Abstract
Purpose
Determine the rate of local failure (LF) using focal conformal, limited margin radiotherapy (RT) and dose escalation for tumors ≥ 8cm (greatest dimension at diagnosis) in children and young adults with Ewing sarcoma (EWS).
Methods and Materials
Eligible patients with EWS were treated on a Phase II institutional trial of focal conformal, limited margin RT using conformal or intensity modulated techniques. The treatment volume incorporated a 1cm constrained margin (CTV1) around the gross tumor. Unresected tumors, <8cm at diagnosis, received a standard dose of 55.8Gy and tumors ≥8cm an escalated dose to 64.8Gy. Patients with microscopic residual disease after resection received adjuvant RT to 50.4Gy. Adjuvant brachytherapy was permitted in selected patients.
Results
Forty-five patients were enrolled, 26 with localized and 19 with metastatic disease. Median (range) age, tumor size and follow-up were 13.0 years (2.9–24.7), 9.0cm (2.4–17.0) and 54.5 months (1.9–122.2), respectively. All patients received systemic chemotherapy. The median (range) RT dose for all patients was 56.1Gy (45–65.5). Seventeen patients received adjuvant, 16 standard dose and 12 escalated dose RT. Failures included 1 local, 10 distant and 1 local/distant. The estimated 10-year cumulative incidence of LF was 4.4% ± 3.1%, with no statistical difference seen between RT treatment groups and no local failures in the escalated dose RT treatment group.
Conclusions
Treatment with focal conformal, limited margin RT, including dose escalation for larger tumors, provides favorable local tumor control in EWS.
Introduction
Ewing Sarcoma (EWS) is a small round cell neoplasm characterized by a recurring translocation involving the EWS gene on chromosome 22 and the ETS family of transcription factors. EWS accounts for approximately 3% of pediatric cancers and 40% of pediatric bone cancers. Roughly 25% of patients present with metastatic disease, the most significant poor prognostic factor. Outcomes for patients with localized disease have improved, with recently reported survival rates of 75%. Patients with metastatic disease continue to do poorly with survival rates around 25%1–8. Additional reported poor prognostic factors include primary disease site (axial), older age and larger tumor size. Data from our institution showed local control rates of 90% for smaller tumors (<8cm in greatest single dimension), but only 52% for larger tumors (≥8cm in greatest dimension)4,9.
Treatment for EWS consists of a multidisciplinary approach, including systemic chemotherapy and local control (surgery and/or radiotherapy [RT]). A challenge of using RT for the treatment of pediatric sarcomas is the difficulty in balancing the administration of high RT doses needed to achieve a good therapeutic response with protecting nearby developing tissues. Historically, conventional RT was used as definitive RT to patients with EWS, but this approach delivers high doses to surrounding normal tissues. Focal conformal RT techniques, in conjunction with limited margin target volumes, may allow for sparing of normal tissues while still maintaining adequate therapeutic doses of RT to the tumor site10–14.
When comparing local control strategies, definitive RT has been reported to have higher rates of local failure compared to surgery, although overall disease control remained similar8,15. For tumors in locations not amenable to surgery, RT is the only local control method2,16,17. Recommendations for treatment planning of definitive RT historically included the pretreatment tumor volume, determined at diagnosis, with a 2.0–2.5cm margin and for adjuvant RT, a 1.5–2.0cm margin9,14. Recent studies have attempted to decrease this margin, including the Children’s Oncology Group which used a 1.5cm margin in their AEWS0031 protocol18 and are currently investigating using a 1.0cm margin.
We designed a prospective, phase II study to determine local tumor control rates using focal conformal, limited-margin RT for children and young adults with primary musculoskeletal tumors, including EWS. We designed the trial to explore the safety and efficacy of using dose-escalated RT as definitive local control for tumors ≥8cm in greatest dimension at diagnosis, a cohort that historically has high rates of local failure1,4–6,19. This trial was not powered to compare the standard and dose escalated cohorts but rather to evaluate the efficacy and toxicity profiles of this approach.
Methods and Materials
Patient Eligibility and Radiation Treatment Plan
Patients aged ≤25-years-old, diagnosed with localized or metastatic EWS and requiring RT as part of their therapy, were eligible to participate in an institutional review board approved Phase II study of limited-margin RT (NCT00186992). Receipt of prior RT was allowed for cases requiring emergent RT to the primary site of disease for ≤1 week, if the dose was accounted for in analysis. After an initial treatment period with chemotherapy, RT to the primary site of disease was delivered in conjunction with chemotherapy as dictated by the treating oncologist. Chemotherapy was then continued after the completion of RT. Patients with residual tumor (microscopic or macroscopic) after surgical resection of their primary tumor were eligible for adjuvant RT. Metastatic site irradiation was not defined in the protocol, but followed the standard at the time of treating residual visible metastatic disease, either at the time of local control or near the completion of all chemotherapy.
Focal conformal RT target volumes were based on the International Committee on Radiation Units and Measurements (ICRU) report 50 and were generated based on CT and MRI planning datasets, obtained in the treatment position. MRI datasets were co-registered to the CT to help fully define target volumes. If available, pre-therapy and pre-surgery imaging datasets were also co-registered or fused to assist in defining the target volumes. In the post-operative setting, the gross tumor volume (GTV) was defined as the postoperative tumor bed, following the collapsed tumor bed and respecting fascial planes and adjacent bones. In the definitive setting, the GTV included residual gross tumor, initially involved bone, and adjacent soft tissues such as pleural or fascial surfaces felt to be initially infiltrated, but would not regress back to the involved bone with the soft tissue mass. Clinical target volume (CTV1) consisted of the GTV plus a 1cm margin, respecting anatomic barriers that limit tumor spread including adjacent bones, non-infiltrated organs and fascial planes that appeared intact on initial imaging. CTV2 added no margin to the GTV (CTV2=GTV). The planning target volumes (PTVs) ranged from 5–10mm. There was no PTV for brachytherapy cases.
Radiation was delivered via conformal RT, intensity-modulated RT (IMRT), interstitial brachytherapy or a combination of these modalities. External beam radiation was delivered with 6- or 15-MV photons, in 1.8Gy fractions. Brachytherapy was delivered with iridium-192 low or high dose rate sources. RT was categorized into 3 dosing groups based on tumor size at diagnosis (measured in the greatest dimension) and the degree of surgical intervention. Patients treated with definitive RT received 45 Gy to CTV1 (and its respective PTV), and either 55.8 Gy (standard dose for tumors <8cm) or 64.8Gy (escalated dose for tumors ≥8cm) to CTV2 (and its respective PTV). For patients receiving adjuvant RT, 50.5 Gy was delivered to CTV1 (and its respective PTV), with no CTV2. Post-operative brachytherapy was dosed at 15 to 20Gy. When brachytherapy was combined with external beam RT, the total cumulative radiation dose was 45 to 58.6Gy. Any deviations to these protocol dosing guidelines were made based on the need for protection of adjacent normal tissues.
Assessments
After completion of RT, patients were followed every 3 months for 1 year, every 6 months for the second year and then yearly for a total of 10 years. Follow-up visits included history, physical exam, functional evaluation with physical/occupational therapy, MRI of the primary tumor site and chest CT for the first 5 years. PET CT was performed every 6 months for 1 year, then again at 2 years post-therapy. A bone scan was ordered as clinically indicated by the PET CT findings.
Radiation specific treatment related toxicity data was collected weekly during therapy and at each follow-up visit according to the Common Toxicity Criteria, version 2.0. A toxicity event was classified as short term if it occurred within 90 days of undergoing RT. If a toxicity event occurred more than 90 days from the start of RT, it was classified as long term. If a patient developed any event or failure, they were taken off study and no longer followed for the development of another event/failure or for late toxicity from RT.
Statistical Analysis
The primary end point of this trial was evaluation of the rate of local failure for the entire cohort. Subgroup analysis was performed based on patient disease status and RT dosing group to explore for any possible relationships, but this trial was not powered to compare outcomes for the dose-escalated subgroup with the standard dose population. Local failure was defined as tumor recurrence or progressive disease within the original CTV, as seen on imaging or biopsy. Recurrence at the edge of the CTV was classified as a local failure as well. Distant failure was defined as the development of any new metastatic disease. Three patients were not included in RT dosing subgroup analysis as they received RT treatment prior to the implementation of escalated dose RT. They were included in the category of standard dose RT for patient population and toxicity analysis.
Survival curves were estimated using the Kaplan-Meier method and compared by the Log-rank test. Cumulative incidence curves of local failure were estimated using the competing risks method and compared by Gray’s test, with competing risks being the development of distant failure or secondary malignancy. Event-free survival (EFS) and failure-free survival (FFS) analysis was conducted based on patient disease status. EFS was defined as the interval from the date of enrollment on study to the date of development of local failure, distant failure or secondary malignancy, or to the date of last follow-up. FFS was defined as the interval from the date of enrollment on study to the date of development of local/distant failure.
Results
Patient Population
Forty-five patients with EWS were enrolled on this study and received RT from January 2003 to May 2013. At the time of analysis, 13 patients had been taken off study due to development of an event or failure. Patient characteristics are summarized in Table 1. The median age at the time of study enrollment was 13.0 years (range, 2.9 – 24.7 years). The median follow-up of all patients remaining on study at the time of analysis was 54.5 months (range, 1.9 – 122.2 months), with 30 patients on study for at least 36 months and 7 patients for 108 months. There were no patient deaths while on-study. No patients were lost to follow-up.
Table 1.
Patient Characteristics
| Patient Characteristic | No. | % |
|---|---|---|
|
| ||
| Sex | ||
| Female | 24 | 53.3% |
| Male | 21 | 46.6% |
|
| ||
| Race | ||
| White | 37 | 82.2% |
| Black | 5 | 11.1% |
| Other | 3 | 6.7% |
|
| ||
| Age | ||
| <14 years | 25 | 55.6% |
| ≥14 years | 20 | 44.4% |
|
| ||
| Local or Metastatic | ||
| Local | 26 | 57.8% |
| Metastatic | 19 | 42.2% |
|
| ||
| Primary Tumor Size at Diagnosis in Greatest Dimension | ||
| <8cm | 20 | 44.4% |
| ≥ 8cm | 25 | 55.6% |
|
| ||
| Osseous vs. Extra Osseous | ||
| Osseous | 35 | 77.8% |
| Extra Osseous | 10 | 22.2% |
|
| ||
| Site Category | ||
| Pelvis | 16 | 35.6% |
| Trunk | 13 | 28.9% |
| Head and Neck | 5 | 11.1% |
| Extremity | 11 | 24.4% |
|
| ||
| Planned Definitive Surgical Resection | ||
| Upfront Resection | 7 | 41.2% |
| Delayed Resection | 10 | 58.8% |
|
| ||
| Metastatic Patients (n=19) | ||
| Primary tumor size ≥ 8cm at diagnosis | 14 | 73.6% |
| Pelvic location | 9 | 47.4% |
| Surgical resection | 4 | 21% |
| Metastatic site: | ||
| Lung | 10 | 52.6% |
| Bone Marrow | 8 | 42.1% |
| Pleura | 2 | 10.5% |
| Lymph Node | 4 | 21% |
| Bone | 9 | 47.4% |
Patients received an average of 18.9 weeks of systemic chemotherapy prior to undergoing RT. Forty-three patients received a regimen of alternating cycles of vincristine, doxorubicin, cyclophosphamide with ifosfamide and etoposide, every 2 (10 pts.) or 3 (33 pts.) weeks depending on the era. One patient had relapsed disease at time of enrollment and received a chemotherapy regimen of vincristine, temozolamide and irinotecan. One patient had progressive disease prior to beginning local control with RT, and changed from a chemotherapy regimen of VDC/IE to cyclophosphamide and topotecan. Six patients had upfront surgical resection, 10 patients had delayed surgical resection and 1 patient received RT prior to planned surgical intervention, but progressed during the pre-operative time period. The median (range) RT dose for all patients was 56.1Gy (45.0–65.5). Seventeen patients received adjuvant RT (50.6Gy, 45.0–58.6), including 3 patients who received brachytherapy (all for involved soft tissue surgical margins), 1 dosed at 45.0Gy with a low-dose rate technique delivered over 5 days, and 2 given in conjunction with external beam RT (brachytherapy delivered with a high-dose rate technique – 13.6Gy/4 fractions) to a total dose of 58.6Gy. Twelve patients received escalated dose RT (64.4Gy, 60.0–65.5). Sixteen patients received standard dose RT (55.7Gy, 54.0–58.6); this includes 3 patients who had tumors ≥8 cm at diagnosis, but were treated on study prior to the implementation of escalated dose RT. Dose ranges accounted for alterations of the prescription to protect normal tissues.
Ten of the 19 patients with metastatic disease received RT to at least one metastatic site, typically if the site remained visible on CT or MR imaging. Whole lung RT was only used in 2 patients at the completion of therapy, while auto-transplant was used in 11 patients. Two patients received whole pleural surface RT in conjunction with their primary site RT to a dose of 30Gy. All 4 patients with bone involvement requiring RT received it at the same time as thier primary site RT.
From 2003–2007 patient had their set up verified by orthogonal EPID/x-ray imaging twice weekly. After 2007 CBCT was routinely used for daily set-up.
Outcomes
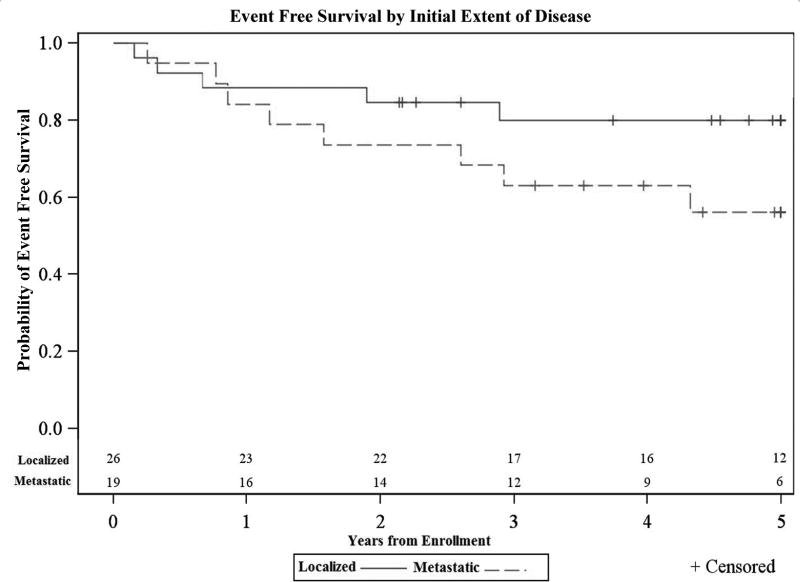
In this 45 patient cohort, 13 (28.9%) experienced events and 12 (26.7%) had failures. In the localized group there were 5 events: 3 distant failures, 1 local/distant failure and 1 local failure. Both local failures occurred within the RT field. In the metastatic patient group there were 8 events: 7 distant failures and 1 secondary malignancy. The 5-year EFS was 79.9% ± 9.9% for the localized group and 56.1% ±14.1% for the metastatic group. This difference in EFS was not statistically significant (p=0.14) (Figure 1). Subgroup FFS analysis was performed within the localized and metastatic cohorts using tumor size at diagnosis (<8cm vs. ≥8 cm, in greatest dimension), age at diagnosis (<14 vs. ≥14-years-old), osseous status (osseous vs. extra osseous primary disease) and site of primary disease (pelvis vs. other). No statistically significant difference in FFS was seen within the four subgroups, in either cohort.
Figure 1.
Event free survival estimate of EWS by disease status. The 5-year EFS was 79.9% ± 9.9% for the localized group and 56.1% ± 14.1% for the metastatic group (p=0.14).
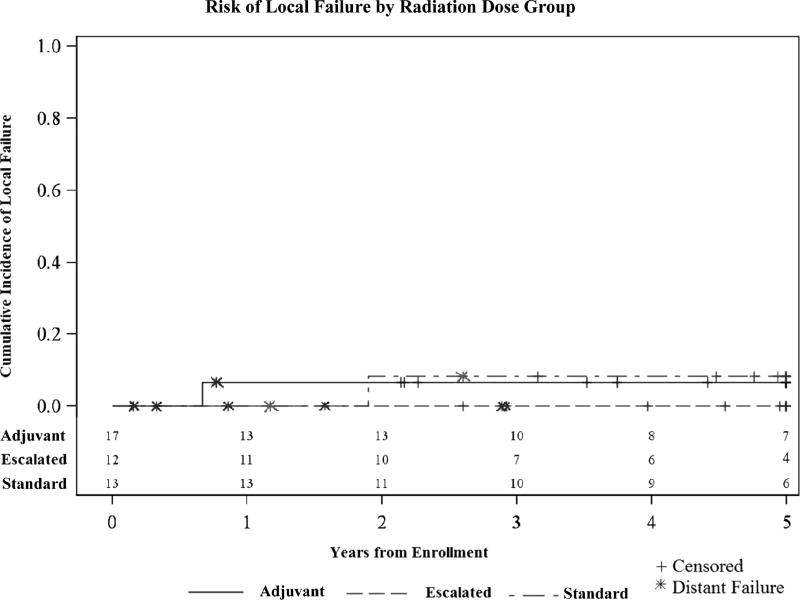
The estimated 10-year cumulative incidence (CI) of local failure (LF) among the entire cohort of 45 patients was 4.4% ± 3.1%. The 10-year CI of LF within the standard and adjuvant dose RT cohorts was 7.7% ± 7.7% and 5.9% ± 5.9%, respectively. No local failures occurred in the escalated RT dose cohort. The difference in LF among the three RT dosing groups was not statistically significant (p=0.65) (Figure 2). No local failures were seen in the 3 patients that were treated with standard dose RT prior to the implementation of escalated dose RT. Within the adjuvant RT group, 3 failures occurred in patients that underwent upfront surgical resection (2 distant, 1 local/distant) and 1 failure in the single patient that received pre-operative RT (distant).
Figure 2.
Cumulative incidence of local failure in EWS patients by RT treatment dosing group at 10-years follow-up. In the adjuvant and standard RT groups, the CI of LF was 5.9%±5.9% and 7.7%±7.7% respectively. No local failures occurred in the escalated dose RT treatment group (p=0.65).
The patient with local/distant failures was a 16-year-old male with localized EWS of the right sacroiliac region (14.2cm in greatest dimension at diagnosis). His treatment consisted of systemic chemotherapy, delayed surgical resection and adjuvant RT (50.4Gy). He was found to have local failure 4.5 months after completion of RT. The patient with only local failure was an 18-year-old male with localized EWS of the ilium (4.5cm in greatest dimension at diagnosis). His treatment included systemic chemotherapy and standard dose RT (55.8Gy). He was found to have local failure 9 months after completion of RT.
Toxicity
The highest graded short and long term toxicities observed for each patient, in each toxicity category, is summarized in Table 2. The highest graded short and long term toxicities observed for each patient, across all toxicity categories, is summarized according to RT dosage group in Table 3. All patients on study (n=45) experienced at least one short term toxicity. No short term toxicities related to recall reaction, arthritis or early osteonecrosis were seen. As expected, the most common short term toxicity was radiation-induced dermatitis (82%). Of the 8 patients who developed grade III short term toxicities, six had radiation-induced dermatitis and two had pain at the radiation site. One short term grade IV toxicity was observed, a 7th rib fracture in the RT field in a 13-year-old male with primary disease of the right thorax and pulmonary metastatic disease at diagnosis, who received adjuvant RT (50.4Gy) after upfront surgical resection.
Table 2.
Short and Long Term Toxicity Events1
| Toxicity Grade2 | I | II | III | IV |
|---|---|---|---|---|
|
| ||||
| Short Term (n=45) | ||||
| Weight Gain/Loss | 2 (4%) | - | - | - |
| Dermatitis3 | 18 (40%) | 19 (42%) | 6 (13%) | - |
| Muscle Weakness | - | 1 (2%) | - | - |
| Myositis | 6 (13%) | 1 (2%) | - | - |
| Musculoskeletal Other3 | 2 (4%) | - | - | 1 (2%) |
| Pain from Radiation3 | 14 (31%) | 6 (13%) | 2 (4%) | - |
|
| ||||
| Long Term (n=44)4 | ||||
| Bone5 | 16 (36%) | 15 (34%) | 1 (2%) | 2 (4%) |
| Joint5 | 11 (25%) | 10 (23%) | 2 (4%) | - |
| Lung | 4 (9%) | 2 (4%) | - | - |
| Skin5 | 23 (52%) | 11 (25%) | 1 (2%) | - |
| Spinal cord | 2 (4%) | - | - | - |
| Subcutaneous Tissue5 | 21 (48%) | 7 (16%) | 3 (7%) | - |
| Secondary Malignancy5 | - | - | - | 1 (2%) |
Highest graded toxicity observed for each patient, in each toxicity category; 45/45 patients developed at least 1 short term toxicity; 40/44 patients developed at least 1 long term toxicity.
Common Toxicity Criteria, Version 2.0, including the RTOG/EORTC Late Radiation Morbidity Scoring Scheme.
Grade III short term toxicities included radiation dermatitis and pain at the site of radiation. The single grade IV short term toxicity was a fracture at the site of radiation therapy.
One patient taken off study < 3months after completion of RT due to distant failure.
Grade III long term toxicities included severe pain/tenderness (bone), pain with severe limitation of movement (joint), marked atrophy (skin) and severe induration/loss of subcutaneous tissue (subcutaneous tissue). Grade IV long term toxicities included a fracture at the site of radiation therapy and development of secondary osteosarcoma within the radiation field.
Table 3.
Short and Long Term Toxicities by Radiation Therapy Dosage Group1
| Toxicity Grade2 | I | II | III | IV |
|---|---|---|---|---|
|
| ||||
| Short Term (n=45) | ||||
| Adjuvant RT (n=17) | 9 (52.9%) | 7 (41.2%) | - | 1 (5.9%) |
| Standard dose RT (n=16) | 6 (37.5%) | 6 (37.5%) | 4 (56.3%) | - |
| Escalated dose RT (n=12) | 3 (25%) | 6 (50%) | 3 (25%) | - |
|
| ||||
| Long Term (n=44)3 | ||||
| Adjuvant RT (n=17) | 4 (23.5%) | 7 (41.2%) | 2 (23.5%) | 1 (5.9%) |
| Standard dose RT (n=15) | 6 (37.5%) | 9 (60%) | - | - |
| Escalated dose RT (n=12) | 2 (16.7%) | 5 (41.7%) | 3 (25%) | 1 (8.3%) |
Highest graded short and long term toxicities observed for each patient within each RT dosing group, across all toxicity categories.
Common Toxicity Criteria, Version 2.0, including the RTOG/EORTC Late Radiation Morbidity Scoring Scheme.
One standard dose RT patient taken off study < 3months after completion of RT due to distant failure.
Forty-four patients (n=44) were evaluated for long term toxicities. Of the 40 patients that developed long term toxicities, 7 were grade III and 3 were grade IV. No patients developed renal or hepatic long term toxicities. The grade III long term toxicities were related to bone (1), joint (2), skin (1) and subcutaneous tissues (3). Two long term grade IV toxicities involved development of necrosis/spontaneous fracture in a bone, with both instances occurring within the RT field. The first patient was a 6.8-year-old male who had upfront resection of localized EWS of the right thorax. Six months after completion of adjuvant RT (50.4Gy), he was found to have a healing right 9th rib fracture. The second patient was a 21-year-old female with primary disease of the right fibula and pulmonary metastatic disease. Twenty months after completion of escalated dose RT (64.8Gy), she was found to have a displaced right fibula fracture and a probable healing right tibia stress fracture. The third long term grade IV toxicity was the development of a secondary malignancy in a 9-year-old female with primary disease of the left sacrum and pulmonary metastatic disease. Four years after completion of standard dose RT (55.8Gy), she developed secondary osteosarcoma within the original RT treatment field.
Discussion
Local control modalities for the treatment of EWS have been an area of debate for several decades. Early multi-institutional studies reported rates of local control of 70–75% for patients treated with RT alone and >90% for those treated with surgery, with or without adjuvant RT. These early results may be attributable to the fact that smaller lesions are typically more amenable to surgical resection and that the quality of RT varies between studies8,14,15,20. As RT techniques and systemic chemotherapy regimens have evolved, rates of local control for patients treated with definitive RT have improved with some recent studies showing no statistical difference in local failure rates between modalities21,22. There still remains a lack of consensus14,15,21. Larger tumor size at diagnosis has historically been a poor prognostic factor and influenced local control, with LF rates reported as high as 50% for patients with larger tumors at diagnosis4,9,23–26. The relationship between tumor size, RT dosing and local control has been previously explored and, with standard or de-escalated dose radiation, cohorts with large tumors at diagnosis had higher rates of local failure4,8,27–29. Dose escalation, thus, may be warranted for these cases, but delivery of such high doses of RT prior the use of image-guided techniques has been limited by exposures to surrounding normal tissues.
We describe here the outcomes of a phase II prospective clinical trial of pediatric and young adult patients with EWS treated with focal conformal limited-margin RT, in conjunction with systemic chemotherapy +/− surgical resection. Importantly, the CTV used in this study was limited to 1cm around the GTV, constrained by anatomic boundaries, and dose-escalated RT was administered for larger tumors (≥8cm in greatest dimension at diagnosis). Another advancement incorporated into this trial was the systematic use of image fusion. The ability to co-register pre-therapy, pre-surgery, and pre-RT imaging datasets, including PET and MR, was a significant improvement in defining the actual tumor. At the time of initiation of this trial, these target volumes, anatomic constraints and dose escalation were previously untested using a conformal or IMRT approach.
With this reduced treatment volume and dose-escalation schema, the estimated 10-year CI of local failure in this study was 4.4% ± 3.1%, with both incidences of local failure occurring within the RT treatment volume. Compared to previous trials, this is an improvement in the rate of local control while also using smaller RT volumes9,24,27, suggesting that the use of a 1cm CTV margin constrained by the local anatomy may be acceptable15,27,30. Additionally, the use of IMRT allowed patients with larger tumors at diagnosis to receive definitive local control therapy with escalated dose RT to an average of 64.4Gy, with no local failures observed in this treatment group. Contemporary patient populations treated with other conformal therapies, such a scattered proton RT, appear to have similar outcomes with reported local control rates of 88% in non-recurrent patients30. Proton beam RT may allow an even greater ability to dose-escalate radiation in those patients with larger unresectable tumors and a high risk for local failure, with a favorable toxicity profile. In our trial the combination of well-defined, limited target volumes, constrained to pathways of spread specific to the patient’s anatomy, and the incorporation of dose escalated conformal or IMRT may be responsible for these favorable results.
Long term toxicities from RT are important to monitor as efforts to improve local control rates continue16,26,31. Dose reduction has traditionally been used to try to decrease RT-related toxicities; our trial attempted to decrease treatment volume, while maintaining intensified RT dosing for patients with larger tumors at diagnosis. RT was generally well tolerated, but grade III and IV toxicities were observed despite limited treatment margins with the highest percentage of long-term grade III and IV toxicities occurring in the escalated dose RT group. This reinforces the need to continue to monitor for toxicities even in the era of increasing use of proton therapy for local control of EWS30.
The development of secondary malignancies after RT is a long term effect that can occur decades after initial treatment. Patients with EWS are at a particularly high risk8,32–35, especially after receiving higher doses of RT33,36,37. In a recent analysis from the childhood cancer survivor study, the 30-year cumulative incidence of subsequent neoplasms in survivors of EWS was 10.1%35. This increased risk must be carefully weighed with the need for higher RT dosing to improve rates of local control. In our study, only one secondary malignancy has occurred (at approximately 5-years after study enrollment); however, our median follow-up at time of analysis was only 54.5 months. Since this risk increases with time, our rate of secondary malignancies may further increase, particularly in the escalated dose RT treatment group33,36. Further improvements in treatment technique, including scanned intensity modulated proton therapy, and the possibility of imaging defined sub-regions of target volumes, so called biologic GTVs or bGTVs, could further reduce this risk of secondary malignancy while maintaining high rates of local control.
In conclusion, we found that treatment with focal conformal, limited-margin RT (CTV1= GTV + 1cm) for pediatric and young adult patients with EWS, including dose escalation for unresected tumors ≥8cm in greatest dimension at diagnosis, provides favorable local tumor control with an acceptable toxicity profile. Based on these outcomes, this RT approach is being used in our current institutional trial using even smaller treatment volumes (5mm CTV) for all RT dosing groups, dose escalation for larger tumors, proton beam irradiation and standardized chemotherapy. We hope this approach will further reduce long term toxicities while maintaining high rates of local control.
Summary.
Treatment with conformal, limited-margin RT for pediatric patients with EWS, including dose escalation for unresected tumors ≥8cm in greatest dimension at diagnosis, yields favorable local tumor control and has a favorable toxicity profile.
Acknowledgments
Supported in part by Cancer Center Grant CA23099 and Cancer Center Support CORE Grant P30 CA 21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior Presentations: SIOP Annual Meeting 2014
Disclaimers: The authors declare no potential conflict of interest.
References
- 1.Rodriguez-Galindo C, Liu T, Krasin MJ, et al. Analysis of prognostic factors in ewing sarcoma family of tumors: review of St. Jude Children's Research Hospital studies. Cancer. 2007;110:375–84. doi: 10.1002/cncr.22821. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig JA. Ewing sarcoma: historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Curr Opin Oncol. 2008;20:412–8. doi: 10.1097/CCO.0b013e328303ba1d. [DOI] [PubMed] [Google Scholar]
- 3.Perkins SM, Shinohara ET, DeWees T, et al. Outcome for children with metastatic solid tumors over the last four decades. PLoS One. 2014;9:e100396. doi: 10.1371/journal.pone.0100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai Y, Kun LE, Brooks MT, et al. Ewing's sarcoma: local tumor control and patterns of failure following limited-volume radiation therapy. Int J Radiat Oncol Biol Phys. 1991;21:1501–8. doi: 10.1016/0360-3016(91)90325-x. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann C, Ahrens S, Dunst J, et al. Pelvic Ewing sarcoma: a retrospective analysis of 241 cases. Cancer. 1999;85:869–77. doi: 10.1002/(sici)1097-0142(19990215)85:4<869::aid-cncr14>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol. 2008;19:814–20. doi: 10.1093/annonc/mdm521. [DOI] [PubMed] [Google Scholar]
- 7.Arvand A, Denny CT. Biology of EWS/ETS fusions in Ewing's family tumors. Oncogene. 2001;20:5747–54. doi: 10.1038/sj.onc.1204598. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Galindo C, Spunt SL, Pappo AS. Treatment of Ewing sarcoma family of tumors: current status and outlook for the future. Med Pediatr Oncol. 2003;40:276–87. doi: 10.1002/mpo.10240. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson SS. Ewing sarcoma: radiation dose and target volume. Pediatr Blood Cancer. 2004;42:471–6. doi: 10.1002/pbc.10472. [DOI] [PubMed] [Google Scholar]
- 10.Krasin MJ, Davidoff AM, Xiong X, et al. Preliminary results from a prospective study using limited margin radiotherapy in pediatric and young adult patients with high-grade nonrhabdomyosarcoma soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2010;76:874–8. doi: 10.1016/j.ijrobp.2009.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merchant TE. Conformal Therapy for Pediatric Sarcomas. Semin Radiat Oncol. 1997;7:236–245. doi: 10.1053/SRAO00700236. [DOI] [PubMed] [Google Scholar]
- 12.Hua C, Gray JM, Merchant TE, et al. Treatment planning and delivery of external beam radiotherapy for pediatric sarcoma: the St. Jude Children's Research Hospital experience. Int J Radiat Oncol Biol Phys. 2008;70:1598–606. doi: 10.1016/j.ijrobp.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Krasin MJ, Wiese KM, Spunt SL, et al. Jaw dysfunction related to pterygoid and masseter muscle dosimetry after radiation therapy in children and young adults with head-and-neck sarcomas. Int J Radiat Oncol Biol Phys. 2012;82:355–60. doi: 10.1016/j.ijrobp.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaspar N, Hawkins DS, Dirksen U, et al. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 15.DuBois SG, Krailo MD, Gebhardt MC, et al. Comparative evaluation of local control strategies in localized Ewing sarcoma of bone: a report from the Children's Oncology Group. Cancer. 2015;121:467–75. doi: 10.1002/cncr.29065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGovern SL, Mahajan A. Progress in radiotherapy for pediatric sarcomas. Curr Oncol Rep. 2012;14:320–6. doi: 10.1007/s11912-012-0235-y. [DOI] [PubMed] [Google Scholar]
- 17.Raney RB, Asmar L, Newton WA, Jr, et al. Ewing's sarcoma of soft tissues in childhood: a report from the Intergroup Rhabdomyosarcoma Study, 1972 to 1991. J Clin Oncol. 1997;15:574–82. doi: 10.1200/JCO.1997.15.2.574. [DOI] [PubMed] [Google Scholar]
- 18.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:4148–54. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulussen M, Ahrens S, Dunst J, et al. Localized Ewing tumor of bone: final results of the cooperative Ewing's Sarcoma Study CESS 86. J Clin Oncol. 2001;19:1818–29. doi: 10.1200/JCO.2001.19.6.1818. [DOI] [PubMed] [Google Scholar]
- 20.Ahrens S, Hoffmann C, Jabar S, et al. Evaluation of prognostic factors in a tumor volume-adapted treatment strategy for localized Ewing sarcoma of bone: the CESS 86 experience. Cooperative Ewing Sarcoma Study. Med Pediatr Oncol. 1999;32:186–95. doi: 10.1002/(sici)1096-911x(199903)32:3<186::aid-mpo5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 21.Yock TI, Krailo M, Fryer CJ, et al. Local control in pelvic Ewing sarcoma: analysis from INT-0091--a report from the Children's Oncology Group. J Clin Oncol. 2006;24:3838–43. doi: 10.1200/JCO.2006.05.9188. [DOI] [PubMed] [Google Scholar]
- 22.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 23.Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol. 2000;18:3108–14. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 24.Schuck A, Ahrens S, Paulussen M, et al. Local therapy in localized Ewing tumors: results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int J Radiat Oncol Biol Phys. 2003;55:168–77. doi: 10.1016/s0360-3016(02)03797-5. [DOI] [PubMed] [Google Scholar]
- 25.Paulino AC, Nguyen TX, Mai WY, et al. Dose response and local control using radiotherapy in non-metastatic Ewing sarcoma. Pediatr Blood Cancer. 2007;49:145–8. doi: 10.1002/pbc.20904. [DOI] [PubMed] [Google Scholar]
- 26.Indelicato DJ, Keole SR, Shahlaee AH, et al. Definitive radiotherapy for ewing tumors of extremities and pelvis: long-term disease control, limb function, and treatment toxicity. Int J Radiat Oncol Biol Phys. 2008;72:871–7. doi: 10.1016/j.ijrobp.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 27.La TH, Meyers PA, Wexler LH, et al. Radiation therapy for Ewing's sarcoma: results from Memorial Sloan-Kettering in the modern era. Int J Radiat Oncol Biol Phys. 2006;64:544–50. doi: 10.1016/j.ijrobp.2005.07.299. [DOI] [PubMed] [Google Scholar]
- 28.Krasin MJ, Rodriguez-Galindo C, Billups CA, et al. Definitive irradiation in multidisciplinary management of localized Ewing sarcoma family of tumors in pediatric patients: outcome and prognostic factors. Int J Radiat Oncol Biol Phys. 2004;60:830–8. doi: 10.1016/j.ijrobp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Krasin MJ, Rodriguez-Galindo C, Davidoff AM, et al. Efficacy of combined surgery and irradiation for localized Ewings sarcoma family of tumors. Pediatr Blood Cancer. 2004;43:229–36. doi: 10.1002/pbc.20095. [DOI] [PubMed] [Google Scholar]
- 30.Rombi B, DeLaney TF, MacDonald SM, et al. Proton radiotherapy for pediatric Ewing's sarcoma: initial clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1142–8. doi: 10.1016/j.ijrobp.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, Zhang Q, Eisenberg BL, et al. Significant Reduction of Late Toxicities in Patients With Extremity Sarcoma Treated With Image-Guided Radiation Therapy to a Reduced Target Volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial. J Clin Oncol. 2015;33:2231–8. doi: 10.1200/JCO.2014.58.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strong LC, Herson J, Osborne BM, et al. Risk of radiation-related subsequent malignant tumors in survivors of Ewing's sarcoma. J Natl Cancer Inst. 1979;62:1401–6. [PubMed] [Google Scholar]
- 33.Tucker MA, D'Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–93. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 34.Navid F, Billups C, Liu T, et al. Second cancers in patients with the Ewing sarcoma family of tumours. Eur J Cancer. 2008;44:983–91. doi: 10.1016/j.ejca.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–95. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuttesch JF, Jr, Wexler LH, Marcus RB, et al. Second malignancies after Ewing's sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol. 1996;14:2818–25. doi: 10.1200/JCO.1996.14.10.2818. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz B, Benadjaoud MA, Clero E, et al. Risk of second bone sarcoma following childhood cancer: role of radiation therapy treatment. Radiat Environ Biophys. 2014;53:381–90. doi: 10.1007/s00411-013-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]