Abstract
Transient Receptor Potential (TRP) proteins form cation channels characterized by a wide variety of activation triggers. Here, we overview a group of TRP channels that respond to reactive redox species to transduce physiological signals, with a focus on TRPA1 and its role in oxygen physiology. Our systematic evaluation of oxidation sensitivity using cysteine-selective reactive disulphides with different redox potentials reveals that TRPA1 has the highest sensitivity to oxidants/electrophiles among the TRP channels, which enables it to sense O2. Proline hydroxylation by O2-dependent hydroxylases also regulates the O2-sensing function by inhibiting TRPA1 in normoxia; TRPA1 is activated by hypoxia through relief from the inhibition and by hyperoxia through cysteine oxidation that overrides the inhibition. TRPA1 enhances neuronal discharges induced by hyperoxia and hypoxia in the vagus to underlie respiratory adaptation to changes in O2 availability. This importance of TRPA1 in non-carotid body O2 sensors can be extended to the universal significance of redox-sensitive TRP channels in O2 adaptation.
Keywords: TRP channels, oxygen, hypoxia, vagus, carotid body
Redox sensitive TRP channels
The TRP protein encoded by the transient receptor potential gene was originally identified in Drosophilia melanogaster.1) In mammals, 28 members of the TRP superfamily have been identified, which are categorized into six subfamilies TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPP (polycystic kidney disease) and TRPML (mucolipin).2,3) TRP proteins are assembled into homo- or hetero-tetramers to form cation-permeable channels.4–7) Most of the TRP channels are polymodal sensors characterized by a wide variety of activation triggers that act from outside and inside the cell. The TRPC channels are typical receptor-activated Ca2+-permeable cation channels regulated by messengers, including diacylglycerol (DAG) and phosphatidylinositol-4,5-bisphophate (PIP2) and Ca2+, metabolized and/or mobilized upon stimulation of metabotropic receptors coupled to phospholipase C (PLC).8,9) It has been demonstrated that TRPC3, for example, amplifies receptor-induced Ca2+ and DAG/protein kinase C signalling via Ca2+ entry-mediated translocation and secondary activation of phospholipase Cγ2 in B lymphocytes.10,11) Some members of the TRPV channels such as the capsaicin receptor TRPV1 are characterized by robust sensitivity to heat to regulate pain sensation.12,13) Some TRPM channels are characterized by the presence of a functional protein domain at their C-termini: TRPM6 and TRPM7 possess a serine/threonine-protein kinase and TRPM2 possesses a nudix-type motif 9 (NUDT9)-homology (NUDT9-H) domain.14) TRPM8 and TRPA1 are responsive to cold temperature.15,16) Number of studies have revealed controversy concerning temperature sensitivity of TRPA1 channels, raising a possibility that TRPA1 is more important in heat sensation than cold sensation.17–19) TRPA1 is also robustly activated by electrophiles following covalent modification of cysteine (Cys) residues within the ankyrin repeat domain (ARD).20,21)
Accumulating evidence suggests that redox reactive species, including reactive oxygen species (ROS), reactive nitrogen species (RNS) and other electrophilic molecules, serve as signaling molecules that regulate biological and physiological processes.22) Dysregulation of redox reactive species is responsible for oxidative damage to membrane lipids, proteins and DNA.23) Thus, physiological and patho-physiological cellular responses are strongly modulated by the balance between the levels of intracellular antioxidants and redox reactive species. The group of TRP channels function as sensors of redox reactive species and as efficient actuators of electric and ionic signals.24) The TRPM2 channel was the first redox-sensitive TRP channel to be identified. Its activation of TRPM2 is triggered by H2O2 through the production of nicotinamide adenine dinucleotide and its metabolites, ADP-ribose and cyclic ADP-ribose.25,26) TRPM2 mediates H2O2-activated Ca2+ or cation influx that drives cell death25) and insulin secretion in pancreatic β-cells.27,28) Furthermore, studies using Trpm2 gene knockout (KO) mice have revealed that H2O2-activated Ca2+ influx through TRPM2 widely contributes to inflammatory responses via chemokine production in monocytes and macrophages,29,30) neutrophil adhesion during myocardial ischaemia/reperfusion injury,31) and NLRP3 inflammasome activation in macrophages.32) TRPM2 also controls irradiation-activated Ca2+ influx that causes irreversible loss of salivary gland function.33) Interestingly, TRPM2 shows characteristic temperature dependence, being activated around body temperature.27,34–36) This is considered reasonable, because both redox reaction and temperature strongly affect protein function through regulation of conformation stability.
TRPM7, characterized by its unique “chanzyme” structure comprising a kinase domain as well as a transmembrane ion channel pore permeable to cations such as Ca2+, Mg2+, Ni2+, Zn2+ and trace metals,37–39) has been suggested to have general biological importance shared by different types of cells.38,39) Tymianski’s group in collaboration with MacDonald’s group demonstrated the activation of TRPM7 by anoxia in cultured neurons subjected to oxygen–glucose deprivation via RNS production.40) However, it is not clarified yet how RNS and other redox species activate TRPM7 channels.
Number of TRP channels that are expressed in various types of cells have been revealed to utilize Cys residues to sense changes in the redox environment. It is in general understood that Cys residues of proteins are highly susceptible to oxidative modification and chemical changes owing to their sulphhydryl group, when compared with other amino acid residues that make up proteins.41) The pKa of Cys residues exposed on the surface is around 7.5, which is close to the physiological pH.42) This means that marginal perturbation of the pH or electrostatic interaction with the surrounding environment can easily affect the population of Cys residues in the thiol and thiolate form.42) Thiolate is highly nucleophilic and thus is more susceptible to electrophiles and oxidizing agents than thiol, conferring the protein with the sensitivity to detect changes in the redox status and other physicochemical parameters.
The first evidence of redox sensing via Cys residues of TRP channels in the sensory neurons was obtained from a study demonstrating that cooperative binding of resiniferatoxin, an ultra-potent agonist of TRPV1, to dorsal root ganglion changed in the presence of the reductant dithiothreitol and the oxidant 5,5′-dithiobis-(2-nitrobenzoic acid).43,44) Subsequent studies found that oxidation and reduction enhanced the activity of rat TRPV1 in response to heat in a recombinant expression system.45,46) We found that TRPV1 is also activated by nitric oxide (NO) via nitrosylation.47) It has been suggested that these TRPV1 channels are activated by the oxidation of both extracellular and intracellular Cys residues.24,47,48) Mutation of Cys616 and Cys621 in rat TRPV1, which are located in the pore-forming domain between the fifth and sixth transmembrane regions, caused significant suppression of the TRPV1 activation by reactive disulphide, NO and H2O2.47) In chicken TRPV1, restoration of individual Cys back after mutation of all Cys residues identified multiple N- and C-terminal reversion mutations, which restore the sensitivity to oxidation.48) In addition, the activation of chicken TRPV1 was proposed to be a consequence of dimerization through the formation of a disulphide bond.49) Because all of the above models of oxidation-induced TRPV1 activation are based on Cys mutagenesis, it is still possible that the suppression of activation is due to non-specific structural disruption of the protein.50) This point is particularly critical for the models that attribute the sensitivity to the presence of the structural disulphide bond.44) Recently, by employing a combination of non-reducing SDS-PAGE, electrophysiology and mass spectrometry, we identified the formation of subunit dimers carrying a stable inter-subunit disulphide bond between Cys258 and Cys742 of human TRPV1.51) These results suggest that Cys258 residues are heterogeneously modified in the TRPV1 tetrameric complex; namely, there are such residues with a free thiol for oxidation sensing and those involved in disulphide bond formation to assist subunit dimerization.
TRPC5 was initially characterized as a Ca2+-permeable cation channel activated upon PLC activation,52,53) and regulated by Ca2+, calmodulin and PIP2.54–58) We have demonstrated that H2O2 and NO activate TRPC5 via the modification of Cys residues.47,59) Both recombinant and native TRPC5 in bovine aortic endothelial cells induce robust Ca2+ influx in response to the application of NO or H2O2.47) By systematically subjecting all of the Cys mutants of TRPC5 to physiological measurements and biotin-switch nitrosylation assays, Cys553 and Cys558 in the putative pore-forming region between the fifth and sixth transmembrane regions were identified as candidate nitrosylation sites.47) It was also hypothesized that a disulphide bond between Cys553 and Cys558 is cleaved by reducing agents such as dithiothreitol or that the antioxidant thioredoxin applied from the extracellular side induces opening of the TRPC5 channel.60) A recent study suggested that inter-subunit disulphide bonds involving Cys553 and Cys558 contribute to the formation of the tetrameric complex of TRPC5.61) Thus, multiple groups agree on the importance of Cys553 and Cys558, but differ on how these residues contribute to redox sensitivity and play roles in regulating TRPC5 channels. There are several possible explanations for this discrepancy. Firstly, the above Cys residues may adopt intermediary oxidation states such as sulphenic acid, which is susceptible to both oxidation and reduction. Secondly, TRPC5 may be composed of subunits with differentially oxidized Cys553 and Cys558. Thirdly, TRPC5 may have to go through the activated state to recover from the oxidation-induced inactivated state upon reducing treatments. Interestingly, the activation of TRPC5 by glutathionylation of Cys176 and Cys178 residues has also been reported.62) Further investigation that clarifies the exact modification status of the Cys residues is necessary to explain these discrepancies.
In vascular endothelial cells, TRPC5 activated by NO via Cys nitrosylation enhances Ca2+ influx and induces NO production by endothelial-type NO synthase (eNOS), raising the possibility that TRPC5 mediates a positive feedback loop of NO production upon vasodilator stimulation in endothelial cells.47,63) TRPC1 and TRPC4, the closest relatives of TRPC5, as well as the thermosensor channels TRPV1, TRPV3 and TRPV4, carry Cys residues corresponding to Cys553 and Cys558 in the TRPC5 protein.47) Indeed, our results indicated that TRPV1 is nitrosylated. TRPV1 also shows sensitivity to phenylarsine oxide and allicin from garlic through the covalent modification of Cys residues located in the C-terminal and N-terminal regions.48,64)
TRPA1, mainly expressed in peptidergic C-fibres including somatosensory and vagal nerves,65,66) is characterized by its unique sensitivity to diverse pungent chemicals, including allyl isothiocyanate (AITC),20,67) allicin,68,69) cinnamaldehyde,67) caffeine,70) nicotine,71) heavy metals such as Zn2+ and Cu2+,72,73) acroelin,74) formalin75) and N-ethyl-maleimide.68) Specific Cys residues of TRPA1 are capable of reacting with many of these compounds via electrophilic Michael addition. Also, Ca2+,76,77) receptor stimulation67,78) and the lipid electrophile 15-deoxy-Δ12,14-prostaglandin J279–82) are known to activate TRPA1. In support of the idea that TRPA1 is activated by electrophiles through modification of these three Cys residues, a mass spectrometric analysis revealed that the irreversible alkylating agent iodoacetamide modifies Cys415, Cys422 and Cys622 localized in the N-terminal ARD of mouse TRPA1.68) In a separate study using Cys mutagenesis in human TRPA1, Cys621, Cys641 and Cys665 were suggested to mediate TRPA1 activation by Cys-modifying electrophiles.83) These same Cys residues are also targeted by ROS and RNS.50,79,81,84–86) It is important to note that ROS/RNS also elicits oxidation of unsaturated fatty acids, which results in the production of reactive electrophilic lipid derivatives such as 4-hydroxynonenal, 4-hydroxexanal, 4-oxononenal and nitro-oleic acid, which are activators of TRPA1.87–89)
Quantification of redox sensitivity in TRP channels
For systematic evaluation of the sensitivity of TRP channels, a congeneric series of reactive disulphide compounds turned out to be a powerful approach.90) Aromatic disulphides are generally regarded as reactive disulphides. Their reactivity (i.e. eletrophilicity) is largely dependent on the substituents attached to them, providing a quantitative basis to evaluate the oxidation sensitivity of TRP channels upon examination for the responsiveness to respective reactive disulphides.
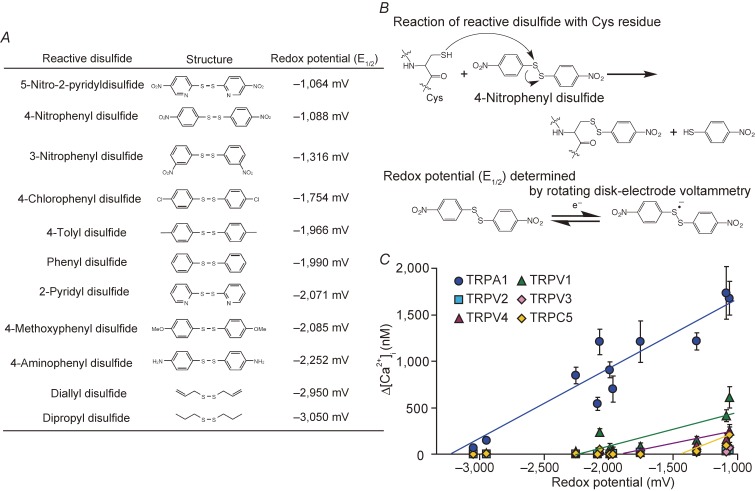
An electrochemical analysis using rotating disk-electrode voltammetry has been applied to a number of chemical compounds including reactive disulphides to quantitatively assess their oxidation (electrophilic) properties. Half-wave potential (E1/2) values of reactive disulphides, which are defined as the midpoint of the rise of current in a voltammogram, were determined. These values were obtained using a glassy carbon working electrode, a platinum wire counter electrode and an Ag/Ag+ reference electrode in the DMSO solution. This is different from the measurement condition of the standard redox potential (E0) values, which is determined by employing the standard hydrogen electrodes in the aqueous solution. However, because of the relative nature, E1/2 values are deemed to be an appropriate descriptor of E0 of these compounds,91) and thus these values are used to represent redox potentials in our experiments (Fig. 1A). With respect to the reaction mechanism underlying redox-sensitive activation of TRP channels, reactive disulphides are electrophilically attacked by Cys residues of TRP channel proteins (Fig. 1B). This differs from the chemical process in which redox potentials are defined to obtain a measure of the tendency of chemical species to acquire electrons. However, the parameters of electrophilicity and redox potential strongly correlate with the electron density of the disulphide bond, suggesting that redox potential is a useful reference to quantify the reactivity of reactive disulphides to a Cys group or, in other words, the sensitivity of TRP channels to oxidative Cys modification.
Figure 1.
Quantification of oxidation sensitivity of TRP channels. A) Chemical structures of reactive disulphides and the redox potentials (E1/2) determined by rotating disk-electrode voltammetry. E1/2 is an empirical value that is defined as the midpoint of the rise of current in voltammogram and, as such, it differs from the standard reduction potential (E0) of the compound. E1/2 values of 5 mM reactive disulphides dissolved in dehydrated DMSO were measured in 0.1 M Bu4NBF4/DMSO using a glassy carbon working electrode, a platinum wire counter electrode and an Ag/Ag+ reference electrode at room temperature. Modified from Takahashi et al.90) B) The chemical reaction of a reactive disulphide compound with a Cys sulfhydryl (upper) and the single electron redox reaction, for which we determined E1/2 values by rotating disk-electrode voltammetry (lower). C) Oxidation sensitivity of TRP channels. Plots of maximum [Ca2+]i rises (Δ[Ca2+]i) induced by 10 µM reactive disulphides in HEK293 cells expressing redox-sensitive TRP channels against redox potentials of respective substances described in A). Modified from Takahashi et al.90)
The plotting of increases in intracellular Ca2+ concentration ([Ca2+]i) via each recombinantly expressed redox-sensitive TRP channels against the E1/2 values of reactive disulphides used for stimulation revealed a positive correlation.90) Respective TRP channels showed characteristic redox sensitivity, when the reactivity of TRP channels was quantitatively evaluated, and threshold redox potentials for each TRP channel were determined by looking at the intercepts of straight lines obtained by fitting with least-squares method against the x-axis (Fig. 1C). Usability of this approach has been reported also in several other experiments. For example, reactivity toward zinc finger proteins in retroviral nucleocapsid was determined using a series of aromatic disulphide for the rational design of electrophilic drugs directed against retroviral zinc fingers.91) With respect to cellular environments, the general redox environment and carotid body (CB) chemoreceptor function have been evaluated by reactive disulphides and other oxidative agents.92) As a future study, E0 values of reactive disulphides should be calculated to obtain the threshold of such values for more generalized evaluation of the redox sensitivity of TRP channels. This should enhance the significance of the chemical–biological approach to quantitatively assess redox properties not only for proteins but also for cellular environments.
TRPA1 is capable of detecting O2 via oxidative Cys modification
Our quantitative analysis of the reactivity of redox-sensitive TRP channels revealed that TRPA1 has the highest sensitivity to disulphides among the TRPs that we tested, predicting that only TRPA1 responds to a relatively inert electrophile, diallyl disulphide, with a redox potential of −2,950 mV.90) Interestingly, the redox potential of O2 was determined to be −2,765 mV by rotating disk-electrode voltammetry, which is less negative than the threshold redox potential value for TRPA1 (approximately −3,400 mV), but is more negative than those of other redox-sensitive TRP channels. As we predicted, only TRPA1 induced [Ca2+]i responses to hyperoxic solution prepared by bubbling with O2 gas, although O2 differs from reactive disulphides in that it snatches an electron from a Cys sulphhydryl group rather than electrophilically attacking the group.93) Other redox-sensitive TRPs (TRPC1, TRPC4, TRPM2 and TRPM7)47) that by themselves do not respond to reactive disulphides including 5-nitro-2-PDS (10 µM) failed to respond to hyperoxia. Diphenylene iodonium (DPI), a potent inhibitor of O2−•-producing enzymes, failed to affect hyperoxia-induced TRPA1 responses, suggesting directness of O2 action on Cys residues. Single TRPA1 channel currents were significantly enhanced by hyperoxic solution, when applied from the intracellular side of cell-free excised inside-out patches and from the extracellular side of cell-free excised outside-out patches. In inside-out patches, single-channel currents induced by hyperoxia for a relatively short period (2 min) were maintained after the readministration of normoxia and reversed by the reduced form of intracellular antioxidant glutathione and dithiothreitol (DTT). This is consistent with the observation that hyperoxia-induced single TRPA1 channel currents were reversed by normoxia in cell-attached patches, which maintains an intact cellular configuration. Thus, TRPA1 has a pronounced susceptibility to Cys oxidation, such that it is directly activated by O2 via glutathione-sensitive oxidation of Cys residues to function as a hyperoxia sensor.
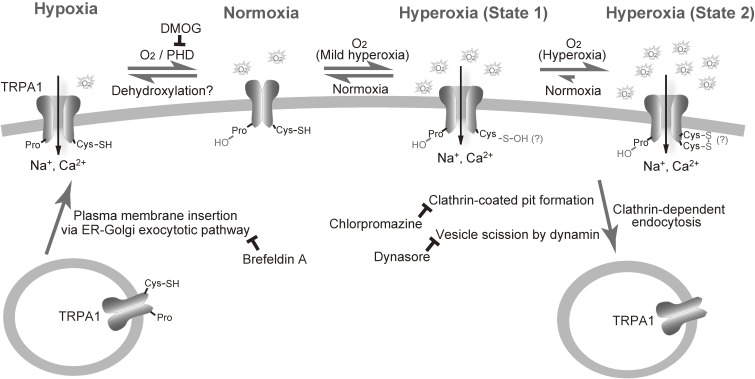
To identify Cys residue(s) required for hyperoxia-induced TRPA1 activation, we individually mutated each of the 29 Cys residues in human TRPA1 to serines and tested the responsiveness of these mutants to hyperoxia as well as to diallyl disulphide, which has a redox potential similar to that of O2, in inducing [Ca2+]i rises.90) The mutants with impaired responses to hyperoxia were further assessed using 2-aminoethyl diphenylborinate (2-APB), which activates TRPA1 independently of Cys modifications, and also by the patch clamp method at fixed membrane potentials and under a defined and optimized intracellular composition, using pipette solution containing polytriphosphate and Ca2+, which sensitizes the activity and prevents the inactivation of TRPA1, respectively.94,95) Other than those non-functional TRPA1 mutants, which failed to show significant responses to 2-APB, the mutants for Cys633 and Cys856 showed abolished responses to hyperoxia, suggesting that they are the main target sites of O2 in hyperoxia (Fig. 2). Incorporation of a derivative of reactive disulphide 5,5′-dithiobis(2-nitrobenzoic acid)47) into green fluorescent protein-tagged TRPA1 (GFP-TRPA1) was abolished by C633S but not by C856S. Notably, coexpression of wild-type TRPA1 with the double mutant of Cys633 and Cys856 nearly abolished hyperoxia-induced but enhanced 2-APB-induced responses, raising the possibility that the hyperoxia response of TRPA1 channels requires all four subunit proteins of tetrameric channel complexes2,96) to carry oxidizable Cys633 and/or Cys856. Thus, free sulphhydryls of Cys633 and Cys856 act as nucleophiles to directly attack electrophiles such as O2 and reactive disulphides, and this oxidative modification is maintained at Cys633. This unique property of Cys633 and Cys856 is underlain by their high reactivity as electron donors. In this context, it is important to note that relatively weak electron acceptors such as diallyl disulphide and 4-tolyl disulphide target the same Cys residues as O2 to activate TRPA1, while reactive disulphides such as 4-nitrophenyl disulphide and 5-nitro-2-PDS with higher potency also act on additional Cys residues. Multiple but different Cys residues have been identified for sensing other covalently modifying substances such as cinnamaldehyde and synthetic Cys-modification reagent N-methyl maleimide (NMM).68,81,83) The reported differences may be due to different stability of the immediate reaction products: O2 may generate an unstable oxidized Cys product, which may turn into a more stable disulphide bond, while cinnamaldehyde and NMM immediately form stable Michael addition adducts.
Figure 2.
Molecular mechanism underlying oxygen-sensing in TRPA1 channel. In hypoxia, a decrease in O2 concentrations relieves TRPA1 from the prolyl hydroxylation, which activates TRPA1. The relief can be achieved by insertion of unmodified TRPA1 proteins to the plasma membrane and internalization of hydroxylated TRPA1 proteins. In hyperoxia, O2 oxidizes Cys633, Cys856 or both, thereby activating TRPA1. TRPA1 may at least take two oxidized state upon hyperoxia: a reversibly oxidized state (State 1) and a relatively stable oxidized state (State 2). Oxidized TRPA1 proteins can be also internalized. Modified from Takahashi et al.90)
Our single-channel recording in cell-excised membrane patches suggests that glutathione plays a role in the regulation of TRPA1 responses to hyperoxia, as already mentioned above.90) Furthermore, glutathionylation of TRPA1 observed in normoxia was augmented after 5 min of hyperoxia, but became undetectable after 20 min of it. The double mutation of Cys633 and Cys856 disrupted hyperoxia-induced glutathionylation. These findings imply that hyperoxia induces changes in oxidative modifications at Cys633 and Cys856, as assessed by glutathione sensitivity (Fig. 2). Interestingly, perfusion of glutathione via a patch pipette solution nearly abolished hyperoxia-induced whole-cell currents for those Cys mutants, which showed impaired responses to hyperoxia but intact responses to 2-APB. These findings further raise the interesting possibility that Cys173, Cys192, Cys641, Cys665, Cys786 and Cys834 protect the O2 reactivity of Cys633 and Cys856 from glutathione to exert antioxidant activity that reverses the TRPA1 activation by O2.
Hypoxia sensing of TRPA1 is underlain by proline hydroxylation
Hyperoxia is not the only range of O2 availability covered by the O2-sensing function of TRPA1 channels.90) Hypoxic solutions prepared by bubbling with N2 gas also induced a robust Ca2+ influx response via TRPA1; TRPA1 activation showed an inverted bell-shaped O2-dependence curve with a minimum at PO2 of 137 mmHg (18%), which is slightly below the atmospheric PO2 of 152 mmHg (20%), regardless of the presence of bicarbonate/CO2. Such O2-dependence enabled TRPA1 to detect subtle changes (from 18% to 20% O2) in the O2 availability at sea level (see also below). Other redox-sensitive TRPs except for TRPM7 failed to respond to hypoxia.40) Meanwhile, hypoxia induced TRPA1 currents in the whole-cell mode of the patch clamp method, but not in the excised inside-out patch mode, suggesting the involvement of intracellular components in the hypoxia-induced activation of TRPA1.
Which factors regulate the hypoxia-induced response of TRPA1? Prolyl hydroxylases (PHDs) are central to hypoxia-sensing that is responsible for regulation of activation of the transcription factor, hypoxia inducible factor (HIF).97,98) The PHD family comprises subtypes PHD1, PHD2 and PHD3, which require O2 as a cofactor for their enzymatic activity. Michaelis constant (Km) values of PHDs for O2, representing the substrate concentration at which half of the enzyme’s active sites are occupied, are 230–250 µM (175–190 mmHg) and close to the atmospheric O2 concentration of 200 µM (152 mmHg),98) suggesting that physiological reductions in O2 concentration result in decreased levels of protein hydroxylation by PHDs. Our alignment of the amino-acid sequences for the prolyl hydroxylation motif in HIF-1 and HIF-297,98) with that of TRPA1 revealed the consensus sequences flanking Pro394 in the N-terminal cytoplasmic ankyrin repeat of human TRPA1.90) Also, mass spectrometry analysis of the consensus TRPA1(386–405) peptide substrate treated with PHD2 and O2, immunoblot analyses with the antibody raised against a synthetic TRPA1 subfragment carrying hydroxylated Pro394 as well as coimmunoprecipitation of TRPA1 with PHDs suggested that the TRPA1 protein is indeed hydroxylated by PHDs. Interestingly, recombinant TRPA1 channels were activated by the inhibition of endogenous PHDs by dimethyloxalylglycine (DMOG) in HEK cells. Overexpression of catalytically dead mutants for PHDs, cotransfection of siRNAs for PHDs and substitution of Pro394 with an alanine residue (P394A) elevated the constitutive activity of TRPA1 and abolished its responses to hypoxia, but not those to hyperoxia. In addition, the overexpression of PHD2 suppressed TRPA1 responses to mild hypoxia (14% O2), whereas excess intracellular PHD2 applied through a patch pipette failed to affect TRPA1 current activation induced by hyperoxia. These findings led us to propose a mechanism in which the hydroxylation of Pro394 by PHDs inhibits TRPA1 channels in normoxia, while a decrease in O2 concentration diminishes PHD activity, to relieve TRPA1 from inhibition, leading to channel activation in hypoxia.90) We also proposed that direct O2 action overrides the PHD-mediated inhibition via pronounced sensitivity of TRPA1 to Cys-mediated oxidation in hyperoxia.
It is known that TRPA1 has multiple activation triggers.21,68,70,81,83,84,99,100) Zn2+ that binds Cys residues in proteins activates TRPA1.73) In TRPV1, Cys nitrosylation significantly enhances sensitivity to protons,47) which also activate TRPA1.81,101) ROS-producing enzymes can also be involved in the O2 sensitivity of TRPA1. However, as far as we tested, the potent O2−•-producing enzyme inhibitor DPI failed to suppress hyperoxia-induced TRPA1 responses (see above). Interestingly, our preliminary experiments90) suggested that the well-known effect of cold temperature of enhancing the dissolution of O2 contributes to the cold-sensitive activation of TRPA1. Therefore, other modes of activation sensitivity may be coupled with redox-sensitivity to finely regulate the O2-sensing function of TRPA1.
Reversibility of hypoxia- and hyperoxia-induced protein modifications in TRPA1 channels
To validate the physiological significance of our hypothesis that Cys oxidation and Pro hydroxylation of TRPA1 proteins underlie their O2-sensing function of TRPA1, it is essential to understand how these protein modifications are reversed, because rapid on/off responses are important attributes of acute O2 adaptation.102)
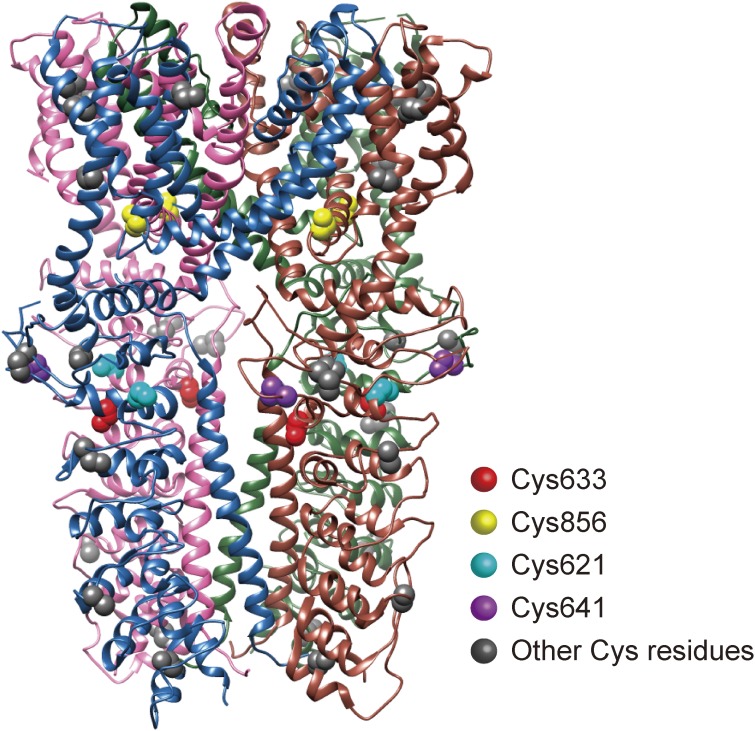
The reversibility of recombinant TRPA1 activation in normoxia after hyperoxia depended on the duration and severity of the preceding hyperoxia.90) TRPA1 activity was retained after the induced conditions of hyperoxia (86% O2) for 19 min were switched back to normoxia (20% O2), and was reversed by the ROS scavenger N-acetylcysteine (NAC) or the reducing agent DTT during the readministration of normoxia. In contrast, TRPA1 responses were reversed in normoxia after mild hyperoxia (28% O2) or after hyperoxia (86% O2) applied for a shorter time period (5 min), and the observed reversibility was unaffected by NAC or DTT. This suggests that a transition of oxidation state is induced in Cys633 and Cys856 (Fig. 2). A possible chemical process behind this is that sulphhydryl groups of Cys633 and Cys856 are initially oxidized by hyperoxia into glutathione-sensitive sulphenic acid, with the subsequent conversion into relatively stable, glutathione-insensitive disulphide bonds.103) It is still unclear whether these disulphide bonds are formed intermolecularly or intramolecularly by TRPA1 proteins. Because our results also indicated the dominant inhibitory effect of the C633S·C856S double mutant, it was likely that oxidation has to be introduced at all four subunits of the TRPA1 channel complex for full activation in response to hyperoxia. Recently, the 3-dimensional structure of the TRPA1 channel has been resolved at the atomic level.104) Although the TRPA1 channel complex with a partial ARD structure lacking the Cys residues in the N-terminal and C-terminal ends and Pro394 was subjected to the analysis, the obtained data at least suggested that a disulphide bond is unlikely to be formed between Cys633 and Cys856 (Fig. 3). Cys633 or Cys856 is also unlikely to form a disulphide bond with Cys641 or Cys655. For further discussion of the precise mechanism involved, there is a need for a structural information on the TRPA1 channel complex with the complete ARD structure or mass-spectrometry information on TRPA1 protein complexes in oxidized and reduced forms.
Figure 3.
Location of Cysteine residues responsible for the sensing of hypoxia, hyperoxia, ROS and carbonyl electrophiles in TRPA1 proteins. The 3-dimensional structure illustration is based upon the protein database 3J9P.105)
After induced conditions of hypoxia (10% O2 for 19 min) were switched back to normoxia (20% O2), TRPA1 activity was retained and reversed by NAC or DTT.90) As observed for the protocol of switching back from hyperoxia to normoxia, the reversibility of hypoxia-induced TRPA1 responses was observed when hypoxia was applied for a shorter time period (5 min) or after mild hypoxia at a higher O2 concentration (14%), and the observed reversibility was unaffected by NAC or DTT. Thus, the duration and severity of preceding hypoxia control the reversibility of TRPA1 activity in normoxia. It is likely that ROS are produced by this ischemia–reperfusion-like protocol and thus induce irreversibility in the TRPA1 response via Cys oxidation in normoxia after severe hypoxia.
In HIF-1α, protein hydroxylation at Pro residues by PHD1–3, which utilize molecular O2 and 2-oxoglutarate as substrates, is known to target HIF-1α to the 26S proteasome for degradation in normoxia.97) Physiological reductions in O2 concentration result in increased protein levels of HIF-1α,98) to elicit systemic adaptive responses such as an increase in red blood cell mass and stimulation of the growth of new blood vessel.105) In TRPA1 channels, we proposed that a decrease in O2 concentrations diminishes PHD activity and relieves TRPA1 from inhibition, leading to its activation in hypoxia (see above). However, the molecular mechanisms underlying the relief process are still elusive. The relief can be achieved by the insertion of unmodified TRPA1 proteins into the plasma membrane through rapid vesicle fusion, or by dehydroxylation through an unidentified enzymatic process. Our analyses of TRPA1 protein translocation to and from the plasmamembrane using evanescent wave microscopy, which illuminates only the subcellular area from the surface of the cell to a depth of less than 100 nm by total internal reflection fluorescence, indicated that the fusion construct GFP-TRPA1 was instantaneously augmented near the cell surface upon hypoxia.90) Using inhibitors of clathrin-dependent endocytosis and ER-Golgi-dependent exocytotic protein translocation, we further demonstrated that the turnover of TRPA1 proteins in the plasma membrane is actively maintained to regulate TRPA1 activity in normoxia and hypoxia, and that the inhibition of PHDs by DMOG decelerates their internalization (Fig. 2). This observation raised the possibility that the insertion of unmodified TRPA1 and the internalization of hydroxylated TRPA1 underlie the relief of PHD-mediated inhibition of TRPA1 channel activity in the plasma membrane. However, we also observed different dwelling times for single TRPA1 channels of the open state in normoxia and hypoxia.90) Different gating behaviours can be more compatible with the activation/deactivation of TRPA1 channels regulated through dehydroxylation of Pro394 by an unidentified enzyme and hydroxylation of Pro394 by PHDs, although the observed behaviours do not necessarily contradict the translocation scenario, in which TRPA1 proteins inserted into the plasma membrane remain dehydroxylated in hypoxia, but immediately become hydroxylated in normoxia. Interestingly, it has been documented that hypoxia is associated with a variety of K+ channels. To trigger the hypoxic inhibition of K+ channels, the importance of mitochondria as the largest consumers of O2 for controlling cytosolic O2 concentration as well as the depletion of intracellular ATP and carbon monoxide production by heme oxygenase has been suggested.106) These processes can be involved in hypoxia-induced activation of TRPA1 in addition to the relief from PHD-mediated inhibition to secure rapid reversibility (on/off) of adaptive responses to changes in O2 availability.
Significance of non-carotid body chemosensors illustrated by TRPA1 in O2 sensing and adaptation
In mammals, the respiratory and cardiovascular systems rapidly adjust themselves to maintain O2 delivery to the most critical organs, such as the brain, according to changes in O2 availability. It is understood that the stimulation of breathing by hypoxia is a reflex triggered mainly by the carotid bodies (CBs) located at the bifurcation of the common carotid arteries.107) It has been also proposed that sensory and vagal afferent neurons detect hypoxia in organs, such as the airway, lungs, and heart, by projecting nerve endings throughout the body, under conditions of low O2 supply.108–111) Enhanced discharges in vagal afferents induce respiratory, cardiac and vascular responses.111–113) However, compared with the CBs, the characteristics and mechanisms of hypoxia detection are still poorly defined in non-CB chemoreceptors including sensory and vagal neurons.111)
Recently, our understanding of the function of non-CB chemoreceptors was greatly expanded by the demonstration that the TRPA1 channel is an essential element for O2 sensing in non-CB chemoreceptors.90,114) In wild-type mice, we showed that exposure to hyperoxic (100% O2) or hypoxic (10%, 13% and 15% O2) gas via a tracheal cannula significantly enhanced the discharge of afferents in the cervical vagal trunk and in the superior laryngeal vagal branch innervating the mucosa of the larynx, using a multifibre neurogram.90) Strikingly, in Trpa1 gene knockout mice, the enhancement of nerve discharges by hyperoxia and mild hypoxia (15% O2) was abolished but that by severe hypoxia (10% and 13% O2) was only delayed.90) Pokorski et al. showed that TRPA1 antagonism using chemical inhibitors abolished the respiratory responses to mild hypoxia (13% O2), but not to severe hypoxia (7% O2), in conscious mice.114) These findings suggest that different O2 signalling mechanisms respond to varying degrees of hypoxic stimulus. In mild hypoxia, the respiratory responses are likely to depend on TRPA1 channels, as TRPA1 deficiency and antagonism abolished these responses. Considering that TRPA1 channels are abundantly expressed in vagal and sensory neurons,95) the responses to mild hypoxia are attributable mainly to non-CB chemoreceptors including vagal nerves. Conversely, during relatively severe hypoxia, TRPA1 channels in non-CB chemoreceptors may play, at the most, a minimal role in regulating the respiratory responses compared with hypoxia-sensitive inhibition of K+ channels in the CBs, in agreement with findings from studies using Trpa1-deficient mice.90) Interestingly, Pokorski et al. reported that the respiratory response to hyperoxia (100% O2) was not appreciably influenced by the TRPA1-selective inhibitor HC-030031, in contrast to our Trpa1-knock out approach.114) Although the basis of this discrepancy between the two studies is yet to be clarified, it is possible that HC-030031 only inhibits the activation state elicited by hypoxia in TRPA1 channels. Thus, the finding of O2 sensitivity of TRPA1 underscores the importance of non-CB chemosensitive mechanisms in respiratory responses to hypoxia and hyperoxia in mammals.
It is generally accepted that respiratory drive is promoted by hypoxia and suppressed by hyperoxia. In explaining how activation of the same vagal TRPA1 channels responsible for enhanced discharges controls respiration in the opposite directions in response to hypoxia and hyperoxia, multiple possibilities, which are not mutually exclusive, can be raised. First, distinct patterns of single-channel opening by TRPA190) may generate different downstream electrical and Ca2+ signals in hypoxia and hyperoxia. Second, hypoxic inhibition of K+ channels may play a key role in determining the directionality of respiratory adaptation.106,115,116) In the CB glomus cells, it has been documented that hypoxia inhibits a variety of K+ channels, leading to the activation of voltage-dependent Ca2+ channels, exocytosis and excitation of the carotid sinus nerve, while hyperoxia reduces depolarization and inhibits exocytosis.106,115) As mentioned in the preceding section, to trigger the hypoxic inhibition of K+ channels, the roles of mitochondria function, intracellular ATP depletion and the inhibition of carbon monoxide production by heme oxygenase have been suggested to regulate K+ channel inhibition. Therefore, it is likely that K+ channels are inhibited by hypoxia but not by hyperoxia in the vagus. This elicits membrane potential depolarization at different levels, which may in turn cause distinct patterns of action potentials and regulate respiration in opposite directions in hypoxia and hyperoxia. The mechanisms involved in K+ channel inhibition can also modulate TRPA1 activity. Third, other oxidation-sensitive TRP channels may contribute to the opposite directionality in respiratory regulation. Hyperoxia may enhance the production of ROS and electrophiles, which are capable of activating respective oxidation-sensitive TRP channels in different manners depending on the level of O2. These are all interesting possibilities, the solution of which should widen and deepen our understanding of the physiology of TRPA1 channels and other redox-sensitive TRP channels.
Previous studies have reported that ventilatory responses to hypoxia after denervation of peripheral O2 chemoreceptors varies among different unanesthetized animal models.117–122) This may suggest existence of O2 sensing systems in the central nervous system (CNS). For instance, the medulla and hypothalamus have been suggested to contain O2 sensors.116) However, the cellular identity of such sensors and molecular mechanisms underlying CNS O2 sensitivity remain elusive. Astrocytes are the most abundant CNS glial cells and contact neuronal somata, dendrites, spines, and presynaptic terminals.123) Astrocytes were traditionally considered as a rather passive CNS cellular component that assist neuronal circuits to maintain their function by providing nutritional and structural support.124) In the last two decades, however, significant evidence has been accumulated that astrocytes play active roles in the regulation of synaptic strength and information processing.124,125) In particular, it has been reported by several groups that astrocytes are involved in central chemosensory mechanisms that maintain cardiorespiratory homeostasis.124,126,127) Despite low protein levels of TRPA1 channels in astrocytes, responses mediated by TRPA1 have previously been confirmed in astrocytes.128–130) Also, TRPV4 channels in astrocytes are activated following osmotic challenges and are up-regulated in ischaemic conditions.131–134) Astrocytes ubiquitously express several isoforms of TRPC channels, of which heteromers assembled from TRPC1, 4 and/or 5 subunits likely act as stretch-activated channels.134–136) Thus, it is possible that redox-sensitive TRP channels contribute to O2 sensitivity of astrocytes in the CNS.
Molecular evolution of O2-sensing mechanism in TRPA1
During the 4.6 billion-year history of the planet, fluctuations in the composition of the atmosphere have played a central role in driving the evolution of living organisms.137) Approximately 3 billion years ago, in the Earth’s oceans, cyanobacteria evolved the ability to harness the energy inherent in sunlight through the process of photosynthesis.138) This lead to the slow rise in atmospheric O2 over the following 3 billion years.137) While the oxidative chemical properties of O2 led to the extinction of the vast majority of unicellular anaerobic life, the eukaryotes formed through symbiosis between different families of bacterial cells withstood the toxic effects of O2 and even utilized its chemical energy to generate cellular ATP much more efficiently than anaerobic metabolism through oxidative phosphorylation.139) As a consequence of this ancient endosymbiotic event, mitochondria today are responsible for the generation of the majority of cellular ATP in metazoans.140)
Most multicellular organisms evolved a mechanism that induces the expression of genes encoding proteins that increase O2 supply and modulate metabolic activity in hypoxic tissues and organisms.137) As a central pathway that coordinates this chronic O2-sensing system, the HIF pathway is highly conserved across species including mammals as well as more ancient lineages such as nematodes and corals.105,137,138) These molecular evolutionary studies suggest that the chronic O2-sensing system mediated by transcriptional regulation had already evolved in the earliest animal species in response to increasing O2 concentration in the Precambrian era (from 4.6 billion to 542 million years ago), during which time the atmospheric concentration of O2 rose from 0% to over 20%.141) In contrast to the analyses of HIF, there have been no studies on the molecular evolutional processes of acute O2-sensing systems responsible for rapid cardiorespiratory adjustments to ensure O2 delivery to the most critical organs, such as the brain and the heart. Characterization of the transition of acute O2-sensing systems over the course of biological evolution may provide major insights in the field of evolutionary biology.
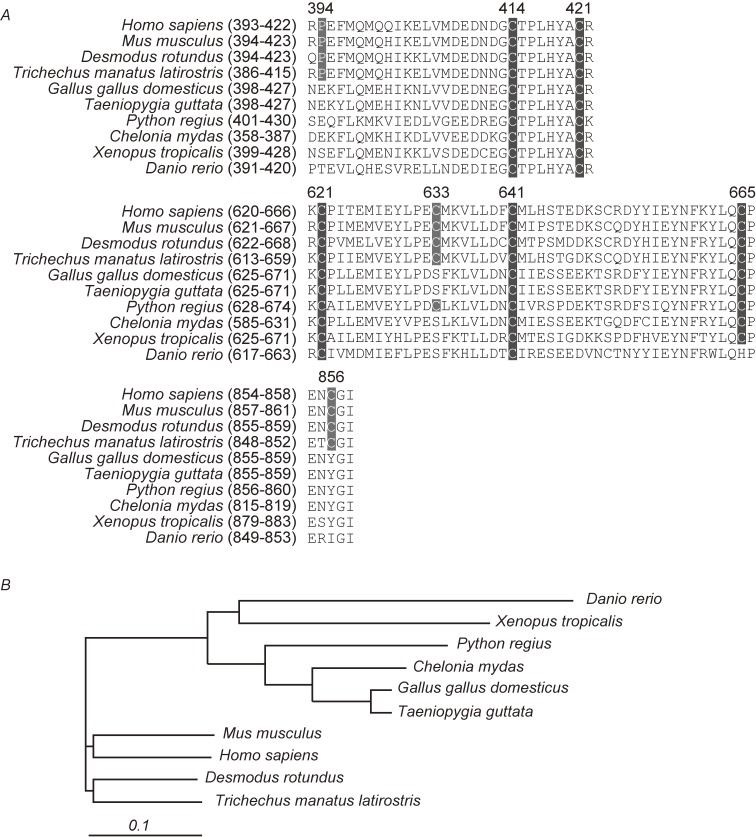
Our evolutionary genetic analysis of TRPA1 shows that hypoxia-sensitive Pro residue (Pro394 in humans) and hyperoxia-sensitive Cys residues (Cys633 and/or Cys856 in human TRPA1) are conserved in Mus musculus (mouse, mammal), Desmodus rotundus (mammal) and Trichechus manatus latirostris (mammal), but not in Gallus gallus domesticus (chicken, avian), Taeniopygia guttata (avian), Python regius (squamate), Chelonia mydas (testudine), Xenopus toropicalis (amphibian) and Danio rerio (bony fish) (Fig. 4). In contrast, Cys414, Cys421, Cys621 and Cys641, which are the action sites of ROS, RNS and electrophiles, are conserved in most vertebrate animals. Indeed, our functional assay revealed that chicken TRPA1 is activated by ROS but not by hypoxia and hyperoxia (unpublished data). Approximately 250 million years ago, when the O2 level was reduced, presumably by the volcanic eruption across the globe, the common ancestor of the mammals acquired the diaphragm through atrophy of the costal bone, enabling it to perform efficient ventilation and abdominal breathing.142) During this time of low O2 concentrations, the body plan of the dinosaur evolved a novel air-sac system,143) which was inherited in a modified form by their descendants, the birds. Thus, it is possible that mammals acquired the distinctive TRPA1-mediated O2-sensing mechanism to rapidly regulate cardiorespiratory systems in response to changes in O2 availability, when a drastic reduction of O2 availability occurred approximately 250 million years ago. Understanding the impact of TRPA1 on cardiorespiratory responses to O2 in both mice and chickens would greatly enhance our knowledge concerning evolutionary processes in mammals in comparison with those in birds, reptiles and dinosaurs.
Figure 4.
Alignments of the amino acid sequences containing the residues responsible for the sensing of hypoxia, hyperoxia, ROS and carbonyl electrophiles in vertebrate TRPA1 channel proteins. A) The Amino acid sequences used for the alignments are from NP_015628 for Homo sapiens, NP_808449 for Mus musculus, AEL30803 for Desmodus rotundus, XP_012409888 for Trichechus manatus latirostris, NP_001305389 for Gallus gallus domesticus, XP_002197858 for Taeniopygia guttata, ADD82928 for Python regius, EMP32288 for Chelonia mydas, NP_001121434 for Xenopus tropicalis and NP_001007066 for Danio rerio. Residue numbers are according to the primary sequence of the TRPA1 (Homo sapiens). B) Phylogenetic tree of TRPA1 channels based on their homology. The scale represents evolutionary distance calculated by Clustal analysis.
Conclusion
In this review, we have mainly discussed the important roles played by TRPA1 channels in the O2-sensing function of vagal neurons. Nevertheless, we can extrapolate this specific finding to a more universal significance of TRP channels in oxygen physiology. Indeed, TRPA1 showed the most abundant expression in sensory neurons,21) but was also detected in numerous tissues,144,145) suggesting the general importance of O2-sensing mediated by this channel. In addition to TRPA1, we found that TRPM7, which was originally known for activation by anoxia,40) is capable of responding to milder hypoxic conditions prepared by bubbling N2 gas into the assay solution.90) It has been shown that TRPM7 is expressed ubiquitously in different types of cells.38,39) Assuming that hypoxia-induced activation is a common feature shared by TRPA1 and TRPM7 in different tissues and cell types, decreases in local O2 levels in vivo by changes in body architecture during development and by changes in climate can modulate TRPA1 and TRPM7 function to modify ionic homeostasis and/or downstream signaling cascades.
Interestingly, TRPA1 activation shows an inverted bell-shaped O2-dependence curve with a minimum at PO2 of 137 mm Hg (18%), which is slightly below the atmospheric PO2 of 152 mm Hg (20%).90) This suggests that so-called normoxia in the atmosphere actually costitues hyperoxia from the perspective of O2-dependent TRPA1 channel function. It has been reported that Caenorhabditis elegans exhibits strong avoidance of hyperoxia through detection by sensory neurons,146) and insects breathe discontinuously to avoid O2 toxicity in hyperoxia.147) Although the physiological relevance of hyperoxia sensing is still elusive in vertebrates including mammals, it is extremely important to identify and characterize oxidation-sensitive TRP channels that are involved in hyperoxia-sensing molecular processes in vertebrate sensory systems.
We suggest that O2-sensing TRP channels play key roles in the molecular mechanisms that underlie the O2-sensing ability of chemoreceptor (or chemoreceptor-like) cells that are ubiquitous in a variety of tissues and organs. It is possible that TRP-mediated O2 sensors detect local O2 availability and contribute to the fine tuning of local O2 levels, which cannot be done by the CB alone. Information on the detected local O2 availability may be transmitted to peripheral organs and tissues through neurons and/or humoral factors to control O2 delivery. TRPA1 and other redox-sensitive TRP channels may transmit positive signals to enhance O2 delivery, when O2 is necessary, and negative signals to suppress excessive O2 delivery responsible for harmful ROS production, when there is an excess of O2. The latter mechanism may maintain the O2 availability of certain organs/tissues and their subareas at levels lower than the atmospheric O2 level. Indeed, it has been suggested that hypoxic levels are important in maintaining the required cellular conditions of certain types of cell in vivo.148–151) It should be noted that TRPA1 and other redox-sensitive TRP channels can also detect substances other than O2. Studying the roles of redox-sensitive TRP channels in controlling O2 levels in vivo in consideration of their conventional activation triggers should open up a new area in the study of oxygen physiology.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Oxygen biology: a new criterion for integrated understanding of life” (No. 26111004) of The Ministry of Education, Culture, Sports, Science and Technology, Japan.
Profile
Yasuo Mori was born in Nagoya, Aichi Prefecture in 1960 and graduated from Kyoto University School of Engineering in 1983. After he received Ph. D. from Kyoto University School of Medicine in 1983, he worked at the same school, and moved to University of Cincinnati College of Medicine and then to National Institute for Physiological Sciences. He became professor at Center for Integrative Bioscience, Okazaki National Research Institutes (presently National Institutes of Natural Sciences) in 2001, and currently is professor at Kyoto University School of Engineering. His main research field has been molecular physiology of calcium-permeable channels including voltage-dependent calcium channels that trigger neurotransmitter release in neurons, and Transient Receptor Potential (TRP) calcium-permeable cation channels that mediate different calcium signaling pathways in response to changes of the environment and the condition in vivo. His study lead to identification of a group of TRP channels that act as in vivo sensors for molecular oxygen and its derivative reactive species. For these achievements, he received Setsuro Ebashi Award.
References
- 1).Montell C., Rubin G.M. (1989) Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2, 1313–1323. [DOI] [PubMed] [Google Scholar]
- 2).Clapham D.E. (2003) TRP channels as cellular sensors. Nature 426, 517–524. [DOI] [PubMed] [Google Scholar]
- 3).Clapham D.E., Julius D., Montell C., Schultz G. (2005) International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol. Rev. 57, 427–450. [DOI] [PubMed] [Google Scholar]
- 4).Fischer M.J., Balasuriya D., Jeggle P., Goetze T.A., McNaughton P.A., Reeh P.W., Edwardson J.M. (2014) Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch. 466, 2229–2241. [DOI] [PubMed] [Google Scholar]
- 5).Hofmann T., Schaefer M., Schultz G., Gudermann T. (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. U.S.A. 99, 7461–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Liao M., Cao E., Julius D., Cheng Y. (2013) Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Strubing C., Krapivinsky G., Krapivinsky L., Clapham D.E. (2003) Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J. Biol. Chem. 278, 39014–39019. [DOI] [PubMed] [Google Scholar]
- 8).Nishida M., Hara Y., Yoshida T., Inoue R., Mori Y. (2006) TRP channels: molecular diversity and physiological function. Microcirculation 13, 535–550. [DOI] [PubMed] [Google Scholar]
- 9).Vazquez G., Wedel B.J., Aziz O., Trebak M., Putney J.W., Jr. (2004) The mammalian TRPC cation channels. Biochim. Biophys. Acta 1742, 21–36. [DOI] [PubMed] [Google Scholar]
- 10).Nishida M., Sugimoto K., Hara Y., Mori E., Morii T., Kurosaki T., Mori Y. (2003) Amplification of receptor signalling by Ca2+ entry-mediated translocation and activation of PLCgamma2 in B lymphocytes. EMBO J. 22, 4677–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Numaga T., Nishida M., Kiyonaka S., Kato K., Katano M., Mori E., Kurosaki T., Inoue R., Hikida M., Putney J.W., Jr., Mori Y. (2010) Ca2+ influx and protein scaffolding via TRPC3 sustain PKCbeta and ERK activation in B cells. J. Cell Sci. 123, 927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- 13).Patapoutian A., Peier A.M., Story G.M., Viswanath V. (2003) ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat. Rev. Neurosci. 4, 529–539. [DOI] [PubMed] [Google Scholar]
- 14).Scharenberg A.M. (2005) TRPM2 and TRPM7: channel/enzyme fusions to generate novel intracellular sensors. Pflugers Arch. 451, 220–227. [DOI] [PubMed] [Google Scholar]
- 15).McKemy D.D., Neuhausser W.M., Julius D. (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58. [DOI] [PubMed] [Google Scholar]
- 16).Peier A.M., Moqrich A., Hergarden A.C., Reeve A.J., Andersson D.A., Story G.M., Earley T.J., Dragoni I., McIntyre P., Bevan S., Patapoutian A. (2002) A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715. [DOI] [PubMed] [Google Scholar]
- 17).Kurganov E., Saito S., Tanaka Saito C., Tominaga M. (2017) Requirement of extracellular Ca2+ binding to specific amino acids for heat-evoked activation of TRPA1. J. Physiol. 595, 2451–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Moparthi L., Kichko T.I., Eberhardt M., Hogestatt E.D., Kjellbom P., Johanson U., Reeh P.W., Leffler A., Filipovic M.R., Zygmunt P.M. (2016) Human TRPA1 is a heat sensor displaying intrinsic U-shaped thermosensitivity. Sci. Rep. 6, 28763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Vriens J., Nilius B., Voets T. (2014) Peripheral thermosensation in mammals. Nat. Rev. Neurosci. 15, 573–589. [DOI] [PubMed] [Google Scholar]
- 20).Jordt S.E., Bautista D.M., Chuang H.H., McKemy D.D., Zygmunt P.M., Hogestatt E.D., Meng I.D., Julius D. (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265. [DOI] [PubMed] [Google Scholar]
- 21).Story G.M., Peier A.M., Reeve A.J., Eid S.R., Mosbacher J., Hricik T.R., Earley T.J., Hergarden A.C., Andersson D.A., Hwang S.W., McIntyre P., Jegla T., Bevan S., Patapoutian A. (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829. [DOI] [PubMed] [Google Scholar]
- 22).Finkel T. (2011) Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Cross C.E., Halliwell B., Borish E.T., Pryor W.A., Ames B.N., Saul R.L., McCord J.M., Harman D. (1987) Oxygen radicals and human disease. Ann. Intern. Med. 107, 526–545. [DOI] [PubMed] [Google Scholar]
- 24).Kozai D., Ogawa N., Mori Y. (2014) Redox regulation of transient receptor potential channels. Antioxid. Redox Signal. 21, 971–986. [DOI] [PubMed] [Google Scholar]
- 25).Hara Y., Wakamori M., Ishii M., Maeno E., Nishida M., Yoshida T., Yamada H., Shimizu S., Mori E., Kudoh J., Shimizu N., Kurose H., Okada Y., Imoto K., Mori Y. (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 9, 163–173. [DOI] [PubMed] [Google Scholar]
- 26).Perraud A.L., Takanishi C.L., Shen B., Kang S., Smith M.K., Schmitz C., Knowles H.M., Ferraris D., Li W., Zhang J., Stoddard B.L., Scharenberg A.M. (2005) Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J. Biol. Chem. 280, 6138–6148. [DOI] [PubMed] [Google Scholar]
- 27).Togashi K., Hara Y., Tominaga T., Higashi T., Konishi Y., Mori Y., Tominaga M. (2006) TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 25, 1804–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Uchida K., Dezaki K., Damdindorj B., Inada H., Shiuchi T., Mori Y., Yada T., Minokoshi Y., Tominaga M. (2011) Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes 60, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Knowles H., Heizer J.W., Li Y., Chapman K., Ogden C.A., Andreasen K., Shapland E., Kucera G., Mogan J., Humann J., Lenz L.L., Morrison A.D., Perraud A.L. (2011) Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proc. Natl. Acad. Sci. U.S.A. 108, 11578–11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Yamamoto S., Shimizu S., Kiyonaka S., Takahashi N., Wajima T., Hara Y., Negoro T., Hiroi T., Kiuchi Y., Okada T., Kaneko S., Lange I., Fleig A., Penner R., Nishi M., Takeshima H., Mori Y. (2008) TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 14, 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Hiroi T., Wajima T., Negoro T., Ishii M., Nakano Y., Kiuchi Y., Mori Y., Shimizu S. (2013) Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 97, 271–281. [DOI] [PubMed] [Google Scholar]
- 32).Zhong Z., Zhai Y., Liang S., Mori Y., Han R., Sutterwala F.S., Qiao L. (2013) TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat. Commun. 4, 1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Liu X., Cotrim A., Teos L., Zheng C., Swaim W., Mitchell J., Mori Y., Ambudkar I. (2013) Loss of TRPM2 function protects against irradiation-induced salivary gland dysfunction. Nat. Commun. 4, 1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Kashio M., Sokabe T., Shintaku K., Uematsu T., Fukuta N., Kobayashi N., Mori Y., Tominaga M. (2012) Redox signal-mediated sensitization of transient receptor potential melastatin 2 (TRPM2) to temperature affects macrophage functions. Proc. Natl. Acad. Sci. U.S.A. 109, 6745–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Song K., Wang H., Kamm G.B., Pohle J., Reis F.C., Heppenstall P., Wende H., Siemens J. (2016) The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353, 1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Tan C.H., McNaughton P.A. (2016) The TRPM2 ion channel is required for sensitivity to warmth. Nature 536, 460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Monteilh-Zoller M.K., Hermosura M.C., Nadler M.J., Scharenberg A.M., Penner R., Fleig A. (2003) TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 121, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Nadler M.J., Hermosura M.C., Inabe K., Perraud A.L., Zhu Q., Stokes A.J., Kurosaki T., Kinet J.P., Penner R., Scharenberg A.M., Fleig A. (2001) LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595. [DOI] [PubMed] [Google Scholar]
- 39).Runnels L.W., Yue L., Clapham D.E. (2001) TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047. [DOI] [PubMed] [Google Scholar]
- 40).Aarts M., Iihara K., Wei W.L., Xiong Z.G., Arundine M., Cerwinski W., MacDonald J.F., Tymianski M. (2003) A key role for TRPM7 channels in anoxic neuronal death. Cell 115, 863–877. [DOI] [PubMed] [Google Scholar]
- 41).Marino S.M., Gladyshev V.N. (2012) Analysis and functional prediction of reactive cysteine residues. J. Biol. Chem. 287, 4419–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Marino S.M., Gladyshev V.N. (2010) Cysteine function governs its conservation and degeneration and restricts its utilization on protein surfaces. J. Mol. Biol. 404, 902–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Szallasi A., Blumberg P.M. (1993) [3H]resiniferatoxin binding by the vanilloid receptor: species-related differences, effects of temperature and sulfhydryl reagents. Naunyn Schmiedebergs Arch. Pharmacol. 347, 84–91. [DOI] [PubMed] [Google Scholar]
- 44).Szallasi A., Lewin N.A., Blumberg P.M. (1993) Vanilloid (capsaicin) receptor in the rat: positive cooperativity of resiniferatoxin binding and its modulation by reduction and oxidation. J. Pharmacol. Exp. Ther. 266, 678–683. [PubMed] [Google Scholar]
- 45).Susankova K., Tousova K., Vyklicky L., Teisinger J., Vlachova V. (2006) Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol. Pharmacol. 70, 383–394. [DOI] [PubMed] [Google Scholar]
- 46).Vyklicky L., Lyfenko A., Susankova K., Teisinger J., Vlachova V. (2002) Reducing agent dithiothreitol facilitates activity of the capsaicin receptor VR-1. Neuroscience 111, 435–441. [DOI] [PubMed] [Google Scholar]
- 47).Yoshida T., Inoue R., Morii T., Takahashi N., Yamamoto S., Hara Y., Tominaga M., Shimizu S., Sato Y., Mori Y. (2006) Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2, 596–607. [DOI] [PubMed] [Google Scholar]
- 48).Chuang H.H., Lin S. (2009) Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc. Natl. Acad. Sci. U.S.A. 106, 20097–20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Wang S., Chuang H.H. (2011) C-terminal dimerization activates the nociceptive transduction channel transient receptor potential vanilloid 1. J. Biol. Chem. 286, 40601–40607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Miyamoto T., Dubin A.E., Petrus M.J., Patapoutian A. (2009) TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One 4, e7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Ogawa N., Kurokawa T., Fujiwara K., Polat O.K., Badr H., Takahashi N., Mori Y. (2016) Functional and structural divergence in human TRPV1 channel subunits by oxidative cysteine modification. J. Biol. Chem. 291, 4197–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Okada T., Shimizu S., Wakamori M., Maeda A., Kurosaki T., Takada N., Imoto K., Mori Y. (1998) Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J. Biol. Chem. 273, 10279–10287. [DOI] [PubMed] [Google Scholar]
- 53).Philipp S., Hambrecht J., Braslavski L., Schroth G., Freichel M., Murakami M., Cavalie A., Flockerzi V. (1998) A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 17, 4274–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Blair N.T., Kaczmarek J.S., Clapham D.E. (2009) Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J. Gen. Physiol. 133, 525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Gross S.A., Guzman G.A., Wissenbach U., Philipp S.E., Zhu M.X., Bruns D., Cavalie A. (2009) TRPC5 is a Ca2+-activated channel functionally coupled to Ca2+-selective ion channels. J. Biol. Chem. 284, 34423–34432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Ordaz B., Tang J., Xiao R., Salgado A., Sampieri A., Zhu M.X., Vaca L. (2005) Calmodulin and calcium interplay in the modulation of TRPC5 channel activity. Identification of a novel C-terminal domain for calcium/calmodulin-mediated facilitation. J. Biol. Chem. 280, 30788–30796. [DOI] [PubMed] [Google Scholar]
- 57).Shimizu S., Yoshida T., Wakamori M., Ishii M., Okada T., Takahashi M., Seto M., Sakurada K., Kiuchi Y., Mori Y. (2006) Ca2+-calmodulin-dependent myosin light chain kinase is essential for activation of TRPC5 channels expressed in HEK293 cells. J. Physiol. 570, 219–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Trebak M., Lemonnier L., DeHaven W.I., Wedel B.J., Bird G.S., Putney J.W., Jr. (2009) Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflugers Arch. 457, 757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Takahashi N., Kozai D., Mori Y. (2012) TRP channels: sensors and transducers of gasotransmitter signals. Front. Physiol. 3, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Xu S.Z., Sukumar P., Zeng F., Li J., Jairaman A., English A., Naylor J., Ciurtin C., Majeed Y., Milligan C.J., Bahnasi Y.M., Al-Shawaf E., Porter K.E., Jiang L.H., Emery P., Sivaprasadarao A., Beech D.J. (2008) TRPC channel activation by extracellular thioredoxin. Nature 451, 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Hong C., Kwak M., Myeong J., Ha K., Wie J., Jeon J.H., So I. (2015) Extracellular disulfide bridges stabilize TRPC5 dimerization, trafficking, and activity. Pflugers Arch. 467, 703–712. [DOI] [PubMed] [Google Scholar]
- 62).Hong C., Seo H., Kwak M., Jeon J., Jang J., Jeong E.M., Myeong J., Hwang Y.J., Ha K., Kang M.J., Lee K.P., Yi E.C., Kim I.G., Jeon J.H., Ryu H., So I. (2015) Increased TRPC5 glutathionylation contributes to striatal neuron loss in Huntington’s disease. Brain 138, 3030–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Foster M.W., Hess D.T., Stamler J.S. (2006) S-nitrosylation TRiPs a calcium switch. Nat. Chem. Biol. 2, 570–571. [DOI] [PubMed] [Google Scholar]
- 64).Salazar H., Llorente I., Jara-Oseguera A., Garcia-Villegas R., Munari M., Gordon S.E., Islas L.D., Rosenbaum T. (2008) A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat. Neurosci. 11, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Bautista D.M., Movahed P., Hinman A., Axelsson H.E., Sterner O., Hogestatt E.D., Julius D., Jordt S.E., Zygmunt P.M. (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. U.S.A. 102, 12248–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Kobayashi K., Fukuoka T., Obata K., Yamanaka H., Dai Y., Tokunaga A., Noguchi K. (2005) Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 493, 596–606. [DOI] [PubMed] [Google Scholar]
- 67).Bandell M., Story G.M., Hwang S.W., Viswanath V., Eid S.R., Petrus M.J., Earley T.J., Patapoutian A. (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857. [DOI] [PubMed] [Google Scholar]
- 68).Macpherson L.J., Dubin A.E., Evans M.J., Marr F., Schultz P.G., Cravatt B.F., Patapoutian A. (2007) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545. [DOI] [PubMed] [Google Scholar]
- 69).Macpherson L.J., Geierstanger B.H., Viswanath V., Bandell M., Eid S.R., Hwang S., Patapoutian A. (2005) The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 15, 929–934. [DOI] [PubMed] [Google Scholar]
- 70).Nagatomo K., Kubo Y. (2008) Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc. Natl. Acad. Sci. U.S.A. 105, 17373–17378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Talavera K., Gees M., Karashima Y., Meseguer V.M., Vanoirbeek J.A., Damann N., Everaerts W., Benoit M., Janssens A., Vennekens R., Viana F., Nemery B., Nilius B., Voets T. (2009) Nicotine activates the chemosensory cation channel TRPA1. Nat. Neurosci. 12, 1293–1299. [DOI] [PubMed] [Google Scholar]
- 72).Gu Q., Lin R.L. (2010) Heavy metals zinc, cadmium, and copper stimulate pulmonary sensory neurons via direct activation of TRPA1. J. Appl. Physiol. (1985) 108, 891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Hu H., Bandell M., Petrus M.J., Zhu M.X., Patapoutian A. (2009) Zinc activates damage-sensing TRPA1 ion channels. Nat. Chem. Biol. 5, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74).Bautista D.M., Jordt S.E., Nikai T., Tsuruda P.R., Read A.J., Poblete J., Yamoah E.N., Basbaum A.I., Julius D. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282. [DOI] [PubMed] [Google Scholar]
- 75).McNamara C.R., Mandel-Brehm J., Bautista D.M., Siemens J., Deranian K.L., Zhao M., Hayward N.J., Chong J.A., Julius D., Moran M.M., Fanger C.M. (2007) TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. U.S.A. 104, 13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Doerner J.F., Gisselmann G., Hatt H., Wetzel C.H. (2007) Transient receptor potential channel A1 is directly gated by calcium ions. J. Biol. Chem. 282, 13180–13189. [DOI] [PubMed] [Google Scholar]
- 77).Zurborg S., Yurgionas B., Jira J.A., Caspani O., Heppenstall P.A. (2007) Direct activation of the ion channel TRPA1 by Ca2+. Nat. Neurosci. 10, 277–279. [DOI] [PubMed] [Google Scholar]
- 78).Dai Y., Wang S., Tominaga M., Yamamoto S., Fukuoka T., Higashi T., Kobayashi K., Obata K., Yamanaka H., Noguchi K. (2007) Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J. Clin. Invest. 117, 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Andersson D.A., Gentry C., Moss S., Bevan S. (2008) Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 28, 2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Maher M., Ao H., Banke T., Nasser N., Wu N.T., Breitenbucher J.G., Chaplan S.R., Wickenden A.D. (2008) Activation of TRPA1 by farnesyl thiosalicylic acid. Mol. Pharmacol. 73, 1225–1234. [DOI] [PubMed] [Google Scholar]
- 81).Takahashi N., Mizuno Y., Kozai D., Yamamoto S., Kiyonaka S., Shibata T., Uchida K., Mori Y. (2008) Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2, 287–298. [DOI] [PubMed] [Google Scholar]
- 82).Trevisani M., Siemens J., Materazzi S., Bautista D.M., Nassini R., Campi B., Imamachi N., Andre E., Patacchini R., Cottrell G.S., Gatti R., Basbaum A.I., Bunnett N.W., Julius D., Geppetti P. (2007) 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. U.S.A. 104, 13519–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Hinman A., Chuang H.H., Bautista D.M., Julius D. (2006) TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. U.S.A. 103, 19564–19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84).Bessac B.F., Sivula M., von Hehn C.A., Escalera J., Cohn L., Jordt S.E. (2008) TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Invest. 118, 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).Sawada Y., Hosokawa H., Matsumura K., Kobayashi S. (2008) Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur. J. Neurosci. 27, 1131–1142. [DOI] [PubMed] [Google Scholar]
- 86).Taylor-Clark T.E., Undem B.J. (2010) Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J. Physiol. 588, 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87).Jian W., Lee S.H., Mesaros C., Oe T., Elipe M.V., Blair I.A. (2007) A novel 4-oxo-2(E)-nonenal-derived endogenous thiadiazabicyclo glutathione adduct formed during cellular oxidative stress. Chem. Res. Toxicol. 20, 1008–1018. [DOI] [PubMed] [Google Scholar]
- 88).Lee S.H., Blair I.A. (2000) Characterization of 4-oxo-2-nonenal as a novel product of lipid peroxidation. Chem. Res. Toxicol. 13, 698–702. [DOI] [PubMed] [Google Scholar]
- 89).Trostchansky A., Rubbo H. (2008) Nitrated fatty acids: mechanisms of formation, chemical characterization, and biological properties. Free Radic. Biol. Med. 44, 1887–1896. [DOI] [PubMed] [Google Scholar]
- 90).Takahashi N., Kuwaki T., Kiyonaka S., Numata T., Kozai D., Mizuno Y., Yamamoto S., Naito S., Knevels E., Carmeliet P., Oga T., Kaneko S., Suga S., Nokami T., Yoshida J., Mori Y. (2011) TRPA1 underlies a sensing mechanism for O2. Nat. Chem. Biol. 7, 701–711. [DOI] [PubMed] [Google Scholar]
- 91).Topol I.A., McGrath C., Chertova E., Dasenbrock C., Lacourse W.R., Eissenstat M.A., Burt S.K., Henderson L.E., Casas-Finet J.R. (2001) Experimental determination and calculations of redox potential descriptors of compounds directed against retroviral zinc fingers: Implications for rational drug design. Protein Sci. 10, 1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Agapito M.T., Sanz-Alfayate G., Gomez-Nino A., Gonzalez C., Obeso A. (2009) General redox environment and carotid body chemoreceptor function. Am. J. Physiol. Cell Physiol. 296, C620–C631. [DOI] [PubMed] [Google Scholar]
- 93).Wallace T.J., Schriesheim A., Bartok W. (1963) The base-catalyzed oxidation of mercaptans. III. Role of the solvent and effect of mercaptan structure on the rate determining step1,2. J. Org. Chem. 28, 1311–1314. [Google Scholar]
- 94).Kim D., Cavanaugh E.J. (2007) Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J. Neurosci. 27, 6500–6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95).Nagata K., Duggan A., Kumar G., Garcia-Anoveros J. (2005) Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 25, 4052–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96).Voets T., Talavera K., Owsianik G., Nilius B. (2005) Sensing with TRP channels. Nat. Chem. Biol. 1, 85–92. [DOI] [PubMed] [Google Scholar]
- 97).Schofield C.J., Ratcliffe P.J. (2004) Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 5, 343–354. [DOI] [PubMed] [Google Scholar]
- 98).Webb J.D., Coleman M.L., Pugh C.W. (2009) Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell. Mol. Life Sci. 66, 3539–3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99).Bessac B.F., Jordt S.E. (2008) Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100).Gracheva E.O., Ingolia N.T., Kelly Y.M., Cordero-Morales J.F., Hollopeter G., Chesler A.T., Sanchez E.E., Perez J.C., Weissman J.S., Julius D. (2010) Molecular basis of infrared detection by snakes. Nature 464, 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101).Wang Y.Y., Chang R.B., Liman E.R. (2010) TRPA1 is a component of the nociceptive response to CO2. J. Neurosci. 30, 12958–12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102).Lopez-Barneo J., Pardal R., Ortega-Saenz P. (2001) Cellular mechanism of oxygen sensing. Annu. Rev. Physiol. 63, 259–287. [DOI] [PubMed] [Google Scholar]
- 103).Ghezzi P. (2005) Regulation of protein function by glutathionylation. Free Radic. Res. 39, 573–580. [DOI] [PubMed] [Google Scholar]
- 104).Paulsen C.E., Armache J.P., Gao Y., Cheng Y., Julius D. (2015) Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105).Semenza G.L., Wang G.L. (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 12, 5447–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106).Weir E.K., Lopez-Barneo J., Buckler K.J., Archer S.L. (2005) Acute oxygen-sensing mechanisms. N. Engl. J. Med. 353, 2042–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107).Heymans J.F., Bouckaert J.J., Dautrebande L. (1931) Sinus carotidien et reflexes respiratoires; sensibilitédes sinus carotidiens aux substances chimiques. Action stimulante respiratoire réflexe du sulfure de sodium, du cyanure de potassium, de la nicotine et de la lobéline. Arch. Int. Pharmacodyn. Ther. 40, 54–91. [Google Scholar]
- 108).De Sanctis G.T., Green F.H., Remmers J.E. (1991) Ventilatory responses to hypoxia and hypercapnia in awake rats pretreated with capsaicin. J. Appl. Physiol. (1985) 70, 1168–1174. [DOI] [PubMed] [Google Scholar]
- 109).Gruss M., Ettorre G., Stehr A.J., Henrich M., Hempelmann G., Scholz A. (2006) Moderate hypoxia influences excitability and blocks dendrotoxin sensitive K+ currents in rat primary sensory neurones. Mol. Pain 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110).Howe A., Pack R.J., Wise J.C. (1981) Arterial chemoreceptor-like activity in the abdominal vagus of the rat. J. Physiol. 320, 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111).Longhurst J.C., Tjen A.L.S.C., Fu L.W. (2001) Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion. Mechanisms and reflexes. Ann. N. Y. Acad. Sci. 940, 74–95. [DOI] [PubMed] [Google Scholar]
- 112).Kubin L., Alheid G.F., Zuperku E.J., McCrimmon D.R. (2006) Central pathways of pulmonary and lower airway vagal afferents. J. Appl. Physiol. (1985) 101, 618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113).Meller S.T., Gebhart G.F. (1992) A critical review of the afferent pathways and the potential chemical mediators involved in cardiac pain. Neuroscience 48, 501–524. [DOI] [PubMed] [Google Scholar]
- 114).Pokorski M., Takeda K., Sato Y., Okada Y. (2014) The hypoxic ventilatory response and TRPA1 antagonism in conscious mice. Acta Physiol. (Oxf.) 210, 928–938. [DOI] [PubMed] [Google Scholar]
- 115).Gonzalez C., Almaraz L., Obeso A., Rigual R. (1994) Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol. Rev. 74, 829–898. [DOI] [PubMed] [Google Scholar]
- 116).Neubauer J.A., Sunderram J. (2004) Oxygen-sensing neurons in the central nervous system. J. Appl. Physiol. 96, 367–374. [DOI] [PubMed] [Google Scholar]
- 117).Angelova P.R., Kasymov V., Christie I., Sheikhbahaei S., Turovsky E., Marina N., Korsak A., Zwicker J., Teschemacher A.G., Ackland G.L., Funk G.D., Kasparov S., Abramov A.Y., Gourine A.V. (2015) Functional oxygen sensitivity of astrocytes. J. Neurosci. 35, 10460–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118).Bisgard G.E., Forster H.V., Klein J.P. (1980) Recovery of peripheral chemoreceptor function after denervation in ponies. J. Appl. Physiol. 49, 964–970. [DOI] [PubMed] [Google Scholar]
- 119).Curran A.K., Rodman J.R., Eastwood P.R., Henderson K.S., Dempsey J.A., Smith C.A. (2000) Ventilatory responses to specific CNS hypoxia in sleeping dogs. J. Appl. Physiol. (1985) 88, 1840–1852. [DOI] [PubMed] [Google Scholar]
- 120).Davenport H.W., Brewer G., Chambers A.H., Goldschmidt S. (1947) The respiratory responses to anoxemia of unanesthetized dogs with chronically denervated aortic and carotid chemoreceptors and their causes. Am. J. Physiol. 148, 406–416. [DOI] [PubMed] [Google Scholar]
- 121).Miller M.J., Tenney S.M. (1975) Hypoxia-induced tachypnea in carotid-deafferented cats. Respir. Physiol. 23, 31–39. [DOI] [PubMed] [Google Scholar]
- 122).Olson E.B., Jr., Vidruk E.H., Dempsey J.A. (1988) Carotid body excision significantly changes ventilatory control in awake rats. J. Appl. Physiol. (1985) 64, 666–671. [DOI] [PubMed] [Google Scholar]
- 123).Ventura R., Harris K.M. (1999) Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 19, 6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124).Marina N., Teschemacher A.G., Kasparov S., Gourine A.V. (2016) Glia, sympathetic activity and cardiovascular disease. Exp. Physiol. 101, 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125).Halassa M.M., Fellin T., Haydon P.G. (2009) Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology 57, 343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126).Gourine A.V., Llaudet E., Dale N., Spyer K.M. (2005) ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436, 108–111. [DOI] [PubMed] [Google Scholar]
- 127).Marina N., Tang F., Figueiredo M., Mastitskaya S., Kasimov V., Mohamed-Ali V., Roloff E., Teschemacher A.G., Gourine A.V., Kasparov S. (2013) Purinergic signalling in the rostral ventro-lateral medulla controls sympathetic drive and contributes to the progression of heart failure following myocardial infarction in rats. Basic Res. Cardiol. 108, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128).Kimura Y., Mikami Y., Osumi K., Tsugane M., Oka J., Kimura H. (2013) Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J. 27, 2451–2457. [DOI] [PubMed] [Google Scholar]
- 129).Shigetomi E., Jackson-Weaver O., Huckstepp R.T., O’Dell T.J., Khakh B.S. (2013) TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J. Neurosci. 33, 10143–10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130).Shigetomi E., Tong X., Kwan K.Y., Corey D.P., Khakh B.S. (2011) TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 15, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131).Benfenati V., Amiry-Moghaddam M., Caprini M., Mylonakou M.N., Rapisarda C., Ottersen O.P., Ferroni S. (2007) Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience 148, 876–892. [DOI] [PubMed] [Google Scholar]
- 132).Benfenati V., Caprini M., Dovizio M., Mylonakou M.N., Ferroni S., Ottersen O.P., Amiry-Moghaddam M. (2011) An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc. Natl. Acad. Sci. U.S.A. 108, 2563–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133).Butenko O., Dzamba D., Benesova J., Honsa P., Benfenati V., Rusnakova V., Ferroni S., Anderova M. (2012) The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS One 7, e39959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134).Verkhratsky A., Reyes R.C., Parpura V. (2014) TRP channels coordinate ion signalling in astroglia. Rev. Physiol. Biochem. Pharmacol. 166, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135).Grimaldi M., Maratos M., Verma A. (2003) Transient receptor potential channel activation causes a novel form of [Ca2+]i oscillations and is not involved in capacitative Ca2+ entry in glial cells. J. Neurosci. 23, 4737–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136).Malarkey E.B., Ni Y., Parpura V. (2008) Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia 56, 821–835. [DOI] [PubMed] [Google Scholar]
- 137).Taylor C.T., McElwain J.C. (2010) Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology (Bethesda) 25, 272–279. [DOI] [PubMed] [Google Scholar]
- 138).Semenza G.L. (2007) Life with oxygen. Science 318, 62–64. [DOI] [PubMed] [Google Scholar]
- 139).Rich P.R. (2003) The molecular machinery of Keilin’s respiratory chain. Biochem. Soc. Trans. 31, 1095–1105. [DOI] [PubMed] [Google Scholar]
- 140).Margulis L., Bermudes D. (1985) Symbiosis as a mechanism of evolution: status of cell symbiosis theory. Symbiosis 1, 101–124. [PubMed] [Google Scholar]
- 141).Berner R.A. (2009) Phanerozoic atmospheric oxygen: New results using the GEOCARBSULF model. Am. J. Sci. 309, 603–606. [Google Scholar]
- 142).Kitaoka H., Chihara K. (2010) The diaphragm: a hidden but essential organ for the mammal and the human. Adv. Exp. Med. Biol. 669, 167–171. [DOI] [PubMed] [Google Scholar]
- 143).O’Connor P.M., Claessens L.P. (2005) Basic avian pulmonary design and flow-through ventilation in non-avian theropod dinosaurs. Nature 436, 253–256. [DOI] [PubMed] [Google Scholar]
- 144).Jaquemar D., Schenker T., Trueb B. (1999) An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J. Biol. Chem. 274, 7325–7333. [DOI] [PubMed] [Google Scholar]
- 145).Mori Y., Takahashi N., Polat O.K., Kurokawa T., Takeda N., Inoue M. (2016) Redox-sensitive transient receptor potential channels in oxygen sensing and adaptation. Pflugers Arch. 468, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146).Gray J.M., Karow D.S., Lu H., Chang A.J., Chang J.S., Ellis R.E., Marletta M.A., Bargmann C.I. (2004) Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430, 317–322. [DOI] [PubMed] [Google Scholar]
- 147).Hetz S.K., Bradley T.J. (2005) Insects breathe discontinuously to avoid oxygen toxicity. Nature 433, 516–519. [DOI] [PubMed] [Google Scholar]
- 148).Endo H., Okuyama H., Ohue M., Inoue M. (2014) Dormancy of cancer cells with suppression of AKT activity contributes to survival in chronic hypoxia. PLoS One 9, e98858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149).Olsson R., Carlsson P.O. (2011) A low-oxygenated subpopulation of pancreatic islets constitutes a functional reserve of endocrine cells. Diabetes 60, 2068–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150).Pan X., Suzuki N., Hirano I., Yamazaki S., Minegishi N., Yamamoto M. (2011) Isolation and characterization of renal erythropoietin-producing cells from genetically produced anemia mice. PLoS One 6, e25839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151).Suda T., Takubo K., Semenza G.L. (2011) Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 9, 298–310. [DOI] [PubMed] [Google Scholar]