Abstract
A 10-y-old cranially implanted rhesus macaque (Macaca mulatta) involved in visual research was presented for dull mentation and weight loss. Physical examination revealed alopecia and poor body conditioning, and bloodwork revealed marked hypercortisolemia (23 μg/dL). Differential diagnoses for hypercortisolemia, weight loss, and alopecia included Cushing and pseudo-Cushing syndromes. To further evaluate hypercortisolemia, we compared the urine cortisol:creatinine ratio (UCCR) at baseline and after low-dose dexamethasone suppression (LDDS) testing in the presenting animal and healthy naïve and implanted working monkeys. At baseline, UCCR was 10 times higher in the presenting macaque (118.1 ± 7.1) than in naïve animals (12.5 ± 12.8) and 3 times higher than in healthy implanted working macaques (44.4 ± 6.9); however, levels were suppressed similarly by dexamethasone in both the presenting animal and healthy controls. In addition, healthy implanted working macaques had significantly higher baseline UCCR levels than naïve controls, suggesting chronic stress in working animals. Abdominal ultrasonography and radiographs of the presenting animal revealed marked bilateral adrenal mineralization but no overt adrenal tumor or hyperplasia. Overall, these results excluded endogenous Cushing syndrome and prompted us to evaluate different causes of pseudo-Cushing syndrome, including depression. Using videorecordings to evaluate behavior, we used published criteria for macaque models of depression models, including huddling, to make a presumptive diagnosis of depression. The macaque was treated with fluoxetine (2 mg/kg PO daily), provided increased environmental enrichment, and followed over time by regular UCCR assessment and videorecordings. The animal improved clinically and behaviorally, and UCCR returned to levels observed in working implanted macaques (44.4) after 8 wk of treatment. This case highlights the potential effect of research-related work on stress and pathologic behaviors in macaques and demonstrates the utility of UCCR and LDDS for screening behavioral and hypothalamic–pituitary–adrenal abnormalities in these animals.
Abbreviations: UCCR, urine cortisol:creatinine ratio; LDDS, low-dose dexamethasone suppression; HPA, hypothalamic–pituitary–adrenal; SSRI, selective serotonin-reuptake inhibitor
Macaques are the most commonly used NHP models in neuroscience. These models are invaluable in understanding how the brain generates complex cognitive behaviors, such as memory and visual perception.20 Essential methodologic advantages of these models are the ability to train macaques to perform specific and complex tasks for rewards and that their behavioral performance can be correlated by using single-neuron recording.20 Data from several hundred neurons can be recorded over several months to years in a single animal, thus allowing detailed analysis of a brain region from very few subjects. To achieve adequate motivation and performance on behavioral tasks, macaques typically undergo progressive training and various levels of water or food restriction. The scientific validity of these behavioral experiments strongly relies on high animal welfare and normal behavior throughout their entire research career.
Major depression is one of the most common mental disorders in the United States.21 Current research suggests that depression is caused by a combination of genetic, biologic, environmental, and psychologic factors.1 Development of the condition can particularly be influenced by early childhood experiences, individual personality, major life changes, trauma, anxiety, chronic or disabling medical conditions and chronic stress.1 Diagnosing the human disorder is complex and is based on persistent alterations in mood and the concomitant presence of 5 of 9 symptoms, including depressed mood, anhedonia, weight loss or gain, changes in sleep patterns, observable agitation or retardation, fatigue, feelings of worthlessness, decreased concentration, and recurrent thoughts of death, during a 2-wk period.1
Depressive-like behavior has been well described in rhesus macaques (Macaca mulatta) and is characterized by aberrancies in behavior and physiology that closely mimic the human disease.2,3,25,33 As such, macaque models present several of the 9 depressive-like behaviors observed in humans, including anhedonia, weight change, change in activity levels, psychomotor agitation and retardation, and fatigue.3 Moreover, these symptoms arise within a typical social context. The development of depressive-like behaviors in macaques appears to be related to a number of factors including rearing conditions, isolation from social groups, social competition, and the psychosocial stress associated with these events.23,32 The spontaneous prevalence of depressive-like behaviors in outdoor socially grouped rhesus macaques is 0.5%, climbs to 7.2% for indoor housed macaques, and further increases to 18.9% in individually housed indoor macaques,13 thus suggesting the potential negative influence of laboratory housing on animal wellbeing. Furthermore, no studies have evaluated the effect of intense research-related activities, such as those described in neuroscience, on chronic stress and the development of depressive-like behaviors in these models. In the context of a predisposing genetic and psychologic background, enrollment in such studies, as well as recurring water or food restrictions, might arguably constitute a major life change, be experienced as stressful, trigger anxiety, and eventually lead to depressive-like behaviors in predisposed macaques.
Here, we present the first recorded case of spontaneous depressive-like behaviors in a 10-y-old male rhesus macaque with a cephalic implant that was working in neurovisual science. The animal presented with obtundation, moderate bilateral alopecia, emaciation, slumped posture, and decreased research-related task performance. The animal was diagnosed with depressive-like behaviors in light of findings regarding hypercortisolemia, behavioral indices, and response to antidepressant treatment.
Case Report
All macaques in this report were maintained with adherence to recommendations of the Guide14 in an AAALAC-accredited institution. Comprehensive physical examination, including CBC, serum biochemistry, and urine and fecal analysis were performed semiannually. All primates were seronegative for SIV, simian retrovirus type D, and simian T-cell leukemia virus and were free of tuberculosis. Macaques were housed indoors under standard husbandry conditions in quad-unit stainless steel caging (70 × 32 × 78.5 in., Lab Products or Allentown) and had weekly access to custom, large (5.8 × 6.25 × 8 ft; total floor space, 45.8 ft2) enrichment units. Animals were provided daily with various toys (for example, chains, kongs, rattles, swings, hammocks, challenger balls), foraging items (for example, dried bean mix, rice, oatmeal, parrot mix, cereals, sunflower seeds, and dried fruits) on boards, in destructible items, or in puzzles as well as fresh fruits and vegetables. Fruits and vegetables were not provided on days of fluid restriction. All animals were enrolled in an IACUC-approved protocol that assessed the neurocircuitry involved in visual pathways and that required single housing (IACUC-approved exemption). Eight were research-naïve and 4 were working under fluid restriction and surgically implanted with a head-fixation post and cephalic recording chamber. All fluid restrictions were performed in accordance with Salk Institute IACUC policies. While on restriction, each macaque received 10 to 20 mL of fluids per kilogram body weight daily and was not fluid-restricted for more than 5 d each week. Fluid rewards were given for successful completion of visual-based tasks, such as the acquisition of a target on a video screen during recording sessions with the animal. Even though the macaques typically learned to meet their entire daily fluid requirement during a working session, several precautions were taken to avoid the possibility of acute or chronic dehydration or clinical disease due to fluid restriction. The laboratory and animal care staff monitored the animal's health daily and maintained accurate records on total daily food and fluid consumption (including treats in the laboratory) as well as on body weight and urine specific gravity. A full physical examination (including CBC, biochemistry, and urine analysis) was performed by the attending veterinarian prior to enrollment in the study. Clearance for continued participation was renewed at each semiannual physical examination. Sick animals or those on treatment were prohibited from being enrolled in fluid-restriction studies. Wound margins and wells of implanted macaques were maintained regularly by the laboratory.
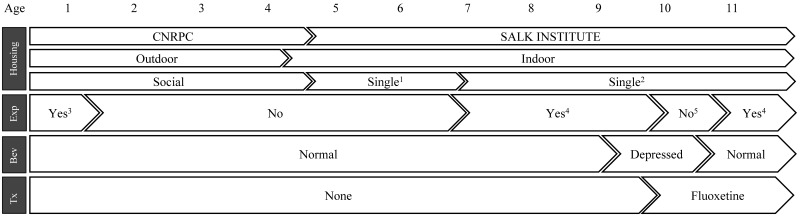
An adult male Indian-origin rhesus macaque (M. mulatta; age, 10 y; weight, 10.1 kg) initially was reported for changes in behavior and body weight loss. The sick animal belonged to a colony of 12 captive-born macaques that originated from the California National Primate Research Center (University of California, Davis, CA). Historically, the presenting animal was part of the CNPRC outdoor breeding colony (Figure 1). During infancy, the animal was injected twice (at 3 and 4 mo of age) with dexamethasone for an acute behavioral study and experienced 1 episode of diarrhea that was successfully treated. The macaque experienced additional episodes of diarrhea and 5 traumatic injuries to the fingers and tail during his last year at UC Davis. All clinical events were treated successfully. The macaque was brought to indoor housing at UC Davis (at 2.5 mo prior to acquisition by the Salk Institute) and paired with a female. The animal was then imported as a single monkey to the Salk Institute (age, 4 y 7 mo; Figure 1). The macaque remained in the general colony for a period of 2 y during which attempts for pairing with existing adult males (no females available) were unsuccessful. He was then enrolled in an experimental protocol requiring single housing (IACUC-approved exemption) and implanted at 7 y old with a chamber. He worked 2.5 y without showing any abnormal behavior, including hair pulling, stereotypical, or self-injurious behaviors prior to the presenting signs. The animal had experienced waxing and waning weight fluctuations in the months after surgery that periodically necessitated removal from the study, but the animal's weight (12.5 kg) and body condition score5 (2.5 to 3 on a scale of 5) rapidly stabilized. In the months preceding presentation, the research team reported a decrease in focus and completion of tasks but could not provide objective data to support their impressions.
Figure 1.
Sequential lifetime events in the presenting animal. Bev, behavior; Exp, involved in research experiments; Tx, treatment.
At the time of the initial report, a cageside examination prior to sedation and physical examination revealed dull mentation characterized by disinterest and apparent apathy to his surroundings. The animal was motivated to rise and ambulate normally, had a normal appetite and fluid intake, normal bowel and urinary voiding, and no other obvious neurologic signs. The animal was sedated with ketamine (10 mg/kg IM) for a full physical examination, CBC analysis, serum biochemistry, urine analysis, and fecal flotation and smear. On physical examination, the macaque had poor body conditioning5 (1.5/5) and marked bilateral symmetric alopecia of the antebrachium and lumbosacral region with no pruritus or evidence of inflammation. Inhouse abdominal ultrasonography was performed and revealed a tortuous left renal artery and loss of detail in the region of the left adrenal, and the right adrenal was not readily identified. Circulating cortisol, ACTH, and free T4 measurements were included in the initial serum biochemistry to assess possible endocrinopathies that could explain the symmetric alopecia (hyperadrenocorticism) and weight loss (hyperthyroidism). The animal recovered from sedation uneventfully and was subsequently removed from study for further diagnostic workup. Blood work, urine analysis, and fecal evaluations were unremarkable, with the exception of elevated resting cortisol levels (23.0 µg/dL, reference range 15.9 ± 2.6 µg /dL).26 ACTH and free T4 concentrations were normal.
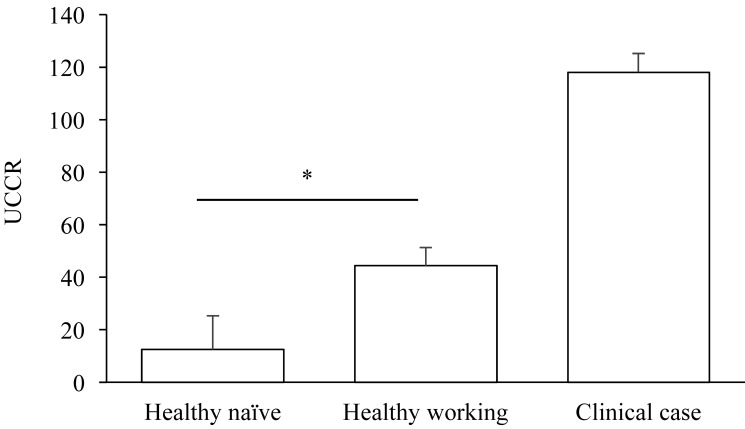
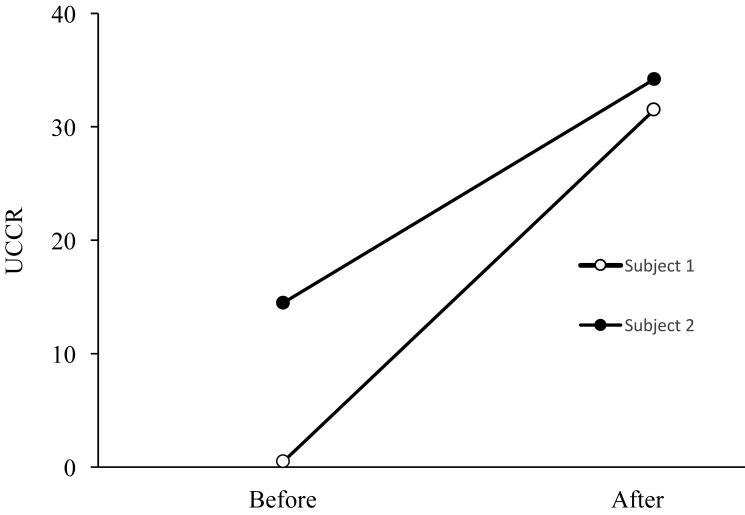
Serial urinary cortisol:creatinine ratios (UCCR) were determined for the patient and 11 healthy control (males; age, 13.1 ± 3.9 y ; weight, 13.4 ±2.2 kg) including 3 working implanted macaques (12.9 ± 3.8 y; 13.1 ± 1.9 kg) and 8 nonworking (13.7 ± 4.6 y, 14.2 ± 3.4 kg) naïve animals to determine the source of hypercortisolemia. UCCR is commonly used to assess hyperadrenocorticism in human and canine populations29 and accurately reflects steroid levels integrated over periods of hours.22 As importantly, collection is relatively noninvasive and integrated results are less likely to be affected by the stress of short-term handling.22 Urine was collected from clean empty urine pans at the same times in the morning to minimize variations due to circadian rhythm. All samples were clear and free of particulate (fecal, feed, or bedding) contamination. The urine specific gravity was not ascertained at the time. UCCR was determined by assaying creatinine concentration (model A480, Beckman Coulter, Brea, CA) and cortisol concentration (Immulite 2000, Siemens Healthineers Global, Erlangen, Germany). At baseline, UCCR was 10 times higher in the patient (118.1 ± 7.1) as compared with naïve macaques (12.5 ± 12.8) and 3 times higher than in healthy implanted working macaques (44.4 ± 6.9), suggesting that the patient's hypercortisolemia was unrelated to the stress of collection (Figure 2). We also noted that healthy implanted working macaques had significantly (Student t test, P < 0.01, JMP software, SAS Institute, Cary, NC) higher baseline UCCR levels as compared with naïve colony members, suggesting that implantation or research activities (or both) expose these animals to increased levels of stress. To further assess this hypothesis, we evaluated UCCR in 2 naïve healthy macaques, 3 mo prior and 3 mo after enrollment in research activities and surgical implantation. Both animals were housed singly before and after surgery. As we expected, UCCR markedly increased once both animals were enrolled in research (Figure 3).
Figure 2.
Urine cortisol:creatinine ratios (UCCR) in healthy naïve nonworking macaques (n = 8), healthy implanted working macaques (n = 3), and the clinical case (n = 1). Samples were collected between 1000 and 1300 on the same day of the week to minimize the influence of circadian rhythm. Collection was facilitated by placement of clean cage pans, and samples were evaluated only when they were free of particulate matter. *, Significant (P < 0.05) difference between values.
Figure 3.
Urine cortisol:creatinine ratios (UCCR) in 2 naïve healthy macaques, 3 mo before and 3 mo after enrollment in research activities and surgical implantation. Samples were collected between 1000 and 1300 on the same day of the week to minimize the influence of circadian rhythm. Collection was facilitated by placement of clean cage pans, and samples were evaluated only when they were free of particulate matter.
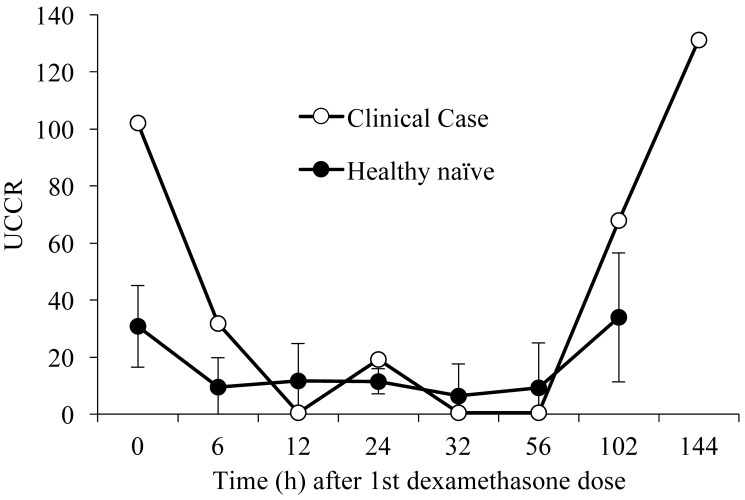
At the time, the top differential diagnoses for the hypercortisolemia, alopecia, weight loss, and abnormal mentation in our index macaque were hyperadrenocorticism and pseudo-Cushing syndrome. Low-dose dexamethasone suppression (LDDS) testing using UCCR as the dependent variable was performed on the patient to rule out hyperadrenocorticism and 3 healthy naïve controls for comparison. Baseline UCCR were determined prior to the administration of 0.5 mg/kg dexamethasone tablets (Dexium, Bimeda US, Oakbrook Terrace, IL) given by mouth at the morning, midday, and evening of the first day.9,10 UCCR were determined at 6, 12, 24, 32, 56, 102, and 144 h after the first dexamethasone dose (Figure 4). LDDS testing revealed similar patterns of suppression in both healthy controls and the patient, with UCCR suppressed by 12 h followed by a plateau and then a release from suppression at 102 h for the healthy controls and 144 h for the sick animal. Moreover, during the time when cortisol was suppressed (between 6 and 56 h), none of cortisol values in the sick animal exceeded 50% of the basal values, an important criterion when interpreting LDDS testing in canines.8 Although neither LDDS nor UCCR should be used as a sole criterion to exclude hyperadrenocorticism in human patients,9 these results combined with the overall clinical presentation of our macaque as well as the CBC and serum biochemistry results further supported our working assumption that this patient did not have hyperadrenocorticism.
Figure 4.
Low-dose dexamethasone suppression (LDDS) test in health naïve controls (n = 3) and the clinical case (n = 1). The LDDS test was performed by administration of dexamethasone (0.5 mg/kg) given orally 3 times daily for 1 d, followed by collection of urine at 0, 6, 12, 24, 32, 56, 102, and 144 h after the first dose of dexamethasone. Dexamethasone suppressed the cortisol concentration initially, a period of suppression followed, and subsequently cortisol levels in both the controls and ill macaque were released from suppression.
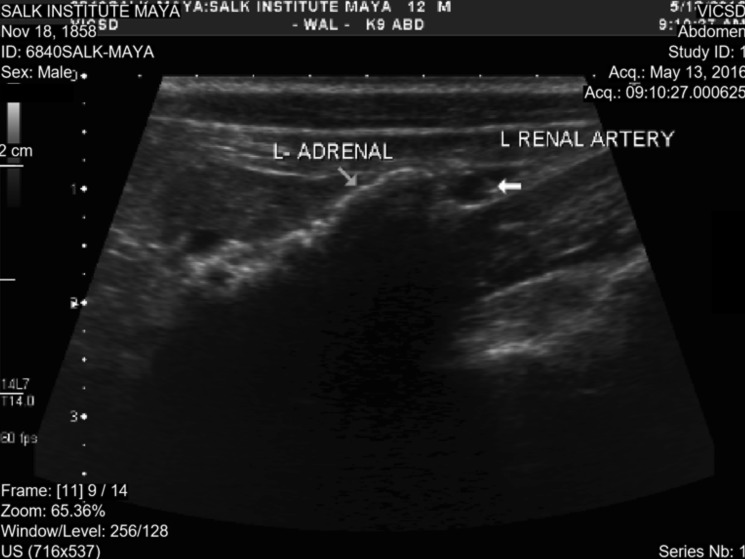
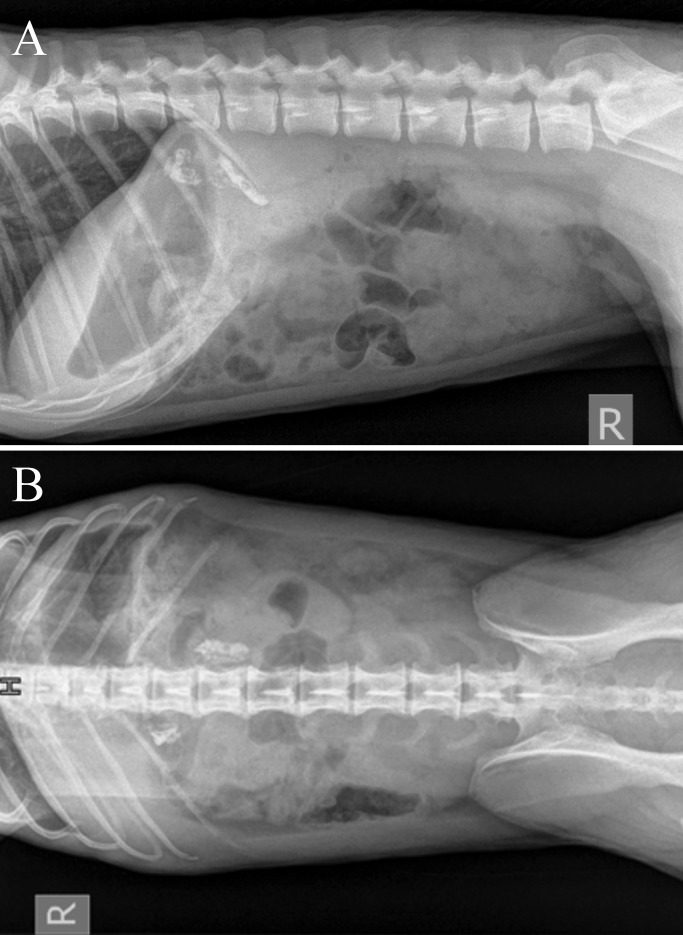
The macaque subsequently was evaluated sonographically (Figure 5) and radiographically (Figure 6) by a board-certified veterinary radiologist to further rule out adrenal hyperplasia or tumors. Ultrasonography revealed a hyperechoic texture with an associated shadowing artifact in the region of both adrenal glands, which presentation is consistent with mineralization of the adrenals. This interpretation was verified by radiographs, which revealed severe bilateral mineralization of the adrenals and which is a common nonpathologic finding in research animals.19,30 Neither imaging modality yielded findings consistent with tumor presence or adrenal hyperplasia, thus providing further evidence to rule out hyperadrenocorticism.
Figure 5.
Ultrasound evaluation of the left adrenal gland, revealing hyperechoic material with an associated shadowing artifact consistent with mineralization of the adrenal gland. Similar findings were seen with the right adrenal (not shown). No evidence of tumor or adrenal hyperplasia was observed.
Figure 6.
(A) Right lateral and (B) dorsoventral radiographs of the ill macaque reveal severe, bilateral, mineral opacities in the region of the adrenals.
Pseudo-Cushing syndrome became the primary diagnosis in the absence of HPA dysfunction. The differential for pseudo-Cushing syndrome in veterinary medicine is limited to alopecia X, an alopecia with associated hyperpigmentation occurring in dogs.8 Considering the closer phylogenetic relation of NHP and humans, we decided to rely on the diagnostic algorithm of human pseudo-Cushing to further investigate our case. Several conditions leading to pseudo-Cushing syndrome have been described in the human literature, including severe obesity, malnutrition, polycystic ovarian syndrome, chronic alcoholism, uncontrolled diabetes, extreme physical stress, and different psychiatric disorders including depression.1 Obesity and malnutrition were ruled out in light of the stabilization of weight and body condition score (2.5/5) and normal food intake during the course of the work-up despite persistence of alopecia, dull mentation, hypercortisolemia, and elevated UCCR. Also excluded were polycystic ovarian syndrome (male animal), alcoholism, uncontrolled diabetes (inconsistent with bloodwork and clinical signs) as well as alopecia X (condition never documented in macaques and absence of typical clinical signs). The remaining cause of pseudo-Cushing disease was depressive disorders.
Videorecording has been used to characterize and diagnose depressive-like behaviors in macaques.11,13 Using this methodology and a published ethogram,13 we obtained 5-min videorecordings (3 each day for 2 wk) of the patient and healthy implanted and nonimplanted single-housed animals when humans were not present in the room, to confirm the diagnosis. We used the time and frequency spent in a hunched posture (sitting with head at the same level as or lower than the shoulders; arms and limbs huddled to the center of the body; no movement of the body or the 4 limbs; eyes open or unable to determine whether the eyes are open) as a strong indicator of depressive-like behavior.13 The overall observation time period was chosen to reflect the diagnostic criteria of major depression in humans, which requires the presence of abnormal behaviors for at least 2 wk to confirm the condition.1 The videorecordings revealed a consistent pattern throughout the observation period, when the sick animal was in the hunched posture (Figure 7 A) for more than half of the time recorded in more than 61% of the collected video sessions. As a comparison, none of these behaviors were observed in healthy naïve or implanted controls. As such, a presumptive diagnosis of depressive-like behaviors was made in light of the consistent huddling behavior, general inactivity, weight loss, and hypercortisolemia.2,3,25,32
Figure 7.
Typical posture of patient (A) before and (B) after fluoxetine treatment. Before treatment, the macaque exhibited characteristic and telltale posturing consistent with depressive-like behaviors. The posture was described as collapsed, with head below shoulders.
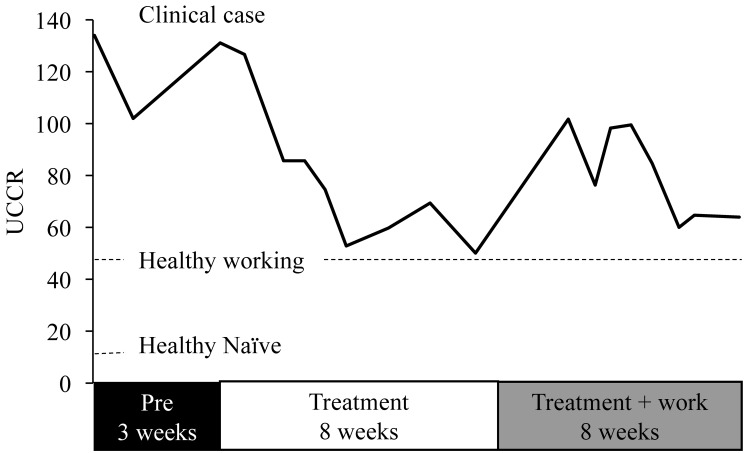
The macaque was started on an antidepressant dose of the selective serotonin-reuptake inhibitor (SSRI) fluoxetine (2 mg/kg PO daily) and provided with additional enrichment (for example, foraging boards, hanging foraging items, destructible enrichment) as well as additional contact time with a trusted caretaker and additional access to large enrichment units. In addition, all research activities were halted until clinical resolution. Response to treatment was monitored by weekly UCCR and videorecording. Urine was collected for UCCR by using clean pans between 1000 and 1300 weekly to minimize the effect of circadian rhythm on outcome. UCCR values slowly declined during the first 6 wk of treatment, reached nadir levels by the 7th week (52.9), and stabilized for 3 wk (Figure 8). The huddling behavior gradually decreased and then ceased, whereas activity (as evidenced by interest in enrichment items, movement within cage, and interest to surroundings) subjectively increased by 7 wk of treatment. The macaque was allowed to return to study after 8 wk of treatment. UCCR values spiked (101.7) then stabilized in the 60s, which is near the average value for our healthy cephalic-implanted macaques. As importantly, the abnormal huddling behavior did not recur (Figure 7 B), alopecia markedly decreased, and the researchers reported a marked improvement on task performance as compared with when the animal was presented for physical examination.
Figure 8.
Effect of fluoxetine on UCCR values over the course of treatment. UCCR values were high (120s) prior to treatment, a slow decline in UCCR occurred during the first 4 wk of treatment, and nadir values were achieved by 5 wk of treatment. At nadir, postural and behavioral indications of depressive-like behavior had diminished, and after 8 wk of treatment, the macaque was returned to study participation. This period is marked by a transient rise in UCCR, followed by subsequent return to near nadir values.
Discussion
Rhesus macaques are among the most commonly used NHP4 in research and are favored particularly for their importance as translational models as well as their hardiness in captive environment.7,12 Nonetheless, these models are susceptible to chronic stress and the development of abnormal behaviors when the laboratory housing environment is substandard7. As such, single housing has often been cited as a source of chronic stress that could lead to physiologic and behavioral changes suggestive of depressive-like behaviors, including crouching, huddling, and overall inactivity.2,3,7,13,16,32 In one report, 1 or 2 cynomolgus macaques per social colony of 17 to 22 members were diagnosed with depressive-like behaviors, for a total prevalence of approximately 5%.32 One group13 determined a prevalence of 0.5% in outdoor socially grouped rhesus macaques; these estimates climbed to 7.2% for all indoor-housed macaques, and further increased to 18.9% of individually housed indoor macaques. These prevalence reports fall somewhere between the 6.7% of the human population that is diagnosed with depression within any 12-mo period and the lifetime prevalence of 16.6%21 and emphasizes the susceptibility of laboratory-housed macaques to major depression. However, no studies have actually determined the influence of chronic behavioral research, which often involves food and water restrictions as motivators, on stress and psychologic wellbeing in those models. Although single housing of our sick animal most certainly contributed to the development of depression, we believe that research activities were a determinant precipitating factor. Indeed, our macaque showed no abnormal behavior for more than 2 y when kept in the naïve colony, despite single housing. In addition, serial UCCR evaluation of naïve and working macaques housed under the same conditions revealed that working, not single housing, was associated with increases in UCCR. This observation was confirmed in a longitudinal study of UCCR in 2 of our animals tested before and after enrollment in research activities. Nonetheless, single-housing and intense research activities probably acted synergistically to induce the depressive-like condition in our animal.
Several studies have explored the pathophysiology of depressive-like behaviors in macaques, with particular emphasis on early life events such as rearing conditions, maternal separation, and variable foraging as a model of maternal stress that affects the maternal relationship with the infant.31 These events are thought to contribute to the development of the depressive-like condition later in life in stress-vulnerable phenotypes. However, the bulk of these studies focus on induced models with little relevance to the clinical diagnosis and treatment of spontaneously arising depressive-like behavior.31 Nonetheless, we cannot exclude that early life experiences in the sick animal, including early involvement in a very brief behavioral study at UC Davis during infancy and abrupt transition from outdoor to indoor housing and transfer to the Salk Institute in late adolescence might have also predisposed this particular macaque to depression.
In humans, diagnosis is guided by the Diagnostic and Statistical Manual of Mental Disorders,1 which details 9 symptoms, of which 5 must be present for a 2-wk period. In translating these criteria to NHP, several objective criteria may be used including changes in weight, activity, focus, posture, psychomotor status, and fatigue; however, challenges are presented in interpreting 2 important human criteria: feelings of worthlessness and thoughts of suicide. Setting the last 2 criteria aside, we created a case definition for the clinically affected macaque that included change in weight, activity (as assessed by videorecording), focus (as assessed by reported performance on research tasks), posture (as assessed by videorecording), psychomotor status (interpreted as mentation change) and included hypercortisolemia on the basis that many human patients demonstrate HPA axis derangement coincident with depression.9,28,33 In light of recent literature describing the reversal of depressive-like states in newly developed macaque models of depression by using antidepressants,24,25 we elected to pursue pharmacologic intervention through the use of fluoxetine.
There is a myriad of treatment options for depression in human healthcare which can be broken into 2 main categories: therapy and medication. Therapy focuses on 3 main approaches: cognitive behavioral therapy, interpersonal therapy, and problem-solving therapy. Because these approaches were deemed impractical in a macaque, we tried to positively influence psychologic wellbeing by providing the animal with inanimate forms of enrichment, increasing positive contact with a trusted caretaker, and allowing more access to large enrichment units.18 At the time, we felt that attempting pair housing would be more stressful and might be detrimental due to the mental status of the patient. Pharmacologic treatment of major depression in humans involves the selection of one or more of the following pharmaceutical classes of drugs: SSRI, other serotonergic antidepressants (serotonin receptor modulators, partial serotonin receptor agonists), serotonin and norepinephrine reuptake inhibitors, tricyclic antidepressants, and monoamine oxidase inhibitors.1,27 We chose fluoxetine, a SSRI, for this case on the basis of published efficacy of antidepressants for reversal of depressive-like behaviors in macaques, as well as availability, ease of administration, and minimal anticipated side-effects for an animal enrolled in a neuroscience protocol.25 This last consideration was a point of concern for the research team, and ultimately the decision to use fluoxetine was made in light of the past use of fluoxetine for self-injurious behavior in our macaque colony that demonstrated no negative effect on research outcome. Moreover, in the current case, fluoxetine treatment not only ameliorated the depressive-like behaviors and blunted the cortisol values seen, but the researchers also reported improvement in the macaque's focus and task performance relative to pretreatment. The response to therapy further confirmed our diagnosis of depressive-like behaviors.
In the current case, the original clinical workup focused on distinguishing potential causes of hypercortisolemia in a macaque showing dull behavior and weight loss. The differential diagnoses included artificial elevations of cortisol due to acute stress and sampling method, hyperadrenocorticism, and pseudo-Cushing syndrome. Using a stress-free collection method (free-catch in clean cage pans) and assessing UCCR minimized the potential confounding effect of stress from capture and sample collection.22 In addition, comparing UCCR levels between healthy controls and the sick macaque suggested that the observed hypercortisolemia was probably not secondary to acute stress. Hyperadrenocorticism and pseudo-Cushing disease share many clinical signs (for example, weight loss, alopecia, and change in activity level), but both can be partially differentiated by using the LDDS test.8-10 In hyperadrenocorticism, the production of cortisol is dysfunctional and cannot be downregulated by suppression of ACTH secretion by the pituitary with dexamethasone. As such, LDDS in hyperadrenocorticism will result in a delayed response to suppression or elevation in cortisol beyond 50% of basal values at given times after suppression.8 In contrast, cases of pseudo-Cushing disease are expected to have normal LDDS test results.
Performing the LDDS in captive macaques poses several challenges: testing is typically done by using serum cortisol values, relies on validated concentrations as cutoffs for the indication of hyperadrenocorticism, and uses results collected at specific time points. We were concerned that serum collection would artificially elevate cortisol due to the stress of the method; we therefore chose to use UCCR as a surrogate dependent variable. However, UCCR yields ratios, not concentrations, and does not accommodate testing of cortisolemia at the time points of a traditional LDDS test, thus introducing an unpredictable variable. In addition, no published concentrations for cortisol after dexamethasone suppression were available for macaques. We, therefore, sought to compare healthy controls with our clinical case and to use the greater than 50% basal values during the 6-h time point as an indicator for hyperadrenocorticism rather than specific concentrations. In our hands, we found that the pattern of cortisol suppression was consistent between the healthy controls and the clinical case. In this case, LDDS yielded suppression below 50% of basal values by 6 h and a pattern of suppression and release from suppression that matched healthy controls, thus suggesting pseudo-Cushing disease in the patient. Although neither LDDS nor UCCR should be used as a sole criterion to exclude hyperadrenocorticism in human patients,9 the results from these tests, combined with the overall clinical presentation as well as CBC analysis, serum biochemistry, and imaging results further comforted our assumption that this patient did not have hyperadrenocorticism, leaving the various etiologies of pseudo-Cushing syndrome to be assessed and ruled out.
We were unable to determine the definitive cause of the depressive-like behaviors in this macaque. We speculate that early life conditions, which included brief but early (3 to 4 mo of age) interventions in infancy and abrupt transition from outdoor to indoor housing in adolescence, may have yielded a stress-vulnerable phenotype, which when faced with the stresses of surgery, fluid-restriction, and single housing may have exceeded the animal's coping abilities and culminated in depressive-like behaviors. The pattern of UCCR during treatment with fluoxetine certainly suggests that the research protocol induced stress. While the macaque was off-study and under treatment, cortisol levels reached baseline (for this animal), however, reintroduction to the study resulted in a temporary spike in cortisol and subsequent fall to baseline. This pattern could be interpreted as protocol-related stress, which treatment appears to mitigate. The observation of elevated UCCR in working implanted macaques relative to naïve, nonworking macaques lends further credence to the hypothesis that the research protocol induced stress in the animal subjects, although to a lesser degree. We hypothesize that our sick animal lacked the coping mechanisms to handle the protocol-related stress, culminating in depressive-like behaviors. In contrast, the other working implanted macaques had sufficient coping mechanisms to maintain normal behavior. Alternatively, the heritable nature of depression might have played a role in this macaque, but the incidence of depression in his lineage was unavailable, thus preventing testing of this hypothesis.6,17 The Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research15 states, “When an animal is unable to completely adapt to a stressor and the resulting stress, an aversive state has developed defined as distress… [which] can manifest as maladaptive behaviors… .” The battery of stressors in the macaque's history were challenging to avoid in the pursuit of neuroscientific research. However, this case serves as a reminder of the importance of using the least amount of fluid restriction necessary to achieve research needs, the importance of pair- or group-housing whenever possible, and in this case, the provision of pharmaceuticals to minimize the adverse consequences of unchecked stressors should depressive-like behaviors ensue. In addition, researchers and veterinarians may find it beneficial to carefully assess animal rearing conditions prior to enrollment in stressful protocols and continuously evaluate animal behavior throughout its research career to minimize the possibility of failure to adapt to research procedures that may lead to depressive behaviors.
In summary, depressive-like behaviors were identified in an adult male rhesus macaque that exhibited hypercortisolemia unrelated to hyperadrenocorticism. Huddling and collapsed posture were the most telltale behaviors that signaled a depression-like etiology and were easily identified by using unattended videorecording. Depressive-like behaviors should be considered a differential when this posturing is observed in NHP, and further assessment of HPA-axis activity is warranted to lend support to the diagnosis. Both behavior and hypercortisolemia were responsive to treatment with fluoxetine, a SSRI, and the macaque returned to study participation. Finally, intensive behavioral research that relies on food or fluid regulation may be stressful and, in certain conditions and predisposed animals, may potentially lead to the development of depressive-like behaviors.
Acknowledgments
We recognize Tarsicio Juarez, Orlando Porcioncula, Joey Garza, and Javier Lopez for their outstanding care of the rhesus macaque: their efforts, observations, and compassion for the animal led to the positive outcome achieved. Also, we thank the research laboratory members for their insights, collaborative attitude, and day-to-day management of the animal—the teamwork between laboratory and veterinary services was critical for successful case resolution.
References
- 1.American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders, 5th ed. Washington (DC): American Psychiatric Association Publishing. [Google Scholar]
- 2.Camus SM, Blois-Heulin C, Li Q, Hausberger M, Bezard E. 2013. Behavioural profiles in captive-bred cynomolgus macaques: towards monkey models of mental disorders? PLoS One 8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camus SM, Rochais C, Blois-Heulin C, Li Q, Hausberger M, Bezard E. 2014. Depressive-like behavioral profiles in captive-bred single- and socially-housed rhesus and cynomolgus macaques: a species comparison. Front Behav Neurosci 8:1–15.24478648 [Google Scholar]
- 4.Carlsson HE, Schapiro SJ, Farah I, Hau J. 2004. Use of primates in research: a global overview. Am J Primatol 63:225–237. [DOI] [PubMed] [Google Scholar]
- 5.Clingerman KJ, Summers L. 2005. Development of a body condition scoring system for nonhuman primates using Macaca mulatta as a model. Lab Anim (NY) 34:31–36. [DOI] [PubMed] [Google Scholar]
- 6.Cohen-Woods S, Craig IW, McGuffin P. 2013. The current state of play on the molecular genetics of depression. Psychol Med 43:673–687. [DOI] [PubMed] [Google Scholar]
- 7.DiVincenti L, Jr, Wyatt JD. 2011. Pair housing of macaques in research facilities: a science-based review of benefits and risks. J Am Assoc Lab Anim Sci 50:856–863. [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman EC, Nelson RW, Reusch C, Scott-Moncrieff JC. 2015. Canine and feline endocrinology, 4th ed. St Louis (MO): Elsevier Saunders. [Google Scholar]
- 9.Findling JW, Raff H. 2005. Screening and diagnosis of Cushing syndrome. Endocrinol Metab Clin North Am 34:385–402. [DOI] [PubMed] [Google Scholar]
- 10.Findling JW, Raff H, Aron DC. 2004. The low-dose dexamethasone suppression test: a reevaluation in patients with Cushing's syndrome. J Clin Endocrinol Metab 89:1222–1226. [DOI] [PubMed] [Google Scholar]
- 11.Gaither AM, Baker KC, Gilbert MH, Blanchard JL, Liu DX, Luchins KR, Bohm RP. 2014. Videotaped behavior as a predictor of clinical outcome in rhesus macaques (Macaca mulatta). Comp Med 64:193–199. [PMC free article] [PubMed] [Google Scholar]
- 12.Hannibal DL, Bliss-Moreau E, Vandeleest J, McCowan B, Capitanio J. 2016. Laboratory rhesus macaque social housing and social changes: implications for research. Am J Primatol 79:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennessy MB, McCowan B, Jiang J, Capitanio JP. 2014. Depressive-like behavioral response of adult male rhesus monkeys during routine animal husbandry procedure. Front Behav Neurosci 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 15.Institute for Laboratory Animal Research, Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research, National Academies Press. 2003. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington (DC): National Academies Press. [Google Scholar]
- 16.Lilly AA, Mehlman PT, Higley JD. 1999. Trait-like immunological and hematological measures in female rhesus across varied environmental conditions. Am J Primatol 48:197–223. [DOI] [PubMed] [Google Scholar]
- 17.Lopresti AL, Hood SD, Drummond PD. 2013. A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep, and exercise. J Affect Disord 148:12–27. [DOI] [PubMed] [Google Scholar]
- 18.Lutz CK, Novak MA. 2005. Environmental enrichment for nonhuman primates: theory and application. ILAR J 46:178–191. [DOI] [PubMed] [Google Scholar]
- 19.Maxie MG. 2007. Pathology of domestic animals, 5th ed. New York (NY): Saunders. [Google Scholar]
- 20.Mitchell JF, Boisvert CJ, Reuter JD, Reynolds JH, Leblanc M. 2014. Correction of refractive errors in rhesus macaques (Macaca mulatta) involved in visual research. Comp Med 64:300–308. [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute of Mental Health. [Internet]. 2015. Major depression among adults. [Cited 18 November 2016]. Available at: https://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml.
- 22.Novak MA, Hamel AF, Kelly BJ, Dettmer AM, Meyer JS. 2013. Stress, the HPA axis, and nonhuman primate wellbeing: a review. Appl Anim Behav Sci 143:135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul IA, English JA, Halaris A. 2000. Sucrose and quinine intake by maternally deprived and control rhesus monkeys. Behav Brain Res 112:127–134. [DOI] [PubMed] [Google Scholar]
- 24.Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. 2007. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci 27:4894–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin D, Chu X, Feng X, Li Z, Yang S, Lu L, Yang Q, Pan L, Yin Y, Li J, Xu L, Chen L, Hu X. 2015. The first observation of seasonal affective disorder symptoms in rhesus macaque. Behav Brain Res 292:463–469. [DOI] [PubMed] [Google Scholar]
- 26.Reinhardt V, Cowley D, Eisele S. 1991. Serum cortisol concentrations of single-housed and isosexually pair-housed adult rhesus macaques. J Exp Anim Sci 34:73–76. [PubMed] [Google Scholar]
- 27.Schatzberg AF, Nemeroff CB. 2017. Textbook of psychopharmacology, 5th ed. Arlington (VA): American Psychiatric Association Publishing. [Google Scholar]
- 28.Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. 2005. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis). Biol Psychol 69:67–84. [DOI] [PubMed] [Google Scholar]
- 29.Smiley LE, Peterson ME. 1993. Evaluation of a urine cortisol:creatinine ratio as a screening test for hyperadrenocorticism in dogs. J Vet Intern Med 7:163–168. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe-Coote S. 2005. The laboratory primate. Boston (MA): Academic Press. [Google Scholar]
- 31.Worlein JM. 2014. Nonhuman primate models of depression: effects of early experience and stress. ILAR J 55:259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu F, Wu Q, Xie L, Gong W, Zhang J, Zheng P, Zhou Q, Ji Y, Wang T, Li X, Fang L, Li Q, Yang D, Li J, Melgiri ND, Shively C, Xie P. 2015. Macaques exhibit a naturally occurring depression similar to humans. Sci Rep 5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu F, Xie L, Li X, Li Q, Wang T, Ji Y, Kong F, Zhan Q, Cheng K, Fang L, Xie P. 2012. Construction and validation of a systematic ethogram of Macaca fascicularis in a free enclosure. PLoS One 7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]