Abstract
The interactions between sugar-containing molecules from the bacteria cell wall and pattern recognition receptors (PRR) on the plasma membrane or cytosol of specialized host cells are the first molecular events required for the activation of higher animal’s immune response and inflammation. This review focuses on the role of carbohydrates of bacterial endotoxin (lipopolysaccharide, LPS, lipooligosaccharide, LOS, and lipid A), in the interaction with the host Toll-like receptor 4/myeloid differentiation factor 2 (TLR4/MD-2) complex. The lipid chains and the phosphorylated disaccharide core of lipid A moiety are responsible for the TLR4 agonist action of LPS, and the specific interaction between MD-2, TLR4, and lipid A are key to the formation of the activated complex (TLR4/MD-2/LPS)2, which starts intracellular signalling leading to nuclear factors activation and to production of inflammatory cytokines. Subtle chemical variations in the lipid and sugar parts of lipid A cause dramatic changes in endotoxin activity and are also responsible for the switch from TLR4 agonism to antagonism. While the lipid A pharmacophore has been studied in detail and its structure-activity relationship is known, the contribution of core saccharides 3-deoxy-d-manno-octulosonic acid (Kdo) and heptosyl-2-keto-3-deoxy-octulosonate (Hep) to TLR4/MD-2 binding and activation by LPS and LOS has been investigated less extensively. This review focuses on the role of lipid A, but also of Kdo and Hep sugars in LPS/TLR4 signalling.
Keywords: lipopolysaccharide, TLR4 (Toll-like receptor 4), MD-2 (myeloid differentiation factor 2), Kdo (3-deoxy-d-manno-octulosonic acid)
1. Innate Immunity Response in Higher Organisms to Pathogen-Associated Molecular Patterns
Mammalian innate immunity relies on a family of pattern recognition receptors (PRRs) to detect conserved microbial molecules, termed pathogen-associated molecular patterns (PAMPs) [1,2]. PRRs engagement by microbial PAMPs activates inflammatory pathways, such as the nuclear factor kappa B (NF-κB) and interferon regulatory factor (IRF) signalling for cytokine transcription and the clearance of the infections. The PRR family includes Toll-like receptor (TLR), C-type lectin receptor (CLR), RIG-I-like receptor (RLR), AIM2-like receptor (ALR), and NOD-like receptors (NLR) containing a Nucleotide-binding domain (NBD) and a Leucine-rich Repeat (LRR) domain. TLR and CLR are localized on the plasma or endosomal membranes, while RLR, ALR, and NLR are cytoplasmic [3,4]. The host receptorial system is redundant in the sense that a collection of structurally diverse PRRs recognizes the same bacterial cell wall component. PRRs then induce various signalling pathways that depend on structurally diverse signalling components [5].
Some of the most inflammatory PAMPs, such as lipopolysaccharide (LPS), lipoproteins, peptidoglycan (PGN), and flagellin, are molecules derived from the bacterial cell wall and are recognized, respectively, by specific PRRs.
Organic chemistry synthesis of chemically homogeneous molecules greatly contributed to the unequivocal determination of the minimal and chemically defined structure that is essential for the immunostimulating actions of LPS [6,7,8]. LPS is composed of three distinct domains (Figure 1) that are covalently linked to each other, which are genetically, biosynthetically, biologically, and chemically distinct: a glycolipid portion termed lipid A, a glycan, and between them a core oligosaccharide.
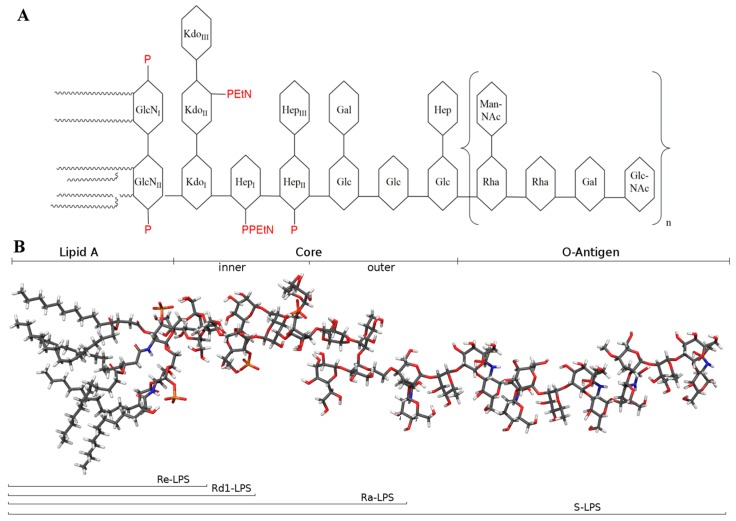
Figure 1.
E. coli lipopolysaccharide (LPS): smooth-lipopolysaccharide (S-LPS) is composed by lipid A, core and O-antigens; truncated rough-LPS (R-LPS) are named Ra, Rd1, and Re depending on the number of sugar units of the core. (A) Schematic representation (B) Chemical structure
The glycan usually is the O-specific polysaccharide (or O-antigen). The membrane-anchoring portion called lipid A is also the biologically active unit (endotoxic principle). The first chemically synthesised lipid A [9], according to the newly elucidated structure, exhibited full activity described for LPS endotoxin.
In the classical nomenclature [10] (Figure 1) LPS is identified by the appearance of the corresponding bacterial colony surface: smooth colonies express complete LPS that has been accordingly termed smooth-lipopolysaccharide (S-LPS). Mutants that express LPS lacking O-antigens form colonies with rough appearance, so that the corresponding truncated LPS variants have been named rough-LPS (R-LPS). However, not all of the mutants lose all O-antigen, so that there are three types of R-LPS (Figure 1). Ra-LPS mutant is composed of the Lipid A and the complete oligosaccharide core, Rd1-LPS mutant is formed by Lipid A and the inner core and the smaller variant, Re-LPS, also called deep rough mutant, only contains two or three Kdo units linked to Lipid A [11,12].
Complete LPS (S-LPS), and its truncated rough variants (R-LPS) including lipid A (Figure 1) are generally defined as endotoxin and have potent pro-inflammatory and immunostimulating action.
Free LPS released from gram-negative cell wall, in form of single molecules or aggregates, is detected by the Toll-like Receptor 4 (TLR4), which is mainly expressed on the surface of haematopoietic cells including monocytes, dendritic cells, and macrophages [11].
A recent review by Kieser and Kagan [5] presents an updated, more complex picture of the LPS recognition by innate immunity cells, suggesting that several membrane and cytosolic receptors are activated by LPS variants. Membrane receptor Brain-specific angiogenesis inhibitor 1 (BAI1) also detects LPS at the bacteria surface and, after promoting phagocytosis, triggers reactive oxygen species (ROS) production and the induction of inflammation. Moreover, LPS that has reached the cytosol is recognized by caspases, initiating formation of the non-canonical inflammasome [12].
From a molecular point of view, glycoconjugate/protein interactions are the key events of activation of innate immunity PRRs, including cytosolic caspases, triggering cytokine production and inflammation. In this review, we will focus on molecular details of sugar-protein interactions in the case of TLR4/MD-2/LPS signalling, the most important and studied pathway in innate immunity and inflammation.
2. Physicochemical Properties of LPS and Molecular Mechanism of LPS/TLR4 Signalling
The amphiphilic character of endotoxin chemical variants (S-LPS, R-LPS or lipid A), results in the formation of micelles in aqueous environment above their critical micellar concentration (CMC) [13]. CMC values between 10−8 M and 10−7 M for deep rough mutant (Re-LPS) [14,15], and between 1.3 and 1.6 μM for E. coli S-LPS [16], were reported. In balanced salts solutions containing physiologic extracellular concentrations of Mg2+ and Ca2+, CMC values of 1 nM or lower are likely [17,18].
From these data and from the fact that LPS aggregates are usually highly stable, aggregated forms of LPS should predominate in the concentration range that is relevant for biological responses. In physiological fluids, LPS aggregates were also found as membrane “blebs”, which are constitutively released from growing Gram-negative bacteria. Transmission electron microscopy (TEM) revealed that blebs exist predominantly as vesicles with an average size of 40–80 nm [19].
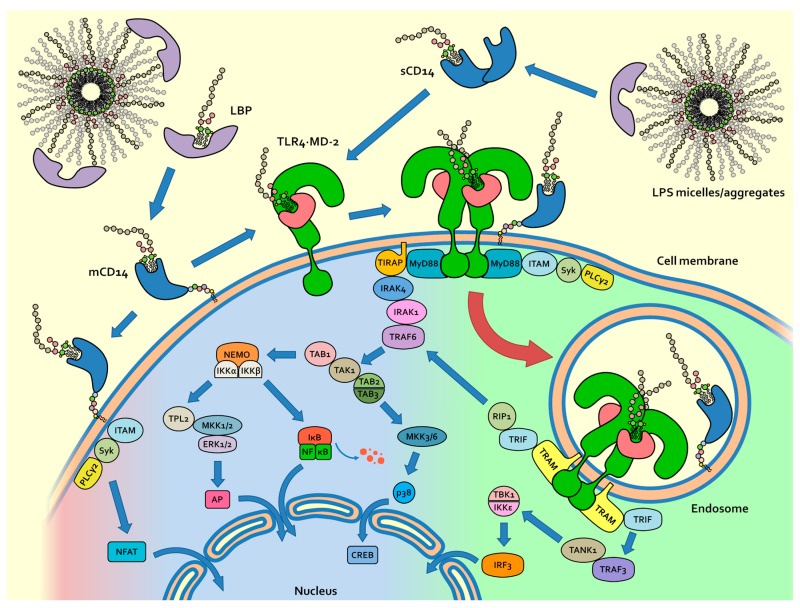
The current view of mammalian endotoxin sensing and signalling (Figure 2) is that it is initiated by the lipid-binding protein (LBP), which is able to extract LPS monomer from the aggregates and to transfer it to the cluster of differentiation 14 (CD14) [20,21]. CD14, then, transfer the LPS monomer to Myeloid Differentiation factor 2 (MD-2) adaptor in the final hexamer complex (TLR4/MD-2/LPS)2 (Figure 2) [22,23,24,25]. This unidirectional flow of LPS from LBP to TLR4/MD-2 is explained by the increasing affinity of each LPS receptor for its ligand [26]. TLR4 crosslinking through the formation of the (TLR4/MD-2/LPS)2 complex is the first step in the inflammatory process. The CD14 receptor, in the membrane-bound form, promotes the formation of hexamer complex (TLR4/MD-2/LPS)2 by binding monomeric LPS and transferring it to MD-2, thus initiating the Myeloid differentiation primary response 88 (MyD88)-dependent intracellular signalling [26,27]. CD14 also plays a fundamental role in the endocytosis of (TLR4/MD-2/LPS)2 and in subsequent intracellular signalling based on triffosome formation [28,29,30,31,32].
Figure 2.
TLR4 activation and signalling from cell membrane and from endosomes: MyD88 and TRIF-dependent pathways are distinct signal pathways leading to cytokine production.
In general, the activation of TLRs by their ligands is the first molecular event of innate immunity, preceding and triggering cytokine production, inflammation and adaptive immune response [33]. From a pharmacological point of view, TLR4 stimulation by non-toxic LPS/lipid A variants is considered as an innovative approach towards potent and selective immunostimulants to be used as vaccine adjuvants and in tumor immunotherapy [34,35]. On the other hand, natural LPS variants or synthetic molecules that inhibit the formation of (TLR4/MD-2/LPS)2 hexamer complex by competing with LPS or other agonists for TLR4/MD-2 and/or CD14 binding are interesting drug candidates targeting diseases that are caused by excessive TLR4 activation by bacterial LPS (sepsis and septic shock) and by endogenous molecules (inflammatory and autoimmune diseases) [36].
The knowledge of the molecular aspects of LPS recognition by CD14 and TLR4/ MD-2 is essential to understand the different TLR4-mediated responses to different LPS/lipid A variants or chemotypes, whose activity varies from toxic (TLR4 agonists), to non-toxic and even LPS with endotoxin-neutralizing activity, corresponding to TLR4 antagonism. The same molecular mechanisms play a fundamental role in innate memory and tolerance to LPS [37]. Finally, the rational design of TLR4 agonists and antagonists as drug candidates relies on the precise knowledge of the molecular aspects of the interaction between lipid A and CD14 and/or MD-2 receptors.
3. Structure-Activity Relationship in Natural and Synthetic Lipid A Variants
The chemical pattern consisting in the disaccharide β(1–6) glucosamine core with two phosphates at C1 and C4′ positions and branched or linear fatty acid chains linked to C2, C3, C2′, and C3′ positions is highly conserved in lipid As of different bacterial species. It is the pharmacophore associated to endotoxic activity. This motif can be found in the natural and synthetic lipid A variants that bind and activate TLR4, thus behaving as agonists. The structural variations of lipid A found in different bacterial species (different acylation patterns, variation in the chemical structure and length of fatty acid chains, covalent modification of phosphate groups), are associated to different biological activity.
Natural Lipid A variants produced by E. coli, Neisseria meningitidis, Campylobacter jejuni, Salmonella minnesota or tiphimurium, Rhodobacter capsulatus or sphaeroides species, as well as biosynthetic precursor lipid IVa and synthetic analogue Eritoran [38] (Figure 3), provided complete information on structure-activity relationship (SAR) of this class of compounds.
Figure 3.
Natural lipid A from Salmonella minnesota, Salmonella typhimurium, Neisseria meningitides (with and without Kdo), Escherichia coli, Campylobacter jejuni, Rhodobacter capsulatus and Rhodobacter sphaeroides; the biosynthetic lipid A precursor: Lipid IVa and the synthetic molecule Eritoran. The numbers of carbon atoms of each fatty acid chain are displayed in blue.
Lipid A variants differ in the acylation pattern, that is the location of fatty acid chains on the disaccharide: 4 + 3 (corresponding to four chains attached to the non-reducing and three chains in the reducing glucosamine) in S. tiphimurium, 4 + 2 in E. coli, C. jejuni and S. minnesota, 3 + 3 in N. meningitidis, 3 + 2 in R. capsulatus and sphaeroides, 2 + 2 in Lipid IVa. Synthetic Eritoran presents a 2 + 2 arrangement as well. Lipid As also presents different composition and length of chains (E. coli contains mainly C14 chains while N. meningitides contains mainly C12 chains).
Crucial information on SAR of lipid A variants has been collected by Boons and co-workers by means of fully synthetic derivatives [39]. This study highlighted that synthetic lipid A from N. meningitidis was significantly more potent than E. coli lipid A. According to these results, the 3 + 3 arrangement of fatty acid chains in N. meningitidis is associated with a stronger endotoxic activity than the 4 + 2 disposition found in E. coli. Moreover, shorter fatty acids chains with 12 instead of 4 carbon atoms are associated to a higher potency in TLR4 stimulation (1–2 orders of magnitude) [39].
It is quite unusual to observe unsaturated fatty acids in lipid A, but it has been reported in Rhodobacter sphaeroides and R. capsulatus LPS and in the Enterobacteriaceae family when they grow at low temperature. Campylobacter jejuni LPS is an exception, in which GlcN are replaced by 2,3-diamino-2,3-dideoxy-d-glucose (GlcN3N) [40] (Figure 3).
In some natural lipid A variants, phosphates in positions C1 and C4′ are covalently functionalized with phosphate, ethanolamine, ethanolamine phosphate, ethanolamine di-phosphate (C. jejuni), and sugars as glycosylamine, 4-amino-4-deoxy-l-arabinopyranose and d-arabinofuranose [41].
In general, while the presence of six fatty acid (FA) chains is associated with endotoxic action (TLR4 agonism), variants with 7 FA, as Salmonella typhimurium, or with less than 6 FA, as in lipid IVa or in the synthetic derivative Eritoran, are on the contrary associated to non-toxic or even anti-endotoxic activity. The anti-endotoxic activity is defined as the competitive antagonist action of lipid A or LPS variants that, when co-administered with endotoxin, neutralize its TLR4 activating effect. A general pharmacophore can be associated to antagonism, consisting of four or five FA that are linked to a disaccharide core bearing in C1 and C4′ positions in one or two negatively charged groups (phosphates or their bioisosteres) [42].
Monosaccharide-based TLR4 modulators allowed for extending the agonism/antagonism rules to the monosaccharide scaffold. The 3:1 ratio between lipid chains and phosphate groups present in lipid A seem an important prerequisite to have agonistic activity both in lipid A variants and in monosaccharides derivatives [43]. Synthetic monosaccharides derivatives with one or two phosphates and two to four fatty acid chains showed in some cases potent TLR4 antagonism in cells and in vivo [44,45,46,47]. In mono- and di-saccharides scaffolds, the phosphate groups have been substituted with bioisosteric, negatively charged carboxylates, and showed similar activities [48,49,50,51].
The elucidation of new lipid A chemical structures deriving from bacteria species is a field in continuous evolution, and SAR in new lipid A types will provide important information for designing new synthetic TLR4 modulators [41,52].
In addition, some natural or synthetic molecules having a chemical structure that is unrelated to lipid A can bind to MD-2 and activate TLR4. The natural compounds Taxol and some opioids activate TLR4 [53,54]. Synthetic pyrimido[5,4-b]indoles [55] and 4-substituted aminoquinazolines [56] were shown to specifically activate TLR4 in a MD-2 dependent and CD14 independent manner, in both mouse and human cells. Synthetic compounds with a linear scaffold instead of glucosamine disaccharide such as ER-112022 [57] are active as TLR4 agonist, thus showing that the disaccharide connecting the two anionic phosphates can be substituted by chemical spacers of different chemical structure and with increased conformational mobility. More recently, new classes of non-LPS-like small molecules, the neoseptins [58,59], Euodenine A and analogues [60], have been reported to be MD-2-dependent TLR4 agonists [5,59,61,62,63].
4. Role of Sugar/Protein and Lipid/Protein Interactions in the Formation and Stability of TLR4/MD-2/Endotoxin Complex
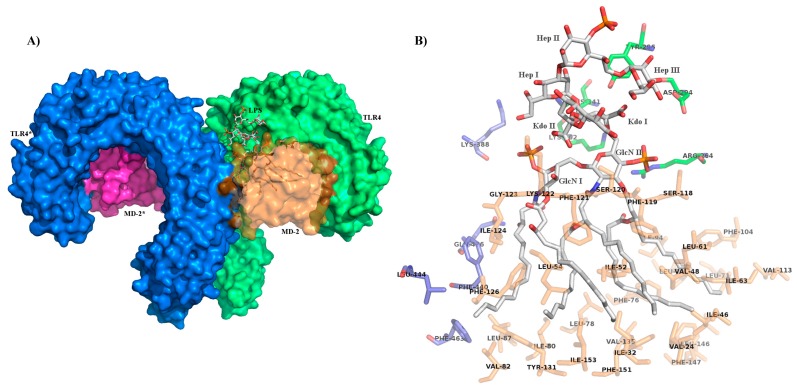
The crystal structures of the hexamer complex (TLR4/MD-2/LPS)2, in which two TLR4 are crosslinked [22] (Figure 4A), has revealed that binding of LPS (Ra-LPS from E. coli) to MD-2 induces agonist-dependent contacts with the C-terminal domain of the second TLR4 molecule of the hexamer (TLR4*). This binding mode is typical of agonists since it is responsible for TLR4 dimerization and activation. Five of the six aliphatic chains of lipid A moiety are deeply inserted into the hydrophobic pocket of MD-2. The FA chain linked to C2 of GlcN I protrudes from MD-2 binding cavity forming, with MD-2 residues (V82, M85, L87, I124, and F126), a binding interface that interacts with hydrophobic residues on the surface of TLR4* (mainly F440*, L444*, and F463*), thus promoting the assembly of the activated hexamer. Additional hydrophilic interactions are also present and involve lipid A’s hydroxyls and phosphates groups. These groups interact with MD-2 and TLR4* residues and stabilize the hexameric complex (Figure 4B: C1 phosphate interacts with K388*, K341 and K362 for the two TLR4 as well as K122 for MD-2; C4′ phosphate is in contact with R264, K362 for TLR4, and K58, S118 for MD-2. Moreover, C4-hydroxyl group of the GlcN I interact with K122 residue).
Figure 4.
Picture of the interactions between LPS, (TLR4/MD-2) complexes (A) (TLR4/MD-2/LPS)2 signalling complex; (B) E. coli Lipid A with inner-core and its interactions with MD-2 (residues in orange) and TLR4 (residues in green) of the same complex and with the second TLR4 molecule, TLR4* (blue), as observed by crystallography (PDBID: 3FXI). LPS is displayed in gray for carbon atoms, red for oxygen, blue for nitrogen and dark orange for phosphate.
Upon lipid A binding, MD-2 experiences a local conformational change, which involves the side chain of F126 and its immediate neighbours [64]. The interaction between lipid A and TLR4/MD-2 has been investigated by NMR in solution and suggests a dynamic role of F126. This phenylalanine residue is located at the rim of the LPS binding cavity of MD-2, and acts as a conformational switch allowing, or not, the formation of activated hexamer [65].
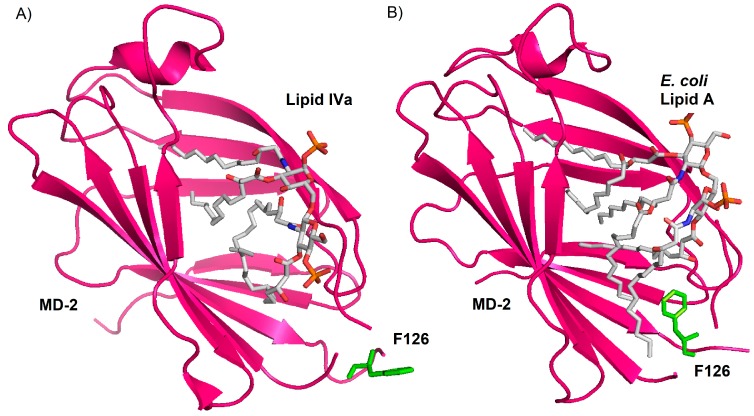
In contrast to hexa-acylated lipid A forms, tetra-acylated lipid IVa is a weak agonist in murine TLR4/MD-2, but is an antagonist in human TLR4/MD-2. The crystal structures of human and mouse TLR4/MD-2 in the complex with tetra-acylated lipid IVa provided the structural basis of this species-specific agonistic or antagonistic activities [66,67]. In human MD-2 complex with lipid IVa, the GlcN-P backbone of lipid IVa was shifted upward and rotated by about 180° in comparison to hexa-acylated LPS [64,68] (Figure 5).
Figure 5.
(A) Cristal structure of human MD-2 with Lipid IVa (PDBID: 2E59); (B) Cristal structure of human MD-2 with E. coli Lipid A (PDBID: 3FXI). Human MD-2 is displayed in purple as ribbon, F126 are displayed in green as sticks and Lipid IVa and Lipid A are displayed according to the color of the atoms as sticks.
This binding mode is not productive and it does not promote the formation of the signalling complex, it is typical of antagonists. Eritoran binds both human and mouse MD-2 with the disaccharide core rotated of 180° respect to agonist lipid A [69]. The interactions of hydrophilic and hydrophobic groups of lipid A with MD-2 and TLR4* have been studied in order to understand the affinity of lipid A variants with the receptor complex, and to explain the switch from agonism to antagonism [61,70,71]. The ligand-based design of new synthetic TLR4 agonist and antagonists has been guided so far by the necessity to reproduce the most important supramolecular interactions among lipid A, MD-2, TLR4, and TLR4* [46,72,73,74].
5. Role of the Sugar Kdo in the TLR4 Interaction of Natural and Synthetic LPS Variants
The LPS inner core (Figure 1) is typically composed by two or three Kdo units, linked via an α-(2 → 6) linkage to the distal GlcN of the lipid A backbone and three units of Hep. The outer core is composed by more common monosaccharides, such as Glucose (Glc) and galactose (Gal) [75]. The inner core structure is reasonably well conserved (Figure 1) [76], and it is therefore an epitope that is capable of inducing a specific antibody to cross-react with the LPS of many different strains of Gram-negative LPS. Immuno-response directed against the inner core has been exploited in vaccines to elicit widely cross-reactive antibodies in human sera [77] and in the production of a monoclonal antibody that is able to bind to a wide range of E. coli and Salmonella LPSs [78,79].
It has long been thought that the inflammatory properties of S-LPS and R-LPS reside only in the lipid A moiety [6,80], and that the nature and number of core saccharides do not have major impact on modulating endotoxicity [81].
From a structural point of view, the inner core sugars, three units of 3-deoxy-d-manno-2-octulosonic acid (Kdo I, II, III) and three units of heptosyl-2-keto-3-deoxy-octulosonate (Hep I, II, III) seem to contribute only marginally to the interactions with receptors. However, Kdo I, Hep I, and Hep III are interacting with the TLR4 residues Y296, K341, and D294, respectively (Figure 4). The fact that the inner core sugar interacts only with TLR4, and not with MD-2 and TLR4* as lipid A, confirms that the presence of the inner core sugars is not essential for the endotoxic activity of LPS [9,80], but also suggests that such additional interaction with TLR4 could be important for increasing LPS binding affinity and specificity to the MD-2/TLR4 heterodimer.
Additionally, recent studies have indicated that Kdo moieties of the inner core are likely to contribute to inflammatory responses [7,82,83,84,85,86,87]. It has also been proved that the N. meningitidis Ra-LPS is a potent agonist of MyD88-dependent and independent cytokines [88].
Several other studies have indicated that Kdo moieties of LPS and/or Ra-LPS importantly contribute to inflammatory responses and to TLR4 activation and signalling [85,89].
Boons and co-workers used chemically pure synthetic lipid A variants, with or without Kdo glycosylation [39], and showed that the activity of N. meningitidis Re-LPS in eliciting TNF-α and IFN-β production in murine macrophages was significantly higher compared to the synthetic N. meningitidis lipid A. As well, the induction of TNF-α was dramatically decreased, to levels like those of the lipid A, when penta-acylated Ra-LPS was subjected to mild acid hydrolysis (pH 4.3), thus separating lipid A from Kdo.
The loss of the two Kdo from penta-acylated meningococcal Re-LPS resulted in a dramatic attenuation in biologic activity [90]. It is worth noting that N. meningitidis Re-LPS is recognized by the CD14, TLR4/MD-2 receptors of both human and murine cells. It suggests that the two Kdo of Re-LPS are not determinant for species-specific differentiation, noted for other LPS structures.
Zughaier et al. compared the responses of human monocyte derived dendritic cell (MDDC) to Neisseria meningitidis LPS, Re-LPS, and lipid A [85]. These three endotoxin variants were obtained from genetically modified bacteria to afford highly controlled serotypes [91,92,93]. It was found that similar to LPS, Re-LPS induced MDDC maturation and significantly up-regulated the expression of CD80 and CD86 co-stimulatory molecules, the MDDC maturation marker, CD83 and HLADR (Human Leukocyte Antigen D Related). On the contrary, lipid A was much less active in inducing the production of the same co-stimulatory molecules. Again, these data suggest that the presence of the two Kdo in Re-LPS greatly increases the activity of lipid A and the authors concluded that Re-LPS is the minimal structure required to induce optimal MDDC maturation and activation.
The same authors produced different endotoxin variants in N. meningitidis and compared their activities in terms of cytokine release (TNF-α) and inflammatory mediators (nitric oxide) in macrophages. By a combination of genetic modification and mild acidic hydrolysis of S-LPS they obtained Rd1-LPS, Re-LPS, and lipid A [94,95,96]. They observed that Rd1-LPS and Re-LPS have similar activities while lipid A is ~10-fold less active. These data suggest that, while Kdo presence is necessary to optimal TLR4 agonism, the Hep units are dispensable [84]. Boons et al. examined the activity of a range of chemically defined, synthetic Lipid As [39,97,98] that were derived from E. coli, S. typhimurium, S. minnesota, and N. meningitidis bacterial strains on mouse macrophages and dendritic cells. It was found that the N. meningitidis Re-LPS was more potent than the Re-LPS from E. coli, which, in turn, were significantly more potent that the synthetic E. coli and N. meningitidis Lipid A [99].
In this case too, the two Kdo units linked to N. meningitidis lipid A were essential to TLR4 activity, even more important than having the two phosphate groups. Monophosphorylated N. meningitidis Lipid A was only slightly less active than the diphosphorylated N. meningitidis Lipid A, and N. meningitidis Re-LPS structures with variable 1 or 4’ phosphorylation were equal agonists. [100].
Muroi and Tanamoto observed similar trend in the case of Salmonella endotoxins activity on human THP-1 cells and TLR4-transfected HEK cells. In this case, the order of endotoxic activity was also S-LPS ≈ Re-LPS >> Lipid A. [82].
Schromm et al. [101] found that the number, nature, and location of negatively charged Kdo monosaccharide units modulate the molecular conformation of E. coli LPS and Lipid A, and that conformation is tightly linked to endotoxicity. Recently, synthetic Re-LPS (containing two or three Kdo) was found to have an enhanced agonist activity compared to Lipid A linked to one Kdo and Lipid A alone [7,42]. A decreased number or lack of Kdo and hydroxymyristic acid is proposed as main contributors to low endotoxic activity of Leptospira interrogans [102], Francisella tularensis [103], Legionella pneumophila [104], and different Rhizobium species LPSs [105].
From a structural point of view, the negatively charged Kdo sugars of LPS may facilitate the binding to leucine-rich repeats (LRR) of TLR4 (most likely to residues 190 to 194 that contain positively charged amino acids [106]) The presence of Kdo moiety plays also a fundamental role in the conformational change of the glucosamine backbone [101]. When linked to Kdo, an upward shift of GlcN I was observed (~4 Å) [22]. This shifted conformation permits to have an additional space for R2 and R3 acyl chains and bring phosphate group closer to TLR4 protein [107].
6. Conclusions and Future Perspectives
While the polysaccharide O-chain seems dispensable to TLR4 activation and signalling, sugars of the core oligosaccharide play a significant role in TLR4 activation. Kdo is important for agonist activity: fully synthetic lipid A containing Kdo units (also called Re-LPS) are always more active than their counterparts lacking Kdo [39]. This increase in the agonist activity is somehow not paralleled by the number of interactions between the Kdo units and MD-2/TLR4/TLR4*, as observed by crystallography: just one interaction per Kdo unit. It should however be considered that the number of interactions actually observed with X-ray may not be exhaustive, since Kdo and Hep carbohydrates protrude from MD-2 binding site, the high rotational freedom of core oligosaccharide can hamper the observation of the interactions with the TLR4/MD-2 complex. The Kdo role in TLR4 antagonism has not, or has only partially been investigated. In analogy with agonists, also antagonists should benefit from additive interaction of Kdo units with TLR4/MD-2 complex. Thanks to the production of lipid A variants with different number of Kdo units linked by chemical [7] or enzymatic [108] processes, it can be assessed that Re-LPS is the minimal chemical motif required for the maximal activation of (TLR4/MD-2/LPS)2 complex.
The synthesis of lipid A mimetics, bearing one to three Kdo units linked with non-natural bonds resistant to enzymatic hydrolysis, would provide a new generation of TLR4 antagonists that should improve potency and specificity.
Another important parameter that determines the efficiency of agonist and antagonist presentation to the TLR4/MD-2 receptor complex is the tendency of the ligands to form supramolecular aggregates in solution. The amphiphilic character of natural lipid As and synthetic lipid A analogues favors the formation of aggregates in solution. The stability of aggregates determines, in turn, the affinity of the ligands for LBP, the first receptor of the extracellular LPS/TLR4 signal pathway. In this context, the addition of hydrophilic sugar moieties to lipid A analogues could improve water solubility, increase the CMC values of molecules, and favor interaction with LBP.
In addition, the role of Kdo and Hep sugars in the binding with LBP and CD14 receptors has still to be investigated. Due to the important function of these two LPS-binding proteins in determining the efficiency of agonist or antagonist presentation to the final TLR4/MD-2 complex, an increase in affinity due to the presence of additive sugars could greatly influence the type and intensity of TLR4 response. CD14 has a leading role in the process of endocytosis of TLR4/MD-2 complex ending by intracellular signalling through the TRAM/TRIF complex formation. The differential affinity of different LPS forms for CD14 (complete S-LPS versus R-LPS lacking the O-chain) was clearly evidenced.
This review presents some examples in which the presence of Kdo in natural or synthetic lipid A variants (mono- or di-saccharides) improves the TLR4 activity if compared to simple lipid A or complete LPS with O-chain. The synthesis and the biological characterization of lipid A analogues glycosylated with Kdo and/or Hep (or mimetics of these sugars) is still largely unexplored.
We suggest that glycosylated lipid A analogues can provide in the next future a new generation of synthetic molecules to be developed as drugs targeting TLR4 signal.
Acknowledgments
This study was financially supported by the H2020-MSC-ETN-642157 project “TOLLerant”. We acknowledge the Italian Ministry for Foreign Affairs and International Cooperation (MAECI) and Roberto Cighetti for Figure 2.
Abbreviations
| AIM2 | Interferon-inducible protein (Absent In Melanoma 2) |
| ALR | AIM2-Like Receptor |
| CD14 | Cluster of Differentiation 14 |
| CD80 | Cluster of Differentiation 80 |
| CD83 | Cluster of Differentiation 83 |
| CD86 | Cluster of Differentiation 86 |
| CLR | C-type Lectin Receptor |
| CMC | Critical Micellar Concentration |
| FA | Fatty acid |
| Gal | Galactose |
| Glc | Glucose |
| GlcN | Glucosamine |
| GlcN3N | 2,3-diamino-2,3-dideoxy-d-glucose |
| GlcNAc | N-Acetyl-Glucosamine |
| GPI | Glycosylphosphatidylinositol |
| HEK | Human Embryonic Kidney Cell 293 |
| Hep | heptosyl-2-keto-3-deoxy-octulosonate |
| HLADR | Human Leukocyte Antigen D Related |
| IL | Interleukin |
| INF | Interferon |
| IRF | Interferon Regulatory Factor |
| IRF3 | Interferon Regulatory Factor 3 |
| Kdo | 3-Deoxy-d-manno-oct-2-ulosonic acid |
| LBP | Lipid Binding Protein |
| LOS | Lipooligosaccharide (Hep3-Kdo2-lipid A) |
| LPS | Lipopolysaccharide |
| LRR | Leucine-Rich Repeat |
| MC | Mast Cells |
| MD-2 | Myeloid Differentiation factor 2 |
| MDDC | Human Monocyte Derived Dendritic Cell |
| MurNAc | N-Acetyl-Muramic acid |
| MyD88 | Myeloid differentiation primary response gene 88 |
| NBD | Nucleotide-Binding Domain |
| NF-κB | Nuclear Factor-kappa B |
| NLR | NOD-Like Receptors |
| NMR | Nuclear Magnetic Resonance |
| NOD 1 & 2 | Nucleotide-binding Oligomerization Domain receptor 1 & 2 |
| PAMP | Pathogen Associated Molecular Pattern |
| PGN | Peptidoglycan |
| PRR | Pattern Recognition Receptor |
| Ra-LPS | Lipid A + core |
| Re-LPS | (Kdo)2-Lipid A |
| RIG-1 | Retinoic Acid Inducible Gene 1 |
| R-LPS | Rough LPS |
| RLR | RIG-I-Like Receptor |
| SAR | Structure Activity Relationship |
| S-LPS | Smooth LPS |
| TANK | TRAF family member-associated NF-κ-B activator |
| TBK1 | TANK-Binding Kinase 1 (Serine/Threonine-protein Kinase TBK1) |
| TEM | Transmission Electron Microscopy |
| THP-1 | Human Leukemic Monocyte Cell Line |
| TIR | Toll/interleukin-1 receptor |
| TLR4 | Toll Like Receptor 4 |
| TNF | Tumor Necrosis Factor |
| TRAM | TNF Receptor Associated Modulator |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Janeway C.A., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi O., Akira S. Pattern Recognition Receptors and Inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Chen G., Shaw M.H., Kim Y.-G., Nuñez G. NOD-Like Receptors: Role in Innate Immunity and Inflammatory Disease. Annu. Rev. Pathol. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 4.Song D.H., Lee J.-O. Sensing of microbial molecular patterns by Toll-like receptors. Immunol. Rev. 2012;250:216–229. doi: 10.1111/j.1600-065X.2012.01167.x. [DOI] [PubMed] [Google Scholar]
- 5.Kieser K.J., Kagan J.C. Multi-receptor detection of individual bacterial products by the innate immune system. Nat. Rev. Immunol. 2017;17:376–390. doi: 10.1038/nri.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imoto M., Yoshimura H., Sakaguchi N., Kusumoto S., Shiba T. Total synthesis of lipid A. Tetrahedron Lett. 1985;26:1545–1548. doi: 10.1016/S0040-4039(00)98548-4. [DOI] [Google Scholar]
- 7.Yoshizaki H., Fukuda N., Sato K., Oikawa M., Fukase K., Suda Y., Kusumoto S. First Total Synthesis of the Re-Type Lipopolysaccharide. Angew. Chem. Int. Ed. 2001;40:1475–1480. doi: 10.1002/1521-3773(20010417)40:8<1475::AID-ANIE1475>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Uehara A., Fujimoto Y., Kawasaki A., Kusumoto S., Fukase K., Takada H. Meso-Diaminopimelic Acid and Meso-Lanthionine, Amino Acids Specific to Bacterial Peptidoglycans, Activate Human Epithelial Cells through NOD1. J. Immunol. 2006;177:1796–1804. doi: 10.4049/jimmunol.177.3.1796. [DOI] [PubMed] [Google Scholar]
- 9.Homma J.Y., Matsuura M., Kanegasaki S., Kawakubo Y., Kojima Y., Shibukawa N., Kumazawa Y., Yamamoto A., Tanamoto K., Yasuda T., et al. Structural Requirements of Lipid A Responsible for the Functions: A Study with Chemically Synthesized Lipid A and Its Analogues. J. Biochem. 1985;98:395–406. doi: 10.1093/oxfordjournals.jbchem.a135294. [DOI] [PubMed] [Google Scholar]
- 10.Huber M., Kalis C., Keck S., Jiang Z., Georgel P., Du X., Shamel L., Sovath S., Mudd S., Beutler B., et al. R-form LPS, the master key to the activation of TLR4/MD-2-positive cells. Eur. J. Immunol. 2006;36:701–711. doi: 10.1002/eji.200535593. [DOI] [PubMed] [Google Scholar]
- 11.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Yang J., Zhao Y., Shao F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr. Opin. Immunol. 2015;32:78–83. doi: 10.1016/j.coi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Gutsmann T., Schromm A.B., Brandenburg K. The physicochemistry of endotoxins in relation to bioactivity. Int. J. Med. Microbiol. 2007;297:341–352. doi: 10.1016/j.ijmm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Takayama K., Din Z.Z., Mukerjee P., Cooke P.H., Kirkland T.N. Physicochemical properties of the lipopolysaccharide unit that activisit. J. Biol. Chem. 1990;265:14023–14029. [PubMed] [Google Scholar]
- 15.Takayama K., Mitchell D.H., Din Z.Z., Mukerjee P., Li C., Coleman D.L. Monomeric Re lipopolysaccharide from Escherichia coli is more active than the aggregated form in the Limulus amebocyte lysate assay and in inducing Egr-1 mRNA in murine peritoneal macrophages. J. Biol. Chem. 1994;269:2241–2244. [PubMed] [Google Scholar]
- 16.Yu L., Tan M., Ho B., Ding J.L., Wohland T. Determination of critical micelle concentrations and aggregation numbers by fluorescence correlation spectroscopy: Aggregation of a lipopolysaccharide. Anal. Chim. Acta. 2006;556:216–225. doi: 10.1016/j.aca.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Giardina P.C., Gioannini T., Buscher B.A., Zaleski A., Zheng D.S., Stoll L., Teghanemt A., Apicella M.A., Weiss J. Construction of Acetate Auxotrophs of Neisseria meningitidis to Study Host-Meningococcal Endotoxin Interactions. J. Biol. Chem. 2001;276:5883–5891. doi: 10.1074/jbc.M009273200. [DOI] [PubMed] [Google Scholar]
- 18.Iovine N., Eastvold J., Elsbach P., Weiss J.P., Gioannini T.L. The carboxyl-terminal domain of closely related endotoxin-binding proteins determines the target of protein-lipopolysaccharide complexes. J. Biol. Chem. 2002;277:7970–7978. doi: 10.1074/jbc.M109622200. [DOI] [PubMed] [Google Scholar]
- 19.Post D.M.B., Zhang D., Eastvold J.S., Teghanemt A., Gibson B.W., Weiss J.P. Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J. Biol. Chem. 2005;280:38383–38394. doi: 10.1074/jbc.M508063200. [DOI] [PubMed] [Google Scholar]
- 20.Schromm A.B., Brandenburg K., Rietschel E.T., Flad H.D., Carroll S.F., Seydel U. Lipopolysaccharide-binding protein mediates CD14-independent intercalation of lipopolysaccharide into phospholipid membranes. FEBS Lett. 1996;399:267–271. doi: 10.1016/S0014-5793(96)01338-5. [DOI] [PubMed] [Google Scholar]
- 21.Schumann R.R. Function of lipopolysaccharide (LPS)-binding protein (LBP) and CD14, the receptor for LPS/LBP complexes: A short review. Res. Immunol. 1992;143:11–15. doi: 10.1016/0923-2494(92)80074-U. [DOI] [PubMed] [Google Scholar]
- 22.Park B.S., Song D.H., Kim H.M., Choi B.-S., Lee H., Lee J.-O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 23.Wright S.D., Ramos R.A., Tobias P.S., Ulevitch R.J., Mathison J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 24.Ulevitch R.J., Tobias P.S. Recognition of Gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 1999;11:19–22. doi: 10.1016/S0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Q., Akashi S., Miyake K., Petty H.R. Cutting Edge: Lipopolysaccharide Induces Physical Proximity Between CD14 and Toll-Like Receptor 4 (TLR4) Prior to Nuclear Translocation of NF-κB. J. Immunol. 2000;165:3541–3544. doi: 10.4049/jimmunol.165.7.3541. [DOI] [PubMed] [Google Scholar]
- 26.Gioannini T.L., Teghanemt A., Zhang D., Coussens N.P., Dockstader W., Ramaswamy S., Weiss J.P. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc. Natl. Acad. Sci. USA. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teghanemt A., Prohinar P., Gioannini T.L., Weiss J.P. Transfer of monomeric endotoxin from MD-2 to CD14: Characterization and functional consequences. J. Biol. Chem. 2007;282:36250–36256. doi: 10.1074/jbc.M705995200. [DOI] [PubMed] [Google Scholar]
- 28.Deguine J., Barton G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014;6:97. doi: 10.12703/P6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagan J.C., Su T., Horng T., Chow A., Akira S., Medzhitov R. TRAM couples endocytosis of TLR4 to the induction of interferon beta. Nat. Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzgerald K.A., Rowe D.C., Barnes B.J., Caffrey D.R., Visintin A., Latz E., Monks B., Pitha P.M., Golenbock D.T. LPS-TLR4 Signaling to IRF-3/7 and NF-κB Involves the Toll Adapters TRAM and TRIF. J. Exp. Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brieger A., Rink L., Haase H. Differential Regulation of TLR-Dependent MyD88 and TRIF Signaling Pathways by Free Zinc Ions. J. Immunol. 2013;191:1808–1817. doi: 10.4049/jimmunol.1301261. [DOI] [PubMed] [Google Scholar]
- 32.Zanoni I., Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front. Cell. Infect. Microbiol. 2013;3:32. doi: 10.3389/fcimb.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mata-Haro V., Cekic C., Martin M., Chilton P.M., Casella C.R., Mitchell T.C. The Vaccine Adjuvant Monophosphoryl Lipid A as a TRIF-Biased Agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 35.Johnson D. A Synthetic TLR4-active glycolipids as vaccine adjuvants and stand-alone immunotherapeutics. Curr. Top. Med. Chem. 2008;8:64–79. doi: 10.2174/156802608783378882. [DOI] [PubMed] [Google Scholar]
- 36.Scior T., Alexander C., Zaehringer U. Reviewing and identifying amino acids of human, murine, canine and equine TLR4/MD-2 receptor complexes conferring endotoxic innate immunity activation by LPS/lipid A, or antagonistic effects by Eritoran, in contrast to species-dependent modulation. Comput. Struct. Biotechnol. J. 2013;5 doi: 10.5936/csbj.201302012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seeley J.J., Ghosh S. Molecular mechanisms of innate memory and tolerance to LPS. J. Leukoc. Biol. 2017;101:107–119. doi: 10.1189/jlb.3MR0316-118RR. [DOI] [PubMed] [Google Scholar]
- 38.Shiozaki M., Doi H., Tanaka D., Shimozato T., Kurakata S.I. Syntheses of glucose analogues of E5564 as a highly potent anti-sepsis drug candidate. Bioorg. Med. Chem. 2006;14:3011–3016. doi: 10.1016/j.bmc.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Gaekwad J., Zhang Y., Zhang W., Reeves J., Wolfert M.A., Boons G.J. Differential induction of innate immune responses by synthetic lipid a derivatives. J. Biol. Chem. 2010;285:29375–29386. doi: 10.1074/jbc.M110.115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moran A.P. Biological and serological characterization of Campylobacter jejuni lipopolysaccharides with deviating core and lipid A structures. FEMS Immunol. Med. Microbiol. 1995;11:121–130. doi: 10.1111/j.1574-695X.1995.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 41.Di Lorenzo F., Billod J.-M., Martín-Santamaría S., Silipo A., Molinaro A. Gram-Negative Extremophile Lipopolysaccharides: Promising Source of Inspiration for a New Generation of Endotoxin Antagonists. Eur. J. Org. Chem. 2017;2017:4055–4073. doi: 10.1002/ejoc.201700113. [DOI] [Google Scholar]
- 42.Kusumoto S., Fukase K., Fukase Y., Kataoka M., Yoshizaki H., Sato K., Oikawa M., Suda Y. Structural basis for endotoxic and antagonistic activities: Investigation with novel synthetic lipid A analogs. J. Endotoxin Res. 2003;9:361–366. doi: 10.1177/09680519030090060901. [DOI] [PubMed] [Google Scholar]
- 43.Tamai R., Asai Y., Hashimoto M., Fukase K., Kusumoto S., Ishida H., Kiso M., Ogawa T. Cell activation by monosaccharide lipid A analogues utilizing Toll-like receptor 4. Immunology. 2003;110:66–72. doi: 10.1046/j.1365-2567.2003.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cighetti R., Ciaramelli C., Sestito S.E., Zanoni I., Kubik Ł, Ardá-Freire A., Calabrese V., Granucci F., Jerala R., Martín-Santamaría S., et al. Modulation of CD14 and TLR4/MD-2 activities by a synthetic lipid A mimetic. ChemBioChem. 2014;15:250–258. doi: 10.1002/cbic.201300588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perrin-Cocon L., Aublin-Gex A., Sestito S.E., Shirey K.A., Patel M.C., André P., Blanco J.C., Vogel S.N., Peri F., Lotteau V. TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci. Rep. 2017;7:40791. doi: 10.1038/srep40791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciaramelli C., Calabrese V., Sestito S.E., Pérez-Regidor L., Klett J., Oblak A., Jerala R., Piazza M., Martín-Santamaría S., Peri F. Glycolipid-based TLR4 modulators and fluorescent probes: Rational design, synthesis and biological properties. Chem. Biol. Drug Des. 2016;88:1–13. doi: 10.1111/cbdd.12749. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez Lavado J., Sestito S.E., Cighetti R., Aguilar Moncayo E.M., Oblak A., Lainšček D., Jiménez Blanco J.L., García Fernández J.M., Ortiz Mellet C., Jerala R., et al. Trehalose- and Glucose-Derived Glycoamphiphiles: Small-Molecule and Nanoparticle Toll-Like Receptor 4 (TLR4) Modulators. J. Med. Chem. 2014;57:9105–9123. doi: 10.1021/jm501182w. [DOI] [PubMed] [Google Scholar]
- 48.Fukase Y., Fujimoto Y., Adachi Y., Suda Y., Kusumoto S., Fukase K. Synthesis of Rubrivivax gelatinosus lipid A and analogues for investigation of the structural basis for immunostimulating and inhibitory activities. Bull. Chem. Soc. Jpn. 2008;81:796–819. doi: 10.1246/bcsj.81.796. [DOI] [Google Scholar]
- 49.Artner D., Oblak A., Ittig S., Garate J.A., Horvat S., Arrieumerlou C., Hofinger A., Oostenbrink C., Jerala R., Kosma P., et al. Conformationally constrained lipid a mimetics for exploration of structural basis of TLR4/MD-2 activation by lipopolysaccharide. ACS Chem. Biol. 2013;8:2423–2432. doi: 10.1021/cb4003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seydel U., Oikawa M., Fukase K., Kusumoto S., Brandenburg K. Intrinsic conformation of lipid A is responsible for agonistic and antagonistic activity. Eur. J. Biochem. 2000;267:3032–3039. doi: 10.1046/j.1432-1033.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 51.Fort M.M., Mozaffarian A., Stover A.G., Correia J.D.S., Johnson D.A., Crane R.T., Ulevitch R.J., Persing D.H., Bielefeldt-Ohmann H., Probst P., et al. A Synthetic TLR4 Antagonist Has Anti-Inflammatory Effects in Two Murine Models of Inflammatory Bowel Disease. J. Immunol. 2005;174:6416–6423. doi: 10.4049/jimmunol.174.10.6416. [DOI] [PubMed] [Google Scholar]
- 52.Billod J.-M., Lacetera A., Guzmán-Caldentey J., Martín-Santamaría S. Computational Approaches to Toll-Like Receptor 4 Modulation. Molecules. 2016;21:994. doi: 10.3390/molecules21080994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawasaki K., Akashi S., Shimazu R., Yoshida T., Miyake K., Nishijima M. Mouse Toll-like Receptor 4 MD-2 complex Mediates Lipopolysaccharide-mimetic Soìignal Transduction by Taxol. J. Biol. Chem. 2000;275:2251–2255. doi: 10.1074/jbc.275.4.2251. [DOI] [PubMed] [Google Scholar]
- 54.Kawasaki K., Gomi K., Kawai Y., Shiozaki M., Nishijima M. Molecular basis for lipopolysaccharide mimetic action of TaxolTM and flavolipin. J. Endotoxin Res. 2003;9:301–307. doi: 10.1177/09680519030090050501. [DOI] [PubMed] [Google Scholar]
- 55.Chan M., Hayashi T., Mathewson R.D., Nour A., Hayashi Y., Yao S., Tawatao R.I., Crain B., Tsigelny I.F., Kouznetsova V.L., et al. Identification of Substituted Pyrimido[5,4-b]indoles as Selective Toll-Like Receptor 4 Ligands. J. Med. Chem. 2013;56:4206–4223. doi: 10.1021/jm301694x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nour A., Hayashi T., Chan M., Yao S., Tawatao R.I., Crain B., Tsigelny I.F., Kouznetsova V.L., Ahmadiiveli A., Messer K., et al. Bioorganic & Medicinal Chemistry Letters Discovery of substituted 4-aminoquinazolines as selective Toll-like receptor 4 ligands. Bioorg. Med. Chem. Lett. 2014;24:4931–4938. doi: 10.1016/j.bmcl.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lien E., Chow J.C., Hawkins L.D., McGuinness P.D., Miyake K., Espevik T., Gusovsky F., Golenbock D.T. A novel synthetic acyclic lipid A-like agonist activates cells via the lipopolysaccharide/toll-like receptor 4 signaling pathway. J. Biol. Chem. 2001;276:1873–1880. doi: 10.1074/jbc.M009040200. [DOI] [PubMed] [Google Scholar]
- 58.Morin M.D., Wang Y., Jones B.T., Su L., Surakattula M.M., Berger M., Huang H., Beutler E.K., Zhang H., Beutler B., et al. Discovery and Structure—Activity Relationships of the Neoseptins: A New Class of Toll-like Receptor-4(TLR4) Agonists. J. Med. Chem. 2016;59:4812–4830. doi: 10.1021/acs.jmedchem.6b00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y., Su L., Morin M.D., Jones B.T., Whitby L.R., Surakattula M.M.R.P., Huang H., Shi H., Choi J.H., Wang K.-W., et al. TLR4/MD-2 activation by a synthetic agonist with no similarity to LPS. Proc. Natl. Acad. Sci. USA. 2016;113:1695–1705. doi: 10.1073/pnas.1525639113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neve J.E., Wijesekera H.P., Du S., Jenkins I.D., Ripper J.A., Teague S.J., Campitelli M., Garavelas A., Nikolapoulos G., Le P.V., et al. Euodenine A: A Small-Molecule Agonist of Human TLR4. J. Med. Chem. 2014;57:1252–1275. doi: 10.1021/jm401321v. [DOI] [PubMed] [Google Scholar]
- 61.Peri F., Calabrese V. Toll-like receptor 4 (TLR4) modulation by synthetic and natural compounds: An update. J. Med. Chem. 2014;57:3612–3622. doi: 10.1021/jm401006s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie N., Gomes F.P., Deora V., Gregory K., Vithanage T., Nassar Z.D., Cabot P.J., Sturgess D., Shaw P.N., Parat M.-O. Activation of mu-opioid receptor and Toll-like receptor 4 by plasma from morphine-treated mice. Brain Behav. Immun. 2017;61:244–258. doi: 10.1016/j.bbi.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Shanmugam A., Rajoria S., George A.L., Mittelman A., Suriano R., Tiwari R.K. Synthetic Toll Like Receptor-4 (TLR-4) Agonist Peptides as a Novel Class of Adjuvants. PLoS ONE. 2012;7:e30839. doi: 10.1371/journal.pone.0030839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohto U., Fukase K., Miyake K., Shimizu T. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc. Natl. Acad. Sci. USA. 2012;109:7421–7426. doi: 10.1073/pnas.1201193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu L., Phillips R.L., Zhang D., Teghanemt A., Weiss J.P., Gioannini T.L. NMR studies of hexaacylated endotoxin bound to wild-type and F126A mutant MD-2 and MD-2·TLR4 ectodomain complexes. J. Biol. Chem. 2012;287:16346–16355. doi: 10.1074/jbc.M112.343467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oblak A., Jerala R. The molecular mechanism of species-specific recognition of lipopolysaccharides by the MD-2/TLR4 receptor complex. Mol. Immunol. 2015;63:134–142. doi: 10.1016/j.molimm.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 67.Yoon S., Hong M., Han G.W., Wilson I. A Crystal structure of soluble MD-1 and its interaction with lipid IVa. Proc. Natl. Acad. Sci. USA. 2010;107:10990–10995. doi: 10.1073/pnas.1004153107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scior T., Lozano-Aponte J., Figueroa-Vazquez V., Yunes-Rojas J.A., Zähringer U., Alexander C. Three-Dimensional Mapping of Differential Amino Acids of Human, Murine, Canine and Equine Tlr4/Md-2 Receptor Complexes Conferring Endotoxic Activation By Lipid a, Antagonism By Eritoran and Species-Dependent Activities of Lipid Iva in the Mammalian Lps Se. Comput. Struct. Biotechnol. J. 2013;7:1–11. doi: 10.5936/csbj.201305003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim H.M., Park B.S., Kim J.I., Kim S.E., Lee J., Oh S.C., Enkhbayar P., Matsushima N., Lee H., Yoo O.J., et al. Crystal Structure of the TLR4-MD-2 Complex with Bound Endotoxin Antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Molinaro A., Holst O., Di L.F., Callaghan M., Nurisso A., D’Errico G., Zamyatina A., Peri F., Berisio R., Jerala R., et al. Chemistry of Lipid A: At the Heart of Innate Immunity. Chemistry. 2015;21:500–519. doi: 10.1002/chem.201403923. [DOI] [PubMed] [Google Scholar]
- 71.Peri F., Piazza M. Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists. Biotechnol. Adv. 2012;30:251–260. doi: 10.1016/j.biotechadv.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 72.Hennessy E.J., Parker A.E., O’Neill L.A.J. Targeting Toll-like receptors: Emerging therapeutics? Nat. Rev. Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 73.Gay N.J., Gangloff M. Structure and Function of Toll Receptors and Their Ligands. Annu. Rev. Biochem. 2007;76:141–165. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- 74.Tom J.K., Dotsey E.Y., Wong H.Y., Stutts L., Moore T., Davies D.H., Felgner P.L., Esser-Kahn A.P. Modulation of Innate Immune Responses via Covalently Linked TLR Agonists. ACS Cent. Sci. 2015;1:439–448. doi: 10.1021/acscentsci.5b00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang J.X., Azad M.A.K., Yuriev E., Baker M.A., Nation R.L., Li J., Cooper M.A., Velkov T. Molecular Characterization of Lipopolysaccharide Binding to Human α-1-Acid Glycoprotein. J. Lipids. 2012;2012:1–15. doi: 10.1155/2012/475153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knirel Y.A., Valvano M.A. Bacterial Lipopolysaccharides. Springer; Berlin, Germany: 2011. [Google Scholar]
- 77.Bennett-guerrero E., Mcintosh T.J., Robin G., Snyder D.S., Gibbs R.J., Michael G., Poxton I.R., McIntosh T.J., Barclay G.R., Mythen M.G., et al. Preparation and Preclinical Evaluation of a Novel Liposomal Complete-Core Lipopolysaccharide Vaccine. Infect. Immun. 2000;68:6202–6208. doi: 10.1128/IAI.68.11.6202-6208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Padova F.E., Brade H., Barclay G.R., Poxton L.R., Liehl E., Schuetze E., Kocher H.P., Ramsay G., Schreier M.H., Mcclelland D.B.L., et al. A Broadly Cross-Protective Monoclonal Antibody Binding to Escherichia coli and Salmonella Lipopolysaccharides. Infect. Immun. 1993;61:3863–3872. doi: 10.1128/iai.61.9.3863-3872.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luk J.M., Lind S.M., Tsang R.S., Lindberg A.A. Epitope mapping of four monoclonal antibodies recognizing the hexose core domain of Salmonella lipopolysaccharide. J. Biol. Chem. 1991;266:23215–23225. [PubMed] [Google Scholar]
- 80.Galanos C., Luderitz O., Rietschel E.T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H., et al. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur. J. Biochem. 1985;148:1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- 81.Erridge C., Bennett-guerrero E., Poxton I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002;4:837–851. doi: 10.1016/S1286-4579(02)01604-0. [DOI] [PubMed] [Google Scholar]
- 82.Muroi M., Tanamoto K. The polysaccharide portion plays an indispensable role in Salmonella LPS-induced activation of NF-kB through human TLR-4. Infect. Immun. 2002;70:6043–6047. doi: 10.1128/IAI.70.11.6043-6047.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Demchenko A.V., Wolfert M.A., Santhanam B., Moore J.N., Boons G.J. Synthesis and biological evaluation of Rhizobium sin-1 lipid A derivatives. J. Am. Chem. Soc. 2003;125:6103–6112. doi: 10.1021/ja029316s. [DOI] [PubMed] [Google Scholar]
- 84.Zughaier S.M., Tzeng Y., Zimmer S.M., Carlson R.W., Stephens D.S., Datta A. Neisseria meningitidis Lipooligosaccharide Structure-Dependent Activation of the Pathway Neisseria meningitidis Lipooligosaccharide Structure-Dependent Activation of the Macrophage CD14/Toll-Like Receptor 4 Pathway. Infect. Immun. 2004;72:371–380. doi: 10.1128/IAI.72.1.371-380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zughaier S., Agrawal S., Stephens D.S., Pulendran B. Hexa-acylation and KDO2-glycosylation determine the specific immunostimulatory activity of Neisseria meningitidis lipid a for human monocyte derived dendritic cells. Vaccine. 2006;24:1291–1297. doi: 10.1016/j.vaccine.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 86.Vasan M., Wolfert M.A., Boons G.-J. Agonistic and antagonistic properties of a Rhizobium sin-1 lipid A modified by an ether-linked lipid. Org. Biomol. Chem. 2007;5:2087–2097. doi: 10.1039/b704427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y., Wolfert M.A., Boons G.J. The influence of the long chain fatty acid on the antagonistic activities of Rhizobium sin-1 lipid A. Bioorg. Med. Chem. 2007;15:4800–4812. doi: 10.1016/j.bmc.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zughaier S.M., Zimmer S.M., Datta A., Carlson R.W., Stephens D.S. Differential Induction of the Toll-Like Receptor 4—MyD88-Dependent and -Independent Signaling Pathways by Endotoxins. Infect. Immun. 2005;73:2940–2950. doi: 10.1128/IAI.73.5.2940-2950.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raetz C.R., Garrett T.A., Reynolds C.M., Shaw W.A., Moore J.D., Smith D.C., Ribeiro A., Murphy R.C., Ulevitch R.J., Fearns C., et al. Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4. J. Lipid Res. 2006;47:1097–1111. doi: 10.1194/jlr.M600027-JLR200. [DOI] [PubMed] [Google Scholar]
- 90.Lien E., Means T.K., Heine H., Yoshimura A., Kusumoto S., Fukase K., Fenton M.J., Oikawa M., Qureshi N., Monks B., et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raetz C.R., Whitfield C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brabetz W., Müller-Loennies S., Holst O., Brade H. Deletion of the heptosyltransferase genes rfaC and rfaF in Escherichia coli K-12 results in an Re-type lipopolysaccharide with a high degree of 2-aminoethanol phosphate substitution. Eur. J. Biochem. 1997;247:716–724. doi: 10.1111/j.1432-1033.1997.00716.x. [DOI] [PubMed] [Google Scholar]
- 93.Murphy R.C., Raetz C.R.H., Reynolds C.M., Barkley R.M. Mass Spectrometry Advances in Lipidomica: Collision Induced Decomposition of Kdo2-Lipid A. Prostaglandins Other Lipid Mediat. 2005;77:131–140. doi: 10.1016/j.prostaglandins.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosner M.R., Tang J., Barzilay I., Khorana H.G. Structure of the Lipopolysaccharide Mutant from an Escherichia coli Heptose-less Mutant. J. Biol. Chem. 1979;254:5906–5917. [PubMed] [Google Scholar]
- 95.Zhou Z., Lin S., Cotter R.J., Raetz C.R.H. Lipid A Modifications Characteristic of Salmonella Typhimurium Are Induced by NH 4VO3 in Escherichia Coli K1. Biochemistry. 1999;274:18503–18514. doi: 10.1074/jbc.274.26.18503. [DOI] [PubMed] [Google Scholar]
- 96.Zhou Z., Ribeiro A.A., Lin S., Cotter R.J., Miller S.I., Raetz C.R.H. Lipid A modifications in polymyxin-resistant Salmonella typhimurium. J. Biol. Chem. 2001;276:43111–43121. doi: 10.1074/jbc.M106960200. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y., Gaekwad J., Wolfert M.A., Boons G.J. Modulation of Innate Immune Responses with Synthetic Lipid A Derivatives. J. Am. Chem. Soc. 2007;129:5200–5216. doi: 10.1021/ja068922a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y., Gaekwad J., Wolfert M.A., Boons G.J. Innate immune responses of synthetic lipid a derivatives of Neisseria meningitidis. Chemistry. 2008;14:558–569. doi: 10.1002/chem.200701165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mariathasan S., Weiss D.S., Newton K., McBride J., O’Rourke K., Roose-Girma M., Lee W.P., Weinrauch Y., Monack D.M., Dixit V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 100.Roth R.I., Yamasaki R., Mandrell R.E., Griffiss J.M. Ability of gonococcal and meningococcal lipooligosaccharides to clot Limulus amebocyte lysate. Infect. Immun. 1992;60:762–767. doi: 10.1128/iai.60.3.762-767.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schromm A.B., Brandenburg K., Loppnow H., Zähringer U., Rietschel E.T., Carroll S.F., Koch M.H., Kusumoto S., Seydel U. The charge of endotoxin molecules influences their conformation and IL-6-inducing capacity. J. Immunol. 1998;161:5464–5471. [PubMed] [Google Scholar]
- 102.Vinh T., Adler B., Faine S. Ultrastructure and chemical composition of lipopolysaccharide extracted from Leptospira interrogans serovar copenhageni. J. Gen. Microbiol. 1986;132:103–109. doi: 10.1099/00221287-132-1-103. [DOI] [PubMed] [Google Scholar]
- 103.Vinogradov E., Perry M.B., Conlan J.W. Structural analysis of Francisella tularensis lipopolysaccharide. Eur. J. Biochem. 2002;269:6112–6118. doi: 10.1046/j.1432-1033.2002.03321.x. [DOI] [PubMed] [Google Scholar]
- 104.Petzold M., Thürmer A., Menzel S., Mouton J.W., Heuner K., Lück C. A structural comparison of lipopolysaccharide biosynthesis loci of Legionella pneumophila serogroup 1 strains. BMC Microbiol. 2013;13:198. doi: 10.1186/1471-2180-13-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Russa R., Urbanik-Sypniewska T., Choma A., Mayer H. Identification of 3-deoxy-lyxo-2-heptulosaric acid in the core region of lipopolysaccharides from Rhizobiaceae. FEMS Microbiol. Lett. 1991;68:337–343. doi: 10.1111/j.1574-6968.1991.tb04620.x. [DOI] [PubMed] [Google Scholar]
- 106.Frecer V., Ho B., Ding J.L. Interpretation of biological activity data of bacterial endotoxins by simple molecular models of mechanism of action. Eur. J. Biochem. 2000;267:837–852. doi: 10.1046/j.1432-1327.2000.01069.x. [DOI] [PubMed] [Google Scholar]
- 107.Liu J. Ph.D. Thesis. Faculty of the James T. Laney School of Graduate Studies of Emory University; Atlanta, GA, USA: 2012. Use of Receptor-Based Drug Design and Applications in the Study of Finding Antagonists for MD-2/TLR4, GLP and CXCR4. [Google Scholar]
- 108.Henderson J.C., O’Brien J.P., Brodbelt J.S., Trent M.S. Isolation and chemical characterization of lipid A from gram-negative bacteria. J. Vis. Exp. 2013;966:e50623. doi: 10.3791/50623. [DOI] [PMC free article] [PubMed] [Google Scholar]