Abstract
A common dilemma faced by all animal bioethics committees arises when exceptions are proposed to the use of analgesics in painful procedures. The committee and researcher must weigh the possible confounding effects of including additional drugs (analgesics) in their treatment regimen against the moral obligation to perform humane research. Often neglected in these considerations are the potential confounding effects of unrelieved pain and consistency with pain-relieving practices in human medicine. In this review, we summarize what is currently known regarding the molecular and physiologic effects of pain and analgesics in common animal models used across several therapeutic areas. This work is intended to help provide guidance and assurance that a comprehensive approach has been taken when contemplating how pain relief will be applied in animal research protocols.
Abbreviations: CLP, cecal ligation and puncture; TBSA, total body surface area
Pain has a profound effect on animal wellbeing, and research teams, laboratory animal veterinarians, and IACUC expend considerable energy and time considering how and when to manage pain that may occur in a particular study. Despite these efforts, a recent review of publications of animal models that required surgical procedures revealed that analgesic use was explicitly mentioned in only 29% of papers that had descriptions of anesthetic use.22 One of the conclusions of the review was that potential for unalleviated pain to skew research outcomes and to induce animal suffering may be vastly underestimated. Similarly, peer-reviewed invasive methodology papers and videos used to train graduate students and researchers often do not mention perioperative analgesia (see references 38 and 90 for examples), reinforcing the concept that analgesics cannot be used in the development of specific animal models or that they are not required. On IACUC protocols, a common justification for withholding analgesics is a concern regarding the potential of these compounds to confound results. These models almost always require general anesthesia to induce various lesions, yet minimal concern is expressed regarding the well-documented effects of both injectable and inhalant anesthetics on neurotoxicity, cytokine expression, immune status, endoplasmic reticulum stress, and protein phosphorylation, particularly in the brain and spinal cord.4,8,11,46,47,68,77,134 Clearly, there are gaps and biases in consideration of the pharmacologic effects of one group of drugs compared with another on model outcomes, in knowledge of how the presence of pain affects research outcomes, in the critical assessment of whether analgesics should be withheld for painful procedures, in which species is being used for modeling, and in which analgesics may be used.
In this review, we have summarized the reported effects of unrelieved pain and analgesic administration in several commonly used animal models, including those inducing neuropathic pain, immunology-related studies, and oncology models. Based on this review, we have developed recommendations regarding decision-making for analgesia use in animal models in which pain may be expected to occur. We surmise that this information will be helpful for investigators and IACUC as they contemplate the appropriateness of withholding analgesics. In addition, the presented information likely will spur discussions regarding whether additional treatment groups are needed to compare the effects of analgesia use on model outcomes.
Neuropathic Pain Mechanisms
Reluctance to use analgesics in various animal models may stem from the fear of introducing confounding effects into the model system being analyzed. According to the authors’ cumulative experience (reviewing a vast number of research protocols and programs), this concern is often not rationalized against the effects of uncontrolled pain when analgesics are withheld. Although several different types of pain are recognized,122 reflection on studies performed in neuropathic pain models can provide insight into how the neurologic components of pain might alter the physiologic and immunologic responses of animals being used in research. In other words, studies that have been performed in controlled models of pain can be used to provide an understanding of how other types of studies might be affected by the presence of inadvertent or unintentional pain caused by research manipulations.
The numerous animal models of neuropathic pain all result in development of chronic somatosensory responses related to direct neuronal damage or disease induction.26 Some of the more widely used rodent models of neuropathic pain include mechanical, traumatic, or chemical injury to nerves, spinal cord, and brain; the creation of central or peripheral vascular and ischemic lesions; and metabolic or viral injuries. A common result in all of these models is that signals from intact and injured nociceptors contribute to ongoing nervous signal amplification toward both innocuous and noxious stimuli. The underlying mechanisms for nociceptive alterations are complex and involve changes in expression of synaptic receptors, neurotransmitters, and ion channel density, enhanced neuronal excitability with generation of ectopic action potentials, sprouting of sympathetic neurons near damaged sensory neurons, cell death, disinhibition of neuronal signaling, and remodeling of synaptic connections with changes in central pain circuitry.6,7,136 Any combination of these reactions may be a byproduct in response to the development of the research model or pain associated with progression of disease.
Neuropathic pain and immune system activation.
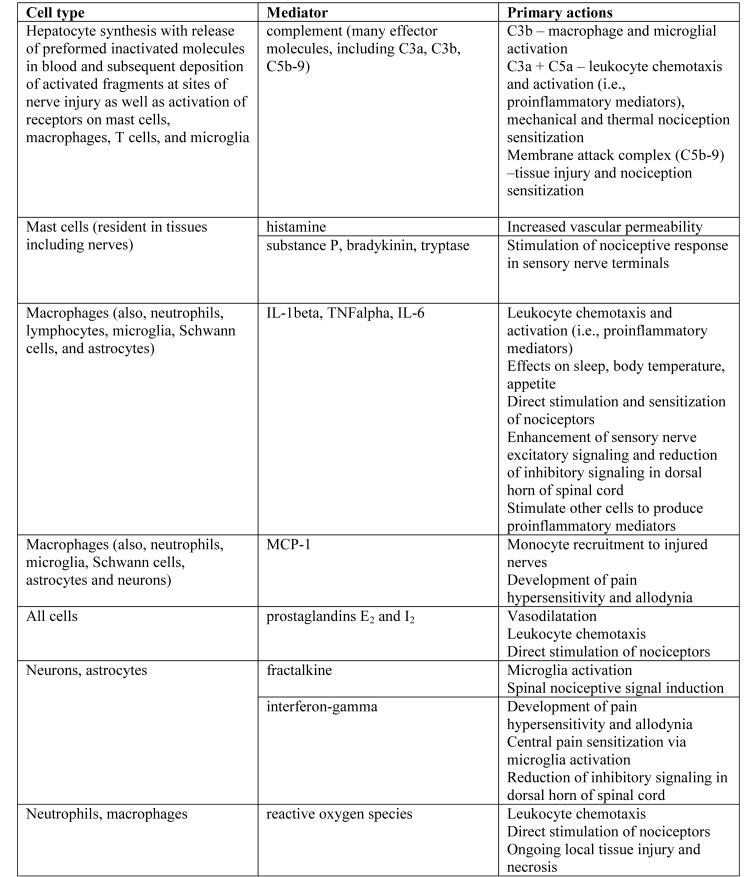
The pathophysiology of chronic pain, particularly neuropathic pain, involves activation of the innate and adaptive immune systems in response to neuronal damage. However, centrally mediated responses to painful stimuli can result in immunosuppression, in part through the hypothalamic–pituitary–adrenal axis (discussed later). Regardless of whether the injury arises peripherally or centrally, complement activation occurs within minutes to hours of injury, resulting in direct cell death as well as activation of macrophages, glia (specifically, Schwann cells in the periphery and astrocytes and microglia in the CNS), and eventual activation and modulation of T cell responses (see Figure 1 for a summary of key inflammatory mediators and their effects).72 In addition, mast cell degranulation occurs at the site of nerve injury, with the release of an array of mediators, including histamine, serotonin, prostaglandins, leukotrienes, various proteases, and proinflammatory cytokines, such as TNF and IL6, which act as chemoattractants to recruit further inflammatory cells to the injured site.44 Macrophages, both those resident in nervous tissue and monocytes recruited to the site of injury, play a key role in the progression of acute and chronic inflammation after nerve injury, through the ongoing release of proinflammatory cytokines, such as TNF and IL1. These cytokines subsequently lead to T cell activation, induction of alterations in the extracellular matrix (which contribute to altered nociception and changes in neuronal circuitry), and glial activation.84,117,123 Tissue damage and inflammation is perpetuated by neutrophil chemotaxis and degranulation, and activation of the adaptive immune system perpetuates ongoing injury and inflammation. The effects of various cytokines and chemokines on the development of neuropathic pain and as potential confounders to experimental studies are addressed further in the following immunology section, and in-depth reviews can be found elsewhere.7,84
Figure 1.
Examples of key inflammatory cell types and mediators involved in neuroimmune activation and inflammation. Modified from references 7 and 36.
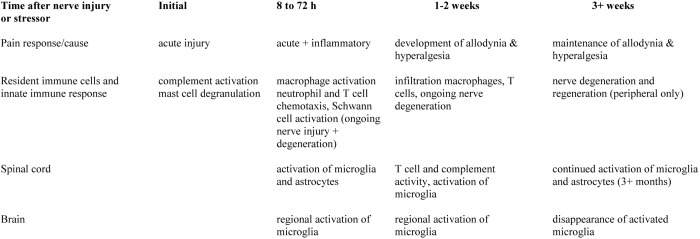
Ongoing cytokine and chemokine expression over time, both directly at the site of injury and in central processing areas, results in activation of glia, alteration of central and peripheral neurotrophic factors, and morphologic alterations in various brain regions, as well as chronic changes in neuroendocrine, neuroimmune, and monoamine responses (summarized in Figure 2) .137 The time course of neuroimmune system activation and subsequent pain development and modulation is important to understand, because it is often a subject of study and is sometimes used as a reason for withholding analgesia. Furthermore, these same mechanisms underlie the development of central pain sensitization or ‘wind-up,’ a chronic condition that can occur regardless of the method underlying pain induction. The long-term changes in neuroplasticity, behavioral responses, neuronal disinhibition, and glial activation that are seen in neuropathic pain models are likely maintained by epigenetic effects on neurons and glial cells in peripheral nerves, the spinal cord, and the brain.31 These responses underscore the need to consider both the immediate effects of unrelieved pain on the model as well as possible long-term effects when a decision is made to withhold analgesics.
Figure 2.
Timeline of neuroimmune system activation and pain response following injury. The timeline is important to consider when evaluating the potential influence of administering analgesics, in particular, whether acute or chronic effects are being studied in a specific model. Modified from references 7 and 84.
Although most of the changes described are observed in animal models of neuronal injury and are seen regardless of how that injury is induced, it would be an overgeneralization to assume that identical changes occur in every animal model that induces pain. However, it is highly likely that many of these changes are present in subclinical and clinical pain occurring in other chronic animal disease models, given the considerable overlap in currently recognized chronic pain mechanisms and classifications.122 The earlier-mentioned mechanisms serve as a reminder that pain, in and of itself, has a profound effect on the body; that—regardless of the research question—immunologic, neurologic, and physiologic systems are inseparably intertwined when using in vivo models; and that these consequences need to be considered seriously in any experimental design in which pain is possible or predicted. In addition to obvious humane considerations, model design needs to account for potential alterations in the parameters or biomarkers measured, the mechanism studied, and the translatability and reproducibility of the model. With regard to model reproducibility, the question of whether unrelieved pain is present or permitted in the corresponding human clinical situation should be considered and when interventions should occur after neuroimmune system activation (as outlined in Figure 2).
The use of analgesics in animal-based models of neuropathic injury and pain models.
The use of analgesics in animal-based models of neuropathic injury and pain is debated, given the marked concern of research groups regarding the potential interference of various classes of analgesic therapeutics in the neuroinflammatory process. In a recent review of reported use of analgesia or analgesic-anesthetic combinations in publications involving animal surgical models, the majority of articles reviewed for neuropathic pain models did not report the use of any analgesic perioperatively or in the days after surgery (only articles involving rodent spinal cord injury or spared nerve injury models were examined).22 Specifically, less than 30% reported analgesic use for mouse spinal surgeries, and less than 10% of articles on rat neuropathy models reported analgesic use.22
The clinical outcomes observed for neuropathic pain include the development of allodynia (pain response to nonpainful stimuli), hyperalgesia (increased response to painful stimuli), spontaneous pain, and behavioral changes, characterized in humans as depression, anxiety, and sleeplessness.7 An onset of headaches is reported in human patients specifically after increases in intracranial pressure with cerebral ischemia or inflammation. It is much more difficult to determine whether headaches also occur in animal models of central neuronal injury, although a number of reliable headache and migraine rodent models do exist, suggesting that the physiologic condition is possible and quantifiable.41 This area requires more research. Behavioral changes associated with chronic neuropathic injury and pain when using animal models have received increasing attention in recent years, given that negative affective and cognitive experiences contribute significantly to the poor quality of life reported by human patients with chronic neuropathic pain.51,71 Potential adverse behavioral changes an important consideration for the IACUC and research team when working with these models because, if present, they may contribute to altered physiology and poor animal wellbeing. This is another area that requires further work.
Timing of evaluation of animal behavior after lesion induction is important, because most neuropathic pain models take at least 3 wk to develop (Figure 2). Studies evaluating behavioral indices in male BALB/C and C57BL/6 mice in the first few weeks after the creation of neuropathic lesions in the spared nerve injury model or complete Freund adjuvant footpad injection model have suggested that no ‘lasting’ changes in affective state are present;128 however, no long-term follow-up was conducted. Because these types of findings may be misinterpreted by the research community and IACUC alike, it is essential that animals used in chronic pain models be assessed after an appropriate interval following lesion induction has occurred. Information is much more clear-cut for rats; there is strong evidence for the development of anxiety-like behaviors, which can be reversed with benzodiazepines, in various chronic neuropathic pain models.33,96 When appropriate behavioral tests are conducted at correct intervals, persistent changes correlating with anxiety-like and depression-like behaviors are seen consistently in a variety of mouse and rat models of neuropathic pain.40,137 Because these adverse effects are unlikely to be treated with analgesics or other drugs, these animal experiences should be weighed carefully during the overall consideration of the pain and distress burden compared with the scientific objectives. Consistent with a cost–benefit approach, when these models are used, endpoint criteria must be well defined in the experimental design.
Both opioids and NSAID have the potential to interfere with the development of neuropathic pain in animal models. By their very nature, NSAID inhibit the activity of cyclooxygenase enzymes (primarily COX1 and COX2), preventing conversion of arachidonic acid into proinflammatory prostaglandins, and these agents are used therapeutically to manage and prevent the onset of pain in humans with neuropathic injuries. There is a risk that using NSAID or opioids acutely after surgery or trauma induction may alter the course of lesion development. For example, the postoperative use of tramadol after partial sciatic nerve damage in rats is reported to reduce the onset of allodynia and led to an increased basic pain threshold at 7 d after lesion induction.65 Both NSAID and opioids may have paradoxical effects on pain outcomes in neuropathic pain models through effects on less well-known mediator pathways. For example, morphine provided to rats for 7 d after the induction of a chronic nerve constriction injury induced increased allodynia.48 This effect was thought to be mediated by an inflammatory pathway, ultimately causing local spinal cord increases in IL1 levels. However, it should not be assumed that any use of analgesic postoperatively will always change the outcome of model development, because the modifications depend on the duration of drug use, the drug dosage, the specific model, and the research question.
Analgesia in central neuropathic and ischemic models.
Multiple factors, including the specific animal model30 and induction of NSAID-sensitive immunologic responses, affect the pathogenesis of brain injury, and the selection of appropriate analgesics in the perioperative period is paramount. For example, the use of carprofen after the induction of traumatic brain injury in mice was shown to result in reduced lesion size and an improved functional outcome.120 Conversely, chronic treatment of rats with high-dose ibuprofen after traumatic brain injury significantly worsened learning ability 4 mo after injury compared with untreated rats, despite similar lesion size and histology.16 Others have evaluated the effect of using short-term therapeutic perioperative doses of buprenorphine compared with meloxicam in a middle cerebral artery occlusion stroke model in male C57BL/6 mice.62 The study addressed whether the use of an analgesic altered the postsurgical infarct size and long-term behaviors. Meloxicam significantly reduced the infarct size, whereas buprenorphine had no effect on infarct volume, and furthermore, had no lasting effect on animal behavior, allowing the researchers to conclude that buprenorphine could be safely used as a perioperative analgesic in this model. This research emphasizes the need for pilot studies to examine the specific outcomes on the model when analgesic withholding is being considered for neuropathic pain models. It is interesting to note that the induction of cerebral ischemia for stroke modeling in NHP routinely includes both a NSAID as well as an opioid at the time of anesthesia induction, local anesthesia around the surgical craniotomy site, and postoperative opioids, as well as antinausea and antiseizure medications.32 This approach better mimics the clinical management of humans with this condition and helps to alleviate unwanted and inadvertent side effects (Figure 2), suggesting that it is a more translatable model for evaluating new therapeutics.
The Use of Analgesics in Immunology Research
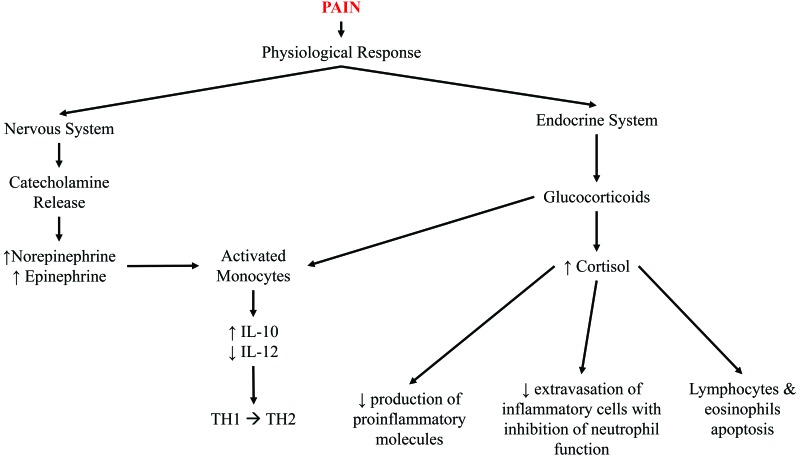
Most analgesics affect the immune system in some fashion, leading to generalized concerns that providing any analgesia will confound immunology studies. Tissue injury does stimulate local inflammatory reactions and cytokine release, as discussed earlier; however, unalleviated pain can also lead to generalized immunosuppression (Figure 3) and commonly occurs in humans and animals undergoing and recovering from surgery.94 Other studies of unalleviated acute and chronic pain and stress in animal models have demonstrated the release of proinflammatory molecules and immunosuppression,107,110,111 indicating that unmanaged pain is potentially as great a confound, if not more so, as administering an analgesic agent.
Figure 3.
Pain is a stressor for the body that leads to emotional, physiologic, and behavioral responses. The physiologic response can lead to immunosuppression by stimulating glucocorticoid and catecholamine release. IL12 is a major inducer of Th1 responses, whereas IL10 antagonizes its effects and favors Th2 responses; both cytokines are predominately produced by activated monocytes. Catecholamines inhibit IL12 and stimulate IL10 production, whereas glucocorticoids inhibit IL12, but do not affect IL10 production. Thus, both glucocorticoids and catecholamines selectively suppress Th1 and favor Th2 responses, thereby suppressing cell-mediated immunity. Cortisol simultaneously 1) decreases the production of proinflammatory molecules (IL1, TNF, GM-CSF, IL2, IL3, IL4, IL5, IL8, prostaglandins, and leukotrienes); 2) reduces extravasation of inflammatory cells with inhibition of neutrophil function; and 3) induces apoptosis in lymphocytes and eosinophils.
Opioids are often the first analgesic considered for refinement in immunology models, because the use of NSAID is predicted to negate many of the attributes the model was designed to manifest. Although the effect is not as obvious, most investigators are aware of the additional potential of opioids to cause immunosuppression, and this consequence is used as justification for withholding analgesics. However, as discussed later, opioid use should not be entirely discounted, because their effects can be transient and variable, depending on the drug and duration of administration. In addition, the use of local anesthetics to augment analgesia should be considered.
Opioids have differing effects depending on the compound and whether the model involves acute or chronic inflammation.88,113 Some opioids transiently affect LPS-induced proinflammatory cytokine serum concentrations in a drug dose- and time-dependent fashion in male Swiss mice.101 Morphine and fentanyl have both been reported to stimulate the hypothalamopituitary–adrenal axis, increase corticosteroid secretion, decrease lymphoproliferation, and decrease NK cell activity.43,80,88,108 In comparison, hydromorphone and buprenorphine have no reported effects on the hypothalamopituitary–adrenal axis, minimal or no known decrease in corticosteroid secretion, and no effect on NK cell activity or lymphoproliferation in Fisher 344 rats or Swiss mice.43,80,88,108 In addition, the administration of buprenorphine has no to minimal effect on proinflammatory cytokine concentrations in male Swiss mice.80,101 Collectively, these findings suggest that buprenorphine, a mixed partial µ-agonist, may have fewer confounding effects on inflammatory responses than other pure µ-opioid receptor agonists, such as morphine and fentanyl.
Opioid immunosuppression appears to be less relevant when these compounds are administered chronically rather than acutely. Continuous infusion of fentanyl significantly decreased lymphoproliferation, NK cell activity, and proinflammatory cytokine concentrations for as long as 3 d, but the effects resolved by day 7, in male Swiss mice.80 In comparison, continuous infusion of buprenorphine for as long as 7 d had no noteworthy measurable effect on immune function at any time point.80 This result suggests that buprenorphine should be used preferentially over fentanyl, given that buprenorphine does not appear to stimulate proinflammatory cytokine plasma concentrations when used acutely or chronically, even at doses much higher than those typically used clinically. Overgeneralization of the immunosuppressive effects of opioids should be avoided when considering which analgesic is most appropriate for use in the experimental design.
The use of analgesics in models of rheumatoid arthritis.
Rheumatoid arthritis is a painful, chronic, autoimmune disease that requires refinement to reduce the suffering and improve the wellbeing of animals used in model development. Whereas NSAID are a mainstay of therapy in humans with this condition, NSAID can modulate the inflammatory response and decrease disease severity in humans and animals.55 Because inflammation underpins disease progression in this model, the use of NSAID adversely affects studies evaluating the inflammatory response associated with arthritis.3,39,55,81,138
Opioids are used infrequently in humans for rheumatoid arthritis pain management but may be used in animal models as an alternative to NSAID. The reported effects of various opioids are quite variable in different animal models, indicating a need for further investigation. During complete Freund adjuvant-induced unilateral paw inflammation in a rodent model, µ- and κ-opioid receptor agonists decrease the severity of inflammation.113 However, morphine accelerated the inflammatory process in Wistar rats, as demonstrated by faster onset and increased severity of adjuvant arthritis, an effect that normalized within 2 wk.35 In Lewis rats, morphine caused a dose-dependent attenuation of adjuvant arthritis progression.133 Further investigations of morphine in LPS-induced arthritis in horses showed an overall decreased severity of inflammation.74,130 As part of these studies, the route of morphine administration was found to be important, with intraarticular injection having the greatest antiinflammatory effect.74 Results with buprenorphine have similarly been mixed. Buprenorphine had no significant effect on the progression of adjuvant arthritis in male Lewis rats,133 but it inhibited inflammation and joint erosion in streptococcal cell wall-induced arthritis in female Lew/SSN rats.131 In addition, prolonged administration of buprenorphine for as long as 31 d inhibits osteoclastic bone resorption in vitro but has proinflammatory effects, as assessed by edema and radiography, in male Lewis rats used in an adjuvant arthritis study.52 These findings highlight the importance of evaluating opioid analgesics in specific animal arthritis models.
Most analgesics affect the immune system in some way, but a number of reports have documented that effective analgesia can be achieved without significant immunomodulatory effects.25,81,133 For example, gabapentin was effective in attenuating allodynia without affecting disease progression during the chronic phase of disease in K/BxN mice, a spontaneous immune complex-mediated model of inflammatory arthritis.25 These results underscore the value of ‘out-of-the-box’ thinking beyond conventionally used analgesics for pain relief in studies where NSAID and opioids may be confounders. In addition, nonpharmacologic measures, such as deep, soft bedding, may be used to enhance the comfort of animals used for arthritis modeling.
Analgesia in sepsis models.
Septicemia is a systemic immune response to an overwhelming infection that can have serious consequences, including multiple organ failure, severe pain, and death.29 In addition to antimicrobial therapy, humans being treated for sepsis generally receive analgesics to provide pain relief.29 This multifactorial disease process has been modeled through a variety of approaches, but the most common model is cecal ligation and puncture (CLP). Whether septicemia itself is painful compared with inducing marked sickness behavior has been debated; however, models using CLP require abdominal surgery, which is painful and which requires at least the administration of short-term analgesia. NSAID are generally avoided, due to their inherent immunomodulatory effects, which can alter animal survival and severity of disease process.127 Selective COX2 inhibitors have specifically been shown to decrease endotoxin-induced mortality in a dose-dependent manner.127 For these reasons, most investigators use an opioid for analgesia in sepsis models.
Opioids can have variable effects on mortality associated with CLP. Morphine and methadone have both been associated with increased mortality in mice,82 whereas this effect has not been seen with buprenorphine in female Hsd:ICR, C57BL/6, or BALB/c mice.27,58,66 Interestingly, the same dose of buprenorphine has been associated with increased mortality in male C57BL/6 mice, but this difference disappeared at a lower buprenorphine dose (0.05 mg/kg compared with 0.1 mg/kg),27 suggesting both potential sex- and dose-associated effects of the analgesic on survival. Whether the lower dose of buprenorphine was clinically efficacious for inducing analgesia in this model is a matter of debate, because the dose has not been associated with analgesia in other surgical models.124 The effects of clinically relevant doses of tramadol on murine mortality have been mixed. Low doses of 20 mg/kg SC are reported to have no effect on survival, whereas doses as high as 80 mg/kg SC were associated with an increased mortality rate.58
Opioids also have variable physiologic effects when used in rodents undergoing CLP. Morphine promoted the dissemination of gram-positive bacteria, directly modulated the gut microbiome, and inhibited bacterial clearance from the abdomen of male C57BL/6 mice.82 In rats, the condition of sepsis and the use of fentanyl both individually caused a statistically significant reduction in gastrointestinal transit time that was synergistic in combination.121 This dysmotility can lead to disruption of nutrient absorption, which can have serious clinical consequences, including death, in septic individuals and should be considered in animals undergoing CLP.121 Tramadol did not have this same gastrointestinal effect when compared with fentanyl in CLP rats.121 However, tramadol did have other transient and mild effects on neutrophil and macrophage counts but did not alter plasma cytokine concentrations in female Hsd:ICR mice that had undergone CLP.58 Buprenorphine had similar effects as tramadol in both Hsd:ICR and BALB/c mice.58,66 In particular, BALB/c mice in proestrus demonstrated increased neutrophils and monocytes after CLP,66 suggesting that the estrous cycle has a greater effect on the CLP model of sepsis than does the analgesic chosen. Collectively, these studies indicate that buprenorphine and tramadol can be used in both mice and rat CLP models, because these drugs have minimal to no effect on mortality or the inflammatory process.
Vaccine development and antibody response.
In most cases, the administration of vaccines does not result in significant pain. However, the administration of infectious agents or neoplastic cells for which vaccines are being developed may result in pain, warranting analgesic use. The Sereny test, a method to evaluate the invasiveness of infectious organisms in guinea pigs, has the potential for causing significant ocular pain. After inoculation of the eye with a bacterial suspension, severe mucopurulent conjunctivitis and keratitis can develop, indicating a positive test result.
Opioids have minimal effects on corneal thickness and immunologic responses in the guinea pig Sereny test. When butorphanol or buprenorphine were administered acutely immediately after clinical signs were noted, the drug had no significant effect on corneal thickness measurements.116 Furthermore, when buprenorphine was administered for the duration of the study, the immunologic response (serum, spleen and lymph node IgG and IgA production) to candidate Shigella vaccines was not disrupted.54 Conversely, morphine administration to domestic pigs prior to vaccination with Bacille–Calmette–Guérin decreased the proliferative responses and cytolytic activity of γδ T cells and decreased NK cell activity.89
In addition, morphine has a strain-associated suppressive effect on antibody production, with suppression seen in C3HeB/FeJ, C3H/HeJ, C57BL/6, and C57BL/6J bgJ/bgJ but not CxBk/ByJ or Balb/cByJ mice.18,19 The use of other analgesics in combination with buprenorphine has had much less influence on antibody production across various strains. Specifically, acetaminophen, meloxicam, and buprenorphine do not decrease the mean or maximal antibody titer after primary or repeated immunization with complete or incomplete Freund adjuvant, but these analgesics were effective in attenuating behavioral signs of pain.69
Because of the very different effects seen in vaccine and antibody production studies, partial µ-opioid receptor agonists, such as buprenorphine, can likely be used. However, focused pilot studies may be needed with specific vaccine studies and antibody production to investigate potential effects.
Ascites production and analgesia.
Although in vitro alternatives should be used when possible, ascites fluid production is still used to produce high concentrations of monoclonal antibodies. This technique is frequently associated with local irritation and visceral pain. The pain arises from the in vivo growth of a hybridoma tumor with the resulting production of ascites fluid,86,98 injection of adjuvants to enhance antibody production,114 or the production of pain-inducing cytokines from hybridoma cells. Because pain is an unwanted side effect, various analgesics have been evaluated for their effects on antibody production. In one study, multimodal analgesia consisting of both clinically relevant doses of meloxicam and buprenorphine did not affect antibody production in male BALB/c mice injected with pristane followed by hybridoma cells, but the treatment was intimated to benefit animals clinically.79 This report suggests that clinically relevant analgesic regimens can be provided to mice to maintain antibody production without the unwanted side effect of pain.
Analgesic use in thermal injury models.
Thermal injuries are associated with significant morbidity and mortality and represent an important human health issue, necessitating the development of animal models to study analgesia and treatment regimens. In thermal injury models, pain is associated with the initial burn, which is compounded through procedural and dressing changes and breakthrough pain. In addition, burn pain intensifies as the total body surface area (TBSA) increases15,103 or when an infection is present.119 It is noteworthy that human burn patients always receive analgesics, suggesting a scientific imperative to use analgesics in animal studies to ensure that results obtained from these studies are relevant and translatable.
Burn pain and the distress that accompanies it have been associated with immunosuppression,10,28,42,45 which can delay healing.104,134 Furthermore, untreated pain impedes wound care, increasing risks for wound infection, decreases range of motion, and is associated with prolonged hospitalizations in humans.103 Pain and stress associated with thermal injuries have similar effects in rodents as in humans. Unalleviated thermally induced pain resulted in slower cutaneous healing93 and increased susceptibility to bacterial infection in female SKH1 mice.104
Unfortunately, there has been a common misconception in the literature that thermal injury resulting in either partial or full-thickness burns is not painful in animals; consequently, animals commonly do not receive analgesics unless they also undergo another procedure considered painful. 56,61,75,90,91,109The misconception that thermal injury is not painful may have arisen from the thought that nerve endings are destroyed and become insensate. 90 However, this notion is contrary to what is known about neuropathic pain (see preceding section and Figure 1) and has never fully been evaluated in these studies. Other researchers have demonstrated significant pain associated with full-thickness thermal injury in rodents, which appears to be maximal on the day after the thermal injury.115 Similarly, studies in cattle have documented associated pain for at least 10 wk after hot-iron branding.125,126 Together, these studies indicate that analgesics must be provided to animals used in burn model studies, and, as indicated in Figure 2, pain after thermal injury should be treated acutely to avoid inadvertently inducing chronic pain and central sensitization effects in these models.
Opioids have variable effects on cytokine indicators of immunosuppression and inflammation depending on the drug, the animal model, and the percentage TBSA injured thermally. After injury of 6.25% TBSA, morphine induced greater immunosuppressive effects than were seen in untreated, thermally injured female C57BL/6 mice. This effect was mitigated when the affected TBSA exceeded 12%,5 indicating that morphine can be used with minimal effect on the immune status in animals undergoing thermal injuries of 12.5% TBSA or greater. In male Sprague–Dawley rats with thermal injuries ranging from 20% to 60% TBSA, twice-daily buprenorphine did not significantly influence proinflammatory cytokine concentrations.9 Similarly, in C3H mice thermally injured at 20% TBSA, continuous buprenorphine infusion for 14 d did not alter burn-induced immunosuppression,64 suggesting that buprenorphine may be preferable to other analgesics for continuous administration in thermal injury models.
Numerous NSAID have been evaluated at clinically relevant doses in burn models, with varying effects. Indomethacin, ibuprofen, and piroxicam have no additional immunosuppressive effect, as determined by a lack of systemic complement consumption, thrombocytopenia, leukocytosis, or leukopenia and the maintenance of bactericidal activity of polymorphonuclear leukocytes in guinea pigs with 30% TBSA thermal injury.12 Indomethacin does not affect local lymph flow or protein leakage in a rabbit hindlimb burn model.20 Similarly, ibuprofen did not affect burn-associated dermal ischemia in a 5% TBSA guinea pig burn model.118 In a murine model, ibuprofen partially restored cell-mediated immunity in female CF1 and BDF1 mice with 15% to 20% TBSA, as did indomethacin, although to a lesser extent.53 In summary, NSAID may have minimal effects on some burn model outcomes; however, adequate analgesia will likely require combination with opioids.
Various topical treatments have been explored in wound-healing models. In a 5% TBSA full-thickness porcine burn model, neither topical amitriptyline nor bupivacaine affected wound healing, wound contraction, or inflammation.102 Similarly, neither lidocaine nor bupivacaine local tissue infiltration altered the rate of cutaneous wound healing in an incisional model in female C57BL/6 mice.132 Although wound neutrophils significantly increased in mice treated with 1% lidocaine, this effect was not seen with 0.5% lidocaine.132 The application of EMLA cream to human burn blisters has not been associated with changes in wound healing; however, EMLA did not alter the area of mechanical or thermal hyperalgesia in these patients.97 Due to their minimal effect on wound healing and lack of systemic effects, topical local analgesics are good additional therapeutic options in combination with other systemic analgesic agents.
Pain and the Use of Analgesics in Oncology Models
Cancer in people or animals can result in nerve injury, inflammation, ischemia, obstruction and compression, and sickness. Any one or a combination of these events can occur, and the outcome is highly influenced by patient genetics, the type of cancer, heterogeneity across cancer subtypes and patients, the size and location of tumor, the presence of metastases, the specific cytokines and chemokines produced by the tumor, and host reactions to the tumor. Because of this variability, characterizing and predicting the nature of pain that may develop when cancer occurs—and the effectiveness of analgesics for treating this pain—is difficult. This is especially true in animals, in which pain and sickness are already difficult to discriminate and treat efficaciously. Because of this complexity, cancer pain has been placed in its own group in the clinical categorization of types of pain for human patients.122
It is generally accepted that most animals with aggressively growing tumors, if left untreated, will at some point experience pain or distress. Therefore, analgesic use must be carefully considered by IACUC. Because analgesics are unlikely to completely alleviate cancer pain and because often scientific objectives may be obtained prior to the onset of pain and distress, most humane guidelines for cancer studies emphasize the adoption of early endpoints.135 Early endpoint definitions typically involve defining removal criteria according to tumor size, characteristics such as ulceration and necrosis for subcutaneous cancer models, and behavioral and body condition scoring indices for internal tumors (orthotopic, metastatic, and transgenic models). These criteria generally work well because the onset of those factors that negatively influence animal wellbeing also alter tumor cell growth and negatively affect the scientific outcomes. However, some procedures in the development and testing of cancer therapeutics in animal models may require additional consideration regarding the alleviation of pain and its potential effect on results, and these are discussed in the following paragraphs.
The development of most orthotopic cancer models requires surgery for tumor cell implantation, and some translational studies involve later tumor resection. As for other surgical procedures, the use of perioperative analgesics is needed to alleviate pain.60 However, some specific effects of opioids and NSAID should be considered when tumor modeling is being conducted. For example, using a laparotomy model combined with intravenous injection of tumor cells, one group compared seeding of tumor cells (metastases) into the lung under conditions of unalleviated pain and in morphine-treated rats.95 The results demonstrated that the number of metastases significantly decreased when pain was alleviated and that morphine significantly lowered serum corticosterone levels. Using the same model, other colleagues confirmed these results with buprenorphine but were unable to replicate suppression of seeding to the lungs in morphine- or fentanyl-treated rats.43 A third group used serum obtained from the surgical pain model and from mice experiencing neuropathic pain to demonstrate that rat mammary cells grew faster in vitro in the ‘pain serum’ when compared with control serum.99 However, human mammary tumor cells (MDA-MB-235) grew at a similar rate regardless of whether they were obtained from animals experiencing pain or not. These studies demonstrate that pain may affect tumor cell behavior, either directly or indirectly through released cytokines and other mediators (Figures 1 and 3), and this response is dependent on the tumor cell type and the type of analgesic used to alleviate the pain.
Other than the studies just described and rodent models of pain due to bone metastases, preclinical studies demonstrating a link between pain and cancer progression are scant. This situation is likely because the onset and intensity of pain is unpredictable in soft tissue cancer models, and thus measuring and controlling these variables is difficult when planning a study. In addition, studies using cancer pain models may provide insights into how pain and the use of analgesic influence cancer cell behavior. Fentanyl, and to a lesser degree, morphine, decreased osteolytic activity and relieved pain when administered to C3H mice that had osteolytic murine sarcoma cells (NCTC 2472) implanted in the femur.37 If clinically relevant, these findings could reinforce the use of morphine in patients with bone metastases but may reduce the use of analgesics preclinical studies investigating bone sparing therapies in this model, because the magnitude of response compared with that of negative controls could be dampened with morphine. However, the issue has not been resolved conclusively, because one study yielded different results when morphine was administered in a similar model using CCL11 sarcoma cells.67 In that study, morphine paradoxically increased bone pain, with concomitant increases in osteolytic activity and expression of the osteolytic mediator 1Lb. In addition, tolerance to morphine doses varies with the mouse strain used, and these differences are likely to affect decisions about application in preclinical models. Clearly, the modulating effects of opioids on the microenvironment of cancer cells is dependent on tumor cell responsiveness, the dose and type of opioid used, and the mouse or rat strain involved.
Because analgesics are commonly used in cancer patients, several studies using rodent tumor models have evaluated the beneficial or adverse effects of these drugs on cancer progression. Although the presence or quantification of pain in these studies is likely to be variable and rarely monitored, review of these studies may provide insights on potential confounding effects in preclinical cancer studies that incorporate analgesics. In a bone orthotopic–metastatic model, the use of buprenorphine did not affect tumor growth, but the pain-relieving effects allowed the investigators to continue their studies longer, thus increasing the opportunity for lung metastases to occur.59 However, the authors of a review article found that, in a majority of studies, opioids had an apoptotic effect on cancer cells,2 and NSAID have been shown to have an apoptotic effect on ovarian,34 breast,78 and hepatic57 tumor cell lines. Of relevance to the metastatic models discussed earlier, cell adhesion and the production of invasive enzymes varied depending on the type of tumor cells and the dose and type of opioid used.2
In addition to having a direct effect on the tumor cells themselves, analgesics may alter the host response to the tumor through alteration of tumor microenvironment. Stimulation of angiogenesis is influenced by opioids, although these effects are highly variable. For example, morphine induced blood vessel formation in vitro.50 In the presence of clinically relevant doses of morphine, MCF7 mammary tumor xenografts in mice had more blood vessels and increased expression of angiogenesis-related signal transduction genes than tumor xenografts from mice not treated with morphine. Contrary to these findings, Lewis lung carcinoma xenografts in athymic mice had decreased blood vessel density and leukocyte cell migration when compared with controls.70 Different cell lines and doses of morphine may have contributed to the differences between these 2 studies. Studies performed with COX1 inhibitors, such as ibuprofen, have shown that NSAID can inhibit ovarian tumor-induced angiogenesis.73In addition, a COX2 inhibitor stunted angiogenesis in the chick embryonic chorioallantoic membrane assay and in murine mammary adenocarcinoma (TA-MTXR) tumors;105 the expression of vascular endothelial growth factor (VEGF), a major stimulator of angiogenesis, also was reduced in this tumor line.
In addition to endothelial cells, immune cell infiltrates comprise part of the tumor microenvironment, and as discussed previously, the use of analgesics can influence tumor-related inflammation. There is growing interest in developing immunomodulating drugs to sensitize and stimulate the immune system to attack tumor cells. As animal models are needed for the preclinical development in immune-mediated cancer therapy, IACUC may encounter requests to exempt analgesic use in animals from these studies. Typically, NSAID are avoided in cancer studies, especially those in which immunosurveillance may influence outcomes. Because several studies 43,70,83 have documented that opioids may have immunosuppressive effects, their use in these types of studies is not clear-cut. Empirical preclinical studies connecting opioid-induced immunosuppression with tumor progression is unavailable, and assumptions are largely based on transitive reasoning (opioid use leads to immunosuppression; immunosuppression leads to tumor progression; therefore opioid use leads to tumor progression).
It is important to reinforce that, in the vast majority of the studies cited earlier, the comparisons did not include evaluation of pain in tumor-bearing animals nor were there separate arms comparing tumor response in unrelieved pain subjects with controls and analgesic-treated animals. Although some evidence suggests that the use of analgesics might be a confound in tumor studies, the effects of not relieving pain are largely unknown. Further well-controlled studies in this area are needed to justify withholding of analgesia in animal tumor models that incur pain.
The timing and period of use of analgesics in cancer model development and testing should be considered. For example, if an investigator proposes to withhold analgesics from an orthotopic tumor protocol early, after surgical implantation, the effect of providing alleviation on research outcomes measured several weeks later should be considered. Early cancer cell growth, which occurs immediately after implantation, is dependent on cell proliferation. Estrogen receptor-positive mammary tumor (MCF7 and MBA-Md-231) cells were susceptible to aspirin-induced inhibition of growth and self-renewal.78 Although never empirically tested, these results might raise concerns that NSAID could negatively affect mammary tumor implantation rates. In contrast, morphine was shown to promote tumor growth and expand cancer stem cell populations in MCF7 and BT549 mammary tumors.87 Paradoxically, if the scientific objective is to generate large numbers of stem cells, the use of morphine could be dually beneficial in this model.
With the increased popularity of multimodal therapeutic approaches for cancer treatment, cancer chemotherapeutics are commonly being tested with newly developed drugs in preclinical animal studies. Therefore, appropriate pilot studies likely are needed to determine whether analgesic use alters responsiveness to chemotherapeutics and whether chemotherapeutics themselves cause pain and sickness. In an in vitro study, the use of morphine increased the resistance of 2 mammary tumor cell lines to doxorubicin and paclitaxel.87 Morphine also partially reversed the growth inhibition, apoptosis, and antiangiogenic response of nasopharyngeal carcinoma cells to cisplatin in vitro.21 However, piroxicam, a widely used NSAID, had the opposite effect on mesothelial cells, instead sensitizing them to the effects of cisplatin.112 Treatment of mesothelial cells with piroxicam upregulated expression of metabolically active genes and downregulated expression of genes associated with RNA processing; these effects are believed to have increased vulnerability to the chemotherapeutic.
Similarly, several chemotherapeutic agents, including platinum agents, taxanes, vinca alkaloids, thalidomide, bortezomib, and ixabepilone, may result in peripheral neuropathy and chronic pain.92 No treatments to date consistently prevent or alleviate chemotherapeutic-mediated pain in humans. Celecoxib, a COX2-selective NSAID, alleviated pain associated with oxaliplatin treatment in mice;63 the drug also enhanced oxaliplatin-mediated killing of human colon cancer line HCT116 in vitro and in vivo.76 Further studies are needed to determine whether analgesics are effective in preventing and treating chemotherapeutic-induced peripheral neuropathic pain without confounding research results in animal models.
In summary, many studies have shown that analgesics can influence tumor growth, metastases, apoptosis, and chemotherapeutic resistance. The presence of analgesics may also alter the tumor microenvironment and host inflammatory response. It is also well established that unalleviated pain alters physiology through central mechanisms and the production of neuropeptides, cytokines, and hormones. Several types of tumor cells have receptors to these molecules that would allow the cells to respond to pain-associated environmental changes.100 Because there is no overarching trend or consensus on how analgesics or unalleviated pain affects many of the parameters being measured in a given cancer study, scientific recommendations for exemption of analgesics are questionable. In such cases, pilot studies should be encouraged, and overall decisions should reflect moral responsibilities to reduce pain and distress, whenever possible. The use of analgesics should be tested as a default. Interestingly, recent reviews that address the potential of analgesics to enhance cancer progression in human patients resulted in a similar conclusion: variability among studies was so high that recommendations regarding withholding analgesics could not be made.2,13,83
Conclusions
Although the examples we have provided suggest that IACUC and research teams are wise to be cautious about analgesic use in various animal models to minimize unnecessary animal use, this caution must be balanced with the ethical imperative to minimize pain and distress in animals used in invasive research models.1,4,14,49,106,114 There is also a scientific imperative to ensure that models are relevant and translatable. Researchers and granting agencies must question whether data obtained from animals treated differently from human patients will contribute meaningfully to current human patient care.
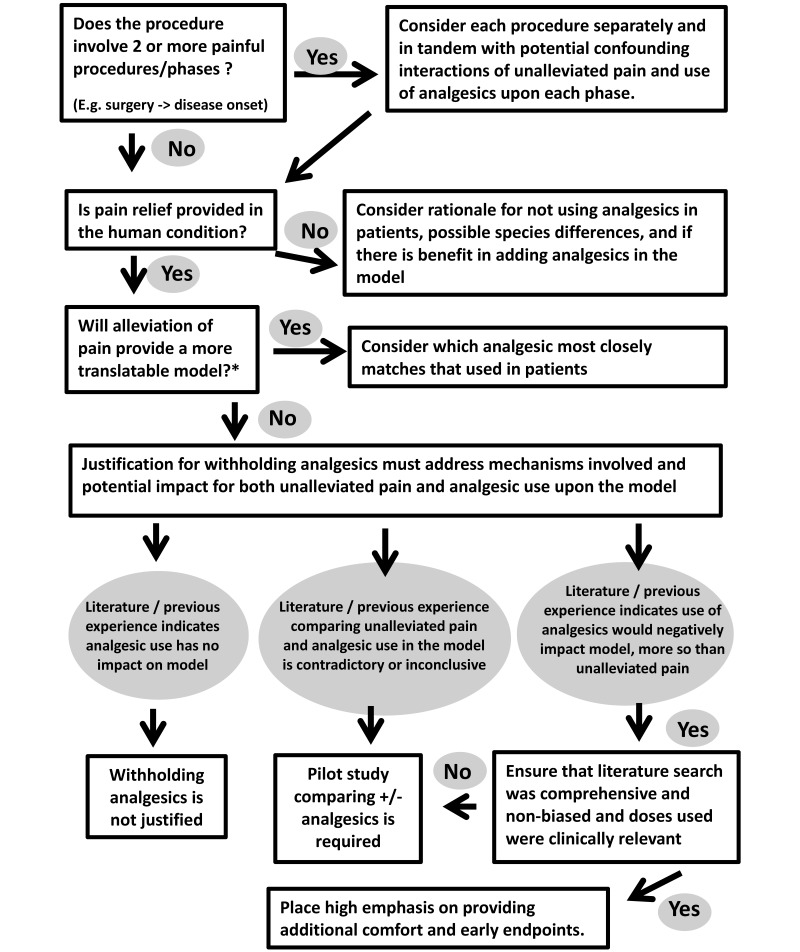
We included Figure 4 to assist investigators and IACUC in designing and evaluating studies that involve pain in animals. In studies requiring surgery to produce models that will subsequently experience pain associated with disease progression, alleviation from surgical pain should be considered separately and in addition to pain caused by the disease model. IACUC should request pilot or validation studies to demonstrate that short-term therapeutic perioperative analgesic use significantly alters the model studied. In addition, the research question must be fine-tuned to ask not whether analgesic use induces any change but whether the change induced is critical to the line of research being studied. The withholding of analgesics should be considered only when sufficient evidence indicates that the mechanism in question or translatability of the model is affected negatively by the specific use of perioperative analgesics. Furthermore, these considerations also should weigh the time span between surgery and onset of disease under study.
Figure 4.
Decision tree for petitions to withhold analgesics in painful procedures involving laboratory animals. To minimize the period during which pain and distress are experienced and regardless of the decision, the experiment should be designed so that the endpoints and objectives are reached as quickly as possible. *, Most studies have both mechanistic and translational components, and the relationship of these 2 objectives need to be considered in regard to alleviation of pain in the model.
Caution must also be applied to avoid overinterpreting results in the literature. Effects revealed in one model are not necessarily broadly generalizable to other models. As such, both research teams and IACUC must ensure that the justification for withholding of analgesia is not based on extrapolations or assumptions from completely different models. Furthermore, many of the studies that have been conducted evaluating the impact of analgesics on model outcome use morphine, a complete µ-agonist, whereas buprenorphine, which is used more commonly than morphine in laboratory animals, is only a partial µ-agonist. Differences in the pharmacokinetics and pharmacodynamics of these agents in human clinical settings23 suggest that these drugs would behave differently in laboratory animals as well. In many of the studies that we have reviewed here, buprenorphine tended to have fewer adverse effects on model outcomes than morphine. These findings suggest that, at minimum, analgesia with buprenorphine should be considered in new or pilot animal studies in which pain is likely to occur. Further model development may warrant buprenorphine use in combination with NSAID and other agents to achieve a more balanced and comprehensive analgesic effect with minimal adverse effects. Personnel involved with model development, pilot study design, and the evaluation of animal outcome must critically review the literature to ensure that relevant and therapeutic doses of analgesics are used to alleviate painful conditions and that appropriate behaviors and other measures are used to evaluate analgesia efficacy. Certainly, some general anesthetic agents provide some perioperative analgesia; however these effects are of short duration only and should be understood to cover only the immediate postoperative period.
In addition to studies needed concerning potential effects of opioids and NSAID on animal models, further research is needed to explore the adjunctive effects of local anesthetics on these models, given that these agents are increasingly being used for pain mitigation in human medicine. Ensuring that animals are housed in an appropriate environment should be assessed for its effects on modulation of pain response and enhancing animal wellbeing in pain models.17,129 Although not reviewed here, ample evidence suggests that widespread adoption of nonpharmacologic refinements to pain management, such as the use of deep bedding, opportunities for exercise, tasty food treats, easy access to food and water, warmth, and social housing with familiar conspecifics leading to social buffering and analgesia, might be implemented to enhance animal wellbeing in these studies.24,85
References
- 1.Abdullahi A, Amini-Nik S, Jeschke MG. 2014. Animal models in burn research. Cell Mol Life Sci 71:3241–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afsharimani B, Cabot P, Parat MO. 2011. Morphine and tumor growth and metastasis. Cancer Metastasis Rev 30:225–238. [DOI] [PubMed] [Google Scholar]
- 3.Agha AM, El-Khatib AS, Al-Zuhair H. 1999. Modulation of oxidant status by meloxicam in experimentally induced arthritis. Pharmacol Res 40:385–392. [DOI] [PubMed] [Google Scholar]
- 4.Al-Mousawi AM, Kulp GA, Branski LK, Kraft R, Mecott GA, Williams FN, Herndon DN, Jeschke MG. 2010. Impact of anesthesia, analgesia, and euthanasia technique on the inflammatory cytokine profile in a rodent model of severe burn injury. Shock 34:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander M, Daniel T, Chaudry IH, Schwacha MG. 2005. Opiate analgesics contribute to the development of postinjury immunosuppression. J Surg Res 129:161–168. [DOI] [PubMed] [Google Scholar]
- 6.Arcourt A, Lechner SG. 2015. Peripheral and spinal circuits involved in mechanical allodynia. Pain 156:220–221. [DOI] [PubMed] [Google Scholar]
- 7.Austin PJ, Moalem-Taylor G. 2010. The neuroimmune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells, and cytokines. J Neuroimmunol 229:26–50. [DOI] [PubMed] [Google Scholar]
- 8.Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Zaja I, Corbett JA, Bosnjak ZJ. 2013. Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg 116:869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber RC, Maass DL, White DJ, Horton JW. 2008. Increasing percent burn is correlated with increasing inflammation in an adult rodent model. Shock 30:388–393. [DOI] [PubMed] [Google Scholar]
- 10.Baumann H, Gauldie J. 1994. The acute-phase response. Immunol Today 15:74–80. [DOI] [PubMed] [Google Scholar]
- 11.Bette M, Schlimme S, Mutters R, Menendez S, Hoffmann S, Schulz S. 2004. Influence of different anaesthetics on proinflammatory cytokine expression in rat spleen. Lab Anim 38:272–279. [DOI] [PubMed] [Google Scholar]
- 12.Bjornson AB, Knippenberg RW, Bjornson HS. 1988. Nonsteroidal antiinflammatory drugs correct the bactericidal defect of polymorphonuclear leukocytes in a guinea pig model of thermal injury. J Infect Dis 157:959–967. [DOI] [PubMed] [Google Scholar]
- 13.Boland JW, McWilliams K, Ahmedzai SH, Pockley AG. 2014. Effects of opioids on immunologic parameters that are relevant to antitumour immune potential in patients with cancer: a systematic literature review. Br J Cancer 111:866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brabb T, Carbone L, Snyder J, Phillips N. 2014. Institutional animal care and use committee considerations for animal models of peripheral neuropathy. ILAR J 54:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browne AL, Andrews R, Schug SA, Wood F. 2011. Persistent pain outcomes and patient satisfaction with pain management after burn injury. Clin J Pain 27:136–145. [DOI] [PubMed] [Google Scholar]
- 16.Browne KD, Iwata A, Putt ME, Smith DH. 2006. Chronic ibuprofen administration worsens cognitive outcome following traumatic brain injury in rats. Exp Neurol 201:301–307. [DOI] [PubMed] [Google Scholar]
- 17.Bushnell MC, Case LK, Ceko M, Cotton VA, Gracely JL, Low LA, Pitcher MH, Villemure C. 2015. Effect of environment on the long-term consequences of chronic pain. Pain 156 Suppl 1:S42–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. 1992. Differential effects of morphine and naltrexone on the antibody response in various mouse strains. Immunopharmacol Immunotoxicol 14:657–673. [DOI] [PubMed] [Google Scholar]
- 19.Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. 1993. Effects of in vivo morphine treatment on antibody responses in C57BL/6 bgJ/bgJ (beige) mice. Life Sci 52:PL43–PL48. [DOI] [PubMed] [Google Scholar]
- 20.Butler K, Lewis GP. 1972. The effect of antiinflammatory agents on the changes in local lymph after thermal injury. Br J Pharmacol 45:644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao LH, Li HT, Lin WQ, Tan HY, Xie L, Zhong ZJ, Zhou JH. 2016. Morphine, a potential antagonist of cisplatin cytotoxicity, inhibits cisplatin-induced apoptosis and suppression of tumor growth in nasopharyngeal carcinoma xenografts. Sci Rep 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carbone L, Austin J. 2016. Pain and laboratory animals: publication practices for better data reproducibility and better animal welfare. PLoS One 11:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Center for Substance Abuse Treatment. 2004. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treatment improvement protocol (TIP) series 40. Rockville (MD): Substance Abuse and Mental Health Services. [PubMed] [Google Scholar]
- 24.Chambers CT, Mogil JS. 2015. Ontogeny and phylogeny of facial expression of pain. Pain 156:798–799. [DOI] [PubMed] [Google Scholar]
- 25.Christianson CA, Corr M, Firestein GS, Mobargha A, Yaksh TL, Svensson CI. 2010. Characterization of the acute and persistent pain state present in K/BxN serum-transfer arthritis. Pain 151:394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colleoni M, Sacerdote P. 2010. Murine models of human neuropathic pain. Biochim Biophys Acta 1802:924–933. [DOI] [PubMed] [Google Scholar]
- 27.Cotroneo TM, Hugunin KM, Shuster KA, Hwang HJ, Kakaraparthi BN, Nemzek-Hamlin JA. 2012. Effects of buprenorphine on a cecal ligation and puncture model in C57BL/6 mice. J Am Assoc Lab Anim Sci 51:357–365. [PMC free article] [PubMed] [Google Scholar]
- 28.D'Elia M, Patenaude J, Hamelin C, Garrel DR, Bernier J. 2004. Corticosterone binding globulin regulation and thymus changes after thermal injury in mice. Am J Physiol Endocrinol Metab 288:E852–E860. [DOI] [PubMed] [Google Scholar]
- 29.Daniels R, Nutbeam T. 2010. ABC of sepsis. Chichester (United Kingdom): Blackwell Publishing. [Google Scholar]
- 30.De Vloo P, Morlion B, van Loon J, Nuttin B. 2017. Animal models for central poststroke pain: a critical comprehensive review. Pain 158:17–29. [DOI] [PubMed] [Google Scholar]
- 31.Descalzi G, Ikegami D, Ushijima T, Nestler EJ, Zachariou V, Narita M. 2015. Epigenetic mechanisms of chronic pain. Trends Neurosci 38:237–246. (Erratum: published 2015 Trends Neurosci 38:579). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiVincenti L., Jr 2013. Analgesic use in nonhuman primates undergoing neurosurgical procedures. J Am Assoc Lab Anim Sci 52:10–16. [PMC free article] [PubMed] [Google Scholar]
- 33.do Nascimento GC, Leite-Panissi CR. 2014. Time-dependent analysis of nociception and anxiety-like behavior in rats submitted to persistent inflammation of the temporomandibular joint. Physiol Behav 125:1–7. [DOI] [PubMed] [Google Scholar]
- 34.Duncan K, Uwimpuhwe H, Czibere A, Sarkar D, Libermann TA, Fisher PB, Zerbini LF. 2012. NSAID induce apoptosis in nonproliferating ovarian cancer cells and inhibit tumor growth in vivo. IUBMB Life 64:636–643. [DOI] [PubMed] [Google Scholar]
- 35.Earl JR, Claxson AW, Blake DR, Morris CJ. 1994. Proinflammatory effects of morphine in the rat adjuvant arthritis model. Int J Tissue React 16:163–170. [PubMed] [Google Scholar]
- 36.Ellis A, Bennett DL. 2013. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth 111:26–37. [DOI] [PubMed] [Google Scholar]
- 37.El Mouedden M, Meert TF. 2007. The impact of the opioids fentanyl and morphine on nociception and bone destruction in a murine model of bone cancer pain. Pharmacol Biochem Behav 87:30–40. [DOI] [PubMed] [Google Scholar]
- 38.Engel O, Kolodziej S, Dirnagl U, Prinz V. [Internet]. 2011. Modeling stroke in mice—middle cerebral artery occlusion with the filament model. J Vis Exp [Cited 09 Sept 2017]. Available at: https://www.jove.com/video/2423/modeling-stroke-mice-middle-cerebral-artery-occlusion-with-filament [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelhardt G, Homma D, Schnitzler C. 1995. Meloxicam: a potent inhibitor of adjuvant arthritis in the Lewis rat. Inflamm Res 44:548–555. [DOI] [PubMed] [Google Scholar]
- 40.Ennaceur A, Chazot PL. 2016. Preclinical animal anxiety research—flaws and prejudices. Pharmacol Res Perspect 4:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erdener SE, Dalkara T. 2014. Modelling headache and migraine and its pharmacological manipulation. Br J Pharmacol 171:4575–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fazal N. 2012T-cell suppression in burn and septic injuries, p 161–190. In: Kapur S, Barbosa Portela M. Immunosuppression—role in health and diseases. Rijeka (Croatia): InTech. [Google Scholar]
- 43.Franchi S, Panerai AE, Sacerdote P. 2007. Buprenorphine ameliorates the effect of surgery on hypothalamus–pituitary–adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav Immun 21:767–774. [DOI] [PubMed] [Google Scholar]
- 44.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. 2005. Mast cells as ‘tunable’ effector and immunoregulatory cells: recent advances. Annu Rev Immunol 23:749–786. [DOI] [PubMed] [Google Scholar]
- 45.Gauglitz GG, Song J, Herndon DN, Finnerty CC, Boehning D, Barral JM, Jeschke MG. 2008. Characterization of the inflammatory response during acute and postacute phases after severe burn. Shock 30:503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ge HW, Hu WW, Ma LL, Kong FJ. 2015. Endoplasmic reticulum stress pathway mediates isoflurane-induced neuroapoptosis and cognitive impairments in aged rats. Physiol Behav 151:16–23. [DOI] [PubMed] [Google Scholar]
- 47.Goto G, Hori Y, Ishikawa M, Tanaka S, Sakamoto A. 2014. Changes in the gene expression levels of microRNAs in the rat hippocampus by sevoflurane and propofol anesthesia. Mol Med Rep 9:1715–1722. [DOI] [PubMed] [Google Scholar]
- 48.Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR. 2016. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci USA 113:E3441–E3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graham SM, McCullough LD, Murphy SJ. 2004. Animal models of ischemic stroke: balancing experimental aims and animal care. Comp Med 54:486–496. [PubMed] [Google Scholar]
- 50.Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. 2002. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res 62:4491–4498. [PubMed] [Google Scholar]
- 51.Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, Rieder A. 2007. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiol Scand 52:132–136. [DOI] [PubMed] [Google Scholar]
- 52.Hall TJ, Jagher B, Schaeublin M, Wiesenberg I. 1996. The analgesic drug buprenorphine inhibits osteoclastic bone resorption in vitro but is proinflammatory in rat adjuvant arthritis. Inflamm Res 45:299–302. [DOI] [PubMed] [Google Scholar]
- 53.Hansbrough JF, Peterson V, Kortz E, Piacentine J. 1983. Postburn immunosuppression in an animal model: monocyte dysfunction induced by burned tissue. Surgery 93:415–423. [PubMed] [Google Scholar]
- 54.Hanson CE, Ruble GR, Essiet I, Hartman AB. 2001. Effects of buprenorphine on immunogenicity and protective efficacy in the guinea pig keratoconjunctivitis model (Sereny test). Comp Med 51:224–229. [PubMed] [Google Scholar]
- 55.Hawkins P, Armstrong R, Boden T, Garside P, Knight K, Lilley E, Seed M, Wilkinson M, Williams RO. 2015. Applying refinement to the use of mice and rats in rheumatoid arthritis research. Inflammopharmacology 23:131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herndon DN, Wilmore DW, Mason AD., Jr 1978. Development and analysis of a small animal model simulating the human postburn hypermetabolic response. J Surg Res 25:394–403. [DOI] [PubMed] [Google Scholar]
- 57.Hossain MA, Kim DH, Jang JY, Kang YJ, Yoon JH, Moon JO, Chung HY, Kim GY, Choi YH, Copple BL, Kim ND. 2012. Aspirin induces apoptosis in vitro and inhibits tumor growth of human hepatocellular carcinoma cells in a nude mouse xenograft model. Int J Oncol 40:1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hugunin KM, Fry C, Shuster K, Nemzek JA. 2010. Effects of tramadol and buprenorphine on select immunologic factors in a cecal ligation and puncture model. Shock 34:250–260. [DOI] [PubMed] [Google Scholar]
- 59.Husmann K, Arlt MJ, Jirkof P, Arras M, Born W, Fuchs B. 2015. Primary tumour growth in an orthotopic osteosarcoma mouse model is not influenced by analgesic treatment with buprenorphine and meloxicam. Lab Anim 49:284–293. [DOI] [PubMed] [Google Scholar]
- 60.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 61.Izamis ML, Uygun K, Uygun B, Yarmush ML, Berthiaume F. 2009. Effects of burn injury on markers of hypermetabolism in rats. J Burn Care Res 30:993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobsen KR, Fauerby N, Raida Z, Kalliokoski O, Hau J, Johansen FF, Abelson KS. 2013. Effects of buprenorphine and meloxicam analgesia on induced cerebral ischemia in C57BL/6 male mice. Comp Med 63:105–113. [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang SP, Zhang ZD, Kang LM, Wang QH, Zhang L, Chen HP. 2016. Celecoxib reverts oxaliplatin-induced neuropathic pain through inhibiting PI3K/Akt2 pathway in the mouse dorsal root ganglion. Exp Neurol 275:11–16. [DOI] [PubMed] [Google Scholar]
- 64.Jobin N, Garrel DR, Bernier J. 2000. Increased burn-induced immunosuppression in lipopolysaccharide-resistant mice. Cell Immunol 200:65–75. [DOI] [PubMed] [Google Scholar]
- 65.Kaneko K, Umehara M, Homan T, Okamoto K, Oka M, Oyama T. 2014. The analgesic effect of tramadol in animal models of neuropathic pain and fibromyalgia. Neurosci Lett 562:28–33. [DOI] [PubMed] [Google Scholar]
- 66.Kennedy LH, Hwang H, Wolfe AM, Hauptman J, Nemzek-Hamlin JA. 2014. Effects of buprenorphine and estrous cycle in a murine model of cecal ligation and puncture. Comp Med 64:270–282. [PMC free article] [PubMed] [Google Scholar]
- 67.King T, Vardanyan A, Majuta L, Melemedjian O, Nagle R, Cress AE, Vanderah TW, Lai J, Porreca F. 2007. Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer. Pain 132:154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kohtala S, Theilmann W, Suomi T, Wigren HK, Porkka-Heiskanen T, Elo LL, Rokka A, Rantamäki T. 2016. Brief isoflurane anesthesia produces prominent phosphoproteomic changes in the adult mouse hippocampus. ACS Chem Neurosci 7:749–756. [DOI] [PubMed] [Google Scholar]
- 69.Kolstad AM, Rodriguiz RM, Kim CJ, Hale LP. 2012. Effect of pain management on immunization efficacy in mice. J Am Assoc Lab Anim Sci 51:448–457. [PMC free article] [PubMed] [Google Scholar]
- 70.Koodie L, Yuan H, Pumper JA, Yu H, Charboneau R, Ramkrishnan S, Roy S. 2014. Morphine inhibits migration of tumor-infiltrating leukocytes and suppresses angiogenesis associated with tumor growth in mice. Am J Pathol 184:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Langley PC, Van Litsenburg C, Cappelleri JC, Carroll D. 2012. The burden associated with neuropathic pain in Western Europe. J Med Econ 16:85–95. [DOI] [PubMed] [Google Scholar]
- 72.Levin ME, Jin JG, Ji RR, Tong J, Pomonis JD, Lavery DJ, Miller SW, Chiang LW. 2008. Complement activation in the peripheral nervous system following the spinal nerve ligation model of neuropathic pain. Pain 137:182–201. [DOI] [PubMed] [Google Scholar]
- 73.Li W, Xu RJ, Lin ZY, Zhuo GC, Zhang HH. 2008. Effects of a cyclooxygenase-1-selective inhibitor in a mouse model of ovarian cancer, administered alone or in combination with ibuprofen, a nonselective cyclooxygenase inhibitor. Med Oncol 26:170–177. [DOI] [PubMed] [Google Scholar]
- 74.Lindegaard C, Gleerup KB, Thomsen MH, Martinussen T, Jacobsen S, Andersen PH. 2010. Antiinflammatory effects of intraarticular administration of morphine in horses with experimentally induced synovitis. Am J Vet Res 71:69–75. [DOI] [PubMed] [Google Scholar]
- 75.Liu L, Li X, Yang J, Chai J, Yu Y, Duan H, Song H, Feng R, Wang T, Yin H, Hu Q, Wang S, Du J. 2015. Comparison of systemic inflammation response and vital organ damage induced by severe burns in different area. Int J Clin Exp Pathol 8:6367–6376. [PMC free article] [PubMed] [Google Scholar]
- 76.Li W, Xu J, Zhao J, Zhang R. 2017. Oxaliplatin and infliximab combination synergizes in inducing colon cancer regression. Med Sci Monit 23: 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loepke AW, Soriano SG. 2008. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg 106:1681–1707. [DOI] [PubMed] [Google Scholar]
- 78.Maity G, De A, Das A, Banerjee S, Sarkar S, Banerjee SK. 2015. Aspirin blocks growth of breast tumor cells and tumor-initiating cells and induces reprogramming factors of mesenchymal to epithelial transition. Lab Invest 95:702–717. [DOI] [PubMed] [Google Scholar]
- 79.Marko ST, Little SF, Benton CG, Kelly R, III, Field AE, Laufer RS. 2014. Effect of analgesics on monoclonal antibody ascites production in mice administered upon recognition of pain. ISRN Immunology 2014:1–8. [Google Scholar]
- 80.Martucci C, Panerai AE, Sacerdote P. 2004. Chronic fentanyl or buprenorphine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain 110:385–392. [DOI] [PubMed] [Google Scholar]
- 81.McQueen DS, Iggo A, Birrell GJ, Grubb BD. 1991. Effects of paracetamol and aspirin on neural activity of joint mechanonociceptors in adjuvant arthritis. Br J Pharmacol 104:178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meng J, Banerjee S, Li D, Sindberg GM, Wang F, Ma J, Roy S. 2015. Opioid exacerbation of gram-positive sepsis, induced by gut microbial modulation, is rescued by IL17A neutralization. Sci Rep 5:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meserve JR, Kaye AD, Prabhakar A, Urman RD. 2014. The role of analgesics in cancer propagation. Best Pract Res Clin Anaesthesiol 28:139–151. [DOI] [PubMed] [Google Scholar]
- 84.Minami M, Katayama T, Satoh M. 2006. Brain cytokines and chemokines: roles in ischemic injury and pain. J Pharmacol Sci 100:461–470. [DOI] [PubMed] [Google Scholar]
- 85.Mogil JS. 2015. Social modulation of and by pain in humans and rodents. Pain 156 Suppl 1:S35–S41. [DOI] [PubMed] [Google Scholar]
- 86.National Research Council. 1999. Monoclonal antibody production. Washington (DC): National Academies Press. [Google Scholar]
- 87.Niu DG, Peng F, Zhang W, Guan Z, Zhao HD, Li JL, Wang KL, Li TT, Zhang Y, Zheng FM, Xu F, Han QN, Gao P, Wen QP, Liu Q. 2015. Morphine promotes cancer stem-cell properties, contributing to chemoresistance in breast cancer. Oncotarget 6:3963–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Odunayo A, Dodam JR, Kerl ME, DeClue AE. 2010. Immunomodulatory effects of opioids. J Vet Emerg Crit Care (San Antonio) 20:376–385. [DOI] [PubMed] [Google Scholar]
- 89.Olin MR, Choi K, Lee J, Peterson PK, Molitor TW. 2007. Morphine modulates γδ lymphocytes cytolytic activity following BCG vaccination. Brain Behav Immun 21:195–201. [DOI] [PubMed] [Google Scholar]
- 90.Orman MA, Ierapetritou MG, Berthiaume F, Androulakis IP. 2011. The dynamics of the early inflammatory response in double-hit burn and sepsis animal models. Cytokine 56:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Orman MA, Nguyen TT, Ierapetritou MG, Berthiaume F, Androulakis IP. 2011. Comparison of the cytokine and chemokine dynamics of the early inflammatory response in models of burn injury and infection. Cytokine 55:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pachman DR, Barton DL, Watson JC, Loprinzi CL. 2011. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther 90:377–387. [DOI] [PubMed] [Google Scholar]
- 93.Padgett DA, Marucha PT, Sheridan JF. 1998. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun 12:64–73. [DOI] [PubMed] [Google Scholar]
- 94.Page GG. 2005. Immunologic effects of opioids in the presence or absence of pain. J Pain Symptom Manage 29 5 Suppl:25–31. [DOI] [PubMed] [Google Scholar]
- 95.Page GG, McDonald JS, Ben-Eliyahu S. 1998. Preoperative vs postoperative administration of morphine: impact on the neuroendocrine, behavioural, and metastatic-enhancing effects of surgery. Br J Anaesth 81:216–223. [DOI] [PubMed] [Google Scholar]
- 96.Parent AJ, Beaudet N, Beaudry H, Bergeron J, Bérubé P, Drolet G, Sarret P, Gendron L. 2012. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behav Brain Res 229:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pedersen JL, Callesen T, Moiniche S, Kehlet H. 1996. Analgesic and antiinflammatory effects of lignocaine–prilocaine (EMLA) cream in human burn injury. Br J Anaesth 76:806–810. [DOI] [PubMed] [Google Scholar]
- 98.Peterson NC. 2000. Behavioral, clinical, and physiologic analysis of mice used for ascites monoclonal antibody production. Comp Med 50:516–526. [PubMed] [Google Scholar]
- 99.Peterson NC, Servinsky MD. 2007. Development of molecular and cellular biomarkers of pain. Comp Med 57:554–562. [PubMed] [Google Scholar]
- 100.Petit T, Davidson KK, Lawrence RA, von Hoff DD, Izbicka E. 2001. Neuropeptide receptor status in human tumor cell lines. Anticancer Drugs 12:133–136. [DOI] [PubMed] [Google Scholar]
- 101.Piersma FE, Daemen MA, Bogaard AE, Buurman WA. 1999. Interference of pain control employing opioids in in vivo immunological experiments. Lab Anim 33:328–333. [DOI] [PubMed] [Google Scholar]
- 102.Pomahac B, Zuhaili B, Kudsi Y, Bleiziffer O, Velander P, Eriksson E, Gerner P. 2007. Safety evaluation of topically applied amitriptyline in porcine full-thickness wounds. Reg Anesth Pain Med 32:377–381. [DOI] [PubMed] [Google Scholar]
- 103.Retrouvey H, Shahrokhi S. 2015. Pain and the thermally injured patient—a review of current therapies. J Burn Care Res 36:315–323. [DOI] [PubMed] [Google Scholar]
- 104.Rojas IG, Padgett DA, Sheridan JF, Marucha PT. 2002. Stress-induced susceptibility to bacterial infection during cutaneous wound healing. Brain Behav Immun 16:74–84. [DOI] [PubMed] [Google Scholar]
- 105.Rosas C, Sinning M, Ferreira A, Fuenzalida M, Lemus D. 2014. Celecoxib decreases growth and angiogenesis and promotes apoptosis in a tumor cell line resistant to chemotherapy. Biol Res 47:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rowe RK, Harrison JL, Thomas TC, Pauly JR, Adelson PD, Lifshitz J. 2013. Using anesthetics and analgesics in experimental traumatic brain injury. Lab Anim (NY) 42:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sacerdote P, Manfredi B, Bianchi M, Panerai AE. 1994. Intermittent but not continuous inescapable footshock stress affects immune responses and immunocyte β-endorphin concentrations in the rat. Brain Behav Immun 8:251–260. [DOI] [PubMed] [Google Scholar]
- 108.Sacerdote P, Manfredi B, Mantegazza P, Panerai AE. 1997. Antinociceptive and immunosuppressive effects of opiate drugs: a structure-related activity study. Br J Pharmacol 121:834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwacha MG, Holland LT, Chaudry IH, Messina JL. 2005. Genetic variability in the immune–inflammatory response after major burn injury. Shock 23:123–128. [DOI] [PubMed] [Google Scholar]
- 110.Shavit Y, Martin FC, Yirmiya R, Ben-Eliyahu S, Terman GW, Weiner H, Gale RP, Liebeskind JC. 1987. Effects of a single administration of morphine or footshock stress on natural killer cell cytotoxicity. Brain Behav Immun 1:318–328. [DOI] [PubMed] [Google Scholar]
- 111.Shurin MR, Kusnecov A, Hamill E, Kaplan S, Rabin BS. 1994. Stress-induced alteration of polymorphonuclear leukocyte function in rats. Brain Behav Immun 8:163–169. [DOI] [PubMed] [Google Scholar]
- 112.Spugnini EP, Cardillo I, Verdina A, Crispi S, Saviozzi S, Calogero R, Nebbioso A, Altucci L, Cortese G, Galati R, Chien J, Shridhar V, Vincenzi B, Citro G, Cognetti F, Sacchi A, Baldi A. 2006. Piroxicam and cisplatin in a mouse model of peritoneal mesothelioma. Clin Cancer Res 12:6133–6143. [DOI] [PubMed] [Google Scholar]
- 113.Stein C, Kuchler S. 2013. Targeting inflammation and wound healing by opioids. Trends Pharmacol Sci 34:303–312. [DOI] [PubMed] [Google Scholar]
- 114.Stills HF., Jr 2005. Adjuvants and antibody production: dispelling the myths associated with Freund's complete and other adjuvants. ILAR J 46:280–293. [DOI] [PubMed] [Google Scholar]
- 115.Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. 2008. Proinflammatory cytokines mediating burn-injury pain. Pain 135:98–107. [DOI] [PubMed] [Google Scholar]
- 116.Swearengen JR, Cockman-Thomas RA, Davis JA, Weina PJ. 1993. Evaluation of butorphanol tartrate and buprenorphine hydrochloride on the inflammatory reaction of the Sereny test. Lab Anim Sci 43:471–475. [PubMed] [Google Scholar]
- 117.Tajerian M, Clark JD. 2015. The role of the extracellular matrix in chronic pain following injury. Pain 156:366–370. [DOI] [PubMed] [Google Scholar]
- 118.Tan Q, Lin Z, Ma W, Chen H, Wang L, Ning G, Zhou X. 2002. Failure of ibuprofen to prevent progressive dermal ischemia after burning in guinea pigs. Burns 28:443–448. [DOI] [PubMed] [Google Scholar]
- 119.Tengvall OM, Bjornhagen VC, Lindholm C, Jonsson CE, Wengstrom Y. 2006. Differences in pain patterns for infected and noninfected patients with burn injuries. Pain Manag Nurs 7:176–182. [DOI] [PubMed] [Google Scholar]
- 120.Thau-Zuchman O, Shohami E, Alexandrovich AG, Trembovler V, Leker RR. 2012. The antiinflammatory drug carprofen improves long-term outcome and induces gliogenesis after traumatic brain injury. J Neurotrauma 29:375–384. [DOI] [PubMed] [Google Scholar]
- 121.Topcu I, Ekici NZ, Isik R, Sakarya M. 2006. The effects of tramadol and fentanyl on gastrointestinal motility in septic rats. Anesth Analg 102:876–881. [DOI] [PubMed] [Google Scholar]
- 122.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavandʼhomme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JW, Wang SJ. 2015. A classification of chronic pain for ICD-11. Pain 156:1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tsuda M. 2015. Microglia in the spinal cord and neuropathic pain. J Diabetes Investig 7:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tubbs JT, Kissling GE, Travlos GS, Goulding DR, Clark JA, King-Herbert AP, Blankenship-Paris TL. 2011. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J Am Assoc Lab Anim Sci 50:185–191. [PMC free article] [PubMed] [Google Scholar]
- 125.Tucker CB, Mintline EM, Banuelos J, Walker KA, Hoar B, Drake D, Weary DM. 2014. Effect of a cooling gel on pain sensitivity and healing of hot-iron cattle brands. J Anim Sci 92:5666–5673. [DOI] [PubMed] [Google Scholar]
- 126.Tucker CB, Mintline EM, Banuelos J, Walker KA, Hoar B, Varga A, Drake D, Weary DM. 2014. Pain sensitivity and healing of hot-iron cattle brands. J Anim Sci 92:5674–5682. [DOI] [PubMed] [Google Scholar]
- 127.Tunçtan B, Altug S, Uludag O, Demirkay B, Abacioglu N. 2003. Effects of cyclooxygenase inhibitors on nitric oxide production and survival in a mice model of sepsis. Pharmacol Res 48:37–48. [PubMed] [Google Scholar]
- 128.Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. 2011. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 152:990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vachon P, Millecamps M, Low L, Thompsosn SJ, Pailleux F, Beaudry F, Bushnell CM, Stone LS. 2013. Alleviation of chronic neuropathic pain by environmental enrichment in mice well after the establishment of chronic pain. Behav Brain Funct 9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Loon JP, de Grauw JC, van Dierendonck M, L'Ami JJ, Back W, van Weeren PR. 2010. Intraarticular opioid analgesia is effective in reducing pain and inflammation in an equine LPS-induced synovitis model. Equine Vet J 42:412–419. [DOI] [PubMed] [Google Scholar]
- 131.Volker D, Bate M, Gentle R, Garg M. 2000. Oral buprenorphine is antiinflammatory and modulates the pathogenesis of streptococcal cell wall polymer-induced arthritis in the Lew/SSN rat. Lab Anim 34:423–429. [DOI] [PubMed] [Google Scholar]
- 132.Waite A, Gilliver SC, Masterson GR, Hardman MJ, Ashcroft GS. 2010. Clinically relevant doses of lidocaine and bupivacaine do not impair cutaneous wound healing in mice. Br J Anaesth 104:768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Walker JS, Chandler AK, Wilson JL, Binder W, Day RO. 1996. Effect of µ-opioids morphine and buprenorphine on the development of adjuvant arthritis in rats. Inflamm Res 45:557–563. [DOI] [PubMed] [Google Scholar]
- 134.Weatherstone KB, Franck LS, Klein NJ. 2003. Are there opportunities to decrease nosocomial infection by choice of analgesic regimen? Evidence for immunity and pain interactions. Arch Pediatr Adolesc Med 157:1108–1114. [DOI] [PubMed] [Google Scholar]
- 135.Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA, Glennie MJ, Kelland LR, Robinson V, Stratford IJ, Tozer GM, Watson S, Wedge SR, Eccles SA, Committee of the National Cancer Research Institute. 2010. Guidelines for the welfare and use of animals in cancer research. Br J Cancer 102:1555–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xie W, Strong JA, Li H, Zhang JM. 2007. Sympathetic sprouting near sensory neurons after nerve injury occurs preferentially on spontaneously active cells and is reduced by early nerve block. J Neurophysiol 97:492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yalcin I, Barthas F, Barrot M. 2014. Emotional consequences of neuropathic pain: insight from preclinical studies. Neurosci Biobehav Rev 47:154–164. [DOI] [PubMed] [Google Scholar]
- 138.Zheng YQ, Wei W, Shen YX, Dai M, Liu LH. 2004. Oral and nasal administration of chicken type II collagen suppresses adjuvant arthritis in rats with intestinal lesions induced by meloxicam. World J Gastroenterol 10:3165–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]