Abstract
Artists and astronomers noticed centuries ago that humans perceive dark features in an image differently from light ones; however, the neuronal mechanisms underlying these dark/light asymmetries remained unknown. Based on computational modeling of neuronal responses, we have previously proposed that such perceptual dark/light asymmetries originate from a luminance/response saturation within the ON retinal pathway. Consistent with this prediction, here we show that stimulus conditions that increase ON luminance/response saturation (e.g., dark backgrounds) or its effect on light stimuli (e.g., optical blur) impair the perceptual discrimination and salience of light targets more than dark targets in human vision. We also show that, in cat visual cortex, the magnitude of the ON luminance/response saturation remains relatively constant under a wide range of luminance conditions that are common indoors, and only shifts away from the lowest luminance contrasts under low mesopic light. Finally, we show that the ON luminance/response saturation affects visual salience mostly when the high spatial frequencies of the image are reduced by poor illumination or optical blur. Because both low luminance and optical blur are risk factors in myopia, our results suggest a possible neuronal mechanism linking myopia progression with the function of the ON visual pathway.
Keywords: area V1, primary visual cortex, receptive field, retina, thalamus
Introduction
Visual information is processed in the brain by separate parallel pathways for signaling luminance increments (ON) and decrements (OFF) in local regions of an image (Hartline, 1938). While ON and OFF pathways combine first in the primary visual cortex (Hubel & Wiesel, 1962), the OFF pathway covers more cortical territory (Jin et al., 2008), makes stronger connections (Jin, Wang, Swadlow, & Alonso, 2011), and drives stronger cortical responses than the ON pathway. OFF-dominated cortical neurons also outnumber ON-dominated neurons by a factor of 2 in layer 4 of cat visual cortex (Y. Wang et al., 2015) and by more than one order of magnitude in the superficial layers of the macaque visual cortex (Yeh, Xing, & Shapley, 2009). The dominance of OFF cortical responses has now been demonstrated in the visual cortex of humans (Zemon, Gordon, & Welch, 1988), cats (Jin et al., 2008, 2011; Liu & Yao, 2014; Rekauzke et al., 2016; Y. Wang et al., 2015), macaques (Xing, Yeh, Gordon, & Shapley, 2014; Yeh et al., 2009; Zurawel, Ayzenshtat, Zweig, Shapley, & Slovin, 2014), tree shrews (Veit, Bhattacharyya, Kretz, & Rainer, 2014), and mice (Tan, Sun, Chen, Kim, & Ji, 2015; but see Polack & Contreras, 2012).
Humans also show pronounced differences in the perception of lights and darks. It has been known since Leonardo da Vinci (MacCurdy, 1938) and Galileo Galilei (1632) that light patches on dark backgrounds look larger than similar-sized dark patches on light backgrounds, a phenomenon known as the irradiation illusion (Helmholtz, 1867). Early psychophysical studies also reported lower thresholds to detect darks than lights (Blackwell, 1946; Bowen, Pokorny, & Smith, 1989; Krauskopf, 1980; Tyler, Chan, & Liu, 1992). However, such threshold differences could be caused by uncontrolled changes in adaptation (Poot, Snippe, & van Hateren, 1997) and are opposite to what would be expected from the higher contrast sensitivity of the ON pathway (Chichilnisky & Kalmar, 2002; Kremkow et al., 2014; Zaghloul, Boahen, & Demb, 2003). On midgray backgrounds, thresholds are lower for darks than lights (Luo-Li, Alais, & Freeman, 2016), probably because midgray backgrounds make the cortex more responsive to dark than light stimuli (Kremkow et al., 2014). Humans also rely more on darks to make judgments about texture variance (Chubb & Nam, 2000), can read dark text on white backgrounds faster than white text on dark backgrounds (Bauer & Cavonius, 1980; Buchner & Baumgartner, 2007), and can detect dark targets on noisy backgrounds more accurately and faster than light targets (Komban et al., 2014; Komban, Alonso, & Zaidi, 2011).
An attractive hypothesis is that both OFF dominance in visual cortex and dark dominance in human vision originate from a common neuronal mechanism: a luminance/response saturation within the ON pathway (Kremkow et al., 2014). The high initial gain of the ON luminance/response function and its early saturation should enlarge light stimuli, making them cover a larger region of the receptive-field surround and more effectively suppressing visual responses. Therefore, the enlargement of light stimuli by the ON luminance/response saturation could explain both the irradiation illusion (light stimuli appear larger) and OFF cortical dominance (large stimuli suppress ON responses more than OFF responses; Komban et al., 2011, 2014; Kremkow et al., 2014; Zhao et al., 2015). We call this enlargement of light stimuli neuronal blur because it is caused by the ON neuronal pathway (Kremkow et al., 2014) and reduces visual acuity. However, unlike optical blur, neuronal blur affects lights more than darks. We predict that the neuronal blur should increase under stimulus conditions that enhance the ON luminance/response saturation (e.g., dark backgrounds) or its effects on light stimuli (e.g., optical blur). The results from this article provide strong support for this general prediction. In addition, the finding that the detection of lights is strongly affected by optical blur and low illumination could explain why optical blur and low light are risk factors in visual diseases such as myopia.
Methods
General methods for all psychophysics visual tasks
Visual stimuli were generated with Psychtoolbox 3 (Brainard, 1997) using custom MATLAB software (Mathworks, Natick, MA). Most stimuli were presented on a 24-in. LCD monitor (BenQ XL2420T, 120 Hz, mean luminance: 156 cd/m2); however, the effect of optical blur on visual salience was measured in a clinical floor with a 20-in. CRT monitor (Mitsubishi SuperBright Diamondtron DP207OSB, 85 Hz, mean luminance: 50 cd/m2). Both monitors were placed at a distance of 1 m from the eye and were gamma corrected. To perform the gamma correction, we first measured the relation between the input voltage and output luminance of the monitor (a power function with a gamma exponent). Then we generated a function in Psychtoolbox 3 that corrected for the gamma nonlinearity and made the relation between input voltage and luminance output linear. The luminance measures were based on the standard V(λ) function established by the Commission Internationale de l'Éclairage (1924), which was used to convert radiant energy into luminous visible energy. The 24-in. monitor subtended 30.3° × 17.0° of visual angle (0.016° × 0.016° per pixel), and the 20-in. monitor 23.7° × 17.7° (0.015° × 0.015° per pixel). The study was approved by the institutional review board at the State University of New York College of Optometry and followed the principles outlined in the Declaration of Helsinki.
Subjects were placed in a dark room in front of the monitor and were monocularly tested with a patch covering the nontested eye. All subjects had either 20/20 vision or vision that was corrected to 20/20. For all visual tasks, subjects used a chin rest to hold their head steady and a numerical keypad to respond to stimuli displayed on the monitor. We used four subjects for measurements of visual acuity (three women and one man, including authors CP and JMA), four for measurements of visual salience with different luminance (one woman and three men, including authors CP, JMA, and RM), and 11 for measurements of visual salience with different optical blur (seven women and four men, including authors JMA and MWD). Some of the experiments required collecting tens of thousands of trials, a task that can be tedious and not very motivating for untrained observers who do not know the purpose of the study. Therefore, we followed a common tradition in human psychophysics and used the authors as the main experimental subjects. Most of the dark/light asymmetries that we report reached significance for each individual subject and were replicated in at least four subjects.
Grating visual acuity
To measure grating visual acuity, we asked subjects to report the orientation (horizontal or vertical) of a half-rectified, square-wave grating followed by a mask. The grating could be light or dark and was presented with different durations (50, 100, 150, or 200 ms) and spatial frequencies (0.5, 1, 2, 4, 8, or 16 c/°) after a 250-ms delay. We used different durations to vary the task difficulty and study any possible dependency of dark/light asymmetries on temporal integration. We did not test durations longer than 200 ms, to avoid eye movements and restrict the measures to single fixations. The mask was made of overlapped horizontal and vertical gratings with the same duration as the grating target. This test of grating acuity was designed to emulate standard measurements of visual acuity in the clinic. Visual acuity is measured in the eye clinic by presenting small letters with sharp borders and asking subjects to discriminate the letters. This letter acuity test frequently requires discriminating the orientation of a gap separating two sharp borders (e.g., discriminate U from C or different orientations of C). Similarly, our rectangular gratings had sharp borders and required subjects to discriminate the orientation of a gap between a limited number of bars. We recognize that the mathematical approximation of spatial frequency from these rectangular gratings is not an accurate description of the sinusoidal grating components. Therefore, we use the term spatial frequency in this article to describe the frequency of rectangular grating bars in visual space, not the frequency of the sinusoidal components of the grating. Notice that this frequency is the fundamental sinusoidal component of the square wave, while the higher harmonics have powers reduced by multiples of 3.
We measured grating visual acuity on three different background conditions: gray, dark, and light. The background/adapting luminance is the monitor luminance before the stimulus is presented. The dark and light gratings had the same Weber contrast on all background conditions and the same mean adaptation luminance when tested on gray backgrounds. On gray background conditions, subjects adapted for 120 s to a midgray screen of 156 cd/m2 and then the square-wave gratings were presented using the same midgray background for light and dark grating bars. On the dark and light background conditions, subjects adapted for 120 s to either a dark background of 0.5 cd/m2 (for gratings with light bars) or a light background of 312 cd/m2 (for gratings with dark bars). Ideally, we would like to cover the entire monitor with grating bars to facilitate the orientation discrimination. However, this can only be done when comparing light and dark bars on midgray backgrounds. Light bars on dark backgrounds appear identical to dark bars on light backgrounds if the gratings cover the entire monitor (with the exception of a phase difference that is irrelevant for orientation discrimination). Therefore, on the dark and light backgrounds, the grating frequency was increased by reducing the size of the grating while keeping the duty cycle constant (from 6° to 0.2° per grating side), an approach that resembles the tests of visual acuity in the eye clinic (Westheimer, 2003; Westheimer, Chu, Huang, Tran, & Dister, 2003). On gray backgrounds, the grating frequency was increased by reducing the width of the grating bars while keeping the grating size and duty cycle constant.
We measured grating visual acuity in four subjects using the full luminance of the monitor (including authors CP and JMA) and in four subjects using stimuli with low luminance (including author CP). The measures under low luminance were obtained by placing a neutral-density filter of 2 in. diameter and 4.0 optical density in front of the eye (NE40A, Thorlabs, Newton, NJ) to reduce the luminance of the stimuli by four orders of magnitude (maximum: 0.0312 cd/m2). Each subject except CP completed 9,600 trials with high luminance and 2,400 trials with low luminance. Subject CP completed 19,200 trials with high luminance and 19,200 trials with low luminance, and also repeated the measurements of low luminance with a pupil of 3-mm diameter placed in front of the eye (4,800 trials; see details on pupil placement later).
Visual salience
Visual salience of light and dark targets was measured with a randomized sequence of square targets presented against a uniform binary-noise background that had the same average number of light and dark pixels (Komban et al., 2011; Zhao et al., 2015). Subjects adapted to a midgray screen for 15 s and then reported the number of squares displayed, which ranged from one to three. The side of the square targets was 6 times larger than the side of the background pixels. Therefore, the probability of forming a false target with random groups of pixels was negligible (p = 0.536). The duration of each trial was determined by the subject's reaction time, and each trial started after the subject responded to the previous trial. Accuracy and reaction times were measured in eight sets of 100 trials presented within the same session (800 trials in total). Between sets, subjects took a break of a few minutes and then adapted to the midgray screen for 15 s again before they started the next set.
To investigate the effect of mean retinal illumination on dark/light asymmetries for visual salience, the stimuli were displayed under two luminance conditions: high and low illumination. In the high-illumination condition, the subjects looked directly at the monitor without any filter and the stimuli were displayed using the maximum luminance range of the monitor (maximum luminance: 312 cd/m2). In the low-illumination condition, the subjects looked at the monitor through a neutral-density filter of 2 in. diameter and 4.0 optical density (NE40A, Thorlabs), which decreased the luminance of the monitor by four orders of magnitude (maximum luminance: 0.0312 cd/m2). We studied the effect of mean retinal illumination on dark/light asymmetries in four subjects (including authors CP, RM, and JMA). Each subject completed 800 trials separated into eight trial blocks in one session.
To measure the effect of optical blur on dark/light visual salience, we blocked accommodation of the dominant eye with cyclopentolate hydrocholoride 1% ophthalmic solution. Efficacy of cycloplegia was tested by verifying sustained blur of visual acuity with a reduced Snellen near point card placed at 1 m from the subject. Optical blur was created by wearing soft contact lenses (1 Day Acuvue Moist or Avaira) that produced −5 D, −3 D, 0 D, +3 D or +5 D of optical blur, taking into account the distance and refractive error of the subject. The order in which contact lenses were given varied randomly between and within subjects. To reduce the optical aberrations due to the pharmacologic dilation of the natural pupils, an artificial pupil of 3.5 mm was used in some experiments. However, the main results could be replicated with and without the artificial pupil, which indicates that the different effects of optical blur on the detection of lights and darks are not due to optical aberrations. The artificial pupil was always centered on the natural pupil and placed as close as possible to the corneal plane (∼9 mm) while still allowing for the subjects to comfortably blink. Notice that the artificial pupil was smaller than the natural pupil, which was pharmacologically enlarged, but not smaller than 3 mm (smaller pupils would reduce the depth of focus and optical blur). The mean luminance of the monitor for the measurements of optical blur was 50 cd/m2. We studied the effect of optical blur in 11 subjects (including authors MWD and JMA), and each subject completed 200 trials separated into two trial blocks in one session.
Dot visual acuity
Dot visual acuity was measured with a randomized sequence of light (312 cd/m2) and dark (0.5 cd/m2) dot targets of different sizes (1, 2, 3, 4, 6, or 8 pixels, 0.016° per side per pixel). The dot targets were superimposed on a circular pedestal of 3.5 cm diameter (3.5°) that acted as a circular local background. The circular pedestal could be light (312 cd/m2 for dark targets), dark (0.5 cd/m2 for light targets), or midgray (156 cd/m2 for both light and dark targets) to replicate the background luminances used for grating visual acuity. Moreover, to study the effect of mean luminance adaptation more precisely for this task, we also tested two intermediate luminance values lighter or darker than midgray (dark-gray pedestal of 46 cd/m2 for light-gray targets of 238 cd/m2, and light-gray pedestal of 238 cd/m2 for dark-gray targets of 46 cd/m2).
Target and pedestal were presented on a red adaptation background of 31.38 cd/m2. The adaptation background was red to make the pedestal visible in all stimulus conditions. For example, if we had used a midgray adaptation background, the pedestal would be visible only when it was black or white, not when it was gray. Target and pedestal were presented together in one of two temporal intervals that were separated by 500 ms and a variable delay (100, 200, 300, or 400 ms). The variable delay was used to manipulate task difficulty. The duration of the target was always half the duration of the pedestal. The task of the subject was to identify the interval in which the dot target was present. Subjects adapted to the red background for 60 s before the interval sequence started, and the red background was maintained for the entire duration of the stimulus presentation. We measured dot visual acuity in four subjects (including authors CP and RM). Each subject completed 1,200 trials for each of the background conditions.
Light/dark adaptation
Our previous work indicates that the ON luminance/response saturation originates at early stages of retinal processing, perhaps as early as the photoreceptor (Kremkow et al., 2014). Therefore, changes in ON luminance/response saturation should be strongly associated with changes in light adaptation at photoreceptor and postreceptor levels (Boynton & Whitten, 1970; Dunn, Lankheet, & Rieke, 2007; B. B. Lee, Pokorny, Smith, Martin, & Valberg, 1990; Purpura, Tranchina, Kaplan, & Shapley, 1990; Schnapf, Nunn, Meister, & Baylor, 1990; Tranchina, Sneyd, & Cadenas, 1991). Our previous work also shows that the ON luminance/response saturation is less pronounced in midgray than black backgrounds (Kremkow et al., 2014). Therefore, to study the contribution of light adaptation to ON luminance/response saturation and dark/light asymmetries, we used multiple backgrounds and always included a gray background (or equivalent uniform noise background) in all psychophysical and cortical measures. The effect of light adaptation was dissected even more precisely in the dot-visual-acuity task by including five different background levels (dark, light, midgray, dark gray, light gray).
Data acquisition and analysis
Reaction-time histograms were plotted using a bin of 200 ms and fitted with an exponential Gaussian function (Komban et al., 2011; Zhao et al., 2015). Statistical significance was assessed with a paired t test when comparing percentages of correct responses between dark and light gratings (e.g., pattern visual acuity) and with a Wilcoxon test when comparing medians (e.g., visual detection with different luminance and optical blur). For all tests, the level of significance was marked as follows: *p < 0.05, **p < 0.01, ***p < 0.001. All error bars are plotted as standard error of the mean.
General methods for physiological recordings
Adult male cats (Felis catus, age 6–12 months, n = 2) were tranquilized with an intramuscular injection of acepromazine (0.2 mg/kg) and anesthetized with an intramuscular injection of ketamine (10 mg/kg). Two intravenous catheters were inserted into each hind limb to administer continuous infusions of propofol (5–6 mg · kg−1 · h−1), sufentanil (10–20 ng · kg−1 · h−1), vecuronium bromide (0.2 mg · kg−1 · h−1), and saline (1–3 ml/h). All vital signs were monitored and carefully maintained within normal physiological limits. Details of the surgical procedures have been described previously (Jin et al., 2008; Kremkow, Jin, Wang, & Alonso, 2016). All procedures were performed in accordance with the guidelines of the U.S. Department of Agriculture, adhered to the Association for Research in Vision and Ophthalmology's statement of the use of animals in ophthalmic and vision research, and were approved by the Institutional Animal Care and Use Committee at the State University of New York, State College of Optometry.
Electrophysiological recordings and data acquisition
We introduced two 32-channel linear multielectrode arrays (0.1-mm interelectrode distance; (Neuronexus, Ann Arbor, MI) in the cat primary visual cortex to record from cortical multiunit activity. The spike recordings were filtered between 250 Hz and 8 kHz, sampled at 40 kHz, and collected by a computer running Omniplex (Plexon, Dallas, TX), as previously described (Jin et al., 2008). The multielectrode arrays were introduced into the cortex with < 5° angle relative to the horizontal plane and centered in layer 4.
Visual stimuli
Custom MATLAB code (with Psychtoolbox extensions (Brainard, 1997) was used to present visual stimuli on two gamma-corrected monitors: a 21-in. CRT monitor (Sony MultiScan G520, 120 Hz, mean luminance: 35 cd/m2) and a 24-in. LCD monitor (BenQ XL2420-B, 120 Hz, mean luminance: 120 cd/m2). The gamma correction was performed using luminance measures based on the standard V(λ) function, as explained earlier. Receptive fields were mapped by stimulus spike-triggered average with sparse noise made of light and dark squares. The squares had sides of 2.8° and were presented at spatial positions separated by 1.4° with an update rate of 30 Hz. The monitor was placed at a distance of 0.57 m from the animal.
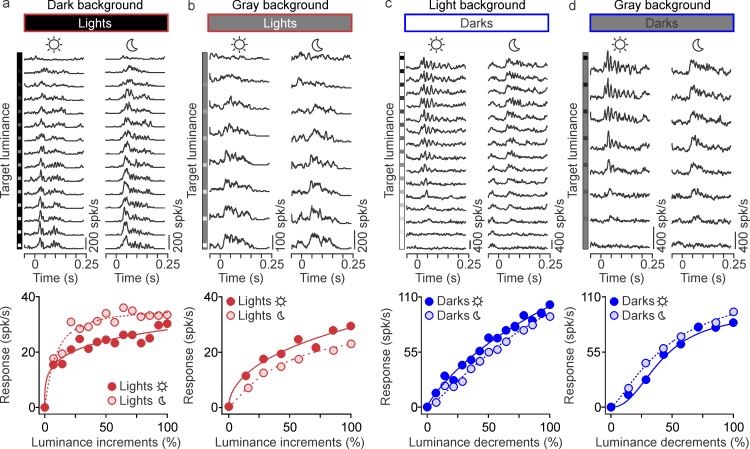
Luminance/response functions
The luminance/response functions of cortical neurons were measured with a square patch of ∼10° per side presented at the center of the cortical-population receptive field (the average of all receptive fields measured at all recording sites). The square patch was presented with 15 different luminance values ranging from 0.03 to 70 cd/m2 (Sony monitor) or 0.27 to 240 cd/m2 (BenQ monitor), divided in equal-luminance intervals. The patch was turned on for 133 ms and off for 133 ms and presented on either dark (0.03 or 0.27 cd/m2), light (70 or 240 cd/m2), or gray (35 or 120 cd/m2) backgrounds. We measured ON responses to light increments on dark or gray backgrounds and OFF responses to light decrements on light or gray backgrounds. To plot the luminance/response function, we measured the mean firing rate between 0 and 133 ms following the stimulus presentation, separately for each of the 15 luminance values and the two different backgrounds. The mean firing rates were then plotted as a function of the luminance increments or decrements and fitted with a Naka–Rushton function (Naka & Rushton, 1966):
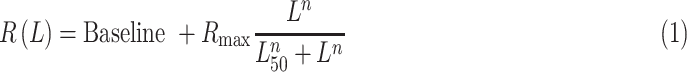 |
where R(L) is the cortical response for luminance L, Baseline is the neuronal baseline activity, Rmax is the maximum response, n is the exponent of the function, and L50 is the luminance that generated 50% of the maximum response. The baseline was calculated as the mean firing rate within 10 ms preceding the stimulus onset. The Naka–Rushton fits were used to extract L50, n, the response at maximum luminance (R100), and the signal-to-noise ratio at 50% luminance (SNR). The SNR was calculated as the maximum response between 1 and 133 ms after the stimulus onset divided by the baseline. To avoid infinite SNR values when the baseline was zero, baselines with values < 1 spike/s were assigned an arbitrary value of 1 spike/sec. Only neurons that had a SNR larger than 7, reasonably good fits (R2 ≥ 0.7), and n between 0 and 10 were included in the analysis.
Perceptual model
We built a computational model to replicate the dark/light asymmetries measured in the psychophysical experiments. The model has three main stages. In the first stage (retinal), images of the stimuli were slightly blurred with a Gaussian function to simulate optical blur. Then they were passed through a luminance/response function that saturated more for ON than OFF visual responses and therefore blurred more light than dark features in the image (neuronal blur). In the second stage (thalamocortical), the blurred images were convolved with a difference-of-Gaussians function to simulate a center–surround receptive field of retinal and thalamic neurons. Because the center–surround structure is similar in retina and thalamus, the convolution was implemented in one single step at the thalamocortical input. The center of the convolution was then multiplied by a variable gain and added to random noise to generate the cortical output. In the third and last stage (perceptual), a correct response was generated when the cortical output crossed threshold. We describe in more detail later the equations underlying the different stages of the model and the parameters used in all the simulations.
The inputs of the model were black-and-white images resembling the stimuli used in the psychophysical experiments (i.e., images of bars, dots, and square patches superimposed on uniform or white noise backgrounds). We first convolved the retinal luminance function L of the images I with the optical point spread function of the eye (PSF), as shown in Equation 2. This convolution created gray levels that were not present in the black-and-white original images and slightly blurred the sharp edges. The PSF was modeled as a Gaussian filter with a standard deviation of 0.5 arcmin (Campbell & Gubisch, 1966):
 |
After it passed through the optics of the eye, we modeled the photoreceptor response fed into ON and OFF pathways with two Naka–Rushton functions (Ron and Roff), as shown in Equation 3. Each R(x, y) provides the response at each image location (x, y) as a function of the pixel luminance L(x, y). The shape of each Naka–Rushton function is determined by the exponent n and the luminance L50 that generates 50% of the maximum response, which changes with background/adaptation luminance and the contrast polarity of the stimulus. The response magnitude is scaled by Rmax. OFF responses are modeled as the absolute value of the function minus 1, because L(x, y) ranges from 0 (black) to 1 (white):
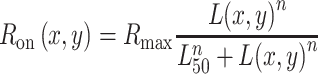 |
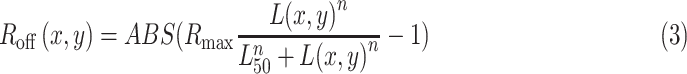 |
Values of L50 and n were lower for ON than OFF pathways (ON: L50 = 0.1, n = 1.6; OFF: L50 = 0.5, n = 2.5), to make the saturation of the luminance/response function more pronounced for ON than OFF visual responses. For the sake of simplicity, Rmax was kept constant and equal for ON and OFF responses (set to a value of 1). The parameter values of L50 and n were based on our measurements of ON and OFF luminance/response functions with visual evoked potentials in humans (Kremkow et al., 2014). These parameters were kept constant across all simulations and stimuli, except for gratings with a gray background. On the gray background, L50 for the ON pathway was 0.3 instead of 0.1, consistent with our physiological and psychophysical measurements. While we made an effort to choose parameter values that resembled those measured in human visual cortex, the purpose of this simple model is not to identify the exact parameters of the luminance/response function in the human fovea. Instead, it is to demonstrate that a difference in luminance/response saturation between ON and OFF pathways can replicate the dark/light asymmetries measured in human vision. Differences in ON and OFF luminance/response saturation can be obtained with other combinations of L50 and n, which may be more appropriate for other stimulus conditions (Rudd, 2013, 2017). However, for the stimuli and luminance conditions used in our physiological measurements from cats, monkeys, and humans (Kremkow et al., 2014), L50 is lower for ON than OFF visual responses, n is larger than 1, and L50 is lower than 0.5. The parameters used in our model are consistent with these measures. The Rmax value is also higher for OFF than ON visual responses, a difference that increases on midgray backgrounds. However, for simplicity, Rmax was set to 1 in our model.
After we fed the photoreceptor response through ON and OFF visual pathways, the response signal was convolved with a center–surround receptive field. Because this center–surround receptive field is similar in retina and thalamus, it was simulated in a single step: a single convolution at the thalamocortical input of the thalamocortical stage (Ton, Toff). Equation 4 describes the cortical responses (Con, Coff) that result from this convolution:
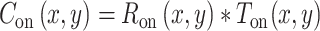 |
 |
The central value of the convolution was used to estimate the cortical response at the target region that was relevant for each task, C(target). This target region was the bar gap in the task for grating acuity and the center of light/dark targets in the other tasks. The value of the convolution at the target region was multiplied by a gain factor g and added to random noise r to obtain the perceptual response P. If this product was larger than an arbitrary perceptual threshold (10% of maximum response), P was set to a value of 1; otherwise, P was set to a random value of 0 or 1 with equal probability. The gain factor simulates the adjustment of neuronal response strength with the stimulus conditions (e.g., higher at low light). The noise simulates the variability of response accuracy near threshold. The P values of 1 and 0 simulate the binary perceptual response, which can be correct or incorrect. As with any binary perceptual decision, the probability of P = 1 is random if the neuronal response does not reach threshold and becomes higher as the value of the convolution increases. For each stimulus condition, the percentage of correct responses was calculated from 100 trials per subject in 200 simulated subjects and the percentages were averaged:
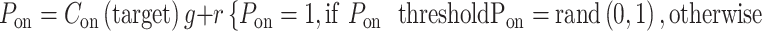 |
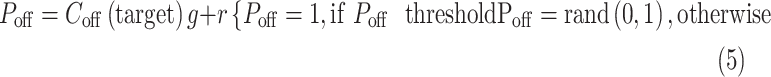 |
It should be emphasized that all the parameter values were always the same for ON and OFF pathways except for L50 and n, which causes the luminance/response function to saturate more for ON than OFF responses. It should also be noted that the larger ON luminance/response saturation made the convolved receptive field larger for the ON than the OFF pathway (Kremkow et al., 2014). Therefore, with a larger ON luminance/response saturation, our model also replicates the differences in receptive-field size between ON and OFF neurons. The number of ON and OFF thalamic inputs is assumed to be the same in the model, as the differences in central retina for beta cells are small in both cats (Wassle, Boycott, & Illing, 1981) and primates (Ahmad, Klug, Herr, Sterling, & Schein, 2003), and could not be demonstrated in cat thalamus (Jin et al., 2008).
The parameter values used in the simulations were as follows: The value of Rmax was always 1 for both ON and OFF responses; n was always 1.6 for ON responses and 2.5 for OFF responses; and L50 was always 0.5 for OFF responses and 0.1 for ON responses, with the exception of bars on a gray background, which were simulated with an L50 of 0.3 for ON responses. The center–surround receptive field was always 2 times larger than the center. The perceptual threshold was always 10% of the maximum response. The noise was modeled as a uniform random distribution that ranged in amplitude from −6% to +6% of maximum response in all simulations except in the simulations of small dots. The standard deviation of the PSF was kept constant at 0.5 arcmin for all simulations except for optical blur. The parameters that changed were the stimuli, receptive-field size, response gain, and random noise. In the simulations for grating visual acuity, we varied the spatial frequency of the stimuli (bar widths: 10 to 168 pixels), the receptive-field size (SD: 4 to 26 pixels), and the response gain (g: 0.4 to 0.7). The different receptive-field sizes and response strengths were varied to simulate the different sizes of the neuronal populations driven by different spatial frequencies. In the simulations of grating visual acuity on a gray background, we also subtracted the background response after Equation 3 to have a response range from 0 to 1, as with light and dark backgrounds. In the simulations for small dots, we varied the dot diameter (9 to 199 pixels) and kept the other parameters constant except for the two smallest dot sizes, which required adjusting the gain (g: 1 to 0.6) and random noise (r: 12% to 3%) to match the data. In the simulations for optical blur, we varied the standard deviation of the Gaussian function simulating optical blur in the retinal stage (PSF SD: 0.5 to 0.75 arcmin) and the receptive-field size (6 to 7 pixels) while keeping the other parameters constant. In the simulations of visual salience at low luminance, we increased the receptive-field size (7 to 11 pixels) and reduced the response gain (0.6 to 0.5), to simulate the reduction in the population response from foveal neurons with small receptive fields at low light.
Results
We have previously shown that ON visual responses saturate more with luminance contrast than OFF visual responses, a difference that is likely to originate in the retina and increases as the background luminance of light targets is reduced (Figure 1a; Kremkow et al., 2014). Based on a computational model, we predicted that this ON luminance/response saturation should cause a spatial enlargement of light stimuli (Figure 1b) that could potentially explain multiple dark/light asymmetries in human vision (Kremkow et al., 2014). We call this spatial distortion neuronal blur because it is caused by neurons and reduces visual acuity. However, unlike optical blur, neuronal blur affects lights more than darks. The main goal of this article is to measure the effects of neuronal blur in human vision using psychophysical methods. According to our model, dark/light asymmetries should become more pronounced under conditions that increase ON luminance/response saturation, such as dark backgrounds. Moreover, because the effect of ON luminance/response saturation is more pronounced at low spatial frequencies (Kremkow et al., 2014), dark/light asymmetries should increase when high spatial frequencies are removed by optical blur or low luminance. The experiments described in the following confirm these main predictions for a large variety of visual tasks, stimulus conditions, and light levels that are common indoors and should be frequently experienced in our daily activities.
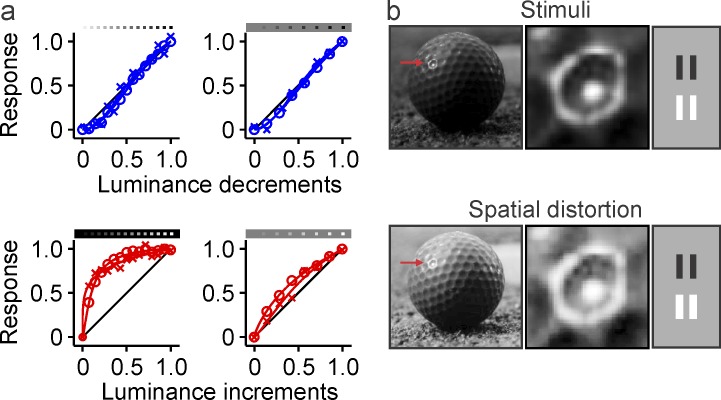
Figure 1.
Neuronal blur. (a) Luminance/response functions measured with dark (blue) and light targets (red), in thalamus (circles) and visual cortex (crosses), on dark/light backgrounds (left) and gray backgrounds (right). Adapted from Kremkow et al., 2014 (figure 3a–3d). The OFF luminance/response function (blue) is roughly linear and independent of background luminance, while the ON luminance/response function saturates with small luminance increments and the saturation is more pronounced on dark than gray backgrounds. (b) The ON luminance/response saturation causes a spatial distortion that we call neuronal blur. The effect of neuronal blur can be seen on the light reflections of a golf ball (left, middle) and light bars (right). The middle panel shows the spatial distortion of the ball reflection in more detail (red arrow in left panel). The neuronal blur of the bars in the right panel is enhanced for illustration purposes.
Grating visual acuity is higher for darks than lights
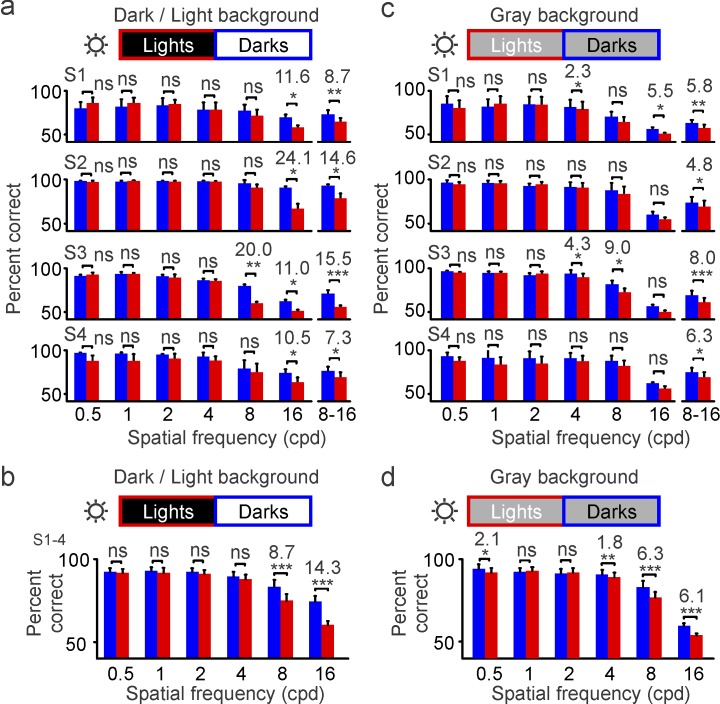
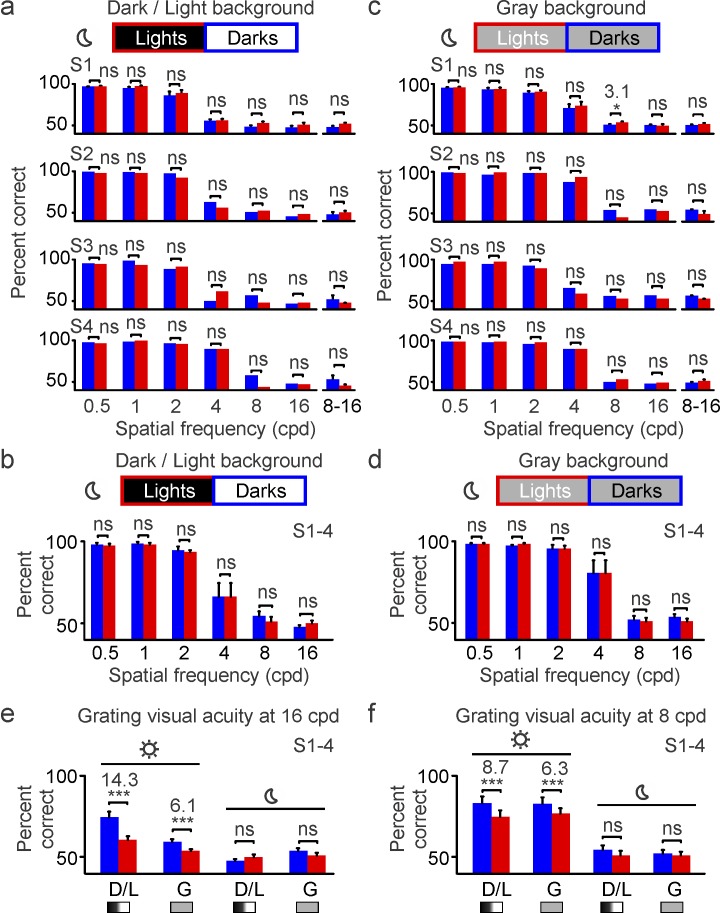
A first prediction from our model is that the enlargement of light stimuli by the ON luminance/response saturation should make it more difficult to discriminate closely spaced bars when they are light than when they are dark. We tested this first prediction with a task that requires pattern discrimination (similar to discriminating letters in an eye chart). Subjects were asked to report the orientation (horizontal or vertical) of a full-contrast grating that was half-wave rectified to be either lighter or darker than the background (Figure 2a). In this task, the percentage of correct responses decreased as we increased the spatial frequency of the grating, a decrease that could be demonstrated using different stimulus durations (50 to 200 ms) either with background luminance different for lights and darks (Figure 2b, dark/light background) or with the same midgray background luminance (Figure 2c). Differences in the percentage of correct responses between darks and lights became apparent at high spatial frequencies (Figure 2b and 2c, shaded areas). When measured at 16 c/° under different levels of background adaptation (Figure 3a, light/dark backgrounds), individual subjects performed 10.5% to 24.1% better with dark than light gratings, with an average difference across subjects of 14.3% (Figure 3b). Therefore, on average, subjects were able to reach their highest visual acuity (discriminate the 16-c/° grating) 14.3% more frequently when the grating was dark than when it was light. Such differences could also be demonstrated in the average of responses to 8–16 c/° for each individual subject (Figure 3a, right), but they could not be demonstrated at lower spatial frequencies. This lack of dark/light asymmetries at low spatial frequencies is consistent with the expected enlargement of light stimuli caused by neuronal blur, which should affect only closely spaced bars (Figure 3a and 3b).
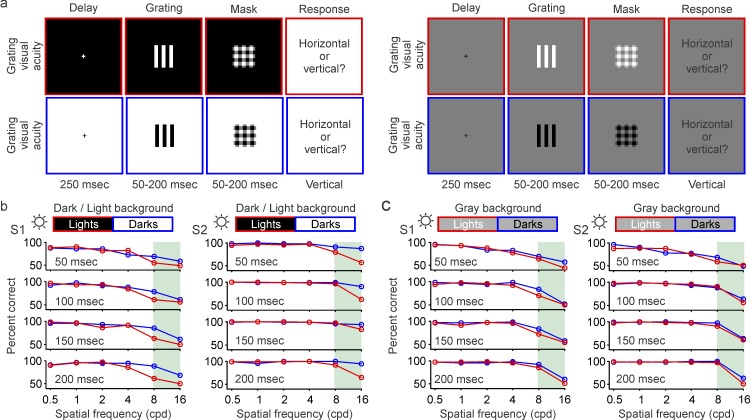
Figure 2.
Grating visual acuity for darks and lights measured under high luminance (maximum: 312 cd/m2). (a) Grating visual acuity was measured with half-rectified gratings that could be either light (top) or dark (bottom), and were presented on dark/light backgrounds (left) or gray backgrounds (right). The gratings were briefly presented after a delay period of 250 ms and were followed by a mask. The subjects had to report the orientation of the grating stimulus, which varied in presentation time (50–200 ms) and spatial frequency (0.5–16 c/°). See videos of the stimuli. (b) Grating visual acuity measured in two different subjects (left: S1; right: S2) as a function of spatial frequency (x-axis) and presentation time (50–200 ms). Light targets were presented on dark backgrounds (red), and dark targets on light backgrounds (blue) using the maximum luminance of the monitor (sun icon). Differences between darks and lights become noticeable at the highest spatial frequencies (shaded green area). (c) Same as (b), but for gray backgrounds.
Figure 3.
Grating visual acuity is higher for darks than lights at high luminance (maximum: 312 cd/m2). Grating visual acuity measured in four different subjects (S1–S4) at high luminance (sun icon). The percentage correct is calculated as an average of four different stimulus durations (trials per spatial frequency: 1,600 for S1 and 800 for S2–S4). (a) Grating visual acuity measured with light targets on dark backgrounds (red) and dark targets on light backgrounds (blue). The bin on the right shows the percentage of correct responses for 8 and 16 c/° combined. (b) Grating visual acuity measured on dark and light backgrounds averaged across four subjects. (c–d) Same as (a–b), but for gray backgrounds. *p < 0.05, **p < 0.01, ***p < 0.001, ns: not significant. Error bars show the standard error of the mean. Notice that the percentage correct is lower in (c–d) than in (a–b) because the midgray background made the contrast lower. However, the reduction on the midgray background is less pronounced for lights than darks because the neuronal blur for lights is also reduced.
Because the ON luminance/response saturation becomes weaker on gray backgrounds (Figure 1a), we also predicted that dark/light asymmetries in grating visual acuity should become less pronounced as the background luminance increases. Consistent with this prediction, gray backgrounds reduced the dark/light differences in visual performance by a factor of 2–4 in individual subjects (Figure 3a and 3c; 11.6% to 5.5% in S1 and 24.1% to 5.3% in S2) and a factor of 2 on average (Figure 3b and 3d; 14.3% to 6.1%, p < 0.001). On gray backgrounds, the dark/light differences were significant for 16 c/° in only the subject who performed the largest number of trials (S1, n = 200 trials; 5.5% difference, p = 0.03), but they were significant in all subjects for the average over 8–16 c/° (Figure 3c; S1: 5.8%, p = 0.001; S2: 4.8%, p = 0.03; S3: 8%, p < 0.001; S4: 6.3%, p = 0.02). These results confirm our first prediction that the enlargement of light stimuli caused by neuronal blur narrows the separation between light grating bars (Figure 1b), making the grating orientation less visible. They also confirm our prediction that as the ON luminance/response saturation becomes weaker on gray backgrounds, the magnitude of dark/light asymmetries is reduced. Importantly, just as is the case for the ON luminance/response saturation (Kremkow et al., 2014), dark/light asymmetries remained significant on gray backgrounds, both at the level of individual subjects (Figure 3c) and in the subject average (Figure 3d). Therefore, we conclude that grating visual acuity is greater for dark than light targets under a wide variety of background conditions that are common indoors and are likely to be experienced in our daily activities.
Low light eliminates dark/light differences in grating visual acuity
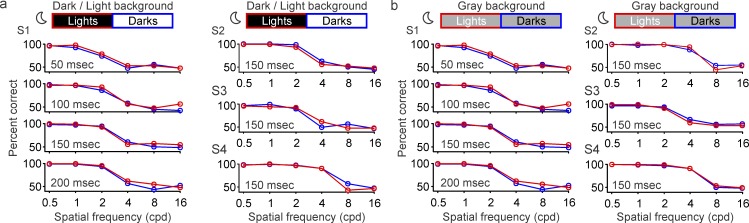
Our second prediction is that reducing vision at high spatial frequencies should eliminate dark/light asymmetries in grating visual acuity because the narrow bar gaps affected by neuronal blur are no longer visible (Figure 1). While low light is known to decrease visual acuity (Johnson, 1976; Johnson & Casson, 1995; Rabin, 1994; Sheedy, Bailey, & Raasch, 1984; Shlaer, 1937; Wilcox, 1932), its effect on dark/light asymmetries remains unknown. Therefore, to test our second prediction, we measured grating visual acuity with a neutral-density filter placed in front of the eye that reduced stimulus luminance by four orders of magnitude (maximum: 0.0312 cd/m2). Under this low retinal illumination (Figure 4a and 4b), the percentage of correct responses fell to chance level for gratings with high spatial frequency (8–16 c/°). With the loss of visual acuity, dark/light asymmetries were eliminated in all individual subjects, an effect that could be demonstrated under different background conditions (Figure 5a–5d). Similar results were obtained when a 3-mm pupil was added to reduce the optical aberrations caused by the enlarged pupil at low light (not shown). Based on these results, we conclude that grating visual acuity is higher for darks than lights but only under photopic illumination (e.g., mean luminance: 156 cd/m2). Differences in grating visual acuity are no longer present when the mean luminance is reduced to 0.0156 cd/m2 (Figure 5e and 5f). These results confirm our prediction that neuronal blur should affect only narrow bar gaps (which are not visible at low light).
Figure 4.
Grating visual acuity for darks and lights measured under low luminance (maximum: 0.0312 cd/m2). (a) Grating visual acuity measured in two different subjects (left: S1; right: S2) as a function of spatial frequency and presentation time. Light targets were presented on dark backgrounds (red) and dark targets on light backgrounds (blue). The mean luminance was reduced by four log units with a neutral-density filter placed in front of the eye (moon icon). (b) Same as (a), but for gray backgrounds.
Figure 5.
Grating visual acuity is similar for darks and lights under low luminance (maximum: 0.0312 cd/m2). (a) Grating visual acuity measured in four different subjects (S1–S4) at low luminance (moon icon). The percentage correct is calculated as an average of four different stimulus durations for S1 (1,600 trials per spatial frequency) and with 150-ms stimulus duration for S2–S4 (200 trials per spatial frequency). There are no differences in grating resolution between darks (blue) and lights (red) at 16 and 8–16 c/° because these spatial frequencies are not visible at low light (percentage correct is at chance level). (b) Grating visual acuity measured on dark and light backgrounds averaged across the four subjects. (c–d) Same as in (a–b), but for gray backgrounds. (e) Comparison of dark and light visual acuity at 16 c/° for high-luminance (sun icon) and low-luminance conditions (moon icon), on dark/light (D/L) and gray (g) backgrounds. (f) Same as (e), but for 8 c/°. *p < 0.05, ***p < 0.001, ns: not significant. Error bars show the standard error of the mean.
Low light reduces visual salience more for lights than darks
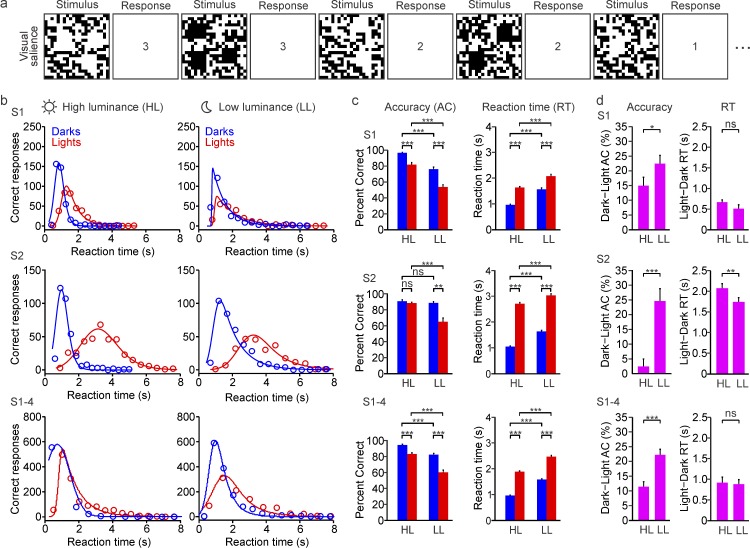
Because the ON luminance/response saturation makes ON responses weaker than OFF responses at low spatial frequencies (Kremkow et al., 2014), our third prediction is that dark/light asymmetries in visual salience should become more pronounced when the high spatial frequencies are removed. We tested this third prediction with a visual-salience task that we have used extensively in the past (Komban et al., 2011, 2014; Wool et al., 2015; Zhao et al., 2015). Subjects were asked to report the number (one, two, or three) of light or dark squares presented on a binary-noise background (Figure 6a), when looking directly at the screen (mean luminance: 156 cd/m2) or through a neutral-density filter that reduced the amount of light by four orders of magnitude (mean luminance: 0.0156 cd/m2). Consistent with our previous results, every subject was more accurate and faster at detecting darks than lights under high luminance (Figure 6b). However, unlike for grating visual acuity, the dark/light asymmetries were not eliminated under low light: They were enhanced (Figure 6c and 6d). Reducing the mean luminance by four orders of magnitude made light targets much more difficult to see than dark targets and increased dark/light differences in visual salience by a factor of 2 (Figure 6d, bottom left; S1–S4: 11.29% to 22.01%). Therefore, we conclude that vision under low light affects the visibility of lights more than darks, as would be expected from the effect of neuronal blur on low spatial frequencies.
Figure 6.
Low luminance reduces visual salience more for lights than for darks. (a) Subjects were asked to report as fast as possible the number of light or dark targets (one, two, or three) presented on a uniform binary-noise background, under high-luminance (mean luminance: 156 cd/m2) and low-luminance conditions (mean luminance: 0.0156 cd/m2). The figure shows a sequence of stimulus frames and correct responses. See videos of the stimuli. (b) Relation between correct responses and reaction time for dark (blue) and light targets (red) measured under high luminance (HL, left) and low luminance (LL, right). The relations are shown for two different subjects (top: S1; middle: S2) and the average of four subjects (S1–S4). Circles: measurements. Lines: Gaussian-exponential fits. (c) Average accuracy (AC, left) and reaction time (RT, right) in the detection of darks (blue) and lights (red), measured under high luminance (HL) and low luminance (LL). The averages are shown for two individual subjects (S1 and S2) and for four subjects combined (S1–S4). (d) Average differences in accuracy (AC, left) and reaction time (RT, right) between darks and lights measured under high luminance (HL) and low luminance (LL). The averages are shown for two individual subjects (S1 and S2) and for four subjects combined (S1–S4). *p < 0.05, **p < 0.01, ***p < 0.001, ns: not significant. Error bars show the standard error of the mean.
Optical blur also reduces visual salience more for lights than darks
Changes in light adaptation affect not only the ON luminance/response saturation but also visual sensitivity. Therefore, it is important to test our third prediction with a stimulus condition, such as optical blur, that reduces vision at high spatial frequencies but does not change the overall stimulus luminance. Because the lens of the eye can only focus objects at the plane of fixation, optical blur dominates all images that we see. Moreover, even at focus, objects are slightly blurred by the optics of the eye, and the blur becomes even more pronounced in visual diseases such as myopia. It is well known that optical blur reduces visual acuity by removing the high spatial frequencies from the image (Bour & Apkarian, 1996; Held, Cooper, & Banks, 2012; Li et al., 2016). However, its effect on dark/light asymmetries has not been measured. Therefore, we tested our third prediction by asking subjects to perform our salience task after pharmacologically blocking lens accommodation and adding contact lenses with different dioptric power.
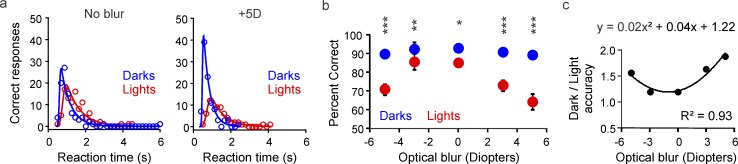
As with low light, optical blur reduced target visibility more for lights than darks (Figure 7a). The difference in detection accuracy between darks and lights increased by a factor of 3 when optical blur was increased by 5 diopters (Figure 7b; from 7.95% at focus to 24.68% at +5 diopters), and the relation could be accurately described with a quadratic function (Figure 7c). Positive defocus had a stronger effect than negative defocus on dark/light asymmetries. However, the difference was only significant at 3 diopters (Figure 7b; 17.97% vs. 6.68% with 3 diopters, p = 0.036; 24.68% vs. 18.79% with 5 diopters, p = 0.189, two-tailed Wilcoxon tests). Therefore, we conclude that removing high spatial frequencies with either optical blur or low luminance affects the visibility of light targets much more than dark ones. This result is consistent with our prediction that the effect of neuronal blur on visual salience should be more pronounced at low spatial frequencies.
Figure 7.
Optical blur reduces visual salience more for lights than for darks. (a) Relation between correct responses and reaction time measured in one subject with dark (blue) and light targets (red), at the focal plane (left) and with +5 diopters (+5D, right). Same task as in Figure 6. (b) Percentage correct of responses for darks (blue) and lights (red) averaged across 11 subjects as a function of optical blur. (c) The ratio between dark and light accuracy increases with optical blur and the relation can be fitted with a quadratic function (top). *p < 0.05, **p < 0.01, ***p < 0.001. Error bars show the standard error of the mean.
Dark/light asymmetries in dot visual acuity depend on background luminance
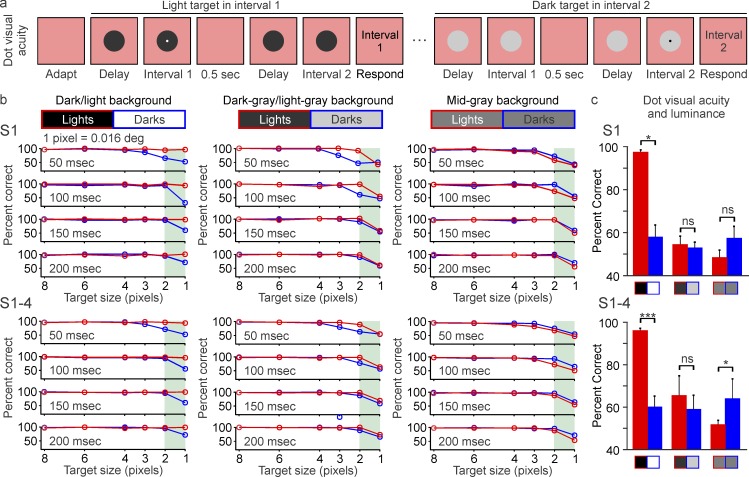
All visual tasks so far described showed a perceptual advantage of darks over lights. Our fourth prediction is that this trend should be reversed for tasks that require the detection of very small targets on dark backgrounds. Because the luminance/response function saturates more (and has higher contrast sensitivity) in ON than OFF visual pathways, neuronal blur should reduce visual acuity for closely spaced light bars but improve it for small light dots. To test this prediction, we asked subjects to detect a small dot in one of two consecutive intervals (Figure 8a). Consistent with our prediction, the smallest dot sizes were more visible when the dot was light on a dark background than when it was dark on a light background. This reduction was independent of stimulus duration (within 50–200 ms) and could be demonstrated in individual subjects (Figure 8b, S1) and in the average across subjects (Figure 8b, S1–S4, shaded areas; 1 pixel = 0.016°). Unlike for the dark/light asymmetries measured earlier, however, the higher acuity for light dots could only be demonstrated on dark backgrounds (Figure 8c). As the background approached a midgray value, the subject average demonstrated a slight but significant higher visual acuity for dark than light dots (Figure 8c, S1–S4). This finding is consistent with the reduction of neuronal blur under gray backgrounds and the higher retinotopic precision for darks than lights in primary visual cortex (Kremkow et al., 2016; K. S. Lee, Huang, & Fitzpatrick, 2016). Therefore, we conclude that small dots are better detected when they are light, but only if the background luminance is low enough to increase both the ON luminance/response saturation and the visual contrast sensitivity. In turn, as the background luminance is increased and the ON luminance/response saturation is reduced, dot visual acuity becomes slightly higher for dark targets.
Figure 8.
Dot visual acuity is higher for lights than darks, but only when lights are presented on dark backgrounds. (a) Subjects were asked to report the interval in which a small dot was presented. The small dot could be light or dark and could be presented on different backgrounds, with different sizes and durations. The intervals were separated by 500 ms and a variable delay that changed with the duration of the target. See videos of the stimuli. (b) Dot visual acuity measured in a single subject (top: S1) and the average of four subjects (bottom: S1–S4). The smallest dots (1 pixel, 0.016°, green shading) were seen better when they were light on dark backgrounds (red) than dark on light backgrounds (blue). This advantage for lights was reduced (middle) and reversed (right) as the background approached a midgray level. (c) Comparison of visual acuity measured with the smallest light and dark dots (1 pixel) on three different backgrounds for a single subject (top: S1) and the average of four subjects (bottom: S1–S4). *p < 0.05, ***p < 0.001, ns: not significant. Error bars show the standard error of the mean.
ON luminance/response saturation is present over a wide range of luminance conditions
All the predictions tested so far were based on measurements of ON and OFF luminance/response functions obtained under photopic conditions at 60 cd/m2 (Kremkow et al., 2014). However, it is possible that the ON and OFF functions become more similar at low luminance, which would invalidate the interpretation of our psychophysical experiments under low light. To test this possibility, we repeated our measurements in cat visual cortex at different mean luminance values (120, 35, 1.2, and 0.35 cd/m2) using the same three different background conditions: light, dark, and midgray. Consistent with our previous results, the ON luminance/response saturation was more pronounced on dark backgrounds (Figure 9a, dark red) than midgray backgrounds (Figure 9b, dark red), and more pronounced for ON (Figure 9a, dark red) than OFF responses (Figure 9c, dark blue). Moreover, the shape of the luminance/response function was more dependent on background luminance for ON (Figure 9a versus 9b, dark red) than OFF responses (Figure 9c versus 9d, dark blue). Importantly, these differences between ON and OFF luminance/response functions were similar across different luminance conditions and could be replicated when we reduced the stimulus luminance by two orders of magnitude. Under low light, ON cortical responses still saturated with small luminance increments (Figure 9a, light red) and the saturation was reduced but still present on the midgray background (Figure 9b, light red). Moreover, OFF cortical responses saturated less with luminance contrast than ON cortical responses also at low luminance (Figure 9c and 9d, light blue).
Figure 9.
The saturation of the ON luminance/response function is preserved under low light. (a) Example cortical responses to light targets presented on dark backgrounds measured under high luminance (sun icon) and low luminance (moon icon). Responses are shown as peristimulus time histograms (top) fitted with Naka–Rushton functions (bottom). (b) Same as (a), but for light targets presented on gray backgrounds. (c) Example cortical responses to dark targets presented on light backgrounds (different cortical recording site from a–b). (d) Same as (c), but for dark targets presented on gray backgrounds.
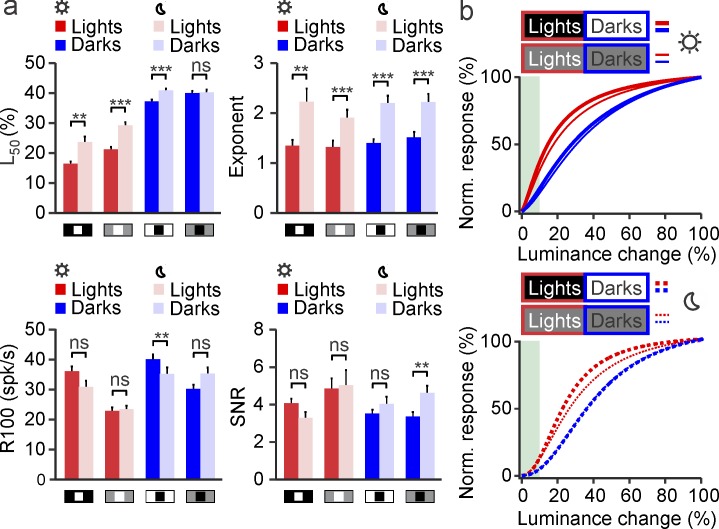
Reducing the stimulus luminance increased the L50 (measured as a percentage of maximum luminance) and the exponent n of the luminance/response function while keeping relatively constant the maximum response and SNR (Figure 10a). The combined increase in both L50 and n shifted the luminance/response function away from luminance contrasts lower than 10%, which may be too small to be resolved at low light (Figure 10b, gray area). Importantly, however, the differences in luminance/response saturation between ON and OFF pathways were well preserved across this wide luminance range (Figure 10b). Therefore, we conclude that, at least in cat visual cortex, the magnitude of the ON luminance/response saturation is strongly dependent on the luminance difference between background and target but is relatively independent of the overall luminance of the display and retinal illumination (within the luminance range tested in our experiments).
Figure 10.
Differences between ON and OFF luminance/response functions are preserved under low luminance in cat visual cortex. (a) Average parameter values for ON (red) and OFF (blue) luminance/response functions measured under high luminance (dark red and dark blue, sun icon) and low luminance (light red and light blue, moon icon). Low light frequently caused an increase in the luminance that generated 50% of maximum response (L50, top left) and the exponent n of the function (top right), but had a more limited effect on the maximum response (R100, bottom left) and signal-to-noise ratio (bottom right). (b) Under high luminance (top, sun icon), the saturation of the average luminance/response function was more pronounced for lights (red) than darks (blue) and for lights on dark (thick red line) than on gray backgrounds (thin red lines). Low light (bottom, moon icon) preserved these differences but caused a shift of the function away from the smallest luminance changes (gray shading), probably because they were too small to be detected under low light. **p < 0.01, ***p < 0.001, ns: not significant. Error bars show the standard error of the mean.
Model of dark/light asymmetries in visual perception
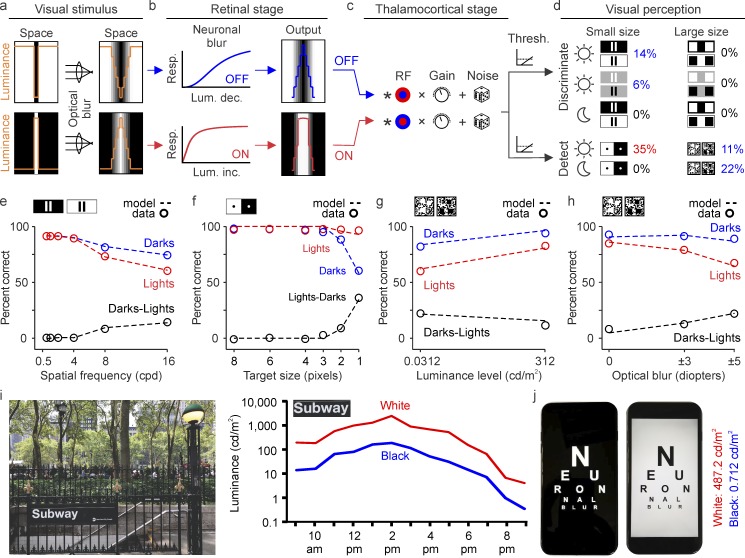
All dark/light asymmetries we have described could be reproduced with a computational model that uses different luminance/response saturation for ON and OFF pathways (see Methods for details). The model has three main stages. In the first stage (retinal stage), the stimulus image is minimally blurred to simulate the optics of the eye (Figure 11a) and then passed through a luminance/response function that saturates the visual responses from ON pathways more than those from OFF pathways (Figure 11b). In the second stage (thalamocortical stage), the ON and OFF responses are convolved with a difference-of-Gaussians function that simulates the center–surround receptive field of the retino-thalamocotical input (retinal and thalamic center–surrounds are combined in a single step). The result from this convolution is multiplied by a variable gain that is the same for both pathways and added to random noise (Figure 11c). In the third stage (perceptual stage), a correct response is generated when the cortical output crosses a threshold that is also the same for both pathways. In all simulations, the only difference between the processing of darks and lights is the luminance/response saturation of ON and OFF pathways. This simple model reproduces the dark/light asymmetries measured in our psychophysical experiments quite accurately. It discriminates the gaps between closely spaced bars 14% better when the bars are dark on a light background than when they are light on a dark background; it makes this difference fall to 6% on a gray background and disappear for wide bars separated by large gaps (Figure 11d and 11e). The model also detects dots 35% better when they are light than dark (Figure 11d and 11f) and detects targets embedded in noise 11% better when they are dark, a difference that increases to 22% under low light (Figure 11d and 11g). The model also replicates changes in dark/light asymmetries with spatial frequency (Figure 11e), dot size (Figure 11f), and optical blur (Figure 11h).
Figure 11.
All dark/light asymmetries can be explained by a model that uses greater luminance/response saturation in ON than OFF visual pathways. (A–D) The main stages of the model are a stimulus input with limited optical blur (a), a retinal stage that passes the stimulus through ON and OFF luminance/response functions with different saturation (b), a thalamocortical stage that convolves the stimulus with center–surround receptive fields of variable gain and noise level (c), and a perceptual stage that discriminates/detects the stimulus when the cortical output crosses a threshold (d). The model reproduces the higher grating resolution for closely spaced dark-bars (blue 14% and 6% on top left) and the lack of differences in resolution for large bars with wide gaps (0% on top right). It reproduces the greater visibility of small light dots (red 35%) and greater salience of dark targets on noisy backgrounds (blue 11% and 22%). (e–h) The model also reproduces changes in dark/light asymmetries measured as a function of spatial frequency (e), target size (f), luminance level (g), and optical blur (h). (i) Outdoor luminance of a black-and-white subway sign (left) measured at different times of the day (right). The luminance of black in this sign is lower than the values of midgray background used in our psychophysical experiments, while the luminance of white is frequently much higher. Therefore, neuronal blur should enlarge the letters in this subway sign throughout the entire day. (j) Indoor luminance measures from an electronic device that is commonly used to read. The luminance measures (right) indicate that neuronal blur should be very pronounced when reading on dark backgrounds in these devices. Luminance measured with Konica Minolta LS-150 (Konica Minolta Sensing Americas, Inc., Ramsey, NJ).
Taken together, our experimental results and model strongly suggest that neuronal blur causes pronounced spatial distortions in human vision that should be often experienced in our daily activities. The spatial distortions should occur every time that a light object is presented on a dark background that has half the luminance of the target (equivalent to our midgray background), and should increase as the background becomes darker. Because the differences in ON and OFF luminance/response saturation are already present at midgray backgrounds of 156 cd/m2, neuronal blur should be experienced outdoors when reading white letters on black backgrounds (Figure 11i) but should become most pronounced indoors, particularly when reading on black backgrounds with certain electronic devices (Figure 11j).
Discussion
Our results indicate that the luminance/response saturation within the ON visual pathway causes a perceptual enlargement of light stimuli that is responsible for multiple dark/light asymmetries in human vision. This spatial distortion (neuronal blur) reduces the spatial resolution for closely spaced light bars, reduces the visibility of light targets embedded in noise, and makes the reduction of light-target visibility more pronounced under low luminance and/or optical blur. Importantly, we demonstrate that low light does not change the general shape of the ON luminance/response function in cat visual cortex (although it slightly shifts it away from the smallest luminance contrasts). Therefore, we conclude that the ON luminance/response saturation affects the spatial resolution and visual salience of light targets and that its effect should be noticed under a wide range of illumination levels that are common in our daily activities and become most pronounced indoors.
Physiological and perceptual dark/light asymmetries
Several studies over the past years have demonstrated that ON and OFF visual pathways are not equally represented in primary visual cortex and that the OFF pathway dominates cortical responses (Jin et al., 2008, 2011; Komban et al., 2014; Kremkow et al., 2014; Liu & Yao, 2014; Rekauzke et al., 2016; Tan et al., 2015; Veit et al., 2014; Y. Wang et al., 2015; Xing et al., 2014; Yeh et al., 2009; Zemon et al., 1988; Zurawel et al., 2014). These ON/OFF asymmetries are already present in the retina of carnivores (Wassle et al., 1981) and primates (Dacey & Petersen, 1992), but they are greatly amplified in visual cortex. In cats, the amplification of OFF cortical dominance is already very pronounced at layer 4, which is the main recipient of thalamic inputs (Y. Wang et al., 2015). In macaques, however, the OFF dominance is much more pronounced in the superficial layers than in layer 4C (Xing, Yeh, & Shapley, 2010; Yeh et al., 2009). A possible interpretation for these differences across species is that cortical OFF dominance becomes most pronounced in the cortical layers where ON and OFF pathways combine, which is layer 4 in cats and the superficial layers in macaques.
Mirroring the ON/OFF asymmetries in cortical function, multiple studies have also demonstrated that darks are perceived better and faster than lights in different visual tasks (Blackwell, 1946; Bowen et al., 1989; Chubb & Nam, 2000; Komban et al., 2011, 2014; Krauskopf, 1980; Luo-Li et al., 2016; Tyler et al., 1992; Zhao et al., 2015). Based on computational modeling, we proposed that both the OFF dominance in visual cortex and dark dominance in human vision originate from the same neuronal mechanism: a luminance/response saturation within the ON visual pathway (Kremkow et al., 2014). The initial high gain of the ON luminance/response saturation should make the lightest gray values surrounding a white target also appear white, effectively enlarging the size of the white target. We previously called this effect neuronal blur because it resembles the size enlargement caused by optical blur, but unlike optical blur, it affects lights differently than darks (Kremkow et al., 2014). We predicted that neuronal blur should have important consequences for human vision, a prediction that is now confirmed here.
Consistent with our prediction, we show that changes in ON luminance/response saturation are strongly associated with changes in visual discrimination and visual salience. Also consistent with our prediction, we show that the effects of neuronal blur on visual salience are enhanced when high-spatial-frequency vision is reduced either by optical blur or low luminance (Burge & Geisler, 2011; Field & Brady, 1997). Optical blur directly filters the high spatial frequencies in the image, while low light reduces the foveal processing of high spatial frequencies (the fovea does not respond well at low light). Because neuronal blur attenuates ON responses more than OFF responses at low spatial frequencies (Kremkow et al., 2014), removing high spatial frequencies should affect the visibility of lights more than darks, which is exactly what we find. At the same time, the neuronal blur should not affect the discrimination of widely spaced bars, because its effects on two-bar discrimination are local and restricted to the bar borders, which is also consistent with our results.
We show that neuronal blur impairs the discrimination of closely spaced light bars because it makes the light bars wider and their separating gaps narrower. At the same time, we show that neuronal blur enlarges tiny light dots presented on dark backgrounds, an effect that is enhanced by the higher contrast sensitivity of the ON pathway (Chichilnisky & Kalmar, 2002; Kremkow et al., 2014; Zaghloul et al., 2003) and dark adaptation (Barlow, Fitzhugh, & Kuffler, 1957; Geisler, 1983; Jackson, Owsley, & McGwin, 1999; J. S. Wang, Estevez, Cornwall, & Kefalov, 2009). Because neuronal blur makes light targets appear larger than dark targets, it explains errors in estimating target size that are generally known as irradiation illusions (Helmholtz, 1867; Kremkow et al., 2014). At the same time, neuronal blur reduces the salience of light targets on noisy backgrounds by expanding the size of light-background regions and reducing the target/background contrast more for lights than darks (Komban et al., 2011, 2014). Unlike optical blur, neuronal blur does not affect perfect black–white edges, because it acts by saturating intermediate gray values. However, all edges projected on the retina are surrounded by gray values because they are slightly blurred by the optics of the eye. Therefore, since dark features in an image are less affected by neuronal blur than are light features, it may be advantageous to have a more precise cortical retinotopy for darks than lights (Kremkow et al., 2016; K. S. Lee et al., 2016). Moreover, the perception of detail should be more disrupted by blurring dark rather than light features in an image (Sato, Motoyoshi, & Sato, 2016). Finally, neuronal blur enlarges the size of light stimuli making them cover a larger region of the receptive-field surround from ON foveal neurons, a mechanism that could explain why the cortex is OFF dominated at cortical regions representing central vision (Jin et al., 2008; Yeh et al., 2009). In addition, because the neuronal blur suppresses ON more than OFF responses to low spatial frequencies, the cortical OFF dominance could be reinforced by the low spatial frequencies that dominate natural scenes (van der Schaaf & van Hateren, 1996) and the optically blurred images formed by the immature infant eye during brain development (Norcia, Tyler, & Hamer, 1990).
The effect of low light in dark/light asymmetries
Reducing the amount of light decreases visual acuity (Cavonius & Robbins, 1973; Fox, Lehmkuhle, & Westendorf, 1976; Shlaer, 1937) and affects ON and OFF cortical responses (Ramoa, Freeman, & Macy, 1985). However, the effect of low light on dark/light asymmetries has been unclear. Our results demonstrate that low light eliminates the dark/light differences in grating visual acuity but enhances the differences in visual salience. We show that the pronounced reduction in visual salience for light targets under low light is caused not by an increase in ON luminance/response saturation but by the loss of high spatial frequencies. Low spatial frequencies are less effective at driving ON than OFF visual responses (Kremkow et al., 2014). Therefore, the removal of high spatial frequencies makes cortical responses more OFF dominated. We have previously demonstrated that the differences in ON and OFF luminance/response saturation are remarkably similar in cats, macaques, and humans (Kremkow et al., 2014), and we now demonstrate that these differences are well preserved across a wide variety of luminance conditions in cat visual cortex. Moreover, we show that the effect of low light on visual salience can be accurately modeled by reducing foveal vision. Therefore, the loss of foveal vision at low luminance can explain both the loss of dark/light asymmetries in visual acuity and the enhancement of dark/light asymmetries in visual salience.
It is well known that adaptation to low light increases visual sensitivity and reduces visual thresholds, a fact already noticed by Weber and Fechner in the 1800s (Fechner, 1860). However, it is difficult to understand how an increase in visual sensitivity could explain all the dark/light asymmetries in visual resolution and visual salience that we describe. For example, changes in visual sensitivity could make small dots easier to detect on very dark backgrounds, but it is unclear how it would make dark targets more salient than light ones under different luminance conditions. Increased visual sensitivity to light scatter could explain why visual resolution is lowest for light targets on very dark backgrounds, but it is unclear how it would make the resolution still lower for light than dark targets on midgray backgrounds, since the overall luminance (background plus target) is slightly higher for light than dark targets. Finally, optical blur does not change the overall luminance of the stimulus but it causes a pronounced increase in dark/light asymmetries for visual salience, a result that cannot be explained by differences in dark adaptation but is easily explained by neuronal blur. Therefore, while changes in visual sensitivity can be used to correctly predict dark/light asymmetries in some visual tasks, such as dot visual acuity, they predict asymmetries opposite to the ones that we observe in grating resolution under gray backgrounds, and fail to predict pronounced asymmetries in visual salience under optical blur. Conversely, neuronal blur can accurately predict dark/light asymmetries for all the visual tasks that we tested.
It is important to emphasize that making the luminance/response saturation larger for ON than OFF visual responses is sufficient to explain a large number of dark/light asymmetries in human vision, as demonstrated by our model. However, ON and OFF differences in luminance/response saturation can be generated by differences in the exponent of the Naka–Rushton function (Rudd, 2013, 2017), the luminance that generates half-maximum response, or both parameters together (Kremkow et al., 2014). Our measurements in cat visual cortex favor a difference in both parameters, but the precise shapes of the ON and OFF luminance/response functions can change across species and luminance conditions. Our results also provide support for the notion that humans see the details of dark features more accurately than those of light features in visual scenes because light features are blurred by the ON luminance/response saturation. This ON luminance/response saturation may be useful to constrain the range of perceived brightness in natural scenes (Frazor & Geisler, 2006), but it also limits the spatial resolution of light targets. These limitations may be tolerable if darks are more common than lights in natural scenes (Cooper & Norcia, 2015; Ratliff, Borghuis, Kao, Sterling, & Balasubramanian, 2010) and if differences among the brightest luminance values provide limited information about scene content. There is certainly little advantage in knowing that two specular highlights of a brightly illuminated sea have different luminance; however, small differences in eye shade are very important to interpreting facial expression.
Neuronal blur may also have long-term implications in vision if it is enhanced in a sustained manner by certain visual behaviors or visual diseases. We notice that neuronal blur is increased by visual conditions that are associated with myopia progression, such as optical blur and low light (Rose et al., 2008; Wallman & Winawer, 2004). Therefore, if myopia progression is caused by a deficit in retinal dopamine (Iuvone, Tigges, Stone, Lambert, & Laties, 1991; Pardue et al., 2008), which is exclusively released by ON retinal cells (Zhang, Zhou, & McMahon, 2007), visual environments that increase neuronal blur (Cohen, Belkin, Yehezkel, Solomon, & Polat, 2011) may lead to weaker ON responses, lower dopamine release, and greater myopia progression. Such a potential link between neuronal blur and visual disease deserves to be rigorously evaluated in future studies.
Conclusion
We demonstrate that a large variety of dark/light asymmetries in human vision can be explained by a spatial distortion of light stimuli due to the luminance/response saturation of the ON visual pathway. We call this spatial distortion neuronal blur because it has a neuronal origin and blurs light stimuli. We show that the differences in luminance/response saturation between ON and OFF pathways are well preserved under a wide variety of luminance conditions that are common indoors. Moreover, we show that the ON luminance/response saturation affects the visual salience of light features mostly in images with low luminance and optical blur, two stimulus conditions that have been shown to be risk factors in myopia. Therefore, our results suggest a possible neuronal mechanism linking myopia progression with the function of the ON visual pathway.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants EY05253 (JMA), EY027361 (JMA), EY07556 (QZ), and EY027157 (RM). We thank Christine Sukis for her help with the experiments using optical blur.
Commercial relationships: none.
Corresponding author: Jose-Manuel Alonso.
Email: jalonso@sunyopt.edu.
Address: Department of Biological and Visual Sciences, State University of New York College of Optometry, New York, NY, USA.
Contributor Information
Carmen Pons, Email: carmen.pons.t@gmail.com.
Reece Mazade, Email: rmazade@sunyopt.edu.
Jianzhong Jin, Email: jjin@sunyopt.edu.
Mitchell W. Dul, Email: mdul@sunyopt.edu.
Qasim Zaidi, Email: qz@sunyopt.edu.
Jose-Manuel Alonso, Email: jalonso@sunyopt.edu.
References
- Ahmad, K. M., Klug, K., Herr, S., Sterling, P., Schein, S.. (2003). Cell density ratios in a foveal patch in macaque retina. Visual Neuroscience, 20 2, 189– 209. [DOI] [PubMed] [Google Scholar]
- Barlow, H. B., Fitzhugh, R., Kuffler, S. W.. (1957). Change of organization in the receptive fields of the cat's retina during dark adaptation. The Journal of Physiology, 137 3, 338– 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, D., Cavonius, C.. (1980). Improving the legibility of visual display units through contrast reversal. Grandjean E., Vigliani E. (Eds.), Ergonomic aspects of visual display terminals (pp 137– 142). London: Taylor & Francis. [Google Scholar]
- Blackwell, H. R. (1946). Contrast thresholds of the human eye. Journal of the Optical Society of America, 36 11, 624– 643. [DOI] [PubMed] [Google Scholar]
- Bour, L. J., Apkarian, P.. (1996). Selective broad-band spatial frequency loss in contrast sensitivity functions: Comparison with a model based on optical transfer functions. Investigative Ophthalmology & Visual Science, 37 12, 2475– 2484. [PubMed] [Article] [PubMed] [Google Scholar]
- Bowen, R. W., Pokorny, J., Smith, V. C.. (1989). Sawtooth contrast sensitivity: Decrements have the edge. Vision Research, 29 11, 1501– 1509. [DOI] [PubMed] [Google Scholar]
- Boynton, R. M., Whitten, D. N.. (1970, Dec 25). Visual adaptation in monkey cones: Recordings of late receptor potentials. Science, 170 3965, 1423– 1426. [DOI] [PubMed] [Google Scholar]
- Brainard, D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10 4, 433– 436. [PubMed] [Google Scholar]
- Buchner, A., Baumgartner, N.. (2007). Text–background polarity affects performance irrespective of ambient illumination and colour contrast. Ergonomics, 50 7, 1036– 1063. [DOI] [PubMed] [Google Scholar]
- Burge, J., Geisler, W. S.. (2011). Optimal defocus estimation in individual natural images. Proceedings of the National Academy of Sciences, USA, 108 40, 16849– 16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, F. W., Gubisch, R. W.. (1966). Optical quality of the human eye. The Journal of Physiology, 186 3, 558– 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavonius, C. R., Robbins, D. O.. (1973). Relationships between luminance and visual acuity in the rhesus monkey. The Journal of Physiology, 232 2, 239– 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichilnisky, E. J., Kalmar, R. S.. (2002). Functional asymmetries in ON and OFF ganglion cells of primate retina. The Journal of Neuroscience, 22 7, 2737– 2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb, C., Nam, J. H.. (2000). Variance of high contrast textures is sensed using negative half-wave rectification. Vision Research, 40 13, 1677– 1694. [DOI] [PubMed] [Google Scholar]
- Cohen, Y., Belkin, M., Yehezkel, O., Solomon, A. S., Polat, U.. (2011). Dependency between light intensity and refractive development under light-dark cycles. Experimental Eye Research, 92 1, 40– 46. [DOI] [PubMed] [Google Scholar]
- Commission Internationale de l'Éclairage. (1924). Commission Internationale de l'Eclairage proceedings. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Cooper, E. A., Norcia, A. M.. (2015). Predicting cortical dark/bright asymmetries from natural image statistics and early visual transforms. PLoS Computational Biology, 11 5, e1004268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey, D. M., Petersen, M. R.. (1992). Dendritic field size and morphology of midget and parasol ganglion cells of the human retina. Proceedings of the National Academy of Sciences, USA, 89 20, 9666– 9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, F. A., Lankheet, M. J., Rieke, F.. (2007). Light adaptation in cone vision involves switching between receptor and post-receptor sites. Nature, 449 7162, 603– 606. [DOI] [PubMed] [Google Scholar]
- Fechner, G. T. (1860). Elemente der Psychophysik, Volume 1 [Translation: Elements of psychophysics, Volume 1]. Leipzig, Germany: Breitkopf und Härtel.
- Field, D. J., Brady, N.. (1997). Visual sensitivity, blur and the sources of variability in the amplitude spectra of natural scenes. Vision Research, 37 23, 3367– 3383. [DOI] [PubMed] [Google Scholar]
- Fox, R., Lehmkuhle, S. W., Westendorf, D. H.. (1976, Month DD). Falcon visual acuity. Science, 192 4236, 263– 265. [DOI] [PubMed] [Google Scholar]
- Frazor, R. A., Geisler, W. S.. (2006). Local luminance and contrast in natural images. Vision Research, 46 10, 1585– 1598. [DOI] [PubMed] [Google Scholar]
- Galilei, G. (1632). Dialogo sopra i due massimi sistemi del mondo, tolemaico e copernicano. Florence, Italy: Battista Landini. [Google Scholar]
- Geisler, W. S. (1983). Mechanisms of visual sensitivity: Backgrounds and early dark adaptation. Vision Research, 23 12, 1423– 1432. [DOI] [PubMed] [Google Scholar]
- Hartline, H. K. (1938). The response of single optic nerve fibers of the vertebrate eye to illumination of the retina. American Journal of Physiology, 121, 400– 415. [Google Scholar]
- Held, R. T., Cooper, E. A., Banks, M. S.. (2012). Blur and disparity are complementary cues to depth. Current Biology, 22 5, 426– 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmholtz, H. (1867). Handbuch der physiologischen Optik. Karsten G. (Ed.), Allgemeine Enzyklopädie der Physik (Vol. 2, 186– 193). Leipzig, Germany: Voss. [Google Scholar]
- Hubel, D. H., Wiesel, T. N.. (1962). Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. The Journal of Physiology, 160, 106– 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone, P. M., Tigges, M., Stone, R. A., Lambert, S., Laties, A. M.. (1991). Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Investigative Ophthalmology & Visual Science, 32 5, 1674– 1677. [PubMed] [Article] [PubMed] [Google Scholar]
- Jackson, G. R., Owsley, C.,& McGwin, G., Jr.. (1999). Aging and dark adaptation. Vision Research, 39 23, 3975– 3982. [DOI] [PubMed] [Google Scholar]
- Jin, J., Wang, Y., Swadlow, H. A., Alonso, J. M.. (2011). Population receptive fields of ON and OFF thalamic inputs to an orientation column in visual cortex. Nature Neuroscience, 14 2, 232– 238. [DOI] [PubMed] [Google Scholar]
- Jin, J., Weng, C., Yeh, C. I., Gordon, J. A., Ruthazer, E. S., Stryker, M. P.,… Alonso, J. M.. (2008). On and off domains of geniculate afferents in cat primary cisual cortex. Nature Neuroscience, 11 1, 88– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, C. A. (1976). Effects of luminance and stimulus distance on accommodation and visual resolution. Journal of the Optical Society of America, 66 2, 138– 142. [DOI] [PubMed] [Google Scholar]
- Johnson, C. A., Casson, E. J.. (1995). Effects of luminance, contrast, and blur on visual acuity. Optometry and Vision Science, 72 12, 864– 869. [DOI] [PubMed] [Google Scholar]
- Komban, S. J., Alonso, J. M., Zaidi, Q.. (2011). Darks are processed faster than lights. The Journal of Neuroscience, 31 23, 8654– 8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komban, S. J., Kremkow, J., Jin, J., Wang, Y., Lashgari, R., Li, X.,et al. (2014). Neuronal and perceptual differences in the temporal processing of darks and lights. Neuron, 82 1, 224– 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauskopf, J. (1980). Discrimination and detection of changes in luminance. Vision Research, 20 8, 671– 677. [DOI] [PubMed] [Google Scholar]
- Kremkow, J., Jin, J., Komban, S. J., Wang, Y., Lashgari, R., Li, X.,et al. (2014). Neuronal nonlinearity explains greater visual spatial resolution for darks than lights. Proceedings of the National Academy of Sciences, USA, 111 8, 3170– 3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremkow, J., Jin, J., Wang, Y., Alonso, J. M.. (2016). Principles underlying sensory map topography in primary visual cortex. Nature, 533 7601, 52– 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, B. B., Pokorny, J., Smith, V. C., Martin, P. R., Valberg, A.. (1990). Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. Journal of the Optical Society of America A, 7 12, 2223– 2236. [DOI] [PubMed] [Google Scholar]
- Lee, K. S., Huang, X., Fitzpatrick, D.. (2016). Topology of ON and OFF inputs in visual cortex enables an invariant columnar architecture. Nature, 533 7601, 90– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. W., So, K., Wu, T. H., Craven, A. P., Tran, T. T., Gustafson, K. M.,et al. (2016). Monocular blur alters the tuning characteristics of stereopsis for spatial frequency and size. Royal Society Open Science, 3 9, 160273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K., Yao, H.. (2014). Contrast-dependent OFF-dominance in cat primary visual cortex facilitates discrimination of stimuli with natural contrast statistics. European Journal of Neuroscience, 39 12, 2060– 2070. [DOI] [PubMed] [Google Scholar]
- Luo-Li, G., Alais, D., Freeman, A. W.. (2016). Orientation discrimination requires coactivation of on- and off-dominated visual channels. Journal of Vision, 16 15: 18, 1– 13, doi:10.1167/16.15.18. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- MacCurdy, E. (1938). The notebooks of Leonardo da Vinci. London: Jonathan Cape. [Google Scholar]
- Naka, K. I., Rushton, W. A.. (1966). S-potentials from colour units in the retina of fish (Cyprinidae). The Journal of Physiology, 185 3, 536– 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcia, A. M., Tyler, C. W., Hamer, R. D.. (1990). Development of contrast sensitivity in the human infant. Vision Research, 30 10, 1475– 1486. [DOI] [PubMed] [Google Scholar]
- Pardue, M. T., Faulkner, A. E., Fernandes, A., Yin, H., Schaeffel, F., Williams, R. W.,et al. (2008). High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Investigative Ophthalmology & Visual Science, 49 2, 706– 712. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack, P. O., Contreras, D.. (2012). Long-range parallel processing and local recurrent activity in the visual cortex of the mouse. The Journal of Neuroscience, 32 32, 11120– 11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poot, L., Snippe, H. P., van Hateren, J. H.. (1997). Dynamics of adaptation at high luminances: Adaptation is faster after luminance decrements than after luminance increments. Journal of the Optical Society of America A, 14 9, 2499– 2508. [DOI] [PubMed] [Google Scholar]
- Purpura, K., Tranchina, D., Kaplan, E., Shapley, R. M.. (1990). Light adaptation in the primate retina: Analysis of changes in gain and dynamics of monkey retinal ganglion cells. Visual Neuroscience, 4 1, 75– 93. [DOI] [PubMed] [Google Scholar]
- Rabin, J. (1994). Luminance effects on visual acuity and small letter contrast sensitivity. Optometry and Vision Science, 71 11, 685– 688. [DOI] [PubMed] [Google Scholar]
- Ramoa, A. S., Freeman, R. D., Macy, A.. (1985). Comparison of response properties of cells in the cat's visual cortex at high and low luminance levels. Journal of Neurophysiology, 54 1, 61– 72. [DOI] [PubMed] [Google Scholar]
- Ratliff, C. P., Borghuis, B. G., Kao, Y. H., Sterling, P., Balasubramanian, V.. (2010). Retina is structured to process an excess of darkness in natural scenes. Proceedings of the National Academy of Sciences, USA, 107 40, 17368– 17373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekauzke, S., Nortmann, N., Staadt, R., Hock, H. S., Schoner, G., Jancke, D.. (2016). Temporal asymmetry in dark-bright processing initiates propagating activity across primary visual cortex. The Journal of Neuroscience, 36 6, 1902– 1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, K. A., Morgan, I. G., Ip, J., Kifley, A., Huynh, S., Smith, W.,et al. (2008). Outdoor activity reduces the prevalence of myopia in children. Ophthalmology, 115 8, 1279– 1285. [DOI] [PubMed] [Google Scholar]
- Rudd, M. E. (2013). Edge integration in achromatic color perception and the lightness-darkness asymmetry. Journal of Vision, 13 14: 18, 1– 30, doi:10.1167/13.14.18. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Rudd, M. E. (2017). Lightness computation by the human visual system. Journal of Electronic Imaging, 26 3, 031209. [Google Scholar]
- Sato, H., Motoyoshi, I., Sato, T.. (2016). On-Off asymmetry in the perception of blur. Vision Research, 120, 5– 10. [DOI] [PubMed] [Google Scholar]
- Schnapf, J. L., Nunn, B. J., Meister, M., Baylor, D. A.. (1990). Visual transduction in cones of the monkey Macaca fascicularis. The Journal of Physiology, 427, 681– 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy, J. E., Bailey, I. L., Raasch, T. W.. (1984). Visual acuity and chart luminance. American Journal of Optometry and Physiological Optics, 61 9, 595– 600. [DOI] [PubMed] [Google Scholar]
- Shlaer, S. (1937). The relation between visual acuity and illumination. The Journal of General Physiology, 21 2, 165– 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, Z., Sun, W., Chen, T. W., Kim, D., Ji, N.. (2015). Neuronal representation of ultraviolet visual stimuli in mouse primary visual cortex. Scientific Reports, 5, 12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranchina, D., Sneyd, J., Cadenas, I. D.. (1991). Light adaptation in turtle cones: Testing and analysis of a model for phototransduction. Biophysical Journal, 60 1, 217– 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, C. W., Chan, H., Liu, L.. (1992). Different spatial tunings for ON and OFF pathway stimulation. Ophthalmic and Physiological Optics, 12 2, 233– 240. [DOI] [PubMed] [Google Scholar]
- van der Schaaf, A., van Hateren, J. H.. (1996). Modelling the power spectra of natural images: Statistics and information. Vision Research, 36 17, 2759– 2770. [DOI] [PubMed] [Google Scholar]
- Veit, J., Bhattacharyya, A., Kretz, R., Rainer, G.. (2014). On the relation between receptive field structure and stimulus selectivity in the tree shrew primary visual cortex. Cerebral Cortex, 24 10, 2761– 2771. [DOI] [PubMed] [Google Scholar]
- Wallman, J., Winawer, J.. (2004). Homeostasis of eye growth and the question of myopia. Neuron, 43 4, 447– 468. [DOI] [PubMed] [Google Scholar]
- Wang, J. S., Estevez, M. E., Cornwall, M. C., Kefalov, V. J.. (2009). Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nature Neuroscience, 12 3, 295– 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Jin, J., Kremkow, J., Lashgari, R., Komban, S. J., Alonso, J. M.. (2015). Columnar organization of spatial phase in visual cortex. Nature Neuroscience, 18 1, 97– 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle, H., Boycott, B. B., Illing, R. B.. (1981). Morphology and mosaic of on- and off-beta cells in the cat retina and some functional considerations. Proceedings of the Royal Society of London Series B: Biological Sciences, 212 1187, 177– 195. [DOI] [PubMed] [Google Scholar]
- Westheimer, G. (2003). Visual acuity with reversed-contrast charts: I. Theoretical and psychophysical investigations. Optometry and Vision Science, 80 11, 745– 748. [DOI] [PubMed] [Google Scholar]
- Westheimer, G., Chu, P., Huang, W., Tran, T., Dister, R.. (2003). Visual acuity with reversed-contrast charts: II. Clinical investigation. Optometry and Vision Science, 80 11, 749– 752. [DOI] [PubMed] [Google Scholar]
- Wilcox, W. W. (1932). The basis of the dependence of visual acuity on illumination. Proceedings of the National Academy of Sciences, USA, 18 1, 47– 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wool, L. E., Komban, S. J., Kremkow, J., Jansen, M., Li, X., Alonso, J. M.,et al. (2015). Salience of unique hues and implications for color theory. Journal of Vision, 15 2: 10, 1– 11, doi:10.1167/15.2.10. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, D., Yeh, C. I., Gordon, J., Shapley, R. M.. (2014). Cortical brightness adaptation when darkness and brightness produce different dynamical states in the visual cortex. Proceedings of the National Academy of Sciences, USA, 111 3, 1210– 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, D., Yeh, C. I., Shapley, R. M.. (2010). Generation of black-dominant responses in V1 cortex. The Journal of Neuroscience, 30 40, 13504– 13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, C. I., Xing, D., Shapley, R. M.. (2009). “Black” responses dominate macaque primary visual cortex v1. The Journal of Neuroscience, 29 38, 11753– 11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul, K. A., Boahen, K., Demb, J. B.. (2003). Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. The Journal of Neuroscience, 23 7, 2645– 2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemon, V., Gordon, J., Welch, J.. (1988). Asymmetries in ON and OFF visual pathways of humans revealed using contrast-evoked cortical potentials. Visual Neuroscience, 1 1, 145– 150. [DOI] [PubMed] [Google Scholar]
- Zhang, D. Q., Zhou, T. R., McMahon, D. G.. (2007). Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. The Journal of Neuroscience, 27 3, 692– 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L., Sendek, C., Davoodnia, V., Lashgari, R., Dul, M. W., Zaidi, Q.,et al. (2015). Effect of age and glaucoma on the detection of darks and lights. Investigative Ophthalmology & Visual Science, 56 11, 7000– 7006. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawel, G., Ayzenshtat, I., Zweig, S., Shapley, R., Slovin, H.. (2014). A contrast and surface code explains complex responses to black and white stimuli in V1. The Journal of Neuroscience, 34 43, 14388– 14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.