Abstract
The term Horner syndrome refers to the clinical presentation of oculosympathoparesis, comprising miosis, ptosis, and facial anhydrosis. To date, there are 2 reports of postoperative Horner syndrome in pigs. In this species the cervical sympathetic chain and cranial cervical sympathetic ganglion are consistently within the carotid artery sheath. This case study describes the sudden onset of Horner syndrome in 2 pigs, from a study cohort of 8, after the placement of a vascular graft between the carotid artery and external jugular vein. Anesthesia and surgery was uneventful in all the pigs in the study, but 2 pigs demonstrated clinical signs including ptosis, enophthalmos and prolapse of the nictitating membrane immediately after recovery from anesthesia. Horner syndrome was diagnosed in light of the clinical signs. These clinical signs persisted throughout the 2-mo study period and did not appear to improve or deteriorate in that time. Gross examination of the surgery site at the end of the study did not reveal an obvious lesion in the carotid artery sheath. The risk of Horner syndrome after surgery involving the carotid artery in pigs had not been reported prior to this study. Without specific measures to protect the cervical sympathetic ganglion during surgery, the incidence of postoperative Horner syndrome was 25% in our population of pigs. Although the welfare implications of this syndrome are minimal, concerted effort to avoid intraoperative damage to the cervical ganglion is essential for future work.
Although pigs are one of the major animal species used in translational research and surgical teaching,13,20,21 data are sparse regarding the proportion of pigs involved in research and teaching conducted under general anesthesia that recover from the procedure, and even fewer reports address the frequency of postoperative complications.6 In this context, reporting adverse or unexpected events, especially those that occur during the postoperative period, is essential to ensure that ongoing refinements to the management of anesthesia, surgery, and postoperative care can be made on a global scale.14
The term Horner syndrome refers to the clinical presentation of oculosympathoparesis, which causes miosis, ptosis, and facial anhydrosis.1,8 Johann Fredrich Horner, a Swiss ophthalmologist, first reported the syndrome in humans in 1869.1 The syndrome is also reported to occur in small laboratory animals;8 dogs and cats, often as a complication of surgery for middle ear disease;10 and in horses, sheep, cattle, and goats after surgical transection of the sympathetic innervation of the head.19 There are 2 reports of postoperative Horner syndrome in pigs.8,15
After placement of a woven aortic stent and aortic catheterization, an adult female pig developed clinical signs consistent with Horner syndrome 5 d after surgery.15 In that case, the pig remained in good health but was euthanized 4 wk after surgery, without any resolution of clinical signs. In a second case report, an 18-d-old female pig underwent surgery on the carotid artery sheath and developed clinical signs of Horner syndrome within 12 h.8 After 3 d, the pig was euthanized due to lack of change in clinical signs. A subsequent anatomic investigation in pigs revealed that the cervical sympathetic chain and cranial cervical sympathetic ganglion were consistently within the carotid sheath.8 This anatomic location is different from that in humans, where these structures are outside the carotid sheath.8 Consequently, these structures may be vulnerable to injury during surgery involving the carotid artery in pigs.
This case study describes the sudden onset of Horner syndrome in 2 pigs, from a study cohort of 8, after placement of a vascular graft between the carotid artery and external jugular vein.
Case Report
The surgical study was approved by the Animal Ethics Committee of the University of Western Australia, in accordance with the Code of Practice for the Care and Use of Animals for Scientific Purposes.2 The pigs (Sus scrofa) were female Large White × Duroc × Landrace pigs and had been sourced commercially as ‘bacon pigs’ (CM Farms, Nambeelup, Western Australia) and acclimated to the Large Animal Facility at the University of Western Australia for 2 wk prior to surgery. The pigs were housed in raised communal pens (4 × 5 meters), fed a maintenance diet (Pig Grower, West Feeds, Welshpool, Western Australia, Australia) with fresh pumpkin and apples, and allowed free access to tap water. Environmental enrichment was provided as music during the day, various toys for play, and daily human interaction. The room was maintained at 22 ± 2 °C and on a 12:12-h light:dark cycle. Prior to general anesthesia, food was withheld for 12 h, but free access to water was allowed. The pigs were clinically normal prior to surgery, when examined by a veterinarian. Eight pigs underwent surgery according to the approved protocol and by using standard anesthesia and surgical technique. The first (44 kg) and fifth (48 kg) pigs developed Horner syndrome that was apparent immediately after recovery from anesthesia.
Anesthesia was induced by using a combination of zolazepam and tiletamine (2 mg/kg of each drug; Zoletil 100, Virbac Australia, Milperra, New South Wales, Australia) and xylazine (2 mg/kg; Ilium Xylazil 100 mg/mL, Troy Laboratories Australia, Glendenning, New South Wales, Australia) by intramuscular injection in the neck. Approximately 10 min later, an auricular vein was catheterized for the delivery of propofol (1 to 2 mg/kg; Propofol Lipuro 1%, Braun, Melsungen, Germany) by intravenous injection to achieve a depth of anesthesia sufficient for endotracheal intubation with a cuffed endotracheal tube (internal diameter, 7 mm; Portex, Smiths Medical, Minneapolis, MN). General anesthesia was maintained with isoflurane (1 mL/mL, Attane Isoflurane, Bayer, Pymble, New South Wales, Australia) in oxygen delivered by a circle-breathing system.
Volume-cycled mechanical ventilation (Datex Ohmeda ADU, GE Healthcare, Uppsala, Sweden) was initiated immediately after tracheal intubation and adjusted to target normocapnia (end-tidal CO2, 35 to 45 mm Hg). The initial tidal volume was 9 to 10 mL/kg, and the respiratory rate was 10 breaths per minute. Positive end-expiration pressure was set at 5 cmH2O. Alterations to the ventilator setting were made to maintain normocapnia by changing the tidal volume, the respiratory rate, or both. Intraoperative monitoring included invasive arterial blood pressure from a catheter in an auricular artery connected to a pressure transducer, oxyhemoglobin saturation and pulse rate from a pulse oximeter placed on a pinna, capnography, pharyngeal temperature, and electrocardiography (Surgivet V9203, Smiths Medical, and Carescape B650, GE Healthcare). These parameters were measured continuously and recorded every 5 min on a paper anesthetic record. Hartmann solution (Compound Sodium Lactate, Fresenius Kabi Australia, Kurig-Gai, New South Wales, Australia) was administered at 10 mL/kg/h intravenously into an auricular vein during anesthesia. A circulating warm-air blanket was used for active warming (model CWS 4000, Cocoon Convective warming system, Care Essentials, North Geelong, Victoria, Australia).
The surgical procedures involved a longitudinal incision of the skin lateral to the ventral midline of the neck and the placement of vascular allografts. The vascular graft was used to create a shunt between the carotid artery and external jugular vein (Figures 1 and 2). A second surgical procedure was performed on each pig whereby an arterial vascular graft was placed to create a bypass in the femoral artery. A simple continuous suture pattern with polypropylene suture material (6-0 Prolene, Ethicon, Johnson and Johnson, Somerville, NJ) was used for vessel anastomoses. The surgery was always performed on the right side, and the pigs were in dorsal recumbency during anesthesia and surgery. To facilitate surgical access to the neck and groin, pancuronium (0.1 mg/kg; Pancuronium Injection BP 2 mg/mL, AstraZeneca, Macquarie Park, New South Wales, Australia) was administered intravenously as required (twice for pig 1; 4 times for pig 5), and neuromuscular blockade was monitored by using a train- of-four pattern of stimulation (Innervator 252, Fisher and Paykel Healthcare, East Tamaki, Auckland, New Zealand). Neostigmine (0.04 mg/kg; Neostigmine injection BP, 0.5 mg/mL, AstraZeneca Australia) and glycopyrrolate (0.01 mg/kg; Robinul, 0.2 mg/mL, Aspen Pharma, St Leonards, New South Wales, Australia) were administered intravenously to reverse neuromuscular blockade after surgery. Heparin was administered once, prior to clamping of the carotid artery (2000 IU IV, DBL Heparin Sodium Injection, 1000 IU/mL, Hospira Australia, Melbourne, Victoria, Australia).
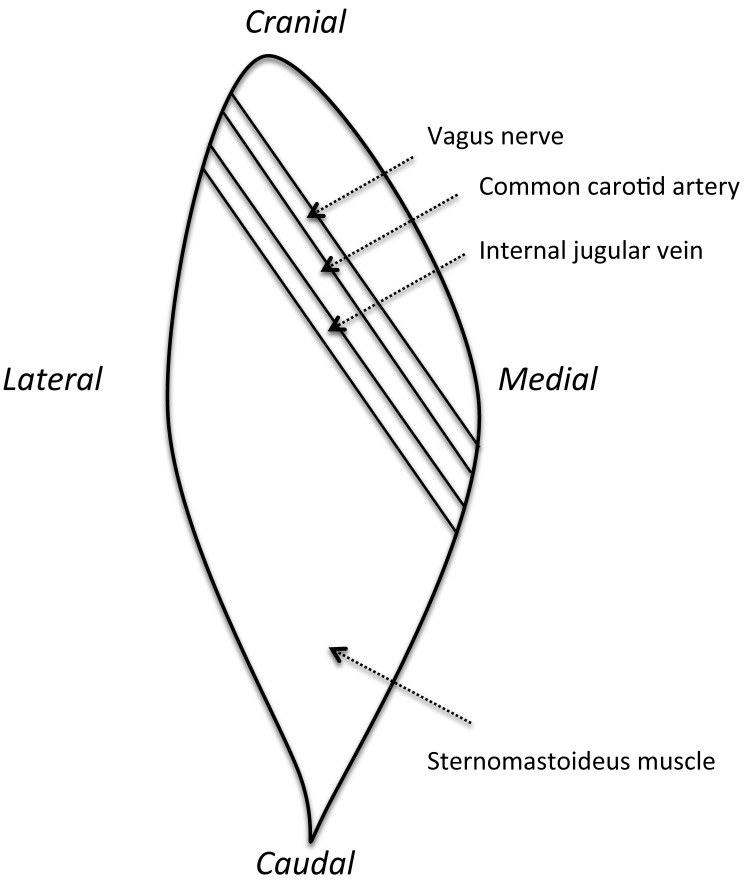
Figure 1.
Sketch of the surgery site in the right side of the neck, showing the locations of the carotid artery, internal jugular vein, vagus nerve, and sternomastoideus muscle.
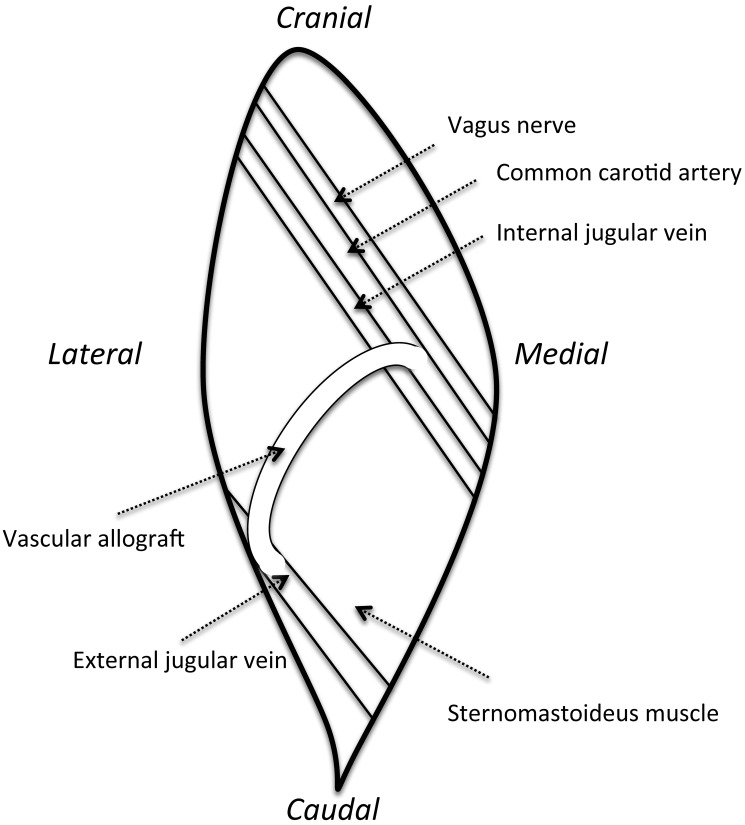
Figure 2.
Sketch of the surgery site in the right side of the neck, showing the location of the vascular allograft between the carotid artery and external jugular vein.
The anesthesia time for pig 1 was 6 h and 10 min and for pig 5 was 5 h and 40 min; the mean anesthesia time for all 8 pigs was 5 h. No physiologic abnormalities, such as hypotension, hypoxemia, bradycardia, tachycardia, hypercapnia, and cardiac arrhythmias occurred in any pig. The lowest body temperature in pig 1 was 35.7 °C and in pig 5 was 36.5 °C. The carotid artery was clamped for 30 min in pig 1 and for 50 min in pig 5; The mean duration of clamping of the artery among all 8 pigs was 40 min.
For analgesia, the pigs received morphine (0.1 mg/kg, Morphine Sulfate Injection BP 10 mg/mL, Hospira Australia) by intramuscular injection every 4 h during surgery, bupivacaine (2 mg/kg, Bupivacaine Injection BP 0.5%, Pfizer Australia, West Ryde, New South Wales, Australia) subcutaneously along the wound margins at the end of surgery, meloxicam (0.5 mg/kg, Ilium Meloxicam, 5 mg/mL, Troy Laboratories Australia) subcutaneously at the end of surgery, and tramadol (2 mg/kg, Tramal 100, CSL Biotherapies, Parkville, Victoria, Australia) by intramuscular injection at the end of surgery. Prophylactic antibiotics were administered once at the beginning of surgery (7 mg/kg SC; Betamox 150 mg Suspension, Norbrook Laboratories, Tullamarine, Victoria, Australia).
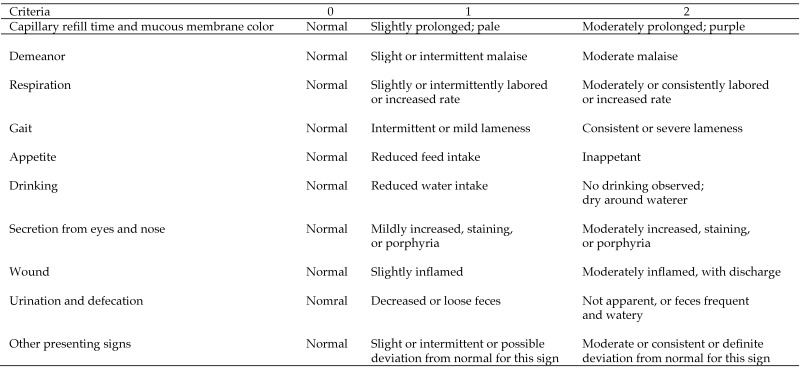
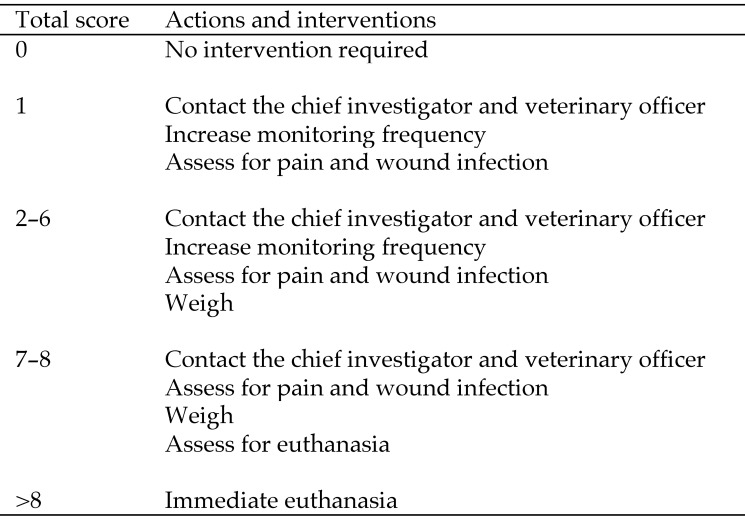
The pigs were observed continuously during the first 4 h after surgery. After that, they were observed at least twice daily for the first 3 d and then once daily for 2 mo. In the postoperative period, wellbeing was assessed by attributing a score to a range of clinical observations (Figure 3). The total score triggered an action according to the Animal Ethics Committee's approved interventions (Figure 4).
Figure 3.
Scoring system for postprocedural monitoring. Pigs were scored according to this scheme twice daily for the first 3 postoperative days and then once daily for the remainder of the study period (2 mo).
Figure 4.
Actions and interventions initiated according to the postprocedural monitoring score (Figure 3).
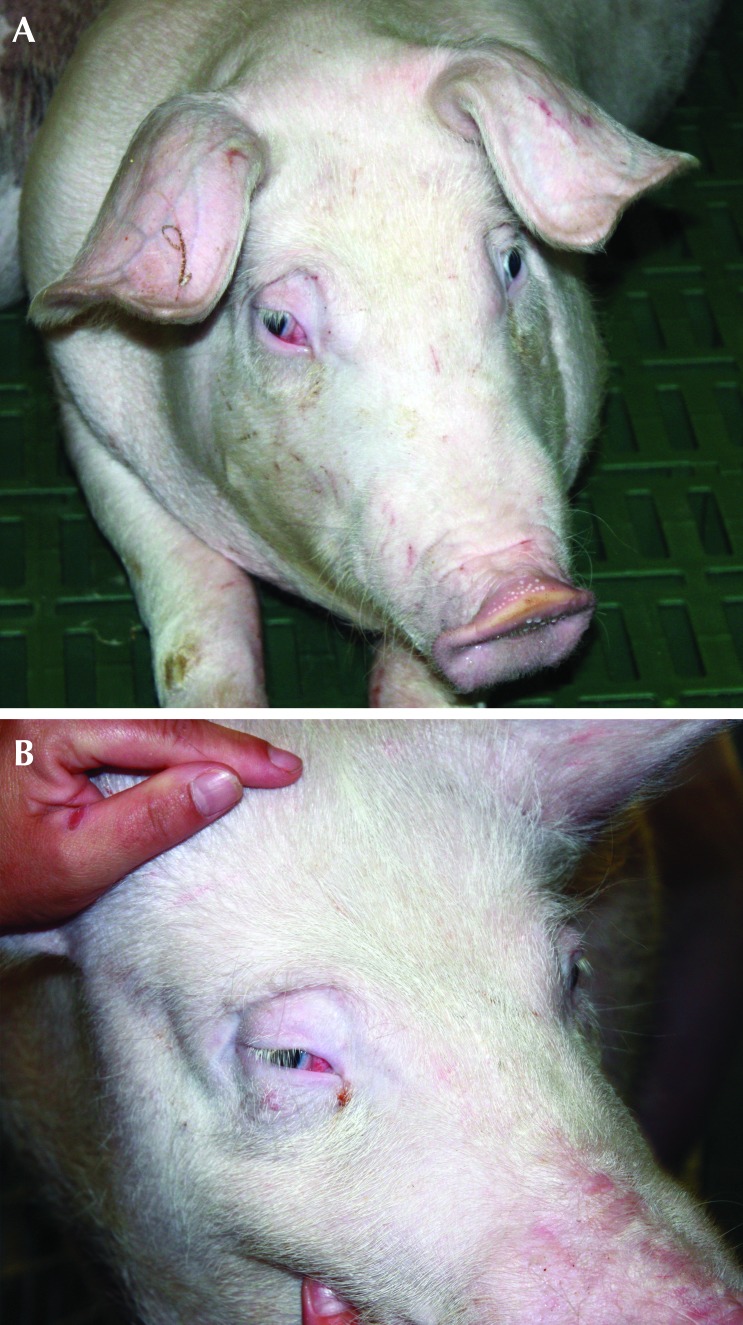
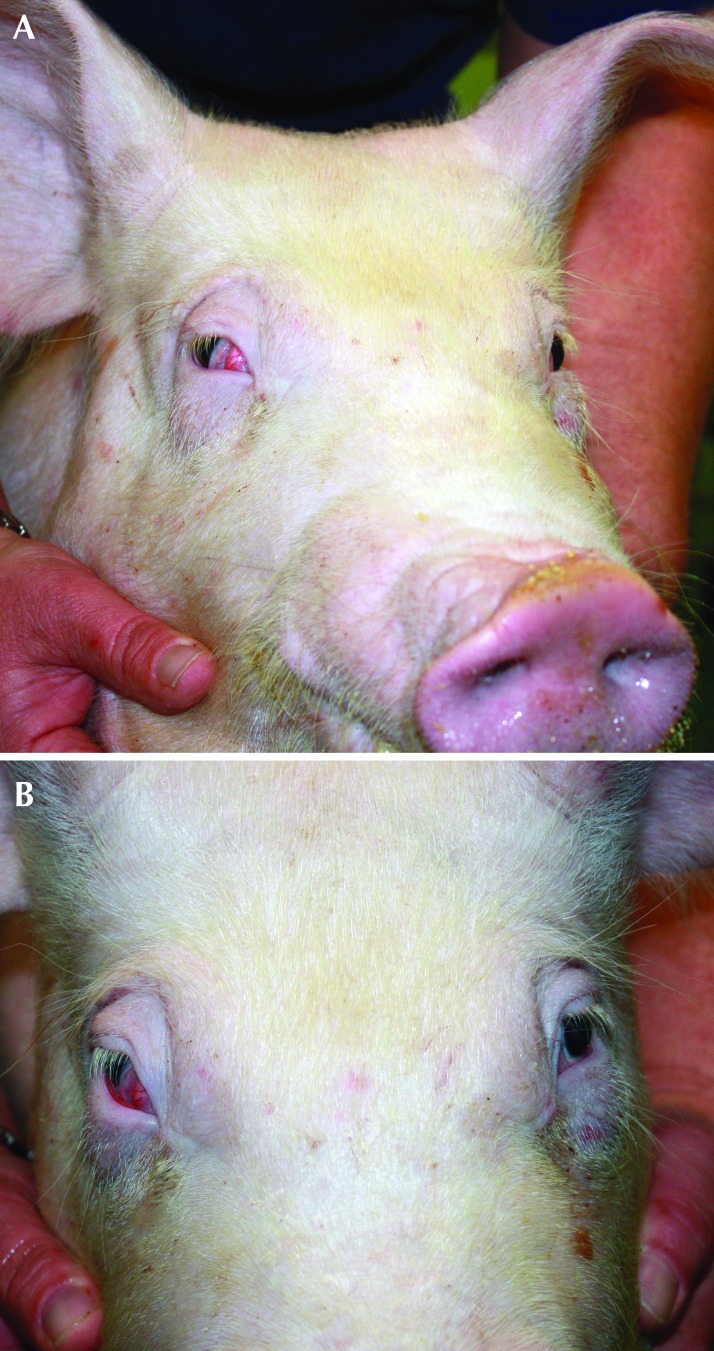
The immediate recovery from anesthesia and surgery was uneventful for all pigs, including the animals that developed Horner syndrome. The exception was that these pigs both developed clinical signs on the right side that were consistent with Horner syndrome (Figures 5 and 6). The clinical signs included ptosis, enophthalmos and prolapse of the nictitating membrane. Thorough ophthalmologic examination was not possible, but the diagnosis of Horner syndrome was made according to the clinical signs, absence of ocular discharge, and apparent comfort of the eye and surrounding structures. These clinical signs persisted throughout the entire 2-mo study period and did not appear to improve or deteriorate during that time.
Figure 5.
(A) Pig 1 the day after surgery and at 7 wk after surgery. (B) Ptosis and prolapse of the nictitating membrane of the right eye is apparent.
Figure 6.
(A) Pig number 5 at 7 wk after surgery. (B) Ptosis and prolapse of the nictitating membrane of the right eye is apparent.
The pigs were euthanized at the end of the study, as planned (2 mo after surgery). Pig 1 weighed 83 kg, and pig 5 weighed 88 kg. Prior to euthanasia, the pigs were anesthetized as previously described, and surgery was performed to harvest the graft sites. After the administration of zolazepam, tiletamine, and xylazine and the onset of heavy sedation, a more thorough examination of the eye was attempted. In both pigs, the pupil of the right eye was slightly smaller than the left eye, and the pupillary light response was intact. Topical phenylephrine (3 drops; Minims Phenylephrine Hydrochloride 10%, Chauvin Pharmaceuticals, London, England, United Kingdom) was applied to the right eye. Fifteen minutes later the pupils were examined again, but the pupil size appeared unchanged.
During the surgery to harvest tissues, the site of anastomosis of the vascular graft and the carotid artery was examined closely by using Doppler ultrasonography and then visually after surgical exposure. Neither gross lesions nor intraluminal abnormalities were present to explain the apparent interruption of the oculosympathetic pathway. All pigs had fibrous adhesions around the vascular graft site. This fibrous tissue did not differ macroscopically between the unaffected and affected pigs. Pigs were euthanized by using intravenous pentobarbitone (160 mg/kg; Lethabarb, 325 mg/mL, Virbac, Australia).
Discussion
Here we report that 2 of 8 (25%) pigs undergoing surgery involving the carotid artery developed ipsilateral Horner syndrome immediately after surgery. In the 2 mo after surgery, there was no evidence of resolution of the clinical signs.
Horner syndrome describes the condition where there is interference to the sympathetic nerve supply to the head, resulting in a characteristic set of clinical signs. The sympathetic nerve travels from the brain through the spinal cord to the level of the T1 or T3 thoracic vertebra. Here the nerve leaves the spine, courses through the brachial plexus, and up through the neck to the cervical ganglion and the eye. Horner syndrome is characterized as being first-, second-, or third-order, depending upon the location of the lesion. First-order Horner syndrome arises from lesions between the brain and T1 or T3 vertebra; second-order lesions are located between the T1 or T3 vertebra and the cervical ganglion, and third-order abnormalities arise from lesions between the cervical ganglion and the eye.7
The diagnosis of Horner syndrome is based on clinical signs. Given the difficulty of examining the eyes of conscious pigs, we were unable to quantify the size of the pupils. Nevertheless, despite these limitations, and given the recent history of surgery involving the carotid artery, the diagnosis of Horner syndrome stands. In humans this diagnosis is made with consideration of a combination of the history, clinical examination findings, and pharmacologic testing to localize the interruption to the oculosympathetic pathway.11 In pigs the diagnosis has also been made after review of the history and clinical signs.8,15 A single report describes ptosis, enophthalmos, and prolapse of the nictitating membrane in an adult pig.15 Miosis was not apparent, but variation in the severity of these individual abnormalities is acknowledged.15 In our current case, the clinical signs were ptosis, enophthalmos, and prolapse of the nictitating membrane. Miosis was difficult to evaluate, in part because achieving physical restraint of the pig for close examination was difficult, and ptosis and prolapse of the nictitating membrane obscured a view of the globe. Thorough ocular examination in a darkened room was infeasible, given the automated light:dark cycle in the room in which the pigs were housed. Finally, evaluation of the eye after sedation is hampered by the side effects of the sedative drugs. For example, ketamine, and presumably tiletamine, may cause mydriasis.9
Pharmacologic isolation of the lesion by using topical phenylephrine has been described in dogs: 10% phenylephrine is applied topically to both eyes, and the time until dilation of the pupils is recorded.3 The pupil of a normal eye and one in first-order Horner syndrome dilate in 60 to 90 min. For a second-order lesion, the pupil dilates in 45 min, and in a third-order case, the pupil dilates in 20 min. The increased sensitivity to phenylephrine in second- and third-order cases is thought to be due to denervation hypersensitivity.3
A lesion caused by surgery of the carotid artery would be expected to cause second-order Horner syndrome. Pharmacologic testing was attempted during anesthesia of the pigs at the end of the study (prior to tissue harvest and euthanasia) but was unrewarding. However, given the clinical history and the pathognomonic symptoms, the lack of response to phenylephrine was not deemed to rule out Horner syndrome. In these pigs, miosis was difficult to evaluate and thus the time for pupillary dilation was not reliably observed. In addition, perhaps 15 min was insufficient time for a change to occur.18
The clinical signs of Horner syndrome vary between species. In humans, the classic description includes ptosis, miosis, and anhydrosis.11 In animals, however, ptosis and prolapse of the nictitating membrane are the most consistent signs.10,18,19 This variation in presentation also occurs between animals species and has been demonstrated after surgical transection of the sympathetic nervous innervation of the head in horses, cows, sheep, and goats.19 The site of transection was preganglionic in all 4 species and ganglionic–postganglionic in 2 additional horses. In horses, the most prominent clinical sign was unilateral sweating over the face and proximal neck, particularly at the base of the ear. In cows, distension of the vasculature and cutaneous heat of the pinna along with reduced sweat production over the nostril on the affected side were present. And lastly, in goats and sheep, slight ptosis of the upper eyelid on the affected side was apparent.19 The authors concluded that the clinical presentation of Horner syndrome was not only varied, but also subtle.19 The pigs in the current case report presented similarly to previous reports in this species, and identifying the abnormality was relatively easy once the animals had recovered from anesthesia.
Given that the clinical signs did not affect the wellbeing of the pigs in this study, and because there was no evidence of discomfort, it was not considered justified to sedate or anesthetize the pigs for the sole purpose of further investigating this postoperative complication. In addition, sedation or anesthesia is likely to have confounded any such examination. The pigs did not experience any other complications associated with the surgical vascular grafts and completed the study as planned.
The prognosis for recovery from Horner syndrome depends on the mechanism of injury and ranges from months to years in humans.11,12 In dogs with idiopathic Horner syndrome, cases resolve in a median time of 15 wk.18 The present case study demonstrated no spontaneous recovery in a 2-mo period, which is the longest reported duration of clinical signs in pigs.
In pigs, the cervical sympathetic chain lies within the carotid sheath and ascends between the cervical vagus nerve and the internal jugular vein. These cranial cervical sympathetic ganglia lie on the medial and ventral surfaces of the internal carotid artery, close to the bifurcation of the common carotid artery and at the level of the first to second cervical vertebrae.8 Awareness of this neuroanatomy of pigs may help surgeons to identify and preserve important structures during surgery in the neck region of pigs. Although the lesions that caused the clinical signs in the 2 pigs in the current study could not be identified at the end of the study, it likely is easier to recognize the ganglia prior to surgery than at 2 mo after the placement of a graft, when new tissue incorporates the graft into the local area. The fibrous tissue at the site of the vessel graft was similar is gross appearance in all of the pigs. Histopathologic examination of this tissue is unlikely to elucidate a specific cause of the clinical signs as the fibrous adhesions were extensive in all animals. One group described a firm mass consisting of a thick-walled cyst with a small strand of suture material within caseous material and attributed this lesion to the clinical signs of Horner syndrome in a single pig.15 Lesions of this appearance were not observed in the current study. In addition, pressure injury from the retractors used during the procedure and subsequent tissue scaring during healing may have contributed to the development of Horner syndrome in these 2 pigs.
One of the challenges in this study, and a major limitation of this report, was the ability to perform a close and thorough examination of the eye. Although training pigs for a range of husbandry and clinical procedures, such as weighing and blood sampling, is increasingly common, and rewarding,16 our pigs were not trained to allow close examination of the eye. This particular procedure wasn't expected to be necessary, but the value of training pigs for basic handling by personnel cannot be underestimated. Positive reinforcement training methods are a refinement in animal handling that can improve animal welfare, animal husbandry, and veterinary care.16 The pigs in the current study were acclimated to the facility and personnel for 2 wk prior to surgery, but, in retrospect, more targeted training would have been valuable. Nevertheless, the pigs were relaxed and comfortable walking into the transport trolley (with weighing scales) and for basic clinical examinations, including the measurement of rectal temperature by using a digital thermometer. This training was essential, given that the end-of-experiment weight of the pigs exceeded 80 kg. A growing body of literature reviews the perioperative management of pigs in a biomedical research environment, but to date, little information in these reviews, or the literature in general, is available regarding training strategies and outcomes in pigs in a laboratory setting.3-6,13
The risk of Horner syndrome after surgery involving the carotid artery in pigs was unknown to us prior to initiating this study. Without specific measures to protect the cervical sympathetic ganglion during surgery, the incidence of Horner syndrome among our 8 pigs was 25%. Although the welfare implications of this syndrome are minimal, concerted effort to avoid damage to ganglion during surgery is essential for future work.
Acknowledgments
We thank the technical staff at the University of Western Australia for the expert care and husbandry of the pigs.
References
- 1.Abbas A, Manjila S, Singh M, Belle V, Chandar K, Miller JP. 2015. Johann Friedrich Horner and the repeated discovery of oculosympathoparesis: whose syndrome is it? Neurosurgery 77:486–491. [DOI] [PubMed] [Google Scholar]
- 2.Bistner S, Rubin L, Cox TA, Condon WE. 1970. Pharmacologic diagnosis of Horner syndrome in the dog. J Am Vet Med Assoc 157:1220–1224. [PubMed] [Google Scholar]
- 3.Bradbury AG, Argyle S, Eddleston M, Clutton RE. 2015. Prophylactic use of antimicrobials in surgical pig models; a literature review (2012 to 2014). Vet Rec 177:16–21. [DOI] [PubMed] [Google Scholar]
- 4.Bradbury AG, Clutton RE. 2016. Are neuromuscular blocking agents being misused in laboratory pigs? Br J Anaesth 116:476–485. [DOI] [PubMed] [Google Scholar]
- 5.Bradbury AG, Clutton RE. 2016. Review of practices reported for preoperative food and water restriction of laboratory pigs (Sus scrofa). J Am Assoc Lab Anim Sci 55:35–40. [PMC free article] [PubMed] [Google Scholar]
- 6.Bradbury AG, Eddleston M, Clutton RE. 2016. Pain management in pigs undergoing experimental surgery; a literature review (2012 to 2014). Br J Anaesth 116:37–45. [DOI] [PubMed] [Google Scholar]
- 7.deLahunta A, Glass E. 2009. Lower motor neuron: general visceral efferent system. p 173–180. In: Veterinary neuroanatomy and clinical neurology, 3rd ed. New York (NY): Elsevier. [Google Scholar]
- 8.Ding P, Tufano RP, Campbell-Malone R, Feng W, Kim SJ, German RZ. 2011. Horner syndrome after carotid sheath surgery in a pig: anatomic study of cervical sympathetic chain. Comp Med 61:453–456. [PMC free article] [PubMed] [Google Scholar]
- 9.Dugdale A. 2010. Considerations for ocular surgery, p 323. In: Dugdale A. Veterinary anaesthesia principles to practice. West Sussex (United Kingdom): Wiley–Blackwell. [Google Scholar]
- 10.Garosi LS, Lowrie ML, Swinbourne NF. 2012. Neurological manifestations of ear disease in dogs and cats. Vet Clin North Am Small Anim Pract 42:1143–1160. [DOI] [PubMed] [Google Scholar]
- 11.Giannaccare G, Gizzi C, Fresina M. 2016. Horner syndrome following thyroid surgery: the clinical and pharmacological presentations. J Ophthalmic Vis Res 11:442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Aguado R, Morales-Angulo C, Obeso-Aguera S, Longarela-Herrero Y, Garcia-Zornoza R, Acle Cervera L. 2012. Horner's syndrome after neck surgery. Acta Otorrinolaringol Esp 63:299–302. [DOI] [PubMed] [Google Scholar]
- 13.Ison SH, Clutton RE, Di Giminiani P, Rutherford KMD. 2016. A review of pain assessment in pigs. Front Vet Sci 3:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2012. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage 20:256–260. [DOI] [PubMed] [Google Scholar]
- 15.Lembo TM, Wright KC, Cromeens DM, Price RE. 2001. Iatrogenic Horner's syndrome in an experimental pig. Contemp Top Lab Anim Sci 40:33–35. [PubMed] [Google Scholar]
- 16.National Centre for the replacement refinement and reduction of animals in research. [Internet]. 2017. Training animals. [Cited 11 July 2017]. Available at: https://www.nc3rs.org.uk/training-animals - Animal%20welfare. [PMC free article] [PubMed]
- 17.National Health and Medical Research Council. [Internet]. 2013. Australian code of practice for the care and use of animals for scientific purposes 8th ed. [Cited 11 July 2017]. Available at: https://www.nhmrc.gov.au/guidelines-publications/ea28.
- 18.Simpson KM, Williams DL, Cherubini GB. 2013. Neuropharmacological lesion localization in idiopathic Horner's syndrome in Golden Retrievers and dogs of other breeds. Vet Ophthalmol 18:1–5. [DOI] [PubMed] [Google Scholar]
- 19.Smith JS, Mayhew IG. 1977. Horner's syndrome in large animals. Cornell Vet 67:529–542. [PubMed] [Google Scholar]
- 20.Swindle MM, Makin A, Herron AJ, Clubb FJ, Jr, Frazier KS. 2012. Swine as models in biomedical research and toxicology testing. Vet Pathol 49:344–356. [DOI] [PubMed] [Google Scholar]
- 21.Swindle MM, Smith AC. 2015. Swine in the laboratory: surgery, anesthesia, imaging and experimental techniques, 3rd ed. Boca Raton (FL): CRC Press. [Google Scholar]