Abstract
Background:
The aim of the present meta-analysis is to determine the efficacy of tetracycline group of antibiotics as local drug delivery agents in the treatment of chronic periodontitis.
Materials and Methods:
MEDLINE, EBSCO, Cochrane database, and Google Scholar were used to identify studies in English published up to January 31, 2017. An additional hand search of relevant journals and of the bibliographies of the paper identified was also performed. Articles retrieved were screened using specific inclusion criteria by two independent reviewers. Randomized control trials investigating the effect of tetracycline group of antibiotics as local drug delivery agents in chronic periodontitis were included in the study.
Results:
Ten relevant articles were selected for the meta-analysis, of which five articles were retrieved after electronic search, three articles were included after hand search, and two unpublished articles were included. The number of patients in studies ranged from 13 to 140 sites with mean age ranging from 20 to 75. A total of 588 sites were treated using tetracycline group of antibiotics as local drug delivery agents in the treatment of chronic periodontitis. The meta-analysis showed standard difference in mean −1.02 mm (95% confidence interval [CI] 0.28, 1.75) for clinical gain in attachment in favor of tetracycline group. Standard difference in mean for probing depth (PD) was 1.20 mm (95% CI 0.57, 1.87) in tetracycline group.
Conclusion:
The results of this meta-analysis showed a significant improvement in periodontal parameters such as CAL, PD, and sulcular bleeding index in favor of tetracycline as local drug delivery compared to placebo.
Keywords: Chronic periodontitis, local drug delivery, meta-analysis, nonsurgical periodontal therapy, tetracycline
INTRODUCTION
Periodontal flora plays the most important role in initiation and progression of periodontal diseases. The presence of diverse microorganism renders the use of different antimicrobials in treatment of chronic periodontitis as an adjunct to mechanical debridement. Antibacterial agents have become an integral part of the therapeutic armamentarium, but systemic use of antibiotics is discouraged due to its side effects.[1]
The tetracycline groups of drug are among the most widely used agents to treat periodontal disease. These are used both systematically and also as local drug delivery agent which have the advantage of avoiding the harmful effect of systemic administration including the development of resistant flora, suppression of normal flora and poor patient compliance.[2]
Local deliveries of antibacterial agents into periodontal pockets have been extensively studied since 1979.[3] This mode of drug delivery avoids most of the problems associated with systemic therapy, limiting the drug to its target site, and hence achieving a much higher concentration. Local drug delivery agents (LDD) in periodontology has gained acceptance and popularity compared to systemic drugs due to decreased risk in development of resistant flora, opportunist infection, and side effects.[4]
Tetracycline in different form holds great promise in controlling the progression of periodontal disease by their ability to reduce microbial burden, to block collagenase activity, and to potentially inhibit bone loss.[5,6] Meta-analysis published in 2003[7] reported a significant mean reduction in probing depth (PD) in favor of local tetracycline therapy and suggested more advantage with fibers compared to other devices. Contradictory to these results, meta-analysis in 2013[8] concluded that there is no significant improvement and suggested use of this evidence with caution as high degree of heterogeneity and high risk of bias in the included trials.
Recent trials[2,9,10,11,12,13,14,15] evaluated sustained release system incorporating tetracycline gave promising results except one study,[2] but pooling of data of such trials and its quality assessment is required before implementing in clinical practice.
MATERIALS AND METHODS
Search strategy
A systematic search was performed using search terms (periodontitis, periodontal pocket, tetracycline, doxycycline, minocycline, LDD) and searched four electronic databases, namely, MEDLINE, EBSCO, Cochrane database, and Google Scholar up to January 31, 2017. Limits used were “humans.” We searched only for randomized controlled trials. Additional studies were retrieved through hand search of the references from relevant articles, manual search of journals, and studies cited for the products approvals. Authors independently reviewed all titles retrieved and abstracts were screened where title was unclear. The abstracts thus found relevant were selected for full text reading. The articles selected for full text reading were read and evaluated by the individual reviewer independently for assessing the risk of bias and retrieving the information relevant for the review and meta-analysis.
Study eligibility criteria
Inclusion criteria were all randomized control trial (RCTs) where tetracycline is used as LDD in patients with periodontitis and one of the clinical outcome variables is clinical attachment level (CAL) or PD. Studies with aggressive periodontitis and smokers were excluded from the study. Primary outcome of interest were CAL and PD. Secondary outcomes considered were gingival index (GI) and sulcular bleeding index (SBI).
Data extraction and assessment of quality of trial
Data were extracted individually by two authors. The details of place, sample size, age, intervention, comparator, follow-up, and outcome were recorded from included studies. Risk of bias was assessed according to Cochrane assessment of risk of bias tool[16] based on randomization, blinding, allocation concealment, loss to follow-up, and intention to treat analysis. Quality of included trials was categorized as high risk, moderate risk, and low risk if more than two, two, or one criterion were not fulfilled, respectively.
Statistical analysis
We used comprehensive meta-analysis version 2 for pooling the results. Standard difference of mean was used as the outcome measure for pooling the data. Change in the clinical parameters from baseline to follow-up was considered for meta-analysis. Results of the studies with data of baseline and follow-up, change in the parameter were calculated. I2 was used as a measure of heterogeneity. I2 >50% was considered statistically significant heterogeneity. Random effects’ model was used for pooling the mean difference when pooled results with fixed effect model and random effect model were different. A sensitivity analysis was also carried out to find if any study had grossly impacted the overall pooled result. None of the study was excluded on the basis of risk of bias.
RESULTS
Studies retrieved
Ten relevant studies were retrieved after systematic search of literature.[2,9,10,11,13,17,18,19,20] Seven articles were retrieved after electronic search of medical database and included for reading full text[2,11,12,14,19,20,21] and four articles were included after hand search.[9,10,13,18] Unpublished data of two studies were obtained from approval letter available at center for drug evaluation and research, USA.[17] Three articles were excluded for the reason as no random concealed allocation or blinding done,[14] cases of aggressive periodontitis were also included[12] and results of control group not available [Figure 1].[21]
Figure 1.
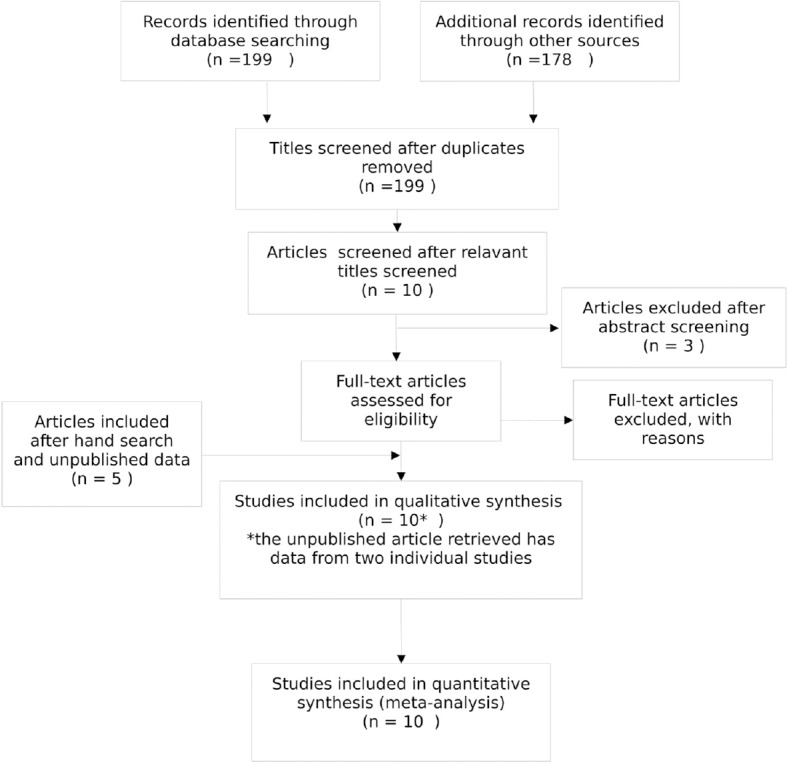
Systematic Search
Risk of bias in included trials
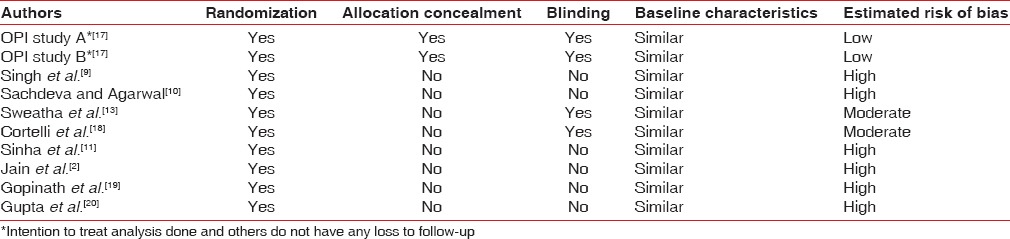
Using Cochrane tool for assessing risk of bias, two studies showed low risk of bias and two studies showed moderate risk of bias. Remaining six had high risk of bias [Table 1]. All included trials had random allocation, but only two had concealed allocation.[17] Only four trials had adequate information about blinding.[13,17,18] Two of the trial has issues related to loss to follow-up, but intention to treat analysis was done for the same.[17] In all studies, baseline characteristics were similar.
Table 1.
Risk of bias in included trials
Study characteristics
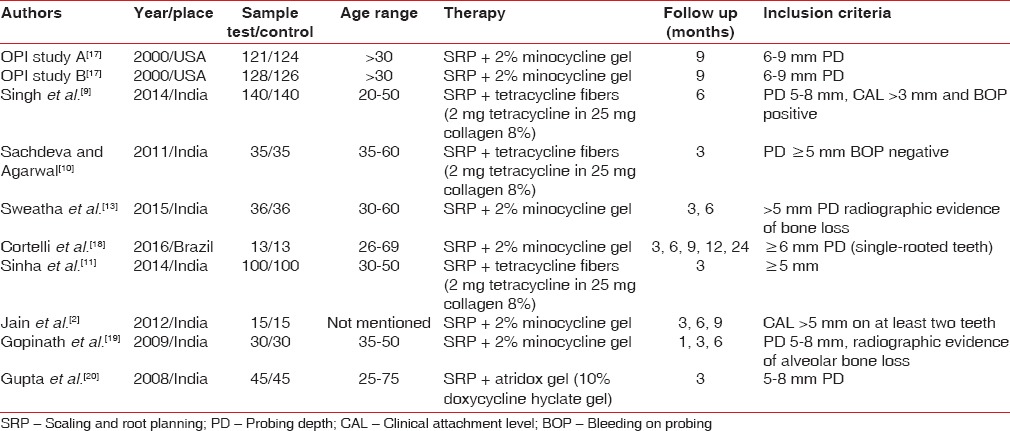
Two out of ten included studies were conducted in USA[17] and one in Brazil;[18] others were conducted in India.[2,9,10,11,13,19,20] The number of sites in studies ranged from 13 to 140. The age of the subjects included ranged from 20 to 75 years. PD was considered for inclusion of participants in all studies and it ranged from 5 to 9 mm. In all of the studies, an initial course of periodontal therapy was completed, including oral hygiene instructions and full mouth scaling and root planning. Of ten included studies, only one had used doxycycline as LDD,[20] six studies had used 2% minocycline gel[2,13,17,18,19] where remaining three had used 8% tetracycline fibers.[9,10,11] Follow-up period varies from 3 to 9 months [Table 2]. Seven studies evaluated PD after 3 months, five did follow-up for 6 months and four followed up the participants for 9 months. Only six studies evaluated attachment gain where four had follow-up for up to 3 months and three had follow-up results after 6 months also.
Table 2.
Details of included studies
Outcomes
Change in attachment level
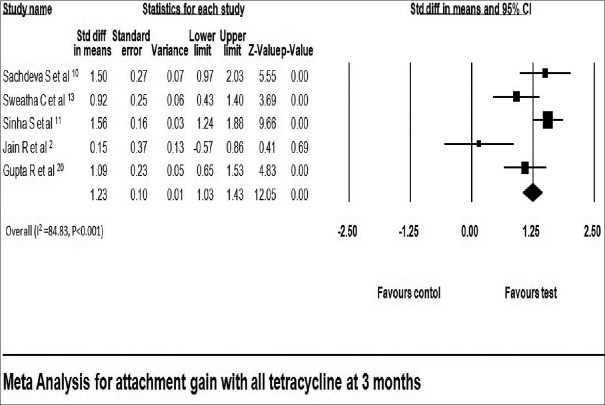
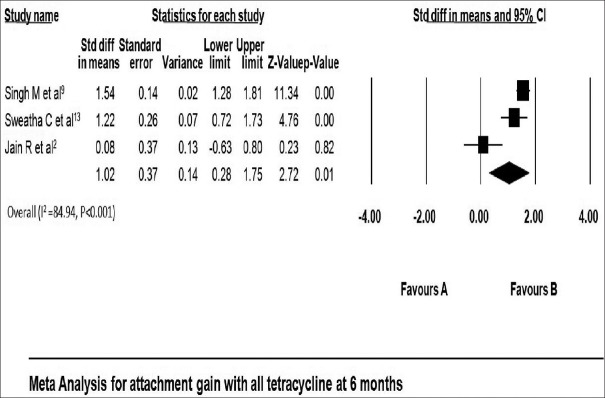
All included studies reported gain in attachment in both test and control group. Gain in attachment reported in tetracycline LDD group ranged from 1.73 ± 0.90 mm[20] to 3.40 ± 0.70 mm[13] at 3 months and 3.20 ± 0.90 mm[9] to 4.06 ± 0.67 mm[13] at 6 months [Table 3]. Attachment gain was significant in all tetracycline group compared with that of placebo at 3 months using fixed effect model (standard difference in mean: 1.23 mm; 95% confidence interval [CI]: 1.03–1.43) [Figure 2]. Gain in attachment was significant after 6 months in all tetracycline group compared to placebo using random effect model (standard difference in mean: 1.02 with 95% CI 0.28–1.75) [Figure 3].
Table 3.
Results of included studies
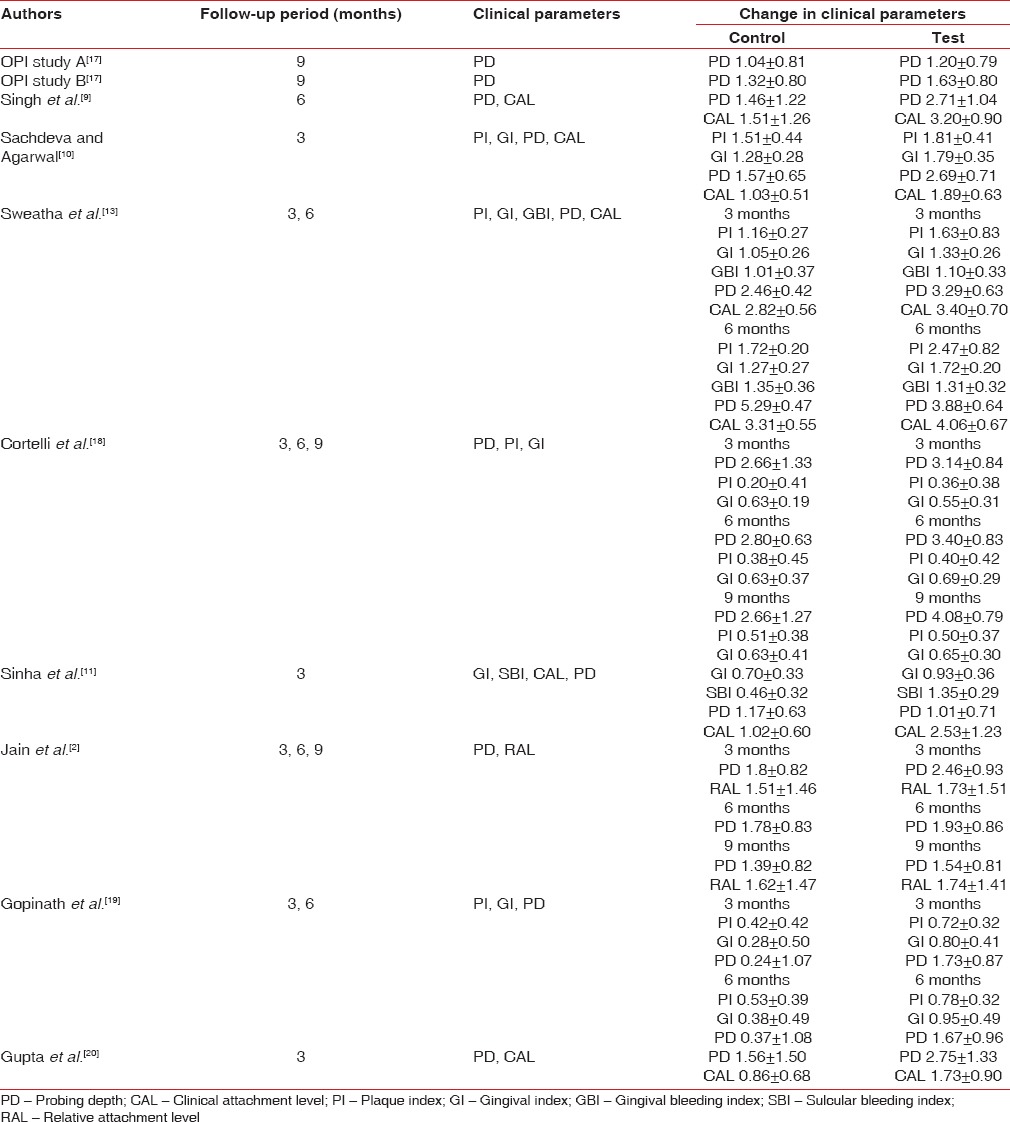
Figure 2.
Change in clinical attachment level at 3 months
Figure 3.
Change in clinical attachment level at 6 months
Change in probing depth
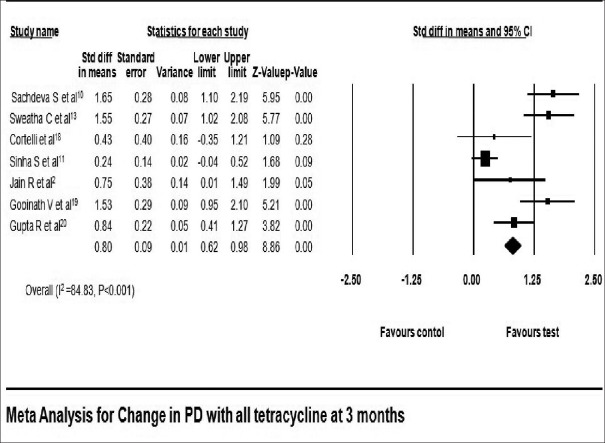
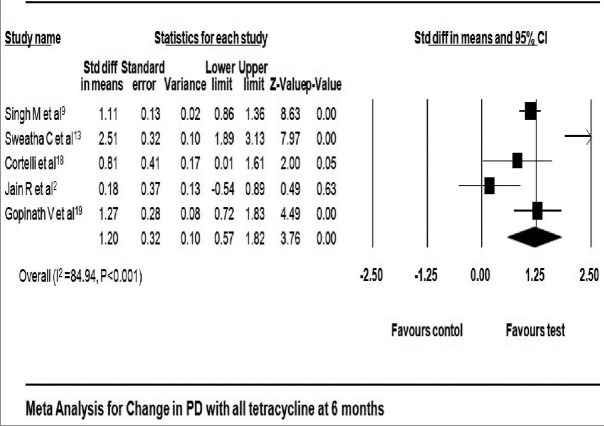
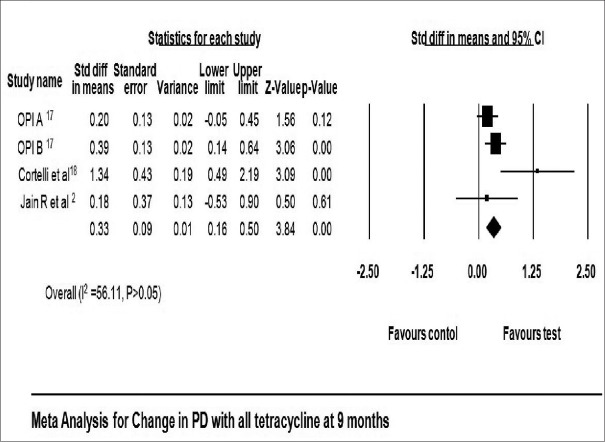
All studies included in this meta-analysis had reported a significant reduction in PD at 3 months,[2,10,13,18,19,20] 6 months,[2,9,13,18,19] and 9 months[2,17,18] in favor of tetracycline as LDD. Reduction in PD reported in tetracycline group ranged from 1.01 ± 0.71 mm[11] to 3.29 ± 0.63 mm[13] at 3 months, 1.67 ± 0.96 mm[19] to 3.88 ± 0.64 mm[13] at 6 months, and 1.20 ± 0.79 mm[17] to 4.08 ± 0.79 mm[18] at 9 months [Table 3]. Reduction in PD local tetracycline group compared to control group was 0.80 mm with 95% CI 0.62–0.98 at 3 months (fixed effect model) [Figure 4] and 1.20 mm with 95% CI 0.57–1.87 at 6 months (fixed effect model) [Figure 5]. The pooled analysis for PD at 9 months reported change of 0.33 mm with 95% CI 0.16–0.50 using fixed effect model [Figure 6].
Figure 4.
Change in probing depth at 3 months
Figure 5.
Change in probing depth at 6 months
Figure 6.
Change in probing depth at 9 months
Others
8% tetracycline fibers are used for local drug in three studies[9,10,11] where clinical parameters and follow-up were different, so data cannot be pooled. Singh et al.[9] evaluated PD and CAL after 6 months and found statistically significant change in favor of test [Table 3]. Sachdeva and Agarwal[10] also evaluated PD and CAL, but follow-up period was 3 months. They found significant improvement in both test and control groups. Sinha et al.[11] has evaluated effect of tetracycline fibers on PD and CAL after 3 months and found a significant change in CAL in favor of test [Table 3]. GI was evaluated at 3 months,[10,11,13,18,19] and significant improvement is observed in both LDD and placebo group, but significant difference between the groups was not observed. Similarly at 6 months, three studies[13,18,19] evaluated GI and no significant difference between the groups was found. Similarly at 3 months, SBI/gingival bleeding index was not showing significant difference between the groups[11,13] and even at 6 months.[13]
DISCUSSION
The use of local antimicrobial agent is to prevent or control microbial-induced inflammation in an effective concentration and be maintained long enough for the desired effect to be accomplished without causing any side effect.
The present systematic review and meta-analysis are aimed to evaluate the clinical effectiveness of local antimicrobial agent tetracycline in the management of chronic periodontitis.
The scientific literature in English up to and including January 2017 was searched for RCT. To the best of our knowledge, till date, there is no published meta-analysis evaluating the clinical effectiveness of tetracycline group of local antimicrobial agent in the management of chronic periodontitis.
The assessment of the quality revealed high risk of bias in majority of included studies. The result of the systematic analysis did not demonstrated any significant difference among different studies in relation to study design, inclusion and exclusion criteria, outcome variables, patients’ follow-up duration.
The CAL and PD were used as primary variables and PI and SBI were used as secondary outcome variables. All the variables showed overall positive effects.
Summary of main results
Gain in CAL and reduction in PD are the major clinical outcome to determine the success of local drug delivery system. All the included RCTs observed statistical significance for CAL in tetracycline and placebo group while reduction in PD was significantly more in tetracycline group compared to placebo.
Attachment gain is the most desirable outcome in success of periodontal therapy. Significant attachment gain was observed in all tetracycline groups at three and 6 months (1.23 mm: 1.03, 1.43 and 1.02 mm: 0.28, 1.75 respectively). These results are clinically significant also. Significant heterogeneity was observed for CAL at three and 6 months (I2 = 74.8, P = 0.003 and I2 = 85.98, P = 0.001, respectively). For the pooled results at 3 months, fixed effect model was used as fixed and random effect model showed similar results while for 6 months, random effect model was used as both models showed difference in pooled observation.
Reduction in PD is one of the desirable outcomes of any periodontal therapy and is most important for the maintenance there after. In all tetracycline group, significant reduction in PD was observed at 3, 6, and 9 months. For pooling the data of results, fixed effect model was used as despite of significant heterogeneity, results of fixed effect model and random effect model were similar.
SBI and GI were secondary outcome variable for this meta-analysis. Pooling of data for the SBI was not possible because only two studies have measured the outcome and one of those have used 2% minocycline[13] and others have used 8% tetracycline.[11] Significant improvement in these inflammatory markers was observed at three and 6 months, but difference between tetracycline and placebo groups was not observed. GI was observed in five studies[10,11,13,18,19] where significant difference between test and control group was not found.
Analysis of study design
All the ten included trials reported adequate randomization while allocation was concealed only in two trials.[17] Four trials[13,17,18] had reported proper blinding and others were not clear. All the included trials reported similar baseline value for periodontal parameters evaluated. Using Cochrane tool for assessing risk of bias, two trials[17] had low risk of bias and two[13,18] had moderate risk of bias.
Limitation of the present meta-analysis
One of the primary limitations of this analysis is the inclusion of only English RCTs. Despite of significant heterogeneity, the data were pooled. The reviewers were not able to procure data from any ongoing trials.
CONCLUSION
The systematic appraisal of the evidences on efficacy of tetracycline group as LDD on CAL and PD has confirmed the benefits compared to placebo. This meta-analysis showed 1.02 mm (0.28, 1.75) gain in CAL and reduction of 1.20 mm (0.57, 1.87) in PD after 6 months in favor of all tetracycline
Clinical implications
This meta-analysis showed significant improvements in clinical periodontal parameters such as CAL and PD for moderate to severe chronic periodontitis cases in favor of tetracycline as LDD compared to placebo. Tetracycline as LDD is effective, user-friendly, and cost-effective so can be more widely used in nonsurgical periodontal therapy.
Implication for future research
Efficacy of tetracycline as LDD can be studied and compared with other drugs and for other form of periodontitis. Different vehicles and concentration are also needed to be studied in larger high quality RCTs. The effect of tetracycline as LDD on the periodontal pathogens can also be studied. Evidence-based systemic review and meta-analysis can be performed to include data published in language other than English.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Greenwell H Committee on Research, Science and Therapy. American Academy of Periodontology. Position paper: Guidelines for periodontal therapy. J Periodontol. 2001;72:1624–8. doi: 10.1902/jop.2001.72.11.1624. [DOI] [PubMed] [Google Scholar]
- 2.Jain R, Mohamed F, Hemalatha M. Minocycline containing local drug delivery system in the management of chronic periodontitis: A randomized controlled trial. J Indian Soc Periodontol. 2012;16:179–83. doi: 10.4103/0972-124X.99259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodson JM, Haffajee A, Socransky SS. Periodontal therapy by local delivery of tetracycline. J Clin Periodontol. 1979;6:83–92. doi: 10.1111/j.1600-051x.1979.tb02186.x. [DOI] [PubMed] [Google Scholar]
- 4.Kalsi R, Vandana KL, Prakash S. Effect of local drug delivery in chronic periodontitis patients: A meta-analysis. J Indian Soc Periodontol. 2011;15:304–9. doi: 10.4103/0972-124X.92559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drisko CH. Non-surgical pocket therapy: Pharmacotherapeutics. Ann Periodontol. 1996;1:491–566. doi: 10.1902/annals.1996.1.1.491. [DOI] [PubMed] [Google Scholar]
- 6.Greenstein G, Polson A. The role of local drug delivery in the management of periodontal diseases: A comprehensive review. J Periodontol. 1998;69:507–20. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 7.Pavia M, Nobile CG, Angelillo IF. Meta-analysis of local tetracycline in treating chronic periodontitis. J Periodontol. 2003;74:916–32. doi: 10.1902/jop.2003.74.6.916. [DOI] [PubMed] [Google Scholar]
- 8.Matesanz-Pérez P, García-Gargallo M, Figuero E, Bascones-Martínez A, Sanz M, Herrera D, et al. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J Clin Periodontol. 2013;40:227–41. doi: 10.1111/jcpe.12026. [DOI] [PubMed] [Google Scholar]
- 9.Singh M, Shreehari AK, Garg PK, Singh S. Clinical efficacy of chlorhexidine chips and tetracycline fibers as an adjunct to non surgical periodontal therapy. Eur J Gen Dent. 2014;3:134–9. [Google Scholar]
- 10.Sachdeva S, Agarwal V. Evaluation of commercially available biodegradable tetracycline fiber therapy in chronic periodontitis. J Indian Soc Periodontol. 2011;15:130–4. doi: 10.4103/0972-124X.84381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha S, Kumar S, Dagli N, Dagli RJ. Effect of tetracycline HCl in the treatment of chronic periodontitis - A clinical study. J Int Soc Prev Community Dent. 2014;4:149–53. doi: 10.4103/2231-0762.142011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javali MA, Vandana KL. A comparative evaluation of atrigel delivery system (10% doxycycline hyclate) atridox with scaling and root planing and combination therapy in treatment of periodontitis: A clinical study. J Indian Soc Periodontol. 2012;16:43–8. doi: 10.4103/0972-124X.94603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweatha C, Srikanth C, Babu MR. A comparative study of the effect of minocycline microspheres as an adjunct to scaling and root planing versus scaling and root planing alone in the treatment of chronic periodontitis. Int J Recent Sci Res. 2015;6:3540–50. [Google Scholar]
- 14.Ahamed S, Jalaluddin M, Khalid I, Moon N, Shaf TK, Ali FM, et al. The use of controlled release locally delivered 10% doxycycline hyclate gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis: Clinical and microbiological results. J Contemp Dent Pract. 2013;14:1080–6. doi: 10.5005/jp-journals-10024-1455. [DOI] [PubMed] [Google Scholar]
- 15.Rao SK, Setty S, Acharya AB, Thakur SL. Efficacy of locally-delivered doxycycline microspheres in chronic localized periodontitis and on Porphyromonas gingivalis. J Investig Clin Dent. 2012;3:128–34. doi: 10.1111/j.2041-1626.2011.00110.x. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. New Jersey, USA: Wiley; 2008. pp. 187–241. [Google Scholar]
- 17.Center for Drug Evaluation and Research. Approval Letter: Arestin (Minocycline Hydrochloride) Microspheres, 1gm. [Last accessed on 2017 Jan 26]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/50781_Arestin_medr.pdf .
- 18.Cortelli JR, Querido SM, Aquino DR, Ricardo LH, Pallos D. Longitudinal clinical evaluation of adjunct minocycline in the treatment of chronic periodontitis. J Periodontol. 2006;77:161–6. doi: 10.1902/jop.2006.040409. [DOI] [PubMed] [Google Scholar]
- 19.Gopinath V, Ramakrishnan T, Emmadi P, Ambalavanan N, Mammen B, Vijayalakshmi, et al. Effect of a controlled release device containing minocycline microspheres on the treatment of chronic periodontitis: A comparative study. J Indian Soc Periodontol. 2009;13:79–84. doi: 10.4103/0972-124X.55844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta R, Pandit N, Aggarwal S, Verma A. Comparative evaluation of subgingivally delivered 10% doxycycline hyclate and xanthan-based chlorhexidine gels in the treatment of chronic periodontitis. J Contemp Dent Pract. 2008;9:25–32. [PubMed] [Google Scholar]
- 21.Singh S, Roy S, Chumber SK. Evaluation of two local drug delivery systems as adjuncts to mechanotherapy as compared to mechanotherapy alone in management of chronic periodontitis: A clinical, microbiological, and molecular study. J Indian Soc Periodontol. 2009;13:126–32. doi: 10.4103/0972-124X.60224. [DOI] [PMC free article] [PubMed] [Google Scholar]