Abstract
Epithelial cells constitute a physical barrier that aids in protecting the host from microbial pathogens. Polarized epithelial cells contain distinct apical and basolateral membrane domains separated by intercellular junctions, including tight junctions (TJ), which contribute to the maintenance of apical–basal polarity. Polarity complexes also contribute to the establishment of TJ formation. Several pathogens perturb epithelial TJ barrier function and structure in addition to causing a loss of apical–basal polarity. Here, we review the impact of pathogenic bacteria on the disruption of cell–cell junctions and epithelial polarity.
Keywords: enteropathogenic Escherichia coli, tight junctions, EPEC, apical–basal polarity
Polarized epithelial cells
Epithelial cells play a critical role in the defense against microorganisms separating the internal compartments of the host from the external components. Tight junctions (TJ), localized at the most apical region of the lateral membrane, are responsible for sealing the intercellular space of epithelial cells.1 TJs control paracellular permeability by regulating the flux of water and solutes across the epithelium and help maintain apical–basal polarity by restricting the intermixing of apical and lateral plasma membrane components.2,3
TJs comprise transmembrane proteins, including claudins, occludin, tricellulin, MarvelD3, and JAM-A, which contribute to TJ strand formation, control the paracellular seal, regulate barrier function, and participate in adhesion and cell transmigration of the immune system, among other functions. In addition to transmembrane proteins, TJ also contain adaptor proteins (ZO-1, ZO-2, ZO-3, cingulin, MAGI-1 -3, MUPP-1) that link transmembrane proteins to the cytoskeleton. Cytoskeletal proteins (ARP2/3, N-WASP, cortactin, and VASP), regulators of actin organization (RhoA, Rac, and the CDC42 family of small GTPases), transcription factors, and nonmuscle myosin II (NMII), among other proteins, localize to TJs and modulate different signaling pathways.3
TJs are crucial for the establishment and maintenance of epithelial apical-basal polarity, which is controlled by three polarity complexes. The Crumbs complex consists of Crumbs (CRB), protein associated with Lin-7 (PALS1), and Pals1-associated tight junction protein (PATJ). The Par complex is formed by partitioning defective homologue 3 and 6 (PAR3/PAR6), atypical protein kinase C (aPKC), and CDC42. The third complex is comprised of Scribble (Scrib)/lethal giant larvae (Lgl)/disc large (Dlg). Apical–basal polarity contributes to cell morphology, directional vesicle transportation, ion and solute transport, and specific localization of proteins and lipids to different membrane domains.4,5
The PAR6/aPKC complex is of special interest; PAR6 acts as a scaffolding protein interacting with all polarity complexes, thus allowing aPKC to phosphorylate those polarity proteins that are substrates for the kinase. The interaction of PAR6 with CDC42-GTP activates aPKC to phosphorylate PAR3, leading to recruitment of TJ proteins and the establishment of cell polarity.6–8 PAR6 interacts with PALS1 and CRB3, the latter being a target protein of aPKC, and this complex is important for TJ formation.9–12 PAR6 interacts with the lateral polarity protein LGL, allowing aPKC phosphorylation and LGL exclusion from the apical membrane, further defining apical–basal polarity and promoting epithelial junction formation.13,14 These data suggest that there is complex interplay between TJ and polarity proteins to establish and maintain TJ structure and function and apical–basal polarity, leading to cellular structural integrity and functionality.
EPEC and TJ disruption
Enteropathogenic Escherichia coli (EPEC) delivers bacterial effector proteins into host intestinal epithelial cells through a type III secretion system (T3SS), causing diarrhea. EPEC disrupts intestinal epithelial TJ architecture, leading to altered fence and gate functions of intestinal epithelial cells.15–22 Disruption of TJ mediated by EPEC involves several events in the host cells, leading to dissociation of protein–protein interactions; disorganized distribution of TJ proteins claudin-1, occluding, and ZO-1; and loss of barrier function correlating with the presence of aberrant strands in the lateral membrane.23 EPEC effectors, specifically EspF, Map, NleA, and EspG, have been widely studied with respect to their specific effects on TJ perturbation.
EspF redistributes occludin from the TJ, decreases transepithelial electrical resistance (TER), and increases paracellular permeability of T84 monolayers.16 EspF also perturbs TJs in vivo, as demonstrated in a mouse model of infection. Ileum and colon from EPEC-infected mice show a dramatic redistribution of occludin and diminished barrier function, suggesting a role in EPEC pathogenicity.16,18,19
Map modulates epithelial barrier function and contributes to EPEC-induced diarrhea. Map interacts with Na+/H+ exchanger regulatory factors I and II (NHERF1/2) and regulates ion channels in the intestine.24,25 Deletion of map attenuates the EPEC-induced drop in TER in Caco-2 monolayers.17 Infection of mice with a map deletion Citrobacter rodentium strain, a murine pathogen similar to EPEC, significantly reduces diarrhea caused by wild-type C. rodentium, suggesting that Map plays a crucial role in the flux of ions and water in the intestine.24, 25 Constitutive expression of Map in MDCKII cells increases the permeability to charged and non-charged molecules, indicating a failure in gate function.26
NleA redistributes ZO-1 and occludin and contributes to the decrease in TER in polarized intestinal cell monolayers.22 This effector binds to and inhibits the COPII protein complex, which participates in the packaging and trafficking of proteins from the ER to the Golgi.27 Using a murine model of C. rodentium infection, it was demonstrated that ablation of the NleA interaction with COPII components prevents the redistribution of ZO-1 and occludin and impairs the increased paracellular permeability caused by the wild-type strain. This suggests that NleA may affect TJs by preventing newly synthesized TJ proteins from reaching the apical membrane.28
EspG also contributes to TER loss and regulates the size-selective paracellular permeability of epithelial cells.20,21 Disruption of microtubule networks by EspG was shown to induce the cytoplasmic accumulation of occludin and delay TJ recovery, suggesting that EspG prevents TJ repair.29 Although altered barrier function has been an area of intense interest, the impact of EPEC on loss of cell polarity following TJ disruption may be another aspect of EPEC pathogenesis.
EPEC causes loss of apical–basal polarity
The impact of EPEC on host intestinal epithelial polarity has only been studied indirectly. Using T84 intestinal epithelial cells, Muza-Moons et al. demonstrated that EPEC infection leads to a progressive redistribution of two basolateral proteins, β1-integrin and Na+/K+ ATPase, to the apical compartment. β1-integrin is a cellular adhesion molecule that participates in anchoring polarized epithelial cells to the basement membrane. Among other functions, integrins have an important role in the establishment of polarity. For example, ablation of β1-integrin causes loss of polarity, leading to defective arterial lumen formation and asymmetric cell division in skin epithelia.30, 31 β1-Integrin also controls the orientation of epithelial polarity and thus the formation of lumens in differentiated acini.32 The EPEC outer membrane protein and major adherence factor intimin interacts with the EPEC translocated intimin receptor (Tir). Interestingly, intimin has also been demonstrated to be capable of interacting with the host cell protein β1-integrin, although this protein is basolaterally positioned in polarized epithelia and thus not accessible to luminal EPEC. However, EPEC infection allows β1-integrin to gain an apical position and become available for interaction with intimin. EPEC infection of polarized epithelial monolayers with a tir deletion strain has no impact on TER compared with wild type. However, in monolayers in which polarity has been altered and β1-integrin is apically positioned, the drop in TER is similar to that caused by wild-type EPEC, demonstrating a functional role for altered polarity in EPEC pathogenesis.33
Effective intestinal ion transport is dependent on cell polarity. Na+/K+ ATPase exchanges three intracellular sodium and two extracellular potassium ions across the basolateral membrane.34 This electrochemical gradient is important for intestinal and colon electrolyte and water transport.35 Additionally, acute enteritis and chronic ileal inflammation in an animal model decrease the activity and expression of Na+/K+ ATPase, leading to diminished electrolyte transport and absorption of nutrients.36–44 EPEC infection affects several different intestinal transporters, including sodium hydrogen exchanger 3 (NHE3) and downregulated in adenoma (DRA), contributing to the loss of ions and water manifested as diarrhea.45–47 EPEC also perturbs the basolateral localization of Na+/K+ ATPase, as well as possibly other ion transporters and channels, redistributing it to the apical membrane domain,23,33 which likely contributes to EPEC pathophysiology.
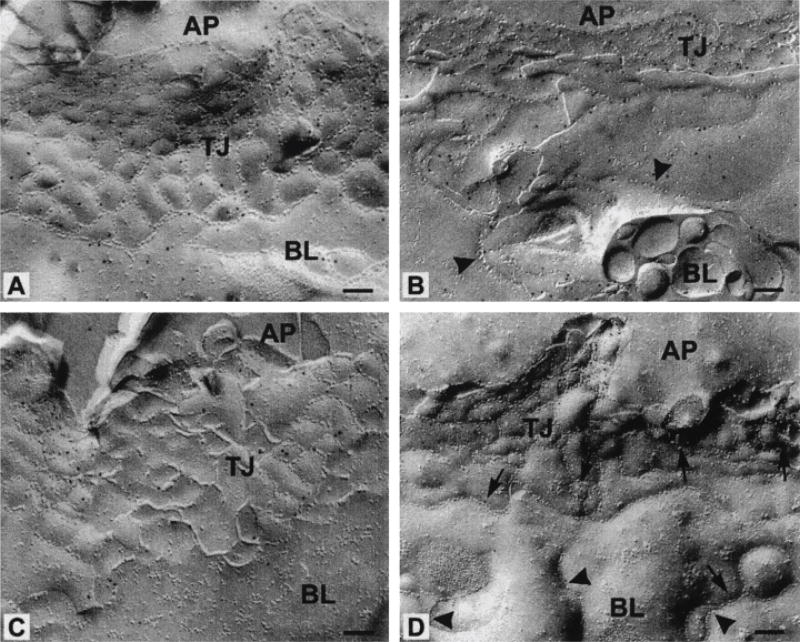
In addition to the redistribution of basolateral proteins, EPEC infection induces the relocalization of ZO-1, occludin, and claudin-1 from the TJ region to the lateral membrane and cytoplasmic compartment, which is the structural/molecular correlate to the loss of fence function. Freeze–fracture replicas of EPEC-infected monolayers revealed aberrant strands extending down the lateral membrane surface and below the TJ area, indicating a prevalent alteration in TJ architecture (Fig. 1). These structural changes correlate with both increased paracellular permeability and decreased TER.23 The redistribution of both basolateral and TJ proteins and the appearance of aberrant TJ strands indicate loss of apical–basal polarity. EPEC-induced perturbation of TJ structure and apical–basal polarity may allow the free diffusion of other cytoplasmic and membrane proteins to incorrect cellular domains, further contributing to EPEC pathogenesis.
Figure 1.
EPEC induces formation of aberrant TJ strands in the lateral membrane. Freeze–fracture images of control uninfected T84 cells immuno-gold labelled for claudin-1 and occludin (A and C, respectively). The apical (AP) and the basolateral (BL) membrane domains of EPEC-infected T84 cells show few (arrows) and ectopic (arrowheads) TJ strands along the lateral membrane, as indicated in the immuno-gold label for claudin-1 and occludin (B and D, respectively). This image was reproduced from Ref. 23 with the express written permission of the publisher.
The impact of EPEC impact on Par polarity complex
Little is known about the direct impact of EPEC on specific polarity complexes. EPEC infection of T84 cells increases PKCζ enzymatic activity and induces its translocation from the cytoplasm to an insoluble fraction containing membrane proteins.48 The activity of aPKC kinase increases upon CDC42 binding to PAR6/aPKC, a critical step in the regulation of Par polarity complex. Interestingly, the EPEC effector Map activates CDC42 GTPase, leading to the formation of filopodia,49,50 and CDC42 activation may also play a role in altering aPKC activity. The 14-3-3 family of cytosolic adaptor proteins also participates in cell signaling. Phosphorylation of PAR3 via aPKC facilitates its binding to 14-3-3, and disruption of this interaction leads to a loss in epithelial cell polarity, suggesting that 14-3-3/PAR3 regulates the activity of Par polarity complex.51,52 Interestingly, our lab reported that EspF associates with 14-3-3ζand cytokeratin-18 (CK-18) in T84 monolayers,53 supporting the hypothesis that EPEC modulates Par complex through its effectors (Fig. 2B).
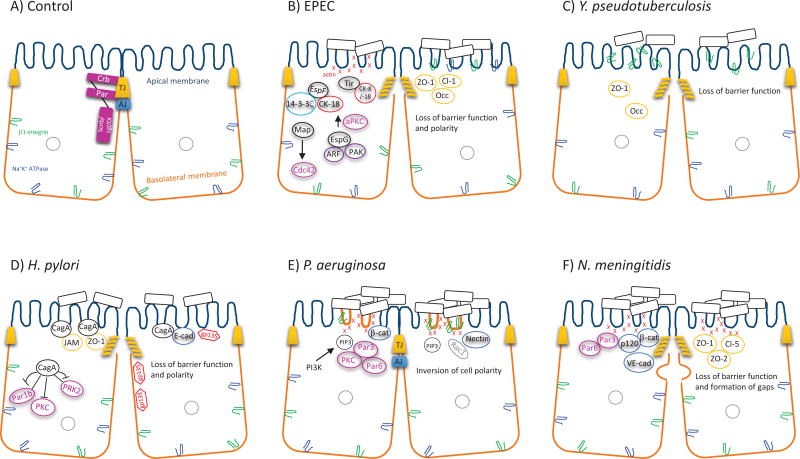
Figure 2.
Model demonstrating the potential effect of pathogens on apical–basal polarity. A monolayer of epithelial cells forms a barrier separating the internal contents of the body from the external environment. The membranes of polarized epithelial cells have differing protein and lipid compositions. The apical membrane faces the lumen, and the basolateral domain is in contact with the underlying basement membrane. (A) The apical polarity complexes Crb and Par localize to TJs, while Scrib/Lgl/Dl resides below the TJ along the lateral membrane. (B) EPEC, through a T3SS, injects bacterial effectors (EspF, Map, EspG, Tir, etc.) into host cells. The effectors redistribute TJ proteins (ZO-1, claudin-1, and occludin), interact with and recruit the IF proteins (CK-8/18) to the pedestal formation, modulate the activity of Par polarity proteins (aPKC and CDC42), and redistribute basolateral proteins (β1-integrin and Na+/K+ ATPase) to the apical domain. All of these events result in a loss of both barrier function and apical–basal polarity. (C) Y. pseudotuberculosis redistributes ZO-1 and occludin from the TJ region and β1-integrin from the basolateral membrane, leading to disrupted barrier function. (D) H. pylori disrupts the organization of apical junctions via the interaction of CagA with ZO-1, JAM, and E-cadherin, causing their recruitment to the apical membrane. CagA binds to PAR1b, PKC, and PRK2, perturbing cell polarity, as demonstrated by the redistribution of the apical protein gp135 to the basolateral compartment. (E) P. aeruginosa increases the activation of PI3K and the generation of membrane PIP3-rich structures, which accumulate F-actin, RAC1, basolateral constituents (β1-integrin, β-catenin, and nectin), and Par polarity proteins (PAR3/PAR6/PKC). The altered composition of the apical membrane suggests that P. aeruginosa inverts epithelial cell polarity. (F) N. meningitidis forms bacterial aggregates in the apical domain of endothelial cells, which leads to an “ectopic early junction–like domain” enriched in F-actin, Par proteins (PAR3 and PAR6), adherens junction proteins (VE-cadherin, β-catenin, and p-120), as well as TJ proteins (ZO-1, ZO-2, and claudin-5). N. meningitidis perturbs cell–cell junctions through the formation of gaps through the lateral membrane of infected cells.
EspG disrupts microtubules and delays recovery of disrupted TJ caused by EPEC infection.29 EspG binds to ADP-ribosylation factor (ARF) and the RAC/CDC42 binding site of p21-activated kinase (PAK).54,55 The PAK family acts downstream of RAC1 and CDC42 GTPases and regulates cytoskeletal dynamics and cell motility. Although it has been demonstrated that EspG interaction with ARF and PAK does not contribute to microtubule destruction,29 its role in apical–basal polarity has not been investigated.
In addition to EPEC effectors possibly affect Par polarity complex directly, there is likely an indirect effect caused by pedestal formation.56 For example, during EPEC infection, Tir is injected directly into host cells, where it is phosphorylated and initiates the recruitment of actin polymerization at the site of bacterial attachment.57,58 It has been demonstrated that Tir interacts and recruits CK-8 and CK-18 to EPEC-induced pedestals.56 Cytokeratins are integral components of intermediate filaments (IF) and play an important role in epithelial polarity. For example, CK-8 knockout mice show a downregulation of aPKC, loss of syntaxin 3 and apical membrane proteins (alkaline phosphatase, sucrose isomaltase, and cystic fibrosis transmembrane conductance regulator), disorganized microtubules, ion-transport defects, and mistargeting of ion transporters to their cellular compartments.59`61 The 14-3-3 proteins bind to CK-8 and CJ-18, and CK-18 Ser33 phosphorylation is crucial for its association with 14-3-3.62,63 Importantly, EspF interacts with CK-18, and EPEC infection increases the solubility of CK-18, leading to a dramatic alteration in the architecture of the IF network in EPEC-infected epithelial cells.53 Deletion of espF partially impaired the ability of EPEC to induce CK-18 solubility and IF morphology.53 EspF forms a complex with 14-3-3ζ/CK-18 in a time-dependent manner. Therefore, we speculate that EspF plays a role in disruption of cell polarity through its binding partners 14-3-3ζ and CK-18 (Fig. 2B). Together, these data support the hypothesis that EPEC effectors cooperate to perturb cell polarity complexes by targeting, in a spatially and temporally regulated manner, multiple steps in this complex process.
Impact of other bacterial pathogens on Par polarity complex and TJ function
Yersinia pseudotuberculosis induces the transmigration of polymorphonuclear leukocytes (PMNs) across the intestinal epithelium. Perturbation of TJs, either by inducing PMN transmigration with the chemoattractant formylated Met–Leu–Phe or by calcium chelation with EDTA, redistributes β1-integrin to the apical surface of intestinal epithelia, where it serves as a receptor for cell invasion.64,65 In polarized epithelial MDCK cells, infection with Y. pseudotuberculosis induces bacterial binding to β1-integrin in the apical membrane, dissociation of ZO-1 and occludin from the TJ, and F-actin reorganization. Consequently, occludin redistributes along the lateral membrane of infected cells, and this event is accompanied by decreased TER and increased permeability (Fig. 2C).66
Helicobacter pylori also disrupts the organization and assembly of apical junctions and causes loss of apical–basolateral polarity in epithelial cells. CagA, an effector protein of H. pylori, localizes to the sites of TJ formation and associates with ZO-1 and JAM (Fig. 2D).67,68 CagA perturbs cell polarity, as demonstrated by the redistribution of glycoprotein 135 (gp135) from the apical to the basolateral membrane.68 CagA interacts with and recruits PAR1b from the cytosol to the plasma membrane, and this association inhibits PAR1 kinase activity and prevents aPKC-mediated PAR1 phosphorylation, causing junctional and polarity defects, inhibiting tubulogenesis and cell differentiation, and initiating epithelial-to-mesenchymal transition (EMT).69–72 CagA also interacts with and inhibits protein kinase C-related kinase 2 (PRK2), which acts downstream of Rho GTPases and is known to affect cytoskeletal rearrangement and cell polarity (Fig. 2D).73
Pseudomonas aeruginosa forms bacterial aggregates on the apical membrane of epithelial cells just before translocation of T3SS-secreted effectors and the associated cytotoxicity.74,75 Activation of phosphatidylinositol 3-kinase (PI3K) and protein kinase B/Akt (Akt) are necessary for P. aeruginosa entry from the apical surface.76 The binding of P. aeruginosa to the apical domain alters the composition of the membrane, transforming it from an apical surface to one with basolateral constituents. This transformation is accompanied by the recruitment of PI3K, the generation of PIP3, and the recruitment of actin into the membrane protrusions (Fig. 2E).77 Interestingly, gp135 and podocalyxin, both apical markers, were absent from the PIP3-rich protrusions. In contrast, the basolateral markers p58, β-catenin, and β1-integrin were present in the apical PIP3-rich structures (Fig. 2E).77 The dramatic rearrangement of membrane composition involves the recruitment of Par polarity complex to the membrane protrusions, suggesting that P. aeruginosa may affect apical–basal polarity.78
Neisseria meningitidis interacts with endothelial cells and perturbs the blood–brain barrier. N. meningitidis recruits components of adherens junctions (VE-cadherin, p120-catenin and β-catenin), TJs (ZO-1, ZO-2, and claudin-5), and Par polarity proteins (PAR3 and PAR6) underneath adherent microcolonies (Fig. 2F).79 N. meningitidis infection causes the formation of an ectopic domain containing filopodia-like structures that is enriched in junctional proteins and is called the “ectopic early junction–like domain.” Interestingly, downregulation of CDC42, a component of Par polarity complex, inhibits the recruitment of PAR3, PAR6, VE-cadherin, p-120 catenin, and actin to the ectopic early junction–like domain. In addition, inhibition of PAR6 and PKCζ reduces the recruitment of p120-catenin, VE-cadherin, actin, and PAR3 to these domains.79 N. meningitidis increases endothelial permeability by inducing the formation of gaps between infected cells. These findings suggest that recruitment of Par polarity complex proteins is associated with disorganized cell–cell junctions and opening the paracellular route, allowing the bacteria to cross the brain endothelial monolayer.79 All of these studies illustrate the sophisticated interplay between pathogen-induced damage and the host response, underscoring apical–basal polarity as a key target of both pathogen and host defense systems.
Conclusions
Several studies highlight the importance of cell–cell junctions in the maintenance of apical–basal polarity and vice versa, indicating the existence of cross talk between these two molecular complexes. Many publications have demonstrated the effect of EPEC on epithelial cell disruption; however, very little is known about the impact of EPEC on apical–basal polarity. We discussed how EPEC and other pathogens may modulate epithelial polarity through dysregulation of multiple factors, including disruption of the intestinal barrier, redistribution of adhesion and polarity proteins, and recruitment of polarity complexes to infection sites, all of which lead to impaired apical–basal polarity. Alterations in the integrity of TJs and cell polarity may be an important step in the pathogenesis of infectious diseases. Future research is warranted to explore the many ways that microorganisms alter epithelial cell homeostasis.
Acknowledgments
This work was supported by a National Institutes of Health grant (DK097043 to GH) and an Edward Hines JR VA Hospital grant (BX002687 to GH).
References
- 1.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J. Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int. Rev. Cytol. 2006;248:261–298. doi: 10.1016/S0074-7696(06)48005-0. [DOI] [PubMed] [Google Scholar]
- 3.Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin. Cell Dev. Biol. 2014;36:157–165. doi: 10.1016/j.semcdb.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tepass U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu. Rev. Cell Dev. Biol. 2012;28:655–685. doi: 10.1146/annurev-cellbio-092910-154033. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 2014;15:225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joberty G, Petersen C, Gao L, et al. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki A, Yamanaka T, Hirose T, et al. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J. Cell Biol. 2001;152:1183–1196. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson A, Driessens M, Aspenstrom P. The mammalian homologue of the Caenorhabditis elegans polarity protein PAR-6 is a binding partner for the Rho GTPases Cdc42 and Rac1. J. Cell. Sci. 2000;113(Pt 18):3267–3275. doi: 10.1242/jcs.113.18.3267. [DOI] [PubMed] [Google Scholar]
- 9.Hurd TW, Gao L, Roh MH, et al. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat. Cell Biol. 2003;5:137–142. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- 10.Lemmers C, Michel D, Lane-Guermonprez L, et al. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol. Biol. Cell. 2004;15:1324–1333. doi: 10.1091/mbc.E03-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotillos S, Diaz-Meco MT, Caminero E, et al. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J. Cell Biol. 2004;166:549–557. doi: 10.1083/jcb.200311031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei Z, Li Y, Ye F, et al. Structural basis for the phosphorylation-regulated interaction between the cytoplasmic tail of cell polarity protein crumbs and the actin-binding protein moesin. J. Biol. Chem. 2015;290:11384–11392. doi: 10.1074/jbc.M115.643791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plant PJ, Fawcett JP, Lin DC, et al. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat. Cell Biol. 2003;5:301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- 14.Yamanaka T, Horikoshi YT, Sugiyama Y, et al. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr. Biol. 2003;13:734–743. doi: 10.1016/s0960-9822(03)00244-6. [DOI] [PubMed] [Google Scholar]
- 15.Simonovic I, Rosenberg J, Koutsouris A, et al. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell. Microbiol. 2000;2:305–315. doi: 10.1046/j.1462-5822.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- 16.McNamara BP, Koutsouris A, O'Connell CB, et al. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Invest. 2001;107:621–629. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean P, Kenny B. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol. Microbiol. 2004;54:665–675. doi: 10.1111/j.1365-2958.2004.04308.x. [DOI] [PubMed] [Google Scholar]
- 18.Shifflett DE, Clayburgh DR, Koutsouris A, et al. Enteropathogenic E. coli disrupts tight junction barrier function and structure in vivo. Lab. Invest. 2005;85:1308–1324. doi: 10.1038/labinvest.3700330. [DOI] [PubMed] [Google Scholar]
- 19.Guttman JA, Li Y, Wickham ME, et al. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell. Microbiol. 2006;8:634–645. doi: 10.1111/j.1462-5822.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 20.Tomson FL, Viswanathan VK, Kanack KJ, et al. Enteropathogenic Escherichia coli EspG disrupts microtubules and in conjunction with Orf3 enhances perturbation of the tight junction barrier. Mol. Microbiol. 2005;56:447–464. doi: 10.1111/j.1365-2958.2005.04571.x. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzawa T, Kuwae A, Abe A. Enteropathogenic Escherichia coli type III effectors EspG and EspG2 alter epithelial paracellular permeability. Infect. Immun. 2005;73:6283–6289. doi: 10.1128/IAI.73.10.6283-6289.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thanabalasuriar A, Koutsouris A, Weflen A, et al. The bacterial virulence factor NleA is required for the disruption of intestinal tight junctions by enteropathogenic Escherichia coli. Cell. Microbiol. 2010;12:31–41. doi: 10.1111/j.1462-5822.2009.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muza-Moons MM, Schneeberger EE, Hecht GA. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell. Microbiol. 2004;6:783–793. doi: 10.1111/j.1462-5822.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- 24.Simpson N, Shaw R, Crepin VF, et al. The enteropathogenic Escherichia coli type III secretion system effector Map binds EBP50/NHERF1: implication for cell signalling and diarrhoea. Mol. Microbiol. 2006;60:349–363. doi: 10.1111/j.1365-2958.2006.05109.x. [DOI] [PubMed] [Google Scholar]
- 25.Martinez E, Schroeder GN, Berger CN, et al. Binding to Na(+) /H(+) exchanger regulatory factor 2 (NHERF2) affects trafficking and function of the enteropathogenic Escherichia coli type III secretion system effectors Map, EspI and NleH. Cell. Microbiol. 2010;12:1718–1731. doi: 10.1111/j.1462-5822.2010.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh AP, Aijaz S. Generation of a MDCK cell line with constitutive expression of the Enteropathogenic E. coli effector protein Map as an in vitro model of pathogenesis. Bioengineered. 2015;6:335–341. doi: 10.1080/21655979.2015.1096456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Thanabalasuriar A, Chaworth-Musters T, et al. The bacterial virulence factor NleA inhibits cellular protein secretion by disrupting mammalian COPII function. Cell. Host Microbe. 2007;2:160–171. doi: 10.1016/j.chom.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Thanabalasuriar A, Kim J, Gruenheid S. The inhibition of COPII trafficking is important for intestinal epithelial tight junction disruption during enteropathogenic Escherichia coli and Citrobacter rodentium infection. Microbes Infect. 2013;15:738–744. doi: 10.1016/j.micinf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Glotfelty LG, Zahs A, Hodges K, et al. Enteropathogenic E. coli effectors EspG1/G2 disrupt microtubules, contribute to tight junction perturbation and inhibit restoration. Cell. Microbiol. 2014;16:1767–1783. doi: 10.1111/cmi.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zovein AC, Luque A, Turlo KA, et al. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev. Cell. 2010;18:39–51. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akhtar N, Streuli CH. An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat. Cell Biol. 2013;15:17–27. doi: 10.1038/ncb2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muza-Moons MM, Koutsouris A, Hecht G. Disruption of cell polarity by enteropathogenic Escherichia coli enables basolateral membrane proteins to migrate apically and to potentiate physiological consequences. Infect. Immun. 2003;71:7069–7078. doi: 10.1128/IAI.71.12.7069-7078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirk KL, Halm DR, Dawson DC. Active sodium transport by turtle colon via an electrogenic Na-K exchange pump. Nature. 1980;287:237–239. doi: 10.1038/287237a0. [DOI] [PubMed] [Google Scholar]
- 35.Charney AN, Kinsey MD, Myers L, et al. Na+-K+-activated adenosine triphosphatase and intestinal electrolyte transport. Effect of adrenal steroids. J. Clin. Invest. 1975;56:653–660. doi: 10.1172/JCI108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allgayer H, Kruis W, Paumgartner G, et al. Inverse relationship between colonic (Na+ + K+)-ATPase activity and degree of mucosal inflammation in inflammatory bowel disease. Dig. Dis. Sci. 1988;33:417–422. doi: 10.1007/BF01536025. [DOI] [PubMed] [Google Scholar]
- 37.Ejderhamn J, Finkel Y, Strandvik B. Na,K-ATPase activity in rectal mucosa of children with ulcerative colitis and Crohn's disease. Scand. J. Gastroenterol. 1989;24:1121–1125. doi: 10.3109/00365528909089265. [DOI] [PubMed] [Google Scholar]
- 38.Rachmilewitz D, Karmeli F, Sharon P. Decreased colonic Na-K-ATPase activity in active ulcerative colitis. Isr. J. Med. Sci. 1984;20:681–684. [PubMed] [Google Scholar]
- 39.Sundaram U, West AB. Effect of chronic inflammation on electrolyte transport in rabbit ileal villus and crypt cells. Am. J. Physiol. 1997;272:G732–41. doi: 10.1152/ajpgi.1997.272.4.G732. [DOI] [PubMed] [Google Scholar]
- 40.Sundaram U, Wisel S, Rajendren VM, et al. Mechanism of inhibition of Na+-glucose cotransport in the chronically inflamed rabbit ileum. Am. J. Physiol. 1997;273:G913–9. doi: 10.1152/ajpgi.1997.273.4.G913. [DOI] [PubMed] [Google Scholar]
- 41.Sundaram U, Wisel S, Fromkes JJ. Unique mechanism of inhibition of Na+-amino acid cotransport during chronic ileal inflammation. Am. J. Physiol. 1998;275:G483–9. doi: 10.1152/ajpgi.1998.275.3.G483. [DOI] [PubMed] [Google Scholar]
- 42.Saha P, Arthur S, Kekuda R, et al. Na-glutamine co-transporters B(0)AT1 in villus and SN2 in crypts are differentially altered in chronically inflamed rabbit intestine. Biochim. Biophys. Acta. 2012;1818:434–442. doi: 10.1016/j.bbamem.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Coon S, Kekuda R, Saha P, et al. Glucocorticoids differentially regulate Na-bile acid cotransport in normal and chronically inflamed rabbit ileal villus cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G675–82. doi: 10.1152/ajpgi.00176.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandle GI, Higgs N, Crowe P, et al. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology. 1990;99:97–105. doi: 10.1016/0016-5085(90)91235-x. [DOI] [PubMed] [Google Scholar]
- 45.Hecht G, Hodges K, Gill RK, et al. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G370–8. doi: 10.1152/ajpgi.00432.2003. [DOI] [PubMed] [Google Scholar]
- 46.Hodges K, Alto NM, Ramaswamy K, et al. The enteropathogenic Escherichia coli effector protein EspF decreases sodium hydrogen exchanger 3 activity. Cell. Microbiol. 2008;10:1735–1745. doi: 10.1111/j.1462-5822.2008.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gill RK, Borthakur A, Hodges K, et al. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J. Clin. Invest. 2007;117:428–437. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savkovic SD, Koutsouris A, Hecht G. PKC zeta participates in activation of inflammatory response induced by enteropathogenic E. coli. Am. J. Physiol. Cell. Physiol. 2003;285:C512–21. doi: 10.1152/ajpcell.00444.2002. [DOI] [PubMed] [Google Scholar]
- 49.Alto NM, Shao F, Lazar CS, et al. Identification of a bacterial type III effector family with G protein mimicry functions. Cell. 2006;124:133–145. doi: 10.1016/j.cell.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 50.Huang Z, Sutton SE, Wallenfang AJ, et al. Structural insights into host GTPase isoform selection by a family of bacterial GEF mimics. Nat. Struct. Mol. Biol. 2009;16:853–860. doi: 10.1038/nsmb.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hurd TW, Fan S, Liu CJ, et al. Phosphorylation-dependent binding of 14-3-3 to the polarity protein Par3 regulates cell polarity in mammalian epithelia. Curr. Biol. 2003;13:2082–2090. doi: 10.1016/j.cub.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 52.Izaki T, Kamakura S, Kohjima M, et al. Phosphorylation-dependent binding of 14-3-3 to Par3beta, a human Par3-related cell polarity protein. Biochem. Biophys. Res. Commun. 2005;329:211–218. doi: 10.1016/j.bbrc.2005.01.115. [DOI] [PubMed] [Google Scholar]
- 53.Viswanathan VK, Lukic S, Koutsouris A, et al. Cytokeratin 18 interacts with the enteropathogenic Escherichia coli secreted protein F (EspF) and is redistributed after infection. Cell. Microbiol. 2004;6:987–997. doi: 10.1111/j.1462-5822.2004.00416.x. [DOI] [PubMed] [Google Scholar]
- 54.Germane KL, Spiller BW. Structural and functional studies indicate that the EPEC effector, EspG, directly binds p21-activated kinase. Biochemistry. 2011;50:917–919. doi: 10.1021/bi1020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selyunin AS, Alto NM. Activation of PAK by a bacterial type III effector EspG reveals alternative mechanisms of GTPase pathway regulation. Small GTPases. 2011;2:217–221. doi: 10.4161/sgtp.2.4.16704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batchelor M, Guignot J, Patel A, et al. Involvement of the intermediate filament protein cytokeratin-18 in actin pedestal formation during EPEC infection. EMBO Rep. 2004;5:104–110. doi: 10.1038/sj.embor.7400038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goosney DL, de Grado M, Finlay BB. Putting E. coli on a pedestal: a unique system to study signal transduction and the actin cytoskeleton. Trends Cell Biol. 1999;9:11–14. doi: 10.1016/s0962-8924(98)01418-4. [DOI] [PubMed] [Google Scholar]
- 58.Kenny B, DeVinney R, Stein M, et al. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 59.Mashukova A, Oriolo AS, Wald FA, et al. Rescue of atypical protein kinase C in epithelia by the cytoskeleton and Hsp70 family chaperones. J. Cell. Sci. 2009;122:2491–2503. doi: 10.1242/jcs.046979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ameen NA, Figueroa Y, Salas PJ. Anomalous apical plasma membrane phenotype in CK8-deficient mice indicates a novel role for intermediate filaments in the polarization of simple epithelia. J. Cell. Sci. 2001;114:563–575. doi: 10.1242/jcs.114.3.563. [DOI] [PubMed] [Google Scholar]
- 61.Toivola DM, Krishnan S, Binder HJ, et al. Keratins modulate colonocyte electrolyte transport via protein mistargeting. J. Cell Biol. 2004;164:911–921. doi: 10.1083/jcb.200308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liao J, Omary MB. 14-3-3 Proteins Associate with Phosphorylated Simple Epithelial Keratins during Cell Cycle Progression and Act as a Solubility Cofactor. J. Cell Biol. 1996;133:345–357. doi: 10.1083/jcb.133.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ku NO, Michie S, Resurreccion EZ, et al. Keratin binding to 14-3-3 proteins modulates keratin filaments and hepatocyte mitotic progression. Proc. Natl. Acad. Sci. U. S. A. 2002;99:4373–4378. doi: 10.1073/pnas.072624299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Isberg RR, Leong JM. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 65.McCormick BA, Nusrat A, Parkos CA, et al. Unmasking of intestinal epithelial lateral membrane beta1 integrin consequent to transepithelial neutrophil migration in vitro facilitates inv-mediated invasion by Yersinia pseudotuberculosis. Infect. Immun. 1997;65:1414–1421. doi: 10.1128/iai.65.4.1414-1421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tafazoli F, Holmstrom A, Forsberg A, et al. Apically exposed, tight junction-associated beta1-integrins allow binding and YopE-mediated perturbation of epithelial barriers by wild-type Yersinia bacteria. Infect. Immun. 2000;68:5335–5343. doi: 10.1128/iai.68.9.5335-5343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amieva MR, Vogelmann R, Covacci A, et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagnoli F, Buti L, Tompkins L, et al. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16339–16344. doi: 10.1073/pnas.0502598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeaiter Z, Cohen D, Musch A, et al. Analysis of detergent-resistant membranes of Helicobacter pylori infected gastric adenocarcinoma cells reveals a role for MARK2/Par1b in CagA-mediated disruption of cellular polarity. Cell. Microbiol. 2008;10:781–794. doi: 10.1111/j.1462-5822.2007.01084.x. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki A, Hirata M, Kamimura K, et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr. Biol. 2004;14:1425–1435. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 71.Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr. Biol. 2004;14:736–741. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 72.Saadat I, Higashi H, Obuse C, et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330–333. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 73.Mishra JP, Cohen D, Zamperone A, et al. CagA of Helicobacter pylori interacts with and inhibits the serine-threonine kinase PRK2. Cell. Microbiol. 2015;17:1670–1682. doi: 10.1111/cmi.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balachandran P, Dragone L, Garrity-Ryan L, et al. The ubiquitin ligase Cbl-b limits Pseudomonas aeruginosa exotoxin T-mediated virulence. J. Clin. Invest. 2007;117:419–427. doi: 10.1172/JCI28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soong G, Parker D, Magargee M, et al. The type III toxins of Pseudomonas aeruginosa disrupt epithelial barrier function. J. Bacteriol. 2008;190:2814–2821. doi: 10.1128/JB.01567-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kierbel A, Gassama-Diagne A, Mostov K, et al. The phosphoinositol-3-kinase-protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol. Biol. Cell. 2005;16:2577–2585. doi: 10.1091/mbc.E04-08-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kierbel A, Gassama-Diagne A, Rocha C, et al. Pseudomonas aeruginosa exploits a PIP3-dependent pathway to transform apical into basolateral membrane. J. Cell Biol. 2007;177:21–27. doi: 10.1083/jcb.200605142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tran CS, Eran Y, Ruch TR, et al. Host cell polarity proteins participate in innate immunity to Pseudomonas aeruginosa infection. Cell. Host Microbe. 2014;15:636–643. doi: 10.1016/j.chom.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coureuil M, Mikaty G, Miller F, et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–87. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]