Abstract
Advancing age is the major risk factor for the development of chronic diseases and is accompanied with changes in metabolic processes and mitochondrial dysfunction. Mitochondrial sirtuins (SIRT3-5) are part of the sirtuin family of NAD+-dependent deacylases and ADP-ribosyl transferases. The dependence on NAD+ links sirtuin enzymatic activity to the metabolic state of the cell, poising them as stress sensors. Recent insights have revealed that SIRT3-5 orchestrate stress responses through coordinated regulation of substrate clusters rather than a few key metabolic enzymes. Additionally, mitochondrial sirtuin function has been implicated in the protection against age-related pathologies including neurodegeneration, cardiopathologies and insulin resistance. In this review, we highlight the molecular targets of SIRT3-5 and discuss their involvement in aging and age-related pathologies.
Keywords: mitochondrial sirtuins, metabolism, sirtuin networks, stress response, aging
Decreased Mitochondrial Function in Aging
Aging is accompanied by degeneration of multiple organ systems leading to mortality. This decline in organ systems is associated with a loss of cellular homeostasis in fundamental pathways, such as genome fidelity, proteostasis, and nutrient sensing [1]. Intriguingly, mitochondria are at the center of multiple pathways in homeostasis due to their central role in bioenergetics, catabolic and anabolic metabolism, generation of reactive oxygen species (ROS), apoptosis, and signal transduction. These organelles are dynamic and reprogram metabolism in response to cellular stress. Unsurprisingly, mitochondrial dysfunction has been linked to numerous aspects of aging, including decreased activity of metabolic enzymes, impaired respiratory capacity and increased oxidative damage [2]. Recent advances have demonstrated that decreased nicotinamide adenine dinucleotide (NAD+) contributes to cellular and mitochondrial decline during aging [3]. NAD+ functions as a cofactor for numerous metabolic enzymes, and is a co-substrate for the sirtuin family of deacylases [3–5]. In this review, we discuss recent advances in the identification of mitochondrial sirtuin (SIRT3-5) substrates and describe the mitochondrial programs that are regulated by SIRT3-5 in response to mitochondrial stress. Finally, we highlight the most recent work connecting SIRT3-5 to aging and age-related diseases.
Mitochondrial Sirtuins are NAD+-Dependent Enzymes
Sirtuins are NAD+-dependent enzymes conserved from bacteria to humans [6]. Mammals contain seven sirtuin enzymes (SIRT1-7). While the catalytic core domain contains amino acid residues invariant throughout evolution, the N- and C-terminal regions are structurally divergent and contribute to differences in subcellular localization, enzymatic activity and substrate specificity [7]. Three sirtuins - SIRT3, SIRT4 and SIRT5 localize to the mitochondrial matrix, positioning these enzymes within the metabolic hub of the cell. SIRT3-5 contain an N-terminal mitochondrial signal sequence that dictates sirtuin localization to these organelles; here, they coordinate numerous facets of mitochondrial biology with important implications for aging and disease [4,7]. For instance, SIRT3 boosts mitochondrial oxidative metabolism in response to nutrient stress and membrane depolarization [8,9]. Consequently, loss of SIRT3 has been mechanistically linked to decreased heart function and neurodegeneration [10,11].
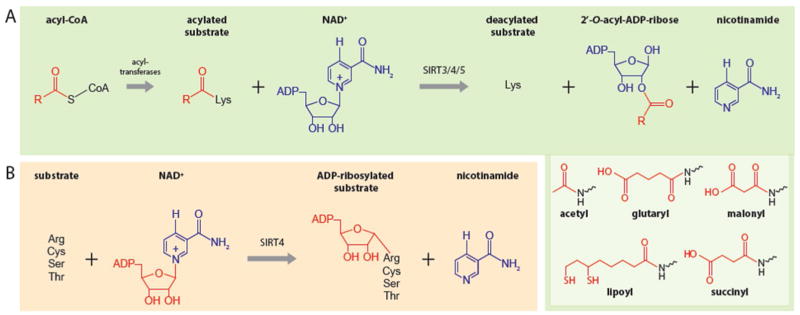
Elucidating the enzymatic activity of sirtuins is a dynamic area of investigation. Initially, sirtuins were identified as NAD+-dependent deacetylases or mono ADP-ribosyltransferases. Recent work has revealed that sirtuins catalyze a wide range of NAD+-dependent reactions, including deacetylation, deacylation, and ADP-ribosylation [3–5]. Hence, sirtuins are often classified as ‘deacylases’ to account for the notion that they are able to remove a variety of long acyl moieties from substrates including succinyl, malonyl, and lipoyl groups, as well as bulkier palmitoyl and myristoyl modifications [12]. During catalysis, sirtuins use NAD+ to remove the acyl group from lysine to form 2′-O-acyl-ADP-ribose and nicotinamide (Figure 1A). Additionally, some sirtuins, including SIRT4 and SIRT6, display ADP-ribosyl transferase activity, used to transfer ADP-ribose from NAD+ to the substrate, thus yielding nicotinamide as a product (Figure 1B). The dependence on NAD+ as a co-substrate positions sirtuins as critical sensors of a cell’s metabolic and redox states [4].
Figure 1. Mitochondrial Sirtuins Are NAD+-Dependent Deacylases and ADP-Ribosyl Transferases.
A. mitochondrial sirtuins remove acyl moieties by positioning NAD+ to nucleophilically attack the acylated lysine. As a result, NAD+ is cleaved and 2′-O-acyl-ADP ribose and nicotinamide are formed in the process. The best characterized acyl modifications removed by mitochondrial sirtuins are shown in the inset. B. SIRT4 catalyzes the transfer of ADP-ribose from NAD+ to arginine, cysteine, serine and threonine substrates. Here, NAD+ is used by SIRT4 to nucleophilically attack the substrate yielding nicotinamide as a byproduct.
The biology of NAD+ is amongst the most dynamic areas of aging research. The ratio between NAD+ and its reduced counterpart NADH is intricately tied to cellular and mitochondrial metabolism, and NAD+/NADH ratios and levels are affected by dozens of cellular reactions. In mitochondria, NADH is generated from glycolysis and from the tricarboxylic (TCA) cycle. NAD+ can be regenerated through numerous reactions, including oxidation of NADH by complex I. The donated electrons feeding into the electron transport chain (ETC) will ultimately contribute to the build-up of a proton gradient that will be used by ATP synthase to produce ATP. In addition, NAD+ can be generated through de novo synthesis, salvage pathways or through oxidation of NADH by lactate dehydrogenase (LDH) in glycolytic cells. NAD+ levels decline during aging, and there is compelling evidence that genetic manipulation of NAD+ biosynthesis or supplementation with NAD+ precursors can extend lifespan in model organisms spanning yeast, worms and mice [13–18]. While mitochondria appear to be protected against stress-induced decreases in NAD+, they can only retain optimal NAD+ levels for a short period of time [19–21]. As mitochondrial sirtuin activity may decline in conditions of decreased NAD+-- for instance during stress and aging -- the inability to regulate appropriate responses to acute mitochondrial stress can severely impair cellular homeostasis [22]. Indeed, a number of studies have reported that restoration of NAD+ levels during stress or aging may directly activate mitochondrial sirtuins to restore homeostasis [14,21]. Due to the scope of this review, we do not focus in depth on the benefits of NAD+ restoration and lifespan improvement, unless directly linked to the discussion of mitochondrial sirtuins. For more in depth reviews on NAD+ and aging, see [3,22,23].
Mitochondrial Sirtuins Regulate Protein Networks to Orchestrate the Stress Response
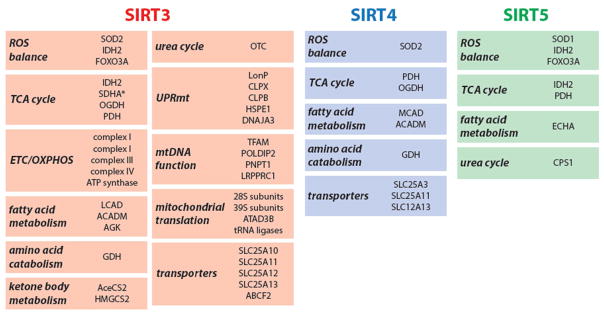
While initial studies focused on identifying individual substrates for mitochondrial sirtuins, recent work has focused on elucidating the networks associated with sirtuins. An emerging idea from these studies is that sirtuins do not regulate the activity of a few key substrates, but rather, regulates functional clusters of targets to orchestrate a coordinated, physiological response [9,24–27]. For example, efforts to systematically identify mitochondrial sirtuins’ substrates have defined changes in the landscape of protein acylation in the presence and absence of SIRT3 or SIRT5; these studies have mainly replied on genetic knockout (KO) mouse models [24–27]. Such approaches have yielded an overview of mitochondrial sirtuin substrates but have not provided information on the complex and dynamic interplay between different mitochondrial sirtuins. Recently, our laboratory used a proteomics approach to map the landscape of mitochondrial protein-protein interactions involving SIRT3, SIRT4 and SIRT5 [9]. The resulting high-confidence interactome provides a unique view of the specificity and coordination of mitochondrial sirtuins, illustrating the complexity and specificity of mitochondrial sirtuin-substrate binding [9]. These data identify unique, as well as overlapping binding partners. Among mitochondrial sirtuins, SIRT3 was found to associate the most with various interacting proteins, whereas SIRT4 and SIRT5 associated with fewer proteins, suggesting that the latter might regulate a narrower spectrum of mitochondrial pathways [9]. SIRT3 associated with proteins involved in amino acid metabolism, fatty acid oxidation, the TCA cycle and ETC/OXPHOS complexes, further highlighting SIRT3’s central role in metabolism (discussed below). Unexpectedly, the SIRT3 interactome revealed associations with mtDNA replication, transcription and translation, suggesting that SIRT3 might control ETC complex formation via the transcriptional and translational control of ETC subunit expression (Figure 2). SIRT4 bound to proteins from similar pathways used by SIRT3, including glutamate dehydrogenase (GDH). Interestingly, only a few enzymes involved in fuel utilization and energy production bound to SIRT5 specifically when compared to control datasets (Figure 2) [9].
Figure 2. Mitochondrial Sirtuins Coordinate the Stress Response Through Control of Substrate Networks.
Recent research has revealed that mitochondrial sirtuins regulate networks of mitochondrial proteins [9]. Shown are the different mitochondrial programs (bold italic) that are under the control of SIRT3, SIRT4 and SIRT5. Note the diversity of substrates and interaction partners, most notably for SIRT3, and the overlap between substrates and functional groups of substrates between sirtuins. The proteins shown are either experimentally validated as substrates or high-confidence sirtuin interacting proteins. For the complete mitochondrial sirtuin networks, see [9].
The sirtuin interactome revealed a dynamic regulation of SIRT3 protein binding in healthy versus stressed mitochondria. For example, under homeostatic conditions, SIRT3 associated with ATP5O, an ATP synthase subunit [9]. However, upon mitochondrial membrane depolarization, the matrix pH decreased from 8.0 to ~6.7, resulting in lowered binding affinity between SIRT3 and ATP5O [9]. Under low pH conditions, SIRT3 dissociated from ATP synthase, deacetylating substrates in the mitochondrial matrix to promote oxidative metabolism and restore the proton gradient[9]. In the future, the mitochondrial sirtuin interactome may serve as a roadmap to deepen our understanding of mitochondrial sirtuin biology. Further research is required to validate these interacting proteins as bona fide sirtuin substrates and/or regulators, and determine the functional significance of these associations; nevertheless, the interactome may potentially facilitate uncovering additional mechanisms that control sirtuin function during cellular homeostasis and stress.
Mitochondrial Sirtuins Protect against Age-Related Diseases
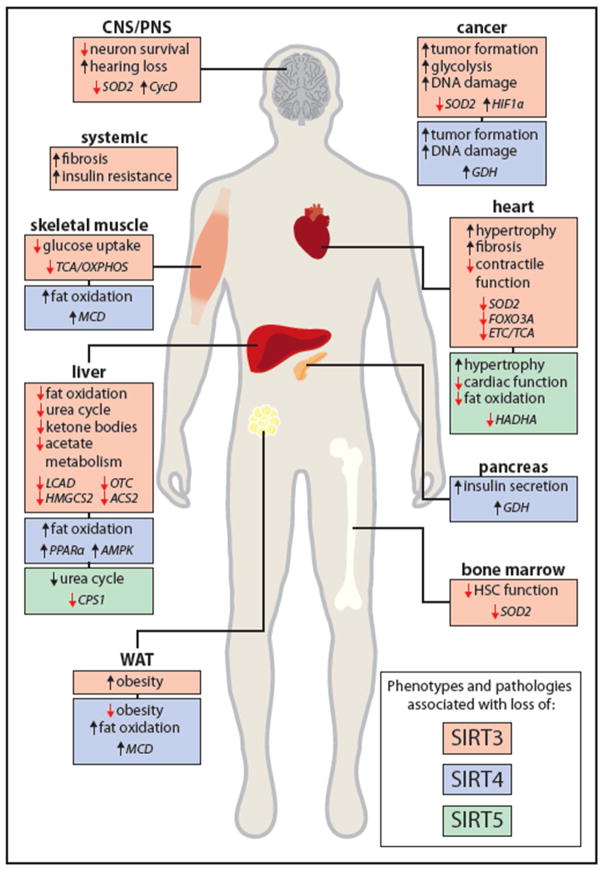
The use of genetic models has contributed greatly to our understanding of sirtuin biology during stress and aging. Loss of mitochondrial sirtuin function, especially SIRT3, has been linked to a number of age-related pathologies including cancer, insulin resistance, heart disease, fibrosis and neurodegeneration [28] (Figure 3). In the next sections, we highlight the molecular mechanisms and sirtuin substrates that underlie the development of age-related pathologies when mitochondrial sirtuin function is compromised. Due to space contraints, we focus primarily on mammalian model systems.
Figure 3. Loss of SIRT3, SIRT4 and SIRT5 Can Cause Age-Related Pathologies.
Loss of SIRT3 (red), SIRT4 (blue) and SIRT5 (green) have been associated with the development of age-related diseases including cardiopathologies, insulin resistance, reduced immune function and neurodegeneration. The major phenotype associated with loss of the specific mitochondrial sirtuin is described per organ system together with the identified substrates (italicized) responsible for the development of the disease. See the section “Mitochondrial Sirtuins Protects Against Age-Related Diseases” for more detail on the different sirtuin substrates and phenotypes associated with loss of sirtuin function. WAT: white adipose tissue, CNS: central nervous system. PNS: pheriperal nervous system.
Mitochondrial Sirtuins and Oxidative Stress
Aging and age-related diseases, including cancer, diabetes and neurodegeneration are connected with increased levels of reactive oxygen species (ROS). ROS are normal byproducts of metabolism. On the one hand, high levels of ROS undermine cellular integrity by damaging lipids, proteins and DNA. On the other hand, balanced ROS levels fulfill important signaling functions and are critical for cellular homeostasis [29]. Numerous studies have linked sirtuin function to both arms of ROS: the regulation of ROS-mediated signaling, as well as the detoxification of damaging ROS.
SIRT3 balances ROS levels via three main mechanisms. First, SIRT3 can directly induce clearance of ROS by deacetylation and activation of superoxide dismutase (SOD2) [30,31]. SOD2 converts superoxide to H2O2, which is neutralized by glutathione [32]. In addition, SIRT3 controls ROS levels in a more indirect fashion by promoting ETC function. Indeed, SIRT3 deacetylates all ETC complexes (including ATP synthase) in order to promote efficient electron transport leading to reduced ROS production and maximizing ATP production [33–38]. In particular, SIRT3 modulates the efficiency of complex I and complex II subunit SDH-A, the two sites where donated electrons from NADH and FADH2 are being fed into the ETC [33,34,36]. Moreover, SIRT3 contributes to glutathione production indirectly by activating the TCA cycle enzyme isocitrate dehydrogenase (IDH2) [39]. IDH2 then oxidizes isocitrate to α-ketoglutarate, thus producing NADPH which is used as a reducing agent to generate glutathione and via this route, contributes to dampening increased ROS levels [40]. Finally, mouse and human SIRT3 controls ROS by inducing a transcriptional antioxidant program through activation of FOXO3A, inducing the transcription of nuclear and mitochondrial genes involved in antioxidant programs and ECT function [41,42]. In sum, SIRT3 can rapidly balance ROS levels through post-translational modifications and in parallel, activate a long-term transcriptional program to protect cells against oxidative damage.
SIRT3 regulation of ROS impacts numerous physiologies linked with aging. For example, Sirt3 expression declines with advanced age in murine bone-marrow residing hematopoietic stem cells (HSCs) [43]. Specifically, Sirt3-deficient aged mice display elevated ROS levels leading to reduced HSC numbers accompanied by diminished self-renewal and reconstitution potentials, thus leading to a decline in immune function [43]. In cancer (murine mammary tumors), Sirt3 loss in mouse embryonic fibroblasts has been associated with increased ROS levels and genomic instability [35]. Moreover, SIRT3 loss in murine and human cells can result in glycolytic metabolic reprogramming of cancers due to ROS-mediated stabilization of HIF1a, thereby contributing to the Warburg effect [44,45].
In contrast to SIRT3, the regulation of ROS by SIRT4 and SIRT5 has been much less studied. However, these sirtuins may contribute to ROS homeostasis based on their targets. For example, SIRT5 may act in parallel to SIRT3 via deacetylation of FOXO3A and desuccinylation of IDH2 [46,47]. Likewise, SIRT4 may affect ROS via pyruvate dehydrogenase complex (PDH), glutamate dehydrogenase (GDH) and fatty acid oxidation through malonyl-CoA decarboxylase (MCD) [48–50]. While this will ultimately lead to decreased donation of electrons to the ETC and reduce the production of ROS, recent studies suggest that Sirt4 activity may increase ROS levels in murine cardiomyocytes [51,52]. In sum, decline of sirtuin activity during aging may compromise the signaling function of balanced ROS levels to impair mitochondrial and cellular homeostasis.
Mitochondrial Sirtuins Regulate Metabolic Plasticity
Mitochondrial sirtuins and their metabolic targets are best studied for their function in metabolic adaptation to nutrient stress such as fasting and caloric restriction (CR). As shown in mice, during times of low energy, Sirt3 expression in liver is induced to promote a metabolic switch to fatty acid oxidation (FAO) and ketone body production to provide alternative energy sources [53]. Activation of enzymes involved in fatty acid oxidation and ketogenesis, such as long-chain acyl coenzyme A dehydrogenase (LCAD) and 3-hydroxy-3-methylglutaryl CoA synthase 2 (HMGCS2), is required for ATP and ketone body production during periods of nutrient stress [53]. Another mouse study showed that Sirt3 not only promotes ketone body production in the liver, but also positively regulates ketone body utilization in the brain, emphasizing a crucial role for SIRT3 in metabolic fuel switching [54]. Increased levels of acetate observed under low nutrient conditions may also provide an additional source of energy regulated by SIRT3. For example, deacetylation and activation of acetyl-CoA synthetase 2 (AceCS2) by human SIRT3 promotes acetate recycling into acetyl-CoA for energy production in extrahepatic tissues [55,56]. Mouse Sirt3 has also been shown to upregulate the urea cycle through deacetylation and activation of ornithine transcarbamoylase (OTC), suggesting a role for SIRT3 in increased amino acid catabolism and ammonia detoxification during periods of metabolic stress [53].
The physiological consequences of SIRT3 loss in the setting of metabolic stress and aging-related disease models have been well studied in vivo. Despite the fact that mice lacking Sirt3 are phenotypically normal under basal conditions [57], Sirt3 deficient mice placed on a high fat diet (HFD) display accelerated development of metabolic syndrome including: accelerated insulin resistance, obesity, hyperlipidemia and steatohepatitis [58]. In mouse muscle, Sirt3 is necessary to preserve glucose oxidation and mitochondrial function thereby opposing the metabolic switch observed during HFD-induced insulin resistance [37,59,60]. In addition, Sirt3 has been directly linked to cardiomyocyte homeostasis by the observation that aged Sirt3-KO mice develop cardiac hypertrophy, a pathological condition caused by enlargement of the heart muscle that can lead to heart failure [41,61,62]. Additionally, loss of Sirt3 in mice has been reported to induce fibrosis in the heart, lung, kidneys and liver [62]. These findings are relevant because fibrosis -- the buildup of excess fibrous tissue in organs -- is typical of the aging process [63]. Together, these findings show that SIRT3 can coordinate multiple response nodes to metabolic stress. SIRT3 regulates metabolic changes upon low nutrient availability and alters metabolic fuel preference in energy-demanding tissues during stress. Therefore, SIRT3 loss may potentially contribute to the development of numerous age-related pathologies including insulin resistance, cardiac dysfunction, fibrosis and cancer (Figure 3).
SIRT4 inhibits mitochondrial catabolism of amino acids and fatty acids [48–50,64–66]. Sirt4 has been shown to repress glutamate dehydrogenase (GDH) activity and suppresses insulin secretion in mouse models [48]. Sirt4 also suppresses FAO via deacetylation and inhibition of malonyl CoA decarboxylase (MCD) activity [49]. Like SIRT3, SIRT4 can also regulate mitochondrial derived signaling; in mice, Sirt4 has been shown to represses fat oxidation by inhibiting peroxisome proliferator-activated receptor α (PPARα) activity [66], as well as AMPK signaling [65]. Furthermore, Sirt4-KO mice exhibit increased FAO, protection against diet-induced obesity, and increased exercise tolerance [49]. Recently, SIRT4 was found to decrease the activity of the pyruvate dehydrogenase (PDH) complex through lipoamidase activityin human cell lines and mouse models [50]. Inhibition of PDH by SIRT4 suppressed pyruvate decarboxylation and subsequent generation of acetyl-CoA[50]. Interestingly, SIRT4 expression is induced by different stresses including DNA damage, reduced mammalian target of rapamycin (mTOR) signaling, increased endoplasmic reticulum (ER) and oxidative stress, thus providing another link between metabolism and stress responses [64,67,68].
SIRT5, a major mitochondrial succinyltransferase, plays an important role in metabolic adaptations, as shown during periods of increased amino acid catabolism in mouse liver [69]. Experiments from human cell lines and mice indicate that Sirt5, in the liver, can promote the detoxification of ammonia by deacylating and activating carbamoyl phosphate synthetase 1 (CPS1), the first and rate-limiting enzyme in the urea cycle [69–71]. SIRT5, like SIRT3, also appears to play a major role in regulation of heart function [72]. For instance, in mice, Sirt5-mediated desuccinylation and activation of Hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase trifunctional protein subunit alpha (HADHA) is necessary to maintain FAO and energy production during periods of energy demand in heart tissue [72]. Accordingly, Sirt5 deficient mice develop hypertrophic cardiomyopathy and show reduced cardiac function during aging [72]. In agreement with these data, Sirt5-KO mice display protein hypersuccinylation in muscle and liver tissue and exhibit FAO defects, indicating that SIRT5 may possess uncharacterized targets [25]. In addition, in mice, Sirt5 can regulate other metabolic pathways such as glycolysis, the TCA cycle, and amino acid metabolism, although the relevance and the specific molecular targets involved remains unclear [27,73].
Together, emerging data demonstrate that mitochondrial sirtuins are important mediators of metabolic plasticity - the dynamic metabolic adaptation to periods of stress. As discussed in this section, SIRT3-5 activity can rewire various metabolic pathways to dynamically switch between different fuels and mediate disposal of catabolic byproducts. Loss of SIRT3-5 and subsequent deregulation of glucose, fatty acid and protein metabolism in response to nutrient stress is fundamental to the development of age-related pathologies. Future studies elucidating the upstream regulation of sirtuins, as well as the coordination of their activity will deepen our understanding of mitochondrial metabolism. In addition, it will be interesting to examine whether control of metabolism mediates cross-talk with other compartments of the cell, such as the cytosol and nucleus.
Mitochondrial Sirtuins Promote Genome Fidelity
The integrity of both the nuclear and mitochondrial genome is essential to maintain tissue homeostasis. To combat the potential devastating consequences of DNA damage, the cell has evolved an intricate signaling network termed the DNA damage response (DDR) [74]. Defects in DNA repair pathways induce DNA damage accumulation in both mitochondrial and nuclear compartments and can induce premature aging in combination with the development of various malignancies [74,75]. For instance, studies in mice have demonstrated that DNA damage accumulation in aged HSCs can underlie diminished stem cell function and tissue decline [76,77]. Cells need to tightly orchestrate their cellular metabolic response under genotoxic conditions to support DNA repair. In the cytosol, a central kinase in the DDR signaling cascade, ataxia-telangiectasia mutated (ATM) activates the pentose phosphate pathway (PPP) to support DNA repair through the generation of nucleotide precursors and AMPK signaling [78,79].
Mitochondrial sirtuins may regulate DNA fidelity via ROS and generation of DNA lesions, and also by controlling a bona fide metabolic checkpoint. SIRT3 and SIRT5 can also contribute to genomic stability through the regulation of ROS levels (see section “Mitochondrial Sirtuins and Oxidative Stress”). Sirt3-mediated deacetylation and activation of SOD2 has been shown to protect cells against genomic instability and oncogene-induced transformation in mouse embryonic fibroblasts [80]. Moreover, in mouse mitochondria, Sirt4 has been found to activate a metabolic checkpoint to halt cell cycle progression and ensure DNA damage repair [64]. Interestingly, the Sirt4 transcript can be induced in response to stressors, such as ionizing radiation, topoisomerase inhibitors, or even mTOR inhibition in mouse cells [64,67,68]. Under these conditions, Sirt4 restricts amino acid catabolism through inhibition of GDH to halt the cell cycle and allow for DNA repair to occur [64,68]. Consequently, loss of Sirt4 impairs the clearance of nuclear DNA damage and leads to genomic instability [64]. The physiological consequence of Sirt4 loss of function and genomic instability is clear; Sirt4 loss of function is associated with spontaneous lung tumors in mice and decreased survival in animals with B cell lymphoma [64]. However, the mechanism by which Sirt4 and GDH inhibition promote genome fidelity is still not well understood. It will be interesting to investigate whether other pathways regulated by SIRT4 mediate direct protection against DNA damage.
The interactions between NAD+-consuming enzymes and DNA damage goes beyond what occurs in mitochondria. It is intriguing that members of another NAD+-consuming class of enzymes, poly(ADP-ribose) polymerase 1/2 (PARP1/2) intricately connects mitochondrial and cellular homeostasis to genomic stability [81]. PARP1/2 sense single-strand and double-strand (ds) DNA breaks and catalyze the NAD+-dependent addition of poly-(ADP)ribose to proteins, including histones and PARP1 itself, near dsDNA lesions [74]. PARP1/2 activity induces chromatin remodeling through recruitment of histone deacetylases complexes to the site of DNA damage. Additionally, the poly(ADP-ribosyl) chains functions as a scaffold for the binding of DNA damage repair enzymes [82]. In addition, the nuclear and cytocolic sirtuins SIRT1 and SIRT6 are directly involved in DNA damage sensing and repair in the nucleus, which implies that sirtuin function in different cellular compartments can impinge on genomic stability (reviewed in [83]). Accumulation of DNA damage during aging may potentially activate PARP1/2 and nuclear sirtuins leading to decreased cellular NAD+ levels. While NAD+ levels in the mitochondria remain stable in response to genotoxic stress, prolonged NAD+ depletion can ultimately lead to mitochondrial dysfunction [21].
In sum, mitochondrial sirtuins support DDR and limit DNA damage through distinct mechanisms, including activation of a metabolic checkpoint in response to genotoxic stress by SIRT4 and by balancing ROS levels through SIRT3. While the mechanisms of DNA damage sensing have not been deeply examined in mitochondria, loss of SIRT4 and SIRT3 has important implications in the development of genomic instability and tumorigenesis [83,84]. Future studies should aim to elucidate additional mechanisms by which sirtuins and glutamate metabolism control DNA damage responses.
Mitochondrial Sirtuins and Aging: Hearing Loss and Neurodegeneration
Recent research has linked loss of mitochondrial sirtuin function to major aspects of the aging process: neurodegeneration in the central and peripheral nervous systems (Figure 3), as well as other disorders such as hearing loss. For instance, Sirt3 dysfunction has been implicated in age-related hearing loss (AHL) [40], a major sensory disorder in aged humans [85]. Whereas wild type mice displayed caloric restriction (CR)-mediated protection against AHL, Sirt3 deficient animals developed AHL, characterized by loss of cochlear cells in the auditory part of the inner ear. The molecular mechanism underlying CR-dependent protection against AHL was mapped to Sirt3-mediated deacetylation and activation of Idh2, with subsequent reduction in ROS levels in cochlear cells [40]. Furthermore, Sirt3 function also seems to protect against noise-induced hearing loss (NIHL), at least in mice [86]. Chronic and/or intense exposure to noise in mice can induce NIHL through degeneration of neurons connecting the cochlea to the central nervous system and therefore, are critical for proper hearing [87]. Interestingly, stimulation of NAD+ levels by dietary supplementation with the NAD+ precursor nicotinamide riboside was shown to be sufficient to rescue animals from NIHL, whereas Sirt3-deficient animals did not respond to this treatment. In line with these observations, cultured neurons from Sirt3-deficient mice have been reported to display increased sensitivity to cell death in response to oxidative and metabolic stress, as well as excitotoxicoly -- a phenomenon where neurons are damaged and killed in response to overstimulation [88]. In terms of neurodegenerative brain diseases, Sirt3-deficient mice have been shown to display increased neuronal damage in models approximating Huntington’s Disease and epilepsy [88]. Neuronal damage in these mice appears to occur through hyperacetylation of SOD2 and cyclophilin D, a component of the mitochondrial permeability transition pore [88]. Of note, neuronal Sirt3 levels were increased after mild in vitro excitation with the neurotransmitter glutamate and following voluntarily exercise, which suggests that modulating SIRT3 levels via glutamate could have therapeutic potential in neurodegenerative disorders. However, future work is warranted to fully validate these hypotheses.
Together, these studies indicate that SIRT3 expression can be important for neuronal survival and function under stress. In addition, these results suggest that elevating SIRT3 activity, for instance through CR-mediated expression, NAD+ restoration, or excitation, could be contemplated for therapeutic approaches aiming to combat the age-related decline in sirtuin activity in the central and peripheral nervous systems. The involvement of other mitochondrial sirtuins, SIRT4 and SIRT5 in neuroprotection remains understudied and consequently, represents a rich area for future research. It is conceivable that SIRT5 could directly protect neurons by balancing ROS levels and indirectly, through control of systemic ammonia levels; however, the causal link between SIRT5 loss and neurodegeneration remains undetermined [69].
Concluding Remarks
Mitochondrial sirtuins SIRT3-5 function as sensors of many distinct stress inputs and integrate this information in a coordinated response to maintain cellular homeostasis. While recent efforts have provided novel insights into the complex network of sirtuin substrates and their interactions in mitochondria, many outstanding questions and clinical opportunities remain (see Outstanding Questions and Box 1). For instance, the post-translational modifications that directly impinge on sirtuin activity or the mechanisms of sirtuin substrate specificity are mostly uncharacterized. Nonetheless, it is evident that SIRT3, SIRT4 and SIRT5 regulate vast clusters of different proteins to induce a stress response. In addition, the overlap between substrate specificity and/or enzymatic activity adds an additional layer of complexity to achieving cellular homeostasis, as different sirtuins may have opposing effects on their substrates. The use of genetic mouse models has benefited our understanding of mitochondrial sirtuin biology and has implicated loss of SIRT3-5 in the development of age-related pathologies. Additionally, decreased NAD+ levels during aging can reduce sirtuin activity, which in turn, may contribute to the aging process. Conservation or restoration of the cellular and mitochondrial pool of NAD+ may therefore represent a promising strategy to boost sirtuin activity. Because mitochondrial sirtuins impact many facets of mitochondrial metabolism and signal transduction, targeting sirtuin activation could be potentially exploited as a therapeutic strategy aiming to combat age-related mitochondrial decline.
Outstanding Questions.
Which mechanisms directly control the activity of mitochondrial sirtuins?
While NAD+ levels influence the activity of mitochondrial sirtuins, post-translational modifications on sirtuins themselves have been identified including: phosphorylation, acetylation and methylation (www.phosphosite.org). However, our understanding of how post-translational modification regulates sirtuin activity or the physiological relevance of such modification is poorly understood.
What determines the substrate specificity of different mitochondrial sirtuins?
Proteomics studies in mice and mammalian cell lines have revealed the landscape of acylation and sirtuin interactions in mitochondria, identifying novel sirtuin substrates. These approaches have shown that clusters of enzymes that share similar biological functions are controlled by mitochondrial sirtuins. However, our knowledge of the molecular mechanisms by which mitochondrial sirtuins recognize and bind their specific substrates is currently incomplete.
What is the significance of long-chain acylation in mitochondria?
In vitro analysis of sirtuin activity has revealed that mitochondrial sirtuins can remove long-chain acyl modifications. In contrast to acetylation, the effect of these long-chain acyl modifications on the activity of mitochondrial proteins and their physiological relevance is incompletely understood.
Can we harness the therapeutic potential of mitochondrial sirtuins?
Mitochondrial sirtuins modulate a number of age-related diseases, including cancer, diabetes, and neurodegeneration, suggesting that increasing (or in certain instances inhibiting) their activity may be beneficial to patients.
Can we develop compounds that target mitochondrial sirtuin activity or their interactions with other proteins?
Such tools might be beneficial in discovery, and harbor the potential to improve the clinical outcomes of a wide range of diseases.
Box 1. Clinician’s Corner.
Age is the major risk factor for the development of chronic diseases. The current demographic shift towards an aged population emphasizes the need for a better understanding of the molecular pathways underlying aging. Loss of mitochondrial sirtuin function in genetic mouse models has been demonstrated to contribute to the development of different age-related diseases including fibrosis, neurodegeneration, insulin resistance and various cardiopathologies.
Mitochondrial dysfunction is a hallmark of aging and is characterized by decreased expression of metabolic enzymes, a decrease in respiratory capacity and increased levels of ROS. While mitochondrial aging is presumably caused by multiple coinciding factors, the central role of SIRT3-5 in regulating mitochondrial metabolism places them as important therapeutic candidates for restoring mitochondrial function during aging.
Mitochondrial sirtuins (SIRT3-5) are members of the sirtuin protein family of deacylases and ADP-ribosyltransferases which depend on NAD+ as a co-substrate. Therapeutic and/or preventive strategies to induce sirtuin function could therefore potentially improve the health span of aging individuals. For instance, caloric restriction induces expression of Sirt3 and has been demonstrated to extend health span in mice, at least partially, in a Sirt3-dependent manner.
Recent efforts have focused on the dietary supplementation with NAD+ precursors as a strategy to induce sirtuin activity. However, the significance and applicability of these strategies to human patients remain unknown.
Trends Box.
Mitochondrial sirtuins SIRT3, SIRT4, and SIRT5 are NAD+-dependent deacetylases, deacylases and ADP-ribosyl transferases. Their dependence on NAD+ directly links their enzymatic activity to the metabolic state of the cell.
In mammalian tissues, mitochondrial sirtuin expression and/or activity may decline with age and contributes to mitochondrial dysfunction, a major hallmark of aging. Additionally, loss of function studies in genetic mouse models have linked decreased sirtuin function to the development of age-related diseases including neurodegenetation, insulin resistance and heart disease.
Supplementation of NAD+ improves healthspan and restores mitochondrial homeostasis in model systems.
Recent insights have revealed that mitochondrial sirtuins affect many facets of mitochondrial biology through the regulation of vast networks of metabolic and non-metabolic enzymes, thus ensuring that mitochondrial homeostasis is achieved during stress conditions.
Acknowledgments
This work is supported by NIH Grant DK103295 and the Glenn Foundation for Medical Research.
Glossary
- Sirtuin family of deacylases and ADP-ribosyl transferases
The sirtuin protein family is a seven member family (SIRT1-7) that possesses NAD+-dependent deacylase and ADP-ribosyl transferase activity. Through post-translational-modifying substrates, sirtuins control a wide range of cellular processes including metabolism, DNA damage repair and gene transcription.
- Tricarboxylic acid (TCA) cycle
A series of chemical reactions that generate energy (in the form of NADH and FADH2) from glucose, fatty acids and proteins under aerobic conditions.
- Complex I
Multiprotein complex that resides in the mitochondrial inner membrane and catalyzes the transfer of electrons from NADH to other electron transport chain complexes. The transfer of electrons is coupled to the translocation of protons to the mitochondrial inner membrane space.
- Electron Transport Chain (ETC)
Series of complexes that transfer electrons from NADH and FADH2 in order to build the proton gradient that is ultimately used by ATP synthase to generate energy in the form of ATP.
- Mitochondrial membrane depolarization
Loss of the proton gradient through loss of oxidative phosphorylation or mitochondrial membrane permeability.
- Complex II
Protein complex residing in the mitochondrial inner membrane that functions in the TCA cycle, where it catalyzes the conversion of succinate to fumarate (also known as succinate dehydrogenase (SDH)) and in the ETC, where it catalyzes the transfer of electrons from FADH2 to the other ECT complexes.
- Fatty acid oxidation (FAO)
The catabolism of fatty acids to generate acetyl-CoA, which enters the TCA cycle, and NADH and FADH2.
- Urea cycle
Series of chemical reactions that mediate the detoxification of ammonia into urea. The liver is the major organ using the urea cycle.
- Metabolic syndrome
A group of conditions that include increased blood pressure, elevated blood sugar levels, increased visceral fat and elevated circulating triglycerides. Together, these symptoms increase the risk for heart disease, diabetes and stroke.
- Steatohepatitis
A type of fatty liver disease characterized by fat accumulation and inflammation in the liver.
- Warburg effect
phenomenon where cells (such as cancer cells) display which involves increased glycolysis even in the presence of oxygen.
- Mammalian target of rapamycin (mTOR)
mTOR is a serine/threonine kinase and is part of two distinct complexes mTORC1 and mTORC2. mTOR is best known for its central function in nutrient sensing and growth control.
- DNA damage response
A network of signaling proteins that collectively coordinate the cellular response to DNA damage to mediate DNA damage repair, halt cell cycle progression or induce apoptosis.
- Ataxia-telangiectasia mutated (ATM)
A serine/threonine kinases that plays a central role in the DDR and is responsible for the activation of numerous downstream targets in response to DNA damage. ATM substrates include p53, CHK1/2 and BRCA1.
- TCA cycle anaplerosis
The collective term for the chemical reactions that replenish TCA intermediates. The opposite is TCA cycle cataplerosis where TCA intermediates are being extracted for biosynthesis.
- Poly(ADP-ribose) polymerases 1/2 (PARP1/2)
PARP1/2 are members of a larger family of NAD+-dependent poly(ADP-ribosyl) transferases that sense single- and double-strand lesions to catalyze the addition of poly(ADP-ribosyl) chains to histones and PARP1 itself to recruit the DNA repair machinery.
- Mitochondrial permeability transition pore (MPTP)
A multiprotein complex that directly links the mitochondrial matrix to the cytosol. Prolonged opening of the MPTP leads to membrane depolarization, mitochondrial swelling and induction of apoptosis.
- Long-chain acyl modifications
A type of post-translational protein modification based on the transfer of long-chain acyl chains to substrate lysines. Long-chain acyl chains include myristoyl (14-carbon) and palmitoyl (16-carbon) in contrast to the shortest acyl modification, acetyl (2-carbon).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.López-Otín C, et al. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun N, et al. The Mitochondrial Basis of Aging. Mol Cell. 2016;61:654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 4.German NJ, Haigis MC. Sirtuins and the Metabolic Hurdles in Cancer. Curr Biol. 2015;25:R569–83. doi: 10.1016/j.cub.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25:138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vassilopoulos A, et al. The human sirtuin family: evolutionary divergences and functions. Hum Genomics. 2011;5:485–496. doi: 10.1186/1479-7364-5-5-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sebastián C, et al. From sirtuin biology to human diseases: an update. J Biol Chem. 2012;287:42444–42452. doi: 10.1074/jbc.R112.402768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschey MD, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang W, et al. Mitochondrial Sirtuin Network Reveals Dynamic SIRT3-Dependent Deacetylation in Response to Membrane Depolarization. Cell. 2016;167:985–1000. e21. doi: 10.1016/j.cell.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winnik S, et al. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 2015;36:3404–3412. doi: 10.1093/eurheartj/ehv290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci. 2013;5:48. doi: 10.3389/fnagi.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman JL, et al. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem. 2013;288:31350–31356. doi: 10.1074/jbc.C113.511261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshino J, et al. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantó C, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes AP, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mouchiroud L, et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 18.Mills KF, et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ying W, et al. NAD+ as a metabolic link between DNA damage and cell death. J Neurosci Res. 2005;79:216–223. doi: 10.1002/jnr.20289. [DOI] [PubMed] [Google Scholar]
- 20.Alano CC, et al. Differences among cell types in NAD(+) compartmentalization: a comparison of neurons, astrocytes, and cardiac myocytes. J Neurosci Res. 2007;85:3378–3385. doi: 10.1002/jnr.21479. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai SI, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantó C, et al. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hebert AS, et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell. 2013;49:186–199. doi: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rardin MJ, et al. Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc Natl Acad Sci USA. 2013;110:6601–6606. doi: 10.1073/pnas.1302961110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rardin MJ, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18:920–933. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishida Y, et al. SIRT5 Regulates both Cytosolic and Mitochondrial Protein Malonylation with Glycolysis as a Major Target. Mol Cell. 2015;59:321–332. doi: 10.1016/j.molcel.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonnell E, et al. SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol Metab. 2015;26:486–492. doi: 10.1016/j.tem.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu X, et al. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, et al. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011;12:534–541. doi: 10.1038/embor.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Remmen H, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 33.Ahn BH, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cimen H, et al. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010;49:304–311. doi: 10.1021/bi901627u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HS, et al. SIRT3 Is a Mitochondria-Localized Tumor Suppressor Required for Maintenance of Mitochondrial Integrity and Metabolism during Stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finley LWS, et al. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS ONE. 2011;6:e23295. doi: 10.1371/journal.pone.0023295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jing E, et al. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci USA. 2011;108:14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman M, et al. Drosophila Sirt2/mammalian SIRT3 deacetylates ATP synthase β and regulates complex V activity. J Cell Biol. 2014;206:289–305. doi: 10.1083/jcb.201404118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu W, et al. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem. 2012;287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Someya S, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundaresan NR, et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peserico A, et al. A novel AMPK-dependent FoxO3A-SIRT3 intramitochondrial complex sensing glucose levels. Cell Mol Life Sci. 2013;70:2015–2029. doi: 10.1007/s00018-012-1244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown K, et al. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013;3:319–327. doi: 10.1016/j.celrep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finley LWS, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell. 2011;19:416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bell EL, et al. SirT3 suppresses hypoxia inducible factor 1α and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30:2986–2996. doi: 10.1038/onc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, et al. SIRT5 prevents cigarette smoke extract-induced apoptosis in lung epithelial cells via deacetylation of FOXO3. Cell Stress Chaperones. 2015;20:805–810. doi: 10.1007/s12192-015-0599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou L, et al. SIRT5 promotes IDH2 desuccinylation and G6PD deglutarylation to enhance cellular antioxidant defense. EMBO Rep. 2016;17:811–822. doi: 10.15252/embr.201541643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haigis MC, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 49.Laurent G, et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol Cell. 2013;50:686–698. doi: 10.1016/j.molcel.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathias RA, et al. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell. 2014;159:1615–1625. doi: 10.1016/j.cell.2014.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo Y-X, et al. Sirt4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw138. [DOI] [PubMed] [Google Scholar]
- 52.Lang A, et al. MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secretory phenotype through sirtuin 4/SIRT4. Aging (Albany NY) 2016;8:484–505. doi: 10.18632/aging.100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hallows WC, et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell. 2011;41:139–149. doi: 10.1016/j.molcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dittenhafer-Reed KE, et al. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab. 2015;21:637–646. doi: 10.1016/j.cmet.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwer B, et al. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proceedings of the National Academy of Sciences. 2006;103:10224–10229. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hallows WC, et al. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proceedings of the National Academy of Sciences. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lombard DB, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirschey MD, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jing E, et al. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes. 2013;62:3404–3417. doi: 10.2337/db12-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lantier L, et al. SIRT3 Is Crucial for Maintaining Skeletal Muscle Insulin Action and Protects Against Severe Insulin Resistance in High-Fat-Fed Mice. Diabetes. 2015;64:3081–3092. doi: 10.2337/db14-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hafner AV, et al. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sundaresan NR, et al. SIRT3 Blocks Aging-Associated Tissue Fibrosis in Mice by Deacetylating and Activating Glycogen Synthase Kinase 3β. Mol Cell Biol. 2015;36:678–692. doi: 10.1128/MCB.00586-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mehal WZ, et al. Scraping fibrosis: expressway to the core of fibrosis. Nat Med. 2011;17:552–553. doi: 10.1038/nm0511-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeong SM, et al. SIRT4 Has Tumor-Suppressive Activity and Regulates the Cellular Metabolic Response to DNA Damage by Inhibiting Mitochondrial Glutamine Metabolism. Cancer Cell. 2013;23:450–463. doi: 10.1016/j.ccr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nasrin N, et al. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 2010;285:31995–32002. doi: 10.1074/jbc.M110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laurent G, et al. SIRT4 represses peroxisome proliferator-activated receptor α activity to suppress hepatic fat oxidation. Mol Cell Biol. 2013;33:4552–4561. doi: 10.1128/MCB.00087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeong SM, et al. SIRT4 regulates cancer cell survival and growth after stress. Biochem Biophys Res Commun. 2016;470:251–256. doi: 10.1016/j.bbrc.2016.01.078. [DOI] [PubMed] [Google Scholar]
- 68.Csibi A, et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell. 2013;153:840–854. doi: 10.1016/j.cell.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakagawa T, et al. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du J, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan M, et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014;19:605–617. doi: 10.1016/j.cmet.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadhukhan S, et al. Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proceedings of the National Academy of Sciences. 2016;113:4320–4325. doi: 10.1073/pnas.1519858113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park J, et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013;50:919–930. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoeijmakers JHJ. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 76.Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 77.Rossi DJ, et al. Hematopoietic stem cell quiescence attenuates DNA damage response and permits DNA damage accumulation during aging. Cell Cycle. 2007;6:2371–2376. doi: 10.4161/cc.6.19.4759. [DOI] [PubMed] [Google Scholar]
- 78.Cosentino C, et al. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011;30:546–555. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanli T, et al. AMP-activated protein kinase (AMPK) beyond metabolism: a novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol Ther. 2014;15:156–169. doi: 10.4161/cbt.26726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tao R, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Vos M, et al. The diverse roles and clinical relevance of PARPs in DNA damage repair: current state of the art. Biochem Pharmacol. 2012;84:137–146. doi: 10.1016/j.bcp.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 82.Schreiber V, et al. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 83.Jeong SM, Haigis MC. Sirtuins in Cancer: a Balancing Act between Genome Stability and Metabolism. Mol Cells. 2015;38:750–758. doi: 10.14348/molcells.2015.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finley LWS, Haigis MC. Metabolic regulation by SIRT3: implications for tumorigenesis. Trends Mol Med. 2012;18:516–523. doi: 10.1016/j.molmed.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu XZ, Yan D. Ageing and hearing loss. J Pathol. 2007;211:188–197. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- 86.Brown KD, et al. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 2014;20:1059–1068. doi: 10.1016/j.cmet.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng A, et al. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016;23:128–142. doi: 10.1016/j.cmet.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]