Abstract
IMPORTANCE
Hemoglobin A1c (HbA1c) reflects past glucose concentrations, but this relationship may differ between those with sickle cell trait (SCT) and those without it.
OBJECTIVE
To evaluate the association between SCT and HbA1c for given levels of fasting or 2-hour glucose levels among African Americans.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective cohort study using data collected from 7938 participants in 2 community-based cohorts, the Coronary Artery Risk Development in Young Adults (CARDIA) study and the Jackson Heart Study (JHS). From the CARDIA study, 2637 patients contributed a maximum of 2 visits (2005–2011); from the JHS, 5301 participants contributed a maximum of 3 visits (2000–2013). All visits were scheduled at approximately 5-year intervals. Participants without SCT data, those without any concurrent HbA1c and glucose measurements, and those with hemoglobin variants HbSS, HbCC, or HbAC were excluded. Analysis of the primary outcome was conducted using generalized estimating equations (GEE) to examine the association of SCT with HbA1c levels, controlling for fasting or 2-hour glucose measures.
EXPOSURES
Presence of SCT.
MAIN OUTCOMES AND MEASURES
Hemoglobin A1c stratified by the presence or absence of SCT was the primary outcome measure.
RESULTS
The analytic sample included 4620 participants (mean age, 52.3 [SD, 11.8] years; 2835 women [61.3%]; 367 [7.9%] with SCT) with 9062 concurrent measures of fasting glucose and HbA1c levels. In unadjusted GEE analyses, for a given fasting glucose, HbA1c values were statistically significantly lower in those with (5.72%) vs those without (6.01%) SCT (mean HbA1c difference, −0.29%; 95% CI, −0.35% to −0.23%). Findings were similar in models adjusted for key risk factors and in analyses using 2001 concurrent measures of 2-hour glucose and HbA1c concentration for those with SCT (mean, 5.35%) vs those without SCT (mean, 5.65%) for a mean HbA1c difference of −0.30% (95% CI, −0.39% to −0.21%). The HbA1c difference by SCT was greater at higher fasting (P = .02 for interaction) and 2-hour (P = .03) glucose concentrations. The prevalence of prediabetes and diabetes was statistically significantly lower among participants with SCT when defined using HbA1c values (29.2%vs 48.6% for prediabetes and 3.8% vs 7.3% for diabetes in 572 observations from participants with SCT and 6877 observations from participants without SCT; P<.001 for both comparisons).
CONCLUSIONS AND RELEVANCE
Among African Americans from 2 large, well-established cohorts, participants with SCT had lower levels of HbA1c at any given concentration of fasting or 2-hour glucose compared with participants without SCT. These findings suggest that HbA1c may systematically underestimate past glycemia in black patients with SCT and may require further evaluation.
Hemoglobin A1c (HbA1c) is a practical measure of average glucose levels during the preceding 2 to 3 months.1–4 In 2009, after review of available evidence, an International Expert Committee recommended the use of HbA1c to diagnose diabetes.5 Extensive work has been done by the NGSP (formerly, the National Glycohemoglobin Standardization Program) to standardize methods for measuring HbA1c and to identify methods that provide accurate HbA1c measurement even in the presence of hemoglobin variants.6
Sickle cell trait (SCT) is the most common hemoglobin variant in the United States, with 8% to 10% of black people affected by SCT compared with less than 1% of white people.7,8 Red blood cells of individuals with normal hemoglobin contain approximately 97% HbA, whereas red blood cells of individuals with SCT contain approximately 60% to 70% HbA and 30% to 40% HbS.9 Although data are limited, it is hypothesized that the presence of HbS results in a shorter lifespan for red blood cells.9–12 This would result in less available time for hemoglobin glycation, which in turn may influence the interpretation of HbA1c in relationship to the glucose values they intend to represent. Correct interpretation of HbA1c values in individuals with SCT is important because it directly affects efforts that use HbA1c for screening, diagnosis, and monitoring of diabetes and prediabetes. Accordingly, the objectives of this study were to (1) examine the association between HbA1c and SCT while controlling for other measures of glucose (fasting and 2-hour glucose) levels, (2) compare the prevalence of prediabetes and diabetes by SCT status, and (3) determine if SCT modifies the discriminative ability of HbA1c to identify individuals with prediabetes or diabetes.
Methods
Study Population
This retrospective study pooled data from participants who self-identified as African American from 2 established community-based cohorts, the Coronary Artery Risk Development in Young Adults (CARDIA) study and the Jackson Heart Study (JHS), to examine the association of SCT with HbA1c, controlling for fasting glucose or 2-hour glucose levels. Details regarding the design of each study have been published.13,14 In brief, the CARDIA study enrolled, from March 25, 1985 to June 7, 1986, a stratified sample of 2637 black people and 2478 white people (n = 5115) from Minneapolis, Minnesota; Chicago, Illinois; Birmingham, Alabama; and Oakland, California. After their initial visit, participants were followed up approximately 2, 5, 7, 10, 15, 20, and 25 years later (data collection, 1985-present). The Jackson Heart Study, a single-site study in Jackson, Mississippi, enrolled 5301 African Americans during the years 2000 through 2004. Participants returned for 2 follow-up examinations approximately 5 and 10 years after their initial visit (data collection, 2000–2013). The data used for these analyses were from years 20 and 25 from the CARDIA cohort and from baseline and years 5 and 10 from the JHS cohort. All participants included in analyses provided written informed consent for genetic studies. Institutional review board approval was obtained separately from each participating institution.
Covariate Assessment
Details regarding data collection procedures for CARDIA15,16 and JHS17 have been published. Data on participants’ sex, self-reported race, date of birth, diet, physical activity, smoking status, medical history, and medication use were collected at each visit by trained interviewers. Physical activity was classified as poor (0), intermediate (>0-<150), or ideal (≥150) using minutes per week of moderate to vigorous physical activity. Diet was classified as poor (0–1), intermediate (2–3), or ideal (4–5) using the number of components achieved from the following list: 4.5 or more cups of fruits and vegetables daily; 198 g or more of fish weekly; less than 1500 mg of sodium daily; less than 450 calories each week of sugar-sweetened beverages; and 3 or more servings daily of whole grains. Smoking was classified as poor (current smoker), intermediate (quit <12 months ago) or ideal (never smoked or quit ≥12 months ago). Height and weight were measured by certified study personnel and used to calculate body mass index (BMI), weight in kilograms divided by height in meters squared. Estimated glomerular filtration rate (eGFR) was calculated at years 20 and 25 in CARDIA and at baseline and year 10 in JHS using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.18 Serum ferritin was measured at year 20 in CARDIA and at baseline in JHS. Previous diabetes diagnosis was defined as self-report of a physician’s diagnosis. Current use of diabetes medications was defined as use of diabetes medications in the 2 weeks prior to the examination.
SCT Status
In CARDIA, SCT status was determined with available DNA samples from the 10-year follow-up and afterwards using single-gene, single-nucleotide polymorphism (SNP) genotyping with TaqMan SNP Genotyping Assays (Life Technologies). In JHS, genotype data for rs334 encoding the sickle hemoglobin mutation (HBB p.Glu7Val) was obtained through whole-exome sequencing using data from baseline. Sickle cell trait was defined as the presence of 1 abnormal allele for HbS.
Plasma Glucose Measures
Plasma glucose was measured using the hexokinase method at all visits in both studies except at baseline in JHS, at which time the glucose oxidase method was used. Previous research has shown these 2 methods of glucose measurement to be highly correlated.19,20 Additionally, 2-hour glucose levels were measured in CARDIA and obtained during a standard oral glucose tolerance test, using 75 g of glucose solution. The coefficient of variation for glucose measures ranged from 1.6% to 3.8% (eTable 1 in the Supplement).
HbA1c Measures
Two NGSP-certified assays were used to measure HbA1c, both using high-performance liquid chromatography. In JHS, a Tosoh 2.2 was used at baseline and a Tosoh G7(variant mode) was used at the 5- and 10-year follow-ups. In CARDIA, a Tosoh G7 (variant mode) was used at the 20- and 25-year follow-up. According to the NGSP, neither Tosoh 2.2 nor Tosoh G7 (variant mode) has experienced clinically significant interference in those with SCT.6 The coefficient of variation for HbA1c assays ranged from 1.2% to 1.9% (eTable 1 in the Supplement).
Main Outcome Measure
The main outcome measure, HbA1c, was assessed at multiple time points and pooled cross-sectionally to examine the association between HbA1c, modeled as a continuous variable, and SCT, adjusting for fasting or 2-hour glucose measures. Prespecified secondary outcomes include prediabetes, diabetes, and combined prediabetes or diabetes, which were defined using the following measures based on cutpoints established by the American Diabetes Association21: (1) fasting glucose levels (prediabetes, 100-<126 mg/dL; diabetes, ≥126 mg/dL; and combined prediabetes or diabetes, ≥100 mg/dL), 2-hour glucose levels (prediabetes, 140-<200 mg/dL; diabetes, ≥200 mg/dL; and combined prediabetes or diabetes, ≥140 mg/dL), and HbA1c levels (prediabetes, 5.7%-<6.5%; diabetes, ≥6.5%; combined prediabetes or diabetes, ≥5.7%). (To convert glucose from mg/dL to mmol/L, multiply by 0.0555.)
Statistical Methods
Baseline characteristics of participants with and without SCT were compared using χ2 tests and analysis of variance for discrete and continuous variables, respectively. All visits with concurrent measurement of HbA1c and fasting glucose or HbA1c and 2-hour glucose levels were included in the analyses. Mean HbA1c values were calculated by SCT status across a range of glucose categories in 10-mg/dL increments for fasting glucose (<80-≥150mg/dL) and in 20-mg/dL increments for 2-hour glucose levels (<80-≥200mg/dL).
Generalized estimating equations (GEE) using random effects at the participant level and an exchangeable correlation matrix to account for correlation among repeated measures were used to assess the association of SCT with HbA1c controlling for fasting glucose levels.22 A multistep approach was used to examine the robustness of findings to model specification. First, unadjusted GEE models were fit using HbA1c as the outcome, SCT as the exposure, and glucose as the primary covariate. Next, GEE analyses were adjusted for the following potential confounders that were identified a priori based on the literature: age, sex, BMI, ferritin levels, eGFR, physician-diagnosed diabetes, use of diabetes medications, and study cohort.23–25 In addition, multiplicative SCT × glucose interaction was tested in unadjusted and adjusted models. Analyses were repeated using 2-hour glucose measures in lieu of fasting glucose measures as the main covariate. Model fit was compared using quasi-likelihood under the independence-model criterion.26 Prespecified subgroup analyses were conducted among participants not taking diabetes medications and in groups of participants stratified by cohort. Interaction by cohort and HbA1c assay on the association between SCT and HbA1c was tested.
Next, among a subset of participants with no previous diabetes or current use of diabetes medications, GEE analyses with a Poisson distribution and an identity link function were used to estimate the unadjusted prevalence of prediabetes and diabetes by SCT status.27
In the same subset of participants, GEE analyses with a Poisson distribution and a log link function (sometimes called a modified Poisson model) were used to generate predictive probabilities that were then used in logistic regression models to compare the discriminative ability of HbA1c levels to identify the combined presence of prediabetes or diabetes. 28 A combined outcome of prediabetes or diabetes was used due to consideration of sample size. Area under the receiver operating characteristic (AUROC) curves were calculated for HbA1c levels in those with and without SCT. Unpaired comparisons of the AUROC curves were conducted to assess the discriminatory power ofHbA1c by SCT status.29,30 Complete case analysis was performed for adjusted analyses because data were more than 98% complete, with the exception of ferritin (7% missing). A2-sided P value of ≤.05 was used for level of significance. Analyses were performed using SAS statistical software version 9.4 (SAS Institute Inc).
Results
Baseline Characteristics
A total of 7938 participants were enrolled at baseline, of whom 2637 were in CARDIA and 5301 in JHS. One thousand sixty-five CARDIA and 2253 JHS participants were excluded. Of those excluded in CARDIA, 550 had no available SCT data; 3 had HbSS; 2, HbCC; 41, HbAC; and 469, no concurrent measurements of HbA1c or fasting or 2-hour glucose measurements. Of those excluded in JHS, 2079 had no available SCT data; 2, HbSS; 80, HbAC; and 92, no concurrent measurements of HbA1c and fasting or 2-hour glucose. A total of 4620 participants (58.2%) were included in these analyses—1572 in CARDIA and 3048 in JHS. Other than having a lower mean BMI, the 1019 CARDIA and 2171 JHS participants excluded from the analyses because of missing data had comparable baseline characteristics with those included in the analysis (BMI, 29.4 vs 29.8; P = .01; eTable 2 in the Supplement).
The 367 participants (7.9%) with SCT were older (53.9 vs 52.2 years, P = .007) and had lower eGFR (92.5 vs 97.5 mL/min/1.73 m2, P < .001), and lower HbA1c values (5.7% vs 5.9%, P = .001; Table 1) than did participants without SCT. Additionally, participants with SCT were more likely to report previously diagnosed diabetes (17.2% vs 14.7%, P=.20) and current use of diabetes medications (15.0% vs 12.5%, P=.19), although these findings did not reach statistical significance.
Table 1.
Baseline Characteristics of Study Participants by Sickle Cell Trait Statusa
| Characteristic | CARDIA and JHS | CARDIA | JHS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Overall (n = 4620) | No SCT (n = 4253) | SCT (n = 367) | P Value | No SCT (n = 1467) | SCT (n = 105) | P Value | No SCT (n = 2786) | SCT (n = 262) | P Value | |
| Men, No. (%) | 1785 (38.6) | 1634 (38.4) | 151 (41.1) | .31 | 598 (40.8) | 42 (40.0) | .88 | 1036 (37.2) | 109 (41.6) | .16 |
|
| ||||||||||
| Age, mean (SD), y | 52.3 (11.81) | 51.3 (11.8) | 53.9 (12.2) | .007 | 45.7 (4.3) | 46.1 (4.2) | .31 | 55.6 (13.0) | 57.1 (12.9) | .08 |
|
| ||||||||||
| BMI, mean (SD) | 31.8 (7.4) | 31.8 (7.5) | 31.7 (7.5) | .82 | 31.5 (7.7) | 31.8 (6.9) | .74 | 32.0 (7.6) | 31.7 (7.8) | .57 |
|
| ||||||||||
| Hemoglobin, mean (SD), mg/dLb | 13.0 (1.5) | 13.1 (1.5) | 12.9 (1.4) | .14 | 13.1 (1.5) | 12.9 (1.4) | .14 | |||
|
| ||||||||||
| Ferritin, mean (SD), ng/mLc | 155.9 (169.7) | 156.3 (171.8) | 151.4 (143.7) | .61 | 124.0 (148.0) | 107.2 (98.7) | .32 | 169.7 (179.1) | 165.0 (152.6) | .68 |
|
| ||||||||||
| eGFR, mean (SD), mL/min/1.73 m2 | 97.1 (21.5) | 97.5 (21.3) | 92.5 (23.3) | <.001 | 103.0 (19.7) | 101.6 (19.7) | .46 | 94.6 (21.5) | 88.8 (23.6) | <.001 |
|
| ||||||||||
| Fasting glucose, mean (SD), mg/dL | 101.6 (33.2) | 101.5 (33.2) | 102.7 (34.2) | .49 | 101.1 (30.2) | 106.0 (38.2) | .11 | 101.7 (34.6) | 101.4 (32.4) | .91 |
|
| ||||||||||
| HbA1c, mean (SD), % | 5.9 (1.2) | 5.9 (1.2) | 5.7 (1.2) | .001 | 5.8 (1.0) | 5.7 (1.1) | .44 | 6.0 (1.3) | 5.7 (1.2) | <.001 |
|
| ||||||||||
| 2-h glucose, mean (SD), mg/dLd | 113.9 (44.5) | 113.8 (44.9) | 116.1 (39.0) | .66 | 113.8 (44.9) | 116.1 (39.0) | .66 | |||
|
| ||||||||||
| Diabetes, No. (%)e | 685 (14.9) | 622 (14.7) | 63 (17.2) | .20 | 213 (14.5) | 23 (21.9) | .04 | 409 (14.7) | 40 (15.3) | .82 |
|
| ||||||||||
| Diabetes medications, No. (%)f | 577 (12.75) | 523 (12.53) | 54 (14.97) | .19 | 116 (7.9) | 15 (14.43) | .02 | 407 (15.0) | 39 (15.1) | .95 |
|
| ||||||||||
| Physical Activity, No. (%)g | ||||||||||
|
| ||||||||||
| Poor health | 1584 (34.4) | 1445 (34.1) | 139 (38.1) | .29 | 71 (4.9) | 3 (2.9) | .66 | 1374 (49.4) | 136 (51.9) | .82 |
|
|
|
|
||||||||
| Intermediate health | 1481 (32.2) | 1368 (32.3) | 113 (31.0) | 494 (33.9) | 35 (34.0) | 874 (31.4) | 78 (29.8) | |||
|
|
|
|
||||||||
| Ideal health | 1540 (33.4) | 1427 (33.7) | 113 (31.0) | 892 (61.2) | 65 (63.1) | 535 (19.2) | 48 (18.3) | |||
|
| ||||||||||
| Diet, No. (%)h | ||||||||||
|
| ||||||||||
| Poor health | 2498 (58.6) | 2292 (58.5) | 206 (59.4) | .17 | 595 (52.7) | 40 (47.1) | .18 | 1697 (60.9) | 166 (63.4) | .73 |
|
|
|
|
||||||||
| Intermediate health | 1710 (40.1) | 1577 (40.3) | 133 (38.3) | 512 (45.4) | 41 (48.2) | 1065 (38.2) | 92 (35.1) | |||
|
|
|
|
||||||||
| Ideal health | 54 (1.3) | 46 (1.2) | 8 (2.3) | 22 (2.0) | 4 (4.7) | 24 (0.9) | 4 (1.5) | |||
|
| ||||||||||
| Smoking, No. (%)i | ||||||||||
|
| ||||||||||
| Poor health | 791 (17.4) | 734 (17.5) | 57 (15.8) | .69 | 367 (25.4) | 20 (19.1) | .25 | 367 (13.4) | 37 (14.5) | .75 |
|
|
|
|
||||||||
| Intermediate health | 83 (1.8) | 77 (1.8) | 6 (1.7) | 45 (3.1) | 2 (1.9) | 32 (1.2) | 4 (1.6) | |||
|
|
|
|
||||||||
| Ideal health | 3683 (80.8) | 3385 (80.7) | 298 (82.6) | 1036 (71.6) | 83 (79.1) | 2349 (85.5) | 215 (84.0) | |||
Abbreviations: HbA1c, hemoglobin A1c; BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; CARDIA, Coronary Artery Risk Development in Young Adults study; eGFR, estimated glomerular filtration rate; JHS, Jackson Heart Study; SCT, sickle cell trait.
SI conversion factor: to convert glucose from mg/dL to mmol/L, multiply by 0.0555.
Baseline for this study was the first visit at which a participant had HbA1c and fasting or 2-hour glucose measured concurrently. For CARDIA participants, this was either the year-20 or year-25 follow-up examination. For JHS participants, this was either examination 1, 2, or 3.
Hemoglobin was only measured in 2976 participants, all of whom are from JHS (ie, 64% of the total analytic sample).
Ferritin was only measured in 4278 participants (ie, 93% of the total analytic sample).
Two-hour glucose was only measured in 1222 participants all of whom are from CARDIA and are not taking diabetes medications (ie, 26% of the total analytic sample).
Diagnosed diabetes is defined as self-reported use of diabetes medications or self-reported physician diagnosis.
Diabetes medications is defined as use of a medication for the treatment of diabetes taken in the 2 weeks prior to the study examination.
For the definitions of poor, intermediate, or ideal physical activity and health, see the Methods section.
For the definition of a poor, intermediate, or ideal diet, see the Methods section.
For the definition of poor, intermediate, or ideal smoking status, see the Methods section.
Association of HbA1c With Glucose Measures
For all 4620 participants, each visit with concurrent HbA1c and fasting glucose or HbA1c and 2-hour glucose measures was included, resulting in 9062 concurrent measures of HbA1c and fasting glucose (2583 from CARDIA; 6479 from JHS) from 4620 unique participants and 2001 concurrent measures of HbA1c and 2-hour glucose measures (2001 from CARDIA) from 1323 unique participants (eFigure in the Supplement). The majority of participants (74.6%) had HbA1c and glucose measured at least twice. Using all available observations, the mean HbA1c was 5.7% in those with SCT vs 6.0% in those without SCT, despite similar mean fasting (103.0 vs 102.9 mg/dL; P=.88) and 2-hour glucose values (118.5 vs 113.0 mg/dL; P=.19) for those with SCT vs those without SCT, respectively. Across all categories of fasting and 2-hour glucose measures, mean HbA1c values were lower in those with vs without SCT (Table 2).
Table 2.
Observed Hemoglobin A1c Value Across Categories of Fasting and 2-Hour Glucose, by SCT Status
| No SCT | SCT | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Total No. of Observations | No. of Unique Participants | HbA1c %, Mean (SD) | Total No. of Observations | No. of Unique Participants | HbA1c %, Mean (SD) | |
| Fasting Glucose, mg/dL | ||||||
| <80 | 482 | 429 | 5.6 (0.9) | 46 | 40 | 5.4 (0.6) |
|
| ||||||
| 80–89 | 2207 | 1683 | 5.5 (0.5) | 167 | 130 | 5.3 (0.5) |
|
| ||||||
| 90–99 | 2712 | 2073 | 5.7 (0.5) | 243 | 183 | 5.5 (0.5) |
|
| ||||||
| 100–109 | 1394 | 1154 | 6.0 (0.6) | 120 | 105 | 5.6 (0.6) |
|
| ||||||
| 110–119 | 513 | 460 | 6.3 (0.7) | 58 | 53 | 5.9 (0.6) |
|
| ||||||
| 120–129 | 268 | 241 | 6.8 (0.9) | 22 | 20 | 6.4 (0.8) |
|
| ||||||
| 130–139 | 165 | 154 | 7.0 (0.9) | 9 | 9 | 6.6 (0.5) |
|
| ||||||
| 140–149 | 128 | 124 | 7.4 (1.2) | 11 | 11 | 6.9 (0.9) |
|
| ||||||
| ≥150 | 473 | 379 | 9.3 (2.1) | 44 | 33 | 8.5 (1.9) |
|
| ||||||
| Overall | 8342 | 4253 | 6.0 (1.2) | 720 | 367 | 5.7 (1.0) |
|
| ||||||
| Two-Hour Glucose, mg/dL | ||||||
|
| ||||||
| <80 | 269 | 230 | 5.4 (0.4) | 14 | 14 | 5.2 (0.3) |
|
| ||||||
| 80–99 | 507 | 440 | 5.5 (0.4) | 33 | 30 | 5.2 (0.3) |
|
| ||||||
| 100–119 | 529 | 465 | 5.6 (0.4) | 29 | 26 | 5.4 (0.3) |
|
| ||||||
| 120–139 | 268 | 244 | 5.7 (0.5) | 26 | 23 | 5.5 (0.2) |
|
| ||||||
| 140–159 | 130 | 123 | 5.8 (0.5) | 8 | 8 | 5.3 (0.3) |
|
| ||||||
| 160–179 | 69 | 66 | 5.9 (0.5) | 9 | 8 | 5.6 (0.4) |
|
| ||||||
| 180–199 | 37 | 35 | 6.2 (0.5) | 3 | 3 | 5.6 (0.5) |
|
| ||||||
| ≥200 | 65 | 59 | 7.6 (2.4) | 5 | 5 | 6.5 (1.5) |
|
| ||||||
| Overall | 1874 | 1239 | 5.7 (0.7) | 127 | 84 | 5.4 (0.5) |
Abbreviations: HbA1c, hemoglobin A1c; SCT, sickle cell trait.
SI conversion factor: to convert glucose from mg/dL to mmol/L, multiply by 0.0555.
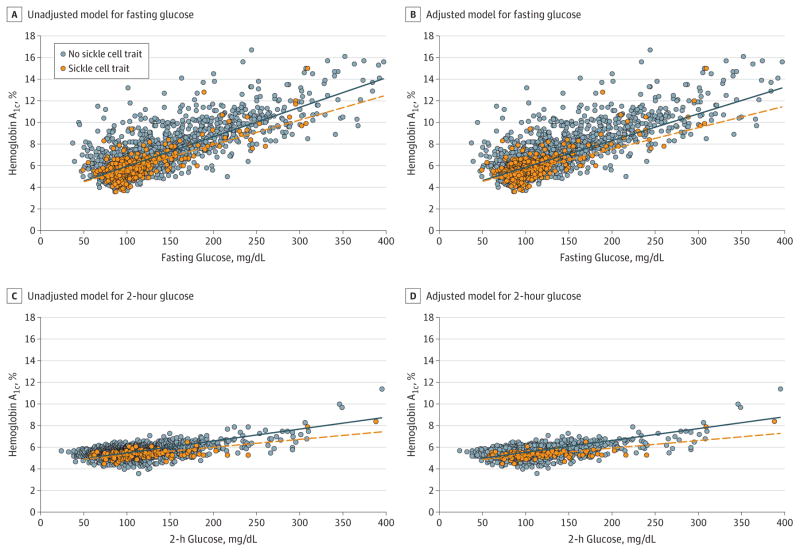
Unadjusted GEE analyses revealed that, at the same fasting glucose concentration, HbA1c values were statistically significantly lower in those with SCT (mean HbA1c, 5.72%) vs those without SCT (mean HbA1c, 6.01%; mean HbA1c difference, −0.29%; 95% CI, −0.35% to −0.23%; P < .001). In adjusted analyses, HbA1c values remained significantly lower in those with SCT (mean HbA1c difference, −0.32%; 95% CI, −0.38% to −0.26%; P < .001). The difference in HbA1c levels by SCT status was greater at higher concentrations of fasting glucose (P = .02 for interaction in unadjusted analyses, Figure 1A; P = .01 in adjusted analyses, Figure 1B). Results were similar in analyses stratified by study cohort and when excluding 1302 observations from participants taking diabetes medications at the time of examination (eTable 3 in the Supplement). There was no evidence of interaction by HbA1c assay (P = .43 for SCT × fasting glucose × HbA1c assay) or by study cohort (P = .63 for SCT × fasting glucose × study cohort).
Figure 1. Scatterplot of Observed Data Model of Hemoglobin A1c vs Fasting and 2-Hour Glucose Measures in Participants With or Without Sickle Cell Trait.
Scatterplot of observed data points along side unadjusted and adjusted regression lines examining the association between sickle cell trait (SCT) and hemoglobin A1c (HbA1c), controlling for fasting or 2-hour glucose values was obtained using generalized estimating equations (GEE) with an exchangeable correlation matrix to account for correlation of repeated measures. All continuous covariates are centered at the population mean. The solid blue lines represent the regression line for those for who did not have SCT and the dashed orange lines for those who had SCT. BMI indicates body mass index; CARDIA, Coronary Artery Risk Development in Young Adults study. To convert glucose from mg/dL to mmol/L, multiply by 0.0555.
A, Included 9062 observations, 720 from participants with SCT and 8342 from participants without SCT. The regression equation: predicted HbA1c = 6.01 + (−0.28 × SCT) + (0.03 × fasting glucose) + (−0.004 SCT fasting glucose).
B, Included 8460 observations, 683 from participants with SCT and 7777 from participants without SCT. The regression equation: predicted HbA1c = 5.93 + (−0.32 × SCT) + (0.03 × fasting glucose) + (−0.005 × SCT × fasting glucose) + (0.04 × 1 if male) + (0.008 × age) + (0.01 × BMI) + (−0.0004 × ferritin) + (0.001 × estimated glomerular filtration rate) + (−0.08 × 1 if a CARDIA participant) + (0.46 × 1 if currently using diabetes medications) + (0.14 × 1 if previous diabetes diagnosis).
C, Included 2001 observations, 127 from participants with SCT and 1874 from participants without SCT. Regression equation: predicted HbA1c = 5.65 + (−0.28 × SCT) + (0.01 × 2-hour glucose) + (−0.004 × SCT × 2-hour glucose).
D, Included 1712 observations, 109 from participants with SCT and 1606 from participants without SCT. Regression equation: predicted HbA1c = 5.66 + (−0.36 × SCT) + (0.01 × 2-hour glucose) × (−0.004 × SCT × 2-hour glucose) + (0.24 × 1 if male) + (0.02 × age) + (0.006 × BMI) + (−0.0006 × ferritin) + (0.0003 × eGFR) + (0.07 × 1 if previous diabetes diagnosis).
Results for 2-hour glucose measures revealed similar HbA1c differences by SCT status. For a given 2-hour glucose level, HbA1c values were statistically significantly lower in those with SCT (mean HbA1c, 5.35%) vs in those without SCT (mean HbA1c, 5.65%), for a mean HbA1c difference of −0.30%(95% CI, −0.39% to −0.21%; P < .001). HemoglobinA1c levels remained significantly lower in those with SCT in adjusted analyses (mean difference in HbA1c, −0.38%;95% CI, −0.49% to −0.28%; P < .001). The difference in HbA1c levels by SCT status was greater at higher 2-hour glucose concentrations (P = .03 for interaction in unadjusted analyses, Figure 1C; P = .03 in adjusted analyses, Figure 1D).
Prevalence of Prediabetes and Diabetes
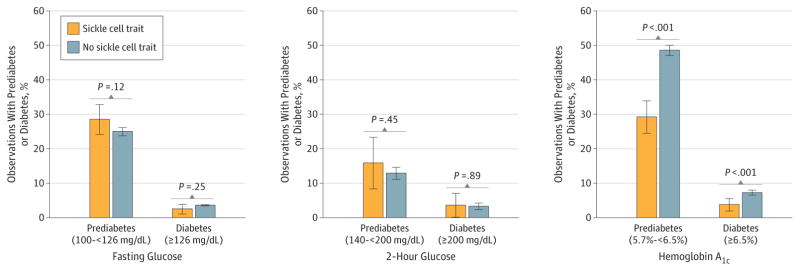
Among a subset of participants with no prior diagnosis of diabetes or current use of diabetes medications (7449 observations for fasting glucose and HbA1c; 1869 observations for 2-hour glucose and HbA1c), the prevalence of prediabetes and diabetes was not significantly different among participants with vs without SCT when defined using fasting glucose (28.6% with vs 25.0% without SCT for prediabetes; P = .12; 2.5% with vs 3.6% without SCT for diabetes; P = .25) or 2-hour glucose values (15.9% with vs 12.9% without SCT for prediabetes, P = .45; and 3.6% with vs 3.3% without SCT for diabetes; P > .89; Figure 2). In contrast, the prevalence of prediabetes and diabetes was statistically significantly lower among participants with SCT when defined using HbA1c values (29.2% with vs 48.6% without SCT for prediabetes and 3.8% with vs 7.3% without SCT for diabetes; P < .001 for all comparisons, Figure 2).
Figure 2. Prevalence of Prediabetes and Diabetes by Sickle Cell Trait Status Among Participants Not Taking Diabetes Medications and With No Prior Diagnosis of Diabetes.
Fasting glucose and hemoglobin A1c analyses included 7499 total observations (6877 observations from participants without sickle cell trait [SCT] and 572 from participants with it). Analyses for 2-hour glucose concentrations were only available from CARDIA participants and included 1869 total observations (1752 observations from participants without SCT and 117 from participants with SCT). For the definition of prediabetes and diabetes by glucose measures, see the Methods section. The prevalence of prediabetes and diabetes by fasting glucose and 2-hour glucose concentration was similar in those with and without SCT (P > .10 for all comparisons). However, the prevalence of prediabetes and diabetes as defined by hemoglobin A1c was significantly higher among participants with vs without SCT (P < .001 for both). Error bars indicate 95% CIs.
Discriminative Ability of HbA1c to Identify Prediabetes or Diabetes
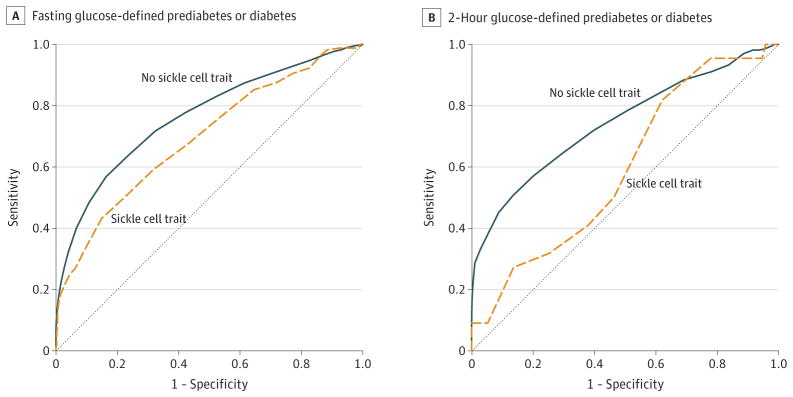
In the same subset of participants without diabetes or diabetes medication use, the discriminative ability of HbA1c to identify the presence of prediabetes or diabetes was statistically significantly lower among participants with SCT (AUROC, 0.70; 95% CI, 0.65–0.74) vs without SCT (AUROC, 0.77; 95% CI, 0.75–0.78; absolute difference, 0.07; 95% CI, 0.02–0.12) when using fasting glucose–defined prediabetes or diabetes (P < .01, Figure 3A). The same held true among participants with SCT (AUROC, 0.60; 10 95% CI, 0.47–0.72) vs those without SCT (AUROC, 0.74; 95% CI, 0.71–0.78; absolute difference, 0.15; 95% CI, 0.02–0.28) when using 2-hour glucose-defined prediabetes or diabetes measures (P = .02, Figure 3B).
Figure 3. Comparison of the Diagnostic Sensitivity of Hemoglobin A1c to Identify Combined Prediabetes or Diabetes by Sickle Cell Trait Status.
A, For fasting glucose of 100mg/dL or higher, the area under the receiver operating characteristic (AUROC) of hemoglobin A1c (HbA1c) was 0.77 (95% CI, 0.75–0.78) among those without sickle cell trait (SCT) and 0.70 (95% CI, 0.65–0.74) among those with SCT. An unpaired comparison of the AUROC curves indicated that the diagnostic ability of HbA1c to identify fasting glucose–defined prediabetes or diabetes was significantly lower among those with SCT than among those without it (P = .007).
B, For 2-hour glucose levels of 140mg/dL or higher, the AUROC of HbA1c was 0.74 (95% CI, 0.71–0.78) among those without SCT and 0.60 (95% CI, 0.47–0.72) among those with SCT. An unpaired comparison of the AUROC curves indicated that the diagnostic ability of HbA1c to identify 2-hour glucose-defined prediabetes or diabetes was significantly lower in those with SCT than in those without it (P = .03). To convert glucose from mg/dL to mmol/L, multiply by 0.0555.
Discussion
In this retrospective cohort study of African Americans participating in 2 large US cohorts, we demonstrated that, at the same fasting or 2-hour glucose concentration, HbA1c is statistically significantly lower among participants with vs without SCT. Moreover, differences in HbA1c concentration by SCT status were greater at higher glucose concentrations. These differences are based on an HbA1c method reported to have no clinically significant interference in individuals with SCT.6,31
Our findings stand in contrast to 2 previous studies. Bleyer and colleagues32 investigated the HbA1c-glucose association in 385 African American in patients, the majority of whom had diabetes and among whom there was an unusually high prevalence of SCT: 109 (28%) had SCT. Despite higher baseline HbA1c values, the authors did not find that SCT significantly altered the relationship between HbA1c and serum glucose. Sumner and colleagues33 examined the sensitivity of HbA1c to detect impaired glucose tolerance in a cohort of 216 African immigrants without diabetes,46(21%)of whom had either HbC traitor SCT. They found no significant difference in the sensitivity of HbA1c by variant hemoglobin status in the detection of prediabetes; however, this study combined participants with SCT and HbC trait, which may have affected findings. Additionally, both of these studies are potentially limited by their small sample size.
We consider 2 ways in which SCT could modify the ability of HbA1c to accurately reflect past glycemia. First, the lifespan of the red blood cells in persons with SCT may be shortened compared with those with normal hemoglobin, resulting in less time available for glycation. However, the evidence to support this hypothesis remains limited and conflicting.9–12 Second, the presence of HbS can result in assay interference with common HbA1c measurement techniques. Current testing on HbA1c laboratory methods uses a relative bias of plus or minus 7.0% to classify clinically significant interference from hemoglobin variants.6 Although the assays used in this study report no clinically significant interference in individuals with SCT, the possibility of minor interference that could potentially explain our findings cannot be ruled out.
Irrespective of the mechanism, our results suggest that currently accepted clinical measures of HbA1c do not reflect recent past glycemia in the same way in African Americans with and without SCT, as evidenced by significantly lowerHbA1c values at the same glucose concentration in those with vs without SCT. These results could have clinically significant implications. As a screening tool, an HbA1c value that systematically underestimates long-term glucose levels may result in a missed opportunity for intervention. In the present study, using standard clinical HbA1c criteria to identify prediabetes and diabetes resulted in identifying 40% fewer cases of prediabetes and 48% fewer cases of diabetes among participants with SCT compared with those without SCT, while glucose-based methods resulted in a similar prevalence regardless of SCT status (Figure 2). The discriminative ability of HbA1c concentration to identify individuals with prediabetes or diabetes was significantly lower in those with vs those without SCT (Figure 3). These findings raise the possibility of benefit from incorporating information on hemoglobin variants into clinical guidelines for interpreting HbA1c values for screening and diagnosis of prediabetes and diabetes. Because black people typically have a higher prevalence of diabetes and experience a number of diabetic complications at higher rates than white people, the cost of inaccurately assessing risk and treatment response is high.34–36
In our study, African Americans with and without SCT had relatively high HbA1c levels with mean values greater than 5%. This is possibly due to the higher values often found in black people than in white people.37,38 Future studies that include biracial populations should further investigate HbA1c differences by race in relation to hemoglobinopathies. We also noted that individuals with SCT had a lower eGFR as was observed previously by Naik and colleagues.39 Future studies should focus on whether a possible delay in the diagnosis and treatment of prediabetes and diabetes in those with SCT could explain their lower kidney function.
Strengths of our study include the availability of detailed information on demographics, medical history, and clinical measures as well as the reproducibility of findings across 2 different versions of the Tosoh HbA1c assay (2.2 and G7) and across 2 different measures of glucose concentration (fasting and 2-hour glucose).
This study has a number of limitations. Despite pooling data from 2 large cohorts, a relatively small number of participants had SCT (367 participants). Although, to our knowledge, this is the largest study to date examining the association between HbA1c, SCT, and other glucose measures, further studies including biracial populations and other hemoglobin variants are needed to confirm and complement our findings. Second, validation of these findings with other HbA1c assays would help explain the potential mechanisms of the disparate HbA1c-glycemia association between those with and without SCT. Third, even though multiple measures of glucose were available and findings were consistent with fasting and 2-hour glucose measures, these measures cannot fully represent average glycemia over the prior 2 to 3 months, which might lower the precision of our estimates. However, our findings were robust in analyses adjusted for potential confounding variables and in various sensitivity analyses suggesting that the likelihood of such bias is low. Fourth, a large number of participants were excluded from analyses due to missing data on SCT status, HbA1c measures, or glucose measures; however, participants excluded were comparable with those in the analytic sample with the exception of a lower BMI at baseline among those excluded. In addition, no clinical outcomes were assessed in this study. Whether the findings in this study have clinical implications is unclear.
Conclusions
Among African Americans from 2 large, well-established cohorts, participants with SCT had lower levels of HbA1c at any given concentration of fasting or 2-hour glucose compared with participants without SCT. These findings suggest that HbA1c may systematically underestimate past glycemia in black patients with SCT and may require further evaluation.
Supplementary Material
Key Points.
Question
Does hemoglobin A1c reflect past glucose concentrations in a similar manner in African Americans with sickle cell trait as it does in those without sickle cell trait?
Findings
In this retrospective cohort study of 4620 African Americans, for any given fasting or 2-hour glucose concentration, individuals with sickle cell trait had significantly lower hemoglobin A1c values, 5.72% vs 6.01%, than those without sickle cell trait.
Meaning
Among African Americans with sickle cell trait, hemoglobin A1c concentration may systematically underestimate past glycemia and should be further evaluated.
Acknowledgments
Funding/Support: CARDIA is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN26820090004 from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005). The JHS is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C from the NHLBI and the National Institute on Minority Health and Health Disparities. Dr Lacy was supported by F31DK105791 (Drs Wu and Wellenius are cosponsors of this grant). Dr Wu was supported by the Center of Innovation in Long-Term Services and Support, Providence VA Medical Center. Dr Carson was supported by K01DK095928. Dr Sumner is supported by the intramural program of NIDDK and NIMHD. Dr Sacks is supported by the Intramural Research Program of the NIH. Dr Naik was supported by NHLBI grant K08HL125100. Joint calling of exome sequence data in JHS was supported by R01HL107816 to S. Kathiresan, who was the principal investigator of the ancillary study that obtained the SCT data on JHS participants.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Disclaimer: The views expressed herein are those of the authors and do not necessarily represent the views of the NHLBI; the NIH; US Department of Veterans Affairs; or the US Department of Health and Human Services.
Additional Contributions: We thank the staff and participants of the CARDIA study and the JHS for their contributions.
Author Contributions: Drs Lacy and Wu had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Lacy, Wellenius, Correa, Wilson, Jacobs, Luo, Gjelsvik, Reiner, Eaton, Wu.
Acquisition, analysis, or interpretation of data: Lacy, Wellenius, Sumner, Correa, Carnethon, Liem, Wilson, Sacks, Jacobs, Carson, Luo, Reiner, Naik, Liu, Musani, Eaton, Wu.
Drafting of the manuscript: Lacy, Correa, Luo, Eaton.
Critical revision of the manuscript for important intellectual content: Wellenius, Sumner, Correa, Carnethon, Liem, Wilson, Sacks, Jacobs, Carson, Luo, Gjelsvik, Reiner, Naik, Liu, Musani, Eaton, Wu.
Statistical analysis: Lacy, Sumner, Jacobs, Luo, Liu, Eaton.
Obtained funding: Lacy, Wellenius, Correa, Wu.
Administrative, technical, or material support: Correa, Wilson, Liu, Musani, Eaton.
Supervision: Wellenius, Sumner, Correa, Carnethon, Luo, Gjelsvik, Naik, Liu, Wu.
Role of the Funder/Sponsor: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ A1c-Derived Average Glucose Study Group. Translating the A1c assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1c. Diabetes Care. 2011;34(S2 suppl 2):S184–S190. doi: 10.2337/dc11-s216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacks DB. A1c versus glucose testing: a comparison. Diabetes Care. 2011;34(2):518–523. doi: 10.2337/dc10-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167(14):1545–1551. doi: 10.1001/archinte.167.14.1545. [DOI] [PubMed] [Google Scholar]
- 5.International Expert Committee. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NGSP. [Accessed November 29, 2016];Factors that interfere with HbA1c test results. http://www.ngsp.org/factors.asp. Updated April 6, 2016.
- 7.Centers for Disease Control and Prevention. [Accessed February 23, 2016];Sickle cell trait fact sheet. http://www.cdc.gov/ncbddd/sicklecell/traits.html.
- 8.Lorey FW, Arnopp J, Cunningham GC. Distribution of hemoglobinopathy variants by ethnicity in a multiethnic state. Genet Epidemiol. 1996;13(5):501–512. doi: 10.1002/(SICI)1098-2272(1996)13:5<501::AID-GEPI6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.McCurdy PR. 32-DFP and 51-Cr for measurement of red cell life span in abnormal hemoglobin syndromes. Blood. 1969;33(2):214–224. [PubMed] [Google Scholar]
- 10.Barbedo MMR, McCurdy PR. Red cell life span in sickle cell trait. Acta Haematol. 1974;51(6):339–343. doi: 10.1159/000208316. [DOI] [PubMed] [Google Scholar]
- 11.Suarez RM, Buso R, Meyer LM, Olavarrieta ST. Distribution of abnormal hemoglobins in Puerto Rico and survival studies of red blood cells using Cr51. Blood. 1959;14(3):255–261. [PubMed] [Google Scholar]
- 12.Weinstein IM, Spurling CL, Klein H, Necheles TF. Radioactive sodium chromate for the study of survival of red blood cells, III; the abnormal hemoglobin syndromes. Blood. 1954;9(12):1155–1164. [PubMed] [Google Scholar]
- 13.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 14.Taylor HA, Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 suppl 6):S6, S4, 17. [PubMed] [Google Scholar]
- 15. [Accessed January 29, 2016];CARDIA year 20 exam protocol. http://www.cardia.dopm.uab.edu/images/more/pdf/CARDIAY20Protocol.pdf.
- 16. [Accessed January 29, 2016];CARDIA year 25 exam protocol. http://www.cardia.dopm.uab.edu/images/more/CARDIA_Y25_Exam_Protocol_v2012-03-01.pdf.
- 17.Carpenter MA, Crow R, Steffes M, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328(3):131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller WG, Myers GL, Ashwood ER, et al. State of the art in trueness and interlaboratory harmonization for 10 analytes in general clinical chemistry. Arch Pathol Lab Med. 2008;132(5):838–846. doi: 10.5858/2008-132-838-SOTAIT. [DOI] [PubMed] [Google Scholar]
- 20.Sacks DB, Arnold M, Bakris GL, et al. National Academy of Clinical Biochemistry; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61–e99. doi: 10.2337/dc11-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Classification and dianosis of diabetes. Diabetes Care. 2016;39(suppl 1):S13–S22. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 22.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 23.Selvin E, Zhu H, Brancati FL. Elevated A1c in adults without a history of diabetes in the US. Diabetes Care. 2009;32(5):828–833. doi: 10.2337/dc08-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim C, Bullard KM, Herman WH, Beckles GL. Association between iron deficiency and A1c levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care. 2010;33(4):780–785. doi: 10.2337/dc09-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang SH, Jung J, Choi EW, Cho KH, Park JW, Do JY. HbA1c levels are associated with chronic kidney disease in a non-diabetic adult population: a nationwide survey (KNHANES 2011–2013) PLoS One. 2015;10(12):e0145827. doi: 10.1371/journal.pone.0145827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardin JW. Generalized Estimating Equations (GEE): Encyclopedia of Statistics in Behavioral Science. Hoboken, NJ: John Wiley & Sons Ltd; 2005. [Google Scholar]
- 27.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 28.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 29.Gonen M. Analyzing Receiver Operating Characteristic Curves With SAS. Cary, NC: SAS Institute Inc; 2007. [Google Scholar]
- 30.McClish DK. Comparing the areas under more than two independent ROC curves. Med Decis Making. 1987;7(3):149–155. doi: 10.1177/0272989X8700700305. [DOI] [PubMed] [Google Scholar]
- 31.Lin CN, Emery TJ, Little RR, et al. Effects of hemoglobin C, D, E, and S traits on measurements of HbA1c by six methods. Clin Chim Acta. 2012;413(7–8):819–821. doi: 10.1016/j.cca.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bleyer AJ, Vidya S, Sujata L, et al. The impact of sickle cell trait on glycated haemoglobin in diabetes mellitus. Diabet Med. 2010;27(9):1012–1016. doi: 10.1111/j.1464-5491.2010.03050.x. [DOI] [PubMed] [Google Scholar]
- 33.Sumner AE, Thoreson CK, O’Connor MY, et al. Detection of abnormal glucose tolerance in Africans is improved by combining A1c with fasting glucose: the Africans in America Study. Diabetes Care. 2015;38(2):213–219. doi: 10.2337/dc14-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 35.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287(19):2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010;304(6):649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziemer DC, Kolm P, Weintraub WS, et al. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010;152(12):770–777. doi: 10.7326/0003-4819-152-12-201006150-00004. [DOI] [PubMed] [Google Scholar]
- 38.Kirk JK, D’Agostino RB, Jr, Bell RA, et al. Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care. 2006;29(9):2130–2136. doi: 10.2337/dc05-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naik RP, Derebail VK, Grams ME, et al. Association of sickle cell trait with chronic kidney disease and albumin uria in African Americans. JAMA. 2014;312(20):2115–2125. doi: 10.1001/jama.2014.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.