilia. Eye (Lond) 2002;16(1):98–106. doi: 10.1038/sj.eye.6700040. [DOI] [PubMed] [Google Scholar]
- 10.Dan Li, Minwen Zhou, Xiaoyan Peng. Homocysteine, methylenetetrahydrofolate reductase C677T polymorphism and risk of retinal vein occlusion: an update meta-analysis. BMC Ophthalmol. 2014;14:147. doi: 10.1186/1471-2415-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahill M, Karabatzaki M, Donoghue C, Meleady R, Mynett-Johnson LA, Mooney D. Thermolabile MTFHR genotip and retinal vascular occlusive disease. Br J Ophthalmol. 2001;85:88–90. doi: 10.1136/bjo.85.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheufele TA, Pieroni CG, Baumal CR. Methylenetetrahydrofolate reductase gene mutation in a 16 year old girl with combined central retinal vein occlusion. Retin Cases Brief Rep. 2007;1(3):134–137. doi: 10.1097/01.ICB.0000279641.53583.66. [DOI] [PubMed] [Google Scholar]