Abstract
Background:
Chlorhexidine (CHX) is the gold standard of all chemical plaque control agents and the most commonly prescribed mouthwash. However, several studies have shown cytotoxic and genotoxic effects of CHX on various eukaryotic cells. In this study, we have used micronuclei as a biomarker of DNA damage in buccal epithelial cells of chronic gingivitis patients who were given adjunct 0.2% CHX for plaque control.
Materials and Methods:
Chronic gingivitis patients who were exclusively on mechanical plaque control methods were taken as control (Group A) (n = 101), and chronic gingivitis patients who along with mechanical plaque control measures were taking 0.2% chlorhexidine mouthwash as adjunct were taken as cases (Group B) (n = 255). The Group B was further divided into 5 subgroups (B1, B2, B3, B4, B5) (n = 51) on increasing duration of usage of CHX from ≤1 week to 24 weeks. Buccal epithelial cells were gently scrapped from the buccal mucosa using soft toothbrush. The epithelial cells were collected in buffer solution and centrifuged at 8000 rpm for 5 min. The buccal epithelial cells were air dried, fixed, and stained with 5% Giemsa stain on preheated glass microscopic slides and observed under microscope to screen 2000 nucleated cells per individual for number of micronucleated cells and micronuclei as genotoxic measure.
Results:
The mean number of micronucleated cells was found to be 0.41 ± 0.71 for Group A as compared values ranging from 1.65 ± 2.09 (Group B1) to 11.7 ± 1.87 (Group B5) in different subgroups of Group B, and similarly, the mean number of micronuclei was found to be 0.48 ± 0.80 for Group A as compared to values ranging from 2.57 ± 1.64 (Group B1) to 14.5 ± 2.49 (Group B5) in different subgroups of Group B using analysis of variance (P < 0.001).
Conclusion:
We conclude that CHX mouthwash is genotoxic to buccal epithelial cells and there is incremental trend in genotoxicity as the duration of usage is increased.
Keywords: Chlorhexidine, chronic gingivitis, genotoxicity, micronucleus
INTRODUCTION
Microbial dental plaque causes chronic gingivitis, which is the inflammation of gingiva; without loss of tooth supporting structures or with reduced but with stable periodontium. The treatment of chronic gingivitis depends on the control of dental plaque.[1,2,3,4]
Dental plaque formation is prevented by mechanical and chemical plaque control measures. Mechanical plaque control methods include toothbrushing and interdental cleaning aids. Mechanical plaque control measures are sometimes inadequate either due to noncompliance of patient or due to improper technique.[5] Chemical plaque control measures such as mouthrinse are commonly prescribed in clinical practice as an adjunct to mechanical plaque control measures in dental caries, gingivitis, periodontitis and halitosis.[6]
Chlorhexidine (CHX) is the most commonly prescribed mouthrinse in clinical practice and is the gold standard among all the chemical plaque control agents known.[7] CHX was initially used as general disinfectant for washing operation sites in surgery, gynecology, and ophthalmology.[8] Dental plaque inhibition by CHX was first reported by Schroeder[9] in 1969, but CHX was established as antiplaque and antigingivitis agent in humans by Loe and Schiott in 1970.[10]
The antiplaque action of chlorhexidine is attributed owing to strong attraction between positively charged chlorhexidine and negatively charged oral bacteria. CHX causes altered cell permeability and leakage of potassium and phosphorous ions in oral bacteria at low concentration, whereas cytolysis and cell death at high concentration.[11,12,13]
CHX is available in three formulations; digluconate, acetate, and hydrochloride. The digluconate is water soluble and is most common formulation. The clinical effectiveness of CHX have been proved in several trials,[7,14,15] however, it has side effects such as discoloration and staining of teeth and oral mucosa, taste alteration, oral erosions, and parotid swelling.[16]
Further, CHX has cytotoxic effect on gingival fibroblasts, epithelial cells, neutrophils, and red blood cells[17,18,19] and also parachloroaniline, a degradation product of chlorhexidine is mutagenic.[20]
Several biomarkers are used to study the effect of various genotoxic agents on oral epithelial cells in malignant transformation and various biomarkers are used in biomonitoring studies to assist in diagnosis, staging, and evaluation of the risk assessment. The biomarkers give insight in the genetic and molecular transformation which are taking place in the process of oral carcinogenesis[21]. One of the most simple and effective biomarker for assessing neoplastic progression in oral mucosal cells is micronuclei.[22] Micronuclei are small cytoplasmic chromatin masses which appear as small nuclei in the cell adjacent to the main nucleus and are formed due to chromosomal damage in basal epithelial cells or due to defect in spindle apparatus during the cell division.[23] There is increase in number of micronuclei in oral epithelial cells when there is malignant transformation from normal mucosa to precancerous and cancerous lesion.[24] The aim of the study was to assess the genotoxic effect of CHX mouthrinse on exfoliated buccal epithelial cells in chronic gingivitis patients using micronucleus (MN) test.
MATERIALS AND METHODS
The study was carried out in patients reporting to the outpatient of Department of Periodontology in collaboration of Interdisciplinary Biotechnology Unit of our Institute.
Inclusion criteria
Systemically healthy chronic gingivitis patients who had periodontal probing depth of 3 mm or less using William's Periodontal probe (Silverline, Hufriedy, USA) and gave their written consent were recruited in the study.
Exclusion criteria
Patients with history of any systemic disease were excluded from the study
Smokers, tobacco and pan masala chewers and alcoholics were excluded from the study
Patients with dental caries and dental restorations and orthodontic appliances were excluded from the study
Patient having any oromucosal lesions, viral diseases, recent vaccinations for the last six were excluded from the study
Patient who undergone any radiodiagnostic examinations in the last six months
The patients enrolled in the study were divided into two groups.
Group A or controls: Chronic gingivitis patients (n = 101) who were exclusively on mechanical plaque control without any adjunctive chemical plaque control measures.
Group B or cases: Chronic gingivitis patients (n = 255) on mechanical plaque control along with adjunct 0.2% CHX mouthwash (aqueous base).
The Group B was further divided into five subgroups based on duration of use of CHX mouthwash as B1 (n = 51): Chronic gingivitis patients on mechanical plaque control along with CHX mouthwash (0.2%) as adjunct for ≤1 week., B2 (n = 51): Chronic gingivitis patients on mechanical plaque control along with CHX mouthwash (0.2%) as adjunct for more than 1 week but ≤2 weeks., B3 (n = 51): Chronic gingivitis patients on mechanical plaque control along with CHX mouthwash (0.2%) as adjunct for more than 2 weeks but ≤4 weeks., B4 (n = 51): Chronic gingivitis patients on mechanical plaque control along with CHX mouthwash (0.2%) as adjunct for more than 4 weeks but ≤12 weeks., B5 (n = 51): Chronic gingivitis patients on mechanical plaque control along with CHX mouthwash (0.2%) as adjunct for more than 12 weeks but ≤24 weeks.
Plaque accumulation and gingival inflammation were measured using plaque index by Silness and Loe,[3] and Gingival index by Loe and Sillness,[25] on six index teeth, namely, maxillary right first molar (16), maxillary right lateral incisor (12), maxillary left first premolar (24), mandibular left first molar (36), mandibular left lateral incisor (32), and mandibular right first premolar (44). If any of these index teeth was missing, then whole mouth index was recorded. The four gingival areas of the tooth were examined for scoring, namely, distofacial, midfacial, mesiofacial, and lingual or palatal. The plaque score and gingival score for individual tooth were calculated by adding all the scores from four gingival areas of tooth and then dividing by four. The individual tooth score was added and divided by number of teeth examined to give the plaque index and gingival index, respectively, for a particular person. The mouth mirror, William periodontal probe, light source were used for scoring. The gingival was air-dried with cotton roll or blast of air before proceeding for scoring.
Micronucleus test
Buccal mucosal cell collection
Soft toothbrush was used for collection of buccal epithelial cells from each participant by gently scraping inside of the check cells. This toothbrush was then swirled into a test tube containing the buffer solution resulting into a cell suspension.[26]
Preparation of buffer solution
0.1M ethylenediaminetetraacetic acid (EDTA), 0.01M Tris HCl, and 0.02M NaCl were dissolved in 1 liter of autoclaved distilled water. The pH of the buffer was adjusted to 7.0 with NaOH.[26]
Chemicals used
Tris hydrochloride (SRL, India), EDTA from (SRL, India), Giemsa stain, sodium chloride, methanol, glycerol, and sodium hydroxide pellets (Merck, India).
Procedure
Epithelial cells collected from the oral mucosa were in the test tube and were washed twice by centrifugation (Sigma 3-30K Refrigerated High-Speed Centrifuge) at 8000 rpm for 5 min using the above-mentioned buffer solution.[27] This step of washing inactivated the endogenous DNAases, removed bacterial load and cell debris that would otherwise complicate the scoring procedure.[28] These cells were then smeared on to clean preheated microscope glass slides and allowed to air dry. The cells were fixed with cold methanol (100%). The slides were kept at 37°C overnight and then stained with 5% Giemsa stain.[27,28]
These slides are observed under microscope (Motic B1-220/223Educational Compound Microscope) to screen 2000 nucleated cells per individual for the presence of MN at the magnification of 100X.[26]
The micronucleated cells were identified as DNA-containing structures which were separated from the main nucleus and had the following characteristics:[26]
Rounded smooth perimeter suggestive of a membrane
The diameter was less than a third (1/3rd) the diameter of the associated nucleus, but large enough to discern shape and color
The staining intensity of MN was similar to that of the nucleus
The texture of MN was similar to that of nucleus
The MN had the same focal plane as the nucleus without overlap or bridge to the associated nucleus.
If a cell contained one MN, it was designated as micronucleated cell with one micronuclei, and if the cell contained two micronuclei, it was designated as micronucleated cell two micronuclei and if the cell contained three micronuclei, it was designated as micronucleated cell containing three micronuclei and so on. The individual scoring was done by adding number of micronucleated cell observed in the slide as total micronucleated cell and total number of micronuclei scored in all micronucleated cells as number of micronuclei.
Thus, total number of micronucleated and total number of micronuclei were taken as genotoxicity measure for a particular subject [Figures 1–4].[29]
Figure 1.
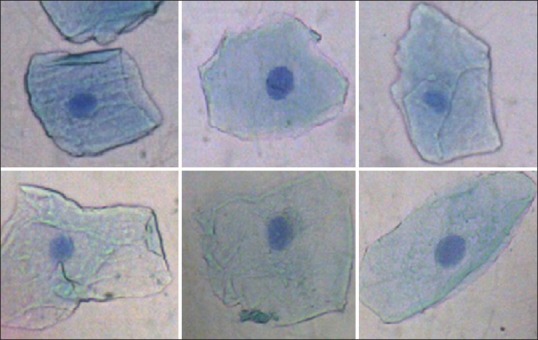
Photomicrograph of buccal cells of control (Group A) showing buccal cells without micronuclei
Figure 4.
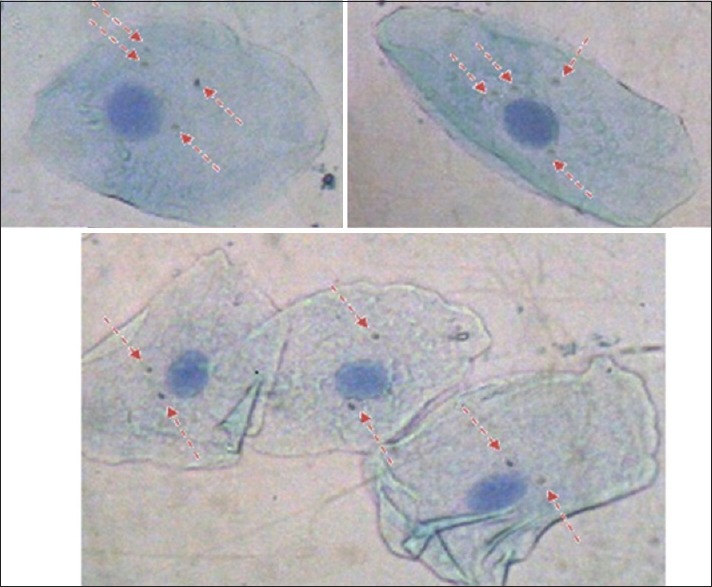
Photomicrograph of buccal cellsof study (GroupB) showing 2 micronucleated cells with 4 micronuclei each and 3 micronucleated cell with 2 micronuclei each (5 micronucleated cells and 14 micronuclie)
Figure 2.
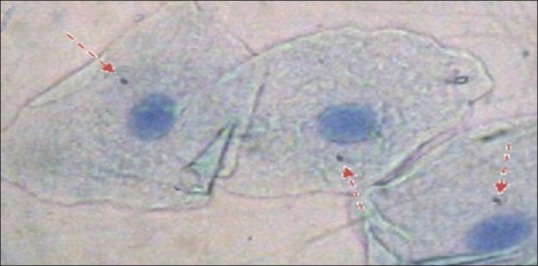
Photomicrograph of buccal cells of study group (Group B) showing 3 micronucleated cells with one micronuclei each (3 micronucleated cells and 3 micronuclei)
Figure 3.
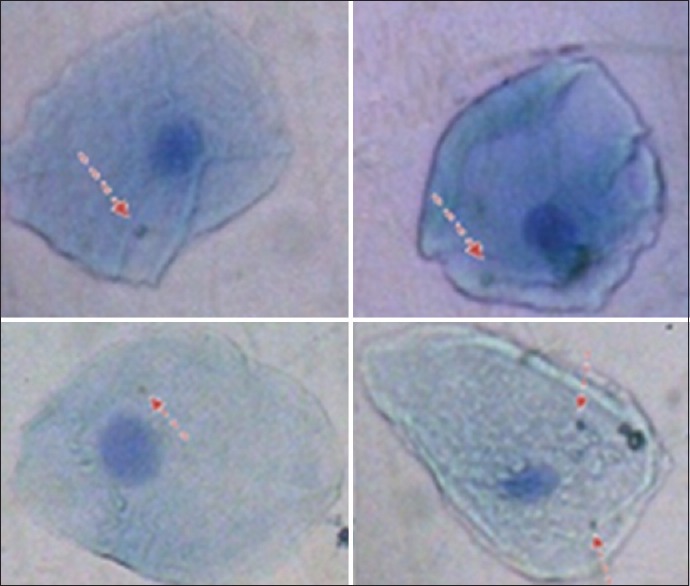
Photomicrograph of buccal cells of study group (Group B) showing micronucleated cells with 1 micronuclei each and one micronucleated cell with 2 micronuclei (4 micronucleated cells and 5 micronuclei)
To remove bias, the slides were coded and were examined under microscope and 2000 cells were screened for micronuclei under microscope at 100X magnification by two examiners.
Statistical analysis
The statistical analysis was done using Statistical Package for Social Sciences Version 15.0, IBM, USA. statistical analysis software. P < 0.05 was taken as statistically significant.
RESULTS
Demography
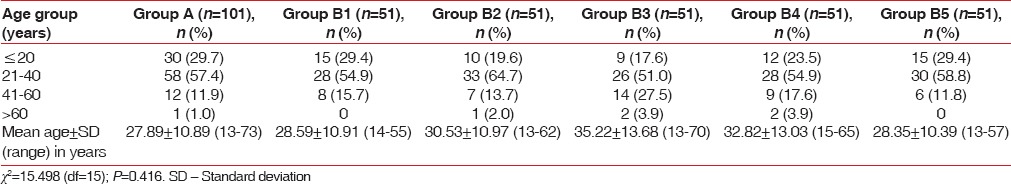
The age of individuals ranged from 13 to 73 years. Majority of cases in all the groups were aged 21–40 years followed by those aged < 20 years and 41–60 years. The mean age of patients in Group A was 27.89 ± 10.89 years (range 13–73 years) whereas the mean age of patients in different subgroups of Group B ranged from 28.35 ± 10.39 (Group B5) to 35.22 ± 13.68 (Group B3) years. There was no significant difference among groups in terms of age (P = 0.416) [Table 1].
Table 1.
Comparison between control and study groups for age
On comparing gender distribution, Group A and Groups B2, B3, and B5, majority of patients were females whereas majority of patients in Group B1 and B4 were males. However, the difference among groups was not significant statistically (P = 0.965) [Table 2].
Table 2.
Comparison between control and study groups for gender distribution

Periodontal health indices
In Group A (control group), mean plaque index was 2.02 ± 0.49. In different subgroups of Group B (study group), mean plaque index ranged from 0.16 ± 0.12 (Group B3) to 0.86 ± 0.51 (Group B1) [Table 3].
Table 3.
Comparison between control and study groups for periodontal health indices

In Group A (control group), mean gingival index was 1.98 ± 0.60. In different subgroups of Group B (study group), mean gingival index ranged from 0.06 ± 0.05 (Group B3) to 0.46 ± 0.53 (Group B1) [Table 3].
Genotoxicity measure
Mean number of micronucleated cells was found to be 0.41 ± 0.71 for Group A as compared values ranging from 1.65 ± 2.09 (Group B1) to 11.7 ± 1.87 (Group B5) in different groups of Group B, thus showing an incremental trend with increasing duration of use of CHX [Table 4 and Figure 5].
Table 4.
Comparison among control and different study subgroups for number of micronucleated cells and total number of micronuclei

Figure 5.
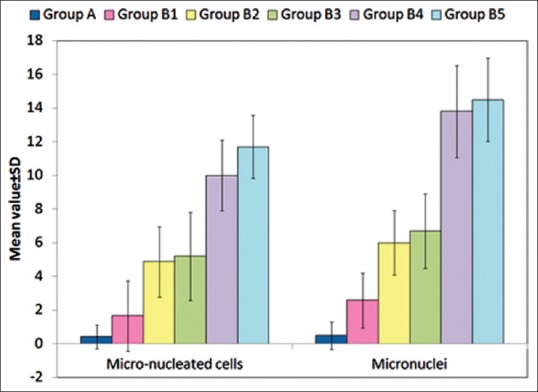
Micronucleated cells and number of micronuclei distribution in control and different study groups
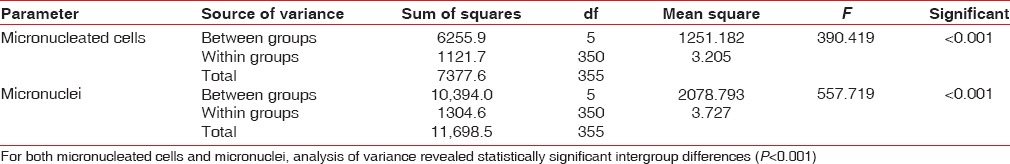
Mean number of micronuclei was found to be 0.48 ± 0.80 for Group A as compared values ranging from 2.57 ± 1.64 (Group B1) to 14.5 ± 2.49 (Group B5) in different subgroups of Group B, thus showing an incremental trend with increasing duration of use of CHX [Table 4 and Figure 5]. For both micronucleated cells and micronuclei, analysis of variance revealed statistically significant intergroup differences (P < 0.001) [Table 5].
Table 5.
Analysis of variance for micronucleated cell and micronuclei count among control and different study subgroups
DISCUSSION
The genotoxicity of CHX was measured in terms of number of micronucleated cells and number of micronuclei observed per 2000 buccal epithelial cells of the individual.
On comparing number of micronucleated cells in Group A (0.41 ± 0.71) with subgroups of Group B; Groups B1, B2, B3, B4 and B5. There was an incremental trend in number of micronucleated cells from B1 (1.65 ± 2.09), B2 (4.88 ± 2.09), B3 (5.20 ± 2.62), B4 (10.0 ± 2.09) to B5 (11.7 ± 1.87) in the sequence A < B1 < B2 ~ B3 < B4 < B5 (P < 0.001) [Tables 4–6 and Figure 5].
Table 6.
Between control (Group A) and study groups (Group B) differences in micronucleated cell and micronuclei count (Tukey honest significant difference test)
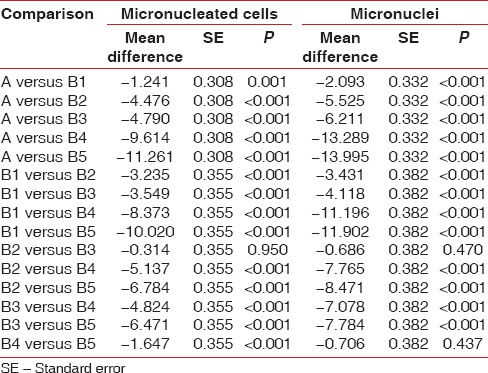
Likewise, on comparing number of MN in Group A (0.48 ± 0.80) with subgroups of Group B; Groups B1, B2, B3, B4 and B5. There was an incremental trend in number of micronuclei cells from B1 (2.57 ± 1.64), B2 (6.00 ± 1.92), B3 (6.69 ± 2.21), B4 (13.8 ± 2.72) to B5 (14.5 ± 2.49) in the sequence of A < B1 < B2 ~ B3 < B4 ~ B5 (P < 0.001) [Tables 4–6 and Figure 5].
In a similar study, Erdemir et al.[30] studied the cytotoxicity of three commercial mouthrinses klorhex (0.2% CHX gluconate), andorex (0.15% benzydamine HCL and 0.12% CHX Gluconate), and tanflex (0.15% benzydamine HCL) on buccal epithelial cells using MN test. Twenty-eight patients aged 16–24 years undergone three mouthrinses’ application and were analyzed before and after 1 week of exposure. Physiologic saline was used for the control group. The MN incidence was scored in the buccal epithelial of each participants. The micronuclei incidence increased in klorhex (0.2% CHX) from 5.57 ± 5.00–11.86 ± 8.51 (P < 0.05), suggesting genotoxic effects of CHX mouthrinse on buccal epithelial cells.
In a more recent study, Carlin et al.[31] compared the DNA damage and cellular death in buccal mucosa cells of patients which were exposed to various commercially available five mouthrinse; Listerine® Cepacol®, Plax alcohol-free®, Periogard®, and Plax Whitening®. Seventy-five individuals were recruited in the study and were divided into five groups containing 15 people each and were exposed to respective commercial mouthrinse. Exfoliated buccal mucosa cells were taken before exposure and after 2 weeks of mouthrinse exposure. Furthermore, blood was also taken from three healthy donors for in vitro study. The result showed that Periogard® (0.12% CHX) caused increase in Micronuclie frequency by 1.8% after 2 weeks of exposure with statistically significant difference (P < 0.05) as compared to before. The single-cell gel (comet) assay demonstrated DNA damage both in blood lymphocytes and buccal mucosa of individuals who were exposed to 0.12% CHX digluconate for 2 weeks.
Likewise, Eren et al.[32] in their study evaluated the genotoxicity of CHX using comet assay (single cell gel electrophoresis, or SCGE). Thirteen individuals were asked to rinse their mouth with 0.12% CHX solution for 18 days. Buccal epithelial cells and peripheral blood lymphocytes were obtained from all participants at baseline and at the end of experiment. A statistically significant amount of DNA damage in both buccal epithelial cells and blood lymphocytes was observed. The same findings were seen by Ribeiro et al.,[33] in peripheral blood and oromucosal cells using MN test and comet assay (single cell gel electrophoresis, or SCGE) in Wistar rats.
These above findings demonstrate genotoxic effects of CHX on buccal epithelial cells. Our results are in agreement with previous various previous studies done by Erdemir et al., 2007, Carlin et al., 2012, and Eren et al., 2002.[30,31,32]
Our findings show that on increasing the duration of CHX use increases the total number of micronucleated cells and micronuclei in buccal epithelial cells of its users. This suggests that CHX causes cumulative genotoxicity and is dependent on time of exposure [Tables 4–6 and Figure 5].
Our observations have been substantiated by Lessa et al.,[34] who in their in vitro study exposed cultured odontoblast-like cells (MDPC-23) to different concentrations of CHX (0.06%, 0.12%, 0, 2%, 1% and 2%) for 60 s, 2 h or 60 s with 24 h recovery period using MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium) assay. They showed that CHX exhibited dose-dependent toxic effect on odontoblast-like cells. The cytotoxic effect of CHX was more pronounced on increasing the contact time of CHX with cells.
Similarly, Giannelli et al.[35] in their in vitro study exposed osteoblastic, endothelial, and fibroblastic cell lines to various concentrations of CHX (0.0025%, 0.005%, 0.0075%, 0.01% and 0.12%) for different times and tested for cell viability and cell death. They found that CHX causes cell damage in concentration and time-dependent manner.
Furthermore, Bonacorsi et al.[36] in their study evaluated CHX-induced cytotoxicity using MTT assay on murine peritoneal macrophages and showed that cytotoxicity of CHX is dependent on concentration and time of exposure.
Likewise, Hidalgo and Dominguez[37] studied the mechanisms underlying CHX-induced toxicity on human fibroblasts using ATP-dependent Luciferin-Luciferase reaction using commercial ATP Bioluminescence Assay Kit (Boehringer Germany). Human fibroblast was exposed to range CHX concentrations of 0.00005%–0.025% for 3, 6, 8, or 24 h in the absence or presence of different concentrations of fetal calf serum (2, 5, and 10%). They observed that CHX causes time-dependent cytotoxicity.
Chang et al.[38] also observed the cytotoxic effects of CHX on human periodontal ligament cells to be concentration and exposure time dependent.
The cytotoxicity of CHX was first shown by Helgeland et al.,[39] who showed cytotoxic effects of CHX on human epithelial cells at very low concentration of 0.05 mM and also inhibition of Na K ATPase on the membrane of erythrocytes. CHX at concentration of 0.01% or greater when exposed to cell cultures of human gingival fibroblasts and HeLa cells causes death of these cells.[17]
Seymour et al. and Watts et al. both investigated the effect of CHX on neutrophil chemotaxis and found a dose-related decrease in the chemotaxis on increasing CHX concentrations from 0.002% to 0.2% in aqueous solutions, and complete cell lysis was seen at 0.2% concentration.[40,41]
Mariotti and Rumpf[42] showed that CHX caused dose-dependent reduction in cellular proliferation of gingival fibroblasts.
Thus, above evidence portrays CHX as a potential genotoxic and cytotoxic agent and also conforms our observations.
The genotoxicity of CHX can be studied by various biomarkers. The biomarkers have been classified on three purposes; the first purpose is to give account about deleterious chemical exposure. Second, to show the biological effects on target tissue and third, to give information about individual susceptibility.[43] There are various assays that have been developed as potential biomarkers in biomonitoring studies which include assessing metaphase chromosomal aberrations, sister chromatid exchanges, and host-cell reactivation. These methods are quite laborious and time-consuming and require highly trained technicians to read and interpret the slides. Therefore, there is great enthusiasm about micronuclei as genotoxic biomonitoring tool.[44]
MN arise from acentric fragments or whole chromosomes which are not included in the main nuclei of the daughter cells. The micronuclei are formed when the cells are exposed to the substances which cause chromosomal breakage (clastogens) and also affect the spindle apparatus (aneugens). MN test in exfoliated buccal mucosa cells have been systematically used in genetic biomonitoring of populations exposed to various genotoxic agents such as tobacco products, pesticides, and alcohol consumption.[22,45]
The advantage of micronuclei is its simplicity, relative ease of scoring, economical in terms of cost, and required person-time resources. Moreover, precision is obtained from scoring large number of cells. MN cell index reflects genomic instability and increased frequency of micronuclei indicates risk of malignancy.[46,47] Micronucleus is multiple endpoint test which indicates chromosomal aberration.[48,49]
We took buccal epithelial cells for assessing genotoxic effect of CHX in our study because it is an easily accessible tissue for sampling cells in a minimally invasive manner and does not cause undue stress to the individuals. It is frequently used in molecular epidemiological studies for investigating the impact of nutrition, lifestyle factors, genotoxin exposure and genotype on DNA damage and cell death.
Furthermore, the buccal epithelial cells are of ectodermal origin and 90% of all cancers are of ectodermal origin in humans. Therefore, buccal mucosa can be used to monitor early genotoxic events which enter the body either through ingestion or inhalation.[50]
The limitations of our study is that it is a cross-sectional study design and it does not give highest level of evidence. Thus, our study calls for undertaking robust study designs such as longitudinal studies and randomized control trials using specialized test such as micronuclei test using DNA specific stains and comet assay.
Our findings question the long-term use of CHX as adjunct chemical plaque control agent and its usage can only be justified after taking into account the risk to benefit ratio. The patient should be educated and motivated for maintaining adequate plaque control which can be achieved by demonstration of proper toothbrushing technique and correct use of interdental plaque control aids and also periodic reevalautions at regular interval recall visits.
CONCLUSION
The study gives us an indication of potential genotoxic effects of CHX mouthwash on buccal epithelial cells, and therefore, it validates the claim for search of an antiplaque agent which has same efficacy as CHX but without genotoxic effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors are thankful to their host institution for providing all help and support in the conduction of the study.
REFERENCES
- 1.Mariotti A. Dental plaque-induced gingival diseases. Ann Periodontol. 1999;4:7–19. doi: 10.1902/annals.1999.4.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177–87. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 3.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 4.Theilade E, Wright WH, Jensen SB, Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res. 1966;1:1–3. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]
- 5.Brecx M. Strategies and agents in supragingival chemical plaque control. Periodontol 2000. 1997;15:100–8. doi: 10.1111/j.1600-0757.1997.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 6.Mandel ID. Chemotherapeutic agents for controlling plaque and gingivitis. J Clin Periodontol. 1988;15:488–98. doi: 10.1111/j.1600-051x.1988.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones CG. Chlorhexidine: Is it still the gold standard? Periodontol 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 8.Davies GE, Francis J, Martin AR, Rose FL, Swain G. 1:6-di-4’-chlorophenyldiguanidohexane (hibitane); laboratory investigation of a new antibacterial agent of high potency. Br J Pharmacol Chemother. 1954;9:192–6. doi: 10.1111/j.1476-5381.1954.tb00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder HE. Formation and inhibition of dental calculus. J Periodontol. 1969;40:643–6. doi: 10.1902/jop.1969.40.11.643. [DOI] [PubMed] [Google Scholar]
- 10.Löe H, Schiott CR. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res. 1970;5:79–83. doi: 10.1111/j.1600-0765.1970.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 11.Hugo WB, Longworth AR. Some aspects of the mode of action of chlorhexidine. J Pharm Pharmacol. 1964;16:655–62. doi: 10.1111/j.2042-7158.1964.tb07384.x. [DOI] [PubMed] [Google Scholar]
- 12.Hugo WB, Longworth AR. Cytological aspects of the mode of action of chlorhexidine diacetate. J Pharm Pharmacol. 1965;17:28–32. doi: 10.1111/j.2042-7158.1965.tb07562.x. [DOI] [PubMed] [Google Scholar]
- 13.Hugo WB, Longworth AR. The effect of chlorhexidine on the electrophoretic mobility, cytoplasmic constituents, dehydrogenase activity and cell walls of escherichia coli and staphylococcus aureus. J Pharm Pharmacol. 1966;18:569–78. doi: 10.1111/j.2042-7158.1966.tb07935.x. [DOI] [PubMed] [Google Scholar]
- 14.Stabholz A, Soskolne WA, Friedman M, Sela MN. The use of sustained release delivery of chlorhexidine for the maintenance of periodontal pockets: 2-year clinical trial. J Periodontol. 1991;62:429–33. doi: 10.1902/jop.1991.62.7.429. [DOI] [PubMed] [Google Scholar]
- 15.Weitz M, Brownstein C, Deasy M. Effect of a twice daily 0.12% chlorhexidine rinse on the oral health of a geriatric population. Clin Prev Dent. 1992;14:9–13. [PubMed] [Google Scholar]
- 16.Flötra L, Gjermo P, Rölla G, Waerhaug J. Side effects of chlorhexidine mouth washes. Scand J Dent Res. 1971;79:119–25. doi: 10.1111/j.1600-0722.1971.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 17.Goldschmidt P, Cogen R, Taubman S. Cytopathologic effects of chlorhexidine on human cells. J Periodontol. 1977;48:212–5. doi: 10.1902/jop.1977.48.4.212. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal S, Piesco NP, Peterson DE, Charon J, Suzuki JB, Godowski KC, et al. Effects of sanguinarium, chlorhexidine and tetracycline on neutrophil viability and functions in vitro . J Periodontal Res. 1997;32:335–44. doi: 10.1111/j.1600-0765.1997.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 19.Gabler WL, Roberts D, Harold W. The effect of chlorhexidine on blood cells. J Periodontal Res. 1987;22:150–5. doi: 10.1111/j.1600-0765.1987.tb01555.x. [DOI] [PubMed] [Google Scholar]
- 20.Van der Bijl P, Gelderblom WC, Thiel PG. On the mutagenicity of parachloroaniline, a breakdown product of chlorhexidine. J Dent Assoc S Afr. 1984;39:535–7. [PubMed] [Google Scholar]
- 21.Schwartz JL. Biomarkers and molecular epidemiology and chemoprevention of oral carcinogenesis. Crit Rev Oral Biol Med. 2000;11:92–122. doi: 10.1177/10454411000110010501. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel HE, Crott JW, Ghandour H, Dallal GE, Choi SW, Keyes MK, et al. Chronic cigarette smoking is associated with diminished folate status, altered folate form distribution, and increased genetic damage in the buccal mucosa of healthy adults. Am J Clin Nutr. 2006;83:835–41. doi: 10.1093/ajcn/83.4.835. [DOI] [PubMed] [Google Scholar]
- 23.Rosin MP. The use of the micronucleus test on exfoliated cells to identify anti-clastogenic action in humans: A biological marker for the efficacy of chemopreventive agents. Mutat Res. 1992;267:265–76. doi: 10.1016/0027-5107(92)90071-9. [DOI] [PubMed] [Google Scholar]
- 24.Casartelli G, Bonatti S, De Ferrari M, Scala M, Mereu P, Margarino G, et al. Micronucleus frequencies in exfoliated buccal cells in normal mucosa, precancerous lesions and squamous cell carcinoma. Anal Quant Cytol Histol. 2000;22:486–92. [PubMed] [Google Scholar]
- 25.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 26.Countryman PI, Heddle JA. The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutat Res. 1976;41:321–32. doi: 10.1016/0027-5107(76)90105-6. [DOI] [PubMed] [Google Scholar]
- 27.Surrallés J, Autio K, Nylund L, Järventaus H, Norppa H, Veidebaum T, et al. Molecular cytogenetic analysis of buccal cells and lymphocytes from benzene-exposed workers. Carcinogenesis. 1997;18:817–23. doi: 10.1093/carcin/18.4.817. [DOI] [PubMed] [Google Scholar]
- 28.Titenko-Holland N, Moore LE, Smith MT. Measurement and characterization of micronuclei in exfoliated human cells by fluorescence in situ hybridization with a centromeric probe. Mutat Res. 1994;312:39–50. doi: 10.1016/0165-1161(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 29.Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in buccal smears: Methods development. Mutat Res. 1992;271:69–77. doi: 10.1016/0165-1161(92)90033-i. [DOI] [PubMed] [Google Scholar]
- 30.Erdemir EO, Sengün A, Ulker M. Cytotoxicity of mouthrinses on epithelial cells by micronucleus test. Eur J Dent. 2007;1:80–5. [PMC free article] [PubMed] [Google Scholar]
- 31.Carlin V, Matsumoto MA, Saraiva PP, Artioli A, Oshima CT, Ribeiro DA, et al. Cytogenetic damage induced by mouthrinses formulations in vivo and in vitro . Clin Oral Investig. 2012;16:813–20. doi: 10.1007/s00784-011-0559-2. [DOI] [PubMed] [Google Scholar]
- 32.Eren K, Ozmeriç N, Sardaş S. Monitoring of buccal epithelial cells by alkaline comet assay (single cell gel electrophoresis technique) in cytogenetic evaluation of chlorhexidine. Clin Oral Investig. 2002;6:150–4. doi: 10.1007/s00784-002-0168-1. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro DA, Bazo AP, da Silva Franchi CA, Marques ME, Salvadori DM. Chlorhexidine induces DNA damage in rat peripheral leukocytes and oral mucosal cells. J Periodontal Res. 2004;39:358–61. doi: 10.1111/j.1600-0765.2004.00759.x. [DOI] [PubMed] [Google Scholar]
- 34.Lessa FC, Aranha AM, Nogueira I, Giro EM, Hebling J, Costa CA, et al. Toxicity of chlorhexidine on odontoblast-like cells. J Appl Oral Sci. 2010;18:50–8. doi: 10.1590/S1678-77572010000100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannelli M, Chellini F, Margheri M, Tonelli P, Tani A. Effect of chlorhexidine digluconate on different cell types: A molecular and ultrastructural investigation. Toxicol In Vitro. 2008;22:308–17. doi: 10.1016/j.tiv.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Bonacorsi C, Raddi MS, Carlos IZ. Cytotoxicity of chlorhexidine digluconate to murine macrophages and its effect on hydrogen peroxide and nitric oxide induction. Braz J Med Biol Res. 2004;37:207–12. doi: 10.1590/s0100-879x2004000200007. [DOI] [PubMed] [Google Scholar]
- 37.Hidalgo E, Dominguez C. Mechanisms underlying chlorhexidine-induced cytotoxicity. Toxicol In Vitro. 2001;15:271–6. doi: 10.1016/s0887-2333(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 38.Chang YC, Huang FM, Tai KW, Chou MY. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:446–50. doi: 10.1067/moe.2001.116812. [DOI] [PubMed] [Google Scholar]
- 39.Helgeland K, Heyden G, Rölla G. Effect of chlorhexidine on animal cells in vitro . Scand J Dent Res. 1971;79:209–15. doi: 10.1111/j.1600-0722.1971.tb02011.x. [DOI] [PubMed] [Google Scholar]
- 40.Seymour KG, Watts TL, Addison IE, Johnson B. An in vivo study of neutrophil locomotion in relation to periodontal disease status and local chlorhexidine. Oral Microbiol Immunol. 1990;5:95–7. doi: 10.1111/j.1399-302x.1990.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 41.Watts TL, Addison I, Johnson B. Effects of chlorhexidine solution on neutrophil locomotion in vitro . J Dent. 1989;17:287–9. doi: 10.1016/0300-5712(89)90041-9. [DOI] [PubMed] [Google Scholar]
- 42.Mariotti AJ, Rumpf DA. Chlorhexidine-induced changes to human gingival fibroblast collagen and non-collagen protein production. J Periodontol. 1999;70:1443–8. doi: 10.1902/jop.1999.70.12.1443. [DOI] [PubMed] [Google Scholar]
- 43.Indulski JA, Lutz W. Molecular epidemiology: Cancer risk assessment using biomarkers for detecting early health effects in individuals exposed to occupational and environmental carcinogens. Rev Environ Health. 1997;12:179–90. doi: 10.1515/reveh.1997.12.3.179. [DOI] [PubMed] [Google Scholar]
- 44.Stich HF, Parida BB, Brunnemann KD. Localized formation of micronuclei in the oral mucosa and tobacco-specific nitrosamines in the saliva of “reverse” smokers, Khaini-tobacco chewers and gudakhu users. Int J Cancer. 1992;50:172–6. doi: 10.1002/ijc.2910500203. [DOI] [PubMed] [Google Scholar]
- 45.Jyoti S, Siddique YH, Khan S, Naz F, Ali F. Effect on micronucleus frequency and DNA damage in buccal epithelial cells of various factors among pan masala and gutkha chewers. Oral Sci Int. 2015;12:9–14. [Google Scholar]
- 46.Neri M, Fucic A, Knudsen LE, Lando C, Merlo F, Bonassi S, et al. Micronuclei frequency in children exposed to environmental mutagens: A review. Mutat Res. 2003;544:243–54. doi: 10.1016/j.mrrev.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 47.He JL, Chen WL, Jin LF, Jin HY. Comparative evaluation of the in vitro micronucleus test and the comet assay for the detection of genotoxic effects of X-ray radiation. Mutat Res. 2000;469:223–31. doi: 10.1016/s1383-5718(00)00077-2. [DOI] [PubMed] [Google Scholar]
- 48.Kirsch-Volders M, Elhajouji A, Cundari E, Van Hummelen P. The in vitro micronucleus test: A multi-endpoint assay to detect simultaneously mitotic delay, apoptosis, chromosome breakage, chromosome loss and non-disjunction. Mutat Res. 1997;392:19–30. doi: 10.1016/s0165-1218(97)00042-6. [DOI] [PubMed] [Google Scholar]
- 49.Miller B, Pötter-Locher F, Seelbach A, Stopper H, Utesch D, Madle S, et al. Evaluation of the in vitro micronucleus test as an alternative to the in vitro chromosomal aberration assay: Position of the GUM working group on the in vitro micronucleus test. Gesellschaft für umwelt-mutations-forschung. Mutat Res. 1998;410:81–116. doi: 10.1016/s1383-5742(97)00030-6. [DOI] [PubMed] [Google Scholar]
- 50.Thomas P, Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, et al. Buccal micronucleus cytome assay. Nat Protoc. 2009;4:825–37. doi: 10.1038/nprot.2009.53. [DOI] [PubMed] [Google Scholar]


