Abstract
The inability to translate findings from studies performed in mouse models to the corresponding human condition is well known, especially those involving infectious, atherosclerotic, and other inflammatory diseases. We hypothesize that mice fail to a mount robust or adequate immune response to infectious agents because of physiologic effects of cold stress due to housing temperatures below the mouse thermoneutral zone (TNZ). This hypothesis was tested by comparing the immune response to the Francisella tularensis live vaccine strain in mice housed at a typical vivarium temperature, which is below the TNZ, with that of mice housed at a temperature near their TNZ. Mice maintained at 28 °C displayed elevated antigen-specific T-cell responses compared with mice housed at 22 °C and survived intranasal challenge that was fatal to immunized mice at 22 °C. These results demonstrate that cold stress due to housing below the mouse TNZ results in a blunted immune response and may compromise their translational value a models for infectious diseases and vaccine development.
Abbreviations: APC, antigen-presenting cells; Ft, Francisella tularensis subsp holarctica; LVS, live vaccine strain; TNZ, thermoneutral zone
The appropriate temperature for housing mice in animal research facilities has been the subject of numerous studies and reviews, but few studies have addressed the effect of ambient temperature on protective immunity in mice. Guidelines from the United States’ National Institutes of Health dictate that mice should be housed at 20 to 26 °C.15 However, to maintain a core temperature of approximately 37 °C, mice begin to produce additional body heat when the ambient temperature drops below approximately 30 °C. Studies on thermal physiology have established that the thermoneutral zone (TNZ) of various mouse strains is 30 to 32 °C, the temperature range of minimal metabolic energy expenditure.11,13 The discrepancy between standard vivarium housing temperatures and the mouse TNZ has largely been ignored by the research community and regulatory agencies but may have profound implications in the translation of mouse models to human disease.
Chronic cold stress results in production and recruitment of brown adipose tissue for adaptive thermogenesis, as a result of norepinephrine release from the sympathetic nervous system.4 This then may result in corticosteroid release from the adrenal cortex, which engages β-adrenergic receptors on many immune cells.26 Consequently, global suppression of the immune system may occur, particularly of proinflammatory cells involved in immune protection8 as well as the expansion of immunosuppressive M2 macrophages.25 Suppressed immune responses due to cold stress in mice housed below their lower critical temperature may account for the high mouse mortality in and failure of numerous mouse models of bacterial, viral, and protozoal infections.19
Several recent studies are consistent with this hypothesis. Mice inoculated intraperitoneally with Klebsiella pneumonia and housed at 34 °C displayed as much as a 105-fold lower bacterial load18 and a 400-fold lower pulmonary pathogen burden after intratracheal inoculation28 than control infected mice maintained at 23 °C. In a pulmonary influenza virus infection model, mice maintained at 30 °C avoided excessive production of proinflammatory cytokines in lung tissue compared with mice maintained at 22 °C or 26 °C.16
Although these results suggest that better immune protection occurs in the absence of cold stress, these studies were all short-term (3 to 7 d), so only innate immune responses would likely be engaged. In addition, with the exception of one study,18 survival did not improve at elevated ambient temperatures, possibly due to collateral inflammation-mediated damage from a robust immune response. In a recent study of immunity to solid tumors, mice maintained at 31 °C developed increased numbers of tumor-infiltrating CD8+ T cells, reduced tumor volume, and increased survival compared with mice housed at 23 °C,21 suggesting that adaptive immunity is improved when cold stress is avoided. Qualitatively similar findings were obtained in a graft-versus-host disease mouse model.23
It is hypothesized that mice commonly fail to mount protective immune responses to infectious agents because of the physiologic effects of cold stress brought on by housing temperatures below the mouse TNZ. Because mice housed well below their TNZ maintain an average core body temperature of 36 to 37.5 °C12,35 due to adaptive thermogenesis,4 the environmental temperatures of both the infectious agent and the mediators of protective immunity are essentially the same regardless of the ambient vivarium temperature. The current study compares the T- and B-cell responses to an attenuated strain of Francisella tularensis (Ft LVS) as well as survival to a potentially lethal dose of Ft LVS in mice maintained at typical vivarium temperatures compared with housing within the mouse TNZ.
Materials and Methods
Mouse husbandry.
Animal housing conditions and experimental design were approved by the IACUC of the University of New Mexico Health Sciences Center (Albuquerque, NM) in accordance with the NIH guidelines15 and AALAS principles in an AAALAC-accredited SPF facility on a 12:12-h light:dark cycle. Every 3 to 4 mo, the facility submits blood from sentinel mice maintained in soiled bedding as well as fecal pelt and cage swabs from research rodent cages for serology and pathogen genetic testing (IDEXX BioResearch, Columbia, MO), respectively. The PCR panels include Citrobacter rodentium, Pasteurella pneumotropica, fur mites (for example, Miobia, Radfordia), and pinworms (Aspicularis, Syphacia). The annual comprehensive serology panel from sentinels screens for antibody to approximately 16 viruses as well as to Mycoplasma pulmonis, Encephalitozoon cuniculi, and Clostridium piliforme. Female BALB/cAnNHsd mice (age, 4 to 5 wk) were obtained from Harlan Sprague–Dawley (now Envigo, Livermore, CA) and acclimated for 1 wk in an ABSL2 facility at approximately 22 °C. Mice were randomly assigned to cages maintained at the normal ambient temperature or to adjacent cages (Wessels induction/warming chambers, WW-CL, Braintree Scientific, Braintree, MA), in which a heating element and temperature probe is imbedded in the center of the cage acrylic floor and thermostatically controlled by using a VE-100 controller (Vivarium Electronics, High Point, NC), modified by the manufacturer for elevated temperatures. Cages were covered with HEPA-filtered microisolation lids in a static airflow environment. Bedding (Teklad TEK-Fresh, Envigo) at an approximate depth of 0.64 cm was changed weekly. Temperature and relative humidity were monitored and recorded continuously at 30-min intervals by using a TR300 sensor (Amprobe Test Tools) protected by a cage of 23-guage galvanized wire mesh with 0.6-cm2 openings that was placed on the cage floor such that the sensor element was elevated 1.3 cm above the bedding, at approximately mouse nose height. The temperature within the acrylic cage floor adjacent to the heating element was set approximately 20 °C higher than the measured air temperature in the cage. Food (Teklad Global Irradiated, Envigo) and water were provided without restriction, supported by the wire cage top. Conscious mice were weighed (PB602-S balance, Mettler Electronics, Toledo, OH) at regular intervals by using 10-s dynamic weighing method.
A preliminary ‘phase 1’ study was undertaken, in which groups of 10 mice were maintained for 18 wk in large cages (23 cm × 46 cm × 18 cm) at an ambient room temperature (mean ± 1 SD) of 21.3 ± 0.2 °C for the control group or in heated cages at 27.5 ± 0.2 °C or at 32.2 °C ± 0.8 °C. However, because the internal measured cage temperature of the 10 control mice cohoused in the unheated cage averaged 23.2 ± 0.2 °C (approximately 2 °C higher than the room air temperature) over the 18-wk period, the housing condition of the 10 control mice was changed to 5 cages of 2 mice per cage for the ‘phase 2’ study, to minimize ambient temperature elevation from body heat and group huddling behavior. These smaller cages (17 cm × 28 cm × 13 cm) provided a floor area of 238 cm2/mouse. In addition, because mice maintained at 32 °C showed similar features to those of mice at 28 °C, only 28 °C was used in the phase 2 study. In the phase 2 study, 12 mice (2 cages of 6 mice per cage) were maintained at 28 °C, providing a floor area of 176 cm2/mouse. Unless otherwise noted, the data reported in the Results section are those from the phase 2 study or from both studies when the 2 protocols can be considered replicate experiments.
Protocol design.
Mice were acclimated to their designate temperatures for 6 wk prior to immunization and individually identified by ear punch. Mice lightly anesthetized with isoflurane underwent intranasal immunization with approximately 200 cfu Ft LVS in 50 μL, a dose calculated from a serially diluted aliquot of stock Ft LVS stored at –70 °C that grew 3.6 × 109 cfu after thawing, as previously described.33 One cage of 4 unimmunized mice was used as controls. At 3 wk after inoculation, 0.2 mL of blood was collected by retroorbital bleeding from the medial canthus of isoflurane-anesthetized mice by using heparinized glass Natelson tubes, which was transferred into 10 U heparin (Sigma, St Louis, MO) and processed as described later. After another 4 wk (13 wk at test temperatures), mice were challenged intranasally with a suspension of 2.6 × 107 cfu Ft LVS in 50 μL PBS; this dose is greater than 104 times the dose of Ft LVS that is fatal to 100% of unimmunized Balb/c mice, for which the LD50 is 300 cfu.10 Bacterial concentration in the challenge suspension was confirmed by plating on cysteine heart agar containing rabbit blood and antibiotics (Remel, Fisher Scientific, Lenexa, KS). Mice were monitored daily for another 2 to 3 wk for morbidity, weight, and mortality. Because immunized mice challenged with this high-dose Ft LVS may recover from a moribund condition, surviving mice were euthanized by CO2 inhalation only after 4 wk of observation.
Isolation of peripheral WBC.
For logistical reasons associated with handling 26 to 28 mice, half of the mice in each group were bled 2 d later than their counterparts, to set up the cell-based assays. Blood was centrifuged at 800 × g for 10 min, and plasma was removed, recentrifuged, and stored frozen. Washed blood cells were suspended in 0.5 mL RPMI1640 medium (serum-free, HyClone, San Angelo, TX) and underlayed with 0.5 mL Lympholyte-M (Cedarlane, Burlington, NC), followed by centrifugation at 1048 × g for 20 min. WBC at the polysucrose–medium interface were removed, washed 3 times as described earlier, and suspended in 0.9 mL medium containing 10% fetal bovine serum (HyClone). Viable PBMC were enumerated using a hemocytometer after diluting 1:5 in PBS containing 0.1% trypan blue. A mean ± 1 SD of 2.2 ± 0.9 × 106 PBMC was obtained from 0.2 mL blood.
Immunoassays.
F. tularensis-specific T cells were determined by using ELISpot assays (Mabtech, Cincinnati, OH), in which response to specific antigen was enumerated by counting the number of IFNγ- and IL2-secreting T cells. The day before each assay, splenocytes for use as antigen-presenting cells (APC) were prepared as previously described30 from an untreated, typically housed Balb/c mouse, γ-irradiated at 30 Gy, and stored at 0 °C. The next day, these APC were pelleted and resuspended in complete medium, counted, and diluted to 3 × 106 viable cells/mL. A portion of the APC preparation was loaded with heat-killed (60 °C, 30 min) Ft LVS at 2 × 107 bacterial cells/mL for 1 h at 37 °C. For each WBC sample, 3 × 105 antigen-loaded APC were added to triplicate wells, and single wells containing control APC were included as the negative control and for monitoring the maximal response to concanavalin A (10 μg/mL; Boehringer Mannheim, Phoenixville, PA). An equal volume (0.1 mL) of each WBC preparation containing an average of 2.2 × 105 cells was added to each well except for the concanavalin A control, to which 2.2 × 104 cells were added; the final volume of all wells was brought to 0.2 mL by using medium. After overnight incubation in 10% CO2 at 37 °C, the IFNγ and IL2 plates were processed according to the manufacturer's instructions. The number of developed spots in each well was counted by using an automated EliSpot reader (Autoimmun Diagnostika, Strasburg, Germany).
Antibody to Ft LVS was assessed by ELISA, based on methodology previously described.1 Briefly, a bacterial pellet was washed twice in PBS followed by centrifugation at 16,000 × g for 20 min and suspended in PBS at 1.0 × 109 cells/mL. Bacteria were heat-killed as described earlier and stored in aliquots at –20 °C. Polystyrene microtiter plates (Immulon 2HB, Fisher ThermoScientific, Waltham, MA) were coated overnight with 0.2 mL of bacterial solution (5.0 × 107 bacteria/mL, in PBS) followed by postcoating with 1 mg/mL gelatin (JT Baker, Phillipsburg, NJ). Plates were gently agitated for 2 h with 0.2 mL mouse test sera (diluted 1:1500) followed by peroxidase-conjugated goat antimouse IgG (H+L; diluted 1:6000; Jackson ImmunoResearch, West Grove, PA) for 1.5 h. Color development after addition of ABTS (Boehringer Mannheim) and H2O2 was measured intermittently for a maximum of 1 h by optical absorbance at 405 μm, and results are reported as OD at 1 h, with samples producing greater than 3.0 OD calculated by extrapolation from earlier time points, as previously described.1 For measuring IgA antiFt LVS antibodies, mouse sera were diluted 1:200, and the secondary antibody was peroxidase-conjugated goat antimouse IgA (diluted 1:500; Southern Biotech, Birmingham, AL).
Plasma corticosterone was determined by using an ELISA kit (Arbor Assays, Ann Arbor, MI) according to the manufacturer's instructions. Plasma samples were pretreated with dissociating solution, and the serum was diluted 1:100 for the assay. Absorbance readings were converted to ng/mL by using the corticosterone standard curve developed in parallel.
Statistical analysis.
Two-tailed student's t tests were used to evaluate the statistical significance of data, in which values within a group were averaged. P values less than 0.05 were considered to indicate significant differences. Survival data were analyzed by using SPSS Statistics, version 23 (IBM SPSS, Armonk, NY), with log rank (Mantel–Cox) for pairwise comparisons and the Fisher exact test. Data are reported as means ± 1 SD in the text and figures.
Results
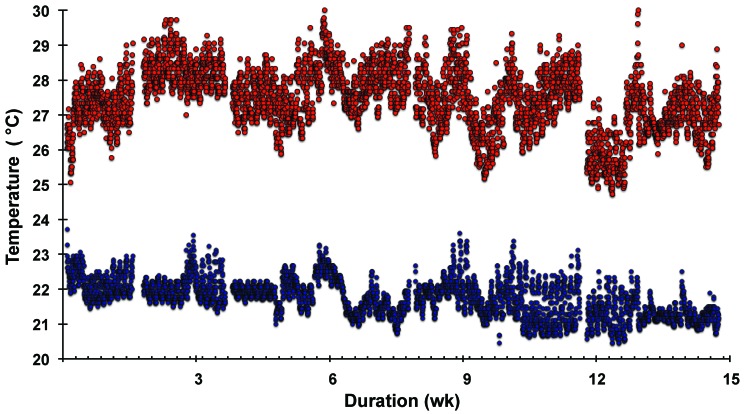
Figure 1 shows the air temperatures in the control and heated cages as measured just above the bedding floor and recorded every 30 min throughout the entire protocol period. Short interruptions in data acquisition are due to removal of the temperature–humidity recording device every few weeks to upload the accumulated data. Regular diurnal temperature fluctuations occurred, with a peak temperature at 0100 to 0400 and a nadir at approximately 1600, spanning approximately 1.9 °C for both the low- and elevated-temperature cages. This difference presumably reflects nocturnal animal activity, consistent with a similar percentage increase in relative humidity during the 0100 to 0400 period (data not shown). The average temperature throughout the entire 15-wk study was 28 °C in the 2 heated cages and was 22 °C in the monitored unheated cage.
Figure 1.
Ambient temperature in unheated (control) and heated mouse cages. The internal cage temperature was measured and recorded continuously every 30 min during the 15-wk exposure period. The 2 warmed cages each contained 6 mice (upper data), and the 5 cages at room temperature each had 2 mice (lower data). The temperature over the course of the study averaged over approximately 2-wk intervals was 27.5 ± 0.5 °C and 27.6 ± 0.6 °C (not shown) for the 2 heated cages; the average temperature was 21.8 ± 0.3 °C for the monitored unheated cage.
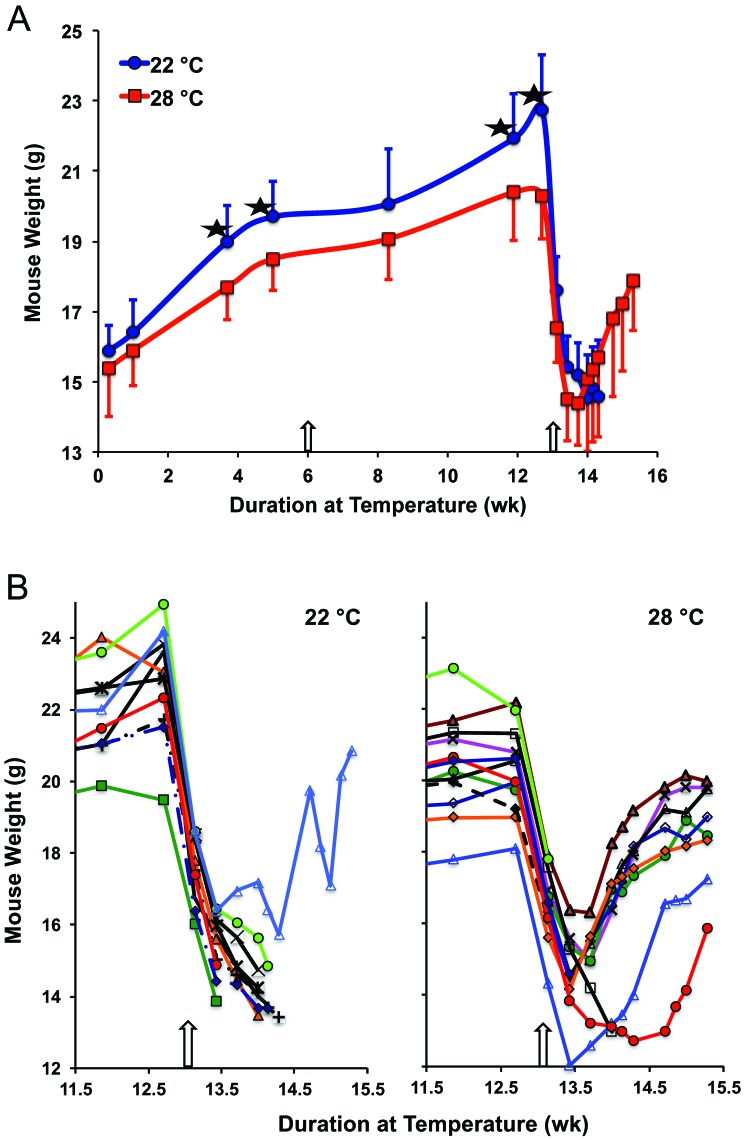
The weight of mice for the duration of the protocol is shown in Figure 2 A. After 4 wk, mice maintained at 28 °C had 6.8% (1.3 g) lower weight than mice at 22 °C (P < 0.006, 95% confidence interval, 0.39 to 2.21 g difference). The lower weight of the mice maintained at elevated temperatures reached a 10.9% difference by 12.7 wk, just prior to challenge with a potentially lethal dose of Ft LVS. In the phase 1 study, mice maintained at 28 °C for 6.4 wk also weighed less than mice at 23 °C (P < 0.04), although a significant difference in weight between these 2 groups was not sustained for the duration of the observation period (data not shown). However, starting at 6.4 wk in the phase 1 study, mice maintained at 32 °C were significantly lighter (0.9 to 1.6 g) than mice at 23 °C (average P value through 18 wk was P < 0.02; data not shown). After mice were challenged with Ft LVS at 12.9 wk, weight dropped precipitously by 2 d after bacteria exposure, with a nadir at 4 to 6 d after challenge for mice that survived (Figure 2 B).
Figure 2.
Effect of ambient temperature on mouse weight. (A) Body weight (mean + 1 SD [for 22 °C]; mean – 1 SD [for 28 °C]) of mice maintained at the 2 indicated temperatures. *, Significant (P < 0.02) difference between values at the indicated time point; the arrow at 6 wk depicts the time when mice were vaccinated with Ft LVS, and that at 12.9 wk shows when mice were challenged with a potentially lethal dose of Ft LVS. (B) Weights of individual mice just before challenge and for the subsequent 2 wk; curtailed data correspond to mice that died.
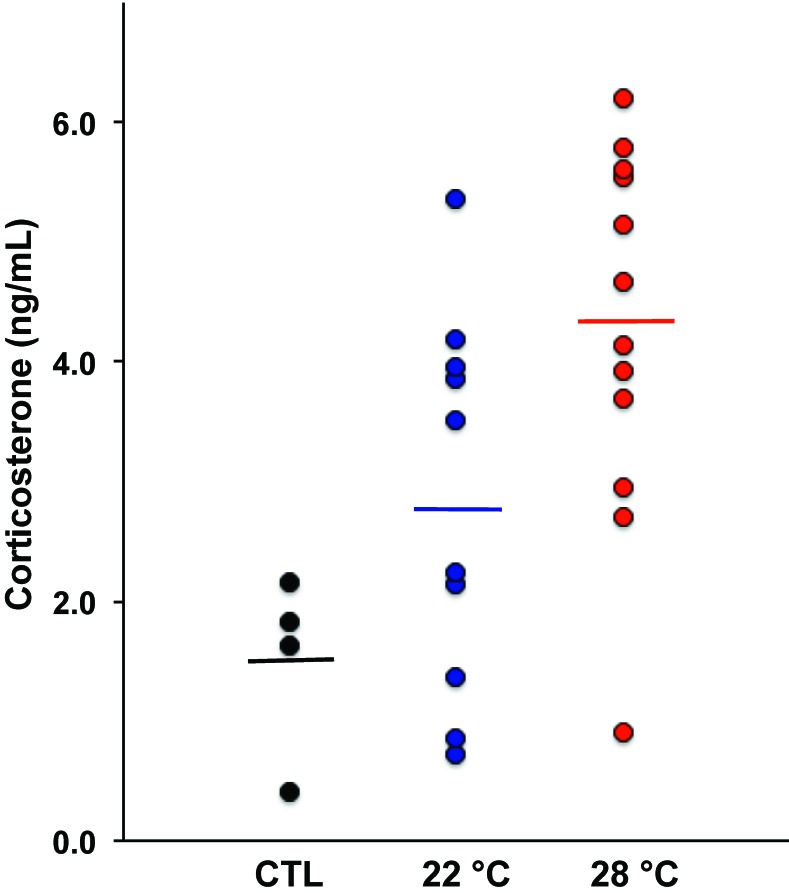
Because maintaining mice below their TNZ was expected to induce a metabolic stress response through activation of the hypothalamic–pituitary–adrenal axis, culminating in secretion of glucocorticoids, plasma corticosterone levels were measured after mice had spent 9 wk at the various temperatures (Figure 3). The average corticosterone level of immunized mice at 28 °C was 1.5-fold higher than for the immunized mice held at 22 °C (P < 0.04; 95% confidence interval, 0.1 to 2.8 ng/mL difference) and 2.8-fold higher than in unimmunized mice (at 22 °C; P < 0.004; 95% confidence interval, 1.0 to 4.5 ng/mL difference). The immunized mice at 22 °C had a 1.9-fold higher corticosterone level than the unimmunized mice held at the same temperature, but this difference did not achieve statistical significance. In the phase 1 study, corticosterone levels did not differ between any of the groups (data not shown), perhaps because the temperatures in the control and heated cages were not separated as widely.
Figure 3.
Plasma corticosterone levels. Each point represents an individual untreated control (CTL) mouse (at 22 °C) or mice in the immunization protocol maintained at the indicated temperature for 9 wk. Inoculated mice at 28 °C had higher corticosterone levels than inoculated mice at 22 °C (P < 0.04) and the CTL mice (P < 0.004). Lines indicate the mean plasma corticosterone concentration in each group.
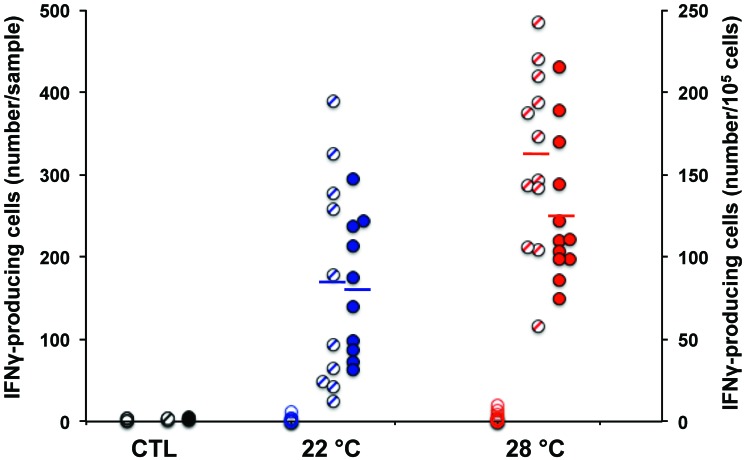
Tularensis-specific cellular and humoral immune responses were measured 3.4 wk after immunization with Ft LVS. Plasma was removed from heparinized blood and stored at -20 °C for subsequent measurement of specific antibodies. Lymphocytes were enriched from a fixed volume of blood by density step-gradient centrifugation and quantified by hemocytometry. Individual cell release of IFNγ during overnight incubation in the presence or absence of killed Ft LVS was measured by EliSpot assay. Approximately 0.1% of cells from immunized mice produced IFNγ in response to LVS in vitro (Figure 4). Mice maintained at 28 °C had 1.6-fold or 1.9-fold more circulating Ft-responsive T cells than mice at 22 °C as assessed by the number of cells per 105 lymphocytes (P < 0.021; 95% confidence interval, 8 to 84) or per volume blood (P < 0.008; 95% confidence interval, 44 to 258), respectively. The nonimmunized control mice had a mean of 1.7 ± 0.9 spot-forming cells per 105 total cells as compared with 104 ± 43 in the immunized mice. In the phase 1 study, mice at 28 °C had 2.8-fold more LVS-specific T cells than mice at 23 °C (P < 0.03). The number of IL2-secreting T cells in response to Ft was 116 ± 41 from mice at 28 °C compared with 75 ± 56 from mice at 22 °C (P < 0.062; data not shown), which, although below statistical significance, included a higher background of IL2-secreting cells (5 ± 2) in the absence of antigen.
Figure 4.
Ft-specific T cell enumeration. T cell response to LVS was measured in vitro at 3.4 wk after immunization of mice with Ft LVS. Values are expressed as the number of IFNγ-secreting spots per volume blood (hatched circles, left axis) or per 105 viable WBC recovered (filled circles, right axis). Open circles are IFNγ-secreting spots in the absence of Ft antigen addition in vitro. CTL mice were unimmunized. Lines indicate the mean number of responding T cells in each group. Mice maintained at 28 °C had more antigen-specific T cells than mice maintained at 22 °C (P < 0.02 per 105 cells and P < 0.008 per sample).
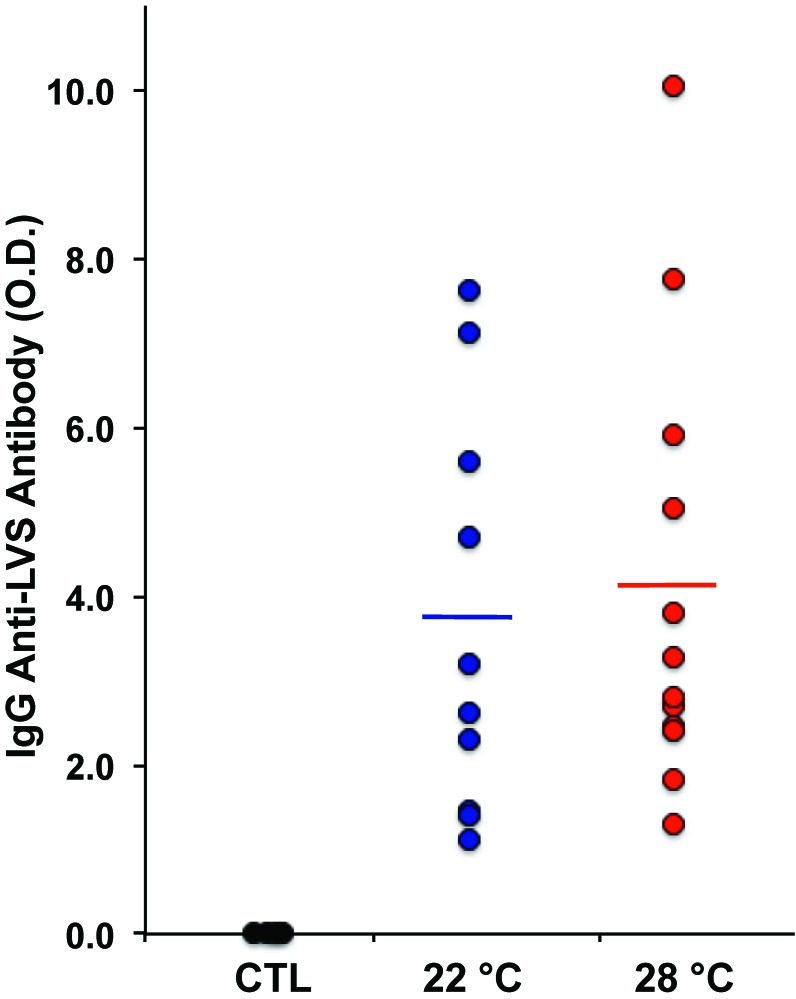
IgG antiLVS antibodies were highly elevated in Ft LVS-immunized mice but did not differ between mice maintained at the 2 temperatures (Figure 5). In the phase 1 protocol mice at 28 °C had a mean of 3.35 ± 1.12 OD450 antiFt compared with 1.80 ± 0.94 OD450 from mice at 23 °C (P < 0.004). In addition, IgA antiFt was detected in 200-fold diluted plasma only from immunized mice, but mean antibody levels did not differ between the mice maintained at the 2 temperatures (0.44 ± 0.48 OD450 compared with 0.39 ± 0.22 OD450, data not shown).
Figure 5.
IgG antibody production in response to F. tularensis LVS. AntiFt antibody activity was measured in 1500-fold diluted plasma collected 3.4 wk after immunization of mice with Ft LVS. Each point represents an individual untreated control (CTL) mouse (at 22 °C) or mice in the immunization protocol maintained at the indicated temperature for 9 wk. Lines show the mean antibody activity in each immunized group.
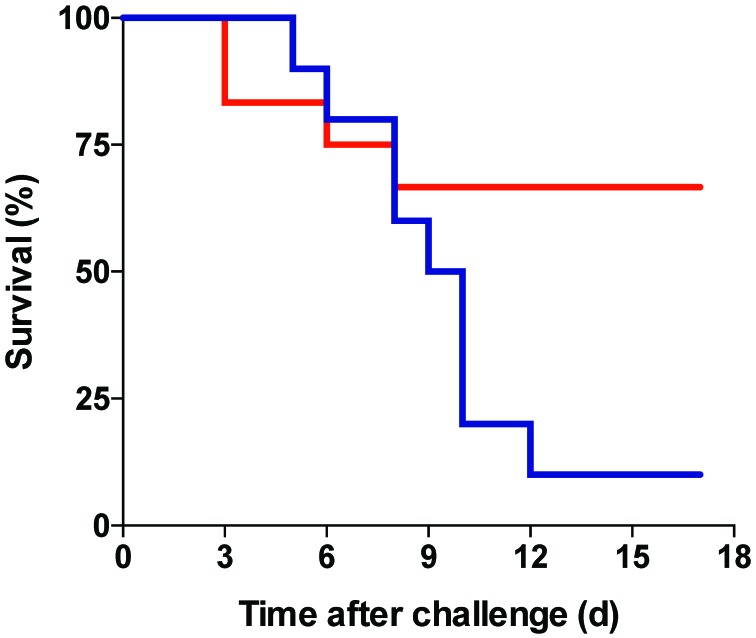
Antigen-naïve Balb/c mice survive intranasal challenge with 102 cfu Ft LVS but succumb within 7 d to intranasal inoculation with 103 cfu Ft LVS.10 In the current study, mice sensitized with 200 cfu Ft LVS were challenged 7 wk later with 2.6 × 107 cfu Ft LVS and monitored daily for morbidity and mortality. Two days after challenge, mice at 22 °C had a 23% weight loss compared with an 18% loss in mice at 28 °C. Mouse weight continued to drop, with maximal losses of 36% and 26%, respectively, 8 d after challenge, when surviving mice that had been housed at 28 °C weighed 15.1 ± 2.0 g compared with 14.5 ± 1.2 g for mice at 22 °C (Figure 2). The lower morbidity of mice at 28 °C was reflected in their prolonged survival (Figure 6). By 12 d after challenge, only 1 of the 10 mice maintained at 22 °C was alive, compared with 8 of the 12 mice at 28 °C (P < 0.041).
Figure 6.
Kaplan–Meier plot of survival of mice after high-dose challenge with Ft LVS. Mice maintained at 28 °C showed greater survival than mice maintained at 22 °C (P < 0.041 by log-rank (Mantel–Cox) 2 analysis and P < 0.012 by 2-tailed, Fisher exact test at termination).
Discussion
This study indicates that mice housed at temperatures below their TNZ had a muted T-cell response and compromised survival in a bacterial infection animal model. F. tularensis is a facultative intracellular pathogen, which is sterilely cleared through cellular immune mechanisms dependent on IFNγ,5 although antibody to Ft contributes substantially to immune protection.9,24 With decreased Ft-specific T cells, mice maintained at 22 °C presumably failed to mount adequate CD4+ T-cell help for macrophage-mediated killing or direct killing of Ft-infected cells by CD8+ T cells. In addition, mice housed at 28 °C gained less weight over a 13-wk period and lost less relative weight in response to a potentially lethal dose of Ft LVS, presumably reflecting the improved immune status and accelerated pathogen clearance of mice housed at a temperature within their TNZ.
In light of the almost 2-fold increase in Ft-specific T cells, it was surprising that neither IgG nor IgA antibody to Ft was consistently elevated in mice maintained at 28 °C, given isotype-switched antibody responses are strictly dependent on CD4+ T cells. Perhaps immunization of mice with Ft LVS at 22 °C elicited a T-cell response that was in excess with regard to help for antibody production, consistent with the remarkably high IgG antiFt LVS activity elicited after just a single immunization. Alternatively, B-cell activity might be relatively unaffected by corticosteroid-mediated immune suppression. Nevertheless, the greater mortality of mice from Ft LVS at 22 °C compared with at 28 °C suggests that, although specific antibodies may contribute to survival,22,24 even a modest 2-fold increase in Ft-specific circulating T cells at 28 °C is more important in this regard. This conclusion is consistent with the finding that depletion of all CD4+ or CD8+ T cells from LVS-vaccinated Balb/c mice abrogated protection from a lethal dose of Ft.33 An additional consideration is that interstitial T cells in the airway parenchema or particular T-cell subsets, such as Th17 cells,29,32 may be more relevant to protection from primary infection with Ft LVS and perhaps are more profoundly affected by subTNZ temperatures than are circulating T cells.
Compared with mice housed at 28 °C, mice maintained at 22 °C for 4 wk were significantly heavier, and they continued to gain more weight over the subsequent 9 wk. This result may seem counterintuitive to the expectation that lower ambient temperatures should result in higher metabolic rate2 and enhanced conversion by thermogenic brown fat of ingested calories to heat rather than to fat to compensate for heat loss to maintain core temperature at approximately 37 °C.3 However, a seminal study34 revealed that both male and female ICR mice were significantly heavier when kept at 22 °C compared with 28 °C, beginning from just a few weeks after birth and maintained continuously for 9 wk, and this difference correlated with significantly greater food intake. Other investigators had similar observations, in that C57BL/6 mice housed at 22 °C gained more weight than mice housed at 30 °C,31 and the intradermal fat of Balb/c mice at 23 °C was 5-fold thicker than that of mice maintained at 31 °C, a cold stress response dependent on the lipoprotein uptake receptor syndecan 1.23
The finding that the mean corticosterone level in mice at 28 °C was the same as or actually higher than that of mice at 22 °C was surprising, given that corticosterone production by the hypothalamus is enhanced in cold-stressed mice.2,19 However, because the glucocorticoid receptor is highly expressed in adipocytes,6 which are increased in cold-stressed mice,20 the steady-state plasma corticosterone might have been unaffected by chronic cold stress at 22 °C as a result of receptor-mediated uptake by adipocytes or nonspecific diffusion into adipose tissue due to its lipophilicity,26 while negatively regulated through inhibition of ACTH release from the pituitary.7
In the earlier study in this facility,33 Balb/c mice vaccinated intranasally with 200 cfu Ft LVS were protected against a respiratory challenge dose of 50,000 cfu LVS that is lethal in unvaccinated mice and against a 2000-cfu dose of Ft biovar A, an antigenically similar but more virulent strain of Ft; however, vaccinated mice succumbed to a 10-fold increased respiratory dose of Ft biovar A. Furthermore, the same vaccination protocol was not protective in C57BL/6 mice.5,33 Balb/c mice were unprotected from intranasal challenge with LVS, unless they were intradermally vaccinated with LVS twice over a 4-wk interval.17 Limited or no protective immunity after vaccination against other respiratory infectious agents has similarly been reported for Chlamydia spp. in C57BL/6 mice14 and for B. anthracis in DBA/2 mice.27 Taken together, these findings are consistent with the view that mice maintained at typical (that is, subTNZ) vivarium temperatures display poor protective immunity in bacterial infection models.
This study has several limitations. Cage temperatures that are increased by using floor-embedded heating elements are not uniform because the static airflow environment results in a gradient of decreasing temperature from the bottom to top of cage, although this pattern also occurs (albeit to a lesser extent) in nonheated cages due to the floor-level heat generated by group-housed mice. Environmental chambers or ventilated microisolation cages, in which delivered air is heated above typical ambient temperatures, would provide more uniform temperatures within the mouse TNZ. In addition, the effect of temperature on protective immunity may be dependent on the nature, amount, or frequency of the immunogen as well as on the sex- and strain-dependent sensitivity of mouse immune systems to suboptimal ambient temperature, as suggested previously.5,33 The current study was essentially a pilot study using a minimal number of animals in 2 protocols, thus leading to uncertainty regarding the reproducibility and magnitude of the observations. Furthermore, the huddling effect of multiple animals in a single cage (as noted in the present study) as well as the type of bedding may elevate the effective internal cage temperature. Finally, mice may gradually adapt to temperatures below their TNZ, as suggested by the increased weight gain in the current study; consequently, possible effects of housing temperature on immune function may be dependent on the duration of exposure to subTNZ temperatures. To test the possibility that insufficient acclimation periods further compromise immune responses, ongoing studies are addressing the B and T cell responses to immunogenic virus-like particles in mice acclimated to subTNZ temperature for only 2 wk.
Because of the mixed success of mice as an animal model for vaccine development, other animal species, particularly rats and rabbits, have been used in vaccine research despite their numerous disadvantages. Although several real or imagined explanations for the difficulties encountered in mouse models of infectious disease have been offered, much of the understanding of cellular and molecular immunology is based on observations and experiments with mouse systems. Discrepancies between in vitro and in vivo studies and between studies performed in different vivaria may be due to the failure to control cold stress. As shown in the current study, simply raising the ambient temperature to the mouse TNZ may substantially expand the successful application of mouse models of infectious disease and vaccine development and thus reduce the number of sentient experimental animals by improving the reproducibility of the experimental conditions. Performing studies at thermoneutrality may enhance the overall translational relevance of murine models to humans, for whom cold stress is generally not a factor.
Acknowledgments
I thank Terry H Wu and Andrew C Hahn (University of New Mexico) for assistance in the conception, design, and execution of the study. Modification of the Wessels warming chamber and reprogramming the V100 controller by Eric H Weyand (Animal Identification and Marking Systems, Hornell, NY) is appreciated. I thank Bryce Chackerian and Meilian Liu (University of New Mexico) for critical reading of the manuscript, as well as the Comparative Medicine journal reviewers for their constructive comments. This project was supported by a competitive grant from the Dedicated Health Research Funds from the School of Medicine, University of New Mexico.
References
- 1.Burlingame RW, Rubin RL. 1990. Subnucleosome structures as substrates in enzyme-linked immunosorbent assays. J Immunol Methods 134:187–199. [DOI] [PubMed] [Google Scholar]
- 2.Cannon B, Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359. [DOI] [PubMed] [Google Scholar]
- 3.Cannon B, Nedergaard J. 2010. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans). Int J Obes (Lond) 34 Suppl 1:S7–S16. [DOI] [PubMed] [Google Scholar]
- 4.Cannon B, Nedergaard J. 2011. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214:242–253. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Shen H, Webb A, KuoLee R, Conlan JW. 2003. Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally, or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine 21:3690–3700. [DOI] [PubMed] [Google Scholar]
- 6.de Kloet AD, Krause EG, Solomon MB, Flak JN, Scott KA, Kim DH, Myers B, Ulrich-Lai YM, Woods SC, Seeley RJ, Herman JP. 2015. Adipocyte glucocorticoid receptors mediate fat-to-brain signaling. Psychoneuroendocrinology 56:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. 1998. Brain corticosteroid receptor balance in health and disease. Endocr Rev 19:269–301. [DOI] [PubMed] [Google Scholar]
- 8.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. 2000. The sympathetic nerve—an integrative interface between 2 supersystems, the brain and the immune system. Pharmacol Rev 52:595–638. [PubMed] [Google Scholar]
- 9.Forestal CA, Malik M, Catlett SV, Savitt AG, Benach JL, Sellati TJ, Furie MB. 2007. Francisella tularensis has a significant extracellular phase in infected mice. J Infect Dis 196:134–137. [DOI] [PubMed] [Google Scholar]
- 10.Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun 59:2922–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon CJ. 1993. Temperature regulation in laboratory rodents. New York (NY): Cambridge University Press. [Google Scholar]
- 12.Gordon CJ. 2009. Quantifying the instability of core temperature in rodents. J Therm Biol 34:213–219. [Google Scholar]
- 13.Gordon CJ. 2012. Thermal physiology of laboratory mice: defining thermoneutrality. J Therm Biol 37:654–685. [Google Scholar]
- 14.Huang J, DeGraves FJ, Lenz SD, Gao D, Feng P, Li D, Schlapp T, Kaltenboeck B. 2002. The quantity of nitric oxide released by macrophages regulates Chlamydia-induced disease. Proc Natl Acad Sci USA 99:3914–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 16.Jhaveri KA, Trammell RA, Toth LA. 2007. Effect of environmental temperature on sleep, locomotor activity, core body temperature, and immune responses of C57BL/6J mice. Brain Behav Immun 21:975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia Q, Lee BY, Clemens DL, Bowen RA, Horwitz MA. 2009. Recombinant attenuated Listeria monocytogenes vaccine expressing Francisella tularensis IglC induces protection in mice against aerosolized type A F. tularensis. Vaccine 27:1216–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Q, Cross AS, Singh IS, Chen TT, Viscardi RM, Hasday JD. 2000. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun 68:1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karp CL. 2012. Unstressing intemperate models: how cold stress undermines mouse modeling. J Exp Med 209:1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasza I, Suh Y, Wollny D, Clark RJ, Roopra A, Colman RJ, MacDougald OA, Shedd TA, Nelson DW, Yen MI, Yen CL, Alexander CM. 2014. Syndecan 1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet 10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, Repasky EA. 2013. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA 110:20176–20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubelkova K, Krocova Z, Balonova L, Pejchal J, Stulik J, Macela A. 2012. Specific antibodies protect γ-irradiated mice against Francisella tularensis infection. Microb Pathog 53:259–268. [DOI] [PubMed] [Google Scholar]
- 23.Leigh ND, Kokolus KM, O'Neill RE, Du W, Eng JW, Qiu J, Chen GL, McCarthy PL, Farrar JD, Cao X, Repasky EA. 2015. Housing temperature-induced stress is suppressing murine graft-versus-host disease through β2-adrenergic receptor signaling. J Immunol 195:5045–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mara-Koosham G, Hutt JA, Lyons CR, Wu TH. 2011. Antibodies contribute to effective vaccination against respiratory infection by type A Francisella tularensis strains. Infect Immun 79:1770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. 2011. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padgett DA, Glaser R. 2003. How stress influences the immune response. Trends Immunol 24:444–448. [DOI] [PubMed] [Google Scholar]
- 27.Peachman KK, Rao M, Alving CR, Burge R, Leppla SH, Rao VB, Matyas GR. 2006. Correlation between lethal toxin-neutralizing antibody titers and protection from intranasal challenge with Bacillus anthracis Ames strain spores in mice after transcutaneous immunization with recombinant anthrax protective antigen. Infect Immun 74:794–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rice P, Martin E, He JR, Frank M, DeTolla L, Hester L, O'Neill T, Manka C, Benjamin I, Nagarsekar A, Singh I, Hasday JD. 2005. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J Immunol 174:3676–3685. [DOI] [PubMed] [Google Scholar]
- 29.Roberts LM, Davies JS, Sempowski GD, Frelinger JA. 2014. IFNγ, but not IL17A, is required for survival during secondary pulmonary Francisella tularensis live vaccine stain infection. Vaccine 32:3595–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin RL, Hermanson TM. 2005. Plasticity in the positive selection of T cells: affinity of the selecting antigen and IL7 affect T-cell responsiveness. Int Immunol 17:959–971. [DOI] [PubMed] [Google Scholar]
- 31.Tian XY, Ganeshan K, Hong C, Nguyen KD, Qiu Y, Kim J, Tangirala RK, Tontonoz P, Chawla A. 2016. Thermoneutral housing accelerates metabolic inflammation to potentiate atherosclerosis but not insulin resistance. Cell Metab 23:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woolard MD, Hensley LL, Kawula TH, Frelinger JA. 2008. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of γ interferon-positive T cells. Infect Immun 76:2651–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, Lyons CR. 2005. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun 73:2644–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamauchi C, Fujita S, Obara T, Ueda T. 1983. Effects of room temperature on reproduction, body and organ weights, food and water intakes, and hematology in mice. Jikken Dobutsu 32:1–11. [DOI] [PubMed] [Google Scholar]
- 35.Zhao ZJ, Chi QS, Cao J, Han YD. 2010. The energy budget, thermogenic capacity and behavior in Swiss mice exposed to a consecutive decrease in temperatures. J Exp Biol 213:3988–3997.21075940 [Google Scholar]