Abstract
Mitogen-activated protein kinase kinase kinases (MAP3Ks), the top components of MAPK cascades, modulate many biological processes, such as growth, development and various environmental stresses. Nevertheless, the roles of MAP3Ks remain poorly understood in cotton. In this study, GhMAP3K65 was identified in cotton, and its transcription was inducible by pathogen infection, heat stress, and multiple signalling molecules. Silencing of GhMAP3K65 enhanced resistance to pathogen infection and heat stress in cotton. In contrast, overexpression of GhMAP3K65 enhanced susceptibility to pathogen infection and heat stress in transgenic Nicotiana benthamiana. The expression of defence-associated genes was activated in transgenic N. benthamiana plants after pathogen infection and heat stress, indicating that GhMAP3K65 positively regulates plant defence responses. Nevertheless, transgenic N. benthamiana plants impaired lignin biosynthesis and stomatal immunity in their leaves and repressed vitality of their root systems. In addition, the expression of lignin biosynthesis genes and lignin content were inhibited after pathogen infection and heat stress. Collectively, these results demonstrate that GhMAP3K65 enhances susceptibility to pathogen infection and heat stress by negatively modulating growth and development in transgenic N. benthamiana plants.
Keywords: cotton, VIGS, ectopic expression, pathogen infection, thermotolerance
1. Introduction
Unlike animals, plants are sessile and cannot change their location to flee unfavourable environmental conditions. Nevertheless, they have evolved a series of sophisticated mechanisms to perceive biotic and abiotic stresses, and to translate this perception into a timely and effectively adaptive response [1,2]. Mitogen-activated protein kinase (MAPK) cascades are highly conserved signal transduction pathways found in all eukaryotes and are often involved in plant response mechanisms [3]. Pathways involving MAPK cascades can trigger different cellular activities, including responses to environmental stresses and programmed cell death and so on [4,5].
MAPK cascades are composed of three functional protein kinases: MAPK kinase kinases, MAPK kinases and MAPKs [3]. These kinases are sequentially activated through phosphorylation by their upstream components [6]. Based on the sequence of their kinase catalytic domain, MAP3Ks are classified into three groups: the MEKK-like, ZIK-like and Raf-like families [7]. While Raf-like MAP3Ks constitute largest MAP3K subfamily, only a small number of Raf-like MAP3Ks have been studied [2].
An increasing number of studies suggest that Raf-like MAP3Ks play significant roles in plant growth and development as well as in the responses of specific plant hormone signal transduction pathways to various environmental stresses [8,9]. The biological importance of Raf-like MAP3Ks has been highlighted by functional analyses. Raf-like MAP3K genes have been reported to participate in multiple biological processes. For example, MAP3Kδ4 is crucial for regulating plant growth and shoot branching [10], and MAP3Kδ4-overexpression increases salt stress tolerance and plays a significant role in the ABA signalling pathway in Arabidopsis [11]. GhMAP3K40 mediates the reduced tolerance to biotic and abiotic stresses in N. benthamiana by negatively regulating growth and development [12]. A growing number of studies demonstrate that Raf-like MAP3Ks are involved in the responses to various environmental stresses and in modulating plant growth and development.
Cotton is suitable for growing in warm seasons, and its growth and yield are seriously threatened by various environmental stresses. Diseases generally cause significant losses in cotton production, particularly at high temperature and high humidity [13]. Disease resistance regulated by SLH1 and RPW8 during plant growth and development is inhibited at high temperature and high humidity [14,15]. Nevertheless, whether this phenomenon affects additional genes remains unclear. Crosstalk mechanisms are involved in the responses to disease, high temperature and high humidity conditions [13]. Three plant hormones, salicylic acid (SA), jasmonate (JA), and ethylene (ET), are known to play significant roles in regulating plant defence responses to various pathogens and abiotic stresses, such as heat [16,17,18,19].
Previous studies have largely focused on MAP2Ks and MAPKs in cotton, while findings regarding MAP3Ks, particularly Raf-like MAP3Ks, are limited. In this study, the expression profiles of GhMAP3K65 under different environmental stresses or treatment with multiple molecules were examined in cotton. Silencing of GhMAP3K65 enhanced resistance to pathogen infection and heat stress in cotton. In contrast, ectopic expression of GhMAP3K65 in N. benthamiana enhanced susceptibility to pathogen infection and heat stress by negatively modulating plant growth and development. Nevertheless, because overexpression of GhMAP3K65 activated the transcription of defence-associated genes, GhMAP3K65 played a positive role in the responses to pathogen infection and heat stress. These findings broaden our horizon that Raf-like MAP3Ks may play significant roles in signal transduction.
2. Results
2.1. Isolation and Sequence Analysis of GhMAP3K65
Based on the conserved Raf-like MAP3K region, the internal GhMAP3K65 fragment was obtained. The 5′-untranslated region (UTR) and 3′-UTR were identified via rapid amplification of cDNA ends polymerase chain reaction (RACE–PCR). Full-length GhMAP3K65 cDNA consisted of 1374 nucleotides, including a 1143 bp open reading frame (ORF), a 68 bp 5′-UTR and a 163 bp 3′-UTR. GhMAP3K65 ORF encoded a protein of 381 amino acid residues, with a predicted molecular mass and isoelectric point of 45.64 and 7.13 kDa, respectively.
As shown in Figure S1a, multiple sequence alignment demonstrated that the amino acid sequence of GhMAP3K65 was similar to that of other typical plant Raf-like MAP3Ks, showing high homology to AtRaf39 from Arabidopsis thaliana (73.30%), BnaRaf39 from Brassica napus L. (71.73%), OsMAP3K27 from Oryza sativa L. (71.20%), and ZmMAP3K50 from Zea mays L. (69.82%). Moreover, GhMAP3K65 possessed typical characteristics of MAPK, including a catalytic protein kinase domain between residues 104–386. The catalytic protein kinase domain of GhMAP3K65 included an ATP-binding site (IAHGTYGTVY), a Ser/Thr kinase active site (IVHRDVKTENMLL), and a conserved sequence (GTLGYMAPEVL). GhMAP3K65 was highly similar to the Raf-like MAP3Ks AtRaf39, BnaRaf39, OsMAP3K27, and ZmMAP3K50 (Figure S1b). These results indicate that GhMAP3K65 is a Raf-like MAP3K gene.
2.2. GhMAP3K65 is Localized to the Cytoplasm and Nucleus
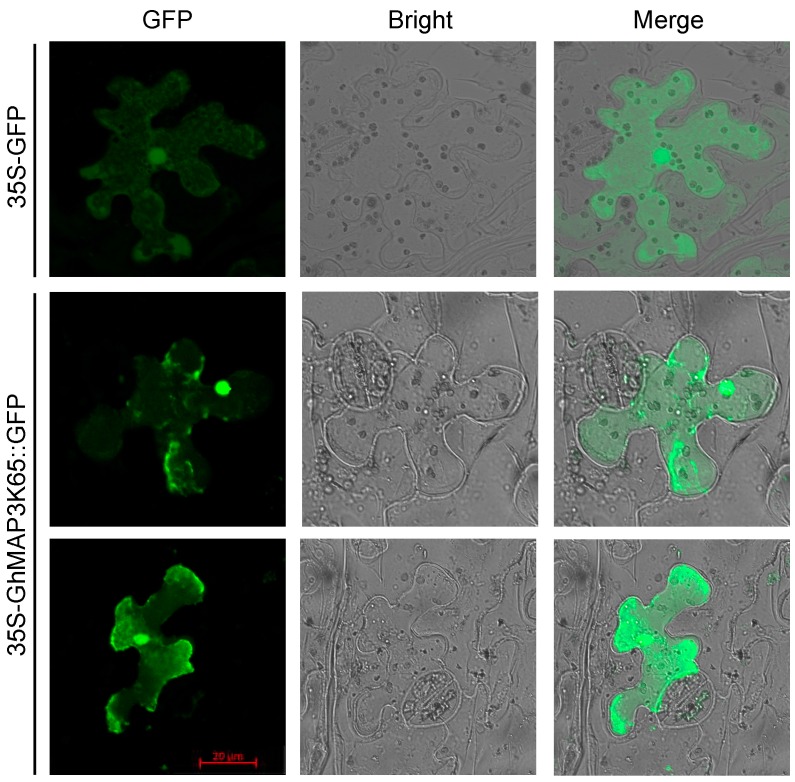
To confirm subcellular localization of GhMAP3K65, GhMAP3K65 ORF without stop codon was fused to 5′-terminal end of green fluorescent protein (GFP) to construct pROKII-GhMAP3K65-GFP, which was regulated by the cauliflower mosaic virus 35S (CaMV35S) promoter. Next, 35S-GFP and 35S-GhMAP3K65::GFP were transformed into N. benthamiana cells, and fluorescence was observed with two-photon laser confocal microscope. As shown in Figure 1, GFP control was localized to the cytoplasm and nucleus and GhMAP3K65::GFP fusion protein was detected throughout cytoplasm and nucleus in N. benthamiana epidermal cells. These results suggest that GhMAP3K65 is both a cytoplasmic- and nuclear-localized protein.
Figure 1.
Subcellular localization of GhMAP3K65 protein transiently expressed in N. benthamiana cells. The 35S-GFP and 35S-GhMAP3K65::GFP were transiently expressed in epidermal cells and the resulting green fluorescence was detected using two-photon laser confocal microscope.
The localization patterns for the 35S-GFP and 35S-GhMAP3K65::GFP proteins were the same. To determine the integrity of 35S-GhMAP3K65::GFP protein, Western blot analyses of the 35S-GFP and 35S-GhMAP3K65::GFP proteins from transiently transformed N. benthamiana plants were examined using a GFP antibody (Figure S2), revealing that the 35S-GhMAP3K65::GFP protein is integral.
2.3. Relative Expression Profiles of GhMAP3K65
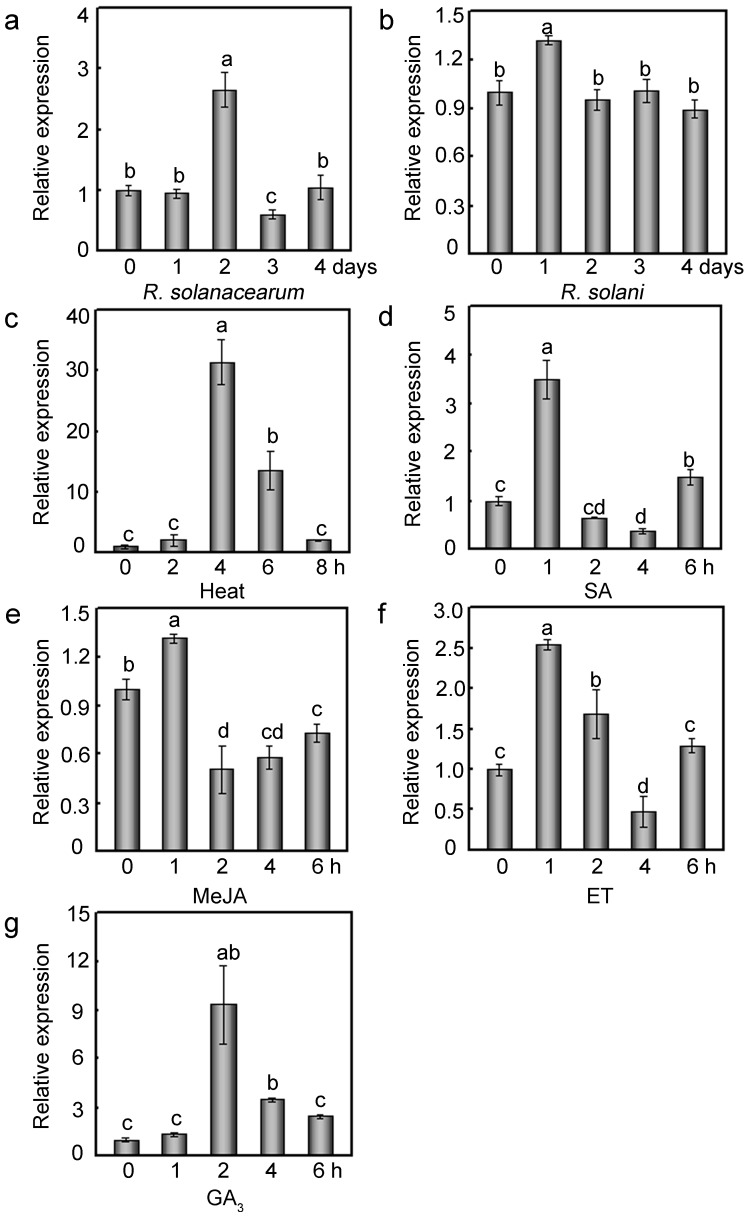
To determine the putative role of GhMAP3K65 in the responses to various environmental stresses, its expression profile was studied after exposure to biotic and abiotic stresses. The GhMAP3K65 expression profile in cotton plants not exposed to treatment was used as a mock control, and no changes in GhMAP3K65 transcript levels were observed during the 0–8 h series (Figure S3). GhMAP3K65 expression was notably increased by Ralstonia solanacearum and mildly induced by Rhizoctonia solani (Figure 2a,b). Heat treatment (42 °C) markedly enhanced GhMAP3K65 expression (Figure 2c). In addition, to explore the mechanism underlying GhMAP3K65 signal transduction in the responses to biotic and abiotic stresses, the effects of diverse signalling molecules on GhMAP3K65 expression were examined. GhMAP3K65 expression was enhanced by SA, methyl jasmonate (MeJA), and ET (Figure 2d–f) and was dramatically induced by gibberellic acid (GA3) (Figure 2g). These results suggest that GhMAP3K65 may be involved in response to biotic and abiotic stresses by mediating plant defence signal transduction pathways.
Figure 2.
Expression profiles of GhMAP3K65 in cotton. Seven-day-old cotton seedlings were examined by the following treatments: (a,b) Seedlings were inoculated with R. solanacearum and R. solani at 8 o’clock a.m. using root-dipping method, and cotton cotyledons were collected every day at 8 o’clock a.m.; (c) Seedlings were exposed to heat stress in a 42 °C incubator at 100% humidity and darkness at 8 o’clock a.m.; (d–g) Cotton cotyledons were sprayed with 2 mM SA, 100 µM MeJA, 5 mM ET, and 500 µM GA3 at 8 o’clock a.m. Regarding the sample collection time points, 8 o’clock a.m. correspond to 0 h, 9 o’clock a.m. correspond to 1 h, etc. The expression profiles of GhMAP3K65 were determined via quantitative real-time PCR (qRT-PCR). Total RNA was extracted from cotton cotyledons at the indicated time points. GhUBI (GenBank accession number: EU304080) was used as an internal control, and the experiments were repeated at least three times. Different letters above the columns indicate significant differences (p < 0.05) according to Duncan’s multiple range test.
2.4. Effects of GhMAP3K65 Silencing on Pathogen Infection and Heat Stress in Cotton
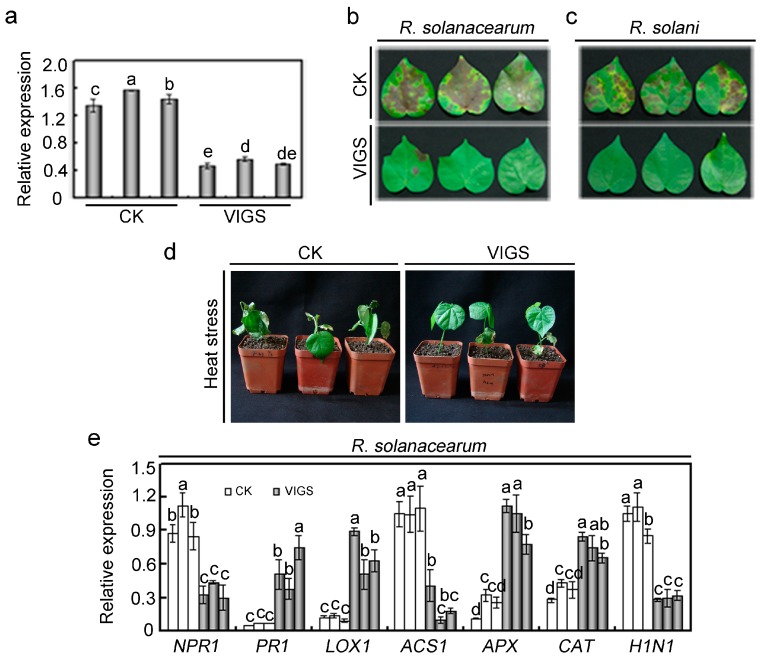
Differential expression profile analysis demonstrated that GhMAP3K65 may play roles in multiple plant defence responses. To evaluate the function of GhMAP3K65 in response to pathogen infection and heat stress, the virus-induced gene silencing (VIGS) technique was used to knock down GhMAP3K65 expression in cotton [20]. The transcript levels of GhMAP3K65 were examined via qRT-PCR in empty vector-treated (CK) and GhMAP3K65-silenced (VIGS) cotton plants. The decreased expression of GhMAP3K65 demonstrated that GhMAP3K65 had been successfully knocked down in cotton (Figure 3a). To confirm pathogen infection and heat stress phenotypes of GhMAP3K65-silenced cotton, CK and VIGS plants were tested. After pathogen infection, VIGS leaves showed enhanced resistance compared with CK (Figure 3b,c). Similarly, CK plants exhibited reduced thermotolerance after heat stress (Figure 3d). We obtained similar results with two additional CK and VIGS plants (Figure S4). These results suggested that silencing of GhMAP3K65 clearly enhanced resistance to pathogen infection and heat stress.
Figure 3.
Loss-of-function analysis of GhMAP3K65 in cotton. (a) Relative GhMAP3K65 transcript levels in vector-treated (CK) and GhMAP3K65-silenced (VIGS) cotton plants were examined via qRT-PCR; (b–d) Representative phenotypes of CK and VIGS plants after R. solanacearum, R. solani and heat stress, respectively; (e–g) The transcript levels of some cotton pathogenesis markers were examined by qRT-PCR 7 days after pathogen infection and 24 h after heat stress, respectively. Different letters above the columns indicate significant differences (p < 0.05) according to Duncan’s multiple range test.
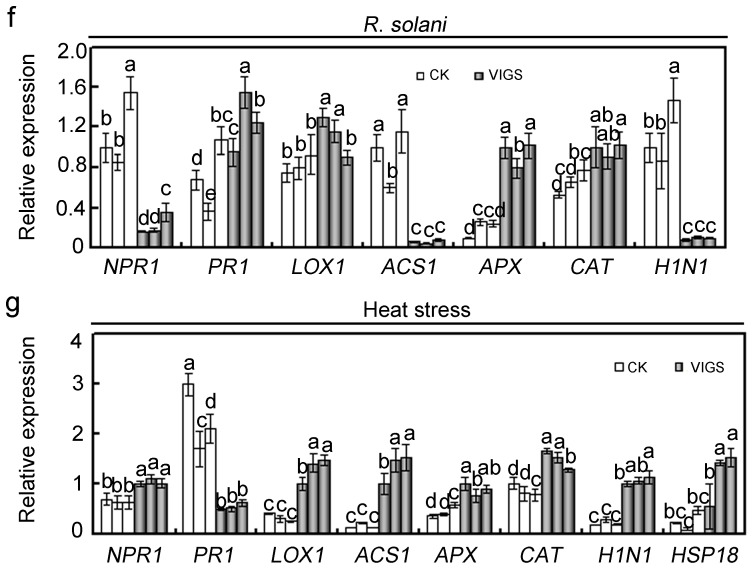
Furthermore, to explore mechanisms underlying effects of GhMAP3K65 silencing on pathogen infection and heat stress, the expression patterns of SA/JA/ET-mediated genes, reactive oxygen species (ROS) detoxification-related genes, hypersensitive response (HR)-related genes, and heat shock-related genes in CK and VIGS plants after pathogen infection and heat stress were examined by qRT-PCR. After pathogen infection, up-regulated genes included PR1 (pathogenesis-related protein 1), APX (ascorbate peroxidase ), CAT (catalase), and LOX1 (lipoxygenase 1), whereas the down-regulated transcription of NPR1 (natriuretic peptide receptor 1), ACS1 (1-aminocyclopropane-1-carboxylate synthase 1), and H1N1 (hairpin-induced 1) was observed (Figure 3e,f). As shown in Figure 3g, the transcript levels of NPR1, LOX1, ACS1, APX, CAT, H1N1, and HSP18 (heat shock protein 18) were significantly increased in VIGS plants compared with those in CK plants, nevertheless the transcript level of PR1 in VIGS plants was obviously decreased relative to wild type (WT) plants after heat stress. These results indicated that GhMAP3K65 may respond to pathogen infection and heat stress by SA/JA/ET and ROS signalling pathways.
2.5. Overexpression of GhMAP3K65 Enhanced Susceptibility to Pathogen Infection and Heat Stress
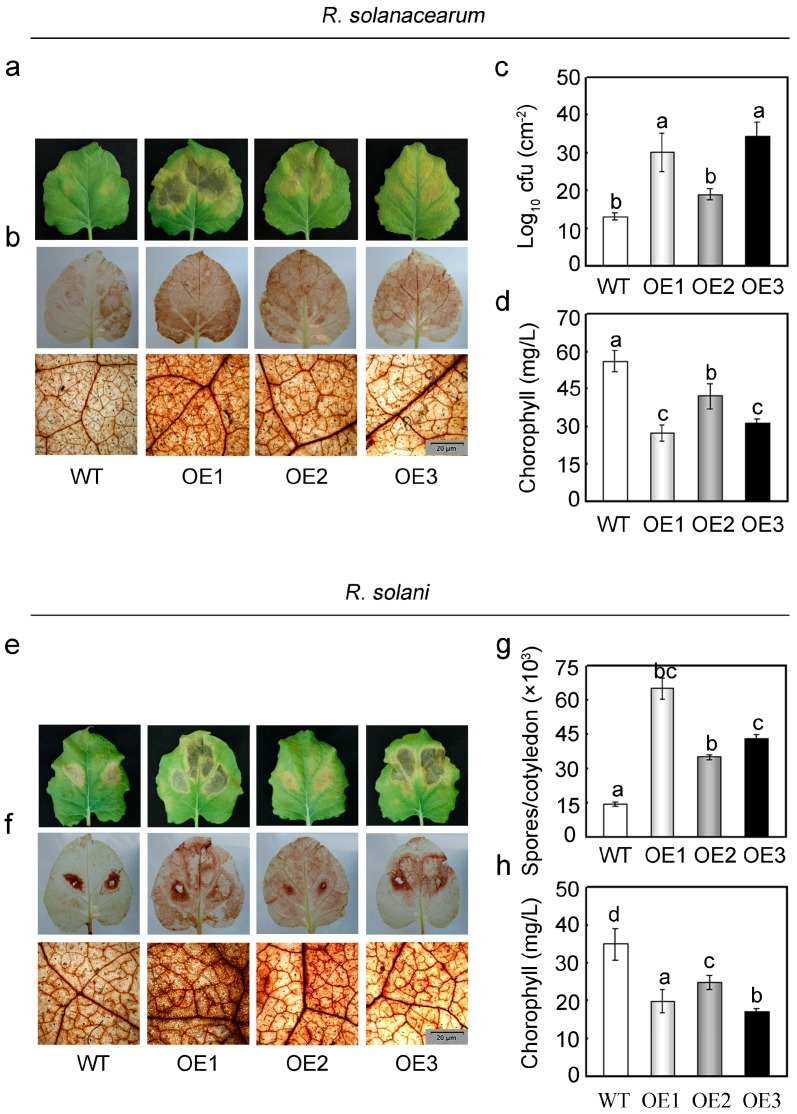
To confirm the results of GhMAP3K65 silencing observed in cotton in a corresponding experimental system, three transgenic N. benthamiana lines (OE1–OE3) were randomly selected for subsequent functional analyses, as transforming cotton is difficult and time-consuming. To explore whether GhMAP3K65-overexpression influences resistance to pathogen infection and heat stress in N. benthamiana, phenotypes of transgenic N. benthamiana plants were examined. For pathogen infection, detached leaves of WT and GhMAP3K65-overexpressing plants were injected with R. solanacearum and R. solani, and those of GhMAP3K65-overexpressing plants showed more severe chlorosis and larger lesions after 7 days (Figure 4a,e). Pathogen infection often results in production of ROS, which play important roles in defence response [21,22]. In diverse ROS, H2O2 is the only species that can cross plant membranes and thus directly functions in cell-to-cell signalling. To examine whether ROS production was relevant to enhanced susceptibility of transgenic N. benthamiana plants, H2O2 accumulation was monitored using 3,3′-diaminobenzidine (DAB) staining. After pathogen infection, H2O2 contents of the overexpressing plants were higher than those of WT plants (Figure 4b,f). These results indicated that GhMAP3K65-overexpression could enhance defence susceptibility by inducing the production of pathogen-induced ROS (mainly H2O2).
Figure 4.
Pathogen infection of GhMAP3K65-overexpressing plants. (a,e) Symptoms in detached leaves of WT and GhMAP3K65-overexpressing plants in response to R. solanacearum and R. solani infection, respectively. The detached leaves were photographed 7 days after infection; (b,f) Symptoms in detached leaves of WT and GhMAP3K65-overexpressing plants following R. solanacearum and R. solani infection, respectively. The accumulation of H2O2 was detected via 3,3′-diaminobenzidine (DAB) staining; (c) Bacterial growth in the leaves of WT and GhMAP3K65-overexpressing plants after R. solanacearum infection; (g) The number of spores per cotyledon after R. solani infection; (d,h) Chlorophyll contents were measured after R. solanacearum and R. solani infection, respectively. The experiments were repeated at least three times. Different letters above the columns indicate significant differences (p < 0.05) according to Duncan’s multiple range test.
To precisely quantify degree of pathogen infection in leaves, colony-forming units (cfu), spores per cotyledon and chlorophyll content assays were performed after pathogen infection. Compared with WT plants, the transgenic N. benthamiana plants exhibited increased bacterial growth, more spores (Figure 4c,g) and lower chlorophyll contents (Figure 4d,h). These results indicated that GhMAP3K65-overexpressing plants suffered more serious damage after pathogen infection.
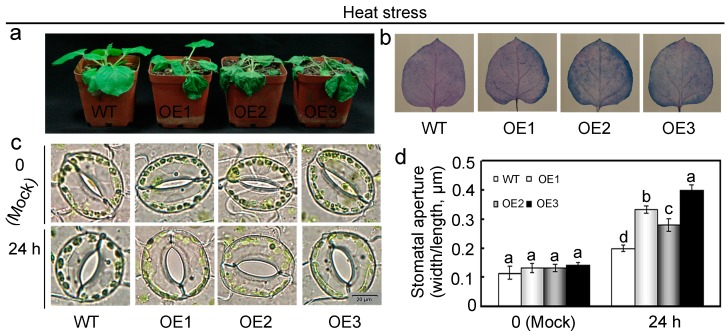
To evaluate the impact of GhMAP3K65 on thermotolerance, WT and transgenic N. benthamiana plants were exposed to heat stress in a 42 °C incubator for 24 h at 100% humidity and darkness. GhMAP3K65-overexpressing plants displayed more severe heat-induced cell death than WT plants (Figure 5a). In addition, local defence response was examined via Trypan blue staining to detect HR-like cell death after heat stress. Compared with WT plants, the transgenic N. benthamiana plants showed significantly increased blue area (HR-like cell death) under heat stress (Figure 5b). Degree of stomatal opening in GhMAP3K65-overexpressing plants was not significantly different from that in WT plants under normal conditions. Nevertheless, extent of stomatal opening observed in GhMAP3K65-overexpressing plants was greater than that in WT plants after heat stress treatment (Figure 5c,d). These results indicated that GhMAP3K65-overexpressing plants suffered greater damage after heat stress.
Figure 5.
Heat stress response of GhMAP3K65-overexpressing plants. (a) Photographs of WT and GhMAP3K65-overexpressing plants grown in soil under heat stress for 24 h; (b) The accumulation of dead cells was detected via Trypan blue staining; (c,d) Stomatal changes under heat stress were observed using a microscope. The experiments were repeated at least three times. The different letters above the columns indicate significant differences (p < 0.05) according to Duncan’s multiple range test.
2.6. Overexpression of GhMAP3K65 Activated Transcription of Defence-Related Genes
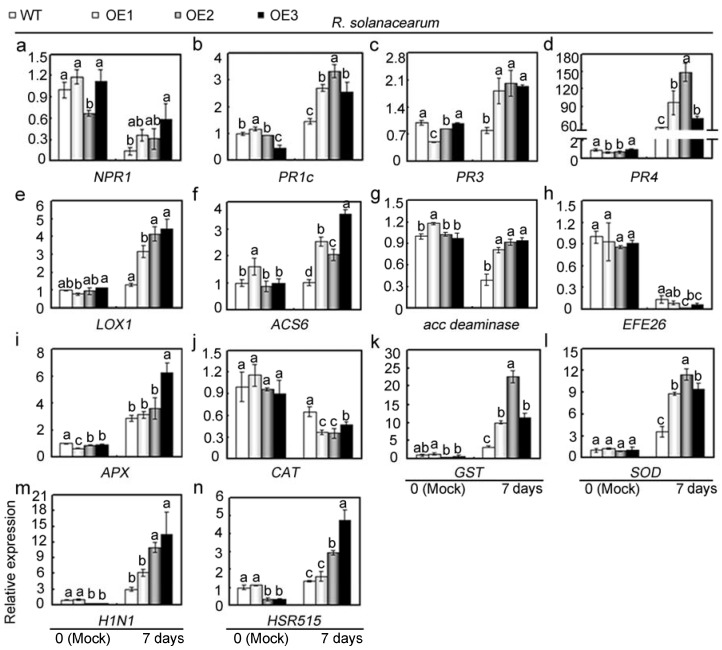
To explore mechanisms underlying GhMAP3K65-regulated diseases and heat stress sensitivity, the expression levels of defence-related genes were examined after pathogen infection and heat stress, respectively. Detached leaves from untreated WT and transgenic N. benthamiana plants were used as mock controls. Following R. solanacearum infection for 3 or 7 days (Figures S5 and Figure 6), the transcription levels of the SA-related genes NPR1, PR1c, PR3, and PR4; the JA-responsive gene LOX1; the ET generation-related genes ACS6, acc deaminase and EFE26 (ethylene forming enzyme 26); the ROS detoxification-related genes APX, CAT, GST (glutathione transferase ), and SOD (superoxide dismutase); and the HR-related genes H1N1 and HSR515 (hypersensitivity-related 515) were examined. Examined tobacco genes have been suggested to be up-regulated after pathogen infection [13,23,24]. A subset of these genes showed higher transcript levels in GhMAP3K65-overexpressing leaves than in WT leaves. At the two tested timepoints, these genes (NPR1, PR1c, PR3, PR4, LOX1, ACS6, acc deaminase, APX, GST, SOD, H1N1, and HSR515) were differentially up-regulated by R. solanacearum infection, while a second set of genes, including EFE26 and CAT, was transcriptionally down-regulated by R. solanacearum at the two tested timepoints. Thus, GhMAP3K65-overexpression influenced the transcription (up- or down- regulation) of 13 defence-related genes examined after R. solanacearum infection. These results indicated that GhMAP3K65-overexpression activates the transcription of defence-related genes, and GhMAP3K65 might therefore play a positive role in response to R. solanacearum.
Figure 6.
Relative transcript levels of defence-related genes in WT and GhMAP3K65-overexpressing plants after R. solanacearum infection. (a–d) Relative transcript levels of the SA-responsive genes NPR1, PR1c, PR3, and PR4. (e) Relative transcript levels of the JA-responsive gene LOX1; (f–h) Relative transcript levels of the ET biosynthesis-associated genes ACS6, acc deaminase and EFE26; (i–l) Relative transcript levels of the ROS detoxification-associated genes APX, CAT, GST, and SOD; (m,n) Relative transcript levels of the HR marker genes H1N1 and HSR515. The experiments were repeated at least three times. Different letters above the columns indicate significant differences (p < 0.05) according to Duncan’s multiple range test.
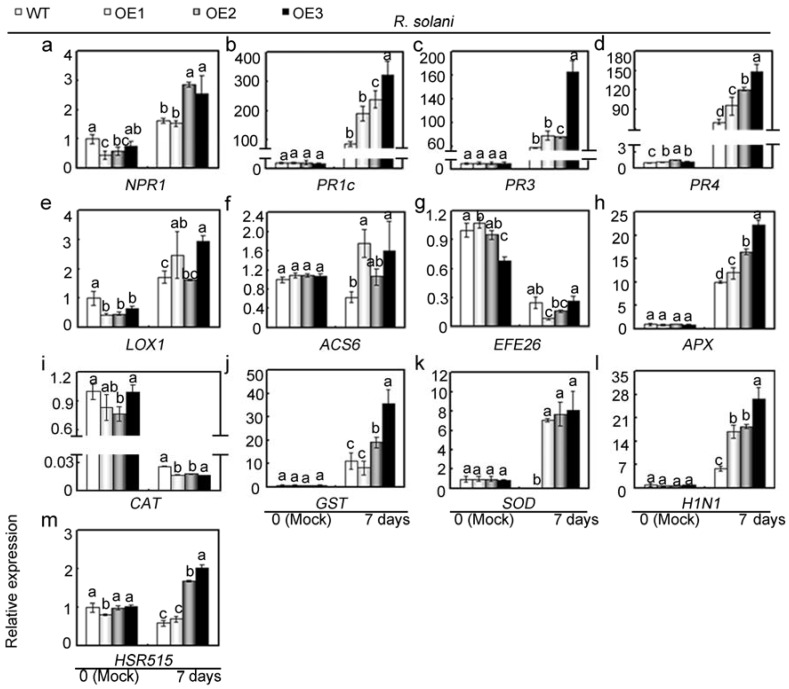
To further elucidate the role of GhMAP3K65 in response to R. solani and the possible underlying mechanisms, we investigated the influence of GhMAP3K65-overexpression on the transcript levels of 13 defence-related genes after 3 or 7 days of R. solani infection (Figures S6 and Figure 7). Under R. solani infection, GhMAP3K65-overexpression differentially influenced the transcription of examined genes. A subset of these genes showed higher transcript levels in GhMAP3K65-overexpressing leaves than in WT leaves. Furthermore, at a minimum of one of the two tested timepoints, these genes (NPR1, PR1c, PR3, PR4, LOX1, ACS6, APX, CAT, GST, SOD, H1N1, and HSR515) were differentially up-regulated by R. solani infection. A second set of genes, including EFE26 and CAT, was transcriptionally down-regulated by R. solani infection at a minimum of one of the two tested timepoints. These results indicated that GhMAP3K65-overexpression activates the transcription of defence-related genes, and GhMAP3K65 might therefore play a positive role in response to R. solani.
Figure 7.
Relative transcript levels of defence-related genes in WT and GhMAP3K65-overexpressing plants under R. solani infection. (a–d) Relative transcript levels of the SA-responsive genes NPR1, PR1c, PR3, and PR4; (e) Relative transcript levels of the JA-responsive gene LOX1; (f,g) Relative transcript levels of the ET biosynthesis-associated genes ACS6 and EFE26; (h–k) Relative transcript levels of the ROS detoxification-associated genes APX, CAT, GST, and SOD; (l–m) Relative transcript levels of the HR-marker genes H1N1 and HSR515. The experiments were repeated at least three times. Different letters above the columns indicate significant differences (p < 0.05) according to Duncan’s multiple range test.
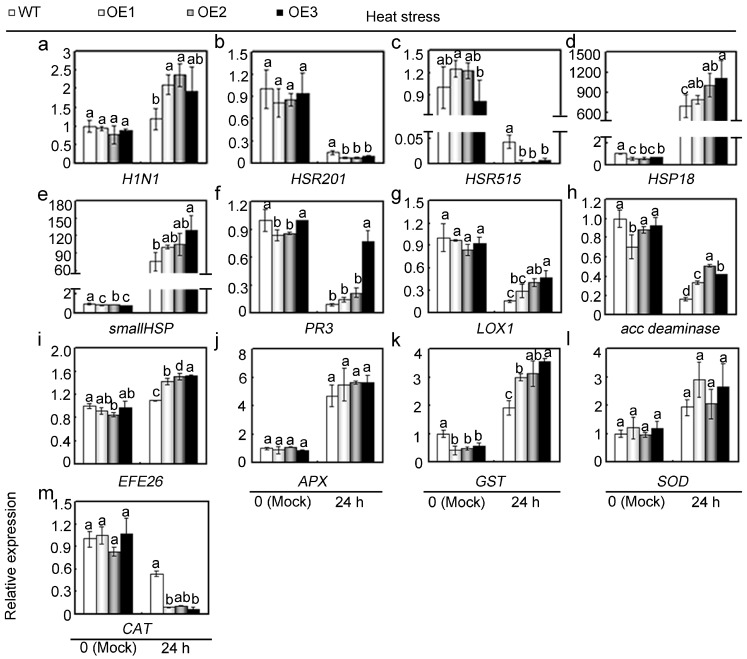
To further ascertain possible mechanisms underlying GhMAP3K65-mediated reduction of thermotolerance observed in GhMAP3K65-overexpressing plants, the transcript levels of 13 defence-related genes were examined in WT and GhMAP3K65-overexpressing plants via qRT-PCR after heat stress for 24 h (Figure 8). Under heat stress, GhMAP3K65-overexpression differentially affected the transcript levels of defence-related genes. Most of these genes showed enhanced transcription levels in GhMAP3K65-overexpressing plants compared with those in WT plants after heat stress. Up-regulated genes included the HR-related gene H1N1; the heat shock-related genes HSP18 and smallHSP; the SA-responsive gene PR3; the JA-responsive gene LOX1; the ET biosynthesis-related genes acc deaminase and EFE26; and the ROS detoxification-related genes APX, GST, and SOD. Downregulated transcription of HSR201, HSR515, and CAT was observed after heat stress, which was caused by GhMAP3K65-overexpression. These results indicated that GhMAP3K65-overexpression activates the transcription of defence-related genes, and GhMAP3K65 might therefore play a positive role in response to heat stress.
Figure 8.
Relative transcript levels of defence-related genes in WT and GhMAP3K65-overexpressing plants under heat stress treatment. (a–c) Relative transcript levels of the HR-marker genes H1N1, HSR201, and HSR515; (d,e) Relative transcript levels of the heat-shock genes HSP18 and smallHSP; (f) Relative transcript levels of the SA-responsive gene PR3; (g) Relative transcript levels of the JA-responsive gene LOX1; (h,i) Relative transcript levels of the ET biosynthesis-associated genes acc deaminase and EFE26; (j–m) Relative transcript levels of the ROS detoxification-associated genes APX, GST, SOD, and CAT. The experiments were repeated at least three times. Different letters above the columns indicate significant differences (p < 0.05) according to Duncan’s multiple range test.
2.7. Overexpression of GhMAP3K65 Impaired Ligin Biosynthesis and Stomatal Immunity in the Leaves of N. benthamiana
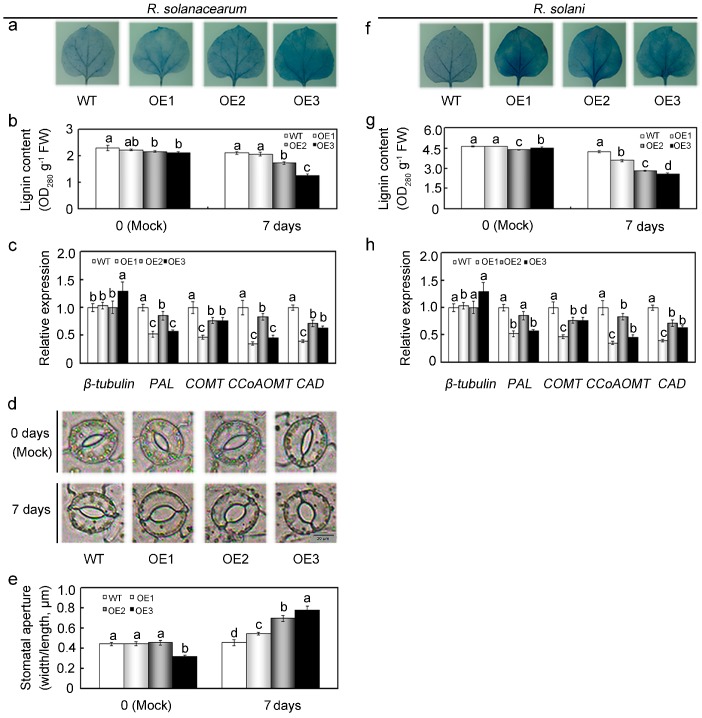
GhMAP3K65-overexpression enhanced susceptibility to pathogen infection and heat stress in transgenic N. benthamiana plants. Nevertheless, GhMAP3K65 activated the transcription of defence-associated genes, which demonstrated that GhMAP3K65 might play a positive role in the responses to pathogen infection and heat stress. Thus, we concluded that GhMAP3K65 might also participate in other biological processes, and our expression profiling results provided some clues in this regard. GhMAP3K65 can be induced significantly and continuously by GA3 (Figure 2g), and expression profiles of a gene often imply its biological function [25]. Phytohormones act as endogenous messengers and organize various signalling transduction pathways, thus allowing plants to respond to stresses [26,27,28], whereas GA plays a significant role in regulating growth and developmental processes in plants [29]. Therefore, we concluded that GhMAP3K65 plays a significant role in modulating plant growth and development. Uninjured leaves were exposed to suspensions of pathogen for 7 days, and cell death in infected leaves was observed via Trypan blue staining. Leaves of transgenic N. benthamiana plants were more deeply stained than those of WT plants after pathogen infection (Figure 9a,f), revealing that GhMAP3K65-overexpressing plants were more significantly infected by pathogen and less competent to withstand pathogen attack. Cell wall is an obstacle that protects cells from pathogen infection. To investigate the potential effects of GhMAP3K65 may have on cell wall traits, lignin content was analyzed from WT and GhMAP3K65-overexpressing plants. No obvious changes were observed in the lignin content of WT and GhMAP3K65-overexpressing lines under normal conditions, whereas the lignin content of GhMAP3K65-overexpressing lines was significantly decreased relative to that of WT plants after pathogen infection (Figure 9b,g). The transcript levels of β-tubulin, encoding tubulin, were not significantly different between WT and GhMAP3K65-overexpressing lines (Figure 9c,h). Lignin can strengthen defensive ability of cell wall, and several key enzymes involved in lignin biosynthesis, including PAL (phenylalanine ammonia-lyase), COMT (caffeic acid 3-O-methyltransferase-like), CCoAOMT (caffeoyl-CoA O-methyltransferase 2), and CAD (cinnamyl alcohol dehydrogenase 1), were therefore examined. The transcript levels of PAL, COMT, CCoAOMT, and CAD were lower in GhMAP3K65-overexpressing plants than in WT plants, indicating that GhMAP3K65-overexpression influences lignin biosynthesis (Figure 9c,h). Stomata have been shown to play an important role in plant defense as a part of the innate immune response [30]. Degree of stomatal opening in GhMAP3K65-overexpressing plants was not significantly different from that in WT plants under normal conditions. Nevertheless, extent of stomatal opening observed in GhMAP3K65-overexpressing plants was greater than that observed in WT plants after R. solanacearum infection (Figure 9d,e). These results indicated that GhMAP3K65-overexpression impaired ligin biosynthesis and stomatal immunity in the leaves of N. benthamiana.
Figure 9.
Ligin biosynthesis and stomatal immunity in the leaves of N. benthamiana impaired by GhMAP3K65-overexpression. (a,f) Leaf symptoms of WT and GhMAP3K65-overexpressing plants after infection with R. solanacearum and R. solani without leaf injuries; (b,g) Lignin content was analyzed from WT and GhMAP3K65-overexpressing lines under pathogen infection; (c,h) The expression levels of the β-tubulin, PAL, COMT, CCoAOMT, and CAD genes were examined in leaves via qRT-PCR; (d,e) Stomatal changes were observed using a microscope after R. solanacearum infection. The experiments were repeated at least three times. Different letters above the columns indicate significant differences (p < 0.05) according to Duncan’s multiple range test.
2.8. Overexpression of GhMAP3K65 Repressed Growth and Development of the Root System
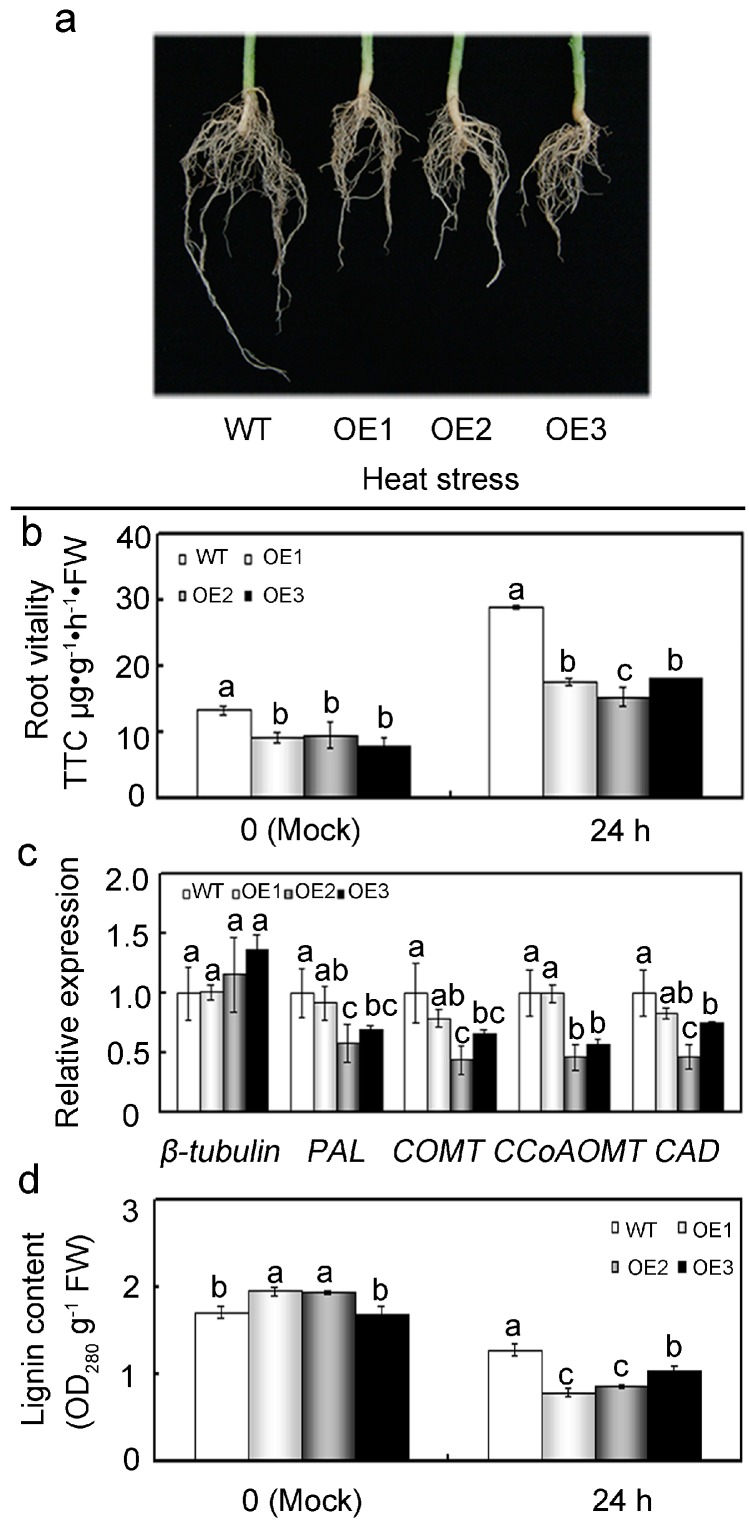
Many Raf-like MAP3Ks participate in growth and development of plant root system. Therefore, root phenotypes of WT and GhMAP3K65-overexpressing plants were monitored (Figure 10a). Root vitality of a plant reflects growth and development of its root system, providing a comprehensive index of root vitality. Root vitality directly affects absorption and utilization of mineral nutrients and water, which play decisive roles in growth and development of entire plant. Root vitality of GhMAP3K65-overexpressing lines was significantly decreased compared with that of WT plants after heat stress relative to mock control (Figure 10b). Several lignin biosynthesis genes were detected in roots, and the transcript levels of PAL, COMT, CCoCOMT, and CAD were found to be lower in GhMAP3K65-overexpressing lines than in WT plants after heat stress (Figure 10c). To investigate the potential effects of GhMAP3K65 may have on cell wall traits, lignin content was analyzed from WT and GhMAP3K65-overexpressing plants. No evident changes were observed in the lignin content of WT and GhMAP3K65-overexpressing lines under normal conditions, whereas lignin content of GhMAP3K65-overexpressing lines was significantly decreased relative to that of WT plants after heat stress (Figure 10d). These results demonstrated that GhMAP3K65-overexpression inhibits growth and development of the root system.
Figure 10.
Defects in roots caused by GhMAP3K65-overexpression. (a) Root system was photographed after being grown in soil for 8 weeks; (b) Root vitality of WT and GhMAP3K65-overexpressing lines; (c) The expression levels of β-tubulin, PAL, COMT, CCoAOMT, and CAD genes were examined in leaves via qRT-PCR; (d) Lignin content was analyzed from WT and GhMAP3K65-overexpressing lines under heat stress. The experiments were repeated at least three times. Different letters above the columns indicate significant differences (p < 0.05) according to Duncan’s multiple range test.
3. Discussion
Many studies have indicated that members of MAPK family participate in defensive reactions to environmental stresses. Expression of numerous MAPK genes is influenced by pathogen infection [31], high salinity [32], cold [33], drought, and heat stress [34]. While numerous MAP3Ks have been identified in various plant genomes, biological functions of only a few have been studied. In this study, multiple sequence alignment and evolutionary tree analyses confirmed that GhMAP3K65 is a Raf-like MAP3K gene. In addition, subcellular localization analysis of GhMAP3K65 suggested that the fusion protein localized in the cytoplasm and nucleus, indicating that GhMAP3K65 may function in both areas. Expression profiles of a gene often imply its biological function [25]. GhMAP3K65 transcription was induced by pathogen infection, heat and diverse signalling molecules in cotton, indicating that GhMAP3K65 may play a role in linking signalling pathways to plant defence responses.
Raf-like MAP3Ks play roles in response to biotic and abiotic stresses. This group of kinases includes GhMAP3K40 (mediates reduced tolerance to biotic and abiotic stresses) [12], DMS1 (mediates drought resistance) [35] and Raf43 (required for tolerance to multiple abiotic stresses) [2]. To broaden a previous analysis of GhMAP3K65, its roles in the responses to pathogen infection and heat stress were examined through loss- and gain-of-function experiments. Silencing of GhMAP3K65 enhanced resistance to pathogen infection and heat stress in cotton. In contrast, overexpression of GhMAP3K65 enhanced susceptibility to pathogen infection and heat stress in N. benthamiana.
Transcript accumulations of HR- and PR-associated genes are representative of plant defence responses and act as markers of pathogen infection. In tobacco plants, H1N1, HSR201, and HSR515 are generally up-regulated in HR provoked after pathogen infection [36]. Because GhMAP3K65-overexpression increased transcript levels of H1N1, HSR515, PR1c, PR3, and PR4, it was expected to play a positive role in response to pathogen infection; however, GhMAP3K65-overexpressing plants instead suffered more severe harm. Plants have been reported to respond to pathogen infection with a burst of ROS. Under biotic stress, ROS act as signalling molecules to activate pathogenesis-related proteins and systemic acquired resistance in cells adjacent to the infection site to prevent further pathogen spread [37,38]. Low levels of ROS serve as signalling molecules that play important roles in plant immunity. However, ROS can also induce high levels of cell death [39]. The accumulation of ROS during plant infections by pathogens has been implicated in susceptible responses against these pathogens [25,40], and H2O2 is a typical ROS. This study demonstrated that GhMAP3K65-overexpressing plants accumulated more H2O2 than WT plants after pathogen infection. However, the transcript levels of ROI-detoxifying enzymes, such as APX, GST, and SOD, were either constitutively higher in overexpressing plants or induced after pathogen infection, indicating that GhMAP3K65 activated the antioxidant system. The results were similar to those achieved using defense-related gene assays—the overexpressing plants were expected to be more resistant to pathogen infection, but they instead suffered more severe damage.
GhMAP3K65-overexpression increased susceptibility of N. benthamiana plants to heat stress. A specific relationship between accumulation of heat shock proteins (HSPs), particularly small HSPs, and thermotolerance was demonstrated previously [41]. We observed that GhMAP3K65-overexpression enhanced the transcription of HSP18 and smallHSP after heat stress compared with that in WT plants. In addition, GhMAP3K65-overexpression was observed to increase the transcript levels of APX, GST, and SOD significantly. Induction of ROS, such as APX, GST, and SOD, reportedly plays a role in thermotolerance [42,43,44]. GhMAP3K65-overexpression enhanced the transcription of heat resistance-related genes, such as HSP18 and smallHSP, and ROI-detoxifying enzymes such as APX, GST, and SOD, which should have demonstrated the positive role of GhMAP3K65 in response to heat stress. However, GhMAP3K65-overexpressing plants suffered more serious damage than WT plants after heat stress.
GhMAP3K65-overexpression enhanced N. benthamiana susceptibility to pathogen infection and reduced its tolerance to heat stress, which was not consistent with increased the transcript levels of defence-related and HSP genes. Similarly, many studies have shown that a single MAP3K may play a role in response to diverse environmental stresses and function in several seemingly different signalling pathways. For instance, GhMAP3K40 functions in response to both biotic and abiotic stresses (e.g., drought, R. solanacearum and R. solani), playing a negative role in response to drought, R. solanacearum and R. solani [12]. Additionally, Raf43 is involved in the regulation of innate immunity in Arabidopsis [2]. Therefore, GhMAP3K65 may play a significant role in response to pathogen infection and heat stress.
While research regarding disparate triggering phenomena and their molecular bases has received increasing interest [45,46]. However, the underlying molecular mechanisms triggered by plant stress remain widely unknown [47]. GhMAP3K65 may play a role in crosstalk between pathogen infection and heat stress signalling pathways mediated by SA, JA, and ET. It is often observed that pathogen infection tends to be more severe under the environmental challenges of higher temperature and humidity. We observed that the transcription of HSR201, HSR515, and CAT is inhibited in the response to heat stress compared with that in WT plants, demonstrating a negative influence of heat stress on plant defence responses. However, GhMAP3K65-overexpression increased the transcript levels of the SA-responsive gene PR3; the JA-responsive gene LOX1; the ET production-associated genes acc deaminase; and EFE26; and the ROS detoxification-associated genes APX, GST, and SOD in response to heat stress compared with their levels in WT plants. Therefore, GhMAP3K65-overexpression appears to reverse the negative influences of high temperature and humidity in the response to pathogen infection.
Previous studies have demonstrated abundant crosstalk between pathogen infection and heat stress signalling pathways [28,48]. R-genes, such as cf-4 and Cf-9 in tomato have been reported to be involved in such crosstalk [49]. In this study, the transcript levels of HSP18 and smallHSP were significantly elevated by GhMAP3K65-overexpression in the response to heat stress, providing strong evidence that heat-induced HSPs play roles in crosstalk between pathogen infection and heat stress mediated by GhMAP3K65.
The expression levels of hormones SA, JA, and ET are constantly elevated in the response to pathogen infection. The balance of these hormones relies on discriminating the pathogen and plays a crucial role in the fine mediation of appropriate plant defence responses [1]. SA mediates fundamental defence responses to biotrophic pathogen infection, while JA generally controls significant defence responses to necrotrophs [50]. The hormones SA, JA, and ET have been demonstrated to induce different PR genes in plants and work either antagonistically or synergistically in defence signalling pathways depending on their concentration [51,52]. In addition to their participation in disease defence signalling pathways, SA, JA, and ET have been implicated in thermotolerance [53,54]. We demonstrated that GhMAP3K65 transcription was induced by exogenous application of the plant hormones SA (2 mM), JA (100 μM), and ET (5 mM) and that GhMAP3K65-overexpression increased the transcript levels of the SA-associated gene PR3, the JA-responsive gene LOX1, and the ET production-related genes acc deaminase and EFE26 in response to heat stress. Hence, we conclude that SA, JA, and ET modulate the expression of GhMAP3K65, influencing the expression of downstream defence- and thermotolerance-associated genes.
GhMAP3K65-overexpressing plants showed enhanced susceptibility to pathogen infection and heat stress. Nevertheless, GhMAP3K65 was demonstrated to play a positive role in plant defence responses. Additionally, GhMAP3K65 transcription was induced by gibberellin. These results promoted evaluation of the putative role of GhMAP3K65 in modulating plant growth and development. After pathogen infection, uninjured leaves of GhMAP3K65-overexpressing plants exhibited more severe cell death than those of WT plants. These results indicated that GhMAP3K65-overexpressing plants are less competent to withstand pathogen attack, which may result from impaired ligin biosynthesis and stomatal immunity in the leaves of N. benthamiana. Lignin confers rigidity to cell walls and is therefore associated with tolerance to biotic and abiotic stresses and the mechanical stability of plants [55]. The cell wall constitutes the first line of plants defence against pathogens, such as bacteria and fungi [54,56]. Lignin solidifies the cell wall, providing a nondegradable barrier to pathogens and is therefore thought to enhance protection against such biotic stresses [57]. An increase in lignification is often observed in response to pathogen attack [57]. Decreased lignin content has been exhibited to hinder plant growth and development [58,59]. However, GhMAP3K65-overexpression decreased lignin content in N. benthamiana plants compared with that in WT plants. Hence, several key enzymes involved in lignin biosynthesis were examined. However, transcription of these lignin biosynthesis genes was suppressed in GhMAP3K65-overexpressing plants, which indicated that GhMAP3K65-overexpression influences lignin biosynthesis. Stomata have traditionally been assumed to merely be passive ports for pathogen entry. However, stomata are now clearly viewed as an integral part of the plant immune system, and regulation of their aperture prevents pathogen entry via leaves [60]. Bacterial pathogens can successfully manipulate stomatal immunity to promote host infection [30]. R. solanacearum enhanced stomatal opening in GhMAP3K65-overexpressing plants compared with that in WT plants. These results showed that GhMAP3K65-overexpression decreased lignin content and lignin biosynthesis, and increased stomatal opening, therefore impairing ligin biosynthesis and stomatal immunity in the leaves of N. benthamiana, which enhanced susceptibility to pathogen infection. In addition, root phenotype and root vitality were significantly different in the WT and GhMAP3K65-overexpressing lines, providing an explanation for the enhanced susceptibility of transgenic N. benthamiana plants to heat stress. Numerous studies have indicated that roots are more sensitive to heat stress than other plant tissues, suggesting that high soil temperature is more detrimental to whole-plant growth than high air temperature [61]. The expression levels of cell wall-associated genes can be altered in response to heat stress treatment. A transcriptomic study carried out in Chinese cabbage (Brassica rapa L.) showed that several cell wall-associated genes could play roles in the acquisition of thermotolerance [62]. Heat stress can mediate changes in secondary cell wall metabolism. Lignin biosynthesis can be altered during the application of elevated temperature. Thus, the expression levels of some key enzymes involved in lignin biosynthesis were examined. Transcription of these lignin biosynthesis genes was inhibited in GhMAP3K65-overexpressing plants, which indicated that GhMAP3K65-overexpression influences lignin biosynthesis. Moreover, GhMAP3K65-overexpression decreased lignin content in N. benthamiana plants compared with that in WT plants. Overall, GhMAP3K65 negatively modulates plant growth and development and therefore results in enhanced susceptibility to pathogen infection and heat stress.
In conclusion, GhMAP3K65 plays roles in the responses to pathogen infection and heat stress mediated by SA, ET, and JA. Although complex regulatory mechanisms involving Raf-like MAP3K proteins in cotton remain unclear, this work provides further insight into the regulatory mechanisms of a Raf-like MAP3K65 protein.
4. Materials and Methods
4.1. Plant Growth and Stress Treatments
Cotton (Gossypium hirsutum L. cv. lumian 22) seeds were germinated in moist gauze, and seedlings were transferred to hydroponic culture for growth in a light chamber at 26 ± 1 °C, with a 16 h light/8 h dark cycle and relative humidity of 60–75%. Seven-day-old cotton seedlings were subjected to diverse treatments described below. For pathogen infection, seedlings were inoculated with suspensions of bacterial pathogen R. solanacearum (OD600 = 0.6–0.8) and conidial suspensions of fungal pathogen R. solani (105 conidia/mL) using root-dipping method. For heat stress, seedlings were exposed to heat stress in a 42 °C incubator for 24 h at 100% humidity and darkness. For signalling molecules, cotton leaves were sprayed with 2 mM SA, 100 µM MeJA, 5 mM ET and 500 µM GA3. Regarding the sample collection time points, 8 o’clock a.m. correspond to 0 h, 9 o’clock a.m. correspond to 1 h, etc. Samples collected at appropriate times were snap-frozen in liquid nitrogen and then stored at −80 °C for subsequent analyses. N. benthamiana seedlings at three- or four-leaf stages were transplanted into pots with wet soil and maintained under glasshouse conditions at 25 °C with a 16 h light/8 h dark photoperiod. Uniformly grown seedlings were used in subsequent studies.
4.2. Isolation of GhMAP3K65 Gene, Vector Construction, and Genetic Transformation
GhMAP3K65 was obtained as described previously [63]. Vector construction and genetic transformation were carried out according to previously described methods [32]. T2 progenies of WT and GhMAP3K65-overexpressing plants were subjected to subsequent functional analyses.
4.3. Subcellular Localization of GhMAP3K65
GhMAP3K65 ORF without stop codon was fused to 5′-terminal end of GFP to construct pROKII-GhMAP3K65-GFP, which was regulated by CaMV35S promoter. Positive control pROKII-GFP construct and pROKII-GhMAP3K65-GFP construct were transformed into Agrobacterium tumefaciens strain GV3101, respectively. Agrobacterium cells collected via centrifugation were resuspended in infiltration buffer (for 100 mL: 1 mL of 1 M MES, pH 5.6; 200 μL of 0.1 M acetosyringone (AS); and 100 μL of 1 M MgCl2) and adjusted to a final OD600 of 1.0. After cultivation for 3 h, Agrobacterium mixture was inoculated into N. benthamiana leaves with 1 mL sterile syringe. Lower epidermis cells were observed using two-photon laser confocal microscope.
4.4. RNA Extraction and Quantitative PCR
Total RNA was obtained from samples using improved cetyltrimethylammonium bromide (CTAB) method [64] and TRIzol reagent (TaKaRa, Dalian, China) and then used for first-strand cDNA synthesis with EasyScript First-strand cDNA Synthetic SuperMix (TransGen Biotech, Beijing, China). To examine GhMAP3K65 transcript levels under different treatments, qRT-PCR was carried out with SYBR PrimeScriptTM RT-PCR Kit (TaKaRa) in 20 µL reaction volume, using CFX96TM Real-time System (Bio-Rad, Hercules, CA, USA) following experimental procedures described previously by Yu et al. [65]. G. hirsutum ubiquitin (UBI) and N. benthamiana β-actin were employed as control genes. The primer pairs used for qRT-PCR are listed in Table S1.
4.5. Virus-Induced Gene Silencing (VIGS) of GhMAP3K65 in Cotton
To silence GhMAP3K65 in cotton, Tobacco Rattle Virus (TRV)-based VIGS system was employed. A fragment of GhMAP3K65 transcribed region was inserted into multiple cloning site of pTRV-RNA2 plasmid to generate pTRV2-GhMAP3K65 constructs. The pTRV1, pTRV2, and pTRV2-GhMAP3K65 constructs were then transformed into Agrobacterium tumefaciens strain GV3101, respectively. Agrobacterium carrying pTRV2 or pTRV2-GhMAP3K65 construct was mixed with pTRV1 strain (1:1 ratio, OD600 = 1.0) and co-infiltrated into cotton cotyledons using 1 mL sterile syringe [20]. VIGS efficiency was assessed via qRT-PCR, and silenced cotton plants were transplanted into soil mixture for subsequent functional analyses. CK and VIGS cotton plants were inoculated with pathogen infection for 7 days using root-dipping method. CK and VIGS cotton plants were exposed to heat stress in a 42 °C incubator for 24 h at 100% humidity and darkness.
4.6. Pathogen Infection
R. solanacearum was incubated overnight at 37 °C in Luria–Bertani (LB) broth in shaker, then collected via centrifugation and resuspended in sterile tap water. R. solani was cultured on potato dextrose agar (PDA) medium in incubator at 28 °C for 2 weeks, after which its spores were suspended in 1% glucose. For pathogen infection, detached leaves of 2-month-old WT and GhMAP3K65-overexpressing plants were inoculated with suspensions of R. solanacearum (OD600 = 0.6–0.8) and conidial suspensions of R. solani (105 conidia/mL) using needleless syringe. Detached leaves from untreated WT and transgenic N. benthamiana plants were used as mock controls. Inoculated leaves were maintained in a glass culture dish and lesions could be observed 7 days after inoculation. Chlorophyll content was determined by described previously [66]. The colony forming units (cfu) of R. solanacearum were determined by described previously [67]. The average number of spores per cotyledon of R. solani was determined by described previously [68].
4.7. Heat Stress Treatment
To evaluate impact of GhMAP3K65 on thermotolerance and to guarantee that necrosis resulting from heat stress was due to increased temperature and not photo-oxidative stress, WT and GhMAP3K65-overexpressing N. benthamiana plants were exposed to heat stress in a 42 °C incubator for 24 h at 100% humidity and darkness [20].
4.8. Histochemical Staining
For DAB staining, N. benthamiana leaves were incubated in DAB solution (1 mg/mL, pH 3.8) for 12 h at 25 °C in the dark. After DAB staining, N. benthamiana leaves were boiled for 10 min to remove chlorophyll and then soaked in 95% ethanol. The Trypan blue staining solution comprised 40 mL of absolute ethanol, 0.01 g of Trypan blue, 5 mL of lactic acid, 5 mL of glycerol, 5 g of phenol and 5 mL of double-distilled water. N. benthamiana leaves were soaked in Trypan blue solution, boiled for 5 min, and then incubated in Trypan blue staining solution for 12 h. In addition, N. benthamiana leaves were soaked in 95% ethanol to remove chlorophyll.
4.9. Structural Defects in Leaves
To explore whether there are structural defects in leaves, uninjured leaves from WT and transgenic N. benthamiana plants were selected and solutions of pathogen were smeared on both sides of the leaves. One week later, the leaves were cut and cell death was detected by Trypan blue staining.
4.10. Lignin Content
Leaves (0.1 g fresh weight (FW)) were triturated using liquid nitrogen, added to a 14-mL centrifuge tube and soaked in 12 mL of 95% ethanol to remove chlorophyll. The supernatant was discarded after centrifugation at 5000 rcf for 10 min. A solution comprising N-hexane:ethanol (2:1, 12 mL) was added to the precipitant, which was then centrifuged at 5000 rcf for 10 min. The precipitant was dried, treated with 2.5 mL of 25% acetyl bromide, incubated at 70 °C for 30 min, and cooled quickly in cold water. Next, 0.9 mL of 2 mol/L NaOH was added to the above reaction solution to stop the reaction, and 0.1 mL of 7.5 mol/L hydrochloric acid hydroxylamine and 4 mL of glacial acetic acid were added before centrifugation at 5000 rcf for 5 min. Next, 3.9 mL of glacial acetic acid was added to 0.1 mL of the supernatant, and the absorbance at 280 nm per gram of fresh sample represented the lignin content. For determining the lignin content of the root system, the same steps used for leaves were taken, except the chlorophyll was removed.
4.11. Determination of Root Vitality by Triphenyl Tetrazolium Chloride (TTC) Method
Root vitality was determined using a root TTC reduction method in tissues following treatment with a red-coloured insoluble complex [69,70]. A TTC solution (0.25 mL of 0.4%) and a small Na2S crystal were added to a 10-mL volumetric flask, which was shaken immediately after the addition of red triphenylformazan (TF) and 95% ethanol. Then, TF (0.25, 0.5, 1.00, 2.00 and 4.00 mL) and 95% ethanol were added to the 10-mL volumetric flask. A blank tube (0 mL of TF) served as the control, and a standard curve was formed. TTC reduction was examined using an improved method described by Zhang et al. [70]. Root segments (0.5 g fresh weight (FW)) were incubated for 6 h in the dark at 37 °C with a mixture comprising 10 mL of 0.4% TTC and 10 mL of tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl, pH 8.4). Next, 2 mL of 1 mol/L H2SO4 was added to the above reaction solution (except the control) to stop the reaction (control: root segments were incubated with 2 mL of 1 mol/L H2SO4, while the other operations were the same). Root segments were subsequently recovered on filter paper and washed with double-distilled water. Water-insoluble red TF was extracted from the root segments using 6 mL of 95% ethanol for 20 min. Absorbances of the extracts were measured at 485 nm to determine root vitality according to the standard curve (i.e., root vitality: TTC μg·g−1·h−1·FW).
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant Number 31471424).
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/18/11/2462/s1.
Author Contributions
Na Zhai and Haihong Jia conceived and designed research. Na Zhai and Dongdong Liu conducted experiments; Shuchang Liu contributed analytical tools; Na Zhai, Haihong Jia, and Manli Ma analyzed data; Xingqi Guo and Han Li guided research; Na Zhai wrote the manuscript. All authors read and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dangl J.L., Jones J.D. Plant pathogens and integrated defense responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 2.Virk N., Li D., Tian L., Huang L., Hong Y., Li X., Zhang Y., Liu B., Zhang H., Song F. Arabidopsis Raf-like mitogen-activated protein kinase kinase kinase gene Raf43 is required for tolerance to multiple abiotic stresses. PLoS ONE. 2015;10:eo133975. doi: 10.1371/journal.pone.0133975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishihama R., Banno H., Shibata W., Hirano K., Nakashima M., Usami S., Machida Y. Plant homologues of components of MAPK (mitogen-activated protein kinase) signal pathways in yeast and animal cells. Plant Cell Physiol. 1995;36:749–757. doi: 10.1093/oxfordjournals.pcp.a078818. [DOI] [PubMed] [Google Scholar]
- 4.Mishra N.S., Tuteja R., Tuteja N. Signalling through MAP kinase networks in plants. Arch. Biochem. Biophys. 2006;452:55–68. doi: 10.1016/j.abb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Opdenakker K., Remans T., Vangronsveld J., Cuypers A. Mitogen-activated protein (MAP) kinases in plant metal stress: Regulation and responses in comparison to other biotic and abiotic stresses. Int. J. Mol. Sci. 2012;13:7828–7853. doi: 10.3390/ijms13067828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin Z., Wang J., Wang D., Fan W., Wang S., Ye W. The MAPKKK gene family in Gossypium raimondii: Genome-wide identification, classification and expression analysis. Int. J. Mol. Sci. 2013;14:18740–18757. doi: 10.3390/ijms140918740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taj G., Agarwal P., Grant M., Kumar A. MAPK machinery in plants: Recognition and response to different stresses through multiple signal transduction pathways. Plant Signal. Behav. 2010;5:1370–1378. doi: 10.4161/psb.5.11.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J.A., Agrawal G.K., Rakwal R., Han K.S., Kim K.N., Yun C.H., Heu S., Park S.Y., Lee Y.H., Jwa N.S. Molecular cloning and mRNA expression analysis of novel rice (Oryzasativa L.) MAPK kinase kinase, OsEDR1, an ortholog of Arabidopsis AtEDR1, reveal its role in defense/stress signalling pathways and development. Biochem. Biophys. Res. Commun. 2003;300:868–876. doi: 10.1016/S0006-291X(02)02944-3. [DOI] [PubMed] [Google Scholar]
- 9.Lin Z., Alexander L., Hackett R., Grierson D. LeCTR2, a CTR1-like protein kinase from tomato, plays a role in ethylene signalling, development and defense. Plant J. 2008;54:1083–1093. doi: 10.1111/j.1365-313X.2008.03481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasayama D., Matsuoka D., Oka M., Shitamichi N., Furuya T., Azuma T., Itoh K., Nanmori T. MAP3Kδ4, an Arabidopsis Raf-like MAP3K, regulates plant growth and shoot branching. Plant Biotechnol. 2011;28:463–470. doi: 10.5511/plantbiotechnology.11.1027a. [DOI] [Google Scholar]
- 11.Shitamichi N., Matsuoka D., Furuya T., Nanmori T. Overexpression of MAP3Kδ4, an ABA-inducible Raf-like MAP3K that confers salt tolerance in Arabidopsis. Plant Biotechnol. 2013;30:111–118. doi: 10.5511/plantbiotechnology.13.0108a. [DOI] [Google Scholar]
- 12.Chen X., Wang J., Zhu M., Jia H., Liu D., Hao L., Guo X. A cotton Raf-like MAP3K gene, GhMAP3K40, mediates reduced tolerance to biotic and abiotic stress in Nicotiana Benthamiana by negatively regulating growth and development. Plant Sci. 2015;240:10–24. doi: 10.1016/j.plantsci.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Rizhsky L., Liang H., Mittler R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 2002;130:1143–1151. doi: 10.1104/pp.006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noutoshi Y., Ito T., Seki M., Nakashita H., Yoshida S., Marco Y., Shirasu K., Shinozaki K. A signal amino acid insertion in the WRKY domain of the Arabidopsis TIR-NBS-LRR-WRKY-type disease resistance protein SLH1 (sensitive to low humidity 1) causes activation of defense responses and hypersensitive cell death. Plant J. 2005;43:873–888. doi: 10.1111/j.1365-313X.2005.02500.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou F., Menke F.L., Yoshioka K., Moder W., Shirano Y., Klessig D.F. High humidity suppresses ssi4-mediated cell death and disease resistance upstream of MAP kinase activation, H2O2 production and defense gene expression. Plant J. 2004;39:920–932. doi: 10.1111/j.1365-313X.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 16.Loake G., Grant M. Salicylic acid in plant defense—The players and protagonists. Curr. Opin. Plant Biol. 2007;10:466–472. doi: 10.1016/j.pbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzo O., Solano R. Molecular players regulating the jasmonate signalling network. Curr. Opin. Plant Biol. 2005;8:532–540. doi: 10.1016/j.pbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Broekaert W.F., Delaure S.L., De Bolle M.F., Cammue B.P. The role of ethylene in host-pathogen interactions. Annu. Rev. Phytopathol. 2016;44:393–416. doi: 10.1146/annurev.phyto.44.070505.143440. [DOI] [PubMed] [Google Scholar]
- 19.Clarke S.M., Cristescu S.M., Miersch O., Harren F.J., Wasternack C., Mur L.A. Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol. 2009;182:175–187. doi: 10.1111/j.1469-8137.2008.02735.x. [DOI] [PubMed] [Google Scholar]
- 20.Dang F.F., Wang Y.N., Yu L., Eulgem T., Lai Y., Liu Z.Q., Wang X., Qiu A.L., Zhang T.X., Lin J., et al. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant Cell Environ. 2013;36:757–774. doi: 10.1111/pce.12011. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez M.E., Pennell R.I., Meijer P.J., Ishikawa A., Dixon R.A., Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–784. doi: 10.1016/S0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- 22.Yoshioka H., Numata N., Nakajima K., Katou S., Kawakita K., Rowland O., Jones J.D., Doke N. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell. 2003;15:706–718. doi: 10.1105/tpc.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen N., Goodwin P.H., Hsiang T. The role of ethylene during the infection of Nicotiana tabacum by Colletotrichum destructivum. J. Exp. Bot. 2003;54:2449–2456. doi: 10.1093/jxb/erg289. [DOI] [PubMed] [Google Scholar]
- 24.Sohn S.I., Kim Y.H., Kim B.R., Lee S.Y., Lim C.K., Hur J.H., Lee J.Y. Transgenic tobacco expressing the hrpNEP gene from Erwinia pyrifoliae triggers defense responses against Botrytis cinerea. Mol. Cells. 2007;24:232–239. [PubMed] [Google Scholar]
- 25.Yan Y., Jia H., Wang F., Wang C., Liu S., Guo X. Overexpression of GhWRKY27a reduces tolerance to drought stress and resistance to Rhizoctonia solani infection in transgenic Nicotiana benthamiana. Front. Physiol. 2015;6:265. doi: 10.3389/fphys.2015.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolters H., Jürgens G. Survival of the flexible: Hormonal growth control and adaptation in plant development. Nat. Rev. Genet. 2009;10:305–317. doi: 10.1038/nrg2558. [DOI] [PubMed] [Google Scholar]
- 27.Huang D., Wu W., Abrams S.R., Cutler A.J. The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J. Exp. Bot. 2008;59:2991–3007. doi: 10.1093/jxb/ern155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita M., Fujita Y., Noutoshi Y., Takahashi F., Narusaka Y., Yamaguchi-Shinozaki K., Shinozaki K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signalling networks. Curr. Opin. Plant Biol. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Singh D.P., Jermakow A.M., Swain S.M. Gibberellins are required for seed development and pollen tube growth in Arabidopsis. Plant Cell. 2002;14:3133–3147. doi: 10.1105/tpc.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnaud D., Hwang I. A sophisticated network of signalling pathways regulates stomatal defenses to bacterial pathogens. Mol. Plant. 2014;129 doi: 10.1093/mp/ssu129. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Zhang L., Lu W., Wang X., Wu C.A., Guo X. Overexpression of cotton GhMKK4 enhances disease susceptibility and affects abscisic acid, gibberellin and hydrogen peroxide signalling in transgenic Nicotiana benthmiana. Mol. Plant Pathol. 2014;15:94–108. doi: 10.1111/mpp.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L., Li Y., Lu W., Meng F., Wu C.A., Guo X. Cotton GhMKK5 affects disease resistance, induces HR-like cell death, and reduces the tolerance to salt and drought stress in transgenic Nicotiana benthamiana. J. Exp. Bot. 2012;63:3935–3951. doi: 10.1093/jxb/ers086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuya T., Matsuoka D., Nanmori T. Membrane rigidification functions upstream of the MEKK-MEKK2-MEKK4 cascade during cold acclimation in Arabidopsis thaliana. FEBS Lett. 2014;588:2025–2030. doi: 10.1016/j.febslet.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 34.Wu L., Zu X., Zhang H., Wu L., Xi Z., Chen Y. Overexpression of ZmMAPK1 enhances drought and heat stress in transgenic Arabidopsis thaliana. Plant Mol. Biol. 2015;88:429–443. doi: 10.1007/s11103-015-0333-y. [DOI] [PubMed] [Google Scholar]
- 35.Ning J., Li X., Hicks L.M., Xiong L. A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 2010;152:876–890. doi: 10.1104/pp.109.149856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czernic P., Huang H.C., Marco Y. Characterization of hsr201 and hsr515, two tobacco genes preferentially expressed during the hypersensitive reaction provoked by phytopathogenic bacteria. Plant Mol. Biol. 1996;31:255–265. doi: 10.1007/BF00021788. [DOI] [PubMed] [Google Scholar]
- 37.Draper J. Salicylate, superoxide synthesis and cell suicide in plant defence. Trends Plant Sci. 1997;2:162–165. doi: 10.1016/S1360-1385(97)01030-3. [DOI] [Google Scholar]
- 38.About-Attia M.A., Wang X., Nashaat Al-Attala M., Xu Q., Zhan G., Kang Z. TaMDAR6 acts as a negative regulator of plant cell death and participates indirectly in stomatal regulation during the wheat stripe rust-fungus interaction. Physiol. Plant. 2016;156:262–277. doi: 10.1111/ppl.12355. [DOI] [PubMed] [Google Scholar]
- 39.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012:2–16. doi: 10.1155/2012/217037. [DOI] [Google Scholar]
- 40.Govrin E.M., Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 2000;10:751–757. doi: 10.1016/S0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- 41.Lujan R., Lledias F., Martinez L.M., Barreto R., Cassab G.I., Nieto-Sotelo J. Small heat-shock proteins and leaf cooling capacity account for the unusual heat tolerance of the central spike leaves in Agave tequilana var. Weber. Plant Cell Environ. 2009;32:1791–1803. doi: 10.1111/j.1365-3040.2009.02035.x. [DOI] [PubMed] [Google Scholar]
- 42.Locato V., Gadaleta C., De Gara L., De Pinto M.C. Production of reactive species and modulation of antioxidant network in response to heat shock: A critical balance for cell fate. Plant Cell Environ. 2008;31:1606–1619. doi: 10.1111/j.1365-3040.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhao F.Y., Liu W., Zhang S.Y. Different responses of plant growth and antioxidant system to the combination of cadmium and heat stress in transgenic and non-transgenic rice. J. Integr. Plant Biol. 2009;51:942–950. doi: 10.1111/j.1744-7909.2009.00865.x. [DOI] [PubMed] [Google Scholar]
- 44.Khanna-Chopra R., Sabarinath S. Heat-stable chloroplastic Cu/Zn superoxide dismutase in Chenopodium murale. Biochem. Biophys. Res. Commun. 2004;320:1187–1192. doi: 10.1016/j.bbrc.2004.06.071. [DOI] [PubMed] [Google Scholar]
- 45.Jaskiewicz M., Conrath U., Peterhansel C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress. EMBO Rep. 2011;12:50–55. doi: 10.1038/embor.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sani E., Herzyk P., Perrella G., Colot V., Amtmann A. Hyperosmotic priming of Arabidopsis seedlings establishes a long-term somatic memory accompanied by specific changes of the epigenome. Genome Biol. 2013;14:R59. doi: 10.1186/gb-2013-14-6-r59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bäurle I. Plant Heat Adaptation: Priming in response to heat stress. F1000Research. 2016;5 doi: 10.12688/f1000research.7526.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narusaka Y., Narusaka M., Seki M., Umezawa T., Ishida J., Nakajima M., Enju A., Shinozaki K. Crosstalk in the responses to abiotic and biotic stresses in Arabidopsis: Analysis of gene expression in cytochrome P450 gene superfamily by cDNA microarray. Plant Mol. Biol. 2004;55:327–342. doi: 10.1007/s11103-004-0685-1. [DOI] [PubMed] [Google Scholar]
- 49.Wang C., Cai X., Zheng Z. High humidity represses cf-4/Avr4- and cf-9/AVR9-dependent hypersensitive cell death and defense gene expression. Planta. 2005;222:947–956. doi: 10.1007/s00425-005-0036-8. [DOI] [PubMed] [Google Scholar]
- 50.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 51.Devadas S.K., Enyedi A., Raina R. The Arabidopsis hrl1 mutation reveals novel overlapping roles for salicylic acid, jasmonic acid and ethylene signalling in cell death and defence against pathogens. Plant J. 2002;30:467–480. doi: 10.1046/j.1365-313X.2002.01300.x. [DOI] [PubMed] [Google Scholar]
- 52.Mur L.A., Kenton P., Atzorn R., Miersch O., Wasternack C. The outcomes of concentration-specific interactions between salicylate and jasmonate signalling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 2006;140:249–262. doi: 10.1104/pp.105.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schramm F., Larkindale J., Kiehlmann E., Ganguli A., Englich G., Vierling E., von Koskull-Doring P. A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J. 2008;53:264–274. doi: 10.1111/j.1365-313X.2007.03334.x. [DOI] [PubMed] [Google Scholar]
- 54.Collinge D.B. Cell wall appositions: The first line of defense. J. Exp. Bot. 2009;60:351–352. doi: 10.1093/jxb/erp001. [DOI] [PubMed] [Google Scholar]
- 55.Frei M. Lignin: Characterization of a multifaceted crop component. Sci. World J. 2013;2013:436517. doi: 10.1155/2013/436517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vance C.P., Kirk T.K., Sherwood R.T. Ligniication as a mechanism of disease resistance. Annu. Rev. Phytopathol. 1980;18:259–288. doi: 10.1146/annurev.py.18.090180.001355. [DOI] [Google Scholar]
- 57.Moura J.C., Bonine C.A., de Oliveira Fernandes Viana J., Dornelas M.C., Mazzafera P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integer. Plant Biol. 2010;52:360–376. doi: 10.1111/j.1744-7909.2010.00892.x. [DOI] [PubMed] [Google Scholar]
- 58.Derikvand M.M., Sierra J.B., Ruel K., Pollet B., Do C.T., Thévenin J., Buffard D., Jouanin L., Lapierre C. Redirection of the phenylpropanoid pathway to feruloyl malate in Arabidopsis mutants deficient for cinnamoyl-CoA reductase 1. Planta. 2008;227:943–956. doi: 10.1007/s00425-007-0669-x. [DOI] [PubMed] [Google Scholar]
- 59.Do C.T., Pollet B., Thévenin J., Sibout R., Denoue D., Barrière Y., Lapierre C., Jouanin L. Both caffeoyl Coenzyme A 3-O-methyltransferase 1 and caffeic acid O-methyltransferase 1 are involved in redundant functions for lignin, flavonoids and sinapoyl malate biosynthesis in Arabidopsis. Planta. 2007;226:1117–1129. doi: 10.1007/s00425-007-0558-3. [DOI] [PubMed] [Google Scholar]
- 60.Gmenez-lbanez S., Boter M., Ortigosa A., García-Casado G., Chini A., Lewsey M.G., Ecker J.R., Ntoukakis V., Solano R. JAZ2 controls stomata dynamics during bacterial invasion. New Phytol. 2017;213:1378–1392. doi: 10.1111/nph.14354. [DOI] [PubMed] [Google Scholar]
- 61.Liu X., Huang B. Root physiological factors involved in cool-season grass response to high soil temperature. Environ. Exp. Bot. 2005;53:233–245. doi: 10.1016/j.envexpbot.2004.03.016. [DOI] [Google Scholar]
- 62.Yang J.Y., Sun Y., Sun A.Q., Yi S.Y., Qin J., Li M.H., Liu J. The involvement of chloroplast HSP100/ClpB in the acquired thermotolerance in tomato. Plant Mol. Biol. 2006;62:385–395. doi: 10.1007/s11103-006-9027-9. [DOI] [PubMed] [Google Scholar]
- 63.Shi J., Zhang L., An H., Wu C., Guo X. GhMPK16, a novel stress-responsive group D MAPK gene from cotton, is involved in disease resistance and drought sensitivity. BMC Mol. Biol. 2011;12:22. doi: 10.1186/1471-2199-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu W., Chu X., Li Y., Wang C., Guo X. Cotton GhMKK1 induces the tolerance of salt and drought stress, and mediates defence responses to pathogen infection in transgenic Nicotiana benthamiana. PLoS ONE. 2013;8:e68503. doi: 10.1371/journal.pone.0068503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu F., Huaxia Y., Lu W., Wu C., Cao X., Guo X. GhWRKY15, a member of the WRKY transcription factor family identified from cotton (Gossypium hirsutum L.) is involved in disease resistance and plant development. BMC Plant Biol. 2012;12:144. doi: 10.1186/1471-2229-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi W., Hao L., Li J., Liu D., Guo X., Li H. The Gossypium hirsutum WRKY gene GhWRKY39-1 promottes pathogen infection defense responses and mediates salt stress tolerance in transgenic Nicotiana benthamiana. Plant Cell Rep. 2014;33:483–498. doi: 10.1007/s00299-013-1548-5. [DOI] [PubMed] [Google Scholar]
- 67.Kaur M., Shang H., Tamplin M., Ross T., Bowman J.P. Culture-dependent and culture-independent assessment of spoilage community growth on VP lamb meat from packaging to past end of shelf-life. Food Microbiol. 2017;68:71–80. doi: 10.1016/j.fm.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 68.Feng J., Zhang H., Strelkov S.E., Hwang S.F. The LmSNF1 gene is required for pathogenicity in the canola blackleg pathogen Leptosphaeria maculans. PLoS ONE. 2014;9:e92503. doi: 10.1371/journal.pone.0092503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fahlgren N., Howell M.D., Kasschau K.D., Chapman E.J., Sullivan C.M., Cumbie J.S., Givan S.A., Law T.F., Grant S.R., Dangl J.L., et al. High-throughput sequencing of Arabidopsis microRNAs: Evidence for frequent birth and death of MIRNA genes. PLoS ONE. 2007;2:e219. doi: 10.1371/journal.pone.0000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Z., Zhang X., Hu Z., Wang S., Zhang J., Wang X., Wang Q., Zhang B. Lack of K-Dependent Oxidative Stress in Cotton Roots Following Coronatine-Induced ROS Accumulation. PLoS ONE. 2015;10:e0126476. doi: 10.1371/journal.pone.0126476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.