Abstract
Expansion of chondrocytes for repair of articular cartilage can lead to dedifferentiation, making it difficult to obtain a sufficient quantity of chondrocytes. Although previous studies have suggested that culture in a three-dimensional environment induces redifferentiation of dedifferentiated chondrocytes, its underlying mechanisms are still poorly understood in terms of metabolism compared with a two-dimensional environment. In this study, we demonstrate that attenuation of transglutaminase 2 (TG2), a multifunctional enzyme, stimulates redifferentiation of dedifferentiated chondrocytes. Fibroblast-like morphological changes increased as TG2 expression increased in passage-dependent manner. When dedifferentiated chondrocytes were cultured in a pellet culture system, TG2 expression was reduced and glycolytic enzyme expression up-regulated. Previous studies demonstrated that TG2 influences energy metabolism, and impaired glycolytic metabolism causes chondrocyte dedifferentiation. Interestingly, TG2 knockdown improved chondrogenic gene expression, glycolytic enzyme expression, and lactate production in a monolayer culture system. Taken together, down-regulation of TG2 is involved in redifferentiaton of dedifferentiated chondrocytes through enhancing glucose metabolism.
Keywords: chondrocytes, redifferentiation, transglutaminase 2, glycolytic metabolism
1. Introduction
Articular cartilage is a unique avascular and aneural tissue in which chondrocytes are embedded in the extracellular matrix (ECM), which is mainly comprised of collagen, glycosaminoglycans (GAGs), and other glycoproteins. Articular chondrocytes maintain articular cartilage homeostasis by remodeling ECM molecules, including type II collagen and proteoglycans. Molecular composition of the ECM also influences the specific functions of articular chondrocytes [1,2,3]. Chondrocytes can exist under low oxygen conditions due to the avascular environment. Thus, glucose metabolism is the main energy source of chondrocytes [4,5,6,7]. Previous studies reported that suppression of glucose metabolism using an inhibitor of glycolytic enzymes can induce dedifferentiation of chondrocytes [8,9].
Since articular cartilage has low self-healing capacity, treatment of cartilage lesions after injury is important to restore cartilage-specific function. Therefore, different therapeutic strategies have been suggested for the treatment of impaired cartilage. Among them, autologous chondrocyte implantation (ACI) is a well-established therapy for patients with knee cartilage damage [10,11]. However, long-term expansion and serial passaging, which are required to obtain a sufficient amount of cells for therapy, lead to dedifferentiation of chondrocytes [12,13]. Dedifferentiation of chondrocytes gives rise to gradual loss of chondrogenic markers and chondrogenic ECM molecules such as type II collagen and GAG. Therefore, many studies have tried to restore the chondrogenic potential of dedifferentiated chondrocytes after serial passages by supplementing growth medium with defined factors [14,15,16,17,18,19], regulating cell density [20,21,22,23], cultivating cells in a more cartilage-like environment [24,25,26]. Three-dimensional (3D) culture systems such as pellet culture have been widely used to induce redifferentiation of dedifferentiated chondrocytes [27,28,29]. In terms of glucose metabolism, however, the molecular mechanism of redifferentiation in a 3D culture system is largely unknown.
Transglutaminase 2 (TG2), a stress-inducible factor, is a ubiquitously expressed multifunctional enzyme that catalyzes the post-translational modification of proteins in a Ca2+-dependent manner [30,31]. In addition to its various enzymatic activities, TG2 is associated with many cellular processes such as cell adhesion [32,33,34], survival, proliferation [35], migration [36,37] and autophagy [38,39]. Recently, it has been reported that TG2 modulates the transcription of critical genes including PPARγ coactivator-1alpha (PGC-1alpha) and cytochrome C that are important for function and biogenesis of mitochondria and regulates cellular energy metabolism [40,41]. Interestingly, increased TG2 expression is related with physiologic maturation to hypertrophy of growth plate chondrocytes. Chondrocyte hypertrophic differentiation is the gradual development process from chondrogenic differentiation to cartilage mineralization, but chondrocyte hypertrophy-like changes play a role in early and late stage osteoarthritis (OA) [42]. Moreover, it was reported that TG2 expression is related to articular chondrocyte hypertrophy and is a biomarker of OA severity [43].
In the present study, we observed gradual up-regulation of TG2 expression in chondrocytes during serial passage. However, elevation of TG2 expression in dedifferentiated chondrocytes was significantly diminished in a pellet culture system. Based on this link between TG2 and redifferentiation of dedifferentiated chondrocytes, we focused on alteration of energy metabolism by TG2 in a pellet culture system.
2. Results
2.1. TG2 Expression is Associated With Dedifferentiation of Chondrocytes
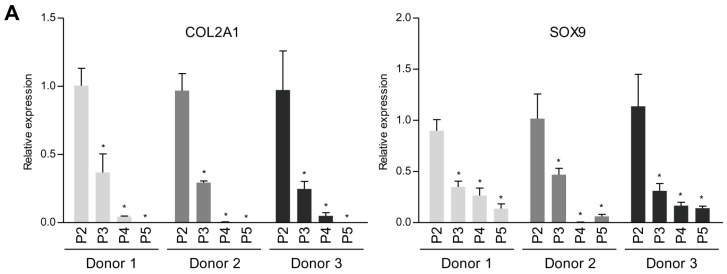
To determine the TG2 expression level during dedifferentiation of chondrocytes, we first analyzed TG2 expression levels after serial passages in monolayer. Interestingly, TG2 expression in human articular chondrocytes from 3 different donors increased in a passage-dependent manner (Figure 1).
Figure 1.
Induction of TG2 in dedifferentiated chondrocytes. Human articular chondrocytes were passed until passage 5 in normal growth medium. (A) COL2A1, SOX9 and (B) TG2 mRNA expression levels in human articular chondrocytes were measured by real-time qPCR. * p < 0.05. n = 3. (C) TG2 protein expression levels in human articular chondrocytes were detected by Western blotting using anti-TG2 polyclonal antibody. Asterisks denote other TGs. TG2 antibody cross-reacts with other transglutaminases. GAPDH and β-actin expressions were examined as a loading control.
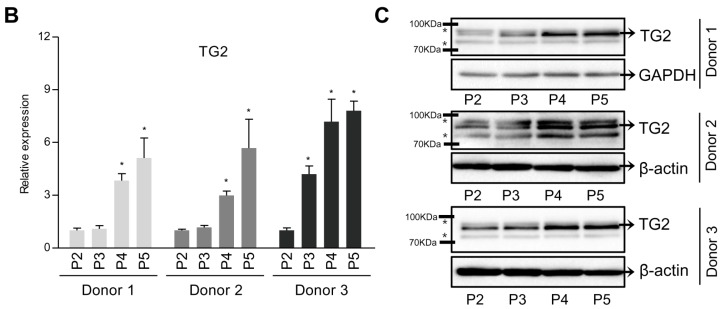
Redifferentiation of dedifferentiated chondrocytes was induced using pellet culture in a 3D environment. Subsequently, SRY (sex determining region Y)-box 9 (SOX9), collagen type II (COL2A1) and aggrecan (ACAN) mRNA expression levels were compared to their counterparts in monolayer culture. As shown in Figure 2A, expression of chondrogenic markers (SOX9, COL2A1 and ACAN) was significantly higher in the pellet culture system (Figure 2A) compared to monolayer culture. Next, TG2 expression in the 3D pellet culture system significantly decreased at both mRNA and protein levels (Figure 2B,C). These results indicate that TG2 expression is highly related to dedifferentiation of chondrocytes after serial passage.
Figure 2.
Changes in chondrogenic marker and TG2 expression in pellet culture system. Human articular chondrocytes were passaged up to 4 in monolayer culture. Cells were detached and cultivated in monolayer or pellet culture system for 14 days. (A) SOX9, COL2A1, ACAN and (B) TG2 mRNA expression levels were measured by real-time qPCR. Data represent mean values ± SD from at least three independent experiments. ML, monolayer. Pellet, pellet culture system. * p < 0.05. n = 3. (C) TG2 protein expression levels were examined by western blotting. Asterisks denote other TGs. β-actin was used as a loading control.
2.2. Alteration of Energy Metabolism in Pellet Culture System
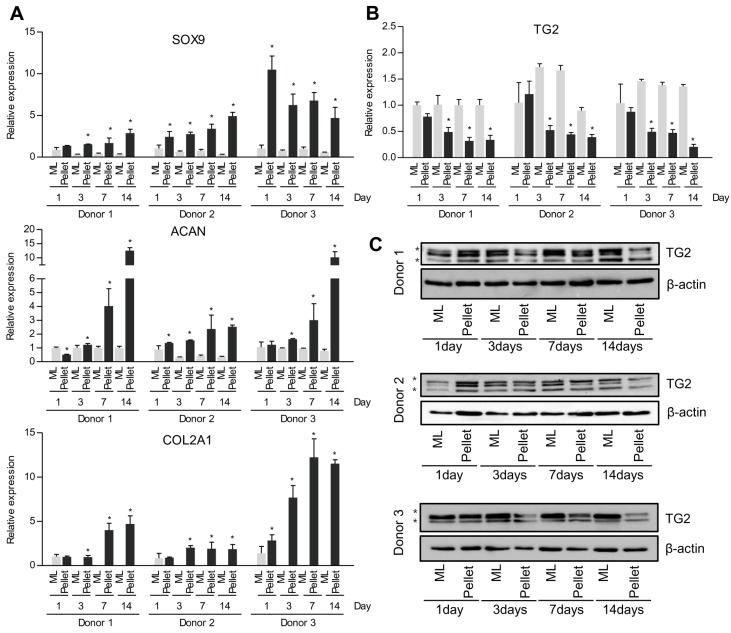
Articular chondrocytes are adapted to low oxygen environments in vivo and generate energy through glycolysis rather than oxidative phosphorylation in mitochondria [6]. We next compared the expression levels of genes involved in the glycolytic pathway between 2D monolayer and 3D pellet culture systems. Expression of glycolysis-related genes such as glucose transporter 1 (GLUT1), hexokinase 2 (HK2), lactate dehydrogenase A (LDHA) and pyruvate kinase muscle isozyme M2 (PKM2) largely increased in pellet culture compared to monolayer culture (Figure 3A,B). In addition, production of lactate, the end product of glycolysis, was much higher in monolayer culture on day 1 of the initial stage, after which it drastically decreased. After 3 days, 3D pellet culture induced higher production of lactate than 2D monolayer culture (Figure 3C). Taken together, these results suggest that metabolic change undergoes during redifferentiation of dedifferentiated chondrocytes in a pellet culture system.
Figure 3.
Alteration of energy metabolism in pellet culture system. Passage 5 human articular chondrocytes were cultured in monolayer or pellet culture system for 14 days. (A) GLUT1, HK2, LDHA and PKM2 mRNA expression levels were quantified by real-time qPCR. Data represent mean values ± SD from at least three independent experiments. * p < 0.05. n = 3. (B) HK2, LDHA and PKM2 protein expression levels were Western blot analysis. β-actin was used as a loading control. (C) Extracellular lactate levels secreted by cells in monolayer or pellet culture systems. Lactate concentration of monolayer culture for 1 day samples was assigned as 100%. ML, monolayer. Pellet, pellet culture system.
2.3. Down-Regulation of TG2 Enhances Glycolytic Pathway in Chondrocytes
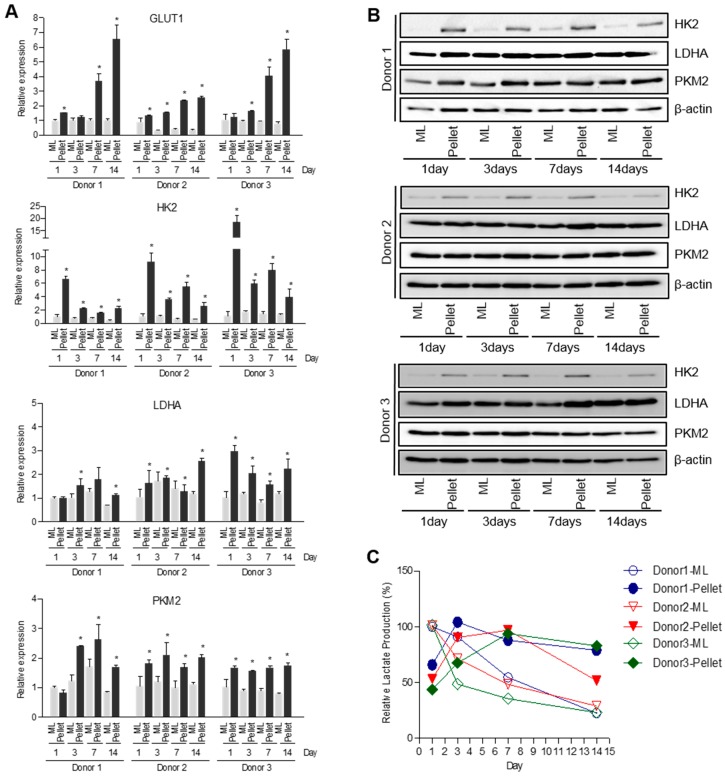
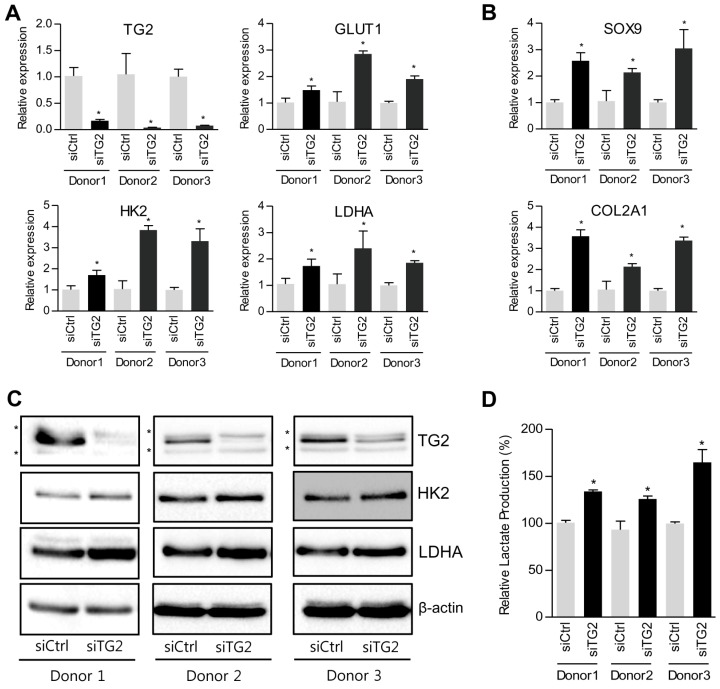
Since TG2 modulates energy metabolism in various cell types [41,44], we hypothesized that TG2 ablation in a pellet culture system can stimulate energy metabolism by regulating expression of glycolysis-related genes. siRNA-mediated knockdown of TG2 expression in dedifferentiated chondrocytes, as confirmed by reduced expression of TG2, induced significant up-regulation of glucose metabolism-associated genes (GLUT1, HK2 and LDHA) as well as chondrocgenic genes (SOX9 and COL2A1) (Figure 4). Furthermore, down-regulation of TG2 in dedifferentiated chondrocytes significantly enhanced production of lactate, demonstrating that down-regulation of TG2 is able to promote redifferentiation of dedifferentiated chondrocytes through improving glucose metabolism.
Figure 4.
Metabolic reprogramming by TG2 knockdown in dedifferentiated chondrocytes. Passage 5 human articular chondrocytes were transfected with TG2-specific siRNA or non-silencing control siRNA. (A) TG2, GLUT1, HK2 and LDHA mRNA levels were quantified by real-time qPCR. (B) The mRNA expression levels of chondrogenic markers (SOX9 and COL2A1) were measured by real-time qPCR. (C) The protein expression levels TG2, HK2 and LDHA were quantified by western blot analysis. Asterisks denote other TGs. β-actin was used as a loading control. (D) Extracellular lactate levels secreted by cells transfected with control siRNA or TG2 siRNA. Lactate concentration of non-silencing control siRNA transfected cell samples was assigned as 100%. Data represent mean values±SD from at least three independent experiments. * p < 0.05. n = 3.
3. Discussion
It is well known that expansion of isolated chondrocytes using conventional monolayer culture method leads to loss of chondrogenic potential, especially dedifferentiation [12,13]. A number of studies have suggested techniques to maintain chondrogenic potential during expansion or induce redifferentiation of dedifferentiated chondrocytes. Among them, three-dimensional (3D) cultures are widely used for redifferentiation of dedifferentiated chondrocytes since they are very simple and low-cost. In terms of glucose metabolism, however, the underlying molecular mechanism of 3D pellet culture remains unclear.
In this study, elevation of TG2 expression was found to be highly associated with dedifferentiation of chondrocytes by serial passage while down-regulation of TG2 in a 3D pellet culture system improved redifferentiation of dedifferentiated chondrocytes through enhancing glycolytic metabolism.
As shown in Figure 1, TG2 expression was drastically augmented in a passage-dependent manner in human articular chondrocytes from 3 donors. It has been reported that TG2 is predominantly localized to the cytosol, although it has been found in mitochondria, the nucleus, cell membrane, and extracellular matrix [45,46]. TG2 is able to modify mitochondrial function, signal transduction targets, and cell surface receptors, which regulate intracellular signaling molecules and extracellular matrix synthesis [47,48]. A previous study demonstrated the PDI function of TG2 in vivo based on analysis of TGM2 knock-out mice, which exhibit abnormalities in the mitochondrial respiratory chain and ATP production [49]. However, it is unclear how TG2 regulates mitochondria-dependent processes.
Since cartilage is an avascular tissue, oxygen deficiency confers a hypoxic environment in vivo (1–5% oxygen tension) [1]. Therefore, articular chondrocytes are well adapted to low oxygen tension, resulting in low mitochondrial mass and low oxygen requirements followed by high glycolytic rates for survival. Previously, monolayer-cultured chondrocytes were shown to have markedly increased mitochondria content compared to freshly isolated chondrocytes. Moreover, chondrocytes undergo drastic alteration of metabolic status from characteristically glycolytic to oxidative energy metabolism during monolayer expansion [50]. Recent studies indicate that TG2’s activity not only can modulate the assembly of respiratory chain complexes in mitochondria but are able to also modulate the transcription of critical genes including PGC-1alpha and cytochrome C that are important for function and biogenesis of mitochondria [40,41]. Elevation of oxidative capacity results from excessive production of reactive oxygen species (ROS), and imbalance between ROS generation and antioxidant processes has been determined to be a cause of oxidative stress-mediated dedifferentiation and senescence of chondrocytes during monolayer expansion [51,52]. Further works need to identify how TG2 is involved in mitochondrial biosynthesis and oxidative stress-induced dedifferentiation and senescence in chondrocytes during monolayer culture.
It has been reported that TG2 expression is mainly controlled at the transcription level [53], and the TG2 promoter has response elements of TNF-α [54,55], IL-1β [56], and IL-6 [57]. Pro-inflammatory cytokines such as TNF-α and IL-1β inhibit expression of master chondrogenic factor SOX9, resulting in down-regulation of chondrocyte-specific markers such as collagen type II [58]. Recently, Johnson et al. reported that TG2 is an essential mediator of chondrocyte hypertrophy and calcification in response to both ATRA and IL-1β using TG2 knockout mice [56]. However, further studies are required to determine the upstream regulator of TG2 expression in dedifferentiated chondrocytes after serial passage.
Elevated TG2 expression of dedifferentiated chondrocytes began to decrease in the 3D pellet culture system (Figure 2). In contrast, expression of glucose metabolism-related genes (GLUT1, HK2, LDHA and PKM2) and lactate production increased in the 3D pellet culture system (Figure 3). GLUT1 is the transmembrane glucose transporter protein that mediate glucose uptake. Since HK2, LDHA and PKM2 are important enzymes that directly modulate glycolysis [59,60], we identified expression of HK2, LDHA and PKM2 in both mRNA and protein levels. Generally, the 3D pellet culture is widely used since it is very simple and favorable to maintain chondrogenic phenotype and improve redifferentiation of dedifferentiated chondrocytes. However, it is not yet clear which factors are involved in the redifferentiation process in a 3D environment. Interestingly, Hunt et al. reported reduction of mitochondrial activity in a 3D culture system [61]. Since TG2 is directly or indirectly implicated in mitochondrial homeostasis [62], cells lacking TG2 alter cellular energy metabolism towards aerobic glycolysis in an attempt to survive [41]. Accordingly, we hypothesized that down-regulation of TG2 expression in the 3D pellet culture system could lead to alteration of cellular energy metabolism. As shown in Figure 4, we observed that down-regulation of TG2 expression in dedifferentiated chondrocytes was responsible for increased expression of metabolic enzymes (HK2, PKM2, LDHA and GLUT1), resulting in enhancement of lactate production. Moreover, chondrogenic markers SOX9 and COL2A1 were restored in dedifferentiated chondrocytes by down-regulation of TG2. These results imply that TG2 reduction in a 3D environment improves redifferentiation of dedifferentiated chondrocytes through energy metabolism rearrangement. Although we did not clarify whether or not TG2 can directly modulate cellular metabolism and induce chondrogenic factors, increased chondrogenic potential can be attributed to metabolism-associated TG2 expression, which should be clarified in the future.
4. Materials and Methods
4.1. Ethics Statement
The study was approved by the institutional review board and ethics committee of CHA Bundang Medical Center (BD2014-07-097). A written informed consent about this experiment was obtained from all subjects.
4.2. Chondrocyte Isolation
Chondrocytes from 3 donors (Donor 1; 58-year-old female, Donor 2; 61-year-old male and Donor 3; 72-year-old male) were isolated from the undamaged region of osteoarthritic (OA) cartilage from total knee arthroplasty. Cartilage was separated from the subchondral bone and cut into 2 × 2-mm pieces using a surgical blade. Cartilage pieces were washed three times with HBSS (Hyclone, Logan, UT, USA) containing 2% antibiotic/antimycotic (Hyclone) and then digested at 37 °C, 5% CO2 in DMEM (Hyclone) containing 0.25 mg/mL of collagenase (Sigma, St. Louis, MO, USA). After 15 h, undigested tissue was removed from cells using a 45 μm cell strainer and plated in culture plates. Isolated cells were cultured in humidified air with 5% CO2 at 37 °C. Culture medium consisted of DMEM/Low glucose (Hyclone), 10% fetal bovine serum (FBS, Hyclone), and 100 units/mL of penicillin-streptomycin (Hyclone).
4.3. Pellet Preparation and Cultivation
For pellet culture, 200,000 cells (passage 4) per pellet were centrifuged at 500 rpm for 5 min in conical tubes in chondrogenic differentiation medium. The tubes were placed in a humidified atmosphere at 37 °C and 5% CO2 with the lid loosely closed. Chondrogenic differentiation medium consisted of high glucose medium, 1% (v/v) insulin transferrin selenium A (Gibco, Grand island, NY, USA), 50 μg/mL of ascorbic acid (Sigma), 100 nM dexamethasone (Sigma), and 10 ng/mL of TGF-β1 (Millipore, Schwalbach, Germany).
4.4. Western Blot Analysis
Cells or pellets were washed twice with PBS (Hyclone) and lysed in RIPA buffer (Cell Signaling Technology, Danvers, MA, USA). Protein concentration was determined using bicinchoninic acid (BCA) protein assay (Thermo Scientific, Rockford, IL, USA). Total protein extracts were separated by SDS-PAGE and electroblotted onto a nitrocellulose membrane. After blocking in 5% non-fat dry milk in Tris-buffered Saline Tween 20 (Biosesang, Gyeonggi-do, Korea), blots were incubated with rabbit polyclonal anti-TG2 antibody (Abcam, Cambridge, UK), rabbit polyclonal anti-Hexokinase 2 antibody, rabbit polyclonal anti-PKM2 antibody, and rabbit polyclonal anti-LDHA antibody (Cell Signaling Technology). After washing, secondary antibody (HRP-conjugated anti-rabbit immunoglobulin) was added and visualized using an ECL prime (GE Healthcare, Little Chalfont, UK).
4.5. RNA Isolation and Real Time Quantitative PCR
Total RNA was isolated using Trizol (Ambion, TX, USA) according to the manufacturer’s instructions, after which cDNA was synthesized with a PrimeScript RT reagent kit (Takara, Tokyo, Japan). Real-time quantitative PCR was performed using Power SYBR® Green PCR Master Mix (Applied Biosystems, San Francisco, CA, USA). ABI StepOnePlus Real-Time PCR System was used for amplification using the following cycle conditions: 95 °C for 10 min × 1 cycle and 95 °C for 15 s, followed by 60 °C for 30 s × 40 cycles. Primer pairs for TG2, ACAN, COL2A1, SOX9, GLUT1, HK2, PKM2, LDHA and 18s rRNA are shown in Table 1. Data were analyzed using the ΔΔCt method, mRNA expression was normalized to 18S rRNA as a housekeeping gene, and gene expression was calculated as a fold change.
Table 1.
Nucleotide sequences of primer pairs for real-time PCR.
| Gene | Human Primer Sequence | |
|---|---|---|
| TG2 | Sense | 5′-GGCACCAAGTACCTGCTCA-3′ |
| Antisense | 5′-AGAGGATGCAAAGAGGAACG-3′ | |
| ACAN | Sense | 5′-GCCTGCGCTCCAATGACT-3′ |
| Antisense | 5′-ATGGAACACGATGCCTTTCAC-3′ | |
| COL2A1 | Sense | 5′-CACGTACACTGCCCTGAAGGA-3′ |
| Antisense | 5′-CGATAACAGTCTTGCCCCACTT-3′ | |
| SOX9 | Sense | 5′-CCCCAACAGATCGCCTACAG-3′ |
| Antisense | 5′-GAGTTCTGGTCGGTGTAGTC-3′ | |
| GLUT1 | Sense | 5′-GGTTGTGCCATACTCATGACC-3′ |
| Antisense | 5′-CAGATAGGACATCCAGGGTAGC-3′ | |
| HK2 | Sense | 5′-TCCCCTGCCACCAGACTA-3′ |
| Antisense | 5′-TGGACTTGAATCCCTTGGTC-3′ | |
| PKM2 | Sense | 5′-CGTCTGAACTTCTCTCATGGAA-3′ |
| Antisense | 5′-ATGGGGTCAGAAGCAAAGC-3′ | |
| LDHA | Sense | 5′-GCAGATTTGGCAGAGAGTATAATG-3′ |
| Antisense | 5′-GACATCATCCTTTATTCCGTAAAGA-3′ | |
| 18S rRNA | Sense | 5′-CTTCCACAGGAGGCCTACAC-3′ |
| Antisense | 5′-CGCAAAATATGCTGGAACTTT-3′ | |
4.6. Lactate Assay
For assessment of lactate production, medium was collected and diluted 1:100 in lactate assay buffer. The amount of lactate present in the media was measured using a Lactate Assay Kit (Biovision, Mountain View, CA, USA) according to the manufacturer’s instructions. The concentrations of lactate were normalized to protein concentration.
4.7. Knockdown Experiment
For the siRNA transfection experiments, cells were seeded in a 12-well plate. After 24 h, TG2 siRNA (Bioneer, Daejeon, Korea) was transfected into cells using Lipofectamine RNA iMAX reagent (Invitrogen, Carlsbad, CA, USA). Transfected cells were incubated for 72 h and subjected to various analyses.
4.8. Statistical Analysis
All data were expressed as the mean ± S.D. Student’s t-test was performed to compare two groups. One-way ANOVA with post-hoc tukey tests was used to analyze multiple groups. The p values less than 0.05 were considered as significant.
5. Conclusions
It is concluded that TG2 is a negative regulator of chondrogenic potential in chondrocytes during expansion. The 3D environment is able to inhibit elevation of TG2 expression of dedifferentiated chondrocytes. On the other hand, regulation of TG2 and/or TG2-dependent signal transduction during monolayer expansion will provide a useful tool to reduce dedifferentiation of chondrocytes.
Acknowledgments
This work was supported by the Ministry of Science, ICT & Future Planning (grant numbers NRF-2016R1A2A1A05004987), the Korea Health Technology R&D Project, in the Korea Health Industry Development Institute (KHIDI) and the Ministry of Health & Welfare, Republic of Korea (grant numbers HI16C0106 and HR16C0002).
Abbreviations
| 3D culture system | Three-Dimensional Culture System |
| ACI | Autologous Chondrocyte Implantation |
| ECM | Extracellular Matrix |
| GAG | Glycosaminoglycan |
| TG2 | Transglutaminase 2 |
| SOX9 | SRY (Sex Determining Region Y)-Box 9 (SOX9) |
| COL2A1 | Collagen Type II |
| ACAN | Aggrecan |
| GLUT1 | Glucose Transporter 1 |
| HK2 | Hexokinase 2 |
| LDHA | Lactate Dehydrogenase A |
| PKM2 | Pyruvate Kinase Muscle isozyme M2 |
| TGF-β1 | Transforming Growth Factor Beta 1 |
Author Contributions
Kyoung-Won Ko designed and performed the experiments, and analyzed the data, and wrote the paper; Sunghyun Park performed the experiments; Bogyu Choi wrote the paper and contributed the discussion; Yoshie Arai and Hojae Bae contributed to discussion; Joong-Myung Lee and Won Chul Choi provided the human articular cartilage tissues; In-Bo Han and Soo-Hong Lee supervised and edited the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Buckwalter J. Articular cartilage. AAOS Instr. Course Lect. 1982;32:349–370. [PubMed] [Google Scholar]
- 2.Poole A.R., Kojima T., Yasuda T., Mwale F., Kobayashi M., Laverty S. Composition and structure of articular cartilage: A template for tissue repair. Clin. Orthop. Relat. Res. 2001;391:S26–S33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 3.Temenoff J.S., Mikos A.G. Tissue engineering for regeneration of articular cartilage. Biomaterials. 2000;21:431–440. doi: 10.1016/S0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 4.Lane J., Brighton C., Menkowitz B. Anaerobic and aerobic metabolism in articular cartilage. J. Rheumatol. 1976;4:334–342. [PubMed] [Google Scholar]
- 5.Mobasheri A., Vannucci S., Bondy C., Carter S., Innes J., Arteaga M., Trujillo E., Ferraz I., Shakibaei M., Martín-Vasallo P. Glucose transport and metabolism in chondrocytes: A key to understanding chondrogenesis, skeletal development and cartilage degradation in osteoarthritis. Histol. Histopathol. 2002;17:1239–1267. doi: 10.14670/HH-17.1239. [DOI] [PubMed] [Google Scholar]
- 6.Stockwell R. Metabolism of cartilage. Cartilage. 2012;1:253–273. [Google Scholar]
- 7.URBAN J. Evidence for a negative Pasteur effect in articular cartilage. Biochem. J. 1997;321:95–102. doi: 10.1042/bj3210095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida T., Kubota S., Aoyama E., Takigawa M. Impaired glycolytic metabolism causes chondrocyte hypertrophy-like changes via promotion of phospho-Smad1/5/8 translocation into nucleus. Osteoarthr. Cartil. 2013;21:700–709. doi: 10.1016/j.joca.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Yu S.-M., Kim H.A., Kim S.-J. 2-Deoxy-D-glucose regulates dedifferentiation through β-catenin pathway in rabbit articular chondrocytes. Exp. Mol. Med. 2010;42:503–513. doi: 10.3858/emm.2010.42.7.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris J.D., Siston R.A., Pan X., Flanigan D.C. Autologous Chondrocyte Implantation. J. Bone Joint Surg. 2010;92:2220–2233. doi: 10.2106/JBJS.J.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knutsen G., Engebretsen L., Ludvigsen T.C., Drogset J.O., Grøntvedt T., Solheim E., Strand T., Roberts S., Isaksen V., Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee: A randomized trail. J. Bone Joint Surg. 2004;86:455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Benya P.D., Padilla S.R., Nimni M.E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15:1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- 13.Von Der Mark K., Gauss V., Von Der Mark H., Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 14.Stoppoloni D., Politi L., Leopizzi M., Gaetani S., Guazzo R., Basciani S., Moreschini O., de Santi M., Scandurra R., d’Abusco A.S. Effect of glucosamine and its peptidyl-derivative on the production of extracellular matrix components by human primary chondrocytes. Osteoarthr. Cartil. 2015;23:103–113. doi: 10.1016/j.joca.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Chua K., Aminuddin B., Fuzina N., Ruszymah B. Insulin-transferrin-selenium prevent human chondrocyte dedifferentiation and promote the formation of high quality tissue engineered human hyaline cartilage. Eur. Cell Mater. 2005;9:58–67. doi: 10.22203/eCM.v009a08. [DOI] [PubMed] [Google Scholar]
- 16.Martin I., Vunjak-Novakovic G., Yang J., Langer R., Freed L. Mammalian chondrocytes expanded in the presence of fibroblast growth factor 2 maintain the ability to differentiate and regenerate three-dimensional cartilaginous tissue. Exp. Cell Res. 1999;253:681–688. doi: 10.1006/excr.1999.4708. [DOI] [PubMed] [Google Scholar]
- 17.Narcisi R., Signorile L., Verhaar J., Giannoni P., Van Osch G. TGFβ inhibition during expansion phase increases the chondrogenic re-differentiation capacity of human articular chondrocytes. Osteoarthr. Cartil. 2012;20:1152–1160. doi: 10.1016/j.joca.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Pei M., Seidel J., Vunjak-Novakovic G., Freed L. Growth factors for sequential cellular de-and re-differentiation in tissue engineering. Biochem. Biophys. Res. Commun. 2002;294:149–154. doi: 10.1016/S0006-291X(02)00439-4. [DOI] [PubMed] [Google Scholar]
- 19.Stewart M.C., Saunders K.M., Burton-Wurster N., Macleod J.N. Phenotypic stability of articular chondrocytes in vitro: The effects of culture models, bone morphogenetic protein 2, and serum supplementation. J. Bone Miner. Res. 2000;15:166–174. doi: 10.1359/jbmr.2000.15.1.166. [DOI] [PubMed] [Google Scholar]
- 20.Hicks D.L., Sage A.B., Schumacher B.L., Sah R.L., Watson D. Growth and phenotype of low-density nasal septal chondrocyte monolayers. Otolaryngol.-Head Neck Surg. 2005;133:417–422. doi: 10.1016/j.otohns.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 21.Mandl E.W., Van Der Veen S.W., Verhaar J.A., Van Osch G.J. Multiplication of human chondrocytes with low seeding densities accelerates cell yield without losing redifferentiation capacity. Tissue Eng. 2004;10:109–118. doi: 10.1089/107632704322791754. [DOI] [PubMed] [Google Scholar]
- 22.Schulze-Tanzil G., de Souza P., Castrejon H.V., John T., Merker H.-J., Scheid A., Shakibaei M. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002;308:371–379. doi: 10.1007/s00441-002-0562-7. [DOI] [PubMed] [Google Scholar]
- 23.Watt F.M. Effect of seeding density on stability of the differentiated phenotype of pig articular chondrocytes in culture. J. Cell Sci. 1988;89:373–378. doi: 10.1242/jcs.89.3.373. [DOI] [PubMed] [Google Scholar]
- 24.Lafont J.E. Lack of oxygen in articular cartilage: Consequences for chondrocyte biology. Int. J. Exp. Pathol. 2010;91:99–106. doi: 10.1111/j.1365-2613.2010.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy C.L., Thoms B.L., Vaghjiani R.J., Lafont J.E. HIF-mediated articular chondrocyte function: Prospects for cartilage repair. Arthritis Res. Ther. 2009;11:213. doi: 10.1186/ar2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanz-Ramos P., Mora G., Ripalda P., Vicente-Pascual M., Izal-Azcárate I. Identification of signalling pathways triggered by changes in the mechanical environment in rat chondrocytes. Osteoarthr. Cartil. 2012;20:931–939. doi: 10.1016/j.joca.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein P., Dong M., Corbeil D., Gelinsky M., Günther K.P., Fickert S. Pellet culture elicits superior chondrogenic redifferentiation than alginate-based systems. Biotechnol. Prog. 2009;25:1146–1152. doi: 10.1002/btpr.186. [DOI] [PubMed] [Google Scholar]
- 28.Lee T.-J., Bhang S.H., La W.-G., Yang H.S., Seong J.Y., Lee H., Im G.-I., Lee S.-H., Kim B.-S. Spinner-flask culture induces redifferentiation of de-differentiated chondrocytes. Biotechnol. Lett. 2011;33:829–836. doi: 10.1007/s10529-010-0488-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z., McCaffery J.M., Spencer R.G., Francomano C.A. Hyaline cartilage engineered by chondrocytes in pellet culture: Histological, immunohistochemical and ultrastructural analysis in comparison with cartilage explants. J. Anat. 2004;205:229–237. doi: 10.1111/j.0021-8782.2004.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckert R.L., Kaartinen M.T., Nurminskaya M., Belkin A.M., Colak G., Johnson G.V., Mehta K. Transglutaminase regulation of cell function. Physiol. Rev. 2014;94:383–417. doi: 10.1152/physrev.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gundemir S., Colak G., Tucholski J., Johnson G.V. Transglutaminase 2: A molecular Swiss army knife. BBA Mol. Cell Res. 2012;1823:406–419. doi: 10.1016/j.bbamcr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaudry C., Verderio E., Jones R., Smith C., Griffin M. Tissue transglutaminase is an important player at the surface of human endothelial cells: Evidence for its externalization and its colocalization with the β 1 integrin. Exp. Cell Res. 1999;252:104–113. doi: 10.1006/excr.1999.4633. [DOI] [PubMed] [Google Scholar]
- 33.Gaudry C.A., Verderio E., Aeschlimann D., Cox A., Smith C., Griffin M. Cell surface localization of tissue transglutaminase is dependent on a fibronectin-binding site in its n-terminal β-sandwich domain. J. Biol. Chem. 1999;274:30707–30714. doi: 10.1074/jbc.274.43.30707. [DOI] [PubMed] [Google Scholar]
- 34.Gentile V., Thomazy V., Piacentini M., Fesus L., Davies P. Expression of tissue transglutaminase in Balb-C 3T3 fibroblasts: Effects on cellular morphology and adhesion. J. Cell Biol. 1992;119:463–474. doi: 10.1083/jcb.119.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nadalutti C., Viiri K., Kaukinen K., Mäki M., Lindfors K. Extracellular transglutaminase 2 has a role in cell adhesion, whereas intracellular transglutaminase 2 is involved in regulation of endothelial cell proliferation and apoptosis. Cell Prolif. 2011;44:49–58. doi: 10.1111/j.1365-2184.2010.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar A., Xu J., Brady S., Gao H., Yu D., Reuben J., Mehta K. Tissue transglutaminase promotes drug resistance and invasion by inducing mesenchymal transition in mammary epithelial cells. PLoS ONE. 2010;5:e13390. doi: 10.1371/journal.pone.0013390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma A., Mehta K. Tissue transglutaminase-mediated chemoresistance in cancer cells. Drug Resist. Updat. 2007;10:144–151. doi: 10.1016/j.drup.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Akar U., Ozpolat B., Mehta K., Fok J., Kondo Y., Lopez-Berestein G. Tissue transglutaminase inhibits autophagy in pancreatic cancer cells. Mol. Cancer Res. 2007;5:241–249. doi: 10.1158/1541-7786.MCR-06-0229. [DOI] [PubMed] [Google Scholar]
- 39.D’Eletto M., Farrace M., Rossin F., Strappazzon F., di Giacomo G., Cecconi F., Melino G., Sepe S., Moreno S., Fimia G., et al. Type 2 transglutaminase is involved in the autophagy-dependent clearance of ubiquitinated proteins. Cell Death Differ. 2012;19:1228–1238. doi: 10.1038/cdd.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altuntas S., D’Eletto M., Rossin F., Hidalgo L.D., Farrace M.G., Falasca L., Piredda L., Cocco S., Mastroberardino P.G., Piacentini M., et al. Type 2 transglutaminase, mitochondria and huntington’s disease: Menage a trois. Mitochondrion. 2014;19:97–104. doi: 10.1016/j.mito.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Rossin F., D’Eletto M., Falasca L., Sepe S., Cocco S., Fimia G., Campanella M., Mastroberardino P., Farrace M., Piacentini M. Transglutaminase 2 ablation leads to mitophagy impairment associated with a metabolic shift towards aerobic glycolysis. Cell Death Differ. 2015;22:408–418. doi: 10.1038/cdd.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van der Kraan P., van den Berg W. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Huebner J.L., Johnson K.A., Kraus V.B., Terkeltaub R.A. Transglutaminase 2 is a marker of chondrocyte hypertrophy and osteoarthritis severity in the Hartley guinea pig model of knee OA. Osteoarthr. Cartil. 2009;17:1056–1064. doi: 10.1016/j.joca.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar S., Donti T.R., Agnihotri N., Mehta K. Transglutaminase 2 reprogramming of glucose metabolism in mammary epithelial cells via activation of inflammatory signaling pathways. Int. J. Cancer. 2014;134:2798–2807. doi: 10.1002/ijc.28623. [DOI] [PubMed] [Google Scholar]
- 45.Csosz E., Bagossi P., Nagy Z., Dosztanyi Z., Simon I., Fesus L. Substrate preference of transglutaminase 2 revealed by logistic regression analysis and intrinsic disorder examination. J. Mol. Biol. 2008;383:390–402. doi: 10.1016/j.jmb.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 46.Facchiano A., Facchiano F. Transglutaminases and their substrates in biology and human diseases: 50 years of growing. Amino Acids. 2009;36:599–614. doi: 10.1007/s00726-008-0124-8. [DOI] [PubMed] [Google Scholar]
- 47.Johnson K.A., van Etten D., Nanda N., Graham R.M., Terkeltaub R.A. Distinct transglutaminase 2-independent and transglutaminase 2-dependent pathways mediate articular chondrocyte hypertrophy. J. Biol. Chem. 2003;278:18824–18832. doi: 10.1074/jbc.M301055200. [DOI] [PubMed] [Google Scholar]
- 48.Nurminsky D., Shanmugasundaram S., Deasey S., Michaud C., Allen S., Hendig D., Dastjerdi A., Francis-West P., Nurminskaya M. Transglutaminase 2 regulates early chondrogenesis and glycosaminoglycan synthesis. Mech. Dev. 2011;128:234–245. doi: 10.1016/j.mod.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernassola F., Federici M., Corazzari M., Terrinoni A., Hribal M.L., de Laurenzi V., Ranalli M., Massa O., Sesti G., McLean W.I., et al. Role of transglutaminase 2 in glucose tolerance: Knockout mice studies and a putative mutation in a MODY patient. FASEB J. 2002;16:1371–1378. doi: 10.1096/fj.01-0689com. [DOI] [PubMed] [Google Scholar]
- 50.Heywood H., Lee D. Monolayer expansion induces an oxidative metabolism and ros in chondrocytes. Biochem. Biophys. Res. Commun. 2008;373:224–229. doi: 10.1016/j.bbrc.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Dai S.M., Shan Z.Z., Nakamura H., Masuko-Hongo K., Kato T., Nishioka K., Yudoh K. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: Possible involvement of caveolin 1–induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006;54:818–831. doi: 10.1002/art.21639. [DOI] [PubMed] [Google Scholar]
- 52.Yu S.-M., Kim S.-J. The thymoquinone-induced production of reactive oxygen species promotes dedifferentiation through the ERK pathway and inflammation through the p38 and PI3K pathways in rabbit articular chondrocytes. Int. J. Mol. Med. 2015;35:325–332. doi: 10.3892/ijmm.2014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffin M., Casadio R., Bergamini C. Transglutaminases: Nature’s biological glues. Biochem. J. 2002;368:377–396. doi: 10.1042/bj20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen R., Gao B., Huang C., Olsen B., Rotundo R.F., Blumenstock F., Saba T.M. Transglutaminase-mediated fibronectin multimerization in lung endothelial matrix in response to TNF-α. Am. J. Physiol. 2000;279:L161–L174. doi: 10.1152/ajplung.2000.279.1.L161. [DOI] [PubMed] [Google Scholar]
- 55.Kuncio G.S., Tsyganskaya M., Zhu J., Liu S.-L., Nagy L., Thomazy V., Davies P.J., Zern M.A. TNF-α modulates expression of the tissue transglutaminase gene in liver cells. Am. J. Physiol. 1998;274:G240–G245. doi: 10.1152/ajpgi.1998.274.2.G240. [DOI] [PubMed] [Google Scholar]
- 56.Johnson K., Hashimoto S., Lotz M., Pritzker K., Terkeltaub R. Interleukin-1 induces pro-mineralizing activity of cartilage tissue transglutaminase and factor XIIIa. Am. J. Pathol. 2001;159:149–163. doi: 10.1016/S0002-9440(10)61682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suto N., Ikura K., Sasaki R. Expression induced by interleukin-6 of tissue-type transglutaminase in human hepatoblastoma hepg2 cells. J. Biol. Chem. 1993;268:7469–7473. [PubMed] [Google Scholar]
- 58.Murakami S., Lefebvre V., de Crombrugghe B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-α. J. Biol. Chem. 2000;275:3687–3692. doi: 10.1074/jbc.275.5.3687. [DOI] [PubMed] [Google Scholar]
- 59.Hunt N.C., Shelton R.M., Grover L.M. Reversible mitotic and metabolic inhibition following the encapsulation of fibroblasts in alginate hydrogels. Biomaterials. 2009;30:6435–6443. doi: 10.1016/j.biomaterials.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 60.Mastroberardino P.G., Farrace M.G., Viti I., Pavone F., Fimia G.M., Melino G., Rodolfo C., Piacentini M. “Tissue” transglutaminase contributes to the formation of disulphide bridges in proteins of mitochondrial respiratory complexes. Biochimi. Biophys.Acta. 2006;1757:1357–1365. doi: 10.1016/j.bbabio.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Nakashima R.A., Paggi M.G., Scott L.J., Pedersen P.L. Purification and characterization of a bindable form of mitochondrial bound hexokinase from the highly glycolytic AS-30D rat hepatoma cell line. Cancer Res. 1988;48:913–919. [PubMed] [Google Scholar]
- 62.Christofk H.R., Vander Heiden M.G., Harris M.H., Ramanathan A., Gerszten R.E., Wei R., Fleming M.D., Schreiber S.L., Cantley L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]