Abstract
The use of animal models in vitamin D deficiency (VDD) research, particularly in regard to maternal deficits, has increased dramatically, yet these studies may be confounded due to ill-conceived experimental timelines. We conducted 2 experiments to (1) characterize the time course of VDD induction and repletion and (2) explore the long-term consequences of VDD on calcium homeostasis and body composition in reproductive-age female mice. Eight-week-old female C57BL/6 mice were randomized to receive either a vitamin D sufficient (VDS) or VDD diet; serum was collected weekly. At week 4, VDD mice were switched to VDS diet, and serum was collected weekly until week 8. Another group of same-age female mice was maintained on VDD diet for 40 wk. Body weights and serum were collected every 2 wk until week 40, when body composition was measured by using echoMRI. Mice did not become VDD until week 3 of the VDD diet and, after decreasing slightly at 4 wk, serum 25-hydroxyvitamin D remained unchanged through 40 wk. Vitamin D repletion to 25-hydroxyvitamin D concentrations considered adequate by the Institute of Medicine took 2 to 3 wk. Prolonged VDD in mice was marked by hypocalcemia and hyperparathyroidism and led to proportional decreases in both lean and fat mass. These data provide guidance in the design of studies using mice as a maternal VDD model, especially those exploring its effects on the developmental origins of health and disease and highlight the importance of monitoring and controlling the calciotropic effects of diet-induced VDD. This study also shows that prolonged VDD in reproductive-age female C57BL/6 mice induces metabolically meaningful changes in absolute, but not relative, body composition.
Abbreviations: 25(OH)D, 25-dihydroxyvitamin D; DOHaD, developmental origin of health and disease; PTH, parathyroid hormone; NRC, National Research Council; VDD, vitamin D deficiency; VDS, vitamin D sufficiency
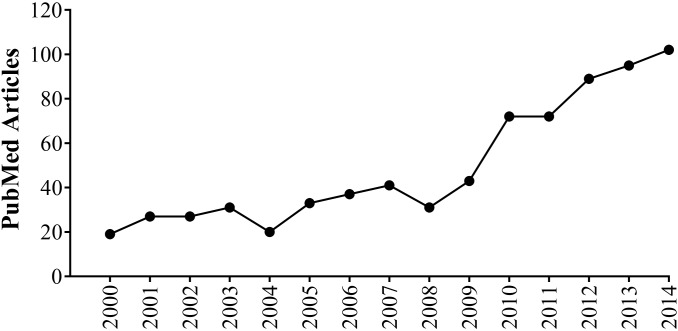
Vitamin D deficiency (VDD) remains one of the most prevalent preventable public health concerns worldwide.32,50,55,70 Evidence has accumulated over the past decade or so that this VDD may be a major contributor to myriad conditions, ranging from obesity and related metabolic diseases3,73 to cancer25,59 and modulation of immune14,66 and brain function.13,37 As the support for associations between this deficiency state and many diseases grows, improving our understanding of the mechanisms for vitamin D's role in health and disease, including potential epigenetic changes, becomes increasingly important.2,72 Furthermore, maternal VDD can result in detrimental developmental origin of health and disease (DOHaD) effects in human and rodent offspring.5,45,48,49,52,53,61,64,71,72 Reproductive-age female rodents are attractive animal models to use in DOHaD investigations because of their low cost, small size, high fertility rates, short gestation periods, and susceptibility to several of the same metabolic diseases that affect humans. Although vitamin D studies using rodent models have been useful, in general, in teasing apart the underlying VDD-induced changes leading to various diseases,40,54,62,63 the scientific literature is fraught with experimental results and interpretations that may be compromised due to inappropriate experimental timelines. To illustrate, the number of PubMed articles featuring keywords relating VDD with rodents has expanded 5-fold over the past 15 y (Figure 1), yet the most recent set of dietary vitamin D recommendations for laboratory animals from the National Research Council (NRC) was published more than 20 y ago.51
Figure 1.
Number of articles including the key words “vitamin D deficiency,” “rodents,” and “animal models.’
VDD studies in animals, including reproductive-age females, need to accommodate the latency period characteristic of dietary fat-soluble vitamins, such as vitamin D, and provide the necessary exposure time, to recapitulate the phenotypic effects observed in VDD human populations. Conversely, secondary systemic complications due to long-term VDD might arise and confound experimental results. For these reasons, the time course and 25-hydroxyvitamin D (25(OH)D) response (the generally accepted best indicator of vitamin D status) to VDS and VDD diets in laboratory rodents warrants investigation, especially in the most widely and frequently used mouse strain for metabolic research, C57BL/6 (‘Black 6’) mice.21 Therefore, our current objectives were: 1) to characterize the time course of VDD induction and repletion in reproductive-age female C57BL/6 mice and 2) to explore the long-term consequences of VDD, particularly in regard to body composition, an indicator of metabolic health.
Materials and Methods
Animals and diets.
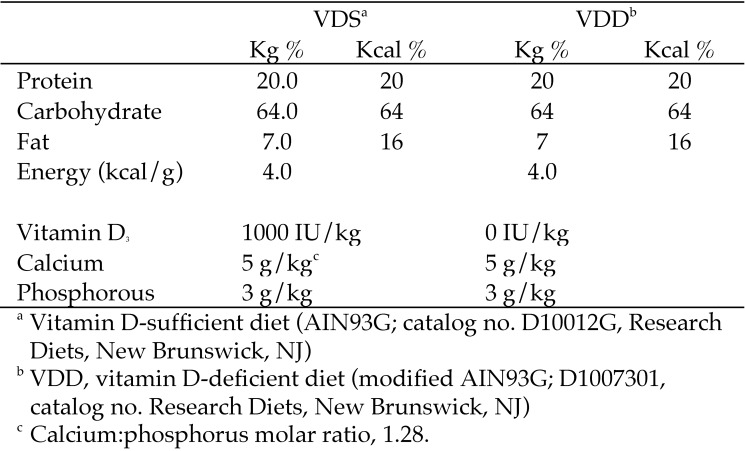
Both experiments were approved and performed in accordance with the University of Missouri IACUC (protocol 7753). All procedures followed the NIH Guidelines for the Care and Use of Laboratory Animals.34 All experiments were conducted as preliminary work for a larger study examining the DOHaD effects of maternal VDD during pregnancy on the long-term metabolic health of offspring. Female C57BL/6J mice (age, 8 wk) were acquired from Jackson Labs (Jackson Labs; Bar Harbor, ME). To determine an effective timeline for inducing and reversing VDD, serum was collected from sexually mature 8-wk-old female C57BL/6 mice, which then Eight-week-old randomized to receive either a vitamin D sufficient (VDS; AIN-93G, D10012G, Research Diets, New Brunswick, NJ) or VDD (modified AIN-93G; D1007301, Research Diets) diet (Figure 2). Other than vitamin D, all nutrients, including calcium and phosphorus, were provided in amounts that meet the NRC nutrient requirements for laboratory animals.51 Mice had free access to diets and water. During the short-term exposure experiment, feed intake was measured by weighing the food daily for 5 consecutive days during weeks 1 and 8. Body weight was measured at baseline and every 2 wk throughout both experiments.
Figure 2.
Nutrient composition and energy density of experimental diets.
Short-term VDD exposure.
Serum was collected at baseline and weekly for 4 wk (weeks 1 through 4) for determination of 25(OH)D, parathyroid hormone (PTH), and calcium concentrations. Immediately after the week 4 collection, VDD mice were switched to a VDS diet, and serum was similarly collected for the subsequent 4 wk (weeks 5 through 8) for determination of 25(OH)D, PTH, and calcium concentrations.
Long-term VDD exposure.
In the long-term study, body weight was measured and serum collected at baseline and every 2 wk thereafter. Three-compartment body composition (lean tissue, fat tissue, and water) was determined by using echoMRI at week 40 of feeding (48 wk of age), just prior to study termination.
Determination of serum vitamin D, calcium, and PTH concentrations.
Whole blood was collected from the saphenous vein and dispensed into serum-separator tubes (catalog no. 201308, Sarstedt, Numbrecht, Germany), centrifuged according to manufacturers’ instructions, and stored at –80 °C. Due to the limited volumes of serum that could be collected at each time point, we alternated which parameters were measured. Circulating serum 25(OH)D concentrations were determined by ELISA (detection range, 10 to 300 nmol/L; intraassay coefficient of variation, 4.2%; catalog no. VID21-K02, Eagle Biosciences; Nashua, NH). Serum calcium concentrations were determined by colorimetric assay (Sigma-Aldrich; St Louis, MO). Serum PTH concentrations were determined by enzyme–linked immunoassay (detection range, 1.47 to 1000 pg/mL; intraassay coefficient of variation, 10%; Sigma–Aldrich).
Statistical analysis.
Baseline comparisons of whole-body weight, 25(OH)D concentrations, and daily feed intake were analyzed by using the Student t test. Between and within dietary group comparisons of circulating 25(OH)D concentrations and body weight were performed by using repeated-measures ANOVA. Diet, time, and the diet×time interaction served as independent variables for the analyses, allowing us to determine whether the effect of diet (main effect; for any given outcome) was dependent on how long the mice were on the diet. In this model, time was a repeated measure, thus making it possible to compare treatment groups at each time point. Data are represented as mean ± SEM. All analyses were done by using SAS 9.4 statistical software (SAS Software, Cary, NC); a P value of 0.05 or less was considered significant.
Results
Vitamin D depletion and repletion in reproductive-age female mice.
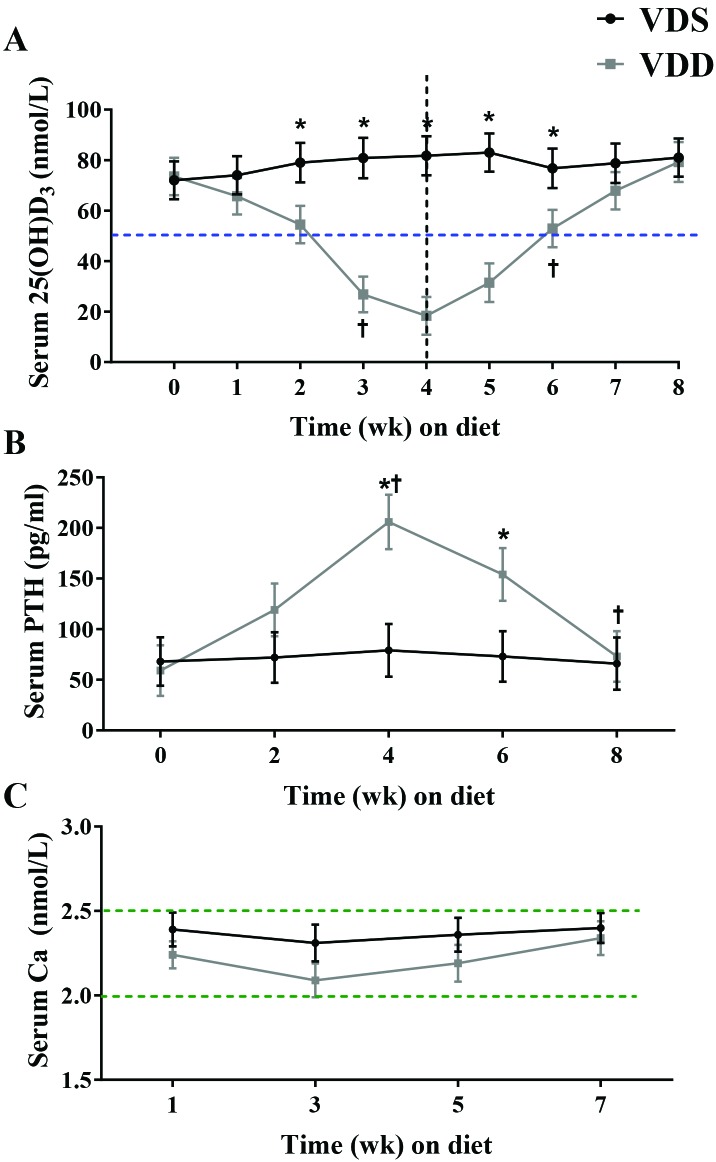
At baseline, neither body weight nor vitamin D status differed between the VDS and VDD groups (19.3 ± 1.3 g compared with 18.8 ± 1.4 g, P = 0.774; Table 1). Likewise, daily feed consumption was similar between the VDS and VDD groups during week 1 (2.6 ± 0.2 g compared with 2.7 ± 0.2 g, P = 0.73) and week 8 (2.9 ± 0.3 g compared with 3.0 ± 0.2 g, P = 0.748; Table 1). Serum 25(OH)D concentrations showed a significant (P < 0.001) interaction between diet and time (Figure 3 A), but the week-to-week change in serum 25(OH)D concentration was significant only between the weeks 2 and 3 of the VDD diet (decrease of 28.2 nmol/L, P = 0.009). Furthermore, the between-group difference in 25(OH)D concentration was not significant until week 2 (53.4 ± 11.4 nmol/L compared with 79 ± 11.2 nmol/L, P = 0.014). Despite having significantly lower 25(OH)D concentrations than the VDS group, VDD mice were not VDD according to the cut-off established by the Institute of Medicine (that is, 25(OH)D less than 50 nmol/L) until week 3. Circulating 25(OH)D concentrations began to level off in the VDD group, with a nonsignificant decrease between weeks 3 and 4 (decrease of 7.5 nmol/L, P = 0.782).60
Table 1.
Baseline body weight, 25(OH)D concentration, and average food intake
| VDS | VDD | P | |
| Whole-body weight (g) | 19.3 ± 1.3 | 18.8 ± 1.4 | 0.774 |
| 25(OH)D (nmol/L) | 73.5 ± 11.5 | 72.0 ± 10.8 | 0.904 |
| Average daily intake (wk 1; g) | 2.6 ± 0.2 | 2.7 ± 0.2 | 0.730 |
| Average daily intake (wk 8; g) | 2.9 ± 0.3 | 3.0 ± 0.2 | 0.748 |
VDD, Vitamin D-deficient; VDS, vitamin D-sufficient
Data are given as means ± SEM. P values represent comparison of dietary groups by using the Student t test.
Figure 3.
Circulating concentrations of (A) 25(OH)D, (B) PTH, and (C) calcium. Female C57BL/6 mice were fed a vitamin D0deficient (VDD; n = 7) diet until week 4 (perpendicular dashed line in panel A) and then switched to a vitamin D-sufficient (VDS; n = 6) diet for another 4 wk. The horizontal dashed line in panel A indicates the cut-off value for VDD in humans. Dotted lines in panel C indicate the hypo- and hypercalcemia thresholds. Data are presented as presents means ± SEM. *, Significant (P < 0.05) difference between-groups at the indicated time point; †, significant (P < 0.05) within-group difference from previous time point.
VDD mice were switched to a VDS diet after the blood collection at week 4 of the VDD diet. Although 25(OH)D concentrations began to increase within the first week on the VDS diet (increase of 13.3 nmol/L, P = 0.236), the change from nadir did not reach statistical significance until the second week (increase of 34.5 nmol/L; P = 0.017), at which point 3 of the 5 mice were VDS. At the end of 3 wk of feeding on the VDS diet, these once-VDD mice were all in adequate status (25[OH]D greater than 50 nmol/L) and had serum concentrations that did not differ from those that received the VDS diet throughout the study (79.3 ± 11.6 nmol/L compared with 80.8 ±11.3 nmol/L, P = 0.846).
Calciotropic hormone response to vitamin D depletion and repletion in reproductive-age female mice.
Serum PTH concentrations demonstrated a significant interaction between diet and time (P < 0.001; Figure 3 B). Serum PTH did not differ between the VDS and VDD groups at baseline (59.1 ± 22.6 compared with 68.3 ± 28.2 pg/mL, P = 0.854). In the VDD group, serum PTH rose between weeks 2 and 4 (increase of 87.2 pg/mL, P = 0.027). At week 4, VDD-fed mice had greater serum PTH concentrations than those fed the VDS diet (205.8 ± 46.6 pg/mL compared with 76.2 ± 29.3 pg/mL, P < 0.001). Serum PTH did not significantly decrease during the first 2 wk of the repletion phase (week 4 to 6, decrease of 51.9 pg/mL, P = 0.168), but concentrations did not decrease significantly between the second and fourth week (decrease of 80.5 pg/mL, P = 0.029). Neither time nor diet their interaction had a significant effect on serum calcium concentrations (Figure 3 C).
Vitamin D status in reproductive-age female mice exposed long-term to VDD.
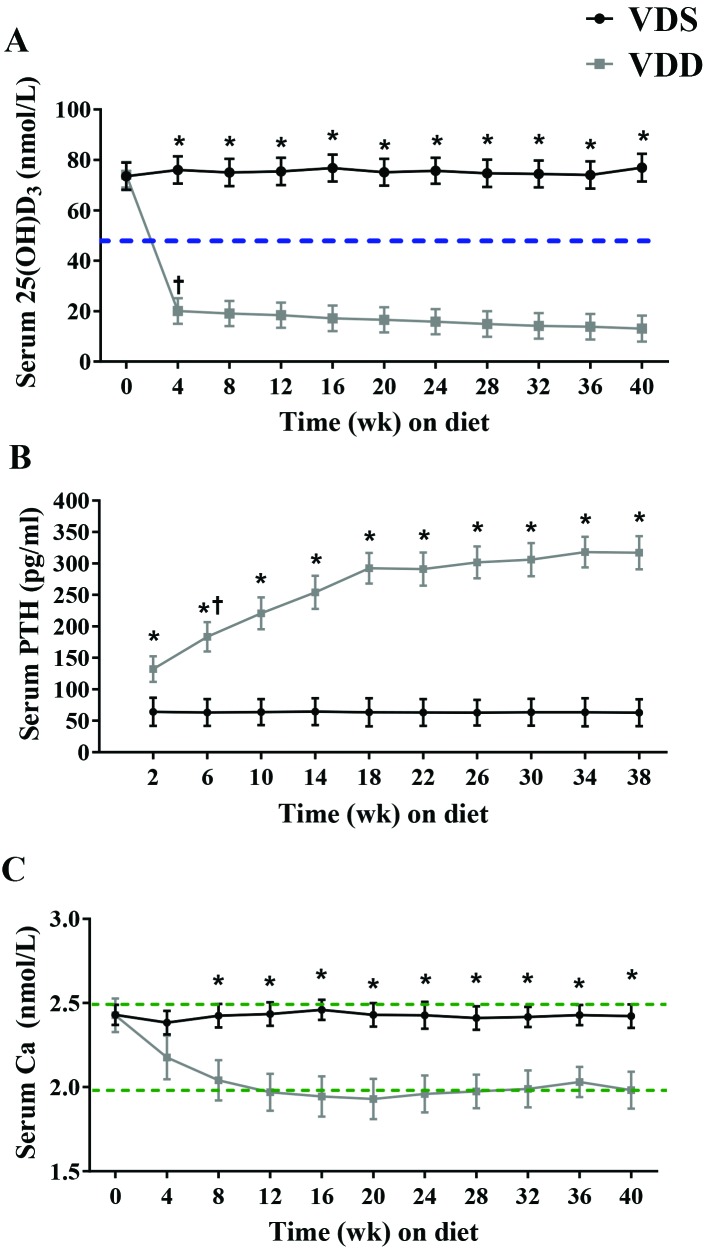
Serum 25(OH)D concentrations at baseline did not differ between the VDS and VDD groups (73.5 ± 11.3 nmol/L compared with 71.4 ± 11.4 nmol/L, P = 0.896; Figure 4 A). Serum 25(OH)D concentrations showed significant (P < 0.001) interaction between diet and time, such that the change between baseline and 4 wk was almost entirely responsible for this interaction. Furthermore, the difference in 25(OH)D concentration between the VDS and VDD groups was significant beginning at week 4, with the VDD group having lower 25(OH)D; this significant difference persisted throughout study.
Figure 4.
Circulating concentrations of (A) 25(OH)D, (B) PTH, and (C) calcium. Female C57BL/6 mice were maintained on a vitamin D-sufficient (VDS; n = 7) or vitamin D-deficient (VDD; n = 6) diet for 40 wk. Dashed line in panel A indicates the cut-off value for VDD in humans. Dotted lines in panel C indicate hypo- and hypercalcemia thresholds. Data are presented as mean ± SEM. *, Significant (P < 0.05) between-group difference at the indicated time point; †, significant (P < 0.05) within-group difference from previous time point.
Calciotropic hormone response in reproductive-age female mice exposed long-term to VDD.
Serum PTH concentrations showed a significant (P < 0.001) interaction between diet and time (Figure 4 B). Serum PTH levels in the VDS group did not change throughout the study (P = 0.963). In the VDD group, serum PTH was significantly higher than in the VDS group beginning at 2 wk (66.2 ± 23.2 pg/mL compared with 131.2 ± 24.4, P = 0.024; Figure 4 B) and stayed significantly higher at each of the subsequent time points. Within the VDD group, serum PTH rose significantly between weeks 2 and 6 (increase of 50.3 pg/mL, P = 0.044); there were no other significant between-week increases for the remainder of the study.
Serum calcium concentrations showed a significant (P = 0.041) interaction between diet and time (Figure 4 C). Although neither group had any significant week-to-week changes in serum calcium concentration, these levels were lower in VDD-fed mice than in VDS mice beginning at week 12 and lasting throughout the experiment.
Growth curve and body composition of reproductive-age female mice exposed long-term to VDD.
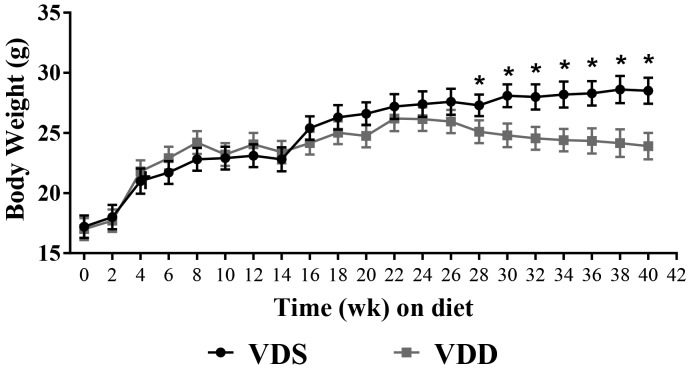
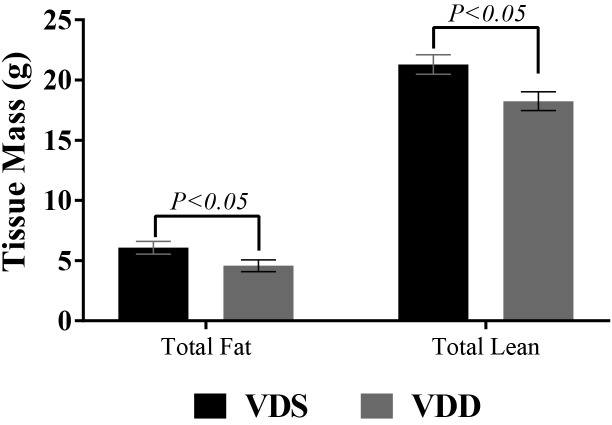
Body weight did not differ between the VDD and VDS groups at baseline (17.0 ± 1.6 g compared with 17.4 ± 1.3 g, P = 0.637; Figure 5). Growth rate increased similarly and in parallel in both groups for the first 18 wk, at which point the growth of VDD mice began to plateau. After 26 wk, the weight of VDD mice began to decrease, and by week 28, VDD mice weighed significantly less than VDS mice (decrease of 2.04 g, P = 0.02). By week 40, VDD mice had lost 12% of their peak weight (week 22) and weighed 4.1 g less than VDS mice (P < 0.001; Figure 6). After 40 wk, VDD mice had less fat (4.7 ± 0.4 g compared with 6.0 ± 0.4 g, P < 0.01) and lean mass (18.1 ± 0.6 g compared with 20.8 ± 0.5 g, P = 0.014; Figure 6) than VDS mice. However, body composition did not differ between groups in terms of percentage body fat. Likewise, total water content did not differ between the VDD and VDS mice (22.7 ± 0.4 compared with 23.1 ± 0.5 g; P = 0.541).
Figure 5.
Growth curves of female C57BL/6 mice fed either a vitamin D-sufficient (VDS; n = 7) or vitamin D-deficient (VDD; n = 6) diet for 40 wk. Data are presented as means ± SEM. *, Significant (P < 0.05) difference between the 2 groups.
Figure 6.
Total fat and lean mass, as determined by echoMRI, of female C57BL/6 mice fed a vitamin D-sufficient (VDS; n = 7) or vitamin D-deficient (VDD; n = 6) diet for 40 wk.
Discussion
In both animal models and humans, it is increasingly becoming apparent that maternal VDD can lead to diseases and disorders in the offspring, for example, those involving reproduction, neurobehavior, and metabolism as well as epigenetic changes.5,45,48,49,52,53,61,64,71,72 C57BL/6 mice, in particular, are among the most commonly used animal models in metabolic disease research.21 However, little is known about the vitamin D needs of these animals, especially reproductive-age females, beyond what is required for normal growth. The escalation in vitamin D research (Figure 1) highlights the importance of determining how the vitamin functions in the model most used in vitamin D-related metabolic health research, including DOHaD studies. Maternal VDD and its deleterious outcomes in offspring have increasingly become an important concern,5,45,48,49,52,53,61,64,71,72 and an estimated 80% of pregnant women have insufficient vitamin D status at some point during pregnancy.9 Therefore in the current study, we sought to characterize the time course of VDD induction (defined by the Institute of Medicine as a 25(OH)D less than 50 nmol/L) and dietary vitamin D repletion, by using the dose recommended by the NRC (1000 IU/kg), in reproductive-age female C57BL/6 mice and to explore the long-term consequences of VDD on body composition, an indicator of metabolic health, in these animals.51,60 Our data demonstrate that: 1)VDD was not achieved until 3 wk on a vitamin D-deplete diet and, after decreasing slightly at 4 wk, vitamin D status remained unchanged through 40 wk; 2) vitamin D repletion occurred slightly more quickly, taking 2 to 3 wk for restoration of serum 25(OH)D concentrations considered by the Institute of Medicine to be adequate for humans; 3) long-term VDD was marked by secondary hypocalcemia and hyperparathyroidism; and 4) long-term VDD led to weight loss with proportional decreases in both lean and fat body compartments.
Serum 25(OH)D, the hydroxy derivative of vitamin D and functional indicator of vitamin D status, has a reported half-life of approximately 20 to 30 d in humans; our observation that it took 3 wk for 25(OH)D concentrations (falling approximately 2 nmol/L daily) to reach a deficient state in reproductive age female mice is consistent with this report.28 Our findings also highlight the importance of well-designed studies that not only ensure sufficient time for vitamin D depletion but also consider the possibility of unchanged, low but stable serum 25(OH)D concentration over an extended time. Our data on calcium homeostasis shed some light in this regard.
The most well-known role of vitamin D involves calcium homeostasis. Because calcium is necessary in nearly every cell and tissue in the body, the serum calcium concentration is maintained within a very tight window. Several mechanisms, involving vitamin D and PTH, exist to help maintain this balance.33 The relationship between serum calcium and serum PTH is of an inverse sigmoidal nature, wherein decreases in serum calcium elicit increases in PTH of a magnitude that is dependent on the deviation from the initial calcium concentration.47 PTH is released into circulation from the chief cells of the parathyroid gland. Acting through both vitamin D-dependent and -independent mechanisms, PTH stimulates target tissues (small intestine, kidney, and bone) to raise serum calcium concentrations and maintain homeostasis.15,16,43,44 Indeed, our short-term VDD study exhibited this calciotropic hormone response. The rise in PTH concentrations in the VDD mice mirrored the decline in serum 25(OH)D, thus keeping serum total calcium within normal range albeit slightly lower than baseline. This decrease is most likely explained by the reduced availability of vitamin D for vitamin D-dependent mechanisms (especially increases in intestinal absorption) of calcium homeostasis.44 Our long-term study showed a similar effect of VDD on serum 25(OH)D concentrations early during vitamin D depletion, but between weeks 8 to 12, serum total calcium decreased significantly, such that concentrations hovered around or were marginally below the normocalcemic minimum throughout the remainder of the study. Serum PTH, however, continued to increase over the entire study, with final concentrations that were 2.5-fold greater than baseline concentrations. These observations reflect the remarkable capacity of calciotropic hormones and related organs to maintain calcium homeostasis in the face of VDD. Therefore, secondary effects (on calciotropic hormones and organs) of VDD need to be considered in any experimental design, especially long-term studies and particularly when attempting to elucidate the noncalciotropic consequences of VDD.
To circumvent health complications associated with the calciotropic consequences of VDD, the use of high-calcium diets should be considered. It is common practice for investigators using vitamin D receptor knockout and CYP27B1-lacking (an enzyme necessary for vitamin D activation) mouse models to use ‘rescue diets’ that maintain normal circulating calcium concentrations.19,36,46 These rescue diets are fortified with calcium (approximately 2%) and phosphorus (approximately 1.25%) and contain additional lactose to promote calcium absorption.17,39 Investigators using a diet-induced VDD model should include a rescue diet to prevent confounding of results.
The potential to mitigate health conditions by restoring sufficient vitamin D status after VDD has garnered much attention. However, this endeavor is muddled by disagreement among the scientific community regarding the concentration of 25(OH)D considered adequate compared with sufficient. For example, the Institute of Medicine defines VDD as serum 25(OH)D concentrations lower than 50 nmol/L, whereas the consensus report from the 14th Vitamin D Workshop states that ‘sufficiency should be defined as concentrations exceeding 75 nmol/L.29,60 Although comparing our current vitamin D repletion results in mice directly with human data is not straightforward due to differences in the dose and mode of vitamin D administration, body size and adiposity, and definition of sufficiency used), human trials demonstrate a repletion time of approximately 4 to 6 wk with oral dosing at physiologic inputs, with 75 nmol/L as the goal.7,27,30 Similarly, our adult female mice achieved circulating 25(OH)D levels that exceeded the Institute of Medicine deficiency threshold after 2 to 3 wk on the VDS diet, and with continued consumption of the VDS diet, our mice indeed attained a vitamin D status considered as sufficient by several scientific organizations by 8 wk.26,29,31 This gradual response in circulating concentrations to dietary vitamin D repletion by using NRC recommended amounts may complicate studies attempting to elucidate the immediate effects of repletion in vivo. Therefore, researchers attempting to address such questions need to either consider this repletion time course or use custom-formulated diets containing slightly increased amounts of vitamin D.12,35 Alternatively, intraperitoneal injection of vitamin D might be used to induce a rapid rise in circulating 25(OH)D.74
In the current study, reproductive0age female mice maintained on a VDD diet demonstrated similar growth patterns to VDS-fed mice until 26 wk, after which VDD mice began rapidly losing weight. At study termination (40 wk of diet; 48 wk of age), VDD-fed mice weighed significantly less than VDS mice. EchoMRI measurement of body composition (fat compared with lean body mass compartments) indicated that the difference in body weight was attributable to widespread losses of both lean and fat tissue. This result is not surprising, given that serum calcium levels fell below 2.0 mmol/L for an extended period of time. Perhaps the observed loss of mass was due to systemic dysregulation and anorexigenic effects associated with hypocalcemia;22 this hypothesis requires further study that includes a test group that receives a calcium rescue diet. Alternatively any speculated anorexigenic effects might be attributed to hormonal disruptions, such as of leptin, neuropeptide Y, or orexin; this question should be addressed in future studies. In addition, follow-up investigations might include dual-energy X-ray absorptiometry scans to confirm how much of the lean mass difference is due to bone mineral compared with muscle loss.
Approximately 30% of the difference in body weight between the VDD and VDS groups is attributable to the loss of fat mass; 64% of the difference in body weight is attributable to lean body mass. The role of vitamin D in adipose tissue is complicated and unclear,1,8,11 but it is well-established that adipose tissue is the primary storage site for fat-soluble vitamins, including vitamin D.41,58 Interestingly, despite the extended VDD, serum 25(OH)D concentrations largely plateaued after the rapid decline during the first 4 wk; therefore sequestered stores might have been mobilized along with fat breakdown.23 Although we are unable to elucidate the tissue-specific effects on lean body mass atrophy, the likely greatest contributor within this compartment is bone loss. As mentioned previously, to maintain serum calcium homeostasis, bone functions the calcium reservoir,56 and reduction in the bone mineral content is well noted in long-term VDD.68 Skeletal muscle is another likely contributor to the observed loss of lean tissue mass. The direct and indirect (effects due to secondary hypocalcemia, hypophosphatemia, or hyperparathyroidism) roles of vitamin D in skeletal muscle are well-characterized,10,18 and vitamin D deficiency has been associated with loss of both skeletal muscle mass and function in both humans and animals.6,20,24,67
A major limitation of both of our experiments was the inability to measure all analytes at the same time points due to constraints on the amount of serum that could safely be collected from the mice. In the long-term study, the primary limitation was the lack of food intake data. Without this information, we are unable to rule out anorexigenic consequences of VDD, which would explain the dramatic weight loss in the VDD mice, as has been documented in some human VDD studies.4,65 Future studies feeding VDD diets on a long-term basis should examine total feed intake and control for potential effects on body weight. The only other known study to examine the long-term effects of VDD in C57BL/6 mice likewise did not measure food intake.69
The well-understood consequences of VDD in lab animals, on which the current NRC recommendations are based, are hypocalcemia and hyperparathyroidism.51 However, since the most recent publication of NRC recommendations in 1995, we have come to understand that vitamin D plays many roles in the body and that animal models that recapitulate VDD-related conditions are needed.32 Here we have characterized the time course of both VDD depletion and repletion in reproductive-age female mice. These data will aid investigators in the design of future work, including studies examining the potential for offspring DOHaD effects originating from maternal VDD. Our results further highlight the importance of monitoring and controlling the calciotropic effects of diet-induced VDD when studying noncalciotropic effects and support for the use of hypocalcemic rescue diets in long-term VDD diet studies. Moreover, we provide evidence in reproductive age C57BL/6 female mice that long-term VDD results in metabolically meaningful changes in absolute, but not relative, body composition (21% reduction in body fat and 13% loss in lean mass). These effects might be an important consideration in studies of vitamin D and metabolic health.42,57
Acknowledgments
This work was funded by the University of Missouri's Department of Nutrition and Exercise Physiology.
References
- 1.Abbas MA. 2017. Physiological functions of vitamin D in adipose tissue. J Steroid Biochem Mol Biol 165:369–381. [DOI] [PubMed] [Google Scholar]
- 2.Alkharfy KM, Al-Daghri NM, Yakout SM, Hussain T, Mohammed AK, Krishnaswamy S. 2013. Influence of vitamin D treatment on transcriptional regulation of insulin-sensitive genes. Metab Syndr Relat Disord 11:283–288. [DOI] [PubMed] [Google Scholar]
- 3.Atabek ME, Eklioglu BS, Akyurek N, Alp H. 2014. Association between vitamin D level and cardiovascular risk in obese children and adolescents. J Pediatr Endocrinol Metab 27:661–666. [DOI] [PubMed] [Google Scholar]
- 4.Barreto-Chang OL, Pearson D, Shepard WE, Longhurst CA, Greene A. 2010. Vitamin D—deficient rickets in a child with cow's milk allergy. Nutr Clin Pract 25:394–398. [DOI] [PubMed] [Google Scholar]
- 5.Belenchia AM, Jones KL, Will M, Beversdorf DQ, Vieira-Potter V, Rosenfeld CS, Peterson CA. 2016. Maternal vitamin D deficiency during pregnancy affects expression of adipogenic-regulating genes peroxisome proliferator-activated receptor γ (PPARγ) and vitamin D receptor (VDR) in lean male mice offspring. Eur J Nutr 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhat M, Kalam R, Qadri SS, Madabushi S, Ismail A. 2013. Vitamin D deficiency-induced muscle wasting occurs through the ubiquitin proteasome pathway and is partially corrected by calcium in male rats. Endocrinology 154:4018–4029. [DOI] [PubMed] [Google Scholar]
- 7.Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. 2013. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab 98:973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumberg JM, Tzameli I, Astapova I, Lam FS, Flier JS, Hollenberg AN. 2006. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J Biol Chem 281:11205–11213. [DOI] [PubMed] [Google Scholar]
- 9.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. 2007. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr 137:447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boland R. 1986. Role of vitamin D in skeletal muscle function. Endocr Rev 7:434–448. [DOI] [PubMed] [Google Scholar]
- 11.Bouillon R, Carmeliet G, Lieben L, Watanabe M, Perino A, Auwerx J, Schoonjans K, Verstuyf A. 2014. Vitamin D and energy homeostasis-of mice and men. Nat Rev Endocrinol 10:79–87. [DOI] [PubMed] [Google Scholar]
- 12.Brouwer DA, van Beek J, Ferwerda H, Brugman AM, van der Klis FR, van der Heiden HJ, Muskiet FA. 1998. Rat adipose tissue rapidly accumulates and slowly releases an orally-administered high vitamin D dose. Br J Nutr 79:527–532. [DOI] [PubMed] [Google Scholar]
- 13.Byrne JH, Voogt M, Turner KM, Eyles DW, McGrath JJ, Burne TH. 2013. The impact of adult vitamin D deficiency on behaviour and brain function in male Sprague–Dawley rats. PLoS One 8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. 2006. Epidemic influenza and vitamin D. Epidemiol Infect 134:1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christakos S. 2012. Mechanism of action of 1,25-dihydroxyvitamin D3 on intestinal calcium absorption. Rev Endocr Metab Disord 13:39–44. [DOI] [PubMed] [Google Scholar]
- 16.Coburn JW, Hartenbower DL, Norman AW. 1974. Metabolism and action of the hormone vitamin D. Its relation to diseases of calcium homeostasis. West J Med 121:22–44. [PMC free article] [PubMed] [Google Scholar]
- 17.Dardenne O, Prud'homme J, Hacking SA, Glorieux FH, St-Arnaud R. 2003. Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1). Bone 32:332–340. [DOI] [PubMed] [Google Scholar]
- 18.DeLuca HF. 2004. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80 6 Suppl:1689S–1696S. [DOI] [PubMed] [Google Scholar]
- 19.Demay MB. 2012. Physiological insights from the vitamin D receptor knockout mouse. Calcif Tissue Int 92:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhesi JK, Jackson SH, Bearne LM, Moniz C, Hurley MV, Swift CG, Allain TJ. 2004. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing 33:589–595. [DOI] [PubMed] [Google Scholar]
- 21.Fontaine DA, Davis DB. 2016. Attention to background strain is essential for metabolic research: C57BL/6 and the international knockout mouse consortium. Diabetes 65:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaggi R, Beltrandi E, Dall'Olio R, Ferri S. 1985. Relationships between hypocalcaemic and anorectic effect of calcitonin in the rat. Pharmacol Res Commun 17:209–215. [DOI] [PubMed] [Google Scholar]
- 23.Gangloff A, Bergeron J, Pelletier-Beaumont E, Nazare JA, Smith J, Borel AL, Lemieux I, Tremblay A, Poirier P, Almeras N, Despres JP. 2015. Effect of adipose tissue volume loss on circulating 25-hydroxyvitamin D levels: results from a 1-y lifestyle intervention in viscerally obese men. Int J Obes (Lond) 39:1638–1643. [DOI] [PubMed] [Google Scholar]
- 24.González-Reimers E, Duran-Castellon MC, Lopez-Lirola A, Santolaria-Fernandez F, Abreu-Gonzalez P, Alvisa-Negrin J, Sanchez-Perez MJ. 2010. Alcoholic myopathy: vitamin D deficiency is related to muscle fibre atrophy in a murine model. Alcohol Alcohol 45:223–230. [DOI] [PubMed] [Google Scholar]
- 25.Grant WB. 2015. Vitamin D deficiency may explain comorbidity as an independent risk factor for death associated with cancer in Taiwan. Asia Pac J Public Health 27:572–573. [DOI] [PubMed] [Google Scholar]
- 26.Harel Z, Flanagan P, Forcier M, Harel D. 2011. Low vitamin D status among obese adolescents: prevalence and response to treatment. J Adolesc Health 48:448–452. [DOI] [PubMed] [Google Scholar]
- 27.Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 2008. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr 87:1738–1742. [DOI] [PubMed] [Google Scholar]
- 28.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. 2003. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77:204–210. [DOI] [PubMed] [Google Scholar]
- 29.Henry HL, Bouillon R, Norman AW, Gallagher JC, Lips P, Heaney RP, Vieth R, Pettifor JM, Dawson-Hughes B, Lamberg-Allardt CJ, Ebeling PR. 2010. 14th vitamin D workshop consensus on vitamin D nutritional guidelines. J Steroid Biochem Mol Biol 121:4–6. [DOI] [PubMed] [Google Scholar]
- 30.Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD. 2008. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93:677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S. 2011. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930. [DOI] [PubMed] [Google Scholar]
- 32.Holick MF, Chen TC. 2008. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87:1080S–1086S. [DOI] [PubMed] [Google Scholar]
- 33.Houillier P, Nicolet-Barousse L, Maruani G, Paillard M. 2003. What keeps serum calcium levels stable? Joint Bone Spine 70:407–413. [DOI] [PubMed] [Google Scholar]
- 34.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 35.Jang HR, Lee JW, Kim S, Heo NJ, Lee JH, Kim HS, Jung JY, Oh YK, Na KY, Han JS, Joo KW. 2010. High dose vitamin D3 attenuates the hypocalciuric effect of thiazide in hypercalciuric rats. J Korean Med Sci 25:1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufmann M, Lee SM, Pike JW, Jones G. 2015. A high-calcium and phosphate rescue diet and VDR-expressing transgenes normalize serum vitamin D metabolite profiles and renal Cyp27b1 and Cyp24a1 expression in VDR null mice. Endocrinology 156:4388–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keeney JT, Forster S, Sultana R, Brewer LD, Latimer CS, Cai J, Klein JB, Porter NM, Butterfield DA. 2013. Dietary vitamin D deficiency in rats from middle to old age leads to elevated tyrosine nitration and proteomics changes in levels of key proteins in brain: implications for low vitamin D-dependent age-related cognitive decline. Free Radic Biol Med 65:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HJ, Lee YM, Ko BS, Lee JW, Yu JH, Son BH, Gong GY, Kim SB, Ahn SH. 2010. Vitamin D deficiency is correlated with poor outcomes in patients with luminal-type breast cancer. Ann Surg Oncol 18:1830–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kollenkirchen U, Fox J, Walters MR. 1991. Normocalcemia without hyperparathyroidism in vitamin D-deficient rats. J Bone Miner Res 6:273–278. [DOI] [PubMed] [Google Scholar]
- 40.Kovacs CS. 2012. The role of vitamin D in pregnancy and lactation: insights from animal models and clinical studies. Annu Rev Nutr 32:97–123. [DOI] [PubMed] [Google Scholar]
- 41.Lawson DE, Douglas J, Lean M, Sedrani S. 1986. Estimation of vitamin D3 and 25-hydroxyvitamin D3 in muscle and adipose tissue of rats and man. Clin Chim Acta 157:175–181. [DOI] [PubMed] [Google Scholar]
- 42.Leibel RL, Rosenbaum M, Hirsch J. 1995. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332:621–628. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Christakos S. 1991. Differential regulation by 1,25-dihydroxyvitamin D3 of calbindin-D9k and calbindin-D28k gene expression in mouse kidney. Endocrinology 128:2844–2852. [DOI] [PubMed] [Google Scholar]
- 44.Lips P. 2012. Interaction between vitamin D and calcium. Scand J Clin Lab Invest Suppl 243:60–64. [DOI] [PubMed] [Google Scholar]
- 45.Magnusson C, Lundberg M, Lee BK, Rai D, Karlsson H, Gardner R, Kosidou K, Arver S, Dalman C. 2016. Maternal vitamin D deficiency and the risk of autism spectrum disorders: population-based study. BJPsych Open 2:170–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthews DG, D'Angelo J, Drelich J, Welsh J. 2016. Adipose-specific Vdr deletion alters body fat and enhances mammary epithelial density. J Steroid Biochem Mol Biol 164:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer GP, Hurst JG. 1978. Sigmoidal relationship between parathyroid hormone secretion rate and plasma calcium concentration in calves. Endocrinology 102:1036–1042. [DOI] [PubMed] [Google Scholar]
- 48.Meems LM, Mahmud H, Buikema H, Tost J, Michel S, Takens J, Verkaik-Schakel RN, Vreeswijk-Baudoin I, Mateo-Leach IV, van der Harst P, Plosch T, de Boer RA. 2016. Parental vitamin D deficiency during pregnancy is associated with increased blood pressure in offspring via Panx1 hypermethylation. Am J Physiol Heart Circ Physiol 311:H1459–H1469. [DOI] [PubMed] [Google Scholar]
- 49.Munger KL, Aivo J, Hongell K, Soilu-Hanninen M, Surcel HM, Ascherio A. 2016. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the finnish maternity cohort. JAMA Neurol 73:515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Makitie O, Ramos-Abad L, Ward L, DiMeglio LA, Atapattu N, Cassinelli H, Braegger C, Pettifor JM, Seth A, Idris HW, Bhatia V, Fu J, Goldberg G, Savendahl L, Khadgawat R, Pludowski P, Maddock J, Hypponen E, Oduwole A, Frew E, Aguiar M, Tulchinsky T, Butler G, Hogler W. 2016. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab 101:394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Research Council.1995. Nutrient requirements of laboratory animals, 4th ed Washington (DC): The National Academies Press. [PubMed] [Google Scholar]
- 52.Nicholas C, Davis J, Fisher T, Segal T, Petti M, Sun Y, Wolfe A, Neal-Perry G. 2016. Maternal vitamin D deficiency programs reproductive dysfunction in female mice offspring through adverse effects on the neuroendocrine axis. Endocrinology 157:1535–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ong YL, Quah PL, Tint MT, Aris IM, Chen LW, van Dam RM, Heppe D, Saw SM, Godfrey KM, Gluckman PD, Chong YS, Yap F, Lee YS, Foong-Fong Chong M. 2016. The association of maternal vitamin D status with infant birth outcomes, postnatal growth and adiposity in the first 2 y of life in a multi-ethnic Asian population: the growing up in Singapore towards healthy outcomes (GUSTO) cohort study. Br J Nutr 116:621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ooi LL, Zheng Y, Zhou H, Trivedi T, Conigrave AD, Seibel MJ, Dunstan CR. 2010. Vitamin D deficiency promotes growth of MCF7 human breast cancer in a rodent model of osteosclerotic bone metastasis. Bone 47:795–803. [DOI] [PubMed] [Google Scholar]
- 55.Palacios C, Gonzalez L. 2014. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol 144PA:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parfitt AM. 1976. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part III of IV parts; PTH and osteoblasts, the relationship between bone turnover and bone loss, and the state of the bones in primary hyperparathyroidism. Metabolism 25:1033–1069. [DOI] [PubMed] [Google Scholar]
- 57.Ravussin Y, Gutman R, Diano S, Shanabrough M, Borok E, Sarman B, Lehmann A, LeDuc CA, Rosenbaum M, Horvath TL, Leibel RL. 2011. Effects of chronic weight perturbation on energy homeostasis and brain structure in mice. Am J Physiol Regul Integr Comp Physiol 300:R1352–R1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenstreich SJ, Rich C, Volwiler W. 1971. Deposition in and release of vitamin D3 from body fat: evidence for a storage site in the rat. J Clin Invest 50:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roskies M, Dolev Y, Caglar D, Hier MP, Mlynarek A, Majdan A, Payne RJ. 2012. Vitamin D deficiency as a potentially modifiable risk factor for thyroid cancer. J Otolaryngol Head Neck Surg 41:160–163. [PubMed] [Google Scholar]
- 60.Ross AC. 2011. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr 14:938–939. [DOI] [PubMed] [Google Scholar]
- 61.Rytter D, Bech BH, Halldorsson TI, Henriksen TB, Grandstrom C, Cohen A, Olsen SF. 2016. Maternal vitamin D status at week 30 of gestation and offspring cardio-metabolic health at 20 y: a prospective cohort study over 2 decades. PLoS One 11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryz NR, Lochner A, Bhullar K, Ma C, Huang T, Bhinder G, Bosman E, Wu X, Innis SM, Jacobson K, Vallance BA. 2015. Dietary vitamin D3 deficiency alters intestinal mucosal defense and increases susceptibility to citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol 309:G730–G742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schoenrock SA, Tarantino LM. 2016. Developmental vitamin D deficiency and schizophrenia: the role of animal models. Genes Brain Behav 15:45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma SS, Jangale NM, Harsulkar AM, Gokhale MK, Joshi BN. 2017. Chronic maternal calcium and 25-hydroxyvitamin D deficiency in Wistar rats programs abnormal hepatic gene expression leading to hepatic steatosis in female offspring. J Nutr Biochem 43:36–46. [DOI] [PubMed] [Google Scholar]
- 65.Shin MY, Kang YE, Kong SE, Ju SH, Back MK, Kim KS. 2015. A case of low bone mineral density with vitamin d deficiency due to prolonged lactation and severe malnutrition. J Bone Metab 22:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smolders J, Damoiseaux J, Menheere P, Hupperts R. 2008. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol 194:7–17. [DOI] [PubMed] [Google Scholar]
- 67.Snijder MB, Lips P, Seidell JC, Visser M, Deeg DJ, Dekker JM, van Dam RM. 2007. Vitamin D status and parathyroid hormone levels in relation to blood pressure: a population-based study in older men and women. J Intern Med 261:558–565. [DOI] [PubMed] [Google Scholar]
- 68.Taitz LS, De Lacy CD. 1962. Parathyroid function in vitamin D deficiency rickets. II. The relationship of parathyroid function to bone changes and incidence of tetany in vitamin D deficiency rickets in South African Bantu infants. Pediatrics 30:884–892. [PubMed] [Google Scholar]
- 69.van der Meijden K, Buskermolen J, van Essen HW, Schuurman T, Steegenga WT, Brouwer-Brolsma EM, Langenbach GEJ, van Ruijven LJ, den Heijer M, Lips P, Bravenboer N. 2016. Long-term vitamin D deficiency in older adult C57BL/6 mice does not affect bone structure, remodeling and mineralization. J Steroid Biochem Mol Biol 164:344–352. [DOI] [PubMed] [Google Scholar]
- 70.van Schoor NM, Lips P. 2011. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab 25:671–680. [DOI] [PubMed] [Google Scholar]
- 71.Vinkhuyzen AA, Eyles DW, Burne TH, Blanken LM, Kruithof CJ, Verhulst F, Jaddoe VW, Tiemeier H, McGrath JJ. 2016. Gestational vitamin D deficiency and autism-related traits: the Generation R Study. Mol Psychiatry [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xue J, Schoenrock SA, Valdar W, Tarantino LM, Ideraabdullah FY. 2016. Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations. Clin Epigenetics 8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yilmaz H, Kaya M, Sahin M, Delibasi T. 2012. Is vitamin D status a predictor glycaemic regulation and cardiac complication in type 2 diabetes mellitus patients? Diabetes Metab Syndr 6:28–31. [DOI] [PubMed] [Google Scholar]
- 74.Zhang ZL, Ding XF, Tong J, Li BY. 2011. Partial rescue of the phenotype in 1alpha-hydroxylase gene knockout mice by vitamin D3 injection. Endocr Res 36:101–108. [DOI] [PubMed] [Google Scholar]