Abstract
Lentil (Lens culinaris; Family: Fabaceae) is a potential functional dietary ingredient which has polyphenol-rich content. Several studies have demonstrated that the consumption of lentil is immensely connected to the reduction in the incidence of diseases such as diabetes, obesity, cancers and cardiovascular diseases due to its bioactive compounds. There has been increasing scientific interest in the study area of lentils as the functional food due to its high nutritive value, polyphenols, and other bioactive compounds. These polyphenols and the bioactive compounds found in lentil play an important role in the prevention of those degenerative diseases in humans. Besides that, it has health-promoting effects. Based on the in vitro, in-vivo and clinical studies, the present review focuses to provide more information on the nutritional compositions, bioactive compounds including polyphenols and health-promoting effects of lentils. Health-promoting information was gathered and orchestrated at a suitable place in the review.
Keywords: polyphenols, lentils, antioxidants, degenerative diseases, health-promoting effects
1. Introduction
Lentil (Lens culinaris; Family: Fabaceae) is an annual indigenous plant from Western Asia and other parts of the world, including North America. Furthermore, this species is now diversified from Hindukush to Afghanistan and Ethiopia to Mediterranean countries [1]. It is well known for its lens-shaped edible seed, which has the most significant dietary compositions, containing macro- and micro-nutrients [2]. Lentils exist as a spectrum of colors, which includes yellow, orange, red, green, brown or black, depending on the cultivar, the composition of the seed coats and cotyledons [3]. The color of dehulled seeds is mainly associated with the cotyledon color, which could be yellow, red or green. While the color of the intact seed is based on the seed coat, it could be tan, brown, green, gray or black. The seed coats of lentil have a higher amount of flavan-3-ols, proanthocyanidins and some flavonols. This suggests that lentil featuring green and gray seed coats might be more promising for a health-promoting diet. According to the Food and Agriculture Organization statistics report in 2014, the global production of the lentils was primarily cultivated and harvested by Canada and India, which were estimated to be 1.99 million and 1.1 million metric tons, followed by Turkey (0.34 million), Nepal (0.22 million) and China (0.125 million) [4]. The evidence demonstrated that the consumption of lentils is highly associated with reductions in the incidence of degenerative diseases including diabetes, cardiovascular disease (CVD) and cancers. There has been an increase in scientific interest of the study of lentils as a functional food due to their high nutritional compositions, nutritive value and the presence of bioactive secondary metabolites. These bioactive compounds in lentils play a vital role in the prevention of degenerative diseases in humans and a significant role in improving health. Based on the explorative studies, the current comprehensive review aims to provide information on the nutritive compositions, bioactive compounds and health-promoting effects of polyphenol-rich lentils and explores their therapeutic values for future clinical studies.
2. Materials and Methods
An electronic search was conducted using PubMed, Science Direct and Google Scholar by finding the keywords “Lentils” AND “bioactive compounds” AND “nutritional compositions” AND “polyphenols” OR “antidiabetic” OR “antioxidants” OR “antimicrobial” in “Title/Abstract/Keywords”, without date restriction, to identify all published studies (in vitro, in vivo, clinical and case-control) that have investigated the connection between lentils and their various beneficial effects. Health-promoting information was gathered and orchestrated in the suitable place in the review.
3. Nutritional Compositions of Edible Lentils
Nutritional compositions of raw, sprouted and cooked lentils are summarized in Table 1. Lentils are known to be an abundant source of protein storage, providing essential and non-essential amino acids to the human body. The predominant proteins in lentils are globulin (47% of the total seed proteins) and an adequate quantity of albumin [5]. Lentils play an important role in crop rotation and the ability to fix atmospheric nitrogen. High quantities of these proteins and essential amino acids in lentils offer an important dietary source for low and middle-income countries [6]. Among 23 pulses, lentils yield the second highest starch percentage of 47.1% and a greater percentage of insoluble dietary fibers [7,8]. Lentils are known to be a good source of prebiotics [9] and have nutritionally important quantities of prebiotic carbohydrates (12.3–14.1 g/100 g of dry lentils) that help to keep up the gut microbial environment and prevent gut-associated diseases [10,11]. Furthermore, lentils are relatively low in fat and sodium, but high in potassium content (1:30 ratio of sodium and potassium) [12]. Given that, it is the best dietary food for patients with obesity and CVD. Lentil seeds are an excellent vegetable source of iron. Studies have shown that the consumption of cooked lentil in the diet prevents iron deficiency anemia [13], iron being a very important mineral, which is required daily, especially for adolescents and pregnant women. Several minerals (zinc, copper, manganese, molybdenum, selenium and boron) and vitamins (thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, folate, α, β and γ tocopherols and phylloquinone) have been well documented in lentils [7,14,15]. Furthermore, lentils have an average quantity of vitamin K of 5 μg/100 g, as reported by the United States Department of Agriculture (USDA) [7]. However, the daily requirement of this vitamin in adults is about 80 μg. The low content of vitamin K renders lentils as safe for patients with CVD upon anticoagulant treatment. Overall, lentils are considered as one of the best dietary sources that has health-promoting effects on various illnesses.
Table 1.
Nutritional compositions of lentils in 100 g of the edible portion [7].
| Nutrients | Unit | Raw | Sprouted | Cooked |
|---|---|---|---|---|
| Water | g | 8.26–9.65 | 51.85–67.34 | 69.64–137.89 |
| Energy | kcal | 343–356 | 82–106 | 116–226 |
| Protein | g | 24.44–25.71 | 6.9–8.96 | 9.02–17.86 |
| Total lipid (fat) | g | 0.92–1.06 | 0.42–0.55 | 0.38–0.75 |
| Carbohydrate | g | 60–64.44 | 17.05–22.14 | 20.13–38.69 |
| Total dietary fiber | g | 10.7–31.4 | - | 7.9–15.6 |
| Total sugars | g | 2.03–2.86 | - | 1.80–3.56 |
| Minerals | ||||
| Calcium | mg | 35–57 | 19–25 | 19–38 |
| Iron | mg | 6.51–7.71 | 2.47–3.21 | 3.33–6.59 |
| Magnesium | mg | 47–69 | 28–37 | 36–71 |
| Phosphorus | mg | 281–335 | 133–173 | 180–356 |
| Potassium | mg | 677–943 | 248–322 | 369–731 |
| Sodium | mg | 3–6 | 8–11 | 123–471 |
| Zinc | mg | 3.27–5.89 | 1.16–1.51 | 1.27–2.51 |
| Vitamins | ||||
| Vitamin C | mg | 3.4–4.5 | 12.7–16.5 | 1.5–3.0 |
| Thiamin | mg | 0.756–0.873 | 0.176–0.228 | 0.169–0.335 |
| Riboflavin | mg | 0.189–0.211 | 0.099–0.128 | 0.073–0.0145 |
| Niacin | mg | 2.605–3.459 | 0.869–1.128 | 1.060–2.099 |
| Vitamin B6 | mg | 0.540–0.698 | 0.146–0.190 | 0.178–0.352 |
| Folate | µg | 479–555 | 77–100 | 181–358 |
| Vitamin B12 | µg | 0.00 | 0.00 | 0.00 |
| Vitamin A, RAE | µg | 2.0–2.5 | 1.8–2.0 | 0 |
| Vitamin A, IU | IU | 32–39 | 35–45 | 8–16 |
| Vitamin E | mg | 0.49–0.55 | 0 | 0.11–0.22 |
| Vitamin K | µg | 4.2–5.0 | 0 | 1.7–3.4 |
| Lipids | ||||
| Total saturated fatty acids | g | 0.154–0.198 | 0.044–0.057 | 0.053–0.105 |
| Total monounsaturated fatty acids | g | 0.0179–0.193 | 0.08–0.104 | 0.064–0.127 |
| Total polyunsaturated fatty acids | g | 0.469–0.526 | 0.169–0.219 | 0.175–0.346 |
4. Bioactive Compounds in Lentils
Various bioactive compounds or secondary metabolites are present in the lentil seed, which are categorized into different functional groups. The bioactive functional groups and their quantity in lentils are listed in Table 2.
Table 2.
List of bioactive functional groups in lentils and their biological functions.
| Bioactive Functional Groups | Individual Components | Quantity in 100 g of Lentils | Biological Functions | Reference |
|---|---|---|---|---|
| Phytosterols | β-sitosterol | 15.0–24.0 mg | Regulate the membrane fluid | [14,16] |
| campesterol | 15.0 mg | |||
| stigmasterol | 20.0 mg | |||
| Active Proteins | ||||
| Trypsin/protease inhibitors | Bowman–Birk trypsin inhibitors | 3–8 trypsin inhibitor unit (TIU)/mg | Anti-nutritional components; decrease the digestibility of dietary proteins; inhibit the cell proliferation in cancer | [17,18] |
| Lectins | Lectins or hemagglutinins | 12.0 mg | Ability to agglutinate red blood cells RBC and strong stimulators of murine B lymphocyte proliferation | [19,20] |
| Defensins | Defensins | 8.0 mg | Participate in the development of innate immunity | [21] |
| Dietary Fibers | Fibers | Insoluble fibers (93–99.7 mg/g) and soluble fibers (<7 mg/g) | Potential effect of hypocholesterolemic, anti-cancer, anti-tumor, antibacterial and hypoglycemic effects | [7,22] |
| Resistant starches | 25.4 g | Significant contributor to gastrointestinal health and gut microbiota | [23] | |
| Polyphenols Flavonoids | Flavonols (e.g., quercetin and kaempferol) | 0.03 to 10.85 and 0.24 to 13.20 mg | Antioxidant potential | [3,24] |
| Flavones, flavanones | Total phenolic content: 26 mg gallic acid equivalents (GAE/100 g fresh wt; total flavonoid content: 21 mg catechin equivalents/100 g, and the condensed tannin content of 870 mg catechin equivalents/100 g | Antioxidant activity and potential effect on cardiovascular disease (CVD), diabetes, osteoporosis and neurodegenerative diseases | [24,25] | |
| Proanthocyanidins or condensed tannins (e.g., prodelphinidins and procyanidins) | ||||
| Flavan-3-ols or flavanols (e.g., catechin and gallocatechin) | 759 mg (GAE)/100 g; glycosides of flavanones: 33.1–186.0 µg; glycosides of flavonols: 9.6–241 µg; dimers procyanidins: 619–1122 µg; trimer procyanidins: 441–498 µg; tetramer procyanidins: 18.5–59.5 µg; galloylated procyanidins 69.3–123 µg | Antioxidant activity | [3,24] | |
| Anthocyanidins (e.g., delphinidin and cyanidin) | ||||
| Polyphenols Non-flavonoids | Hydroxybenzoic acids | Hydroxybenzoic acids: 4.5–28.4 µg | Antioxidant activity and potential effect on diabetes, osteoporosis CVD and neurodegenerative diseases | [24,25] |
| Hydroxycinnamic acids (e.g., p-coumaric acid, ferulic acid and sinapic acid) | Prodelphinidins 369–725 µg; condensed tannins: 870 mg catechins equivalent | Antioxidant activity | [3,24] | |
| Stilbenoids, trans-resveratrol-3-O-glucoside | Glycosides of trans-resveratrol: 5.5–9.3 µg; | Antioxidant activity and potential effect on diabetes and CVD | [24,25] | |
| Phytoestrogens: isoflavones | Formononetin, daidzein, genistein, glycitein, matairesinol, biochanin A, coumestrol, lariciresinol, pinoresinol, secoisolariciresinol, coumestrol | Total isoflavones (9.5 μg), total lignans (26.6 μg) and total phytoestrogens (36.5 μg) | Antioxidant potential | [26] |
| Phytate | Phytic acid | 620 mg | Inhibit the proliferation of colorectal cancer | [27] |
| Triterpenoids | Squalene | 0.7 mg | Chemopreventive potential against colorectal cancer | [28] |
| Saponins | Saponins | 25 mg | Hypoglycemic and antidiabetic potential | [29] |
5. Polyphenols in Lentils
Lentils have the highest total phenolic content in comparison to six other common legumes, such as green pea, chickpea, cowpea, yellow pea, mung bean and peanut [3]. Polyphenols are generally a large group of compounds, classified into different classes, based on the presence of the number of phenolic rings and their structural elements or substituents [30,31]. Two main groups can be identified based on the aromatic rings, which are attached to the heterocyclic rings, known as the flavonoid groups (flavones, flavonols, flavanones, flavanonols, flavanols or catechins, anthocyanins, neoflavonoids and chalcones) and the non-flavonoid groups (simple phenols, phenolic acids, hydroxybenzoic acids, tannins, acetophenones and phenylacetic acids; hydroxycinnamic acids, coumarins, benzophenones, xanthones, stilbenes, lignans and secoiridoids) [31,32]. Various functional polyphenols in the lentils are described according to their classes, subclasses and chemical structures in Table 3.
Table 3.
| Polyphenol | Classes | Sub-Classes | Compound Name | Structure |
|---|---|---|---|---|
| Flavonoids | Flavonoids | Flavanols | (−)-Epigallocatechin | 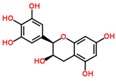 |
| (+)-Catechin-3-O-glucose | 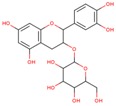 |
|||
| Catechin | 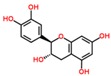 |
|||
| Catechin-7-O-glucoside | 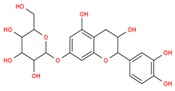 |
|||
| Catechin gallate | 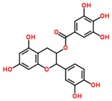 |
|||
| Epicatechin | ||||
| Epicatechin gallate | 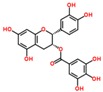 |
|||
| Flavonols | Quercetin-3-O-glucoside | 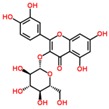 |
||
| Quercetin-3-O-galactoside | 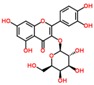 |
|||
| Quercetin-3-O-xyloside | 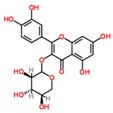 |
|||
| Kaempferol-3-O-rutinoside 7-O-rhamnoside | 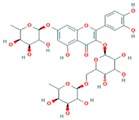 |
|||
| Kaempferol-4′-O-glucoside | 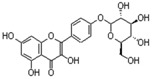 |
|||
| Kaempferol-5-O-glucoside | 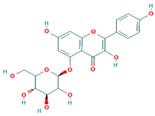 |
|||
| Kaempferol-3-O-glucoside | 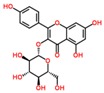 |
|||
| Kaempferol-3-O-rutinoside | 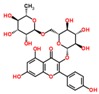 |
|||
| Myricetin-3-O-rhamnoside | 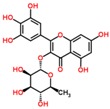 |
|||
| 4″″-Acetylsagittatin A | 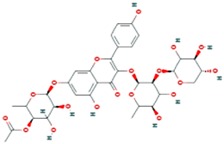 |
|||
| Proanthocyanidins | Procyanidin | 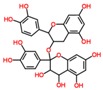 |
||
| Prodelphinidin | 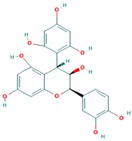 |
|||
| Flavanones | Eriodictyol | 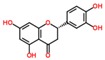 |
||
| Eriodictyol-7-O-rutinoside | 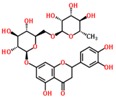 |
|||
| Naringenin | 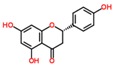 |
|||
| Flavone | Luteolin | 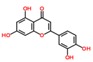 |
||
| Luteolin-4′-O-glucoside | 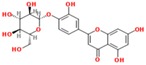 |
|||
| Luteolin-3′,7-diglucoside | 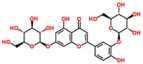 |
|||
| Luteolin-7-O-glucoside | 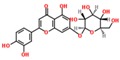 |
|||
| 5,7-dimethoxyflavone | 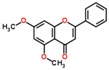 |
|||
| Anthocyanins | Malvidin-3-O-galactoside | 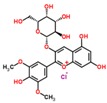 |
||
| Non-flavonoids | Phenolic acids | Hydroxybenzoic acids | Syringic acid | 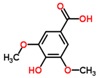 |
| Vanillic acid 4-|A-D-glucoside | 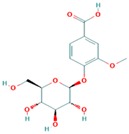 |
|||
| 2,3-Dihydroxy benzoic acid | 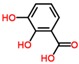 |
|||
| p-hydroxy benzoic acid | 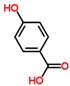 |
|||
| Gallic acid | 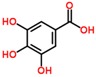 |
|||
| Hydroxycinnamic acid | 3-hydroxy cinnamic acid | |||
| p-Coumaroyl malic acid | 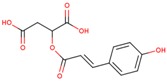 |
|||
| Sinapic acid | 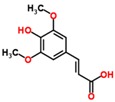 |
|||
| Other polyphenols | Hydroxycoumarin | 4-Hydroxy-6-methyl coumarin | 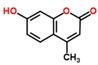 |
6. Health Promoting Effects of Lentils
Polyphenol-rich lentils have potential health benefits as complementary and alternative medicines, which are exerted in the form of antioxidant, antibacterial, anti-fungal, antiviral, cardioprotective, anti-inflammatory, nephroprotective, antidiabetic, anticancer, anti-obesity, hypolipidemic and chemopreventive activities. Furthermore, lentils are useful as a prognostic marker for various cancers including thyroid and hepatic carcinoma. Detailed information on lentil polyphenols’ dose range, route of administration, model used and negative controls is presented based on in vivo, in vitro and clinical research studies according to the title and is depicted in Table 4, Table 5, Table 6, Table 7 and Table 8.
Table 4.
Summary of in vitro, in vivo and clinical studies on the antidiabetic activities of polyphenol-rich lentils.
| Polyphenol-Rich Lentils | Model | Dose and Route of Administration | Negative Control | Investigation | Results | Reference |
|---|---|---|---|---|---|---|
| Total phenolics and flavonoids | In vitro | 50–500 μg/mL | - | Assay of antioxidant activities DPPH, FRAP, ORAC and inhibitory properties against α-glucosidase and pancreatic lipase | Antidiabetic, hypotensive and antioxidant activity | [47] |
| Total phenolics | In vitro | 100.9 mg/g f.m. | 300 mM NaCl | Assay of α-amylase inhibitor activity and expected glycemic index values | Antidiabetic potential | [38] |
| Flavonoids | Male albino rats | 15 g/kg/p.o. of lentil food formulation | Alloxan (150 mg/kg bw | Assay of glucose, urea, serum total protein, total TG and TC | Antidiabetic and hypolipidemic potential | [48] |
| Total phenolics and flavonoids | Male Nile rats | 720 g/kg/p.o. of lentil food formulation | STZ (35 mg/kg i.p.) | Assay of glycemic index, glycemic load and cumulative load, blood glucose (fasting, random and OGTT) and plasma lipid parameters (plasma TC and TG) plus necropsy findings (liver and kidney pathology plus adipose reserves) | Antidiabetic and hypolipidemic potential | [49] |
| Flavonol glycosides and free flavanols | Male Sprague-Dawley rats | 57% raw whole lentil; 52% cooked whole lentil; 51% raw dehulled lentil; 47% cooked dehulled lentil/p.o. | STZ (35 mg/kg i.p.) | Assay of serum glucose and serum lipid levels | Antidiabetic and hypolipidemic potential | [41,42] |
| Total phenols | Human with diabetes | 50 g cooked lentil/p.o. | - | Assay of FBS, TC and glycemic control | Antidiabetic and cardioprotective activity | [41] |
| Total phenols | Human with diabetes | 1 cup cooked lentil/day/p.o. | - | Assay of body weight, HbA1C, TC, BP, heart rate, glycemic control | Antidiabetic and cardioprotective activity | [50] |
| Total phenolics and flavonoids | Obese patients with type 2 diabetes | 60 g lentil sprouts/p.o. daily during 8 weeks | - | Assay of weight, height and waist circumference, lipid profile, | Antidiabetic and hypolipidemic potential | [39] |
DPPH: 2,2-diphenyl-1-picrylhydrazyl; FRAP: ferric reducing antioxidant power assay; ORAC: oxygen radical absorbance capacity; g f.m.: germination fraction matter; p.o.: per oral; i.p.: intraperitoneal; bw: body weight; STZ: streptozotocin; OGTT: oral glucose tolerance test; TG: triglycerides; TC: total cholesterol; FBS: fasting blood sugar; BP: blood pressure; HbA1C: glycated hemoglobin.
Table 5.
Summary of in vitro, in vivo and clinical studies on the antioxidant activities of polyphenol-rich lentils.
| Polyphenol-Rich Lentils | Model | Dose and Route of Administration | Negative Control | Investigation | Results | Reference |
|---|---|---|---|---|---|---|
| Procyanidin and prodelphinidin dimers and trimers; gallate procyanidins; kaempferol derivatives, quercetin glucoside acetate; luteolin derivatives and p-coumaric acid | Human astrocytoma cell line (U-373), renal adenocarcinoma (TK-10), breast adenocarcinoma (MCF-7), melanoma (UACC-62), colon carcinoma (HT29) and hepatocellular carcinoma (HepG2) | 0.06–0.12 µg/µL | H2O2, FeSO4 and FeSO4 + H2O2 | Assay of antioxidant activity by ORAC, DPPH, MTT and intracellular ROS | Antioxidant neuroprotective and anticancer activities | [60] |
| Flavanols and phenolic acids | Human colonic carcinoma cell line (Caco-2) | 20–100 μg/mL | - | Assay of proinflammatory cytokines COX-2, IL-1β and IL-6 in TNF-α | Anti-inflammatory activity | [61] |
| Total phenolics and flavonoids | In vitro | 200 mg sprout extracts | - | Assay of radical activity and expected glycemic index values | Antioxidant and antidiabetic activity | [62] |
| Flavonoids | In vitro | 100 μL, 1 mg/mL | - | Assay of TEAC, DPPH, superoxide radical, hydrogen peroxide, FRAP and inhibition of β-carotene degradation activity; diabetes was assayed on α-amylase and α-glucosidase activity | Antioxidant and antidiabetic potential | [63,64] |
| Total phenolics and flavonoids | In vitro | 55–119 μg/mL | - | Assay of DPPH or ORAC, anti-inflammatory activities on LOX, COX-1, COX-2 pathways | Antioxidant and anti-inflammatory activities | [65] |
| Total phenols, flavonoids and tannins | In vitro | - | - | Assay of DPPH | Antioxidant potential | [66] |
| Total phenolics and flavonoids | In vitro | 25 and 40 μM | Arsenic (10, 25, and 40 μM | Assay of transcriptional upregulation of serine acetyltransferase, O-acetyl serine (thiol)-lyase, γ-glutamylcysteine synthetase and phytochelatin synthase genes; assay of SOD, ascorbate peroxidase, dehydroascorbate reductase, GR and GST | Antioxidant potential | [67] |
| Hydroxybenzoic compounds, protocatechuic, vanillic acid, aldehyde p-hydroxybenzoic, trans-ferulic acid and trans-p-coumaric acid | In vitro | 0.02 and 0.1% of lentil seed extracts | - | Assay of hydroxyl radical scavenging activity | Antioxidant potential | [68] |
| Kaempferol glucoside | In vitro | 0.00625–5 mg/mL | - | Assay of DPPH, TEAC, FRAP and ORAC | Antioxidant potential | [33,69] |
| Total phenolics and flavonoids | In vitro | 0.00625–5 mg/mL | - | Assay of DPPH | Antioxidant potential | [70,71] |
| Flavonol glycosides and free flavanols | In vitro | 100 mg | - | Assay of PRTC, TEAC, ABTS, total phenolics, tocopherols (α-T, β-T, γ-T, δ-T), GSH and L-ascorbic acid | Antioxidant potential | [72,73] |
| Total phenolics and flavonoids | In vitro | 20–100 μg/mL | - | Assay of COX-2 producing PGE (2) inhibitory assay | Anti-inflammatory activity | [74] |
MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; ROS: reactive oxygen species; COX: cyclooxygenase; IL: interleukin; TNF: tumor necrosis factor; TEAC: trolox equivalent antioxidant capacity; LOX: lysyl oxidase; SOD: superoxide dismutase; GR: glutathione reductase; GST: glutathione s-transferase.
Table 6.
Summary of in vitro, in vivo, clinical and intervention/observational studies on the anti-obesity and cardioprotective potentials of polyphenol-rich lentils.
| Polyphenol-Rich Lentils | Model | Dose and Route of Administration | Negative Control | Investigation | Results | Reference |
|---|---|---|---|---|---|---|
| Flavonoids | Human colonic carcinoma cell line (Caco-2) | 1.5, 3, 4.5, 6, 7.5 and 10 mg/mL | - | Assay of LDH, caspase-3, total DNA fragmentation, morphological changes related to apoptosis | Chemo-preventive agents | [75] |
| Free flavanols | Human with hyperhomocysteinemia and coronary artery disease | 500 μg folate and 10 g lentils and other pulses and foods/p.o. | - | Assay of plasma total homocysteine | Cardioprotective activity | [76] |
| Phenolic acids | In vitro | 20–100 μg/mL | - | Assay of platelet aggregation activity | Cardioprotective activity | [74] |
| Total phenolics | Male Wistar rats | 200 and 400 mg/kg/p.o. | Doxorubicin (15 mg/kg bw/i.p. | Assay of BUN, serum creatinine, serum total protein, urinary total protein, and urinary creatinine, SOD, CAT, LPO and GSH in kidney | Nephroprotective potential | [77] |
| Phenolic compounds | Male albino rats | 100, 200, 400 mg/kg/p.o. | - | Assay of blood picture (RBC, WBC and Hb), lipid fraction (total lipid, TC, TG, HDL, LDL and VLDL), liver function (AST, ALT and ALP, bilirubin) and kidney function (uric acid, urea and creatinine), total protein and its fractions (albumin and globulin), lipid peroxidation and antioxidative enzyme activity (SOD, CAT) | Hypolipidemic and antihypercholesterolemic activity | [78] |
| Total phenolics and flavonoids | Male Sprague-Dawley rats | Ten isocaloric and isonitrogenous diets were prepared; 5 of them were cholesterol-free and differed in the content of lentil powder (%): lentil-free (0), raw dehulled (60.5), raw whole (66.6), cooked dehulled (62.5) and cooked whole (65.6); while in the other 5, cholesterol (1%) | High cholesterol feed | Assay of TC, LDL-C, HDL-C, TG, AIP, CRR and atherogenic coefficient | Cardioprotective activity | [79] |
| Total phenolics | Male Wistar rats | 200 g/kg/p.o. for 28 days | - | Assay of hepatic lipase and lipoprotein lipase in epididymal fat, gastrocnemius and heart | Cardioprotective and hypolipoproteinemia activity | [80] |
| Flavonoids | Sprague-Dawley female rats | 100, 200, 400 mg/kg/p.o. | Triton WR-1339 (250 mg/kg/i.v.) | Assay of TC, TG, HDL, LDL and VLDL | Antihyperlipidemic activity | [81] |
| Total phenolics | Human | - | - | Cross-cultural and intervention studies | Cardioprotective activity | [82] |
| Phenolic acids | Human | 13% p.o. | - | Assay of LDL | Hypolipidemic activity | [83] |
| Total phenolics | Human | 120–130 g cooked lentil/day for 30–56 days/p.o. | - | Assay of TC, LDL, TG | Hypolipidemic activity | [84] |
| Phenolic acids | Human with hyperlipidemic patients | 140 g/oral for 4 months’ time | - | Assay of serum TC and TG | Hypolipidemic activity | [85] |
LDH: lactate dehydrogenase; BUN: blood urea nitrogen; CAT: catalase; LPO: lipid peroxidation; WBC: white blood cells; Hb: hemoglobin; HDL: high density lipoprotein; LDL: low density lipoprotein; VLDL: very low density lipoprotein; AST: aspartate transaminase; ALT: alanine transaminase; ALP: alkaline phosphatase; AIP: atherogenic index of plasma; CRR: cardiac risk ratio; i.v.: intravenous.
Table 7.
Summary of the in vitro antimicrobial potentials of polyphenol-rich lentils.
| Polyphenol-Rich Lentils | Model | Dose and Route of Administration | Negative Control | Investigation | Results | Reference |
|---|---|---|---|---|---|---|
| Flavonoids and lectins | Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa | 0.1–1 mL | - | Assay of agar well diffusion method | Antibacterial activity | [92] |
| Flavonoids | Xanthomonas axonopodis pv. phaseoli | 250 mg/mL | - | Assay of disc diffusion method | Antibacterial activity | [90] |
| Ellagic acid, lupeol and leucodelphinidin | Bacillus cereus, S. aureus, P. aeruginosa and E. coli | 250 mg/mL | - | Assay of disc diffusion method | Antibacterial activity | [93] |
| Flavonoids and proteins | Aspergillus niger | - | - | 47-residue, plant defensin was purified by ammonium sulfate precipitation, gel filtration, chromatography and RP-HPLC; complete amino acid sequence, RT-PCR, cloning and cDNA sequence were performed | Antifungal activity | [21,91] |
| Flavonoids and proteins | Fusarium oxysporum | 36 µM | - | Mycelial growth in Mycosphaerella arachidicola | Antifungal activity | [94] |
| Flavonoids, lentil lectin and the diterpene ester | Human peripheral blood mononuclear leucocytes. murine splenocytes and white Swiss inbred C67B1/6 mice | 600 µg/mL | Concanavalin A | Assay of interferon-γ production | Antiviral activity | [95,96] |
pv.: pathovar; RP-HPLC: reverse phase high performance liquid chromatography.
Table 8.
Summary of in vitro, in vivo and clinical studies on the anticancer and chemopreventive effects of polyphenol-rich lentils.
| Polyphenol-Rich Lentils | Model | Dose and Route of Administration | Negative Control | Investigation | Results | Reference |
|---|---|---|---|---|---|---|
| Flavonoids, lentil lectins | Human colon adenocarcinoma HT29 and colonic fibroblast CCD-18Co cells | 19 µM | - | cDNA, encoding a Bowman–Birk protease inhibitor, assessed with an array of molecular masses | Antiproliferative properties in colon cancer | [97] |
| Flavonoids, lentil lectins | Human colon carcinoma cell line CACO-2 | 1.5, 3, 4.5, 6, 7.5 and 10 mg/mL | - | Production of IL-1, IL-6, IL-8 and MCP-1 were measured by ELISA and RT-PCR | Anticancer activity | [105] |
| Flavonoids, lentil lectins | Nasopharyngeal carcinoma CNE1 and CNE2 cell lines | 1–5 mg/mL | - | Assay of MTT, flow cytometry and Western blotting | Anticancer activity | [106] |
| Total phenolics and flavonoids | In vitro | 100 µL | 2,2′-Azobis (2-amidino propane hydrochloride | Assay of DPPH, radical scavenging assay, the hydroxyl radical- and the peroxyl radical-induced DNA strand scission assays | Potent chemopreventive agents | [100] |
| Cooked Lentil seeds with iron | Sprague-Dawley rats | 35 mg/kg/p.o. | Iron-free diet (anemic group) | Assay of body weight, feed intake, Hb, hematocrit, MCV, MCH, MCHC, RBC, WBC and serum iron, platelet count and TIBC | Protective effect on iron deficiency anemia | [13] |
| Kaempferol, quercetin and myricetin | Human | 1 cup cooked lentil/day/p.o. | - | Validated food frequency questionnaires in 1991 and 1995 from 90,630 women in the Nurses’ Health Study II | Protective against breast cancer | [98] |
| Flavonols | Human | 1 cup cooked lentil/day/p.o. | - | Validated food frequency questionnaires | Protective against breast cancer | [107] |
| Total phenolics and flavonoids | Human | 1 cup cooked lentil/day/p.o. | - | Validated food frequency questionnaires in 1976 and 1982 from 78,000 men | Protective against prostate cancer | [108] |
| Total phenolics and flavonoids | Human | 1 cup cooked lentil/day/p.o. | - | Validated food frequency questionnaires in 617 incident cases of prostate cancer | Protects against prostate cancer | [109] |
| Isoflavones-genistein | Human | 1 cup cooked lentil/day/p.o. | - | A validated food frequency questionnaires incident cases of prostate cancer | Protects against prostate cancer | [99] |
| Flavonols, flavones and flavonoid | Human | 1 cup cooked lentil/day/p.o. | - | A validated food frequency questionnaires | Protects against prostate cancer | [110] |
| Flavonoids, lentil seed lectins | Human | - | - | Assay by using a flow cytometer | Screening for colorectal cancer | [111] |
| Flavonoids, lentil seed lectins | Patients with benign thyroid disease and thyroid carcinomas | - | - | Assay of Lens culinaris agglutinin reactive thyroglobulin ratios in sera and wash fluids | Useful for distinguishing between thyroid carcinoma and benign thyroid tumor | [112] |
| Flavonoids, lentil seed lectins | Patients with benign thyroid disease and thyroid carcinomas | - | - | Assay of Lens culinaris agglutinin reactive thyroglobulin ratios in sera and wash fluids | Useful prognostic marker for thyroid cancer | [113] |
MCP: monocyte chemotactic protein; MCV: mean corpuscular value; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; TIBC: total iron binding capacity.
6.1. Anti-Diabetic Activity of Lentils
Świeca et al. [38] observed that the regular consumption of the germinated lentils is beneficial for the prevention and management of diabetes. Lentils have the ability to improve blood glucose, lipid and lipoprotein metabolism in diabetic and healthy human beings [39]. In vitro and in vivo studies of polyphenol-rich lentil seed showing the anti-diabetic potentials are summarized in Table 4. Besides that, the studies that are associated with lentils and diabetic animal models have reported that the high flavonoid and fiber content of lentils play a significant role in the gut motility and prevent the impairment of metabolic control in diabetic rats, so having a promising implication for diabetic patients [40]. The regular consumption of cooked lentils (50 g) among diabetic patients leads to significant reductions of fasting blood sugar (FBS), glycemic load and glycemic index in streptozotocin (STZ)-induced diabetic animals [41,42]. Reductions of the glycemic index from the diet are due to the presence of polyphenols in the lentils that have been linked with health-promoting impacts on metabolic disorders such as diabetes, obesity, coronary heart diseases and CVD [43,44]. Furthermore, in vitro and in vivo studies have also demonstrated that lentils in the diet regulate starch digestibility, glycemic load and the glycemic index, which diminish diabetes complications [45,46]. Thus, a diet including lentils appears to be an effective intervention and management strategy for the prevention of diabetes.
6.2. Antioxidant Potential of Lentils
A wide range of in vitro evidence implies that lentils have the highest total antioxidant capacity when they are compared to chickpeas, common beans and soybeans, which were measured by 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power, oxygen radical absorbing capacity, Trolox equivalent antioxidant capacity and total radical-trapping antioxidant parameters [51,52,53,54]. Evidence has shown that lentils have greater oxygen radical scavenging potential compared to various vegetables and fruits, such as onion, horseradish, potatoes, wheat germ, blueberries and sweet cherries [7]. Lentils have different groups of phenolic compounds such as procyanidin and prodelphinidin dimers and trimers, gallate procyanidins, kaempferol derivatives, quercetin glucoside acetate, luteolin derivatives and p-coumaric acid, hydroxybenzoic compounds, protocatechuic, vanillic acid, aldehyde p-hydroxybenzoic, trans-ferulic acid and trans-p-coumaric acid, compared to other legumes, providing greater antioxidant potentials and health-promoting effects. These phenolic compounds in lentils naturally act as antioxidants and have the ability to restrict the formation of reactive oxygen species, as well as superoxide anion by chelating metal ions or inhibiting enzymes [52,53]. In vitro and in vivo studies of polyphenol-rich lentils that exert antioxidant potentials are summarized in Table 5.
6.3. Anti-Obesity Activity of Lentils
Large prospective epidemiological studies have reported that the intake of phenolic-rich lentils is inversely connected with the incidence of obesity and diabetes [55]. An earlier human study shows that the intake of lentil seed along with pasta and sauce reduces food intake, body weight and waist circumference [56]. Furthermore, lentil seed containing flavonoids and fiber enhances satiety and lowers the amount of food intake, which lead to maintaining body weight in obese subjects [56]. Observational studies have further reported an inverse relationship between the consumption of lentils and the basal metabolic index or risk associated with obesity [57]. Besides that, interventional studies have shown the potential of lentils to inhibit α-glucosidase and pancreatic lipase, which has the ability to decrease glucose and fat digestion and absorption in the intestine. Ultimately, polyphenol-rich lentils control postprandial glucose and fat, which is crucial in the management of diabetes and obesity [58,59]. Flavonoids in lentils have the potential to inhibit the actions of α-glucosidase and lipase, which suggests that dietary lentil consumption could manage post-prandial blood glucose and body weight [37]. In vitro, in vivo, clinical and interventional/observational studies of lentils possessing anti-obesity potentials are summarized in Table 6.
6.4. Cardioprotective Effect of Lentils
Phenolic-rich lentil seed consumption has been inversely linked with the occurrence of various CVDs [43]. Lentils containing polyphenols have the potential to reduce blood pressure by angiotensin I-converting enzyme (ACE) inhibitor activity [86,87]. The recent study observed that bioactive compounds (legumin, vicilin and convicilin) in lentil possess higher antioxidant, ACE-inhibitory and cardioprotective activity [88]. Besides that, the polyphenol-rich lentil seeds have the ability of antihyperlipidemic, hypohomocysteinemic, anti-cholesterolemic and a cardioprotective effect that reduces the risk of hypertension and coronary artery diseases [76,82]. In the hypertensive animal model, administration of lentils actively reduces the total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL) and pathological manifestations of cardio-morphometric analysis. These findings reinforce the importance of lentil seed and its diet prescription as a therapeutic potential for hypertensive patients [78,84]. Al-Tibi et al. [42] observed that treatment with lentil seeds reduces the glycemic index and hyperlipidemic effects in the STZ-induced diabetic animal model. In this study, lentils significantly raised the high density lipoprotein (HDL) levels and reduced blood glucose levels in diabetic rats. Concisely, these studies recommend that the dietary consumption of polyphenol-rich lentils should be on a regular basis, having the potential to decrease the risk of cardiovascular and coronary artery diseases. In vitro and in vivo studies of lentils exerting cardioprotective potentials are summarized in Table 6.
6.5. Antimicrobial Activity of Lentils
Lentils containing flavonoids and lectins have been reported as non-toxic and safe for use in medical diagnostic kits [89]. A bioactive peptide called “defensing”, which is isolated from germinated lentil seeds, possesses a broad spectrum of biological activities, including antimicrobial activities against various infections associated with bacteria and fungi [21,90]. It is a group of “host defense peptides” synthesized in the lentil seeds, which are involved in the development of innate immunity. They are tiny, basic, cysteine-rich peptides, containing antifungal activity, which inhibit the growth of Aspergillus niger [21,91]. Likely, “defensins” can interrupt viral digestive enzymes, such as human immunovirus (HIV)-1 reverse transcriptase, which impacts viral replication. “Defensins” have been further observed to block ion channels and to inhibit protein translation. Therefore, “defensing” in lentil seeds along with phenolic compounds acts as a potential inhibitor of microbial growth. In vitro studies of lentils exerting antimicrobial potentials are summarized in Table 7.
6.6. Anticancer Activity of Lentils
The consumption of lentil seeds reduces the incidence of various cancers including colon, thyroid, liver, breast and prostate [97,98,99]. A large prospective epidemiologic study associated with polyphenol-rich lentils and breast cancer on 90,630 women exhibited an inverse relationship between lentils and the risk of breast cancer [98]. Lentil seeds have a high polyphenolic content that potentially could prevent carcinogens through chemo-preventive activities, including the uptake of carcinogens, activation or formation, detoxification, binding to DNA and fidelity of DNA repair [100,101]. Moreover, lectins in lentils have anticancer properties, which have been observed in various in vitro, in vivo and human studies [20]. These lectins along with phenolic compounds in lentil seeds have been proven as therapeutic agents. They potentially bind to cancer cell membranes/receptors, causing cytotoxicity, apoptosis and autophagy; thereby, they inhibit the growth of tumors [20]. The underlying mechanism of the anticancer potential of lectins and phenolic compounds in lentil is that they bind to ribosomes, which inhibits protein synthesis. Furthermore, this provokes a change of the cell cycle by inducing non-apoptotic G1-phase accumulation mechanisms, G2/M phase cell cycle arrest and apoptosis. In addition to that, this can also activate the caspase cascade in mitochondria and downregulate telomerase activity, which inhibits angiogenesis [20,102]. Thus, lectins and phenolic compounds derived from lentil seeds seem to be promising therapeutic agents against tumorigenesis or cancer cell agglutination and/or aggregation. The lentil seeds and their chemo-preventive potential on colorectal carcinogenesis have been well documented using azoxymethane, significantly reducing the number of dysplastic lesions and neoplasms in the colon of rats [101,103]. In addition, lentils have greater chemopreventive potential when compared to green and yellow peas [104]. This is because lentils contain antioxidant bioactive compounds such as flavonoids (flavanones, flavan-3-ols, flavones, flavonols, anthocyanidins and tannins, including condensed tannins or proanthocyanidins) that are greatly responsible for chemoprevention. This chemo-preventive potential is not constrained to polyphenolic-rich lentils or split seeds. In vitro and in vivo studies of lentil seeds exerting anticancer and chemopreventive potentials are summarized in Table 8.
7. Conclusions
Lentils have been consumed as a part of the diet worldwide and play a significant function in human nutrition as a rich source of bioactive and non-bioactive nutrients. When comparing to pulses, lentils have the highest starch content and insoluble dietary fiber content and high quantities of prebiotic carbohydrates that maintain the gut microbiota, which prevents colon-associated diseases. Lentils are among the cost-effective legumes, and they have lower quantities of fat, sodium and vitamin K, but a high content of potassium. This demonstrates them as a health-promoting source of nutrients, and their intake in the daily diet should increase, as this is related to the prevention of obesity and CVD. Besides these nutrients, lentils have certain bioactive food components, namely “polyphenols”. These polyphenol-rich lentil seeds have antioxidant potential and a primary function in protecting against various diseases such as diabetes, obesity, CVD and cancer. Various rodent studies and large prospective epidemiologic studies have reported that lentil consumption reduces the risk of those chronic diseases, which could be an exceptionally cost-effective approach towards improving health. Due to their nutritional and health-promoting potential, the development of lentil-based functional food products as well as nutraceuticals should be widely promoted.
Acknowledgments
The work was jointly supported by two grants (R201627 and R201714) from Beijing Normal University-Hong Kong Baptist University United International College, Zhuhai, Guangdong, China.
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethyl-benzothiazoline-6-sulphonic acid) |
| AFP | α-fetoprotein |
| AIP | atherogenic index of plasma |
| ALP | alkaline phosphatase |
| ALT | alanine transaminase |
| AST | aspartate transaminase |
| bw | body weight |
| BP | blood pressure |
| BUN | blood urea nitrogen |
| CAT | catalase |
| cDNA | complementary deoxyribonucleic acid |
| COX-1, 2 | cyclooxygenase 1, 2 |
| CVD | cardiovascular diseases |
| CRR | cardiac risk ratio |
| DNA | deoxyribonucleic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ELISA | enzyme-linked immunosorbent assay |
| FBS | fasting blood sugar |
| FRAP | ferric reducing antioxidant power assay |
| GR | glutathione reductase |
| GSH | reduced glutathione |
| GST | glutathione-s-transferase |
| HbA1C | glycated hemoglobin |
| Hb | hemoglobin |
| HDL | high density lipoprotein |
| HPLC | high performance liquid chromatography |
| i.p. | intraperitoneal |
| i.v. | intravenous |
| IL | interleukin |
| kg | kilogram |
| LDH | lactate dehydrogenase |
| LDL | low density lipoprotein |
| LOX | lysyl oxidase |
| LPO | lipid peroxidation |
| MCH | mean corpuscular hemoglobin |
| MCHC | mean corpuscular hemoglobin concentration |
| MCP-1 | monocyte chemotactic protein 1 |
| MCV | mean corpuscular value |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| OGTT | oral glucose tolerance test |
| ORAC | oxygen radical absorbance capacity |
| p.o. | per oral |
| PGE (2)-PRTC | peroxyl radical-trapping capacity |
| RBC | erythrocyte |
| ROS | reactive oxygen species |
| RP | reducing power |
| RT-PCR | reverse transcriptase polymerase chain reaction |
| SOD | super oxide dismutase |
| STZ | streptozotocin |
| TC | total cholesterol |
| TEAC | trolox equivalent antioxidant capacity |
| TG | triglycerides |
| TIBC | total iron binding capacity |
| TNF-α | tumor necrosis factor alpha |
| VLDL | very low density lipoprotein |
| WBC | leucocyte |
Author Contributions
Kumar Ganesan and Baojun Xu conceived of and designed the review. Kumar Ganesan wrote the paper. Baojun Xu critically read and improved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Faris M.A.E., Takruri H.R., Issa A.Y. Role of lentils (Lens culinaris L.) in human health and nutrition: A review. Mediterr. J. Nutr. Metab. 2013;6:3–16. doi: 10.1007/s12349-012-0109-8. [DOI] [Google Scholar]
- 2.Food and Agriculture Organization (FAO) Traditional Food Plants. FAO; Rome, Italy: 1988. [(accessed on 16 July 2017)]. Available online: http://www.fao.org/docrep/W0078E/w0078e12.htm. [Google Scholar]
- 3.Xu B., Chang S.K. Phenolic substance characterization and chemical and cell-based antioxidant activities of 11 lentils grown in the Northern United States. J. Agric. Food Chem. 2010;58:1509–1517. doi: 10.1021/jf903532y. [DOI] [PubMed] [Google Scholar]
- 4.FAOSTAT Food and Agricultural Organization of United Nations: Economic and Social Department: The Statistical Division. [(accessed on 19 July 2017)]; Available online: http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor.
- 5.Lombardi-Boccia G., Ruggeri S., Aguzzi A., Cappelloni M. Globulins enhance in vitro iron but not zinc dialysability: A study on six legume species. J. Trace Elem. Med. Biol. 2013;17:1–5. doi: 10.1016/S0946-672X(03)80037-8. [DOI] [PubMed] [Google Scholar]
- 6.Hoover R., Hughes T., Chung H., Liu Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010;43:399–413. doi: 10.1016/j.foodres.2009.09.001. [DOI] [Google Scholar]
- 7.United States Department of Agriculture (USDA) Agricultural Research Service, National Nutrient Database for Standard Reference Release 28. Nutrient Database Laboratory Home Page. [(accessed on 14 August 2016)]; Available online: https://ndb.nal.usda.gov/ndb/search/list.
- 8.Bednar G.E., Patil A.R., Murray S.M., Grieshop C.M., Merchen N.R., Fahey G.C. Starch and fiber fractions in selected food and feed ingredients affect their small intestinal digestibility and fermentability and their large bowel fermentability in vitro in a canine model. J. Nutr. 2001;131:276–286. doi: 10.1093/jn/131.2.276. [DOI] [PubMed] [Google Scholar]
- 9.Dwivedi S., Sahrawat K., Puppala N., Ortiz R. Plant prebiotics, and human health: Biotechnology to breed prebiotic-rich nutritious food crops. Electr. J. Biotechnol. 2014;17:238–245. doi: 10.1016/j.ejbt.2014.07.004. [DOI] [Google Scholar]
- 10.Fooks L.J., Fuller R., Gibson G.R. Prebiotics, probiotics and human gut microbiology. Int. Dairy J. 1999;9:53–61. doi: 10.1016/S0958-6946(99)00044-8. [DOI] [Google Scholar]
- 11.Johnson C.R., Combs G.F., Thavarajah P. Lentil (Lens culinaris L.): A prebiotic-rich whole food legume. Food Res. Int. 2013;51:107–113. doi: 10.1016/j.foodres.2012.11.025. [DOI] [Google Scholar]
- 12.Padovani R.M., Lima D.M., Colugnati F.A., Rodriguez-Amaya D.B. Comparison of proximate, mineral and vitamin composition of common Brazilian and US foods. J. Food Compos. Anal. 2007;20:733–738. doi: 10.1016/j.jfca.2007.03.006. [DOI] [Google Scholar]
- 13.Soltan S.S.A. The protective effect of soybean, sesame, lentils, pumpkin seeds and molasses on iron deficiency anemia in rats. World Appl. Sci. J. 2013;23:795–807. [Google Scholar]
- 14.Ryan E., Galvin K., O’Connor T.P., Maguire A.R., O’Brien N.M. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum. Nutr. 2007;62:85–91. doi: 10.1007/s11130-007-0046-8. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez C., Frias J., Vidal-Valverde C., Hernandez A. Correlations between some nitrogen fractions, lysine, histidine, tyrosine, and ornithine contents during the germination of peas, beans, and lentils. Food Chem. 2008;108:245–252. doi: 10.1016/j.foodchem.2007.10.073. [DOI] [Google Scholar]
- 16.Kalogeropoulos N., Chiou A., Ioannou M., Karathanos V.T., Hassapidou M., Andrikopoulos N.K. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010;121:682–690. doi: 10.1016/j.foodchem.2010.01.005. [DOI] [Google Scholar]
- 17.De Almeida Costa G.E., da Silva Queiroz-Monici K., Reis S.M.P.M., de Oliveira A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006;94:327–330. doi: 10.1016/j.foodchem.2004.11.020. [DOI] [Google Scholar]
- 18.Guillamon E., Pedrosa M.M., Burbano C., Cuadrado C., de Cortes Sanchez M., Muzquiz M. The trypsin inhibitors present in seed of different grain legume species and cultivar. Food Chem. 2008;107:68–74. doi: 10.1016/j.foodchem.2007.07.029. [DOI] [Google Scholar]
- 19.Freier T.C., Rudiger H.E. Lectin-binding proteins from lentil seeds as mitogens for murine B lymphocytes. Phytochemistry. 1990;29:1459–1461. doi: 10.1016/0031-9422(90)80100-U. [DOI] [Google Scholar]
- 20.De Mejia E.G., Prisecaru V.I. Lectins as bioactive plant proteins: A potential in cancer treatment. Crit. Rev. Food Sci. Nutr. 2005;45:425–445. doi: 10.1080/10408390591034445. [DOI] [PubMed] [Google Scholar]
- 21.Finkina E.I., Shramova E.I., Tagaev A.A., Ovchinnikova T.V. A novel defensin from the lentil Lens culinaris seeds. Biochem. Biophys. Res. Commun. 2008;371:860–865. doi: 10.1016/j.bbrc.2008.04.161. [DOI] [PubMed] [Google Scholar]
- 22.Demirbas A. β-Glucan and mineral nutrient contents of cereals grown in Turkey. Food Chem. 2005;90:773–777. doi: 10.1016/j.foodchem.2004.06.003. [DOI] [Google Scholar]
- 23.Perera A., Meda V., Tyler R. Resistant starch: A review of analytical protocols for determining resistant starch and of factors affecting the resistant starch content of foods. Food Res. Int. 2010;43:1959–1974. doi: 10.1016/j.foodres.2010.06.003. [DOI] [Google Scholar]
- 24.Xu B., Yuan S., Chang S. Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. J. Food Sci. 2007;72:S167–S177. doi: 10.1111/j.1750-3841.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 25.Scalbert A., Manach C., Morand C., Remesy C., Jimenez L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 26.Thompson L.U., Boucher B.A., Liu Z., Cotterchio M., Kreiger N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr. Cancer. 2006;54:184–201. doi: 10.1207/s15327914nc5402_5. [DOI] [PubMed] [Google Scholar]
- 27.Barahuie F., Dorniani D., Saifullah B., Gothai S., Hussein M.Z., Pandurangan A.K., Arulselvan P., Norhaizan M.E. Sustained release of anticancer agent phytic acid from its chitosan-coated magnetic nanoparticles for drug-delivery system. Int. J. Nanomed. 2017;12:2361–2372. doi: 10.2147/IJN.S126245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao C.V., Newmark H.L., Reddy B.S. Chemopreventive effect of squalene on colon cancer. Carcinogenesis. 1998;19:287–290. doi: 10.1093/carcin/19.2.287. [DOI] [PubMed] [Google Scholar]
- 29.Elekofehinti O.O. Saponins: Anti-diabetic principles from medicinal plants—A review. Pathophysiology. 2015;22:95–103. doi: 10.1016/j.pathophys.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Dueñas M., Sun B., Hernández T., Estrella I., Spranger M.I. Proanthocyanidin composition in the seed coat of lentils (Lens culinaris L.) J. Agric. Food Chem. 2003;51:7999–8004. doi: 10.1021/jf0303215. [DOI] [PubMed] [Google Scholar]
- 31.Taylor W.G., Fields P.G., Sutherland D.H. Fractionation of lentil seeds (Lens culinaris Medik.) for insecticidal and flavonol tetraglycoside components. J. Agric. Food Chem. 2007;55:5491–5498. doi: 10.1021/jf0705062. [DOI] [PubMed] [Google Scholar]
- 32.Aguilera Y., Dueñas M., Estrella I., Hernández T., Benitez V., Esteban R.M., Martín-Cabrejas M.A. Evaluation of phenolic profile and antioxidant properties of Pardina lentil as affected by industrial dehydration. J. Agric. Food Chem. 2010;58:10101–10108. doi: 10.1021/jf102222t. [DOI] [PubMed] [Google Scholar]
- 33.Zou Y., Chang S.K., Gu Y., Qian S.Y. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions. J. Agric. Food Chem. 2011;59:2268–2276. doi: 10.1021/jf104640k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang B., Deng Z., Tang Y., Chen P.X., Liu R., Ramdath D.D., Liu Q., Hernandez M., Tsao R. Effect of domestic cooking on carotenoids, tocopherols, fatty acids, phenolics, and antioxidant activities of lentils (Lens culinaris) J. Agric. Food Chem. 2014;62:12585–12594. doi: 10.1021/jf504181r. [DOI] [PubMed] [Google Scholar]
- 35.Żuchowski J., Pecio Ł., Stochmal A. Novel flavonol glycosides from the aerial parts of lentil (Lens culinaris) Molecules. 2014;19:18152–18178. doi: 10.3390/molecules191118152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirali M., Ambrose S.J., Wood S.A., Vandenberg A., Purves R.W. Development of a fast extraction method and optimization of liquid chromatography-mass spectrometry for the analysis of phenolic compounds in lentil seed coats. J. Chromatogr. B. 2014;969:149–161. doi: 10.1016/j.jchromb.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Zhang B., Deng Z., Ramdath D.D., Tang Y., Chen P.X., Liu R., Liu Q., Tsao R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on a-glucosidase and pancreatic lipase. Food Chem. 2015;172:862–872. doi: 10.1016/j.foodchem.2014.09.144. [DOI] [PubMed] [Google Scholar]
- 38.Świeca M., Baraniak B., Gawlik-Dziki U. In vitro digestibility and starch content, predicted glycemic index and potential In Vitro anti-diabetic effect of lentil sprouts obtained by different germination techniques. Food Chem. 2013;138:1414–1420. doi: 10.1016/j.foodchem.2012.09.122. [DOI] [PubMed] [Google Scholar]
- 39.Aslani Z., Mirmiran P., Alipur B., Bahadoran Z., Farhangi M.A. Lentil sprouts effect on serum lipids of overweight and obese patients with type 2 diabetes. Health Promot. Perspect. 2015;5:215–224. doi: 10.15171/hpp.2015.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolever T.M., Katzman-Relle L., Jenkins A.L., Vuksan V., Josse R.G., Jenkins D.J. Glycaemic index of 102 complex carbohydrate foods in patients with diabetes. Nutr. Res. 1994;14:651–669. doi: 10.1016/S0271-5317(05)80201-5. [DOI] [Google Scholar]
- 41.Shams H., Tahbaz F., Entezari M., Abadi A. Effects of cooked lentils on glycemic control and blood lipids of patients with type 2 diabetes. ARYA Atheroscler. 2008;4:1–5. [Google Scholar]
- 42.Al-Tibi A.T.B., Takruri H.R., Ahmad M.N. Effect of dehulling and cooking of lentils (Lens culinaris, L.) on serum glucose and lipoprotein levels in streptozotocin-induced diabetic rats. Malays. J. Nutr. 2010;16:409–418. [PubMed] [Google Scholar]
- 43.Flight I., Clifton P. Cereal grains and legumes in the prevention of coronary heart disease and stroke: A review of the literature. Eur. J. Clin. Nutr. 2006;60:1145–1159. doi: 10.1038/sj.ejcn.1602435. [DOI] [PubMed] [Google Scholar]
- 44.Liu R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007;46:207–219. doi: 10.1016/j.jcs.2007.06.010. [DOI] [Google Scholar]
- 45.Hodge A.M., English D.R., O’Dea K., Giles G.G. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27:2701–2706. doi: 10.2337/diacare.27.11.2701. [DOI] [PubMed] [Google Scholar]
- 46.Chung H.-J., Liu Q., Pauls K.P., Fan M.Z., Yada R. In vitro starch digestibility, expected glycemic index and some physicochemical properties of starch and flour from common bean (Phaseolus vulgaris L.) varieties grown in Canada. Food Res. Int. 2008;41:869–875. doi: 10.1016/j.foodres.2008.03.013. [DOI] [Google Scholar]
- 47.Peñas E., Limón R.I., Martínez-Villaluenga C., Restani P., Pihlanto A., Frias J. Impact of Elicitation on Antioxidant and Potential Antihypertensive Properties of Lentil Sprouts. Plant Foods Hum. Nutr. 2015;70:401–407. doi: 10.1007/s11130-015-0508-3. [DOI] [PubMed] [Google Scholar]
- 48.Tawfeuk H.Z., Hassan N.M., Khalil H.I., Kerolles S.Y. Anti-diabetic effects of dietary formulas prepared from some grains and vegetables on type 2 diabetic rats. J. Agroaliment. Process. Technol. 2014;20:69–79. [Google Scholar]
- 49.Bolsinger J., Landstrom M., Pronczuk A., Auerbach A., Hayes K.C. Low glycemic load diets protect against metabolic syndrome and type 2 diabetes mellitus in the male Nile rat. J. Nutr. Biochem. 2017;42:134–148. doi: 10.1016/j.jnutbio.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Jenkins D., Wolever T., Taylor R.H., Barker H., Fielden H., Baldwin J.M., Goff D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 51.Duenas M., Hernandez T., Estrella I. Assessment of in vitro antioxidant capacity of the seed coat and the cotyledon of legumes in relation to their phenolic contents. Food Chem. 2006;98:95–103. doi: 10.1016/j.foodchem.2005.05.052. [DOI] [Google Scholar]
- 52.Pellegrini N., Serafini M., Salvatore S., Del Rio D., Bianchi M., Brighenti F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol. Nutr. Food Res. 2006;50:1030–1038. doi: 10.1002/mnfr.200600067. [DOI] [PubMed] [Google Scholar]
- 53.Xu B., Chang S.K. Effect of soaking, boiling, and steaming on total phenolic content and antioxidant activities of cool season food legumes. Food Chem. 2008;110:1–13. doi: 10.1016/j.foodchem.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 54.Fratianni F., Cardinale F., Cozzolino A., Granese T., Albanese D., Di Matteo M., Nazzaro F. Polyphenol composition and antioxidant activity of different grass pea (Lathyrussativus), lentils (Lens culinaris), and chickpea (Cicer arietinum) ecotypes of the Campania region (Southern Italy) J. Funct. Foods. 2014;7:551–557. doi: 10.1016/j.jff.2013.12.030. [DOI] [Google Scholar]
- 55.Kris-Etherton P.M., Hecker K.D., Bonanome A., Coval S.M., Binkoski A.E., Hilpert K.F., Etherton T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002;113:71–88. doi: 10.1016/S0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 56.Mollard R., Zykus A., Luhovyy B., Nunez M., Wong C., Anderson G. The acute effects of a pulse-containing meal on glycaemic responses and measures of satiety and satiation within and at a later meal. Br. J. Nutr. 2012;108:509–517. doi: 10.1017/S0007114511005836. [DOI] [PubMed] [Google Scholar]
- 57.McCrory M.A., Hamaker B.R., Lovejoy J.C., Eichelsdoerfer P.E. Pulse consumption, satiety, and weight management. Adv. Nutr. Int. Rev. J. 2010;1:17–30. doi: 10.3945/an.110.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu B.J., Han L.K., Zheng Y.N., Lee J.H., Sung C.K. In vitro inhibitory effect of triterpenoidal saponins from Platycodi Radix on pancreatic lipase. Arch. Pharmacol. Res. 2005;28:180–185. doi: 10.1007/BF02977712. [DOI] [PubMed] [Google Scholar]
- 59.Balasubramaniam V., Mustar S., Khalid N.M., Rashed A.A., Noh M.F.M., Wilcox M.D., Pearson J. Inhibitory activities of three Malaysian edible seaweeds on lipase and α-amylase. J. Appl. Phycol. 2013;25:1405–1412. doi: 10.1007/s10811-012-9964-4. [DOI] [Google Scholar]
- 60.Lopez A., El-Naggar T., Duenas T., Ortega T., Estrella I., Hernandez T., Gomez-Serranillos M.P., Palomino O.M., Carretero M.E. Influence of processing in the phenolic composition and health-promoting properties of Lentils (Lens culinaris L.) J. Food Process. Preserv. 2016 doi: 10.1111/jfpp.13113. [DOI] [Google Scholar]
- 61.Zhang B., Deng Z., Tang Y., Chen P.X., Liu R., Ramdath D.D., Liu Q., Hernandez M., Tsao R. Bioaccessibility, in vitro antioxidant and anti-inflammatory activities of phenolics in cooked green lentil (Lens culinaris) J. Funct. Foods. 2017;32:248–255. doi: 10.1016/j.jff.2017.03.004. [DOI] [Google Scholar]
- 62.Świeca M., Gawlik-Dziki U. Effects of sprouting and postharvest storage under cool temperature conditions on starch content and antioxidant capacity of green pea, lentil and young mung bean sprouts. Food Chem. 2015;185:99–105. doi: 10.1016/j.foodchem.2015.03.108. [DOI] [PubMed] [Google Scholar]
- 63.Talukdar D. In Vitro antioxidant potential and type II diabetes-related enzyme inhibition properties of traditionally processed legume-based food and medicinal recipes in Indian Himalayas. J. Appl. Pharm. Sci. 2013;3:26–32. [Google Scholar]
- 64.Fouad A.A., Rehab F.M. Effect of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris Medik.) sprouts. Acta Sci. Pol. Technol. Aliment. 2015;14:233–246. doi: 10.17306/J.AFS.2015.3.25. [DOI] [PubMed] [Google Scholar]
- 65.Boudjou S., Oomah B.D., Zaidi F., Hosseinian F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013;138:1543–1550. doi: 10.1016/j.foodchem.2012.11.108. [DOI] [PubMed] [Google Scholar]
- 66.Elaloui M., Ghazghazi H., Ennajah A., Manaa S., Guezmir W., Karray N.B., Laamouri A. Phenolic profile, antioxidant capacity of five Ziziphus spina-christi (L.) Willd provenances and their allelopathic effects on Trigonella foenum-graecum L. and Lens culinaris L. seeds. Nat. Prod. Res. 2017;31:1209–1213. doi: 10.1080/14786419.2016.1226830. [DOI] [PubMed] [Google Scholar]
- 67.Talukdar D., Talukdar T. Coordinated response of sulfate transport, cysteine biosynthesis, and glutathione-mediated antioxidant defense in lentil (Lens culinaris Medik.) genotypes exposed to arsenic. Protoplasma. 2014;251:839–855. doi: 10.1007/s00709-013-0586-8. [DOI] [PubMed] [Google Scholar]
- 68.Gharachorloo M., Tarzi B.G., Baharinia M., Hemaci A.H. Antioxidant activity and phenolic content of germinated lentil (Lens culinaris) J. Med. Plants Res. 2012;6:4562–4566. [Google Scholar]
- 69.Świeca M., Baraniak B. Influence of elicitation with H2O2 on phenolics content, antioxidant potential and nutritional quality of Lens culinaris sprouts. J. Sci. Food Agric. 2014;94:489–496. doi: 10.1002/jsfa.6274. [DOI] [PubMed] [Google Scholar]
- 70.Jameel M., Ali A., Ali M. Isolation of antioxidant phytoconstituents from the seeds of Lens culinaris Medik. Food Chem. 2015;175:358–365. doi: 10.1016/j.foodchem.2014.11.130. [DOI] [PubMed] [Google Scholar]
- 71.Bubelov Z., Sumczynski D., Salek R.N. Effect of cooking and germination on antioxidant activity, total polyphenols and flavonoids, fiber content, and digestibility of lentils (Lens culinaris L.) J. Food Process. Preserv. 2017:e13388. doi: 10.1111/jfpp.13388. [DOI] [Google Scholar]
- 72.Fernandez-Orozco R., Zieliński H., Piskuła M.K. Contribution of low-molecular-weight antioxidants to the antioxidant capacity of raw and processed lentil seeds. Nahrung. 2003;47:291–299. doi: 10.1002/food.200390069. [DOI] [PubMed] [Google Scholar]
- 73.Landi N., Pacifico S., Piccolella S., Di Giuseppe A.M., Mezzacapo M.C., Ragucci S., Iannuzzi F., Zarrelli A., Di Maro A. Valle Agricola lentil, an unknown lentil (Lens culinaris Medik.) seed from Southern Italy as a novel antioxidant and prebiotic source. Food Funct. 2015;6:3155–3164. doi: 10.1039/C5FO00604J. [DOI] [PubMed] [Google Scholar]
- 74.Zia-Ul-Haq M., Landa P., Kutil Z., Qayum M., Ahmad S. Evaluation of anti-inflammatory activity of selected legumes from Pakistan: In vitro inhibition of cyclooxygenase-2. Pak. J. Pharm. Sci. 2013;26:185–187. [PubMed] [Google Scholar]
- 75.Busambwa K., Sunkara R., Diby N., Offei-Okyne R., Boateng R., Verghese M. Cytotoxic and apoptotic effects of sprouted and non-sprouted lentil, green and yellow split-peas. Int. J. Cancer Res. 2016;12:51–60. [Google Scholar]
- 76.Pinto X., Vilaseca M.A., Balcells S., Artuch R., Corbella E., Meco J.F., Grinberg D. A folate-rich diet is as effective as folic acid from supplements in decreasing plasma homocysteine concentrations. Int. J. Med. Sci. 2005;2:58–63. doi: 10.7150/ijms.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adikay S., Saisruthi K. Phytoremedial effect of Lens culinaris against doxorubicin-induced nephrotoxicity in male Wistar rats. Int. J. Green Pharm. 2016;10:172–177. [Google Scholar]
- 78.Mahmoud N.E. The Semi-Modified Diets as Antioxidants, Hypolipidemic and Hypocholesterolemic Agents. Food and Agriculture Organization of the United Nations; Rome, Italy: 2011. [(accessed on 14 July 2017)]. Available online: http://agris.fao.org/aos/records/EG2012000695. [Google Scholar]
- 79.Ahmad M.N. The effect of lentil on cholesterol-induced changes of serum lipid cardiovascular indexes in rats. Prog. Nutr. 2017;19:48–56. [Google Scholar]
- 80.Boualga A., Prost J., Taleb-Senouci D., Krouf D., Kharoubi O., Lamri-Senhadji M., Belleville J., Bouchenak M. Purified chickpea or lentil proteins impair VLDL metabolism and lipoprotein lipase activity in epididymal fat, but not in muscle, compared to casein, in growing rats. Eur. J. Nutr. 2009;48:162–169. doi: 10.1007/s00394-009-0777-4. [DOI] [PubMed] [Google Scholar]
- 81.Vohra K., Gupta V.K., Dureja H., Garg V. Antihyperlipidemic activity of Lens culinaris Medikus seeds in Triton WR-1339 induced hyperlipidemic rats. J. Pharmacogn. Nat. Prod. 2016;2:117. doi: 10.4172/2472-0992.1000117. [DOI] [Google Scholar]
- 82.Lukito W. Candidate foods in the Asia-Pacific region for cardiovascular protection: Nuts, soy, lentils, and tempe. Asia Pac. J. Clin. Nutr. 2001;10:128–133. doi: 10.1046/j.1440-6047.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 83.Duane W. Effects of legume consumption on serum cholesterol, biliary lipids, and sterol metabolism in humans. J. Lipid Res. 1997;38:1120–1128. [PubMed] [Google Scholar]
- 84.Bazzano L.A., Thompson A.M., Tees M.T., Nguyen C.H., Winham D.M. Non-soy legume consumption lowers cholesterol levels: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2011;21:94–103. doi: 10.1016/j.numecd.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jenkins D., Wong G.S., Patten R., Bird J., Hall M., Buckley G.C., Little J.A. Leguminous seeds in the dietary management of hyperlipidemia. Am. J. Clin. Nutr. 1983;38:567–573. doi: 10.1093/ajcn/38.4.567. [DOI] [PubMed] [Google Scholar]
- 86.Boye J.I., Roufik S., Pesta N., Barbana C. Angiotensin I-converting enzyme inhibitory properties and SDS-PAGE of red lentil protein hydrolysates. LWT-Food Sci. Technol. 2010;43:987–991. doi: 10.1016/j.lwt.2010.01.014. [DOI] [Google Scholar]
- 87.Hanson M.G., Zahradka P., Taylor C.G. Lentil-based diets attenuate hypertension and large-artery remodelling in spontaneously hypertensive rats. Br. J. Nutr. 2014;111:690–698. doi: 10.1017/S0007114513002997. [DOI] [PubMed] [Google Scholar]
- 88.Garcia-Mora P., Penas E., Frias J., Martinez-Villaluenga C. Savinase, the most suitable enzyme for releasing peptides from lentil (Lens culinaris var. Castellana) protein concentrates with multifunctional properties. J. Agric. Food Chem. 2014;62:4166–4174. doi: 10.1021/jf500849u. [DOI] [PubMed] [Google Scholar]
- 89.Mitchell B.S., Brooks S.A., Leathem A.J., Schumacher U. Do HPA and PHA-L have the same binding pattern in metastasizing human breast and colon cancers? Cancer Lett. 1998;123:113–119. doi: 10.1016/S0304-3835(97)00414-X. [DOI] [PubMed] [Google Scholar]
- 90.Kulshrestha S., Chaturvedi S., Jangir R., Agrawal K. In vitro Evaluation of antibacterial activity of some plant leaf extracts against Xanthomonas axonopodis pv. phaseoli isolated from seeds of lentil (Lens culinaris Medik.) Int. Res. J. Biol. Sci. 2015;4:59–64. [Google Scholar]
- 91.Finkina E.I., Balandin S.V., Serebryakova M.V., Potapenko N.A., Tagaev A.A., Ovchinnikova T.V. Purification and primary structure of novel lipid transfer proteins from germinated lentil (Lens culinaris) seeds. Biochemistry. 2007;72:430–438. doi: 10.1134/S0006297907040104. [DOI] [PubMed] [Google Scholar]
- 92.Nair S.S., Madembil N.C., Nair P., Raman S., Veerabadrappa S.B. Comparative analysis of the antibacterial activity of some phytolectins. Int. Curr. Pharm. J. 2013;2:18–22. doi: 10.3329/icpj.v2i2.13192. [DOI] [Google Scholar]
- 93.Khan D.A., Hassan F., Ullah H., Karim S., Baseer A., Abid M.A., Ubaidi M., Khan S.A., Murtaza G. Antibacterial activity of Phyllanthusemblica, Coriandrum sativum, Culinaris medic, Lawsonia alba and Cucumis sativus. Acta Pol. Pharm. 2013;70:855–859. [PubMed] [Google Scholar]
- 94.Wang H.X., Ng T.B. An antifungal peptide from red lentil seeds. Peptides. 2007;28:547–552. doi: 10.1016/j.peptides.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 95.Rönnblom L., Funa K., Ersson B., Alm G.V. Lectins as inducers of interferon-γ production in human lymphocytes: Lentil lectin is highly efficient. Scand. J. Immunol. 1982;16:327–331. doi: 10.1111/j.1365-3083.1982.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 96.Taylor J.L., Sedmak J.J., Jameson P., Lin Y.G., Grossberg S.E. Markedly enhanced production of γ interferon in murine T lymphocytes treated with lentil lectin and the diterpene ester, mezerein. J. Interferon Res. 1984;4:315–327. doi: 10.1089/jir.1984.4.315. [DOI] [PubMed] [Google Scholar]
- 97.Caccialupi P., Ceci L.R., Siciliano R.A., Pignone D., Clemente A., Sonnante G. Bowman-Birk inhibitors in lentil: Heterologous expression, functional characterization and antiproliferative properties in human colon cancer cells. Food Chem. 2010;120:1058–1066. doi: 10.1016/j.foodchem.2009.11.051. [DOI] [Google Scholar]
- 98.Adebamowo C.A., Cho E., Sampson L., Katan M.B., Spiegelman D., Willett W.C., Holmes M.D. Dietary flavonols and flavonol-rich foods intake and the risk of breast cancer. Int. J. Cancer. 2005;114:628–633. doi: 10.1002/ijc.20741. [DOI] [PubMed] [Google Scholar]
- 99.Perabo F.G., Von Löw E.C., Ellinger J., von Rücker A., Müller S.C. Soy isoflavone genistein in prevention and treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2008;11:6–12. doi: 10.1038/sj.pcan.4501000. [DOI] [PubMed] [Google Scholar]
- 100.Spanou C., Stagos D., Tousias L., Angelis A., Aligiannis N., Skaltsounis A.L., Kouretas D. Assessment of antioxidant activity of extracts from unique greek varieties of Leguminosae plants using In Vitro assays. Anticancer Res. 2007;27:3403–3410. [PubMed] [Google Scholar]
- 101.Faris M.A., Takruri H.R., Shomaf M.S., Bustanji Y.K. Chemopreventive effect of raw and cooked lentils (Lens culinaris L) and soybeans (Glycine max) against azoxymethane-induced aberrant crypt foci. Nutr. Res. 2009;29:355–362. doi: 10.1016/j.nutres.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 102.Scarafoni A., Magni C., Duranti M. Molecular nutraceutics as a mean to investigate the positive effects of legume seed proteins on human health. Trends Food Sci. Technol. 2007;18:454–463. doi: 10.1016/j.tifs.2007.04.002. [DOI] [Google Scholar]
- 103.Shomaf M., Takruri H., Faris M.A.I.E. Lentils (Lens culinaris, L.) attenuatecolonic lesions and neoplasms in Fischer 344 rats. Jordan Med. J. 2011;45:231–239. [Google Scholar]
- 104.Busambwa K., Miller-Cebert R., Aboagye L., Dalrymple L., Boateng J., Shackelford L., Verghese M. Inhibitory effect of lentils, green split and yellow peas (sprouted and non-sprouted) on azoxymethane-induced aberrant crypt foci in Fisher 344 male rats. Int. J. Cancer Res. 2014;10:27–36. doi: 10.3923/ijcr.2014.27.36. [DOI] [Google Scholar]
- 105.Rodríguez-Juan C., Pérez-Blas M., Suárez-García E., López-Suárez J.C., Múzquiz M., Cuadrado C., Martín-Villa J.M. Lens culinaris, Phaseolus vulgaris and Vicia faba lectins specifically trigger IL-8 production by the human colon carcinoma cell line Caco-2. Cytokine. 2000;12:1284–1287. doi: 10.1006/cyto.1999.0731. [DOI] [PubMed] [Google Scholar]
- 106.Chan Y.S., Yu H., Xia L., Ng T.B. Lectin from green speckled lentil seeds (Lens culinaris) triggered apoptosis in nasopharyngeal carcinoma cell lines. Chin. Med. 2015;10:25. doi: 10.1186/s13020-015-0057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bruce W.R., Giacca A., Medline A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol. Biomark. Prev. 2000;9:1271–1279. [PubMed] [Google Scholar]
- 108.Mills P.K., Beeson W.L., Phillips R.L., Fraser G.E. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer. 1989;64:598–604. doi: 10.1002/1097-0142(19890801)64:3<598::AID-CNCR2820640306>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 109.Jain M.G., Hislop G.T., Howe G.R., Ghadirian P. Plant foods, antioxidants, and prostate cancer risk: Findings from case-control studies in Canada. Nutr. Cancer. 1999;34:173–184. doi: 10.1207/S15327914NC3402_8. [DOI] [PubMed] [Google Scholar]
- 110.Wang L., Lee I.M., Zhang S.M., Blumberg J.B., Buring J.E., Sesso H.D. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am. J. Clin. Nutr. 2009;89:905–912. doi: 10.3945/ajcn.2008.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Desilets D.J., Davis K.E., Nair P.P., Salata K.F., Maydonovitch C.L., Howard R.S., Kikendall J.W., Wong R.K. Lectin binding to human colonocytes is predictive of colonic neoplasia. Am. J. Gastroenterol. 1999;94:744–750. doi: 10.1111/j.1572-0241.1999.00946.x. [DOI] [PubMed] [Google Scholar]
- 112.Shimizu K., Nakamura K., Kobatake S., Satomura S., Maruyama M., Kameko F., Tajiri J., Kato R. The clinical utility of Lens culinaris agglutinin-reactive thyroglobulin ratio in serum for distinguishing benign from malignant conditions of the thyroid. Clin. Chim. Acta. 2007;379:101–104. doi: 10.1016/j.cca.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 113.Kanai T., Amakawa M., Kato R., Shimizu K., Nakamura K., Ito K., Hama Y., Fujimori M., Amano J. Evaluation of a new method for the diagnosis of alterations of Lens culinaris agglutinin binding of thyroglobulin molecules in thyroid carcinoma. Clin. Chem. Lab. Med. 2009;47:1285–1290. doi: 10.1515/CCLM.2009.277. [DOI] [PubMed] [Google Scholar]


