Abstract
Two new ergostane-type sterols; (22E)-5α,6α-epoxyergosta-8,14,22-triene-3β,7β-diol (1) and 5α,6α-epoxyergost-8(14)-ene-3β,7α-diol (2) were isolated from the fruiting bodies of king trumpet mushroom (Pleurotus eryngii), along with eight known compounds (3–10). All isolated compounds were evaluated for their inhibitory effects on aromatase. Among them, 4 and 6 exhibited comparable aromatase inhibitory activities to aminoglutethimide.
Keywords: Pleurotus eryngii, sterol, ergostane, aromatase inhibitor
1. Introduction
Estrogen is responsible for breast cancer growth. The target genes of an estrogen receptor are in control of cancer cell development in estrogen-dependent breast tumors. Binding of estrogen receptor to estrogen triggers transcription of its target genes [1]. Aromatase is the rate-limiting enzyme in estrogen biosynthesis [1]. This enzyme converts androgens (testosterone and androtestosterone) into estrogens (estradiol and estrone, respectively) [2]. Aromatase inhibitors (AIs) are adjuvant in hormone treatments commonly prescribed for breast cancers that are hormone receptor-positive in the early stage [3]. However, the currently used AIs have several side effects of menopausal symptoms such as hot flashes, vaginal dryness, sexual dysfunction, musculoskeletal symptoms, osteoporosis, bone fracture, fatigue, mood disturbance, nausea, and vomiting [3]. Therefore, natural compounds obtained from safe food resources might be useful in the search for promoter-specific AIs with few side effects [4].
Pleurotus eryngii (Japanese name: eringi, English name: oyster mushroom or king trumpet) is an edible mushroom. P. eryngii is native to North Africa, Asia, and Europe [5], and also grown commercially in Japan, China, and the US [6]. Previous studies demonstrated the inhibitory effects on human neutrophil elastase (HNE) [7], antioxidant and antimutagenic activities [8], and inhibitory effects on allergic mediators [9] of P. eryngii extracts. P. eryngii contains amino acids, vitamins, and dietary fiber [10]. It also includes polysaccharides [11,12], pleurone [7], ergostane-type sterols [13], and eryngiolide A [14]. These chemical constituents exhibit biological activities such as the antioxidant [11] and antitumor activities [12] of a polysaccharide, HNE-inhibitory effects of pleurone [7], and cytotoxicity against human cancer cell lines of eryngiolide A [14]. We recently reported eringiacetal A, which is an ergostane-type sterol with a cage-shaped structure [15], and a 9,11-seco-ergostane and five ergostane-type sterols [16] from the fruiting bodies of P. eryngii. In a continuing study, we isolated 10 ergostane-type sterols, and elucidated the structures of two new compounds; (22E)-5α,6α-epoxyergosta-8,14,22-triene-3β,7β-diol (1), and 5α,6α-epoxyergost-8(14)-ene-3β,7α-diol (2). In addition, the isolated constituents were evaluated for inhibitory activities on aromatase.
2. Results
2.1. Isolation and Structure Elucidation
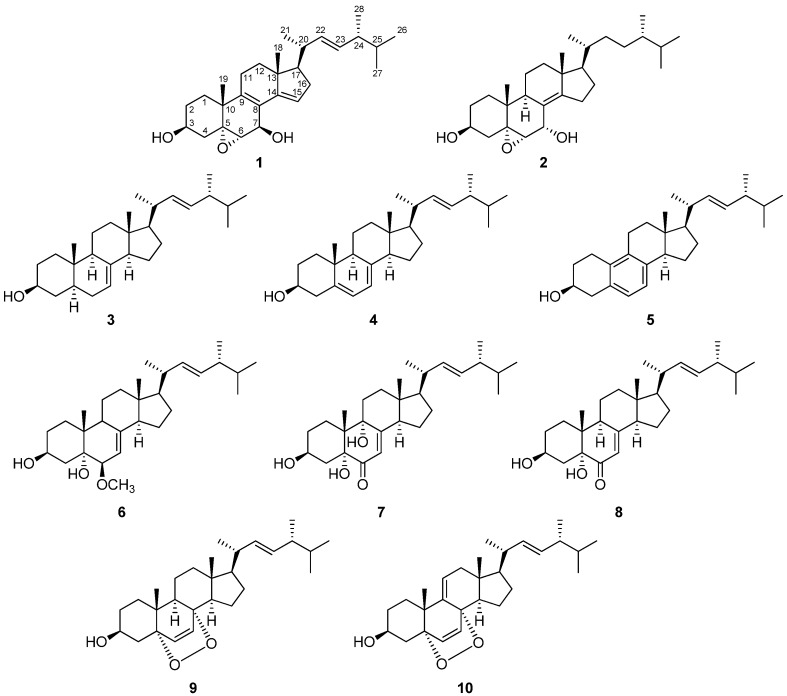
(22E)-Ergosta-7,22-dien-3β-ol (3) [17], (22E)-ergosta-5,7,22-trien-3β-ol (4) [18], (22E)-19-norergosta-5,7,9,22-tetraen-3β-ol (5) [19], ergosterol peroxide (9) [18], and 9,11-dehydroergosterol peroxide (10) [20] were isolated from sample 1, and Compounds 1, 2, (22E)-6β-methoxyergosta-7,22-diene-3β,5α-diol (6) [21], (22E)-3β,5α,9α-trihydroxyergosta-7,22-dien-6-one (7) [21], and (22E)-3β,5α-dihydroxyergosta-7,22-dien-6-one (8) [22] were obtained from sample 2 (Figure 1). Of these, 1 and 2 were new compounds.
Figure 1.
Structures of compounds.
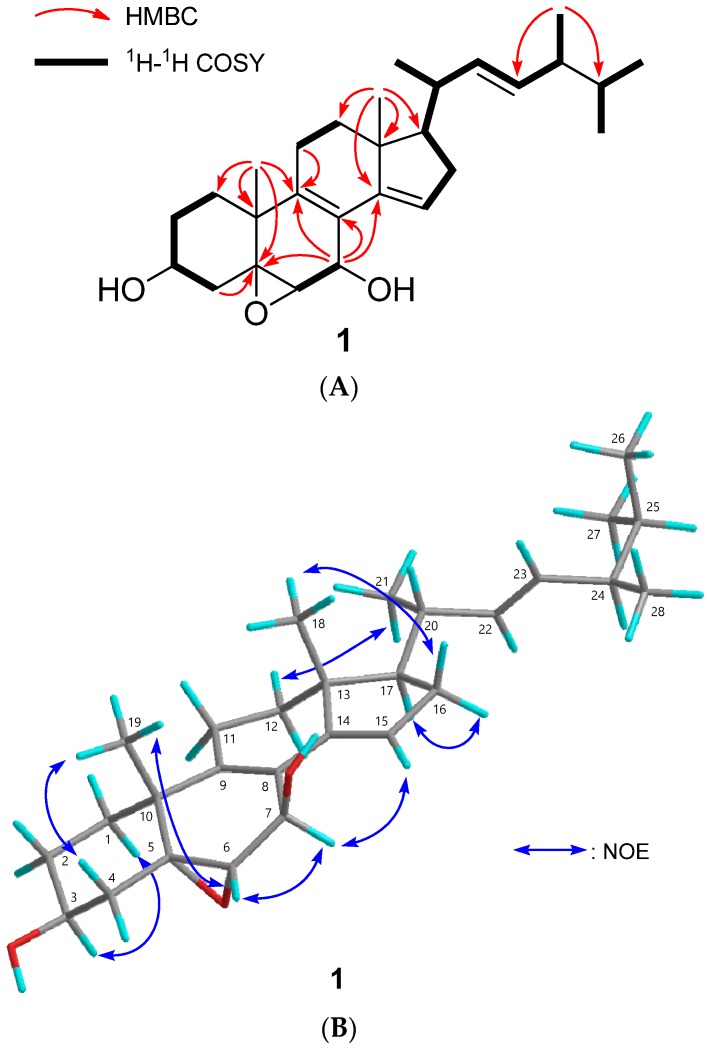
Compound 1 was isolated as an amorphous solid, with a molecular formula of C28H42O3 by HREIMS. The infrared (IR) spectrum indicated the presence of hydroxy groups (νmax 3451 cm−1), and the UV spectrum suggested the presence of a conjugated diene (λmax 242.0 nm). The 1H and 13C NMR spectra (δH and δC in ppm) in CDCl3 displayed signals for two tertiary methyls (δH 0.82 (singlet (s)), 1.30 (s)), four secondary methyls (δH 0.83 (doublet (d)), 0.85 (d), 0.93 (d), 1.04 (d)), three oxymethines (δH 3.24 (d), 3.96 (triplet of triplets (tt)), 4.85 (broad singlet (br s)); δC 59.5 (d), 63.8 (d), 68.4 (d)), an sp3 oxygenated quaternary carbon (δC 63.3 (s)), a tetrasubstituted olefin (δC 122.2 (s), 138.8 (s)), a trisubstituted olefin (δH 5.55 (br s); δC 118.7 (d), 147.7 (s)), and a disubstutited olefin (δH 5.20 (doublet of doublets (dd)), 5.28 (dd); δC 132.4 (d), 135.1 (d)) (Table 1, Figures S1–S4). In the HMBC spectrum, the correlations were observed as follows; Me-19 (δH 0.82 (s))/C-5 (δC 63.3 (s)), C-9 (δC 138.8 (s)); H-7 (δH 4.85 (br s))/C-5 (δC 63.3 (s)), C-9 (δC 138.8 (s)), C-14 (δC 147.7 (s)); H-6 (δH 3.24 (d))/C-7 (δC 63.8 (d)), C-8 (δC 122.2 (s)); Me-18 (δH 0.82 (s))/C-14 (δC 147.7 (s)); Me-28 (δH 0.93 (d))/C-23 and C-25 (Figure 2A and Figure S5). The correlations between H2-1–H2-2–H-3 (δH 3.96 (tt))–H2-4; H-6 (δH 3.24 (d))–H-7 (δH 4.85 (br s)); H-15 (δH 5.55 (br s))–H2-16–H-17–H-20–Me-21; H-20–H-22 (δH 5.20 (dd))–H-23 (δH 5.28 (dd))–H-24–Me-28 (δH 0.93 (d)); Me-26 (δH 0.85 (d))–H-25–Me-27 (δH 0.83 (d)) were observed in the 1H-1H COSY spectrum (Figure 2A and Figure S6). From the above, the planar structure was determined as shown in Figure 2A. The configuration of the hydroxy groups at the C-3 position was determined as β-orientation because of the coupling constant (J) (δH 3.96 (tt, 11.5, 5.4 Hz)). The NOE correlation of Me-19/H-6β (equatrial) suggested that the epoxy group at C-5,6 was α-oriented, and that of H-7α/H-15 suggested that 7-OH was β-oriented (Figure 2B and Figure S7). The geometry of the double bond at C-22 was determined as E from the coupling constants of H-22 (δH 5.20 (dd, J = 15.2, 7.6 Hz)) and H-23 (δH 5.28 (dd, J = 15.2, 7.9 Hz)). Comparison of 13C NMR chemical shifts at C-24 (δC 42.8) and 28 (δC 17.6) with those of 24R (δC 42.9 (C-24) and 17.7 (C-28)) and 24S (δC 43.2 (C-24) and 18.1 (C-28)) methylcholestane-type sterols [23,24] established the stereochemistry of C-24 as R. Therefore compound 1 was determined as (22E)-5α,6α-epoxyergosta-8,14,22-triene-3β,7β-diol (Figure 1, Table S1). Compound 1 was similar to (22E)-5α,6α-epoxy-ergosta-8,14,22-triene-7β,7α-diol [25], except for the absence of a 7α-hydroxy group and the presence of a 7β-hydroxy group. There are differences in δH value measured with C6D6 such as H-7 (7α-hydroxy-type: δH 4.34 (1H, dd, J = 11.2, 2.6 Hz) [25] vs. 7β-hydroxy-type (1): δH 4.74 (br s)), and H-15 (7α-hydroxy-type: δH 6.50 (1H, dd, J = 3.3, 1.8 Hz) [25] vs. 7β-hydroxy-type (1): δH 5.33 (br s)).
Table 1.
1H and 13C NMR Data for Compounds 1 and 2 in CDCl3 (δ in ppm; J in Hz).
| 1 | 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Position | δH | δC | δH | δC | ||||
| 1α | 2.01 | (1H, multiplet (m)) | 31.0 | t | 1.46 | (1H, m) | 32.2 | t |
| 1β | 1.86 | (1H, m) | 1.67 | (1H, m) | ||||
| 2 | 1.68 | (2H, m) | 30.9 | t | α 1.96 | (1H, m) | 31.1 | t |
| β 1.56 | (1H, m) | |||||||
| 3 | 3.96 | (1H, tt, J = 11.5, 5.4) | 68.4 | d | 3.92 | (1H, tt, J = 11.4, 3.0) | 68.7 | d |
| 4α | 1.50 | (1H, m) | 39.0 | t | 1.42 | (1H, m) | 39.6 | t |
| 4β | 2.21 | (1H, m) | 2.13 | (1H, dd, J = 13.2, 11.4) | ||||
| 5 | 63.3 | s | 67.8 | s | ||||
| 6 | 3.24 | (1H, d, J = 2.4) | 59.5 | d | 3.15 | (1H, d, J = 3.5) | 61.3 | d |
| 7 | 4.85 | (1H, br s) | 63.8 | d | 4.43 | (1H, dd, J = 9.6, 3.5) | 65.1 | d |
| 8 | 122.2 | s | 125.1 | s | ||||
| 9 | 138.8 | s | 2.35 | (1H, m) | 38.7 | d | ||
| 10 | 38.3 | s | 35.8 | s | ||||
| 11 | 2.19 | (2H, m) | 22.2 | t | α 1.49 | (1H, m) | 19.0 | t |
| β 1.40 | (1H, m) | |||||||
| 12α | 1.47 | (1H, m) | 35.4 | t | 1.16 | (1H, m) | 36.7 | t |
| 12β | 1.99 | (1H, m) | 1.95 | (1H, m) | ||||
| 13 | 44.6 | s | 43.1 | s | ||||
| 14 | 147.7 | s | 152.7 | s | ||||
| 15 | 5.55 | (1H, br s) | 118.7 | d | α 2.65 | (1H, m) | 25.0 | t |
| β 2.30 | (1H, m) | |||||||
| 16α | 2.27 | (1H, m) | 1.89 | (1H, m) | 26.6 | t | ||
| 16β | 2.08 | (1H, m) | 36.8 | t | 1.41 | (1H, m) | ||
| 17 | 1.55 | (1H, m) | 56.4 | d | 1.21 | (1H, m) | 56.6 | d |
| 18 | 0.82 | (3H, s) | 15.6 | quartet (q) | 0.85 | (3H, s) | 17.9 | q |
| 19 | 1.30 | (3H, s) | 23.6 | q | 0.87 | (3H, s) | 16.5 | q |
| 20 | 2.24 | (1H, m) | 38.8 | d | 1.46 | (1H, m) | 34.9 | d |
| 21 | 1.04 | (3H, d, J = 6.5) | 21.0 | q | 0.93 | (3H, d, J = 6.8) | 19.1 | q |
| 22 | 5.20 | (1H, dd, J = 15.2, 7.6) | 135.1 | d | A 1.03 | (1H, m) | 33.4 | t |
| B 1.44 | (1H, m) | |||||||
| 23 | 5.28 | (1H, dd, J = 15.2, 7.9) | 132.4 | d | A 0.95 | (1H, m) | 30.4 | t |
| B 1.37 | (1H, m) | |||||||
| 24 | 1.88 | (1H, m) | 42.8 | d | 1.21 | (1H, m) | 39.1 | d |
| 25 | 1.48 | (1H, m) | 33.1 | d | 1.58 | (1H, m) | 31.5 | d |
| 26 | 0.85 | (3H, d, J = 6.8) | 19.9 | q | 0.85 | (3H, d, J = 7.1) | 20.5 | q |
| 27 | 0.83 | (3H, d, J = 6.8) | 19.6 | q | 0.78 | (3H, d, J = 7.0) | 17.6 | q |
| 28 | 0.93 | (3H, d, J = 6.8) | 17.6 | q | 0.77 | (3H, d, J = 6.9) | 15.4 | q |
Figure 2.
Structure determination of compound 1. (A) Key HMBC and 1H-1H COSY correlations of compound 1; (B) Key NOE correlations of compound 1. The atoms of C, H, and O were shown in grey, aqua, and red, respectively.
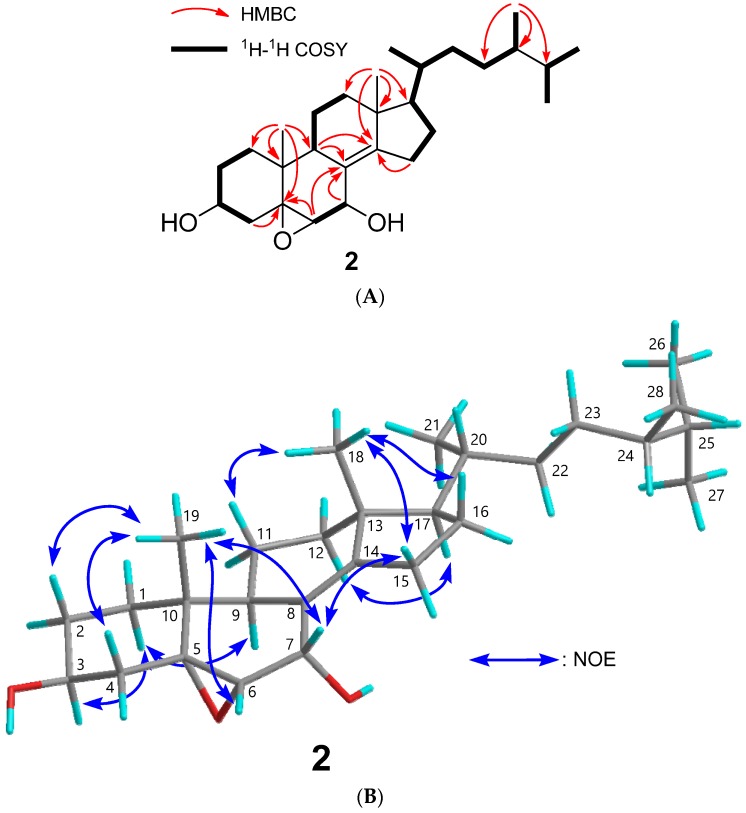
Compound 2 was isolated as an amorphous solid, with a molecular formula of C28H46O3. The IR spectrum suggested the presence of hydroxy groups (3387 cm−1). The 1H, 13C NMR and HSQC spectra indicated the presence of two tertiary methyls (δH 0.85 (s), 0.87 (s)), four secondary methyls (δH 0.77 (d), 0.78 (d), 0.85 (d), 0.93 (d)), two oxymethines (δH 3.92 (tt), 4.43 (dd); δC 65.1 (d), 68.7 (d)), a trisubstituted epoxy (δH 3.15 (d); δC 61.3 (d), 67.8 (s)), and a tetrasubstituted olefin (δC 125.1 (s), 152.7 (s)) (Table 1, Figures S8–S11). Based on the correlations at Me-18/C-14 (δC 152.7 (s)), Me-19/C-5 (δC 67.8 (s)), and H-15/C-8 (δC 125.1 (s)) and C-14 (δC 152.7 (s)) in the HMBC spectrum, and H2-1–H2-2–H-3 (δH 3.92 (tt))–H2-4; H-6 (δH 3.15 (d))–H-7 (δH 4.43 (dd)) in the 1H-1H COSY spectrum (Figure 3A, Figures S12 and S13), oxymethines were at C-3 and C-7 positions, a trisubstituted epoxy group at the C-5, 6 positions, and a tetrasubstituted olefin at the C-8, 14 positions (Figure 3A). The NOE correlation between H-7 and Me-19 demonstrated the configuration of the hydroxy group at the C-7 position as α-orientation (Figure 3B and Figure S14). The NOE correlation between H-4β and Me-19 suggested the orientation of the epoxy group at C-5, 6 was α (Figure 3B and Figure S14). The stereochemistry of C-24 was established as S by comparison of the 1H NMR chemical shift at Me-28 (δH 0.77) with those of 24R (δH 0.802) and 24S (δH 0.781) ergostane-type sterols [26,27]. Therefore, the structure of 2 was established as 5α,6α-epoxyergosta-8(14)-ene-3β,7α-diol (Figure 1, Table S2).
Figure 3.
Structure determination of compound 2. (A) Key HMBC and 1H-1H COSY correlations of compound 2; (B) Key NOE correlations of compound 2. The atoms of C, H, and O were shown in grey, aqua, and red, respectively.
2.2. Evaluation for Aromatase Inhibitory Effects
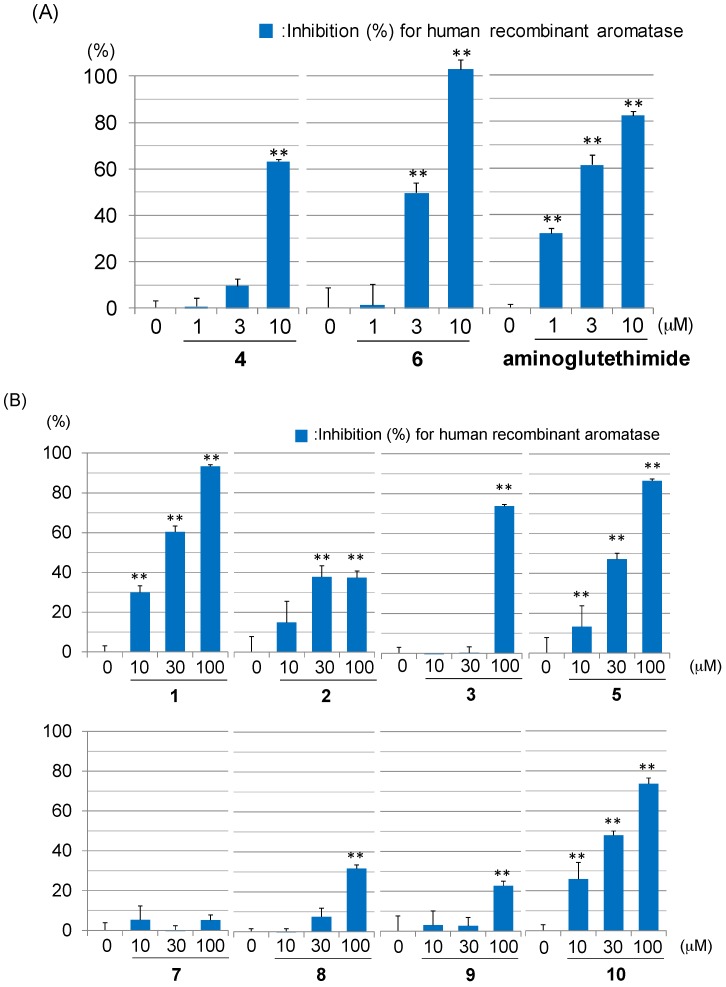
Compounds 1–10 and aminoglutethimide, a positive control, were evaluated for their aromatase inhibitory activities. Compounds 4 and 6 exhibited comparable inhibitory activities (IC50 4: 8.1 µM; 6: 2.8 µM) to aminoglutethimide (IC50 2.0 µM) (Figure 4A). Compounds 1, 3, 5, and 10 showed moderate activities (IC50 1: 17.3 µM; 3: 66.1 µM; 5: 33.8 µM; 10: 32.6 µM) (Figure 4B). Compounds 2, 7, 8, and 9 weakly inhibited aromatase (Figure 4B). Above results suggested that compounds 4 and 6 can be regarded as potential anti-breast cancer agents targeting aromatase. Based on the results in the figures, the following structure-activity relationship of the compounds can be concluded: (i) The double-bond at C-5, 6 intensifies the aromatase inhibitory activity in ergost-7-ene compounds (3 (IC50 66.1 µM) vs. 4 (IC50 8.1 µM)); (ii) 9(11)-double-bond enhances the inhibitory activity in 5α,8α-epidioxyergost-6-ene compounds (9 (IC50 > 100 µM) vs. 10 (IC50 32.6 µM)); (iii) 7-ene-6-one compounds did not show this activity (7 and 8 (IC50 > 100 µM)).
Figure 4.
Inhibitory effects of sterols (1–10) from P. eryngii against human recombinant aromatase. (A) Inhibitory effects of sterols (4, 6) and aminoglutethimide at 1, 3, and 10 µM. (B) Inhibitory effects of sterols (1–3, 5, 7–10) at 10, 30, and 100 µM. Each value represents the mean ± the standard error (S.E.) of three determinations. Significant differences from the vehicle control (0 μM) group shown as ** p < 0.01.
3. Experimental Section
3.1. General Methods
Dibenzylfluorescein (DBF) and Human CYP19 + P450 Reductase SUPERSOMES (human recombinant aromatase) were obtained from BD Biosciences (Heidelberg, Germany). The physical data were obtained by the following instruments: a Yanagimoto micro-melting point apparatus for melting points (uncorrected); a JASCO DIP-1000 digital polarimeter for Optical rotations; a Perkin-Elmer 1720X FTIR spectrophotometer for IR spectra; an Agilent-NMR-vnmrs600 for the 1H and 13C NMR spectra (1H: 600 MHz; 13C: 150 MHz) in CDCl3 with tetramethylsilane as the internal standard; a Hitachi M-4000H double-focusing mass spectrometer for EIMS (70 eV). Column chromatography was carried out by Silica gel (70–230 mesh, Merck, Darmstadt, Germany) and silica gel 60 (230–400 mesh, Nacalai Tesque, Inc., Kyoto, Japan). HPLC was performed by the following systems; system I: Cosmosil 5SL-II column (25 cm × 20 mm i.d.) (Nacalai Tesque, Inc.), hexane/EtOAc (5:1), 8.0 mL/min, 35 °C; system II: Shimpack PREP-ODS (25 cm × 20 mm i.d.) (Shimadzu corp., Kyoto, Japan), MeOH, 8.0 mL/min, 35 °C; system III: Cosmosil 5C18-MS-II column (25 cm × 20 mm i.d.) (Nacalai Tesque, Inc.), MeOH/H2O (95:5), flow rate, 4.0 mL/min, 35 °C; system IV: Cosmosil 5C18-MS-II column, MeOH/H2O (9:1), 4.0 mL/min, 35 °C.
3.2. Materials
The fruiting bodies of P. eryngii were purchased from HOKUTO Corp. They were cultivated in Kagawa, Japan (Sample 1 in 2011, and Sample 2 in 2014). A voucher material has been deposited in the Herbarium of the Laboratory of Medicinal Chemistry, Osaka University of Pharmaceutical Sciences.
3.3. Extraction and Isolation
3.3.1. Sample 1
Sample 1 (fruiting bodies of P. eryngii (21 kg, fresh weight)) was extracted with MeOH under reflux (1 week, 4 times). The MeOH extract (170 g) was then divided into EtOAc and H2O fractions by liquid-liquid partition. The EtOAc fraction (60 g) was separated into 20 fractions (Fr. S1-A to S1-T) with SiO2 column chromatography (CC) (SiO2 (3.5 kg); CHCl3/EtOAc (1:0 to 0:1), and EtOAc/MeOH (5:1, and 0:1)).
Fr. S1-H (836.5 mg), CHCl3/EtOAc (10:1)-eluted fraction, was separated with SiO2 CC to yield 8 fractions, S1-H1 to S1-H8. Preparative HPLC (system I) of S1-H3 (185.7 mg), hexane/EtOAc (5:1)-eluted fraction, provided 7 fractions, S1-H3-1 to SF3-7. S1-H3-4 was identified as 4 (31.8 mg; retention time (tR) 19.2 min). Preparative HPLC (system II) of S1-H3-5 (5.4 mg, tR 36.5 min) provided 3 (1.9 mg; tR 37.5 min). Preparative HPLC (system IV) of S1-H6 (14.3 mg), hexane/EtOAc (3:1)-eluted fraction, provided 10 (1.3 mg, tR 95.4 min) and 9 (1.7 mg, tR 120.2 min).
Fr. S1-I (1072.3 mg), CHCl3/EtOAc (10:1)-eluted fraction, was separated with SiO2 CC to give 8 fractions, S1-I1 to S1-I10. Preparative HPLC (system I) of S1-I5 (45.2 mg), hexane/EtOAc (5:1)-eluted fraction, provided 5 (2.8 mg, tR 42.7 min).
3.3.2. Sample 2
Sample 2 (fruiting bodies of P. eryngii (120 kg, fresh weight)) was extracted with MeOH under reflux (3 days, 4 times). The MeOH extract (2625 g) was divided into EtOAc and H2O fractions by liquid-liquid partition. The EtOAc fraction (240 g) was separated into 37 fractions (Fr. S2-A to S2-Z, and S2-a to S2-k) with SiO2 column chromatography (CC) (SiO2 (2.8 kg); CHCl3/EtOAc (1:0 to 0:1), and MeOH).
Fr. S2-V (3964.9 mg), CHCl3/EtOAc (1:1)-eluted fraction, was separated by SiO2 CC to give 8 fractions, S2-V1 to S2-V21. Preparative HPLC (system III) of Fr. S2-V4 (110.9 mg), hexane/EtOAc (1:1)-eluted fraction, provided 8 (1.5 mg; tR 36.9 min) and 6 (6.3 mg; tR 49.5 min). Preparative HPLC (system IV) of Fr. S2-V6 (451.6 mg), hexane/EtOAc (1:1)-eluted fraction, provided 7 (1.1 mg; tR 59.8 min). Preparative HPLC (system IV) of Fr. S2-V7 (270.5 mg), hexane/EtOAc (1:1)-eluted fraction, provided 2 (3.0 mg; tR 76.8 min). Preparative HPLC (system III) of Fr. S2-V10 (270.5 mg), hexane/EtOAc (1:1)-eluted fraction, provided 1 (1.9 mg; tR 36.3 min).
3.3.3. (22E)-5α,6α-Epoxyergosta-8,14,22-triene-3β,7β-diol (1)
[α]20D −23.6 (c = 0.13, EtOH); IR νmaxKBr cm−1: 3451, 2960, 1697, 1557, 1456; UV λmaxEtOH nm (logε): 206.0 (3.75), 242.0 (3.62); EIMS m/z: 426 [M]+ (71), 315 (27), 300 (57), 172 (83), 69 (100); HREIMS m/z: 426.3127 [M]+ (calcd for 426.3134: C28H42O3); 1H NMR (400 MHz, C6D6) δH ppm: 0.80 (s, H-19), 0.91 (d, 6.8 Hz), 0.93 (d, 6.4 Hz), 1.01 (d, 6.8 Hz), 1.07 (d, 6.4 Hz), 1.12 (s, H-18), 3.11 (d, 2.4 Hz, H-6), 3.83 (tt, 11.6, 4.8 Hz, H-3), 4.74 (br s, H-7), 5.24 (dd, 15.6, 8.4 Hz, H-22), 5.32 (overlapped, H-23), 5.33 (br s, H-15).
3.3.4. 5α,6α-Epoxyergost-8(14)-ene-3β,7α-diol (2)
[α]20D −112.2 (c = 0.13, EtOH); IR νmaxKBr cm−1: 3387, 2959, 2936, 2871, 1466, 1377; EIMS m/z: 430 [M]+ (5), 412 (100), 394 (57), 379 (58), 267 (23), 213 (21); HREIMS m/z: 430.3450 [M]+ (calcd for 430.3447: C28H46O3).
3.4. Inhibitory Effects against Human Recombinant Aromatase
Inhibitory assay against human recombinant aromatase was performed as described previously [28,29].
3.5. Statistics
Values are described as the mean ± standard error of the mean (S.E.M.). Statistical analysis was performed by one-way analysis of variance, followed by Dunnett’s test. Probability (p) values less than 0.05 were regarded as significant.
4. Conclusions
In this study, we isolated two new sterols (1 and 2) and elucidated their structures. They have 5α,6α-epoxy-7-hydroxy ergostane structure. In aromatase inhibitory assay, compounds 4 and 6 possessed comparable inhibitory effects (IC50 4: 8.1 µM; 6: 2.8 µM) against human recombinant aromatase to aminoglutethimide (IC50 2.0 µM). These results suggested that compounds 4 and 6 have potential as anti-breast cancer agents.
Acknowledgments
We thank Katsuhiko Minoura and Mihoyo Fujitake (Osaka University of Pharmaceutical Sciences) for the NMR and MS measurements.
Abbreviations
| NMR | Nuclear magnetic resonance |
| HREIMS | High resolution electron ionization mass spectrometry |
| CDCl3 | Duterated chloroform |
| HMBC | Heteronuclear multiple bond coherence |
| COSY | Correlation spectroscopy |
| NOE | Nuclear overhauser effect |
| HSQC | Hetero nuclear single quantum coherence |
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/18/11/2479/s1.
Author Contributions
Takashi Kikuchi, Takeshi Yamada, Kiyofumi Ninomiya, Toshio Morikawa and Reiko Tanaka designed the experiments; Takashi Kikuchi, Naoki Motoyashiki, Takeshi Yamada and Reiko Tanaka isolated compounds, and elucidated their structures. Kanae Shibatani, Kiyofumi Ninomiya and Toshio Morikawa evaluated aromatase inhibitory effects of compounds. Takashi Kikuchi, Toshio Morikawa and Reiko Tanaka wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hong Y., Li H., Yuan Y.-C., Chen S. Molecular characterization of aromatase. Ann. N. Y. Acad. Sci. 2009;1155:112–120. doi: 10.1111/j.1749-6632.2009.03703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston S.R.D., Dowsett M. Aromatase inhibitors for breast cancer: Lessons from the laboratory. Nat. Rev. Cancer. 2003;3:821–831. doi: 10.1038/nrc1211. [DOI] [PubMed] [Google Scholar]
- 3.Bae K., Yoo H.-S., Lamoury G., Boyle F., Rosenthal D.S., Oh B. Acupuncture for aromatase inhibitor-induced arthralgia: A systematic review. Integr. Cancer Ther. 2015;14:496–502. doi: 10.1177/1534735415596573. [DOI] [PubMed] [Google Scholar]
- 4.Balunas M.J., Kinghorn A.D. Natural compounds with aromatase inhibitory activity: An update. Planta Med. 2010;76:1087–1093. doi: 10.1055/s-0030-1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu K., Yamanaka M., Gyokusen M., Kaneko S., Tsutsui M., Sato J., Sato I., Sato M., Kondo R. Estrogen-like activity and prevention effect of bone loss in calcium deficient ovariectomized rats by the extract of Pleurotus eryngii. Phytother. Res. 2006;20:659–664. doi: 10.1002/ptr.1927. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez Estrada A.E., Royse D.J. Yield, size and bacterial blotch resistance of Pleurotus eryngii grown on cottonseed hulls/oak sawdust supplemented with manganese, copper and whole ground soybean. Bioresour. Technol. 2007;98:1898–1906. doi: 10.1016/j.biortech.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Lee I.-S., Ryoo I.-J., Kwon K.-Y., Ahn J.S., Yoo I.-D. Pleurone, a novel human neutrophil elastase inhibitor from the fruiting bodies of the mushroom Pleurotus eryngii var. ferulae. J. Antibiot. 2011;64:587–589. doi: 10.1038/ja.2011.47. [DOI] [PubMed] [Google Scholar]
- 8.Kang M.Y., Rico C.W., Lee S.C. In vitro antioxidative and antimutagenic activities of oak mushroom (Lentinus edodes) and king oyster mushroom (Pleurotus eryngii) byproducts. Food Sci. Biotechnol. 2012;21:167–173. doi: 10.1007/s10068-012-0021-5. [DOI] [Google Scholar]
- 9.Han E.H., Hwang Y.P., Kim H.G., Choi J.H., Im J.H., Yang J.H., Lee H.-U., Chun S.-S., Chung Y.C., Jeong H.G. Inhibitory effect of Pleurotus eryngii extracts on the activities of allergic mediators in antigen-stimulated mast cells. Food Chem. Toxicol. 2011;49:1416–1425. doi: 10.1016/j.fct.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Kawai J., Ouchi K., Inatomi S., Andoh T. Pleurotus eryngii ameliorates lipopolysaccharide-induced lung inflammation in mice. Evid.-Based Complement. Altern. Med. 2014;2014:532389. doi: 10.1155/2014/532389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang A., Li X., Xing C., Yang J., Sun P. Antioxidant activity of polysaccharide extracted from Pleurotus eryngii using response surface methodology. Int. J. Biol. Macromol. 2014;65:28–32. doi: 10.1016/j.ijbiomac.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z., Xu J., Fu Q., Fu X., Shu T., Bi Y., Song B. Antitumor activity of a polysaccharide from Pleurotus eryngii on mice bearing renal cancer. Carbohydr. Polym. 2013;95:615–620. doi: 10.1016/j.carbpol.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Yaoita Y., Yoshihara Y., Kakuda R., Machida K., Kikuchi M. New sterols from two edible mushrooms, Pleurotus eryngii and Panellus serotinus. Chem. Pharm. Bull. 2002;50:551–553. doi: 10.1248/cpb.50.551. [DOI] [PubMed] [Google Scholar]
- 14.Wang S.-J., Li Y.-X., Bao L., Han J.-J., Yang X.-L., Li H.-R., Wang Y.-Q., Li S.-J., Liu H.-W. Eryngiolide A, a cytotoxic macrocyclic diterpenoid with an unusual cyclododecane core skeleton produced by the edible mushroom Pleurotus eryngii. Org. Lett. 2012;14:3672–3675. doi: 10.1021/ol301519m. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi T., Masumoto Y., In Y., Tomoo K., Yamada T., Tanaka R. Eringiacetal A, 5,6-seco-(5S,6R,7R,9S)-5,6:5,7:6,9-triepoxyergosta-8(14),22-diene-3β,7β-diol, an unusual ergostane sterol from the fruiting bodies of Pleurotus eryngii. Eur. J. Org. Chem. 2015;2015:4645–4649. doi: 10.1002/ejoc.201500382. [DOI] [Google Scholar]
- 16.Kikuchi T., Maekawa Y., Tomio A., Masumoto Y., Yamamoto T., In Y., Yamada T., Tanaka R. Six new ergostane-type steroids from king trumpet mushroom (Pleurotus eryngii) and their inhibitory effects on nitric oxide production. Steroids. 2016;115:9–17. doi: 10.1016/j.steroids.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Keller A.C., Maillard M.P., Hostettmann K. Antimicrobial steroids from the fungus Fomitopsis pinicola. Phytochemistry. 1996;41:1041–1046. doi: 10.1016/0031-9422(95)00762-8. [DOI] [PubMed] [Google Scholar]
- 18.Seo Hyo W., Hung Tran M., Na M., Jung Hyun J., Kim Jin C., Choi Jae S., Kim Jung H., Lee H.-K., Lee I., Bae K., et al. Steroids and triterpenes from the fruit bodies of Ganoderma lucidum and their anti-complement activity. Arch. Pharm. Res. 2009;32:1573–1579. doi: 10.1007/s12272-009-2109-x. [DOI] [PubMed] [Google Scholar]
- 19.Barrero A.F., Oltra J.E., Poyatos J.A., Jimenez D., Oliver E. Phycomysterols and Other Sterols from the Fungus Phycomyces blakesleeanus. J. Nat. Prod. 1998;61:1491–1496. doi: 10.1021/np980199h. [DOI] [PubMed] [Google Scholar]
- 20.Du Z.-Z., Shen Y.-M. A rare new cleistanthane diterpene from the pericarp of Trewia nudiflora. Helv. Chim. Acta. 2006;89:2841–2845. doi: 10.1002/hlca.200690256. [DOI] [Google Scholar]
- 21.Kawagishi H., Katsumi R., Sazawa T., Mizuno T., Hagiwara T., Nakamura T. Cytotoxic steroids from the mushroom Agaricus blazei. Phytochemistry. 1988;27:2777–2779. doi: 10.1016/0031-9422(88)80662-9. [DOI] [Google Scholar]
- 22.Ishizuka T., Yaoita Y., Kikuchi M. Sterol constituents from the fruit bodies of Grifola frondosa (Fr.) S.F. Gray. Chem. Pharm. Bull. 1997;45:1756–1760. doi: 10.1248/cpb.45.1756. [DOI] [PubMed] [Google Scholar]
- 23.Yan X.-H., Liu H.-L., Huang H., Li X.-B., Guo Y.-W. Steroids with Aromatic A-Rings from the Hainan Soft Coral Dendronephthya studeri Ridley. J. Nat. Prod. 2011;74:175–180. doi: 10.1021/np100562n. [DOI] [PubMed] [Google Scholar]
- 24.Li W., Zhou W., Song S.B., Shim S.H., Kim Y.H. Sterol Fatty Acid Esters from the Mushroom Hericium erinaceum and Their PPAR Transactivational Effects. J. Nat. Prod. 2014;77:2611–2618. doi: 10.1021/np500234f. [DOI] [PubMed] [Google Scholar]
- 25.Ohnuma N., Amemiya K., Kakuda R., Yaoita Y., Machida K., Kikuchi M. Sterol constituents from two edible mushrooms, Lentinula edodes and Tricholoma matsutake. Chem. Pharm. Bull. 2000;48:749–751. doi: 10.1248/cpb.48.749. [DOI] [PubMed] [Google Scholar]
- 26.Rubinstein I., Goad L.J., Clague A.D.H., Mulheirn L.J. The 220 MHz NMR spectra of phytosterols. Phytochemistry. 1976;15:195–200. doi: 10.1016/S0031-9422(00)89083-4. [DOI] [Google Scholar]
- 27.Kobayashi M., Krishna M.M., Haribabu B., Anjaneyulu V. Marine sterols. XXV. Isolation of 23-demethylgorgost-7-ene-3β,5α,6β-triol and (24S)-ergostane-3β,5α,6β,7β,15β-pentol from soft corals of the Andaman and Nicobar coasts. Chem. Pharm. Bull. 1993;41:87–89. doi: 10.1248/cpb.41.87. [DOI] [Google Scholar]
- 28.Ninomiya K., Shibatani K., Sueyoshi M., Chaipech S., Pongpiriyadacha Y., Hayakawa T., Muraoka O., Morikawa T. Aromatase Inhibitory Activity of Geranylated Coumarins, Mammeasins C and D, Isolated from the Flowers of Mammea siamensis. Chem. Pharm. Bull. 2016;64:880–885. doi: 10.1248/cpb.c16-00218. [DOI] [PubMed] [Google Scholar]
- 29.Tanabe G., Tsutsui N., Shibatani K., Marumoto S., Ishikawa F., Ninomiya K., Muraoka O., Morikawa T. Total syntheses of the aromatase inhibitors, mammeasins C and D, from Thai medicinal plant Mammea siamensis. Tetrahedron. 2017;73:4481–4486. doi: 10.1016/j.tet.2017.06.016. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.