Abstract
This study was aimed to assess the expression and clinical performance of microRNA-329 (miR-329) in breast cancer. We recruited 134 breast cancer patients and 70 healthy volunteers for this study. MiR-329 expression was estimated by quantitative real-time polymerase chain reaction. A receiver operating characteristic assay was performed to evaluate the diagnostic value of serum miR-329. In addition, the prognostic significance of miR-329 was evaluated through Kaplan–Meier survival and Cox regression analyses. According to quantitative real-time polymerase chain reaction, miR-329 expression was downregulated in cancerous samples compared with healthy and normal controls (P<0.01), and its expression in serum specimens positively correlated with its expression in tissue samples (R=0.493, P<0.001). The decreased expression of miR-329 correlated with lymph node metastasis (P=0.015) and TNM stage (P=0.003). A receiver operating characteristic curve with an area under the curve of 0.932 was constructed, indicating the high diagnostic accuracy of miR-329. From the survival and multivariate Cox assays, we found that downregulated miR-329 expression was associated with poor overall survival (log-rank P<0.001) and served as an independent prognostic factor (hazard ratio =2.987, 95% CI =1.681–5.308, and P<0.001). In silico analysis using The Cancer Genome Atlas confirmed that miR-329 expression was lower in breast cancer cases compared with normal controls (P<0.001) and could be an efficient biomarker for cancer patients. Down-regulated miR-329 expression was an effective diagnostic and prognostic biomarker, which could be used for targeted therapy in patients with breast cancer.
Keywords: miR-329, diagnosis, prognosis, breast cancer, target therapy
Introduction
Breast cancer is a worldwide health burden. It represents one of the most common malignancies among females and leads to a reduced quality of life in cancer patients.1 Statistics published by the World Health Organization indicate that there are ~70 newly diagnosed breast cancer cases among every 100,000 individuals annually in North America, Europe, and other countries.2 Some risk factors have been identified, such as lack of breastfeeding and dietary patterns, which are closely correlated with tumorigenesis of breast cancer.3–5 Therefore, many studies focusing on the treatment of breast cancer have been carried out. Although the mortality rate of this malignancy has been decreased thanks to the advances in therapeutic strategies, the prognoses and outcomes of breast cancer patients remain less than ideal.6,7 Previous data revealed that some patients with breast cancer present metastasis in the initial diagnosis, leading to poor outcomes and high mortality.8 Thus, improvement in diagnosis and prognosis is urgently needed for better management of breast cancer.
To improve cancer treatment, a great deal of cancer-related molecules and genes have been investigated to serve as useful biomarkers in various human malignancies.9,10 MicroRNAs (miRNAs), a series of small endogenous RNAs, are considered to be the crucial members among all the available molecular markers.11,12 They can regulate gene expression by binding to the 3′-untranslated regions of target mRNAs.13 MiRNAs have also been reported to be involved in a wide array of biological processes.14 In human cancers, aberrant expression levels of miRNAs have been detected, indicating that they may have special roles during tumor progression.15 Furthermore, the application of miRNAs has received increasing attention for their crucial clinical significance in cancers.16 Therefore, the identification of more functional miRNAs is critical for cancer treatment. MicroRNA-329 (miR-329) is clinically significant in some human cancers, such as glioblastoma and hepatocellular carcinoma.17,18 In addition, significantly downregulated miR-329 expression has been detected in breast cancer samples.19 Thus, we questioned whether there was a relationship between miR-329 expression and breast cancer diagnosis and prognosis.
In this study, we sought to assess the expression patterns of miR-329 in breast cancer samples and to investigate its correlation with tumor progression. In addition, the diagnostic and prognostic values of miR-329 were explored in patients with breast cancer.
Materials and methods
Patients and specimens collection
All the experimental procedures were approved by the Ethics Committee of Wenzhou Medical University. Informed consent was signed by each participant. A total of 134 patients who were histologically diagnosed with breast cancer at the Second Affiliated Hospital of Wenzhou Medical University between January 2006 and December 2010 were included in this study. In addition, 70 healthy volunteers were recruited in our research to act as healthy controls. All these healthy cases were selected from individuals who had routine physical examinations in the hospital without any history of malignancy. None of the breast cancer patients had ever received any therapy prior to the sampling. Venous blood samples were collected from all the participants and then centrifuged to obtain serum specimens. A total of 134 paired breast cancer tissues and adjacent normal breast tissues were isolated from the breast cancer patients during the surgical resection, immediately stored in liquid nitrogen, and kept at −80°C in an ultrafreezer. Concurrently, clinicopathological characteristics of these patients were summarized for subsequent analysis; they are shown in Table 1. A 5-year follow-up survey was conducted following the surgical resection for each of the patients, and their survival times were recorded.
Table 1.
Association of miR-329 with the clinicopathological features of breast cancer patients
| Features | Total no (n=134) |
miR-329 expression
|
χ2 | P-values | |
|---|---|---|---|---|---|
| Low (n=76) | High (n=58) | ||||
| Age (years) | 0.017 | 0.897 | |||
| ≤50 | 50 | 28 | 22 | ||
| >50 | 84 | 48 | 36 | ||
| Tumor size (cm) | 0.005 | 0.945 | |||
| ≥3.0 | 55 | 31 | 24 | ||
| >3.0 | 79 | 45 | 34 | ||
| LN metastasis | 5.888 | 0.015 | |||
| Negative | 58 | 26 | 32 | ||
| Positive | 76 | 50 | 26 | ||
| PR expression | 0.424 | 0.515 | |||
| Negative | 65 | 35 | 30 | ||
| Positive | 69 | 41 | 28 | ||
| ER expression | 0.009 | 0.925 | |||
| Negative | 63 | 36 | 27 | ||
| Positive | 71 | 40 | 31 | ||
| HER-2 expression | 0.056 | 0.813 | |||
| Positive | 57 | 33 | 24 | ||
| Negative | 77 | 43 | 34 | ||
| TNM | 9.060 | 0.003 | |||
| I–II | 61 | 26 | 35 | ||
| III–IV | 73 | 50 | 23 | ||
Abbreviations: ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; LN, lymph node; miR-329, microRNA-329; PR, progesterone receptor.
Cell culture and exosomes collection
The human breast cancer cell lines MCF-7 and BT474 and a normal breast epithelial cell line MCF-10A were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum. Cell cultures were incubated in a humidified incubator with 5% CO2 at 37°C. The exosomes were isolated from the cell culture medium using ultracentrifugation and then stored at −80°C for further analyses.
RNA extraction and qRT-PCR
The extraction of total RNA was performed by using TRIzol reagent® (Invitrogen, Carlsbad, CA, USA) following manufacturer’s instructions. The expression of miR-329 was measured using quantitative real-time polymerase chain reaction (qRT-PCR). Single-stranded cDNA, which was synthesized from RNA using the AMV reverse transcription system (Promega, Madison, WI, USA), was used as the template for qPCR. The qRT-PCR was performed with SYBR® Green PCR master mix (Applied Biosystems, Foster City, CA, USA), and all the reactions were run on the 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with U6 as the internal control gene. The sequences of primers used in this assay were as follows: miR-329 forward, 5′-GGGAACACACCTGGTTAAC-3′; reverse, 5′-CAGTGCGTGTCGTGGAGT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACATATACT-3′; and reverse, 5′-ACGCTTCACGAATTTGCGTGTC-3′. The final relative miR-329 expression was computed with the 2−ΔΔCt method and normalized to U6.
In silico analysis of miR-329 using TCGA database
The expression levels of miR-329 and clinical information of 755 breast cancer cases were downloaded from The Cancer Genome Atlas (TCGA) database. In addition, the corresponding information of 74 normal controls was collected for the analyses.
Statistical analysis
The differences between the two groups were compared using Student’s t-test. The relationship between miR-329 expression and clinicopathological features was analyzed with a chi-square test. The mean expression value of miR-329 was used to classify high and low miR-329 expression groups. Pearson correlation analysis was used to estimate the degree of dependency between variables. Receiver operating characteristic (ROC) analysis was conducted to evaluate the diagnostic value of serum miR-329 expression. Survival analysis was performed for breast cancer patients using the Kaplan–Meier method and log-rank test. Furthermore, Cox regression analysis was used to explore the prognostic significance of miR-329. All the above statistical analyses were carried out by SPSS Version 18.0 software. The difference was considered statistically significant if the P-value was <0.05.
Results
Baseline characteristics of breast cancer patients and expression patterns of miR-329
A total of 134 histologically diagnosed breast cancer patients were included in this study with the average age of 52.52±16.28 years. Among these patients, 61 cases were diagnosed at a TNM I–II stage, while 73 cases were diagnosed at a TNM III–IV stage. Additionally, clinical characteristics of breast cancer patients are listed in Table 1.
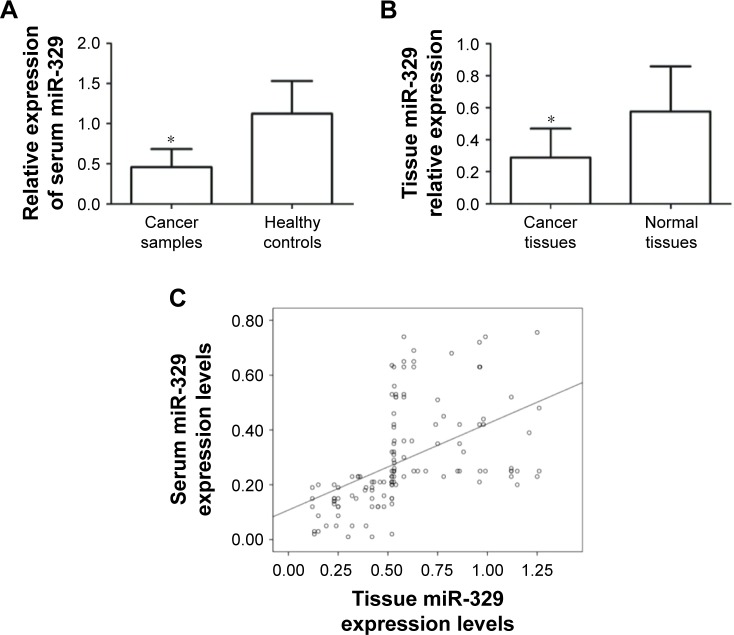
The expression levels of miR-329 in the clinical samples were assessed by qRT-PCR. Serum miR-329 expression was downregulated in breast cancer patients compared with the healthy controls (P=0.0056, Figure 1A). Similarly, the expression of miR-329 was decreased in breast cancer tissues compared to normal controls (P=0.0068, Figure 1B). According to the Pearson correlation, we found that the serum miR-329 expression was positively correlated with tissue miR-329 expression levels (R=0.493, P<0.001, Figure 1C).
Figure 1.
Expression of miR-329 in serum and tissue specimens.
Notes: (A) miR-329 expression was downregulated in the serum samples of breast cancer patients compared with those of healthy controls (*P=0.0056). (B) The expression of miR-329 was decreased in breast cancer tissues compared with adjacent normal tissues (*P=0.0068). (C) The serum expression of miR-329 was positively correlated with the miR-329 tissue expression (R=0.493, P<0.001).
Abbreviation: miR-329, microRNA-329.
Expression levels of miR-329 in exosomes
In addition to serum and tissue expression patterns, miR-329 expression in exosomes collected from breast cancer cell culture medium was assessed in this study. The results shown in Figure 2 indicate that no significant miR-329 expression was detected between exosomes in breast cancer cells compared with normal breast cells (P>0.05).
Figure 2.
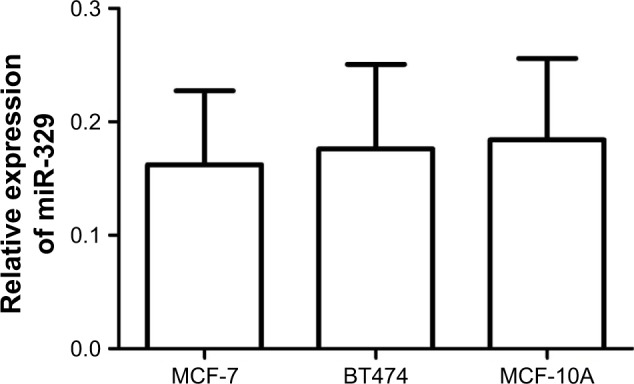
Expression of miR-329 in breast cancer cell-derived exosomes.
Note: No difference in miR-329 expression was found between breast cancer cells and normal breast cells (P>0.05).
Abbreviation: miR-329, microRNA-329.
Relationship between miR-329 expression and clinicopathological data of breast cancer patients
To assess whether miR-329 was involved in the progression of breast cancer, its association with clinical parameters was examined. The results of the chi-square test showed that breast cancer patients with positive lymph node metastasis had a lower miR-329 expression (P=0.015) and that the decreased miR-329 expression could be found in the patients with advanced TNM stages (P=0.003), indicating that miR-329 expression was associated with lymph node metastasis and TNM stage. Conversely, no relationship was detected between miR-329 expression and age, tumor size, and expression of progesterone receptor (PR), estrogen receptor (ER), or human epidermal growth factor receptor 2 (HER-2; P>0.05 for all; Table 1).
Clinical significance of miR-329 in breast cancer diagnosis and prognosis
Considering the deregulation of miR-329 in breast cancer samples, we examined its clinical performance in cancer patients. In the current study, an ROC curve with an area under the curve (AUC) of 0.932 was constructed (Figure 3), suggesting the high diagnostic accuracy of miR-329. At the optimal cutoff value of 0.745, the sensitivity and specificity were 87.1% and 89.6%, respectively. In addition, survival analysis based on expression of miR-329 was conducted for breast cancer patients. From the survival curves, we found that patients with lower miR-329 expression had shorter survival times than those with high levels (log-rank P<0.001, Figure 4). Furthermore, Cox regression analysis was used to verify the prognostic value of miR-329. The analysis revealed that the downregulated miR-329 expression was associated with a poor prognosis and acted as an independent prognostic factor in patients with breast cancer (hazard ratio =2.987, 95% CI =1.681–5.308, and P<0.001, Table 2).
Figure 3.
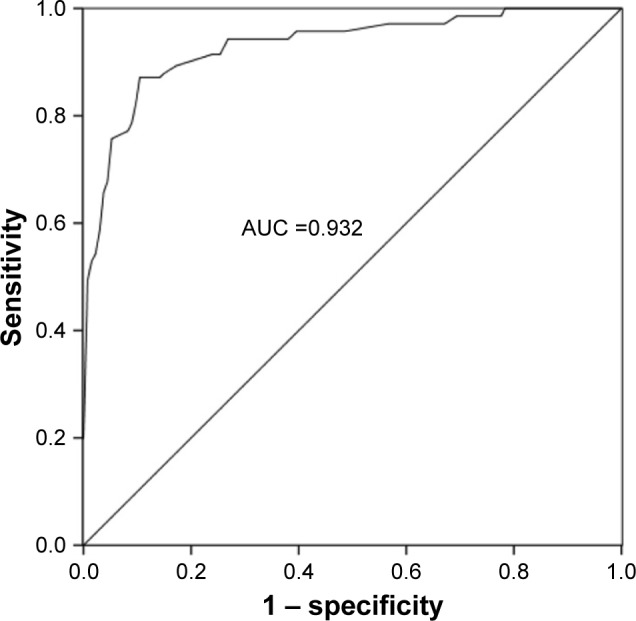
ROC curve based on the expression of miR-329 for breast cancer patients.
Note: The AUC value was 0.932, indicating the high diagnostic value of miR-329 for patients with breast cancer.
Abbreviations: AUC, area under the curve; miR-329, microRNA-329; ROC, receiver operating characteristic.
Figure 4.
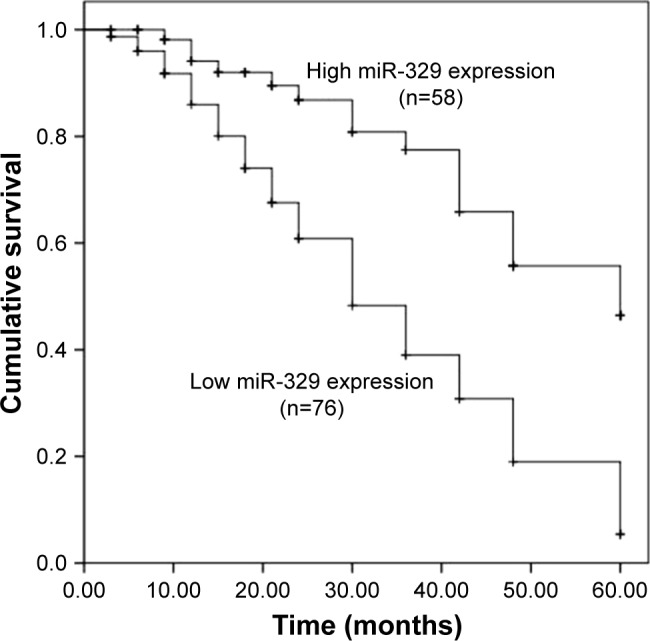
Kaplan–Meier survival analysis for breast cancer patients based on the difference in expression of miR-329.
Note: Decreased miR-329 expression correlated with poor overall survival of cancer patients (log-rank P<0.001).
Abbreviation: miR-329, microRNA-329.
Table 2.
Cox regression analysis for miR-329 in patients with breast cancer
| Characteristics | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| miR-329 (low vs high) | 2.987 | 1.681–5.308 | 0.000 | 2.987 | 1.681–5.308 | 0.000 |
| Age (year) (≥50 vs <50) | 0.849 | 0.505–1.428 | 0.538 | – | – | – |
| Tumor size (cm) (>3.0 vs ≤3.0) | 1.163 | 0.713–1.896 | 0.546 | – | – | – |
| Lymph node metastasis (positive vs negative) | 0.957 | 0.589–1.554 | 0.858 | – | – | – |
| PR expression (positive vs negative) | 1.327 | 0.818–2.152 | 0.252 | – | – | – |
| ER expression (positive vs negative) | 0.956 | 0.592–1.544 | 0.854 | – | – | – |
| HER-2 expression (positive vs negative) | 0.804 | 0.497–1.302 | 0.376 | – | – | – |
| TNM stage (III vs I–II) | 1.319 | 0.796–2.187 | 0.283 | – | – | – |
Note: – indicates no related data.
Abbreviations: ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; HR, hazard ratio; miR-329, microRNA-329; PR, progesterone receptor.
Validation of miR-329 expression and its clinical significance in TCGA
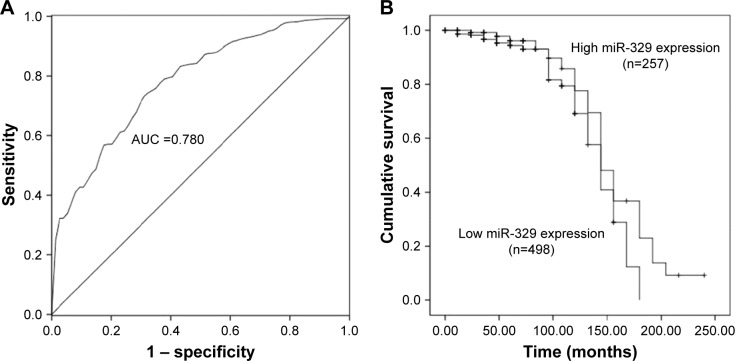
To further verify the expression and clinical significance of miR-329 in breast cancer, we used data collected from TCGA database. As shown in Figure 5, expression of miR-329 was significantly lower in breast cancer cases than in normal controls (P<0.001). Furthermore, the AUC of miR-329 was found to be 0.780 in the ROC analysis with a sensitivity of 72.6% and a specificity of 70.3% at the cutoff value of 0.987 (Figure 6A), which suggested the relatively high diagnostic accuracy of miR-329 in breast cancer patients. Moreover, the Kaplan–Meier survival analysis for the cancer patients revealed that low expression of miR-329 was correlated with poor overall survival (log-rank P=0.033, Figure 6B).
Figure 5.
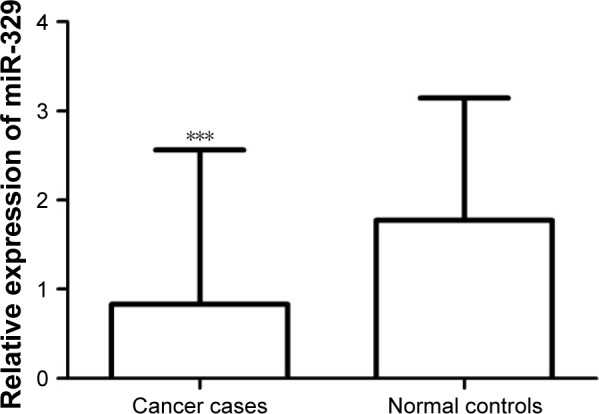
Expression of miR-329 in breast cancer patients as per data collected from TCGA.
Note: Downregulated miR-329 expression was detected in breast cancer cases compared with the normal controls (***P=0.00065).
Abbreviations: miR-329, microRNA-329; TCGA, The Cancer Genome Atlas.
Figure 6.
Clinical significance of miR-329 in breast cancer patients as per data collected from TCGA.
Notes: (A) miR-329 had a relatively high diagnostic performance with an AUC of 0.780. (B) Downregulated miR-329 expression proved to be associated with poor overall survival of breast cancer patients (log-rank P=0.033).
Abbreviations: AUC, area under the curve; miR-329, microRNA-329; TCGA, The Cancer Genome Atlas.
Discussion
According to the statistics, breast cancer is considered a serious health risk for females, and it ranks as the most frequent malignancy in women in some countries.20,21 It is reported that numerous metastatic breast cancer cases in the USA occur in younger women.22 Thus, early detection is critically important for effective treatment of this malignancy. Thus far, considerable progress has been made in cancer treatment;23 however, the prognosis of breast cancer remains dismal, which is mainly due to aggressive biological factors.24 Therefore, diagnosis and prognosis of breast cancer need to be improved urgently. Accumulating data have revealed that a wide array of molecular biomarkers has a relatively high clinical value in various types of malignancies.25–27 Consequently, the poor outcomes of breast cancer emphasize the great need to identify novel diagnostic and prognostic biomarkers.
MiRNAs are considered an important class of the available biomarkers. Emerging studies have revealed that aberrant miRNA expression is involved in tumor progression.28 MiRNAs can suppress target gene expression, which may act as a crucial factor during tumor development.29 Thus, they are described as oncogenes or tumor suppressors that participate in the progression of tumors.30 For example, the tumor suppressor role of microRNA-205 has been observed in renal cell cancer tissues, and it was shown to suppress tumor cell progression.31 MicroRNA-584 was found to correlate with cell invasion and migration in thyroid carcinoma.32 Furthermore, the clinical significance of these miRNAs has also been highlighted in various cancers.33,34 MiR-329, an extensively studied miRNA, has been investigated in some cancers and its clinical value was highlighted in previous studies.18,35,36 A study conducted by Kang et al has revealed that the expression of miR-329 was lower in breast cancer and associated with tumor cell proliferation, migration, and invasion, indicating its potential role for targeted therapy.19 However, the understanding of the clinical significance of miR-329 in breast cancer is limited. In the current study, the expression of miR-329 in breast cancer specimens was found to be abnormal when compared with the noncancerous samples. Thus, we speculated that miR-329 may possess potential clinical value for patients with this malignant disease.
In the present study, expression levels of miR-329 were measured by qRT-PCR. The analysis showed that the expression of miR-329 was significantly lower in breast cancer serum specimens compared to healthy controls. Similarly, downregulated miR-329 expression was also detected in breast cancer tissues compared with the adjacent normal tissues. Moreover, serum miR-329 expression positively correlated with tissue miR-329 expression. In addition to the serum and tissue samples, we investigated miR-329 expression in breast cancer-derived exosomes and found no difference in miR-329 expression patterns between breast cancer cells and normal breast cells. According to the chi-square test, downregulated miR-329 expression was found to be influenced by positive lymph node metastasis and advanced TNM stage. Considering these data, miR-329 might function as a potential tumor suppressor and be involved in the development of breast cancer. In the study by Kang et al, expression of miR-329 was also found to be downregulated in breast cancer tissues and was reported to be involved in tumor progression via regulating the expression of p130Cas.19 Our results were in accordance with the previous data.
Given the potential tumor suppressor role of miR-329, we speculated that there were relationships between miR-329 expression and breast cancer diagnosis and prognosis. Thus, we used ROC analysis to examine its diagnostic value for cancer patients, and we found that the decreased miR-329 expression was a highly sensitive and specific diagnostic marker for breast cancer. Additionally, Kaplan–Meier survival analysis was performed, and it was found that low levels of miR-329 expression were associated with poor overall survival of breast cancer patients. To further confirm its prognostic value, the multivariate Cox regression assay results demonstrated that the downregulated expression of miR-329 was an independent prognostic biomarker in patients with breast cancer. To further confirm the results above, the miR-329 expression levels and clinical information of 755 breast cancer patients were downloaded from the TCGA database. The analysis also showed that the expression miR-329 was downregulated in breast cancer cases compared with the normal controls, and its expression could be used as a potential diagnostic and prognostic biomarker for patients with breast cancer.
Taken together, the data in our study revealed that the downregulated expression of miR-329 was correlated with the progression of breast cancer. Decreased miR-329 expression could serve as an efficient diagnostic and prognostic biomarker and may be used to improve targeted therapy for breast cancer. Although we provide evidence that miR-329 acts as a candidate biomarker for breast cancer, its molecular mechanisms remain unclear and need to be explored in further studies.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.de Deus Moura R, Carvalho FM, Bacchi CE. Breast cancer in very young women: clinicopathological study of 149 patients ≤25 years old. Breast. 2015;24(4):461–467. doi: 10.1016/j.breast.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Miller JW, King JB, Joseph DA, Richardson LC, Centers for Disease Control and Prevention (CDC) Breast cancer screening among adult women behavioral risk factor surveillance system, United States, 2010. MMWR Suppl. 2012;61(2):46–50. [PubMed] [Google Scholar]
- 3.Reeder JG, Vogel VG. Breast cancer prevention. Cancer Treat Res. 2008;141:149–164. doi: 10.1007/978-0-387-73161-2_10. [DOI] [PubMed] [Google Scholar]
- 4.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360(9328):187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 5.Clapp RW, Jacobs MM, Loechler EL. Environmental and occupational causes of cancer: new evidence 2005–2007. Rev Environ Health. 2008;23(1):1–37. doi: 10.1515/reveh.2008.23.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirsh VA, Chiarelli AM, Edwards SA, et al. Tumor characteristics associated with mammographic detection of breast cancer in the Ontario breast screening program. J Natl Cancer Inst. 2011;103(12):942–950. doi: 10.1093/jnci/djr138. [DOI] [PubMed] [Google Scholar]
- 7.Rivera E, Gomez H. Chemotherapy resistance in metastatic breast cancer: the evolving role of ixabepilone. Breast Cancer Res. 2010;12(Suppl 2):S2. doi: 10.1186/bcr2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler J, Schuler M, Gauler TC, et al. Circulating U2 small nuclear RNA fragments as a diagnostic and prognostic biomarker in lung cancer patients. J Cancer Res Clin Oncol. 2016;142(4):795–805. doi: 10.1007/s00432-015-2095-y. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Yang L, Zhao J, et al. Nuclear-enriched abundant transcript 1 as a diagnostic and prognostic biomarker in colorectal cancer. Mol Cancer. 2015;14:191. doi: 10.1186/s12943-015-0455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toraih EA, Mohammed EA, Farrag S, Ramsis N, Hosny S. Pilot study of serum microRNA-21 as a diagnostic and prognostic biomarker in Egyptian breast cancer patients. Mol Diagn Ther. 2015;19(3):179–190. doi: 10.1007/s40291-015-0143-6. [DOI] [PubMed] [Google Scholar]
- 12.Xu S, Yi XM, Zhou WQ, Cheng W, Ge JP, Zhang ZY. Downregulation of miR-129 in peripheral blood mononuclear cells is a diagnostic and prognostic biomarker in prostate cancer. Int J Clin Exp Pathol. 2015;8(11):14335–14344. [PMC free article] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Macfarlane LA, Murphy PR. MicroRNA: biogenesis, function and role in cancer. Curr Genomics. 2010;11(7):537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang XC, Ma Y, Meng PS, Han JL, Yu HY, Bi LJ. miR-433 inhibits oral squamous cell carcinoma (OSCC) cell growth and metastasis by targeting HDAC6. Oral Oncol. 2015;51(7):674–682. doi: 10.1016/j.oraloncology.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Wang MJ, Li Y, Wang R, et al. Downregulation of microRNA-124 is an independent prognostic factor in patients with colorectal cancer. Int J Colorectal Dis. 2013;28(2):183–189. doi: 10.1007/s00384-012-1550-3. [DOI] [PubMed] [Google Scholar]
- 17.Qiu S, Lin S, Hu D, Feng Y, Tan Y, Peng Y. Interactions of miR-323/miR-326/miR-329 and miR-130a/miR-155/miR-210 as prognostic indicators for clinical outcome of glioblastoma patients. J Transl Med. 2013;11:10. doi: 10.1186/1479-5876-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Li W, Guo J, Li G, Chen F, Zhou J. Downregulation of miR-329 promotes cell invasion by regulating BRD4 and predicts poor prognosis in hepatocellular carcinoma. Tumour Biol. 2016;37(3):3561–3569. doi: 10.1007/s13277-015-4109-4. [DOI] [PubMed] [Google Scholar]
- 19.Kang H, Kim C, Lee H, et al. Downregulation of microRNA-362-3p and microRNA-329 promotes tumor progression in human breast cancer. Cell Death Differ. 2016;23(3):484–495. doi: 10.1038/cdd.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller AB. Early detection of breast cancer in the emerging world. Zentralbl Gynakol. 2006;128(4):191–195. doi: 10.1055/s-2006-933488. [DOI] [PubMed] [Google Scholar]
- 21.Mousavi SM, Montazeri A, Mohagheghi MA, et al. Breast cancer in Iran: an epidemiological review. Breast J. 2007;13(4):383–391. doi: 10.1111/j.1524-4741.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 22.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309(8):800–805. doi: 10.1001/jama.2013.776. [DOI] [PubMed] [Google Scholar]
- 23.Diaby V, Tawk R, Sanogo V, Xiao H, Montero AJ. A review of systematic reviews of the cost-effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Breast Cancer Res Treat. 2015;151(1):27–40. doi: 10.1007/s10549-015-3383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Roosmalen W, Le Devedec SE, Golani O, et al. Tumor cell migration screen identifies SRPK1 as breast cancer metastasis determinant. J Clin Invest. 2015;125(4):1648–1664. doi: 10.1172/JCI74440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Zhang Q, Wu L, et al. Serum miR-499 as a novel diagnostic and prognostic biomarker in non-small cell lung cancer. Oncol Rep. 2014;31(4):1961–1967. doi: 10.3892/or.2014.3029. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Jin L, Jiang L, et al. Serum miR-372 is a diagnostic and prognostic biomarker in patients with early colorectal cancer. Anticancer Agents Med Chem. 2016;16(4):424–431. doi: 10.2174/1871520615666150716110406. [DOI] [PubMed] [Google Scholar]
- 27.Su ZX, Zhao J, Rong ZH, Wu YG, Geng WM, Qin CK. Diagnostic and prognostic value of circulating miR-18a in the plasma of patients with gastric cancer. Tumour Biol. 2014;35(12):12119–12125. doi: 10.1007/s13277-014-2516-6. [DOI] [PubMed] [Google Scholar]
- 28.Yin ZL, Wang YL, Ge SF, et al. Reduced expression of miR-503 is associated with poor prognosis in cervical cancer. Eur Rev Med Pharmacol Sci. 2015;19(21):4081–4085. [PubMed] [Google Scholar]
- 29.Luo M, Shen D, Zhou X, Chen X, Wang W. MicroRNA-497 is a potential prognostic marker in human cervical cancer and functions as a tumor suppressor by targeting the insulin-like growth factor 1 receptor. Surgery. 2013;153(6):836–847. doi: 10.1016/j.surg.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Zuberi M, Mir R, Das J, et al. Expression of serum miR-200a, miR-200b, and miR-200c as candidate biomarkers in epithelial ovarian cancer and their association with clinicopathological features. Clin Transl Oncol. 2015;17(10):779–787. doi: 10.1007/s12094-015-1303-1. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Tang ZY, He Y, Liu LF, Li DJ, Chen X. miRNA-205 is a candidate tumor suppressor that targets ZEB2 in renal cell carcinoma. Oncol Res Treat. 2014;37(11):658–664. doi: 10.1159/000368792. [DOI] [PubMed] [Google Scholar]
- 32.Xiang J, Wu Y, Li DS, et al. miR-584 suppresses invasion and cell migration of thyroid carcinoma by regulating the target oncogene ROCK1. Oncol Res Treat. 2015;38(9):436–440. doi: 10.1159/000438967. [DOI] [PubMed] [Google Scholar]
- 33.Zuberi M, Khan I, Mir R, Gandhi G, Ray PC, Saxena A. Utility of serum miR-125b as a diagnostic and prognostic indicator and its alliance with a panel of tumor suppressor genes in epithelial ovarian cancer. PLoS One. 2016;11(4):e0153902. doi: 10.1371/journal.pone.0153902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Gao X, Wei F, et al. Diagnostic and prognostic value of circulating miR-21 for cancer: a systematic review and meta-analysis. Gene. 2014;533(1):389–397. doi: 10.1016/j.gene.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 35.Xiao B, Tan L, He B, Liu Z, Xu R. MiRNA-329 targeting E2F1 inhibits cell proliferation in glioma cells. J Transl Med. 2013;11:172. doi: 10.1186/1479-5876-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun CC, Li SJ, Zhang F, et al. Hsa-miR-329 exerts tumor suppressor function through down-regulation of MET in non-small cell lung cancer. Oncotarget. 2016;7(16):21510–21526. doi: 10.18632/oncotarget.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]