Abstract
Background:
Adequate Vitamin D is essential for dental and skeletal health in children and adult. The purpose of this study was to assess the correlation of serum Vitamin D level with external-induced apical root resorption (EARR) following fixed orthodontic treatment.
Materials and Methods:
In this cross-sectional study, the prevalence of Vitamin D deficiency (defined by25-hydroxyvitamin-D) was determined in 34 patients (23.5% male; age range 12–23 years; mean age 16.63 ± 2.84) treated with fixed orthodontic treatment. Root resorption of four maxillary incisors was measured using before and after periapical radiographs (136 measured teeth) by means of a design-to-purpose software to optimize data collection. Teeth with a maximum percentage of root resorption (%EARR) were indicated as representative root resorption for each patient. A multiple linear regression model and Pearson correlation coefficient were used to assess the association of Vitamin D status and observed EARR. P < 0.05 was considered statistically significant.
Results:
The Pearson coefficient between these two variables was determined about 0.15 (P = 0.38). Regression analysis revealed that Vitamin D status of the patients demonstrated no significant statistical correlation with EARR, after adjustment of confounding variables using linear regression model (P > 0.05).
Conclusion:
This study suggests that Vitamin D level is not among the clinical variables that are potential contributors for EARR. The prevalence of Vitamin D deficiency does not differ in patients with higher EARR. These data suggest the possibility that Vitamin D insufficiency may not contribute to the development of more apical root resorption although this remains to be confirmed by further longitudinal cohort studies.
Keywords: 25-Hydroxycalciferol, orthodontics, root resorption, Vitamin D
INTRODUCTION
External apical root resorption (EARR) is an undesirable multifactorial complication of orthodontic treatments, which is influenced by numerous biological or environmental factors.[1,2,3] EARR demonstrated no significant clinical consequences; however, severe root resorption (more than one-fourth of the root length) might result in considerable deterioration of the tooth structure.[4,5] There is a controversial literature on various promoting factors including genetics (presence of interleukin [IL-1B]), gender, age, tongue thrust, anterior open bite, type of malocclusion, and pretreatment root morphology as host-related or biological factors.[1,6,7] However, more recent research on genetic variants concludes no apparent association between IL-1B and EARR.[6] Environmental factors such as type and duration of fixed orthodontic treatment, extraction, and type of anterior torque force have also demonstrated to leave a considerable impact on perceived EARR.[6]
From all patient-related factors, previous studies demonstrated that systemic factors, including hormone deficiencies and alveolar bone density, could influence EARR.[8,9] Therefore, orthodontists need to be aware of any possible chronic medical conditions that might interfere with overall success of orthodontic treatments. To date, various systematic factors such as different hormonal changes including hyperparathyroidism,[10] hyper and hypothyroidism,[11,12] calcium-deficient,[13] and ovariectomization[14,15,16] have also been implicated as potential risk factors to individualize the prediction of each patient's risk of developing EARR, but the result remains controversial.
Vitamin D deficiency is prevalent in all at risk groups (mentioned above) all around the world, especially in Middle East area despite the adequate sunshine in the region.[17] Vitamin D, an essential fat-soluble vitamin, is synthesized in the skin upon exposure to ultraviolet-B radiation from sunlight (at 290–315 nm wavelengths) and plays a critical role in calcium maintenance, bone growth and development, and in prevention of chronic diseases including cancer, diabetes, obesity, autoimmune disorders, and cardiovascular diseases.[17,18,19,20] Living in areas more vulnerable to air pollution and of higher latitude increases Vitamin D deficiency risk.[21] Several recent reports demonstrate a significant association between periodontal health and the intake of Vitamin D. Evidence has demonstrated that Vitamin D might be beneficial for oral health, not only for its direct positive effect on bone health and mineral metabolism but also due to its indirect action as an anti-inflammatory agent.[22,23,24]
Both dietary Vitamin D excess[6] and deficiency have been suggested to be involved in the pathophysiology of common permanent tooth resorption, which is an inflammatory event, observed in cats.[25] The active metabolite of Vitamin D, 1,25 dihydroxycholecalciferol [1,25(OH) 2D], is considered to be responsible for indirect stimulation of osteoclastogenesis by upregulating the expression of some secondary messengers.[26,27] Because this active metabolite has been considered as the regulatory factor to modulate the formation and activity of osteoclasts and odontoclasts in resorptive processes, it is hypothesized that Vitamin D is involved in the pathophysiology of observed resorption.[25] However, the effect of Vitamin D for apical root resorption is unclear.
On the other hand, there are still no standard criteria for the diagnosis and evaluation of EARR in clinical orthodontics.[28] Panoramic and lateral cephalometrics have been proposed to be more applicable for measurement of EARR considering their significant advantages of less radiation exposure, visualization of complete dentition, and less time consuming for the operator and more patient-friendly compared to very recent micro-computed tomography (CT) three-dimensional methods.[5,29,30,31] However, they still considered to be less accurate than periapical films and overestimate the EARR by 20% of the amount of root loss.[29,32] Periapical films also are prone to be affected by magnification errors, and according to the recent study by Pereira, this could be overcome using the percentage of root/tooth variation instead of direct measurement of root resorption.[28] As the named study, recent advances in the digital image processing and artificial intelligence techniques have made it possible for the computer-assisted superimpositions to be done more accurately and have increased their clinical applicability. Hence, the aim of this study was to assess the correlation of serum Vitamin D level with EARR following fixed orthodontic treatments.
MATERIALS AND METHODS
Samples and examination of charts
In this cross-sectional study, 34 Iranian patients (23.5% male; age range 12–23 years; mean age 16.63 ± 2.84,) who were referred to our Orthodontics Department (Shahid Beheshti University of Medical Sciences, Dental School) were selected using random cluster sampling method. Sample size was determined to be equal to 35 patients considering α = 0.05, β = 0.20 (power equal to 0.80), and r = 0.5 (moderate effect size) with a power and sample size calculation software (version 3.0.43). Considering the possibility of dropouts to be 20% during the study, we enrolled 37 patients.
Adult was defined as a minimum of 12 years of age at start of the treatment and was treated with multibonded Roth appliances with 0.022” × 0.028” bracket slots with a general wire sequence of 0.016” round nickel–titanium (Ni-Ti) to 0.016” round stainless steel to 0.016 × 0.022” rectangular stainless steel archwires. The exclusion criteria were other congenital, systemic, or concomitantly diagnosed serious medical conditions, history of dental trauma, cavity or significant malformation, parafunctional habit, anterior open bite, headgear or hyrax therapy regimens, and fixed orthodontic treatment with permanent tooth extraction. In addition, subjects with known hepatic or renal disease, metabolic bone disease, history of allergy or autoimmune disease, malabsorption, type 1 diabetes, hypercortisolism, malignancy, immobility for more than 1 week, pregnancy, lactation, and medications influencing bone metabolism and any chronic consumption of anti-inflammatory drug calcium, Vitamin D, calcium, and also fish oil supplement and history of sun bathing after starting the orthodontic treatment were not eligible for the study. Those whose radiographs lacked visibility of upper incisors, those with significantly distorted radiographs, crowding of teeth, unclear roots, and those with unilaterally and bilaterally lateral missing teeth in the maxilla were also excluded from the study.
All the related demographic data of the patients were recorded through orthodontic records. The chronological age of the patients was calculated and recorded by subtracting the birth dates from the date on which the radiographs were taken. Written informed consent was obtained from the patients and parents for the entire process of the study including blood samples. All those who refused to take part in the study were excluded from the study. The study protocol was based on the ethical principles governing medical research and human subjects in Helsinki Declaration (2013 version, http://www.wma.net/en/30publications/10policies) and also approved by the Ethical Committee of Dental Research Center of Shahid Beheshti University of Medical Sciences.
Pretreatment malocclusion was recorded according to Angle's and skeletal classification [Table 1]. Treatment time was recorded as the time lapse between appliance placement and removal. The amount of overjet was recorded based on the horizontal distance between upper and lower incisors at the start of the fixed orthodontic treatment. The recording was based on examination of the anamnestic records and interview with the orthodontists.
Table 1.
Pretreatment frequency of each type of malocclusion (n=34)

Examination of periapical radiographs
The original periapical radiographs of all three groups were obtained with the same digital X-ray unit (MinRay, SOREDEX, Helsinki, Finland – (10 kvp at 8 mA, 0.1 S); Processor: ACETON PSPIX, France) at the same distance and using the same exposure settings (70–85 kVp at 10 Ma). All radiographs were exported and saved in JPEG format using the Digora software (version 2.8®, Soredex, Helsinki, Finland). The digital radiographs were then visualized and analyzed through Photoshop CS (Adobe Systems Inc., San Jose, CA, USA). We applied a zoom of up to 150% when necessary. All the periapical radiographs were taken in natural head positions and the examiners used the scanned version of the radiographs.
Root form was scored subjectively as normal, blunt, eroded, pointed, bent and bottle shaped, using the criteria for subjective scoring of root form, presented by Mirabella et al.[45] Presence of a root canal filling was also recorded. Any teeth with considerable malformation in root morphology at pretreatment were excluded from the study.
Root resorption assessment
To assess the amount of root resorption in the upper incisors for each subject, all radiographs were scored by an examiner trained for point registration and blinded to Vitamin D status of the patients. To measure the distances, a proprietary tool was developed on MATLAB's image processing toolbox (MATLAB 7.14 2012a, Mathworks Inc., MA, USA). Using this tool, the operator marked four points including incisal edge, root apex, mesial, and distal crown edges on the target tooth in both pre- and post-treatment X-rays [Figure 1]. The root resorption was then calculated automatically by the software based on the formulation presented in the article by Pereira et al.[28]
Figure 1.
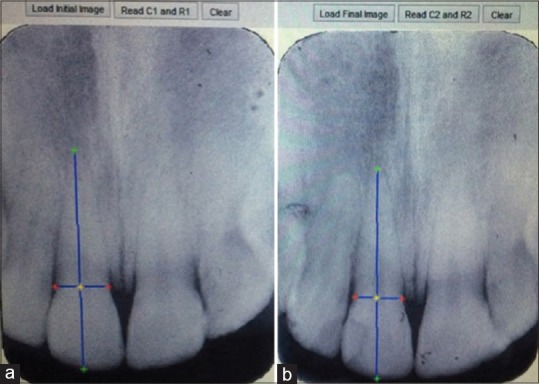
Evaluation method of external apical root resorption. (a) Point selection of a sample incisor teeth, at the start of the treatment (left), (b) point selection of the same sample at the end of the fixed orthodontic treatment (right).
In this method, a correction factor was calculated based on the assumption that crown length had to remain unchanged in the pre- (C1) and post- (C2) treatment X-rays. Therefore, the ratio C1/C2 could determine the inconsistency between crown lengths of the two X-rays and was used to compensate for the enlargement factor. Apical root resorption was then calculated as follows:
CF = C1/C2, (1)
CR2 = R2 × CF, (2)
Root resorption = 1 – (CR2/R1), (3)
Where CF is the correction factor and R1 is the root length in pretreatment X-rays, whereas R2 and CR2 are root lengths and corrected root lengths in posttreatment X-rays, respectively. The point marking process was done on an enlarged version of the X-ray to help reduce the error. Furthermore, the operator repeated her markings five times on each pair of X-rays, recorded the software output after each marking, and used the averaged value as the final root resorption.
Biochemical parameters
Sampling was performed between 8:00 and 9:00 am in a unique laboratory (Fereshte Lab). The Vitamin D status of the patients was made according to precise paraclinical examination in a well-established protocol as samples were centrifuged and stored at −20°C. 25(OH) 2D (was measured by radioimmunoassay (kits EUROIMMUNE, manufactured by Biosource Europe SA, Belgium) using autoanalyzer, Liasys (Italy) (Interassay coefficient of variation, 80%).
The patients were divided into three separate groups considering the Vitamin D status. Mild, moderate, and severe Vitamin D deficiencies were defined as 25-OHD values of 20–30 ng/mL, 10–20 ng/mL, and <10 ng/mL, respectively.[18] We used both the absolute and classification stages of Vitamin D to assess its relation with the observed %EARR.
Statistical analysis
All data were statistically analyzed by the STATA software (version 12.0, StataCorp LP, College Station, Texas, USA). To avoid interobserver error, all the measurements were done by the same operator (AS). After all radiographs were assessed, a random subset of ten radiographs was re-examined after 14 days, to estimate the methodological error by means of percentage of absolute intraobserver agreement. To shadow the effect of seasons of the study period, all the samples were taken over a 1¼-year period and were collected at one-time point (spring - April/June).
The mean score of the calculated %EARR between centrals and laterals was compared using t-test. The Vitamin D was expressed as a mean ± standard deviation and also percentage (%) of each predetermined level. The Pearson coefficient correlation and linear regression model were used to demonstrate any associations between apical root resorption (EARR) and Vitamin D status presented as continuous variables.
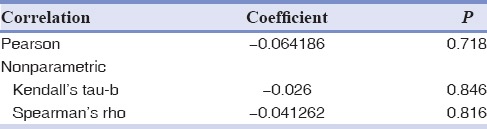
Kolmogorov–Smirnov test statistic was used for testing normality for continuous variables. Since the serum Vitamin D level was defined as an ordinal variable (mild, moderate, and severe), the nonparametric test of Kendall's tau-b and Spearman coefficient correlation were carried out to find any relation with %EARR. P < 0.05 was considered statistically significant.
RESULTS
Dahlberg errors were 0.068 and 0.091 for %EARR in central and lateral teeth, respectively. These values for both central and lateral teeth were considered statistically insignificant (paired t-test; P = 0.359 and 0.412, respectively). The Pearson correlation coefficient of repeated measurements was about 0.665 (P = 0.036) and Cronbach's alpha of 0.798, which demonstrated a high reliability of measurements.
Descriptive data
The radiographs of 34 (23.5% male; age range 12–23 years; mean age 16.63 ± 2.84) participants were studied. Two of them were excluded because of unclear periapical radiographs. One more patient was also excluded because of bilateral missing of maxillary laterals.
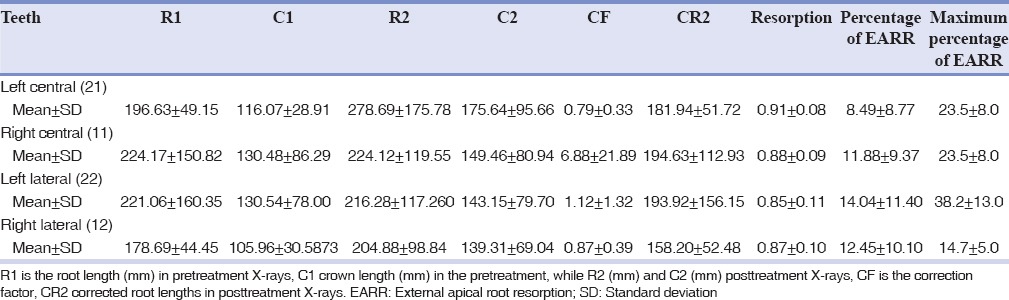
Overall, the mean %EARR was ranged from 8.49 (tooth 21) to 14.04 (tooth 22). Root resorption was higher in laterals than centrals on both right and left sides [Table 2]. Likewise, the maximum %EARR value obtained for each sample ranged from 14.7 (tooth 12) to 38.2 (tooth 22) with the average amount of 18.84 ± 9.78% [Table 2].
Table 2.
Mean and standard deviation of R1, C1, R2, C2, CF, CR2, resorption, percentage external apical root resorption and maximum percentage of external apical root resorption of teeth
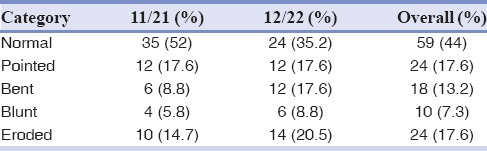
On the field of root morphology, based on the presented category at method and material section, most of the both central and laterals were categorized as having normal shapes [Table 3]. In addition, same percentage of both lateral and central incisors demonstrated pointed shape. An insignificant number of both types was considered to be blunt or eroded at the start of the fixed orthodontic treatment. The mean duration of orthodontic treatment was 3.91 years ± 1.67. The mean level of Vitamin D was 32.95 ± 17.94 (range: 5.0–80.2). The frequency distribution of normal, mild deficiency, moderate deficiency, and severe deficiency of serum Vitamin D level in all patients were 50%, 23.5%, 20.6%, and 5.9%, respectively (mild, moderate, and severe Vitamin D deficiencies were defined as 25(OH) D values of 20–30 ng/mL, 10–20 ng/mL, and <10 ng/mL, respectively).
Table 3.
Root morphology categories
Correlation
The correlation of serum Vitamin D with maximum %EARR of participants was investigated by both Pearson coefficient and logistic regression [Tables 4 and 5]. The Pearson coefficient between these two variables was determined about 0.15 (P = 0.38). Regression analysis revealed that Vitamin D status of the patients demonstrated no significant statistical correlation with EARR, after adjustment of confounding variables using linear regression model (P > 0.05) [Table 5]. In adjusted models, the association (95% confidence interval [CI]) of serum Vitamin D level with EARR was 0.09 (95% CI: −0.10–0.29). In another version, since the serum Vitamin D level was defined as an ordinal variable (mild, moderate, and severe), the nonparametric test of Kendall's tau-b and Spearman coefficient correlation reported no statistical relations between %EARR and Vitamin D levels [Table 6].
Table 4.
Linear logistic regression model analysis for correlation of external apical root resorption and Vitamin D level, after age, sex, treatment duration, and overjet adjustments
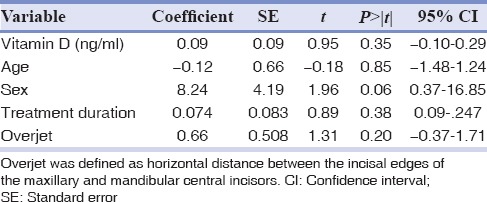
Table 5.
Pearson correlation coefficient among confounding variables
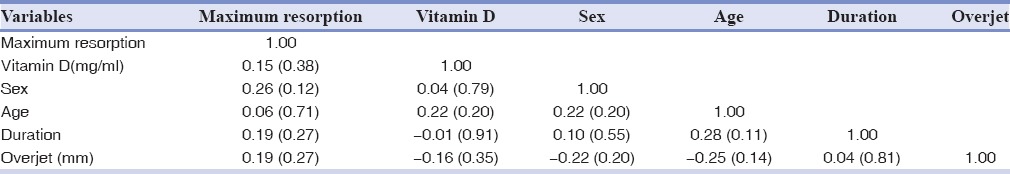
Table 6.
The overall correlation of Vitamin D status (expressed as mild, moderate, and severe) with external apical root resorption percentage
DISCUSSION
The present study showed that patients with lower serum Vitamin D level at the final stage of fixed multibracket orthodontic treatment are not more likely to present higher amount of EARR. To the best of our knowledge, this is the first study on orthodontic patients to show the correlation of the Vitamin D status with observed EARR. This study suggests that Vitamin D level is not among the clinical variables that are potential contributors to EARR. This was observed in an overall analysis that was adjusted for the potentially confounding factors of age at the start of treatment, sex and pretreatment overjet, and treatment duration [Table 4]. The prevalence of Vitamin D deficiency does not differ in patients with higher EARR. These results support previous findings by Booij-Vrieling et al., at 2010, which demonstrated that the Vitamin D status of animals did not differ between the groups of with and without observed resorption, based on the serum levels of 25-hydroxycholecalciferol. However, in the named study, the amount of expression of Vitamin D receptor protein differed among groups with and without observed resorption.[25]
In addition, Seifi et al. found the same data and hypothesized that Vitamin D deficiency may play a key role in the pathophysiology of root resorption following orthodontic tooth movement.[33] On this study, the amount of observed root resorption showed no significant difference after injection of Vitamin D metabolite alone with untreated control group on Wistar rats.[33] However, injection of prostaglandin E2 increases the amount of observed root resorption in comparison to other groups. A group of animals who received both prostaglandin E2 and Vitamin D also demonstrated no significant difference with control group so that these data suggested that administration of Vitamin D might provide a protective role on the root surface during orthodontic tooth movement, and in addition, in those patients that present spontaneous root resorption lesions.[33] The root resorption process involves complex interactions between inflammatory cells, resorbing cells, cytokines, and enzymes.[34] In 2004, Kawakami also showed that the local application of 1,25(OH) 2D3 enhances the reestablishment of supporting tissue, especially alveolar bone of teeth, after orthodontic treatment.[35] Perhaps the effect of Vitamin D on the supporting structures could be verified by the correlation between excess of Vitamin D and EARR. Further investigations are needed to confirm the biological mechanisms underlying the effects of Vitamin D on the orthodontically induced EARR.
Several previous studies have examined the association of potential confounding variables and external apical resorption in orthodontic patients. To eliminate a possible environmental variable, we excluded the patients undergoing headgear or hyrax therapy and extraction regimens.[28] As the previous study investigating the association of nine clinical variables with EARR, this current study adjust for the potential confounding effects of age at the start of treatment, sex, overjet, and treatment duration.[28] As it is stated, adjustment for these potentially confounding factors in model is preferred to well-matching testing groups and may influence the finding of significance or nonsignificance.
The amount of EARR was reported and analyzed by maximum percentage of EARR for each patient, as this is a clinically more meaningful clinical criteria.[28,36] Root resorption was higher in laterals than centrals on both right and left sides which is consistent with previous studies on EARR.[37,38] In the current study, most of the pretreatment periapical radiographs demonstrated normal root morphology although the percentage of pointed and bent root form was higher in laterals.[39] As widely observed in literature, orthodontic movement of teeth with severe root morphology is contraindicated in extreme cases, which might increase their chance of root resorption and consequently decrease the long-term stability of the results.[7]
As it is stated clearly in literature, after cone beam computed tomography (CBCT), which is presented as the most accurate measurement of EARR, periapical radiographies are at the second place.[40] However, these radiographies need extra radiation exposure that is not accepted in most regular clinical settings.[28] As periapical radiographies of maxillary teeth are mostly advised for teeth with a history of trauma or malformation abnormalities seen in panoramic view, the number of patients with pre- and post-radiographies are extremely limited. As in this study, the periapical radiographs were chosen and considering ethical reasons; this could not be conducted prospectively. Since these radiographs are not in a required radiograph of fixed orthodontic treatment. Hence, the inclusion criteria were those patients with initial periapical radiographies and of course without a history of trauma which might incorrectly influence the correlation between EARR and Vitamin D status of orthodontic patients. In fact, the prospective design of the study would be counted as more appropriate although not feasible type of correlational study.
On the other hand, Vitamin D status or level of individuals is a multifactorial element, resulting from a wide range of genetically, biological and environmental factors.[41] It has been reported in the literature that frequency of insufficiency or deficiency of this factor was much higher than presumed status, particularly in Middle-East region.[17,41] This could be the result of first two risk factors than environmental ones including sun exposure or daily diet.[22] The samples were taken over a 1½-year period and were collected at one-time point (spring/summer—April/September) to eliminate the need of data analysis by season.[42] More importantly, season appears to be a small determining component of Vitamin D level, as countries with long winters have less deficiency rates overall compared to sunny countries.[43] The distribution of Vitamin D insufficiency and deficiency in this study is in agreement with other studies in the same target population.[43,44]
The study has limitations, as it is a cross-sectional study. Ideally, it would be better to evaluate the association in a prospective study following patients and matched control before starting orthodontic treatment and to quantify Vitamin D levels over time and determine an individual as hyper- or hypo-Vitamin D with several measurements, so more precisely clarify whether Vitamin D is truly associated with the EARR risk. At the moment, we cannot rule out reverse causality as explaining the findings. Furthermore, there is a possibility that threshold level of serum Vitamin D for root resorption exists. In that case, root resorption initiates and/or progresses only at below specific level of serum Vitamin D. If this hypothesis is true, amount of root resorption does not correlate with the serum Vitamin D level over the threshold level. Therefore, it cannot be concluded as serum Vitamin D level has nothing to do with root resorption with the current study design and results. The dataset in this study was limited to patients referred to the orthodontics department and suffer from the potential for selection bias. We have very limited information on other potential confounding factors such as genetics and treatment-centered factors. Finally, the root morphology was evaluated subjectively using periapical radiographs, but root resorption is a 3D phenomenon, and its morphology should be analyzed with precision using cone beam CT. However, taking CBCT for patients was not considered to be ethical.
CONCLUSION
The data support that considering the observed Vitamin D level range in this study, Vitamin D level of orthodontic patients does not correlate with their manifested EARR after adjusting for age and sex. We can conclude that, in subjects undergoing fixed orthodontic treatments, the variance of the observed EARR is not a result of differences in serum concentrations of 1,25(OH) 2D. Further prospective cohort studies would be required to fully clarify any possible correlation.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
Acknowledgments
This study was extracted from an undergraduate thesis of Azin Sadighnia under supervision of Dr. Azita Tehranchi at Shahid Beheshti School of dentistry. The authors wish to thank the Dentofacial Deformities Research Center, Shahid Beheshti University of Medical Sciences, for support of this project.
REFERENCES
- 1.Sharab LY, Morford LA, Dempsey J, Falcão-Alencar G, Mason A, Jacobson E, et al. Genetic and treatment-related risk factors associated with external apical root resorption (EARR) concurrent with orthodontia. Orthod Craniofac Res. 2015;18(Suppl 1):71–82. doi: 10.1111/ocr.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahida K, Agrawal C, Baswaraj H, Tandur AP, Patel B, Chokshi H. Root resorption: An abnormal consequence of the orthodontic treatment. Int J Contemp Dent. 2015:6. [Google Scholar]
- 3.Weltman B, Vig KW, Fields HW, Shanker S, Kaizar EE. Root resorption associated with orthodontic tooth movement: A systematic review. Am J Orthod Dentofacial Orthop. 2010;137:462–76. doi: 10.1016/j.ajodo.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Zahrowski J, Jeske A. Apical root resorption is associated with comprehensive orthodontic treatment but not clearly dependent on prior tooth characteristics or orthodontic techniques. J Am Dent Assoc. 2011;142:66–8. doi: 10.14219/jada.archive.2011.0030. [DOI] [PubMed] [Google Scholar]
- 5.Chan EK, Darendeliler MA. Exploring the third dimension in root resorption. Orthod Craniofac Res. 2004;7:64–70. doi: 10.1111/j.1601-6343.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- 6.Wu FL, Wang LY, Huang YQ, Guo WB, Liu CD, Li SG, et al. Interleukin-1β +3954 polymorphisms and risk of external apical root resorption in orthodontic treatment: A meta-analysis. Genet Mol Res. 2013;12:4678–86. doi: 10.4238/2013.October.18.6. [DOI] [PubMed] [Google Scholar]
- 7.Valladares Neto J, Rino Neto J, de Paiva JB. Orthodontic movement of teeth with short root anomaly: Should it be avoided, faced or ignored? Dental Press J Orthod. 2013;18:72–85. doi: 10.1590/s2176-94512013000600012. [DOI] [PubMed] [Google Scholar]
- 8.Jung YH, Cho BH. External root resorption after orthodontic treatment: A study of contributing factors. Imaging Sci Dent. 2011;41:17–21. doi: 10.5624/isd.2011.41.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopatiene K, Dumbravaite A. Risk factors of root resorption after orthodontic treatment. Stomatologija. 2008;10:89–95. [PubMed] [Google Scholar]
- 10.Midgett RJ, Shaye R, Fruge JF., Jr The effect of altered bone metabolism on orthodontic tooth movement. Am J Orthod. 1981;80:256–62. doi: 10.1016/0002-9416(81)90289-x. [DOI] [PubMed] [Google Scholar]
- 11.Loberg EL, Engström C. Thyroid administration to reduce root resorption. Angle Orthod. 1994;64:395–9. doi: 10.1043/0003-3219(1994)064<0395:TATRRR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Poumpros E, Loberg E, Engström C. Thyroid function and root resorption. Angle Orthod. 1994;64:389–93. doi: 10.1043/0003-3219(1994)064<0389:TFARR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Goldie RS, King GJ. Root resorption and tooth movement in orthodontically treated, calcium-deficient, and lactating rats. Am J Orthod. 1984;85:424–30. doi: 10.1016/0002-9416(84)90163-5. [DOI] [PubMed] [Google Scholar]
- 14.Sirisoontorn I, Hotokezaka H, Hashimoto M, Gonzales C, Luppanapornlarp S, Darendeliler MA, et al. Tooth movement and root resorption; the effect of ovariectomy on orthodontic force application in rats. Angle Orthod. 2011;81:570–7. doi: 10.2319/101710-607.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arslan SG, Arslan H, Ketani A, Hamamci O. Effects of estrogen deficiency on tooth movement after force application: An experimental study in ovariectomized rats. Acta Odontol Scand. 2007;65:319–23. doi: 10.1080/00016350701678725. [DOI] [PubMed] [Google Scholar]
- 16.Yamashiro T, Takano-Yamamoto T. Influences of ovariectomy on experimental tooth movement in the rat. J Dent Res. 2001;80:1858–61. doi: 10.1177/00220345010800091701. [DOI] [PubMed] [Google Scholar]
- 17.Fanari Z, Hammami S, Hammami MB, Hammami S, Abdellatif A. Vitamin D deficiency plays an important role in cardiac disease and affects patient outcome: Still a myth or a fact that needs exploration? J Saudi Heart Assoc. 2015;27:264–71. doi: 10.1016/j.jsha.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Zubeidi H, Leon-Chi L, Newfield RS. Low Vitamin D level in pediatric patients with new onset type 1 diabetes is common, especially if in ketoacidosis. Pediatr Diabetes. 2016;17:592–8. doi: 10.1111/pedi.12342. [DOI] [PubMed] [Google Scholar]
- 19.Chacko SJ, Pauwaa S, Barengolts E, Ciubotaru I, Kansal MM. Vitamin D attenuates left atrial volume changes in African American males with obesity and prediabetes. Echocardiography. 2016;33:681–5. doi: 10.1111/echo.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trilok Kumar G, Chugh R, Eggersdorfer M. Poor Vitamin D status in healthy populations in India: A review of current evidence. Int J Vitam Nutr Res. 2015;85:185–201. doi: 10.1024/0300-9831/a000228. [DOI] [PubMed] [Google Scholar]
- 21.Kelishadi R, Moeini R, Poursafa P, Farajian S, Yousefy H, Okhovat-Souraki AA, et al. Independent association between air pollutants and Vitamin D deficiency in young children in Isfahan, Iran. Paediatr Int Child Health. 2014;34:50–5. doi: 10.1179/2046905513Y.0000000080. [DOI] [PubMed] [Google Scholar]
- 22.Stein SH, Tipton DA. Vitamin D and its impact on oral health – An update. J Tenn Dent Assoc. 2011;91:30–3. [PubMed] [Google Scholar]
- 23.Anand N, Chandrasekaran SC, Rajput NS. Vitamin D and periodontal health: Current concepts. J Indian Soc Periodontol. 2013;17:302–8. doi: 10.4103/0972-124X.115645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastos Jdo A, Andrade LC, Ferreira AP, Barroso Ede A, Daibert Pde C, Barreto PL, et al. Serum levels of Vitamin D and chronic periodontitis in patients with chronic kidney disease. J Bras Nefrol. 2013;35:20–6. doi: 10.5935/01012800.20130004. [DOI] [PubMed] [Google Scholar]
- 25.Booij-Vrieling HE, Ferbus D, Tryfonidou MA, Riemers FM, Penning LC, Berdal A, et al. Increased Vitamin D-driven signalling and expression of the Vitamin D receptor, MSX2, and RANKL in tooth resorption in cats. Eur J Oral Sci. 2010;118:39–46. doi: 10.1111/j.1600-0722.2009.00707.x. [DOI] [PubMed] [Google Scholar]
- 26.Quinn JM, Horwood NJ, Elliott J, Gillespie MT, Martin TJ. Fibroblastic stromal cells express receptor activator of NF-kappa B ligand and support osteoclast differentiation. J Bone Miner Res. 2000;15:1459–66. doi: 10.1359/jbmr.2000.15.8.1459. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D, Yang YQ, Li XT, Fu MK. The expression of osteoprotegerin and the receptor activator of nuclear factor kappa B ligand in human periodontal ligament cells cultured with and without 1alpha, 25-dihydroxyvitamin D3. Arch Oral Biol. 2004;49:71–6. doi: 10.1016/s0003-9969(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 28.Pereira SA, Lopez M, Lavado N, Abreu JM, Silva H. A clinical risk prediction model of orthodontic-induced external apical root resorption. Rev Port Estomatol Med Dent Cir Maxilofac. 2014;55:66–72. [Google Scholar]
- 29.Sameshima GT, Asgarifar KO. Assessment of root resorption and root shape: Periapical vs. panoramic films. Angle Orthod. 2001;71:185–9. doi: 10.1043/0003-3219(2001)071<0185:AORRAR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Dindaroǧlu F, Doǧan S. Evaluation and comparison of root resorption between tooth-borne and tooth-tissue borne rapid maxillary expansion appliances: A CBCT study. Angle Orthod. 2016;86:46–52. doi: 10.2319/010515-007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bansal P, Nikhil V, Kapur S. Multiple idiopathic external apical root resorption: A rare case report. J Conserv Dent. 2015;18:70–2. doi: 10.4103/0972-0707.148900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mavragani M, Bøe OE, Wisth PJ, Selvig KA. Changes in root length during orthodontic treatment: Advantages for immature teeth. Eur J Orthod. 2002;24:91–7. doi: 10.1093/ejo/24.1.91. [DOI] [PubMed] [Google Scholar]
- 33.Seifi M, Hamedi R, Naziri M. The synergistic effect of Vitamine D and Prostaglandin E2 on orthodontic tooth movement in rats. Iran J Orthod. 2013;8:1–5. [Google Scholar]
- 34.Ne RF, Witherspoon DE, Gutmann JL. Tooth resorption. Quintessence Int. 1999;30:9–25. [PubMed] [Google Scholar]
- 35.Kawakami M, Takano-Yamamoto T. Local injection of 1,25-dihydroxyvitamin D3 enhanced bone formation for tooth stabilization after experimental tooth movement in rats. J Bone Miner Metab. 2004;22:541–6. doi: 10.1007/s00774-004-0521-3. [DOI] [PubMed] [Google Scholar]
- 36.Linge BO, Linge L. Apical root resorption in upper anterior teeth. Eur J Orthod. 1983;5:173–83. doi: 10.1093/ejo/5.3.173. [DOI] [PubMed] [Google Scholar]
- 37.Harris EF, Kineret SE, Tolley EA. A heritable component for external apical root resorption in patients treated orthodontically. Am J Orthod Dentofacial Orthop. 1997;111:301–9. doi: 10.1016/s0889-5406(97)70189-6. [DOI] [PubMed] [Google Scholar]
- 38.Linhartova P, Cernochova P, Izakovicova Holla L. IL1 gene polymorphisms in relation to external apical root resorption concurrent with orthodontia. Oral Dis. 2013;19:262–70. doi: 10.1111/j.1601-0825.2012.01973.x. [DOI] [PubMed] [Google Scholar]
- 39.Kjaer I. Morphological characteristics of dentitions developing excessive root resorption during orthodontic treatment. Eur J Orthod. 1995;17:25–34. doi: 10.1093/ejo/17.1.25. [DOI] [PubMed] [Google Scholar]
- 40.Lund H, Gröndahl K, Hansen K, Gröndahl HG. Apical root resorption during orthodontic treatment. A prospective study using cone beam CT. Angle Orthod. 2012;82:480–7. doi: 10.2319/061311-390.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karampoor S, Zahednasab H, Ramagopalan S, Mehrpour M, Safarnejad Tameshkel F, Keyvani H, et al. 25-hydroxyvitamin D levels are associated with multiple sclerosis in Iran: A cross-sectional study. J Neuroimmunol. 2016;290:47–8. doi: 10.1016/j.jneuroim.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Hovsepian S, Amini M, Aminorroaya A, Amini P, Iraj B. Prevalence of Vitamin D deficiency among adult population of Isfahan City, Iran. J Health Popul Nutr. 2011;29:149–55. doi: 10.3329/jhpn.v29i2.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144(Pt A):138–45. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebrahimi M, Khashayar P, Keshtkar A, Etemad K, Dini M, Mohammadi Z, et al. Prevalence of Vitamin D deficiency among iranian adolescents. J Pediatr Endocrinol Metab. 2014;27:595–602. doi: 10.1515/jpem-2013-0428. [DOI] [PubMed] [Google Scholar]
- 45.Mirabella AD, Šrtun J. Risk factors for apical root resorption of maxillary anterior teeth in adult orthodontic patients. American Journal of Orthodontics and Dentofacial Orthopedics. 1995 Jul;31(108):48–55. doi: 10.1016/s0889-5406(95)70065-x. [DOI] [PubMed] [Google Scholar]


