Abstract
Mucous membrane pemphigoid (MMP) is a heterogeneous group of autoimmune chronic inflammatory, subepithelial blistering disorder, predominantly involving the mucous membranes. It has a female predilection and commonly occurring after the fifth decade of life. The oral mucosa is affected in more than 90% of cases. Dentists could be the first health personnel to identify and diagnose this rare mucocutaneous lesion. Two unique cases of oral MMP with varied clinical presentation, the diagnostic modality, treatment and follow-up are presented.
Keywords: Autoimmune, desquamative gingivitis, mucous membrane pemphigoid, subepithelial
INTRODUCTION
Immune-mediated subepithelial blistering diseases (IMSEBD),[1] includes a number of subepithelial vesiculobullous disorders such as bullous pemphigoid, pemphigoid gestationis, mucous membrane pemphigoid (MMP), dermatitis herpetiformis, and linear IgA disease. MMP is an autoimmune, chronic inflammatory subepithelial blistering disorder that primarily affects the mucous membranes.[2] It is a rare disease, with an incidence of 2–10 cases out of 100,000 individuals.[3] MMP follows less severe course than pemphigus. Autoantibodies such as IgG (97%), C3 (78%) IgA (27%), and IgM (12%)[4] are directed against the various antigens such as targeting bullous antigens 1 and 2, laminin 332, 311, type VII collagen, α6 β4-integrin, nonidentified basal membrane zone antigens[5] in epithelial basement membrane zones/hemidesmosomes, proving MMP's heterogeneity.[6] Elderly females are commonly affected with mean age onset of 50–80 years.[6] The predominant mucosal sites involved are the oral mucosa, ocular mucosa, oropharynx, larynx, and genital region. Skin involvement is restricted to the regions of head, neck, and upper torso.[2] The distinguishing feature of MMP is the scarring of the mucosa after the erosions, and the blisters heal. As scarring was common in the ocular mucosa, it was previously referred to as cicatricial pemphigoid.[6] Long-term complications of MMP include scarring, blindness, dysphagia, laryngeal stenosis, anal or urethral strictures.[2] Intraoral features of MMP include desquamative gingivitis, vesicles, erosions covered by pseudomembranes and ulcers.[4]
Our cases are unique as there was no ocular or cutaneous involvement and in one case the lesion was localized to one site as compared to a generalized mucosal involvement which are seen in these groups of lesions.
CASE REPORTS
Case 1
A 60-year-old female patient presented with 1 year history of intact blisters in mouth which ruptured leaving painful ulcers and peeling of the gingiva. It was accompanied by pain and burning sensation while taking hot and spicy foods. Topical antiseptic gel was prescribed by a physician for the ulcers but on application she did not have any relief. Personal history was noncontributory. She had attained menopause 4 years before and there was no history of systemic disease.
Extraoral examination revealed no ocular, cutaneous, or genital lesions. Intraoral examination revealed generalized gingival erythema, the maxillary gingiva showed loss of stippling from attached gingiva till mucogingival junction was seen in the labial and palatal aspect. Single localized ulcer with areas of erythema in labial mucosa in relation to 13, in palatal mucosa in relation to 23, diffuse irregular ulcers, erosions with pseudomembranes was present on lower labial mucosa from 41 to 44 region, erythematous patches in the right buccal mucosa, right retromolar region were present [Figure 1a–d]. Application of lateral pressure caused gingival bleeding and sloughing of the tissues indicating positive Nikolsky's sign. On pressure, epithelium is peeled off from the lower labial mucosa. The entire oral mucosa was tender on palpation.
Figure 1.
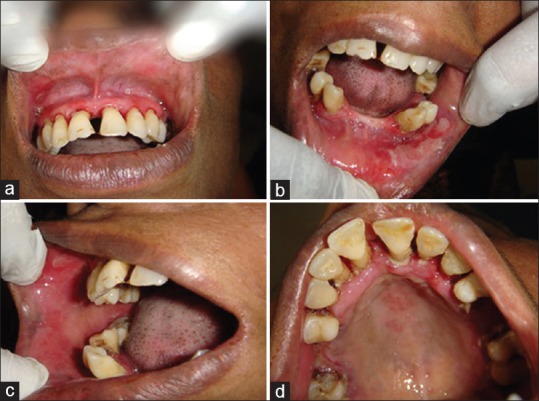
Clinical findings at our patient 1 first visit. Gingival desquamation, localized ulcer in labial mucosa in relation to 13 (a); Diffuse shallow irregular ulcers, erosions with pseudomembranes on lower labial mucosa from 41 to 44 region (b); Erythematous patches in right buccal mucosa, right retromolar region (c); Erythematous patches in palate, localized ulcer in palatal mucosa in relation to 23 was seen (d)
Biochemical investigations such as complete blood count, liver function tests, blood sugar estimation revealed values within the normal range. A perilesional biopsy was performed on the left lower labial mucosa and histopathology revealed hyperparkeratinized stratified squamous epithelium of variable thickness with subepithelial split, basal cell degeneration in few areas. The underlying connective tissue stroma showed inflammatory cell infiltrate, few eosinophils, and vascularity [Figure 2]. The clinical and histopathological features favor a final diagnosis of MMP.
Figure 2.
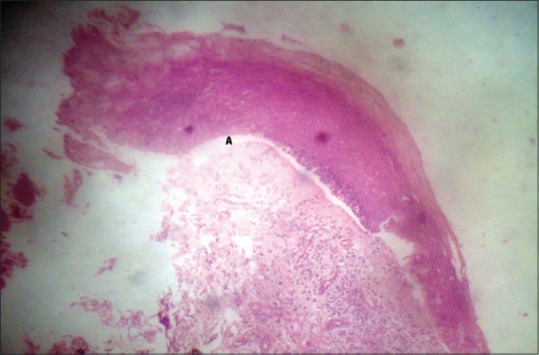
Photomicrograph revealed subepithelial split (A), basal cell degeneration in few areas. The underlying connective tissue stroma shows inflammatory cell infiltrate, few eosinophils, vascularity (H and E, ×40)
The patient was advised to maintain good oral hygiene. Patient was prescribed topical application of high potent steroid-clobetasol propionate. Patient was instructed to apply the cream thrice a day for 2 weeks over the ulcer and retain it for about 30 min after which she could wash her mouth. Tablet betamethasone 0.5 mg was powdered mixed with water and used as a mouth wash twice daily for 10 days.
Vitamin E supplements–Lycopene once daily was prescribed for 1 month. The patient was reviewed every 2 weeks for the first 1 month. The lesions improved considerably with topical steroids within 4 weeks of starting the treatment [Figure 3]. The patient was asked to taper the topical steroid application, but as the lesion exacerbated again patient was instructed to start with systemic steroid 40 mg/day as a single morning dose for 5 days and subsequently was tapered to 5 mg every 5 days till a maintenance dose of 10 mg was reached. There was no adverse drug effect during the treatment. Review after 2 months showed an almost complete healing except for lower labial mucosa in relation to 33, 34, upper gingiva which showed mild erythematous patches [Figure 4]. The patient was maintained on low potent triamcinolone acetonide topical ointments twice application per day on every alternate day and is being routinely followed up.
Figure 3.
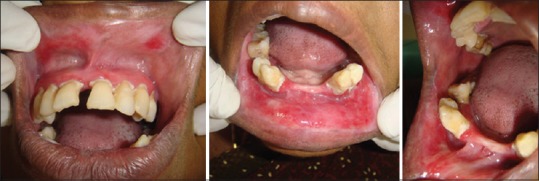
Clinical findings at 4 weeks’ follow-up. Partial resolution of all lesions following topical steroid therapy
Figure 4.
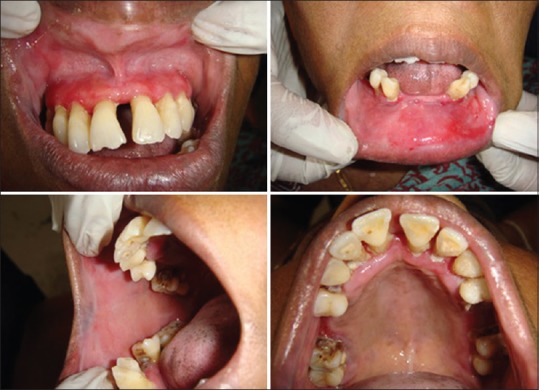
Clinical findings of patient 1 after 2 months follow up. Healing of lesions following systemic steroid therapy, except for the lower labial mucosa in relation to 33, 34, upper gingiva which showed mild erythematous patches
Case 2
A 67-year-old female patient, presented with a history of blisters in the mouth occurring frequently for 3 months. Blisters ruptured to leave an ulcerated area. It was painful while having spicy foods and had not undergone any treatment for them. Patient was completely edentulous but had discontinued wearing the complete dentures since the onset of the present complaint. She had attained menopause 10 years before. History also revealed that there were no ulcers present in any other region of the body. On intraoral examination, there was an intact solitary vesicle in the left upper posterior vestibular region. There was presence of inflammation, localized ulceration, in the right upper alveolus in the anterior region. Areas of diffuse erythema are present in lower alveolus region [Figure 5a–c]. When lateral pressure was applied on the blister, there was formation of new vesicle on adjacent mucosa eliciting positive Nikolsky's sign. All the blood parameters were normal. An incisional perilesional biopsy along with intact vesicle was performed for histopathologic studies. Histology revealed presence, of stratified squamous epithelium, presence of subepithelial cleft, underlying connective tissue stroma showing inflammatory cell infiltrate, few eosinophills, lymphocytes, and vascularity [Figure 6]. The clinical features and histopathology favor a final diagnosis of MMP. Patient was prescribed topical application of high potent steroid-Clobetasol propionate. The patient was instructed to apply the cream thrice a day for 2 weeks over the ulcer. Tablet betamethasone 0.5 mg was powdered mixed with water and used as a mouth wash twice daily for 10 days. Vitamin E supplements–lycopene once daily was prescribed for 1 month. The patient was reviewed every 2 weeks for the first 1 month. The lesions improved considerably with topical steroids within 4 weeks of starting the treatment. The patient was asked to taper the topical steroid application. As there was complete resolution [Figure 7], systemic steroids were not prescribed. The patient is being routinely followed up.
Figure 5.
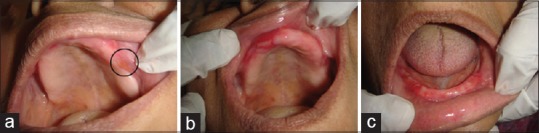
Clinical findings at our patient 2 first visit. Intraoral intact vesicle single in number in the left upper vestibular region posteriorly (a); Presence of inflammation, localized ulceration in the right upper alveolus anteriorly (b); Areas of diffuse erythematous patches in lower alveolus region (c)
Figure 6.
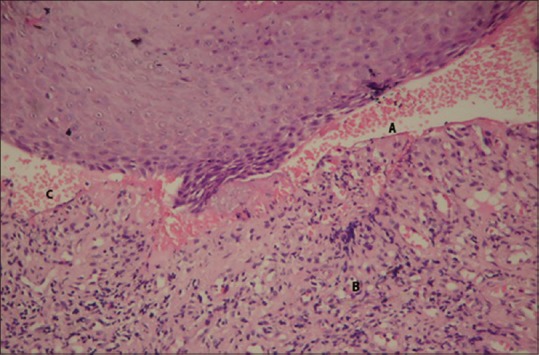
Photomicrograph revealed presence of stratified squamous epithelium, presence of subepithelial cleft (A); underlying connective tissue stroma showing inflammatory cell infiltrate (B); few eosinophils, lymphocytes and vascularity (C) (H and E, ×100)
Figure 7.
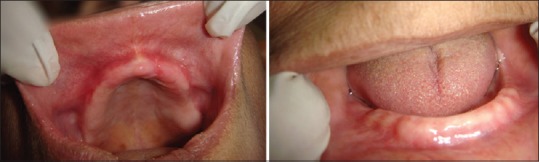
Clinical findings at 4 weeks’ follow-up. Complete resolution of all lesions within 4 weeks of topical steroid therapy
DISCUSSION
Wickmann in 1794 reported the first case of MMP in a female patient.[7] Exact etiology of MMP is unclear. Severe mucosal inflammatory injury, drugs (clonidine, indomethacin, D-penicilamine), viruses, ultraviolet light, and genetic predisposition such as HLA DQB1*0301 are the possible factors that have been reported. The prevalence of HLA-DQB1*03:01 was first described in patients with pure ocular MMP[8] and later proved in all clinical variants.[9] Association of HLA-DQB1*0301 in patients with oral MMP, its role in T-lymphocyte recognition of antigens in the basement membrane zone has been identified.[10,11] The pathogenesis of MMP is probably an autoantibody induced, complement-mediated release of cytokines and leukocyte enzymes by neutrophil sequestration and possibly complement mediated cell lysis, responsible for loss of basal cell-basement membrane adhesion, thus leading to vesicle formation under epithelium.[12] Antibodies against oral mucosal protien such as 168-KDa, α6 integrin is identified purely for Oral MMP.[13] Recently, genotype of the interleukin 4 receptor A-1902 A/A is found in 90% of patients with oral pemphigoid, scarring is low in this group of patients.[14]
Desquamative gingivitis is the main clinical sign in MMP. The clinical feature is erythematous gingiva, loss of stippling, extending apically from gingival margins to alveolar mucosae. They may be mild, small patches to widespread erythema with a glazed appearance.[15] Silverman et al.,[16] Agbo-Godeau et al.,[17] had reported a higher prevalence of gingival involvement of MMP in their study.
Vesicles or bullae may also be present at any other site in oral mucosa, positive for Nikolsky sign. The vesicles break, leading to pseudo membrane-covered irregular erosions which has yellowish slough surrounded by an inflammatory halo. The erosions spread more slowly than pemphigus and are more self-limiting. The hard palate, soft palate, buccal mucosae, alveolar ridge, tongue may be involved.[6]
Our patients exhibited varied clinical presentation. Case 1 had exclusive gingival desquamation and mucosal erosions whereas Case 2 had an intact solitary vesicle and a localized patchy area of erythema. Both were positive for Nikolsky's sign. Both our patients were elderly females in the fifth decade of life and both had attained menopause. There was exclusive oral presentation with no evidence of ocular, cutaneous or genital lesions. Variations in pathogenetic mechanisms, clinical presentations and response to therapy should be due to variations in antigens and antibodies.[12]
Biopsy is the gold standard for diagnosing any oral mucosal lesions. Biopsy in gingiva is usually not indicated as the already existing chronic gingival inflammation may confuse with the diagnosis.[6] The preferred site for biopsy is vesicle or perilesional tissue, not an erosion as it will show only loss of epithelium. Classic histopathologic features include a subepithelial split with a variable inflammatory cells infiltrate containing eosinophils in lamina propria. The histopathological picture of both our cases was consistent with MMP.
Direct immunofluorescence (DIF) is essential for diagnosis and typically shows a continuous, linear deposition of IgG, C3, less commonly IgA, along the basement membrane zone.[5] Standard indirect immunofluorescence (IIF) is usually negative, as serum samples from MMP patients contain antiepithelial–connective tissue junction autoantibodies at low titers. Salt-split skin by IIF is sensitive, help in detecting circulating autoantibodies. Immunoblotting, immunoprecipitation help in difficult cases.[5,6] Target antigen is achieved with enzyme-linked immunosorbent assay systems for BP180 and laminin 332.[18,19]
Since histopathological diagnosis was definite, and in accordance with clinical findings of MMP, immunohistochemistry was not done in our patients due to financial constraints. Differential diagnosis from other IMSEBDs such as bullous pemphigoid, linear IgA disease, and dermatitis herpetiformis was done clinically. The absence of skin lesions excluded the diagnosis of bullous pemphigoid. The absence of triggering factors such as infections and penicillin, absence of “string of bead” sign clinically ruled out linear IgA disease. The absence of pruritic eruption in skin, no association with Gluten-sensitive enteropathy ruled out dermatitis herpetiformis. Absence of big, flaccid bulla, presence of subepithelial split excluded intraepithelial bullous disorder such as pemphigus vulgaris. The presence of exclusive oral lesions with intact bullae, desquamative gingivitis along with subepithelial split histopathologically in elderly females proved our both cases to be only oral MMP.
Excellent oral care was emphasized as it consisted of brushing the teeth twice daily using soft bristle brush, flossing on a daily basis, scaling every 3–6 months.[20] This was emphasized in our Case 1 as the oral hygiene was poorly maintained. This could be because of accelerated periodontal attachment loss due to painful oral hygiene measures and would have become inadequate over the long term. Patient was constantly motivated to maintain good oral hygiene. Sharp edges of teeth in relation to 33, 34 had underwent coronoplasty. Case 2 was completely edentulous. We have advised her to discontinue wearing dentures temporarily until lesion heals, as it may provoke new bullae. Trauma can expose the hidden antigens of BMZ along with chronic inflammatory process of mucosa thus evoking secondary autoimmune response.[5] Topical corticosteroids play an important role in the treatment of oral MMP. For mild nonprogressive oral disease, moderate to high potent topical steroid is recommended as followed in our Case 2. Topical application of high potent steroids with occasional use of oral corticosteroids is the appropriate therapy during exacerbations, for extensive and rapidly progressive oral MMP as followed in our Case 1.
In case of partial response to systemic steroids, adjuvant therapy with azathioprine, cyclophosphamide, dapsone or mycophenolate mofetil has been used effectively in the treatment of MMP. Rituximab, intravenous immunoglobulin, or infliximab are used to reduce autoantibody production thus reducing inflammation. Low-energy laser phototherapy and cryotherapy are also used.[21] Oral Steroids has adverse effects like adrenal suppression hence should not be administered for more than 2 weeks. Adverse effects of topical corticosteroid like candidiasis, reactivation of herpes simplex virus occurs that can be prevented by adding antimycotic therapy like miconazole gel either alone or with antiseptics like chlorhexidine mouthwashes. Our patients during the entire treatment period were continuously monitored for any side effects of prolonged steroid therapy. They did not have any complications and routine biochemical evaluation which was performed during therapy was normal.
CONCLUSION
We had reported two patients of exclusive oral lesions of MMP with different clinical presentations at the time of reporting. Both the patients need to be monitored constantly by dentists, ophthalmologists, and dermatologists. MMP should be considered as a differential diagnosis for any desquamative, vesiculobullous disease occurring in an elderly females. Dentist's role in patients care is vital. The diagnosis is based on thorough history taking, clinical examination, biopsy with histologic and immunofluorescence examination. Amongst the numerous immunomodulatory drugs used in treating Oral mucous membrane pemphigoid-Corticosteroids (both topical and systemic) are used predominantly. Routine follow-up should be done to prevent exacerbations and remissions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chan LS, Fine JD, Briggaman RA, Woodley DT, Hammerberg C, Drugge RJ, et al. Identification and partial characterization of a novel 105-kDalton lower lamina lucida autoantigen associated with a novel immune-mediated subepidermal blistering disease. J Invest Dermatol. 1993;101:262–7. doi: 10.1111/1523-1747.ep12365189. [DOI] [PubMed] [Google Scholar]
- 2.Xu HH, Werth VP, Parisi E, Sollecito TP. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57:611–30. doi: 10.1016/j.cden.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dear JW, Lilitkarntakul P, Webb DJ. Are rare diseases still orphans or happily adopted? The challenges of developing and using orphan medicinal products. Br J Clin Pharmacol. 2006;62:264–71. doi: 10.1111/j.1365-2125.2006.02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scully C, Carrozzo M, Gandolfo S, Puiatti P, Monteil R. Update on mucous membrane pemphigoid: A heterogeneous immune-mediated subepithelial blistering entity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:56–68. doi: 10.1016/s1079-2104(99)70194-0. [DOI] [PubMed] [Google Scholar]
- 5.Bruch-Gerharz D, Hertl M, Ruzicka T. Mucous membrane pemphigoid: Clinical aspects, immunopathological features and therapy. Eur J Dermatol. 2007;17:191–200. doi: 10.1684/ejd.2007.0148. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320–32. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- 7.Wickmanns JE. Ideas on the diagnosis. Vol. 1. Hanover: Helwig; 1894. p. 89. [Google Scholar]
- 8.Ahmed AR, Foster S, Zaltas M, Notani G, Awdeh Z, Alper CA, et al. Association of DQw7 (DQB1*0301) with ocular cicatricial pemphigoid. Proc Natl Acad Sci U S A. 1991;88:11579–82. doi: 10.1073/pnas.88.24.11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgado JC, Turbay D, Yunis EJ, Yunis JJ, Morton ED, Bhol K, et al. A common major histocompatibility complex Class II allele HLA-DQB1*0301 is present in clinical variants of pemphigoid. Proc Natl Acad Sci U S A. 1996;93:8569–71. doi: 10.1073/pnas.93.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrozzo M, Fasano ME, Broccoletti R, Carbone M, Cozzani E, Rendine S, et al. HLA-DQB1 alleles in Italian patients with mucous membrane pemphigoid predominantly affecting the oral cavity. Br J Dermatol. 2001;145:805–8. doi: 10.1046/j.1365-2133.2001.04448.x. [DOI] [PubMed] [Google Scholar]
- 11.Setterfield J, Theron J, Vaughan RW, Welsh KI, Mallon E, Wojnarowska F, et al. Mucous membrane pemphigoid: HLA-DQB1*0301 is associated with all clinical sites of involvement and may be linked to antibasement membrane IgG production. Br J Dermatol. 2001;145:406–14. doi: 10.1046/j.1365-2133.2001.04380.x. [DOI] [PubMed] [Google Scholar]
- 12.Scully C, Lo Muzio L. Oral mucosal diseases: Mucous membrane pemphigoid. Br J Oral Maxillofac Surg. 2008;46:358–66. doi: 10.1016/j.bjoms.2007.07.200. [DOI] [PubMed] [Google Scholar]
- 13.Sami N, Bhol KC, Ahmed AR. Treatment of oral pemphigoid with intravenous immunoglobulin as monotherapy. Long-term follow-up: Influence of treatment on antibody titres to human alpha6 integrin. Clin Exp Immunol. 2002;129:533–40. doi: 10.1046/j.1365-2249.2002.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrozzo M, Dametto E, Fasano ME, Broccoletti R, Carbone M, Rendine S, et al. Interleukin-4RA gene polymorphism is associated with oral mucous membrane pemphigoid. Oral Dis. 2014;20:275–80. doi: 10.1111/odi.12106. [DOI] [PubMed] [Google Scholar]
- 15.Bagan J, Lo Muzio L, Scully C. Mucosal disease series. Number III. Mucous membrane pemphigoid. Oral Dis. 2005;11:197–218. doi: 10.1111/j.1601-0825.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- 16.Silverman S, Jr, Gorsky M, Lozada-Nur F, Liu A. Oral mucous membrane pemphigoid. A study of sixty-five patients. Oral Surg Oral Med Oral Pathol. 1986;61:233–7. doi: 10.1016/0030-4220(86)90367-1. [DOI] [PubMed] [Google Scholar]
- 17.Agbo-Godeau S, de Lima Soares P, Szpirglas H. Cicatricial pemphigoid: Management in stomatology. Rev Stomatol Chir Maxillofac. 2004;105:206–10. doi: 10.1016/s0035-1768(04)72308-6. [DOI] [PubMed] [Google Scholar]
- 18.Bernard P, Antonicelli F, Bedane C, Joly P, Le Roux-Villet C, Duvert-Lehembre S, et al. Prevalence and clinical significance of anti-laminin 332 autoantibodies detected by a novel enzyme-linked immunosorbent assay in mucous membrane pemphigoid. JAMA Dermatol. 2013;149:533–40. doi: 10.1001/jamadermatol.2013.1434. [DOI] [PubMed] [Google Scholar]
- 19.Calabresi V, Carrozzo M, Cozzani E, Arduino P, Bertolusso G, Tirone F, et al. Oral pemphigoid autoantibodies preferentially target BP180 ectodomain. Clin Immunol. 2007;122:207–13. doi: 10.1016/j.clim.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Knudson RM, Kalaaji AN, Bruce AJ. The management of mucous membrane pemphigoid and pemphigus. Dermatol Ther. 2010;23:268–80. doi: 10.1111/j.1529-8019.2010.01323.x. [DOI] [PubMed] [Google Scholar]
- 21.Di Zenzo G, Carrozzo M, Chan LS. Urban legend series: Mucous membrane pemphigoid. Oral Dis. 2014;20:35–54. doi: 10.1111/odi.12193. [DOI] [PubMed] [Google Scholar]


