Abstract
Semaphorin-3E (Sema-3E) is a member of a large family of proteins originally identified as axon guidance cues in neural development. It is expressed in different cell types, such as immune cells, cancer cells, neural cells, and epithelial cells. Subsequently, dys-regulation of Sema-3E expression has been reported in various biological processes that range from cancers to autoimmune and allergic diseases. Recent work in our laboratories revealed a critical immunoregulatory role of Sema-3E in experimental allergic asthma. We further speculate possible immune modulatory function(s) of Sema-3E on natural killer (NK) cells.
Keywords: semaphorins, semaphorin-3E, plexins, nervous system, cardiovascular, allergic asthma, cancer, natural killer cells
1. The Semaphorin Family: Classification and Structure
Semaphorins were first discovered as axon guidance molecules in the nervous system [1,2]. Currently, they represent a large family of proteins that are classified into eight classes (1–7 and V). Classes 1 and 2 are found in invertebrates. Classes 3–7 exist in vertebrates, whereas Class V is unique to viruses [3]. The differences between these classes are related to their sequences and structures. A signature domain, however, called the semaphorin (Sema) domain, is conserved among all members of the semaphorins family. The extracellular Sema domain consists of 500 amino acids and is a cysteine-rich sequence that is crucial for receptor binding specificity and protein functions (Figure 1) [4,5,6,7]. Semaphorin molecules can be membrane-bound (Classes 1, 3, 4, 5, and 6) [8,9], secreted proteins (Classes 2, 3, 4, and V) [10,11], or glycosyl–phosphatidyl–inositol (GPI)-linked proteins (Class 7) [8].
Figure 1.
Classes and structures of semaphorins. Semaphorins are represented in their classification into eighth classes. Class 1 and 2 semaphorins are found in invertebrates. Class 3–7 semaphorins are found in vertebrates. The Sema domains characterize both semaphorins and plexins. Additional domains present in semaphorins and plexins include PSI domains (plexin, semaphorin, and integrin) and immunoglobulin (Ig)-like domains. Class 7 semaphorin (Sema-7) contains a membrane-associated GPI moiety at its carboxyl terminus. Class V (Sema-8) semaphorins are highly similar to the Class 7 semaphorins and are found in DNA viruses. Semaphorins can be either secreted or membrane-bound proteins. Note: Some members of the Class 4 semaphorins can be found in secreted form. Some members of the Class 3 proteins can be found on cell surfaces.
The plexin receptors are large 200 kDa transmembrane proteins that have been identified in vertebrates (Plexins A1–A4, B1–B3, C1, and D1) and two in invertebrates (Plexins A and B) [3]. The extracellular part of the plexin receptors contains a Sema domain, followed by three PSI (plexin–semaphorin–integrin) domains and three IPT (immunoglobulin, plexin, and transcription factors) domains [12]. The PSI domain is a small cysteine-rich domain that is crucial for protein–protein interactions [13]. The IPT domain is required for proper ligand binding to the plexin receptors. The intracellular domain or cytoplasmic tail of the Plexin molecule is highly conserved and plays a crucial role in transmitting the signals following ligand binding. It contains a putative tyrosine phosphorylation sites, a GTPase-binding domain, and a segmented GTPase-activating protein (GAP) domain [14,15,16]. Neuropilins (NRP) receptors, NRP1 and NRP2, are single-pass transmembrane proteins that contain short cytoplasmic tails. The extracellular portion of NRPs contains two repeat complement-binding (CUB) domains (a1 and a2 domains), two coagulation factor-like domains (b1 and b2 domains), and a juxta-membrane meprin/A5/mu-phosphatase (MAM) homology domain (c domain) [17].
Selective binding and signaling of individual Semaphorin members is thought to be determined by the receptor complexes that can exist either as homomeric or heteromeric complexes [5]. Most semaphorin molecules mediate their effector functions by direct binding and signaling of plexins and neuropilins (NRPs) receptors [15]. For example, Sema-4A has 4 types of receptors: the Plexin-D family, the Plexin-B family, Tim-2 (T-cell, immunoglobulin, and mucin domain protein 2), and NRP-1. In most cases, members of the Plexin-A family require neuropilins as ligand-binding partners, whereas members of the other plexin families are directly activated by semaphorins [18]. Binding of Semaphorin-3 family members to neuropilin (NRP) receptors depends on their N-terminus Sema sequences, and whether the 70-amino acid stretch within these sequences will determine binding specificity [6,19]. NRP receptors may lack intrinsic signaling capabilities due to their short cytoplasmic tails [20]; however, both NRP1 and NRP2 were found to be essential for semaphorin-3-induced signal transduction [21,22]. Blocking of NRP1 (receptor of Sema-3a) using anti-NRP1 antibodies resulted in ablation of the axon repulsion of mouse cortical neurons [23]. Knock-out of the NRP-1 gene expression caused semaphorin-3a insensitivity in embryonic DRG neurons [24]. NRP-2 is an essential component for semaphorin-3f function. Blocking of the NRP-2 receptor abolished the semaphorin-3f-induced growth cone collapse of embryonic rat sympathetic neurons, and axon repulsion in neonatal mouse cortical neurons [21]. The development of dopaminergic neurons in the meso-diencephalon was impaired in mice that were deficient in NRP2 [25]. It is therefore unclear what signal transducers are involved in the downstream signaling of the NRP receptors. Depending on the cell type, other membrane-associated proteins such as vascular endothelial growth factor receptor (VEGF) or CD72 could act as co-receptors for specific semaphorin members to mediate its effector functions [13]. For examples, Class 6 semaphorins bind to Class A plexin receptors and carry out different biological activities depending on its VEGF co-receptor [13].
In the neuronal system, semaphorins can mediate repulsive axon guidance, cell migration, invasive growth, and growth cone collapse by several post-translational modifications [26] and oligomerization [27]. The importance of semaphorins in regulating cellular events beyond the nervous system is emerging. Members of the semaphorin family are reported to play important roles in immune, respiratory, and cardiovascular systems, in physiological processes such as angiogenesis, embryogenesis, and in pathological conditions such as airway diseases and tumor formation [28,29,30,31,32,33]. This review focuses on the recent advances in our understanding of the semaphorin-3E (Sema-3E) member of the family in these processes, and its emerging roles in regulating immune responses.
2. Receptors and Signaling of the Semaphorin-3E
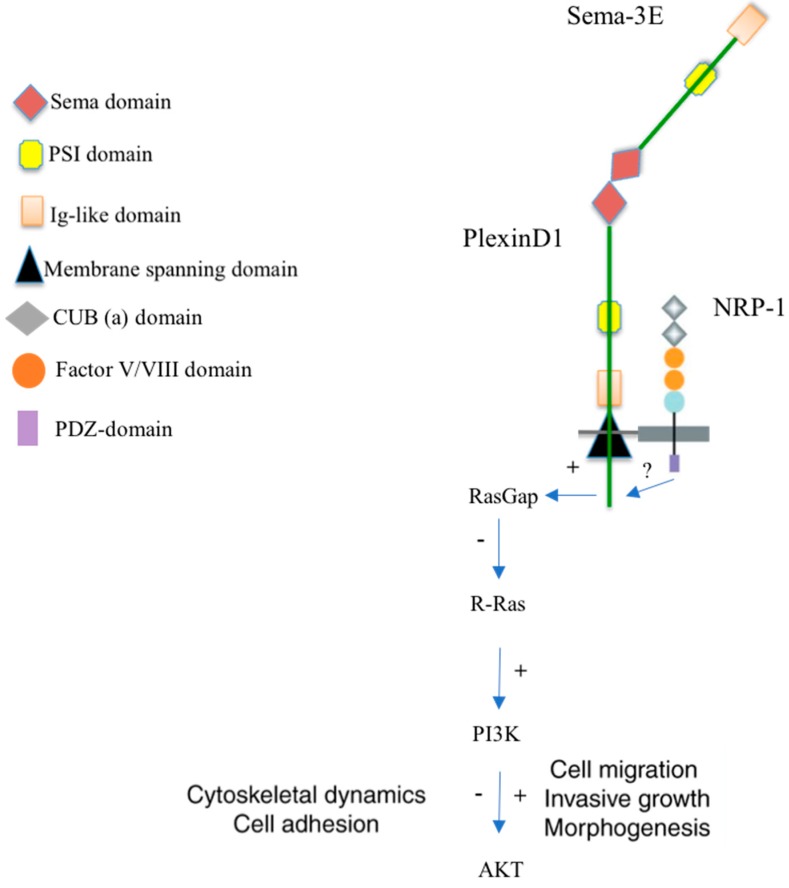
The Sema-3E gene is located on Chromosome 7 and encodes a 85–90 kDa protein. Unlike the other Semaphorin-3 members, Sema-3E can bind to the Plexin-D1 receptor with high affinity, independent of the NRP [4,33,34,35]. Intracellular tail of Plexin-D1 contains two highly conserved intracellular domains—the SEX-PLEXIN domain and the SEMA/PLEXIN domain. The SEMA/PLEXIN domain of Plexin-D1 includes two C region RasGAP domains. Each RasGAP domain includes a short motif of (GTPase)-activating proteins (GAPs) and monomeric GTPases of the R-Ras subfamily (Figure 2). A monomeric Rho GTPase binding domain (RBD) is found within the two C regions. Plexin-D1 acts as a RasGAP to antagonize both an integrin-mediated cell extracellular matrix (ECM) adhesion and PI3K, a modulator of cell survival, growth, and migration signaling. Rho family GTPase 2 (Rnd2) is required for the activation of the RasGAP activity of Plexin-D1. Upon Sema-3E Plexin-D1 stimulation, pre-existing Plexin-D1-Rnd2/RLG (resistance-nodulation division/Release Guard signal) complexes undergo Rnd2/RLG-dependent intracellular conformational changes that translate the concentration and distribution of extracellular Sema-3 cues into an intracellular gradient of distinct Plexin-D1 activities [20,36]. Multifaceted functions of semaphorins may also be mediated by other signaling pathways, such as mitogen-activated protein kinase or phosphatidylinositol 3-kinases. The mechanism underlying how the binding of semaphorins induces a network of downstream signaling has not been fully deciphered.
Figure 2.
The Sema-3E/Plexin-D1 receptor signaling. Sema3E binds its receptor, Plexin-D1. This interaction leads to the activation of the intracellular Plexin-D1 RasGAP (Ras GTPase activating protein) domain and subsequently reduces R-Ras activity [37]. Semaphorin-3E can regulate integrin functions and cytoskeletal dynamics via the intrinsic R-Ras GAP activity of plexins and the recruitment of regulatory molecules, and can thereby affect cell adhesion and migration. These effects can sometimes result in opposing functional responses, depending on the activation/inhibition of PI3K (phosphoinositide 3-kinase), in a cell-type-specific manner. The role of NRP-1 remains unclear. It could function to inhibit repulsive signaling by Plexin-D1, thereby facilitating attractive/growth-promoting responses. (+) Activation; (−) Inhibition; (?) Unknown.
3. Semaphorin-3E in the Nervous System
Sema-3E proteins are present in the neural scar and influence a wide range of molecules and cell types in and surrounding the injured tissue [38]. The Sema-3E–Plexin-D1 axis has a dual function in axonal growth depending on the presence of NRP1. For certain subpopulations of corticofugal and striatonigral neurons that express Plexin-D1 but not NRP1, Sema-3E acts as a repellent. In contrast, in subiculo-mammillary neurons, the presence of receptor complexes of NRP1 in addition to Plexin-D1 switches the Sema-3E signal from repulsion to attraction and/or stimulation of axonal growth [39]. Recently, Sema-3E–Plexin-D1 signaling is involved in synaptic recognition in the spinal cord and striatum. In the spinal cord, Sema-3E–Plexin-D1 plays a role in the specificity of monosynaptic sensory-motor connections [40]. Therefore, in the spinal cord post-synaptic neurons releasing guidance cue, Sema-3E, and repel incoming axons that express Plexin-D1 to prevent inappropriate synapse formation [41]. Ding et al. reported that Sema-3E was secreted by incoming thalamic axons and that Plexin-D1 expressed by one subtype of post-synaptic neuron could specify synaptic specificity [42]. Collectively, Sema-3E–Plexin-D1 signaling determines synaptic recognition and specificity in multiple parts of the nervous system [34].
4. Semaphorin-3E in Cardiovascular Development
Sema-3E was discovered to play crucial roles in cardiovascular development, mainly acting through NRP1 and Plexin-D1 [43]. Many studies have been focused on Sema-3E signaling in vascular patterning and cardiac morphogenesis, and Sema-3E signaling impairment has been associated with various human cardiovascular disorders, such as persistent truncus arteriosus, sinus bradycardia, and anomalous pulmonary venous connections [44]. Sema-3E–Plexin-D1 signaling is required for proper dorsal aortae patterning in the early embryo [45]. This signaling can repress angiogenesis by antagonizing the proangiogenic activity of VEGF [46]. Moreover, Sema-3E affects retinal angiogenic cell fate decisions by regulating cell responsiveness to VEGF and Notch in tip and stalk cells [47].
5. Semaphorin-3E and Cancers
High levels of expression of Sema-3E and Plexin-D1 were observed in human colon cancer, liver metastasis, and melanoma progression [17,48]. Further functional analyses revealed that Sema-3E could regulate invasiveness of tumor cells in a Plexin-D1-dependent manner [49,50]. In breast cancers, expressions of Sema-3E and the Plexin-D1 receptor have been shown to be upregulated in advanced and metastatic human breast tumors. The Sema-3E/Plexin-D1 signaling promoted survival of breast cancer cells. Suppression of such Sema-3E/Plexin-D1 signaling pathway in human and mouse breast cancer cells induced apoptosis in vitro and subsequently reduced metastasis in vivo [48,49,51].
6. Semaphorin-3E in an Allergic Asthma Model
Emerging data suggest that semaphorins and their receptors are key regulators of allergic inflammatory responses in the airways [32]. Of particular interest to us, we observed that expression of Sema-3E was significantly suppressed in the airways of severe asthmatic patients [52] and in an experimental mouse model of asthma [53]. In addition, the surface expression of the Plexin-D1 receptor was reduced in the airway smooth muscle cells from asthmatic patients, thus suggesting the functional importance of Sema-3E/Plexin-D1 signaling in allergic asthma [54]. We reported that Sema-3E inhibited human airway smooth muscle (ASM) cell migration and proliferation by modulation of Rac1, ERK1/2, and Akt pathways [54]. To further examine the role of Sema-3E in the development and maintenance of allergic asthma, we used Sema-3E-deficient mice in experimental models of asthma. We observed that genetic ablation of Sema-3E in mice resulted in increased lung granulocytosis, increased airway hyper-responsiveness, mucus overproduction, collagen deposition, and Th2/Th17 lung inflammation in allergic asthma [53]. The regulatory role of Sema-3E in allergic asthma seems to be mediated by the modulations and/recruitment of pulmonary dendritic cell (DC) subset [53] and neutrophils [55]. Intranasal administration of recombinant Sema-3E alleviated these pathological features of experimental allergic asthma, highlighting the importance of Sema-3E in maintaining a homeostatic balance in the airway [56].
7. The Prospective Role of Semaphorin-3E in Regulating Natural Killer (NK) Cell Functions
NK cells are bone-marrow-derived cells that constitute 10–15% of blood lymphocytes [57]. They migrate to peripheral tissues or inflamed lymph nodes to exert their immune-surveillance functions [58]. They are currently classified as members of the emerging family of the innate lymphoid cells that play important roles in innate immunity and tissue remodeling [59,60]. NK-cell activation and function can be regulated by target cell recognitions, cytokines (such as IL-2, IL-12, IL-15, IL-18) [61], or dendritic cells (DCs) in microenvironments [62,63]. The interaction of NK and DCs (crosstalk) is bi-directional, involving multiple cytokine signals and direct cell–cell contacts [64,65,66,67]. DC-derived cytokine IL-12 is critical in the generation of IFN-γ-producing NK cells. Interestingly, DCs also derive soluble factors such as IL-1 and IL-18, which have implications in terms of the acquisition of IL-12 receptor on NK cells [68]. Mutually, NK cells promote DC maturation and activation by inducing MHC molecule expression and by enhancing the ability to secrete IL-18, IL-12, and P70 via upregulation of CD86 molecules and the activation of Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) and NKp30 signaling [69,70]. As DCs can acquire different abilities to induce immunological tolerance or to stimulate functionally distinct T cell subsets (such as Th1, Th2, and Th17) effectively [71], the regulation of DC maturation/functions by NK cells is important in coordinating innate and adaptive immune responses. NK–DC crosstalk therefore shapes anti-tumor and anti-microbial responses in vivo [64,67,72,73,74,75,76].
Holl et al. reported that Plexin-B2 and Plexin-D1 are reciprocally expressed on plasmacytoid and myeloid DCs. The predominant expression of the Plexin-D1 receptor on bone-marrow-derived DCs (BMDC) can be further modulated by TLR ligands [77]. In addition, splenic DCs expressed high levels of Sema-3E [77]. Plexin-D1-deficient and wild-type DCs exhibited comparable LPS-induced DC maturation, T-cell stimulations, and migrations towards CXCL12/19 gradients. However, the Plexin-D1-deficient DCs were hyper-responsive in their secretion of IL-12/IL-23 p40 (but not IL-6) when these sorted splenic DCs were cultured in vitro at the steady state for 24 h [77]. It will be interesting to examine how modulation of IL-12 production by Plexin-D1/Sema-3E may further regulate NK cell activation, NK cell functions, or NK–DC crosstalk in vitro and in vivo.
Our recent work demonstrated that mouse NK cells expressed Sema-3E receptor (Plexin-D1) on their cell surface [78]. It thus highlighted the possibilities of a direct regulatory effect of Sema-3E on NK-cell functions. We observed also the expression of Sema-3E in bone-marrow-derived DCs was tightly regulated in DC maturation [78]. It will be interesting to examine further, for example, how Sema-3E production by DCs regulates NK cell functions, NK-induced DC maturation, or DC homeostasis in NK–DC crosstalk.
8. Conclusions
Semaphorins were first identified as axon guidance cues in neural development. Recent work has established its multi-faceted role in the cardiovascular system, airway biology, cancers, and immune cell regulation. Future investigations of the role(s) of Sema-3E in regulating NK cells and/or NK–DC crosstalk will provide new insights into the importance of Sema-3E in maintaining the homeostasis of immune cells in physiological and pathological settings.
Acknowledgments
Sam K.P. Kung was supported by research grants from NSERC discovery grant RGPIN-2015-04144, and the Canadian Cancer Society Research Institute Innovation Grant: Innov14-1 #702459. Abdulaziz Alamri was supported by a scholarship from King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare there is no conflict of interest.
References
- 1.Koncina E., Roth L., Gonthier B., Bagnard D. Role of semaphorins during axon growth and guidance. In: Bagnard D., editor. Axon Growth and Guidance. Springer; New York, NY, USA: 2007. pp. 50–64. [DOI] [PubMed] [Google Scholar]
- 2.Wong J., Yu W., O’Connor T.P. Transmembrane grasshopper Semaphorin I promotes axon outgrowth in vivo. Development. 1997;124:3597–3607. doi: 10.1242/dev.124.18.3597. [DOI] [PubMed] [Google Scholar]
- 3.Roney K., Holl E., Ting J. Immune plexins and semaphorins: Old proteins, new immune functions. Protein Cell. 2013;4:17–26. doi: 10.1007/s13238-012-2108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yazdani U., Terman J.R. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey A. Receptor complexes for each of the Class 3 Semaphorins. Front. Cell. Neurosci. 2012;6 doi: 10.3389/fncel.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koppel A.M., Feiner L., Kobayashi H., Raper J.A. A 70 Amino Acid Region within the Semaphorin Domain Activates Specific Cellular Response of Semaphorin Family Members. Neuron. 1997;19:531–537. doi: 10.1016/S0896-6273(00)80369-4. [DOI] [PubMed] [Google Scholar]
- 7.Gu C., Yoshida Y., Livet J., Reimert D.V., Mann F., Merte J., Henderson C.E., Jessell T.M., Kolodkin A.L., Ginty D.D. Semaphorin 3E and Plexin-D1 Control Vascular Pattern Independently of Neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 8.Siebold C., Jones E.Y. Structural insights into semaphorins and their receptors. Semin. Cell Dev. Biol. 2013;24:139–145. doi: 10.1016/j.semcdb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Smith E.P., Shanks K., Lipsky M.M., DeTolla L.J., Keegan A.D., Chapoval S.P. Expression of neuroimmune semaphorins 4A and 4D and their receptors in the lung is enhanced by allergen and vascular endothelial growth factor. BMC Immunol. 2011;12:30. doi: 10.1186/1471-2172-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nkyimbeng-Takwi E., Chapoval S.P. Biology and function of neuroimmune semaphorins 4A and 4D. Immunol. Res. 2011;50:10–21. doi: 10.1007/s12026-010-8201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepelletier Y., Moura I.C., Hadj-Slimane R., Renand A., Fiorentino S., Baude C., Shirvan A., Barzilai A., Hermine O. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur. J. Immunol. 2006;36:1782–1793. doi: 10.1002/eji.200535601. [DOI] [PubMed] [Google Scholar]
- 12.Worzfeld T., Offermanns S. Semaphorins and plexins as therapeutic targets. Nat. Rev. Drug Discov. 2014;13:603. doi: 10.1038/nrd4337. [DOI] [PubMed] [Google Scholar]
- 13.Kumanogoh A., Kikutani H. Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat. Rev. Immunol. 2013;13:802–814. doi: 10.1038/nri3545. [DOI] [PubMed] [Google Scholar]
- 14.Gelfand M.V., Hong S., Gu C. Guidance from above: Common cues direct distinct signaling outcomes in vascular and neural patterning. Trends Cell Biol. 2009;19:99–110. doi: 10.1016/j.tcb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann F., Rougon G. Mechanisms of axon guidance: Membrane dynamics and axonal transport in semaphorin signalling. J. Neurochem. 2007;102:316–323. doi: 10.1111/j.1471-4159.2007.04578.x. [DOI] [PubMed] [Google Scholar]
- 16.Nasarre P., Gemmill R.M., Drabkin H.A. The emerging role of class-3 semaphorins and their neuropilin receptors in oncology. OncoTargets Ther. 2014;7:1663. doi: 10.2147/OTT.S37744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura F., Goshima Y. Neuropilin: From Nervous System to Vascular and Tumor Biology. Springer Science & Business Media; New York, NY, USA: 2002. Structural and functional relation of neuropilins; pp. 55–69. [DOI] [PubMed] [Google Scholar]
- 18.Ito D., Kumanogoh A. The role of Sema4A in angiogenesis, immune responses, carcinogenesis, and retinal systems. Cell Adhes. Migr. 2016;10:692–699. doi: 10.1080/19336918.2016.1215785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuda T., Taniguchi M. Congenital diseases and semaphorin signaling: Overview to date of the evidence linking them. Congenit. Anom. 2015;55:26–30. doi: 10.1111/cga.12095. [DOI] [PubMed] [Google Scholar]
- 20.Guo H.-F., Vander Kooi C.W. Neuropilin Functions as an Essential Cell Surface Receptor. J. Biol. Chem. 2015;290:29120–29126. doi: 10.1074/jbc.R115.687327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H., Chédotal A., He Z., Goodman C.S., Tessier-Lavigne M. Neuropilin-2, a Novel Member of the Neuropilin Family, Is a High Affinity Receptor for the Semaphorins Sema E and Sema IV but Not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/S0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T., Nakamura F., Jin Z., Kalb R.G., Strittmatter S.M. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat. Neurosci. 1998;1:487–493. doi: 10.1038/2203. [DOI] [PubMed] [Google Scholar]
- 23.Castellani V., Chédotal A., Schachner M., Faivre-Sarrailh C., Rougon G. Analysis of the L1-Deficient Mouse Phenotype Reveals Cross-Talk between Sema3A and L1 Signaling Pathways in Axonal Guidance. Neuron. 2000;27:237–249. doi: 10.1016/S0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- 24.Gu C., Rodriguez E.R., Reimert D.V., Shu T., Fritzsch B., Richards L.J., Kolodkin A.L., Ginty D.D. Neuropilin-1 Conveys Semaphorin and VEGF Signaling during Neural and Cardiovascular Development. Dev. Cell. 2003;5:45–57. doi: 10.1016/S1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolk S.M., Gunput R.A., Tran T.S., van den Heuvel D.M., Prasad A.A., Hellemons A.J., Adolfs Y., Ginty D.D., Kolodkin A.L., Burbach J.P., et al. Semaphorin 3F Is a Bifunctional Guidance Cue for Dopaminergic Axons and Controls Their Fasciculation, Channeling, Rostral Growth, and Intracortical Targeting. J. Neurosci. 2009;29:12542–12557. doi: 10.1523/JNEUROSCI.2521-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams R.H., Lohrum M., Klostermann A., Betz H., Püschel A.W. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J. 1997;16:6077–6086. doi: 10.1093/emboj/16.20.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klostermann A., Lohrum M., Adams R.H., Püschel A.W. The chemorepulsive activity of the axonal guidance signal semaphorin D requires dimerization. J. Biol. Chem. 1998;273:7326–7331. doi: 10.1074/jbc.273.13.7326. [DOI] [PubMed] [Google Scholar]
- 28.Takamatsu H., Kumanogoh A. Diverse roles for semaphorin-plexin signaling in the immune system. Trends Immunol. 2012;33:127–135. doi: 10.1016/j.it.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Capparuccia L., Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment—Two sides of a coin. J. Cell Sci. 2009;122:1723–1736. doi: 10.1242/jcs.030197. [DOI] [PubMed] [Google Scholar]
- 30.Tran T.S., Kolodkin A.L., Bharadwaj R. Semaphorin regulation of cellular morphology. Annu. Rev. Cell Dev. Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- 31.Vanderhaeghen P., Cheng H.-J. Guidance molecules in axon pruning and cell death. Cold Spring Harb. Perspect. Biol. 2010;2:a001859. doi: 10.1101/cshperspect.a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Movassagh D.H., Khadem D.F., Gounni P.A.S. Semaphorins and Their Roles in Airway Biology: Potential as Therapeutic Targets. Am. J. Respir. Cell Mol. Biol. 2017 doi: 10.1165/rcmb.2017-0171TR. [DOI] [PubMed] [Google Scholar]
- 33.Klagsbrun M., Shimizu A. Semaphorin 3E, an exception to the rule. J. Clin. Investig. 2010;120:2658–2660. doi: 10.1172/JCI44110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh W.-J., Gu C. The Role and Mechanism-of-Action of Sema3E and Plexin-D1 in Vascular and Neural Development. Semin. Cell Dev. Biol. 2013;24:156–162. doi: 10.1016/j.semcdb.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamagnone L., Artigiani S., Chen H., He Z., Ming G.-L., Song H.-J., Chedotal A., Winberg M.L., Goodman C.S., Poo M.-M., et al. Plexins Are a Large Family of Receptors for Transmembrane, Secreted, and GPI-Anchored Semaphorins in Vertebrates. Cell. 1999;99:71–80. doi: 10.1016/S0092-8674(00)80063-X. [DOI] [PubMed] [Google Scholar]
- 36.Gay C.M., Zygmunt T., Torres-Vázquez J. Diverse functions for the semaphorin receptor PlexinD1 in development and disease. Dev. Biol. 2011;349:1–19. doi: 10.1016/j.ydbio.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uesugi K., Oinuma I., Katoh H., Negishi M. Different Requirement for Rnd GTPases of R-Ras GAP Activity of Plexin-C1 and Plexin-D1. J. Biol. Chem. 2009;284:6743–6751. doi: 10.1074/jbc.M805213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mecollari V., Nieuwenhuis B., Verhaagen J. A perspective on the role of class III semaphorin signaling in central nervous system trauma. Front. Cell. Neurosci. 2014;8:328. doi: 10.3389/fncel.2014.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chauvet S., Cohen S., Yoshida Y., Fekrane L., Livet J., Gayet O., Segu L., Buhot M.-C., Jessell T.M., Henderson C.E., et al. Gating of Sema3E/PlexinD1 Signaling by Neuropilin-1 Switches Axonal Repulsion to Attraction during Brain Development. Neuron. 2007;56:807–822. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pecho-Vrieseling E., Sigrist M., Yoshida Y., Jessell T.M., Arber S. Specificity of Sensory-Motor Connections Encoded by Sema3e-PlexinD1 Recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshida Y. Semaphorin Signaling in Vertebrate Neural Circuit Assembly. Front. Mol. Neurosci. 2012;5:71. doi: 10.3389/fnmol.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding J.B., Oh W.-J., Sabatini B.L., Gu C. Semaphorin 3E–Plexin-D1 signaling controls pathway-specific synapse formation in the striatum. Nat. Neurosci. 2012;15:215–223. doi: 10.1038/nn.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valdembri D., Regano D., Maione F., Giraudo E., Serini G. Class 3 semaphorins in cardiovascular development. Cell Adhes. Migr. 2016;10:641–651. doi: 10.1080/19336918.2016.1212805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epstein J.A., Aghajanian H., Singh M.K. Semaphorin Signaling in Cardiovascular Development. Cell Metab. 2015;21:163–173. doi: 10.1016/j.cmet.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meadows S.M., Ratliff L.A., Singh M.K., Epstein J.A., Cleaver O. Resolution of defective dorsal aortae patterning in Sema3E deficient mice occurs via angiogenic remodeling. Dev. Dynam. 2013;242:580–590. doi: 10.1002/dvdy.23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zygmunt T., Gay C.M., Blondelle J., Singh M.K., Flaherty K.M., Means P.C., Herwig L., Krudewig A., Belting H.G., Affolter M., et al. Semaphorin-PlexinD1 Signaling Limits Angiogenic Potential via the VEGF Decoy Receptor sFlt1. Dev. Cell. 2011;21:301–314. doi: 10.1016/j.devcel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J., Oh W.-J., Gaiano N., Yoshida Y., Gu C. Semaphorin 3E–Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev. 2011;25:1399–1411. doi: 10.1101/gad.2042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luchino J., Hocine M., Amoureux M.-C., Gibert B., Bernet A., Royet A., Treilleux I., Lécine P., Borg J.-P., Mehlen P. Semaphorin 3E suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancers. Cancer Cell. 2013;24:673–685. doi: 10.1016/j.ccr.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Casazza A., Kigel B., Maione F., Capparuccia L., Kessler O., Giraudo E., Mazzone M., Neufeld G., Tamagnone L. Tumour growth inhibition and anti-metastatic activity of a mutated furin-resistant Semaphorin 3E isoform. EMBO Mol. Med. 2012;4:234–250. doi: 10.1002/emmm.201100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blanc V., Nariculam J., Munson P., Freeman A., Klocker H., Masters J., Williamson M. A role for class 3 semaphorins in prostate cancer. Prostate. 2011;71:649–658. doi: 10.1002/pros.21281. [DOI] [PubMed] [Google Scholar]
- 51.Casazza A., Finisguerra V., Capparuccia L., Camperi A., Swiercz J.M., Rizzolio S., Rolny C., Christensen C., Bertotti A., Sarotto I. Sema3E–Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J. Clin. Investig. 2010;120:2684. doi: 10.1172/JCI42118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Movassagh H., Shan L., Chakir J., McConville J.F., Halayko A.J., Koussih L., Gounni A.S. Expression of semaphorin 3E is suppressed in severe asthma. J. Allergy Clin. Immunol. 2017 doi: 10.1016/j.jaci.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 53.Movassagh H., Shan L., Mohammed A., Halayko A.J., Gounni A.S. Semaphorin 3E Deficiency Exacerbates Airway Inflammation, Hyperresponsiveness, and Remodeling in a Mouse Model of Allergic Asthma. J. Immunol. 2017;198:1805–1814. doi: 10.4049/jimmunol.1601514. [DOI] [PubMed] [Google Scholar]
- 54.Movassagh H., Shan L., Halayko A.J., Roth M., Tamm M., Chakir J., Gounni A.S. Neuronal chemorepellent Semaphorin 3E inhibits human airway smooth muscle cell proliferation and migration. J. Allergy Clin. Immunol. 2014;133:560–567. doi: 10.1016/j.jaci.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Movassagh H., Saati A., Nandagopal S., Mohammed A., Tatari N., Shan L., Duke-Cohan J.S., Fowke K.R., Lin F., Gounni A.S. Chemorepellent Semaphorin 3E Negatively Regulates Neutrophil Migration In Vitro and In Vivo. J. Immunol. 2017;198:1023–1033. doi: 10.4049/jimmunol.1601093. [DOI] [PubMed] [Google Scholar]
- 56.Movassagh H., Shan L., Duke-Cohan J.S., Halayko A.J., Uzonna J.E., Gounni A.S. Semaphorin 3E Alleviates Hallmarks of House Dust Mite-Induced Allergic Airway Disease. Am. J. Pathol. 2017;187:1566–1576. doi: 10.1016/j.ajpath.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Campbell K.S., Hasegawa J. Natural killer cell biology: An update and future directions. J. Allergy Clin. Immunol. 2013;132:536–544. doi: 10.1016/j.jaci.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waldhauer I., Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 59.Cella M., Miller H., Song C. Beyond NK Cells: The Expanding Universe of Innate Lymphoid Cells. Front. Immunol. 2014;5:282. doi: 10.3389/fimmu.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N.J., Mebius R.E., et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 61.Fuchs A., Colonna M. Innate lymphoid cells in homeostasis, infection, chronic inflammation and tumors of the gastrointestinal tract. Curr. Opin. Gastroenterol. 2013;29:581–587. doi: 10.1097/MOG.0b013e328365d339. [DOI] [PubMed] [Google Scholar]
- 62.Ferlazzo G., Pack M., Thomas D., Paludan C., Schmid D., Strowig T., Bougras G., Muller W.A., Moretta L., Münz C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc. Natl. Acad. Sci. USA. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wehner R., Dietze K., Bachmann M., Schmitz M. The Bidirectional Crosstalk between Human Dendritic Cells and Natural Killer Cells. J. Innate Immun. 2011;3:258–263. doi: 10.1159/000323923. [DOI] [PubMed] [Google Scholar]
- 64.Boudreau J.E., Bonehill A., Thielemans K., Wan Y. Engineering Dendritic Cells to Enhance Cancer Immunotherapy. Mol. Ther. 2011;19:841–853. doi: 10.1038/mt.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferlazzo G., Morandi B. Cross-Talks between Natural Killer Cells and Distinct Subsets of Dendritic Cells. Front. Immunol. 2014;5:159. doi: 10.3389/fimmu.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laffont S., Seillet C., Ortaldo J., Coudert J.D., Guéry J.-C. Natural killer cells recruited into lymph nodes inhibit alloreactive T-cell activation through perforin-mediated killing of donor allogeneic dendritic cells. Blood. 2008;112:661–671. doi: 10.1182/blood-2007-10-120089. [DOI] [PubMed] [Google Scholar]
- 67.Morandi B., Mortara L., Chiossone L., Accolla R.S., Mingari M.C., Moretta L., Moretta A., Ferlazzo G. Dendritic Cell Editing by Activated Natural Killer Cells Results in a More Protective Cancer-Specific Immune Response. PLoS ONE. 2012;7:e39170. doi: 10.1371/journal.pone.0039170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andrews D.M., Scalzo A.A., Yokoyama W.M., Smyth M.J., Degli-Esposti M.A. Functional interactions between dendritic cells and NK cells during viral infection. Nat. Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 69.Karlhofer F.M., Ribaudo R.K., Yokoyama W.M. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 70.Cooper M.A., Fehniger T.A., Fuchs A., Colonna M., Caligiuri M.A. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 71.Cools N., Ponsaerts P., Van Tendeloo V.F.I., Berneman Z.N. Balancing between immunity and tolerance: An interplay between dendritic cells, regulatory T cells, and effector T cells. J. Leukoc. Biol. 2007;82:1365–1374. doi: 10.1189/jlb.0307166. [DOI] [PubMed] [Google Scholar]
- 72.Marcenaro E., Carlomagno S., Pesce S., Moretta A., Sivori S. NK/DC Crosstalk in Anti-viral Response. In: Lambris J.D., Hajishengallis G., editors. Current Topics in Innate Immunity II. Springer; New York, NY, USA: 2012. pp. 295–308. [DOI] [PubMed] [Google Scholar]
- 73.Nguyen-Pham T.-N., Yang D.-H., Nguyen T.-A.T., Lim M.-S., Hong C.Y., Kim M.-H., Lee H.J., Lee Y.-K., Cho D., Bae S.-Y., et al. Optimal culture conditions for the generation of natural killer cell-induced dendritic cells for cancer immunotherapy. Cell. Mol. Immunol. 2012;9:45–53. doi: 10.1038/cmi.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiao L., Gao X., Joyee A.G., Zhao L., Qiu H., Yang M., Fan Y., Wang S., Yang X. NK Cells Promote Type 1 T Cell Immunity through Modulating the Function of Dendritic Cells during Intracellular Bacterial Infection. J. Immunol. 2011;187:401–411. doi: 10.4049/jimmunol.1002519. [DOI] [PubMed] [Google Scholar]
- 75.Shekhar S., Peng Y., Gao X., Joyee A.G., Wang S., Bai H., Zhao L., Yang J., Yang X. NK cells modulate the lung dendritic cell-mediated Th1/Th17 immunity during intracellular bacterial infection. Eur. J. Immunol. 2015;45:2810–2820. doi: 10.1002/eji.201445390. [DOI] [PubMed] [Google Scholar]
- 76.Shekhar S., Yang X. Natural killer cells in host defense against veterinary pathogens. Vet. Immunol. Immunopathol. 2015;168:30–34. doi: 10.1016/j.vetimm.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holl E.K., Roney K.E., Allen I.C., Steinbach E., Arthur J.C., Buntzman A., Plevy S., Frelinger J., Ting J.P.Y. Plexin-B2 and Plexin-D1 in Dendritic Cells: Expression and IL-12/IL-23p40 Production. PLoS ONE. 2012;7:e43333. doi: 10.1371/journal.pone.0043333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alamri A., Kung S. (Department of Immunology, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada). Personal communication. 2017.