Abstract
Two novel scaffolds, 4-pyridylanilinothiazoles (PAT) and 3-pyridylphenylsulfonyl benzamides (PPB), previously identified as selective cytotoxins for von Hippel–Lindau-deficient Renal Carcinoma cells, were used as templates to prepare affinity chromatography reagents to aid the identification of the molecular targets of these two classes. Structure–activity data and computational models were used to predict possible points of attachment for linker chains. In the PAT class, Click coupling of long chain azides with 2- and 3-pyridylanilinothiazoleacetylenes gave triazole-linked pyridylanilinothiazoles which did not retain the VHL-dependent selectivity of parent analogues. For the PPB class, Sonagashira coupling of 4-iodo-(3-pyridylphenylsulfonyl)benzamide with a propargyl hexaethylene glycol carbamate gave an acetylene which was reduced to the corresponding alkyl 3-pyridylphenylsulfonylbenzamide. This reagent retained the VHL-dependent selectivity of the parent analogues and was successfully utilized as an affinity reagent.
Keywords: Click chemistry, Sonogashira cross coupling, Renal cell carcinoma, Von Hippel–Lindau factor, GLUT-1
1. Introduction
Identification of tumour-selective agents is a top priority in anticancer drug development. One approach is to leverage a genetic abnormality common to a particular tumour type and to identify agents that are selectively cytotoxic to tumour cells with this genetic abnormality. We recently used such a synthetic lethality approach1–3 to discover two novel chemotypes that were selectively cytotoxic to Renal Cell Carcinoma (RCC) cells lacking the von Hippel Lindau factor (VHL) both in vitro and in vivo.4,5
RCCs are refractory to standard chemo- and radiotherapy and advanced RCC has an extremely poor prognosis.6 Although new ‘targeted’ anti-angiogenic agents such as sunitinib and sorafinib have been approved for use against the highly vascularised advanced RCC, these agents provide limited efficacy and patients eventually relapse and succumb to their disease. Thus, there is still a cogent need for drugs with increased efficacy in the treatment of advanced RCC. Common to a majority of RCCs is the loss of function of the von Hippel–Lindau (VHL) tumour suppressor gene.7 The VHL protein regulates a variety of proteins,8 including the activity of the Hypoxia Inducible Factor (HIF) family of transcription factors, by targeting them for degradation. Loss of this control increases HIF activity and increases transcription of a wide range of genes.9 This genetic response mimics the impact of tumour hypoxia and promotes reprogramming of tumor metabolism, progression, invasion, and metastasis, resulting in an aggressive phenotype, poor prognosis and resistance to therapeutic agents,10,11 and so the VHL-deficient RCC cell line also provides a model of tumour cells under hypoxic stress.
The two chemotypes identified in our synthetic lethal screen, 4-pyridylanilinothiazoles (PAT) (1) and 3-pyridylphenylsulfonyl benzamides (PPB) (2) (Fig. 1) displayed selective cytotoxicity for VHL-deficient RCC4 cells compared to RCC/VHL VHL-proficient cells (Table 1) but displayed different phenotypic behaviour. In the first case, PAT cytotoxicity was independent of HIF-1 status. PAT compounds induced autophagy, as measured by LC3 immunostaining and Western blot analysis, and this led to cell death. Functional analysis of the activity of the PAT class using a yeast deletion pool implicated proteins involved in Golgi body processing as important in the induction of autophagy, but failed to unequivocally identify the target protein(s) of the PATs.4
Figure 1.
PAT and PPB chemotypes identified from RCC HTS.
Table 1.
IC50 values and selectivity ratios in Renal Cell Carcinoma cells
| No | RCC4 IC50 (µM) | RCC4/VHL IC50 (µM) | Ratioa |
|---|---|---|---|
| 1b | 2.1 | 40 | 19 |
| 2 | 0.16 | >40c | >250 |
| 7 | 1.7 | 2.6 | 1.5 |
| 8 | >40 | >40 | ND |
| 11 | >40 | >40 | ND |
| 16 | 2.9 | 3.9 | 1.3 |
| 19 | >40 | >40 | ND |
| 30 | 5.8 | >40 | >7 |
| 32 | 7.9 | >40 | >5 |
| 38 | >40 | >40 | ND |
Ratio = IC50 (RCC4/VHL)/IC50 (RCC4).
Data from Ref. 15.
Solubility prevented determination of IC50 values.
In contrast, PPB cytotoxicity was dependent on HIF-1 status and resulted in necrotic cell death. The PPBs decreased glycolysis in a VHL-dependent manner and inhibited the uptake of glucose.5
Further development of these novel chemotypes into viable anticancer agents is critically dependent on the identification of the molecular target of action. In this study we report our synthetic efforts to use structure activity relationships (SAR), in combination with molecular design, to develop chemical biology tools suitable for use in an affinity chromatography approach12 for target identification for both PAT and PPB chemotypes.
2. Molecular design
We expanded on the initial hit compound 1 and explored the SAR for the PAT chemotype to identify more potent and selective analogues, but were hampered in this by the lack of an identified molecular target.13 This handicap led us to use a comparative molecular field analysis (CoMFA) to determine possible bioactive conformations to aid our studies. We identified a positive steric contour (Fig. 2, green volume) adjacent to the pyridine ring as a potential feature for further analogue development and this feature was further explored, along with the orientation of the thiazole B-ring and the roles of the heteroatom substituents, in a subsequent study.14 We used Suzuki–Miyaura and Sonogashira Pd-mediated cross coupling reactions and nucleophilic displacement reactions to prepare series of aryl-, alkynyl-, alkoxy- and alkylamino-substituted pyridines, respectively, to explore the steric and electronic contours adjacent to the pyridyl ring. Although we failed to improve the predictivity of the CoMFA model, we did identify several analogues with increased potency and/or selectivity to 1. The CoMFA model predicts the 2- and 3-positions of the pyridine ring were the most favorable for functionalization with a variety of groups at these positions providing potent and selective compounds. Although, two acetylenic compounds displayed selectivity for VHL-negative cells, a lack of selectivity for higher homologues dissuaded us from using an acetylene linker. Either pyrazole or triazole groups in the 2-position provided similar or improved potency and selectivity compared to the parent 1 [IC50 (RCC4) ca. 2 µM, selectivity (RCC4VHL/RCC4), 6 to 30-fold)14 and suggested a ‘Click’15 strategy to incorporate a long chain linker suitable for affinity chromatography.
Figure 2.
CoMFA model 3D-QSAR conformer model relating PAT conformation to cytotoxicity. Electrostatic fields are contoured at 80% favored (blue) and 20% disfavored (red). Steric fields are contoured at 80% favored (green) and 20% disfavored (yellow).
Our SAR studies on the PPB chemotype identified considerable steric tolerance at the 4-position of the phenyl sulfonamide16 These data suggested molecules with a long chain linker attached to this position may retain activity and may provide a plausible strategy for attachment of linkers required for affinity reagents. The overexpression of the facultative glucose transporter GLUT-1 in VHL-deficient RCC cells, combined with evidence for inhibition of glucose uptake, led us to consider the possibility that the cytotoxicity of the PPBs was mediated through an interaction with GLUT-1.5 We were able to use a homology model of GLUT-117 to identify a binding mode consistent with the SAR (Fig. 3) and our approach to position a long chain linker at the 4-position.
Figure 3.
A representation of PPB S3 (white ball and stick) and fasentin (blue stick) modelled in the central transport channel of GLUT-1. Possible interactions with Trp412 and Arg126 are shown.
3. Results
3.1. Chemistry
3.1.1. PAT synthesis
We elaborated the 2-acetylene PATs using ‘Click’ chemistry to generate a substituted triazole. We had previously demonstrated that Sonogashira cross coupling reaction of bromide 3 with TMS acetylene in the presence of PdCl2(PPh3)2 and CuI as catalysts gave an intermediate silylacetylene which was deprotected to give acetylene 4 in 75% yield (Scheme 1). Formation of the benzyltriazole 5 from acetylene 4 and benzyl azide also proceeded in good yield. Incorporation of a long chain alkyl linker using Click chemistry was initially investigated for a BOC-protected aminohexylazide. Reaction of tert-butyl (6-azidohexyl)carbamate with acetylene 4 gave triazole 6 in 39% yield which was deprotected to give amine 7 suitable for loading onto beads using N-hydroxysuccinimide coupling chemistry. We also wished to incorporate a longer polyethylene glycol chain as a linker and used the commercially available 20-azido-3,6,9,12,15,18-hexaoxaeicosan-1-amine in a Click reaction with acetylene 4 to give triazole 8 in 20% yield.
Scheme 1.
Reagents and conditions: (a) (i) TMS-acetylene, PdCl2(PPh3)2, CuI, NEt3, DMF, 50 °C; (ii) K2CO3, THF/MeOH, 20 °C; (b) azide, CuSO4·5H2O, Na ascorbate, DCM/H2O/EtOH, 20 °C; (c) TFA/DCM, 20 °C; (d) EtI, DMF, 20 °C.
We had shown the aminothiazole NH is necessary for biological activity and so designed an appropriate negative control (Scheme 1). Alkylation of bromide 3 with ethyl iodide in DMF gave 9 in 66% yield. Sonogashira cross coupling reaction with TMS acetylene using PdCl2(PPh3)2 and CuI in DMF/NEt3 gave, after deprotection of the TMS group using potassium carbonate in THF/MeOH, the acetylene 10. Click reaction with 20-azido-3,6,9,12,15,18-hexaoxaeicosan-1-amine gave triazole product 11 in 71% yield.
We also explored the positive steric contour adjacent to the pyridyl ring via the 3-pyridyl position, although this proved more challenging due to the low reactivity at this position. The 3-bromopyridyl analogue 12 underwent Sonogashira cross-coupling reaction with TMS acetylene with subsequent deprotection of the TMS group to give the 3-acetylene 13 in low (10–35%) yields (Scheme 2). In an alternative strategy, 3-bromopyridine 12 was converted to the corresponding 3-iodopyridine 14 by a copper-catalyzed halogen exchange reaction. Sonogashira reaction with the iodide 14 gave acetylene 13 in 52% yield. Since this route adds an additional step and the overall yield was not significantly higher, it was not adopted.
Scheme 2.
Reagents and conditions: (a) (i) TMS-acetylene, PdCl2(PPh3)2, CuI, NEt3, DMF, 50 °C; (ii) K2CO3, THF/MeOH, 20 °C; (b) CuI, NaI, MeNH(CH2)2NHMe, dioxane, 110 °C; (c) CuSO4·5H2O, Na ascorbate, DCM/H2O/DMF, 20 °C, 20 h; (d) azide, CuSO4·5H2O, Na ascorbate, TBTA, DCM/H2O/DMF, 60 °C, 20 h; (e) EtI, DMF, 20 °C.
We had previously demonstrated Click reaction of 3-acetylene 13 with benzyl azide gave benzyl triazole 15 in 55% yield.14 In this instance, Click reaction of acetylene 13 with 20-azido-3,6,9,12,15,18-hexaoxaeicosan-1-amine using CuSO4·5H2O and sodium ascorbate at room temperature, or at 40 °C, gave no product. However, addition of tris(benzyltriazolyl)amine (TBTA) ligand18 and increased reaction temperature (60 °C) gave the triazole PAT derivative 16 in 20% yield.
A negative control was also prepared for the 3-pyridyl isomer based on the observation that N-alkylation of the aniline abolished activity against RCC4 cells.13 Alkylation of 3-bromopyridine 12 with ethyl iodide in DMF gave 17 in 60% yield. Sonogashira cross-coupling reaction with TMS acetylene and subsequent deprotection of the TMS group gave 3-acetylene 18 in 65% yield. Finally, Click reaction in the presence of the TBTA ligand at 60 °C gave triazole product 19 in 41% yield.
3.1.2. PPB synthesis
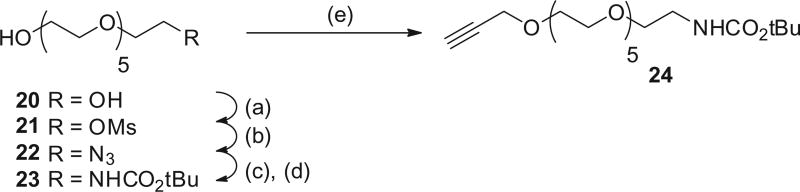
We were able to readily prepare the propargyl-PEG6-carbamate 2419 efficiently from commercially available hexaethylene glycol 20 (Scheme 3). Glycol 20 was selectively mono-mesylated using mesyl chloride in the presence of silver oxide. Treatment of the mesylate 21 with sodium azide gave azide 22 in 99% yield. Reduction of 22 gave the intermediate amine which was protected as the carbamate 23 in 80% yield. Reaction of alcohol 23 with propargyl bromide gave the acetylene 24.
Scheme 3.
Reagents and conditions: (a) MsCl, Ag2O, DCM; (b) NaN3, DMF; (c) H2 (60 psi), Pd/C, EtOH; (d) BOC2O, DCM; (e) MsCl, Et3N, DCM; (f) HCl/MeOH; (g) NaH, propargyl bromide, nBu4N+I−, THF.
Condensation of the protected benzoic acid 2520 with 3-aminopyridine gave the amide 26 which was deprotected and coupled to 4-iodophenylsulfonyl chloride to give the iodide 28 (Scheme 4). Sonogashira coupling was used to attach long chain linkers to the PPB core structure. Thus, reaction of 28 with propargyl-PEG6-carbamate 24 gave the protected acetylene 29 which was readily deprotected to give amine 30.We also explored a more flexible linker with reduction of the acetylene 29 giving carbamate 31 which was deprotected to give amine 32. The negative control reagent was prepared using a phenyl replacement for the 3-pyridyl moiety based on the previously determined SAR which demonstrates increased potency for 3-pyridyl analogues.16 Thus in a similar sequence, coupling of benzoic acid 25 with aniline gave the amide 33 which was deprotected and coupled with 4-iodophenylsulfonyl chloride to give iodide 35. Sonogashira coupling with 24 gave the acetylene 36 which was reduced and deprotected to give amine 38.
Scheme 4.
Reagents and conditions: (a) (COCl)2, DMF, THF, 50 °C, then 3-aminopyridine or aniline; (b) HBr, HOAc, 20 °C; (c) 4-IPhSO2Cl, pyridine, 20 °C (d) PdCl2(PPh3)2, 24, CuI, Et3N, DMF, 50 °C; (e) HCl, MeOH, 20 °C; (f) H2, Pd/C, EtOH, 20 °C; (g) TFA, DCM, 20 °C.
3.2. Biological assays
The potency and selectivity of the potential affinity reagents were evaluated in a XTT growth inhibition assay (as IC50: the drug concentration over a 4 d exposure required to inhibit cell growth by 50%) in the RCC4 VHL negative and the RCC/VHL positive cell lines (Table 1) to identify reagents with sufficient selectivity for target identification studies. Unfortunately, addition of long chain triazole substituents at the 2-position of the PAT scaffold was accompanied by loss of selectivity (7) or loss of potency (8 and 11). This was mirrored for analogues with long chain amines at the 3-position (16 and 19) and these negative results halted our efforts with this class. In contrast, the PPB reagents 30 and 32 retained potency and selectivity, although somewhat attenuated, for the RCC4 cell line, whereas the negative control 38 was inactive as required. Compounds 32 and 38 were conjugated to Affigel-10 resins and used for affinity chromatography on RCC4 cell homogenates using antibodies against GLUT-1, GLUT-2 and GLUT-3 for identification.5 This demonstrated selective retention of GLUT-1 protein by reagent 32, but not reagent 38, confirming GLUT-1 as a molecular target of the PPB chemotype.
4. Discussion
One of the risks of using phenotypic screens for drug discovery is that a molecular target for any hits may not be apparent. Not only does this hinder the ‘hit-to-lead’ development of the class, it undermines any potential clinical development of the agents. Here we have attempted to design chemical biology tools to assist in the identification of two hits identified from the same phenotypic screen. The two classes of compound had quite different properties with the PAT class inducing cell death via autophagy in a VHL-dependent manner and the PPB class inducing necrotic cell death via disruption of glucose metabolism in a HIF-dependent manner. Our pursuit of chemical biology tools to tease out the molecular targets was guided by established SAR and interpretation using molecular modelling. The CoMFA model developed from the SAR of the PAT class had demonstrated a positive steric contour adjacent to the pyridyl ring, but attempts to exploit this putative space gave mixed results. Our efforts to build on analogues bearing pyrazole or triazole substituents at the 2-position were not successful and none of the reagents prepared for the PAT class showed any selectivity for VHL-negative cells. That the CoMFA model could account for only ca 34% of the variation in cytotoxicity data may contribute to our inability to identify PAT reagents with selectivity for VHL-deficient cells.
On the other hand we had accumulated evidence suggesting that glucose metabolism was the target process for the PPB class and this provided a limited number of molecular targets. This was further clarified through glucose uptake experiments which demonstrated that the PPB class did inhibit glucose uptake.5 The biochemical and genetic evidence suggested that GLUT-1 was a potential molecular target and this was confirmed using the PPB affinity chromatography reagents.5 Subsequently, we modelled a series of ‘active’ PPB analogues and the known GLUT-1 inhibitor fasentin21 in the transporter’s central channel to explore potential interactions between the PPB scaffold and GLUT-1. The GLUT-1 homology model (PDB No. 1SUK) had been generated using the glycerol 3-phosphate antiporter GlpT structure as an initial homology template followed by evolutionary homology using glucose-6-phosphate translocase as a template. The resulting structure accounted for the biochemical and mutagenic evidence, as well as identifying binding sites for glucose and several inhibitors.21 The active compounds (S1–S6; Supporting Information) were selected from those displaying <1 µM IC50 values against the RCC4 cell line and a selectivity for the RCC4 over the RCC4/VHL cell line of >100. Some predicted binding modes positioned the 3-pyridyl moiety deep into the central channel towards the extracellular end, occupying a position similar to that predicted for fasentin (Fig. 3). In this orientation, the amide carbonyl group occupies a similar location to amide carbonyl in fasentin, with both groups potentially able to interact with the side chain of Arg126, a residue involved in glucose transport.22 In this mode, the ligand’s central aromatic group is located between the channel aromatic residues Tyr28, Phe72 and Trp412. Additionally, the sulfonyl group may also interact with the indole nitrogen of Trp412, another residue associated with glucose transport.23 The various substituents at the 4-phenyl position were oriented toward the intracellular entrance of the channel, and the tolerance observed for various substituents provides the rationale for appending a long chain linker group at the 4-phenyl position. Other predicted binding modes indicated the potential for an interaction between the ligands pyridyl N and the side chain amine of Lys38. Such an interaction may explain loss of potency and selectivity seen for the phenyl derivative 38. Although the side chain positions were not optimized for ligand binding in the GLUT1 homology model, the data indicate the potential for the active compounds to interact with several key residues that span the solute channel. The proposed binding mode appears to occupy a similar site predicted for both the recently reported thiazolidine 2,4-diones24 and diphenoxybenzoates25–27 that inhibit glucose uptake through binding to GLUT-1. In particular, the thiazolidine 2,4-diones are predicted to make an electrostatic interaction with Trp412 and Arg126 as well as form a π–π stacking interaction with Tyr2824 Similarly, the diphenoxybenzoate WZB114 was modelled binding to Arg126 and Trp412, as well as Asn34. While these interactions remain to be formally tested, it is possible that the hydrophobic and polar groups contributed by these amino acids define a tractable drug target within the solute channel of GLUT-1, and these may provide an opportunity for the identification of new GLUT-1 inhibitors.
This study highlights the successful design and synthesis of chemical biology tools to identify the molecular target of the PPB class, while also showing that success in these endeavours is not always guaranteed. Having identified the molecular target of the PPBs we are now poised to further develop this class towards a tumour-selective anti-tumour agent. The potential opportunity afforded by these agents is increasingly recognised as a novel therapeutic strategy for the treatment of cancer.28,29
5. Experimental
5.1. Chemistry
5.1.1. General procedures
Analyses were carried out in the Campbell Microanalytical Laboratory, University of Otago, Dunedin, NZ. All final products were analysed by reverse-phase HPLC, (Altima C18 5 µm column, 150 × 3.2 mm; Alltech Associated, Inc., Deerfield, IL) using an Agilent HP1100 equipped with a diode-array detector. Mobile phases were gradients of 80% acetonitrile/20% H2O (v/v) in 45 mM ammonium formate at pH 3.5 and 0.5 mL/min. Final compound purity was determined by monitoring at 330 ± 50 nM and was >95%. Melting points were determined on an Electrothermal 2300 Melting Point Apparatus. NMR spectra were obtained on a Bruker Avance 400 spectrometer at 400 MHz for 1H and 100 MHz for 13C spectra. Spectra were obtained in (CD3)2SO unless otherwise specified, and were referenced to Me4Si. Chemical shifts and coupling constants were recorded in units of ppm and Hz, respectively. Assignments were determined using COSY, HSQC, and HMBC two-dimensional experiments. Low resolution mass spectra were gathered by direct injection of methanolic solutions into a Surveyor MSQ mass spectrometer using an atmospheric pressure chemical ionization (APCI) mode with a corona voltage of 50 V and a source temperature of 400 °C. High resolution mass spectra (HRMS) were measured on a Bruker microTOF-QII Hybrid Quadrupole Time of Flight (TOF-Q) mass spectrometer interfaced with either an Electrospray Ionization (ESI) or Atmospheric Pressure Chemical Ionization (APCI) probe allowing positive or negative ions detection. Solutions in organic solvents were dried with anhydrous MgSO4. Solvents were evaporated under reduced pressure on a rotary evaporator. Thin-layer chromatography was carried out on aluminium-backed silica gel plates (Merck 60 F254) with visualization of components by UV light (254 nm) or exposure to I2. Column chromatography was carried out on silica gel (Merck 230–400 mesh).
Compounds 3, 4, 5, 12, 13, 14 and 15 were prepared as previously described.14,15
5.1.2. tert-Butyl 6-(4-{4-[2-(3-toluidino)-1,3-thiazol-4-yl]-2-pyridinyl}-1H-1,2,3-triazol-1-yl)hexylcarbamate (6)
CuSO4·5H2O (16 mg, 0.064 mmol) and sodium ascorbate (26 mg, 0.13 mmol) were added to a solution of acetylene 4 (185 mg, 0.64 mmol) and tert-butyl 6-azidohexylcarbamate (160 mg, 0.70 mmol) in a mixture of DCM/H2O/EtOH (1:1:1, 3 mL). The reaction was stirred at 18 °C for 24 h and was then partitioned between EtOAc (150 mL) and H2O (50 mL). The organic phase was washed with H2O (2 × 10 mL), dried and the solvent was evaporated. The residue was purified by column chromatography, eluting with 2% MeOH/DCM, to give triazole 6 (140 mg, 42%) as a colourless viscous oil: 1H NMR δ 10.31 (s, 1H, NH), 8.62–8.63 (m, 2H, H-5′, H-6″), 8.54 (dd, J = 1.6, 0.7 Hz, 1H, H-3″), 7.80 (dd, J = 5.1, 1.6 Hz, 1H, H-5″), 7.79 (s, 1H, H-5‴), 7.57 (br s, 1H, H-2″ ″), 7.51 (br d, J = 8.1 Hz, 1H, H-6″ ″), 7.25 (t, J = 7.8 Hz, 1H, H-5″ ″), 6.83 (d, J = 7.4 Hz, 1H, H-4″ ″), 6.72 (br s, 1H, NHBoc), 4.43 (t, J = 7.0 Hz, 2H, H-6), 2.89 (q, J = 6.3 Hz, 2H, H-1), 2.35 (s, 3H, CH3), 1.84–1.92 (m, 2H, CH2), 1.36 [s, 11H, C(CH3)3, CH2], 1.27–1.28 (m, 4H, CH2); 13C NMR δ 163.6, 155.5, 150.8, 150.1, 147.6, 147.2, 142.0, 140.9, 138.2, 128.9, 123.2, 122.3, 118.9, 117.7, 115.5, 114.2, 107.7, 77.3, 49.5, 29.6, 29.2, 28.2 (3), 25.6, 25.5, 21.3 (1C not observed); MS m/z 535.5 (MH+, 100%). Anal. Calcd for C28H35N7 O2S·⅓H2O: C, 62.31; H, 6.66; N, 18.17. Found: C, 62.39; H, 6.55; N, 17.94.
5.1.3. 6-(4-{4-[2-(3-Methylphenyl)-1,3-thiazol-4-yl]-2-pyridinyl}-1H-1,2,3-triazol-1-yl)-1-hexanammonium trifluoroacetate (7)
TFA (0.44 mL, 5.8 mmol) was added to a solution of carbamate 6 (125 mg, 0.23 mmol) in DCM (15 mL), and the reaction mixture was stirred at 18 °C for 3 h. The solvent was evaporated and the residue was dried under high vacuum. The residue was triturated with iPr2O, filtered and dried to give the trifluoracetate salt 7 (130 mg, 100%) as an orange solid: mp (iPr2O) 179–181 °C; 1H NMR δ 10.33 (s, 1H, NH), 8.68 (s, 1H, H-5‴), 8.64 (br d, J = 5.3 Hz, 1H, H-6″), 8.57 (d, J = 0.9 Hz, 1H, H-3″), 7.85 (dd, J = 5.3, 1.7 Hz, 1H, H-5″), 7.84 (s, 1H, H-5), 7.67 (br s, 3H, NH3), 7.58 (br s, 1H, H-2′), 7.51 (br d, J = 8.1 Hz, 1H, H-6′), 7.25 (t, J = 7.8 Hz, 1H, H-5′), 6.83 (d, J = 7.5 Hz, 1H, H-4′), 4.47 (t, J = 6.9 Hz, 2H, H-1″ ″); 13C NMR δ 163.7, 158.1 (q, J = 35.0 Hz), 150.1, 149.5, 147.4, 146.6, 142.6, 140.9, 138.3, 128.9, 123.5, 122.4, 119.1, 117.7, 115.7, 114.3, 108.4, 49.5, 29.3, 26.7, 25.3, 25.1 (2), 21.3; MS m/z 435.2 (MH+, 100%). Anal. Calcd for C28H35N7O2S·2.25CF3CO2H: C, 47.86; H, 4.27; N, 14.21. Found: C, 47.98; H, 4.37; N, 14.13.
5.1.4. 4-{2-[1-(20-Amino-3,6,9,12,15,18-hexaoxaicos-1-yl)-1H-1,2,3-triazol-4-yl]-4-pyridinyl}-N-(3-methylphenyl)-1,3-thiazol-2-amine (8)
CuSO4·5H2O (5 mg, 0.02 mmol) and sodium ascorbate (8 mg, 0.04 mmol) were added to a suspension of acetylene 4 (50 mg, 0.17 mmol) and 20-azido-3,6,9,12,15,18-hexaoxaeicosan-1-amine (60 mg, 0.17 mmol) in a mixture of DCM/H2O/EtOH (1:1:1; 1.5 mL), and the reaction mixture was stirred at 18 °C for 20 h. The mixture was then diluted with EtOAc (100 mL) and washed with H2O (3 × 10 mL), washed with brine (10 mL), dried and the solvent was evaporated. The residue was purified by column chromatography, eluting with 0.5% NH4OH/1.5% MeOH/EtOAc, to give triazole 8 (20 mg, 18%) as a tan glass: 1H NMR δ 10.32 (br s, 1H, NH), 8.63 (dd, J = 5.2, 0.6 Hz, 1H, H-6″), 8.58 (s, 1H, H-5‴), 8.55 (dd, J =1.6, 0.7 Hz, 1H, H-3″), 7.78–7.81 (m, 2H, H-5, H-5″), 7.58 (s, 1H, H-2′), 7.50 (br d, J = 8.1 Hz, 1H, H-6′), 7.25 (t, J = 7.8 Hz, 1H, H-5′), 6.83 (d, J = 7.5 Hz, 1H, H-4′), 6.62 (br s, 2H, NH2), 4.63 (t, J = 5.2 Hz, 2H, H-1), 3.90 (t, J = 5.2 Hz, 2H, H-2), 3.56 (m, 2H, OCH2), 3.49–3.52 (m, 2H, OCH2), 3.44–3.48 (m, 16 H, OCH2), 3.33 (t, J = 5.8 Hz, 2H, H-19), 2.63 (br t, J = 5.4 Hz, 2H, H-20), 2.35 (s, 3H, CH3); 13C NMR δ 163.6, 150.7, 150.1, 147.6, 147.2, 142.0, 140.9, 138.2, 128.9, 123.7, 122.3, 118.9, 117.7, 115.5, 114.2, 107.7, 72.8, 69.7 (2), 69.7 (4), 69.6 (2), 69.5 (2), 68.6, 49.6, 41.2, 21.3; MS m/z 642.1 (MH+, 100%); HRMS (ESI) Calcd for C31H44N7O6S (MH+) m/z 642.3068; found m/z 642.3067.
5.1.5. 4-(2-Bromo-4-pyridinyl)-N-ethyl-N-(3-methylphenyl)-1,3-thiazol-2-amine (9)
K2CO3 (97 mg, 0.7 mmol) and EtI (56 µL, 0.7 mmol) were added to a stirred solution of bromopyridine 3 (200 mg, 0.58 mmol) in anhydrous DMF (3 mL) and the reaction mixture was stirred at 18 °C for 20 h. The mixture was partitioned between EtOAc (100 mL) and H2O (50 mL). The organic phase was washed with H2O (3 × 20 mL), brine (20 mL), dried and the solvent was evaporated. The residue was purified by column chromatography, eluting with 20% EtOAc/pet. ether, to give amine 9 (142 mg, 66%) as a colourless oil which was used directly: 1H NMR δ 8.38 (dd, J = 5.1, 0.4 Hz, 1H, H-6′), 8.02 (d, J = 0.8 Hz, 1H, H-3″), 7.85 (dd, J = 5.1, 1.4 Hz, 1H, H-5″), 7.64 (s, 1H, H-5), 7.40 (t, J = 7.7 Hz, 1H, H-5′), 7.29 (br s, 1H, H-2′), 7.26 (br d, J = 7.8 Hz, 1H, H-6′), 7.20 (br d, J = 7.5 Hz, 1H, H-4′), 4.02 (q, J = 7.1 Hz, 2H, CH2CH3), 2.36 (s, 3H, CH3), 1.22 (t, J = 7.1 Hz, 3H, CH2CH3); MS m/z 374.8/376.8 (MH+, 100%).
5.1.6. N-Ethyl-4-(2-ethynyl-4-pyridinyl)-N-(3-methylphenyl)-1,3-thiazol-2-amine (10)
PdCl2(PPh3)2 (21 mg, 0.03 mmol) was added to a purged solution of bromopyridine 9 (203 mg, 0.54 mmol), TMS-acetylene (0.43 mL, 3.0 mmol), and CuI (6 mg, 0.03 mmol) in a mixture of DMF and NEt3 (1:1; 4 mL), and the reaction mixture was stirred at 50 °C for 2 h. The reaction mixture was cooled to 18 °C, and partitioned between EtOAc (200 mL) and H2O (50 mL). The organic phase was washed with brine (50 mL), dried and the solvent was evaporated. The residue was dissolved in a mixture of THF/MeOH (1:1; 20 mL), K2CO3 (75 mg, 0.54 mmol) was added and the mixture was stirred at 18 °C for 1 h. The solvent was evaporated and the residue purified by column chromatography, eluting with 20% EtOAc/pet. ether, to give acetylene 10 (145 mg, 84%) as a colourless oil which was used directly: 1H NMR δ 8.56 (dd, J = 5.2, 0.7 Hz, 1H, H-6″), 7.96 (dd, J = 1.6, 0.7 Hz, 1H, H-3″), 7.82 (dd, J = 5.2, 1.7 Hz, 1H, H-5″), 7.59 (s, 1H, H-5), 7.39 (t, J = 7.7 Hz, 1H, H-5′), 7.29 (br s, 1H, H-2′), 7.25 (br d, J = 7.7 Hz, 1H, H-6′), 7.19 (br d, J = 7.6 Hz, 1H, H-4′), 4.32 (s, 1H, C≡CH), 4.03 (q, J = 7.1 Hz, 2H, CH2CH3), 2.35 (s, 3H, CH3), 1.22 (t, 3H, CH2CH3); 13C NMR δ 169.2, 150.6, 147.0, 144.0, 142.2, 141.9, 139.7, 129.9, 128.2, 127.1, 123.8, 123.3, 119.8, 107.7, 83.3, 80.0, 47.2, 20.8, 12.7; MS m/z 320.7 (MH+, 100%).
5.1.7. 4-{2-[1-(20-Amino-3,6,9,12,15,18-hexaoxaicos-1-yl)-1H-1,2,3-triazol-4-yl]-4-pyridinyl}-N-ethyl-N-(3-methylphenyl)-1,3-thiazol-2-amine (11)
CuSO4·5H2O (10 mg, 0.04 mmol) and sodium ascorbate (16 mg, 0.08 mmol) were added to a suspension of acetylene 10 (120 mg, 0.37 mmol) and 20-azido-3,6,9,12,15,18-hexaoxaeicosan-1-amine (72 mg, 0.21 mmol) in a mixture of DCM/H2O/EtOH (1:1:1; 3 mL), and the reaction mixture was stirred at 18 °C for 20 h. The mixture was diluted with 20% MeOH/DCM (100 mL), SiO2 was added and the solvent was evaporated. The residue was purified by column chromatography, eluting with 0.5% NH4OH (28%)/1.5% MeOH/ EtOAc, to give triazole 11 (90 mg, 71%) as a tan oil: 1H NMR δ 8.59 (dd, J = 5.2, 0.7 Hz, 1H, H-6″), 8.58 (s, 1H, H-5‴), 8.46 (dd, J = 1.6, 0.7 Hz, 1H, H-3″), 7.74 (dd, J = 5.2, 1.7 Hz, 1H, H-5″), 4.58 (s, 1H, H-5), 7.40 (t, J = 7.7 Hz, 1H, H-5′), 7.31 (br s, 1H, H-2′), 7.27 (br d, J = 7.8 Hz, 1H, H-6′), 7.20 (br d, J = 7.6 Hz, 1H, H-4′), 4.62 (t, J = 5.2 Hz, 2H, H-1″ ″), 4.07 (q, J = 7.0 Hz, 2H, CH2CH3), 3.89 (t, J = 5.2 Hz, 2H, H-2″ ″), 3.56 (m, 2H, OCH2), 3.45–3.51 (m, 18 H, OCH2), 3.34 (t, J = 5.8 Hz, 2H, H-19″ ″), 2.64 (t, J = 5.8 Hz, 2H, H-20″ ″), 2.36 (s, 3H, CH3), 1.25 (t, J = 7.0 Hz, 3H, CH2CH3), NH2 not observed; 13C NMR δ 169.2, 150.7, 150.0, 147.9, 147.2, 144.1, 142.2, 139.7, 129.8, 128.1, 127.1, 123.75, 123.71, 119.0, 115.4, 107.1, 72.8, 69.73 (3), 69.72 (4), 69.6 (2), 69.5, 68.6, 49.6, 47.1, 41.2, 20.8, 12.8; MS m/z 672.4 (MH+, 100%); HRMS Calcd for C33H48N7O6S (MH+) m/z 670.3381; found m/z 670.3394.
5.1.8. 20-(4-{4-[2-(3-Methylphenyl)-1,3-thiazol-4-yl]-3-pyridinyl}-1H-1,2,3-triazol-1-yl)-3,6,9,12,15,18-hexaoxaicosan-1-ammonium trifluoroacetate (16)
CuSO4·5H2O (4 mg, 0.09 mmol), sodium ascorbate (6 mg, 0.03 mmol) and TBTA (16 mg, 0.03 mmol) were added to a suspension of acetylene 13 (42 mg, 0.15 mmol) and 20-azido-3,6,9,12,15,18-hexaoxaeicosan-1-amine (32 mg, 0.09 mmol) in a mixture of DMF/H2O (1:1; 2 mL) and the reaction mixture was stirred at 18 °C for 3 h and then at 60 °C for 16 h. Additional acetylene 13 (30 mg, 0.1 mmol) in DCM (1 mL) was added and the mixture was heated at 60 °C for 1 h, at which time all the starting azide has been consumed. The reaction mixture was diluted with MeOH (15 mL), SiO2 (1 g) was added and the solvent was evaporated. The residue was purified by column chromatography, eluting with 0.5% aq NH3 (25%)/1.5% MeOH/EtOAc, to give crude amine 16 (35 mg, 66%). The product was further purified by reverse phase preparative HPLC [gradient (90–2–90%) (H2O/TFA pH 2.56)/(90% CH3CN/H2O)] to give amine 16 as the trifluoroacetate salt (18 mg, 26%) as a tan oil: 1H NMR δ 10.19 (s, 1H, NH), 8.84 (s, 1H, H-2″), 8.69 (d, J = 5.1 Hz, 1H, H-6″), 7.99 (s, 1H, H-5′), 7.83 (d, J = 5.2 Hz, 1H, H-5″), 7.74 (br s, 3H, NH3+), 7.23–7.26 (m, 2H, H-2″ ″, H-6″ ″), 7.13 (t, J = 7.7 Hz, 1H, H-5″ ″), 6.98 (s, 1H, H-5‴), 6.75 (d, J = 7.4 Hz, 1H, H-4″ ″), 4.48 (t, J = 5.3 Hz, 2H, H-20), 3.70 (t, J = 5.3 Hz, 2H, H-19), 3.58 (t, J = 5.3 Hz, 2H, H-2), 3.42–3.56 (m, 20 H, 10 × CH2O), 2.97 (sext, J = 5.5 Hz, 2H, H-1), 2.24 (s, 3H, CH3); 13C NMR δ 162.9, 149.1, 147.9, 146.8, 142.4, 141.9, 140.8, 138.1, 128.7, 124.8, 122.1, 117.3, 114.0, 109.7, 69.69 (8), 69.66 (2), 69.6, 69.55, 69.53, 68.5, 66.6, (2C not observed); MS m/z 642.1 (MH+, 100%); HRMS (ESI) Calcd for C31H44N7O6S (MH+) m/z 642.3068; found m/z 642.3071.
5.1.9. 4-(3-Bromo-4-pyridinyl)-N-ethyl-N-(3-methylphenyl)-1,3-thiazol-2-amine (17)
EtI (56 µL, 0.7 mmol) was added to a mixture of bromopyridine 12 (200 mg, 0.58 mmol) and K2CO3 (97 mg, 0.7 mmol) in anhydrous DMF and the reaction mixture was stirred at 18 °C for 20 h. The mixture was partitioned between EtOAc (150 mL) and H2O (50 mL). The organic layer was washed with H2O (50 mL) washed with brine (50 mL), dried and the solvent was evaporated. The residue was purified by column chromatography, eluting with 20% EtOAc/pet. ether, to give the amine 17 (130 mg, 60%) as a colourless oil: 1H NMR δ 8.79 (s, 1H, H-2″), 8.58 (d, J = 5.0 Hz, 1H, H-6″), 7.87 (br d, J = 5.1 Hz, 1H, H-5″), 7.49 (s, 1H, H-5), 7.39 (t, J = 7.7 Hz, 1H, H-5′), 7.30 (br s, 1H, H-2′), 7.27 (br d, J = 7.8 Hz, 1H, H-6′), 7.19 (br d, J = 7.5 Hz, 1H, H-4′), 3.99 (q, J = 7.1 Hz, 2H, CH2CH3), 2.36 (s, 3H, CH3), 1.22 (t, J = 7.1, 3H, CH2CH3); 13C NMR δ 163.3, 152.7, 148.6, 145.8, 144.2, 141.5, 139.8, 129.9, 128.1, 127.0, 125.1, 123.6, 118.1, 110.0, 47.3, 20.8, 12.8; MS m/z 376.0 (MH+, 100%), HRMS (ESI) Calcd for C17H17BrN3S (MH+) m/z 374.0321; found m/z 374.0324.
5.1.10. N-Ethyl-4-(3-ethynyl-4-pyridinyl)-N-(3-methylphenyl)-1,3-thiazol-2-amine (18)
PdCl2(PPh3)2 (30 mg, 0.04 mmol) was added to a purged solution of bromopyridine 17 (130 mg, 0.35 mmol), TMS-acetylene (0.5 mL, 3.5 mmol), and CuI (8 mg, 0.04 mmol) in a mixture of DMF/NEt3 (1:1; 4 mL), and the reaction mixture was stirred at 50 °C for 2 h. The mixture was cooled to 18 °C and was partitioned between EtOAc (200 mL) and H2O (50 mL). The organic phase was washed with brine (50 mL), dried and the solvent was evaporated. The residue was dissolved in a mixture of THF/MeOH (1:1; 30 mL), K2CO3 (50 mg, 0.35 mmol) was added and the mixture was stirred at 18 °C for 1 h. The solvent was then evaporated and the residue purified by column chromatography, eluting with 20% EtOAc/pet. ether, to give acetylene 18 (72 mg, 65%) as an unstable yellow oil which was used without further purification: 1H NMR δ 8.74 (s, 1H, H-2″), 8.57 (d, J = 5.1 Hz, 1H, H-6″), 8.04 (d, J = 5.3 Hz, 1H, H-5″), 7.76 (s, 1H, H-5), 7.34 (dt, J = 7.4, 1.1 Hz, 1H, H-5′), 7.19 (br s, 1H, H-2′), 7.14–7.19 (m, 2H, H-4′, H-6′), 4.07 (q, J = 7.1 Hz, 2H, CH2CH3), 3.47 (s, 1H, C≡CH), 2.39 (s, 3H, CH3), 1.31 (t, J = 7.1 Hz, 3H, CH2CH3); MS m/z 320.7 (MH+, 100%).
5.1.11. 20-(4-{4-[2-(Ethyl-3-methylanilino)-1,3-thiazol-4-yl]-3-pyridinyl}-1H-1,2,3-triazol-1-yl)-3,6,9,12,15,18-hexaoxaicosan-1-ammonium trifluoroacetate (19)
CuSO4·5H2O (6 mg, 0.022 mmol), sodium ascorbate (10 mg, 0.044 mmol) and TBTA (25 mg, 0.044 mmol) were added to a suspension of acetylene 18 (71 mg, 0.22 mmol) and 20-azido-3,6,9,12,15,18-hexaoxaeicosan-1-amine (80 mg, 0.22 mmol) in a mixture of DMF, DCM and H2O (1:1:1; 3 mL), and the mixture was heated at 50 °C for 3 h. The reaction mixture was cooled to 18 °C, diluted with DCM and MeOH (30 mL), SiO2 (1 g) was added and the solvent was evaporated. The residue was purified by column chromatography, eluting with 0.5% aq NH3 (25%)/1.5% MeOH/EtOAc, to afford crude amine 19 (90 mg). The product was further purified by preparative HPLC [gradient (95–64%) (H2O/TFA pH 2.56)/(90% CH3CN/H2O)] to give the amine 19 as the trifluoroacetate salt (60 mg, 41%) as a tan oil: 1H NMR δ 8.81 (s, 1H, H-2″), 8.69 (d, J = 5.4 Hz, 1H, H-6″), 8.09 (s, 1H, H-5′), 7.89 (d, J = 5.4 Hz, 1H, H-5″), 7.75 (br s, 3H, NH3+), 7.37 (t, J = 7.7 Hz, 1H, H-5″ ″), 7.25 (br s, 1H, H-2″ ″), 7.22 (br d, J = 7.9 Hz, 1H, H-6″ ″), 7.17 (br d, J = 7.6 Hz, 1H, H-4″ ″), 6.73 (s, 1H, H-5″ ′), 4.58 (t, J = 5.2 Hz, 2H, H-20), 3.86 (q, J = 7.1 Hz, 2H, CH2CH3), 3.84 (t, J = 5.2 Hz, 2H, H-19), 3.58 (t, J = 5.3 Hz, 2H, H-2), 3.42–3.56 (m, 20 H, 10 × CH2O), 2.97 (sext, J = 5.5 Hz, 2H, H-1), 2.34 (s, 3H, CH3), 1.11 (t, J = 7.1 Hz, 3H, CH2CH3); 13C NMR δ 168.5, 158.2 (q, J = 36 Hz), 148.4, 147.2, 146.7, 144.1, 142.8, 142.2, 139.7, 129.8, 128.0, 126.9, 125.2, 124.8, 124.2, 123.6, 115.8 (q, J = 292 Hz), 109.7, 69.69 (4), 69.67 (3), 69.59 (2), 69.56, 68.6, 66.6, 49.5, 47.2, 38.6, 20.8, 12.6; MS m/ z 672.4 (MH+, 100%); HRMS (ESI) Calcd for C33H48N7O6S (MH+) m/z 670.3381; found m/z 670.3349.
5.1.12. tert-Butyl 17-hydroxy-3,6,9,12,15-pentaoxaheptadec-1-ylcarbamate (23)
Mesyl chloride (1.78 mL, 23.0 mmol) was added dropwise to a stirred suspension of hexaethylene glycol 20 (5.42 g, 19.2 mmol) and Ag2O (4.67 g, 20.2 mmol) in dry DCM (50 mL) at 20 °C and the mixture was stirred at 20 °C for 3 days. The mixture was filtered through diatomaceous earth and the solvent evaporated. The residue was purified by column chromatography, eluting with a gradient (0–10%) of MeOH/EtOAc, to give 17-hydroxy-3,6,9,12,15-pentaoxaheptadec-1-yl methanesulfonate (21) (3.52 g, 51%) as a colourless oil: 1H NMR (CDCl3) δ 4.36–4.40 (m, 2H, CH2OSO2), 3.76–3.78 (m, 2H, CH2O), 3.70–3.74 (m, 2H, CH2O), 3.64–3.67 (m, 16 H, 8 × CH2O), 3.59–3.62 (m, 2H, CH2O), 3.09 (s, 3H, SO2CH3), 2.80 (br s, 1H, OH); MS m/z 361.6 (MH+, 100%). A mixture of the mesylate 21 (3.52 g, 9.8 mmol) and NaN3 (1.27 g, 19.5 mmol) in dry DMF (20 mL) was stirred at 110 °C for 2 h. The mixture was cooled to 20 °C and the solvent evaporated. The residue was purified by column chromatography, eluting with 10% MeOH/EtOAc, to give 17-azido-3,6,9,12,15-pentaoxaheptadecan-1-ol (22) (2.98 g, 99%) as a colourless oil: 1H NMR (CDCl3) δ 3.71–3.74 (m, 2H, CH2O), 3.65–3.69 (m, 18 H, 9 × CH2O), 3.59–3.62 (m, 2H, CH2O), 3.39 (br t, J = 5.2 Hz, 2H, CH2N3), 2.82 (br s, 1H, OH); MS m/z 308.5 (MH+, 100%). A mixture of azide 22 (2.98 g, 9.7 mmol) and Pd/C (100 mg) in EtOH (50 mL) was stirred under H2 (60 psi) for 1 h. The mixture was filtered through diatomaceous earth and washed with EtOH (3 × 20 mL) and the solvent was evaporated. The crude residue was dissolved in DCM (50 mL) and di-tert-butyl dicarbonate (2.56 g, 11.7 mmol) in DCM (20 mL) was added dropwise and the solution was stirred at 20 °C for 16 h. The solvent was evaporated and residue was purified by column chromatography, eluting with 10% MeOH/EtOAc, to give carbamate 23 (2.97 g, 80%) as a colourless oil: 1H NMR (CDCl3) δ 5.17 (br s, 1H, NHCO2), 3.70–3.74 (m, 2H, CH2O), 3.60–3.68 (m, 18 H, 9 × CH2O), 3.54 (br t, J = 5.1 Hz, 2H, CH2O), 3.31 (br q, J = 5.1 Hz, 2H, CH2N), 2.81 (br s, 1H, OH), 1.44 [s, 9 H, C(CH3)3]; MS m/z 382.5 (MH+, 100%).
5.1.13. tert-Butyl 3,6,9,12,15,18-hexaoxahenicos-20-yn-1-ylcarbamate (24)
NaH (343 mg, 8.56 mmol) was added in small portions to a stirred solution of alcohol 15 (2.97 g, 7.8 mmol) in THF (50 mL) at 0 °C and the resulting mixture stirred at 0 °C for 30 min. Propargyl bromide (0.87 mL, 7.8 mmol) was added followed by nBu4N+I− (29 mg, 78 µmol) and the mixture was stirred at 20 °C for 16 h. The reaction was quenched with satd aq NH4Cl and extracted with EtOAc (4 × 50 mL). The combined organic fraction was washed with brine (50 mL), dried and the solvent evaporated. The residue was purified by column chromatography, eluting with 80% EtOAc/pet. ether, to give the acetylene 24 (2.56 g, 79%) as a colourless oil: 1H NMR (CDCl3) δ 5.05 (br s, 1H, NHCO2), 4.20 (d, J = 2.4 Hz, 2H, CH2C≡C), 3.68–3.71 (m, 4H, 2 × CH2O), 3.64–3.67 (m, 12H, 6 × CH2O), 3.60–3.63 (m, 4H, 2 × CH2O), 3.54 (br t, J = 5.2 Hz, 2H, CH2O), 3.31 (br q, J = 5.2 Hz, 2H, CH2N), 2.42 (t, J = 2.4 Hz, 1H, CH), 1.44 [s, 9 H, C(CH3)3]; MS m/z 420.7 (MH+, 100%); HRMS (ESI) Calcd for C20H38NO8 (MH+) m/z 420.2592, found 420.2590 (0.4 ppm).
5.1.14. Benzyl 4-(pyridine-3-ylcarbamoyl)benzylcarbamate (26)
Oxalyl chloride (4.58 mL, 52.5 mmol) was added dropwise to a solution of 4-(benzyloxycarbonylamino)methyl)benzoic acid 2520 (10.0 g, 35.0 mmol) and DMF (4 drops) in dry THF (150 mL), and the mixture stirred at 50 °C for 4 h. The solvent was evaporated and the residue dissolved in pyridine (80 mL). 3-Aminopyridine (3.62 g, 38.5 mmol) was added and the solution stirred at 20 °C for 48 h. Water (150 mL) was added, the mixture stirred for another 2 h, the precipitate filtered off, washed with water and dried to give the carbamate 26 (7.82 g, 62%) as a white solid: mp (EtOH) 207–210 °C; 1H NMR δ 10.37 (s, 1H, NHCO), 8.92 (d, J = 2.3 Hz, 1H, H-2′), 8.31 (dd, J = 4.7, 1.5 Hz, 1H, H-6′), 8.18 (ddd, J = 8.3, 2.5, 1.5 Hz, 1H, H-4′), 7.93 (br d, J = 8.3 Hz, 2H, H-2, H-6), 7.89 (br t, J = 6.0 Hz, 1H, NHCO2), 7.41 (br d, J = 8.3 Hz, 2H, H-3, H-5), 7.31–7.39 (m, 6 H, H-5′, H-2″, H-3″, H-4″, H-5″, H-6″), 5.06 (s, 2H, CH2O), 4.30 (d, J = 6.2 Hz, 2H, CH2N). Anal. Calcd for C21H19N3O3: C, 69.79; H, 5.30; N, 11.63. Found: C, 69.60; H, 5.40; N, 11.63.
5.1.15. 4-(Aminomethyl)-N-(3-pyridinyl)benzamide dihydrobromide (27)
A suspension of carbamate 26 (2.2 g, 6.09 mmol) in HBr/AcOH (30%, 30 mL) was stirred at 20 °C for 3 h. Et2O (200 mL) was added, the mixture was stirred for another 30 min, the precipitate filtered off, washed with Et2O and dried to give benzamide 27 (2.35 g, 99%) as a white solid: mp (EtOAc) 292–296 °C; 1H NMR δ 11.06 (s, 1H, NHCO), 9.35 (d, J = 2.2 Hz, 1H, H-2′), 8.70 (ddd, J = 8.5, 2.2, 1.1 Hz, 1H, H-4′), 8.64 (br d, J = 5.4 Hz, 1H, H-6′), 8.31 (br s, 3H, NH2·HBr), 8.09 (br d, J = 8.2 Hz, 2H, H-2, H-6), 7.96 (dd, J = 8.6, 5.4 Hz, 1H, H-5′), 7.67 (d, J = 8.4 Hz, 2H, H-3, H-5), 5.95 (br s, 1H, pyrN·HBr), 4.16 (q, J = 5.8 Hz, 2H, CH2N). Anal. Calcd for C13H15Br2N3O: C, 40.13; H, 3.89; N, 10.80. Found: C, 39.99; H, 3.94; N, 10.36.
5.1.16. 4-((4-Iodophenylsulfonamido)methyl)-N-(pyridin-3-yl)benzamide (28)
A mixture of amine 27 (727 mg, 3.2 mmol) and 4-iodobenzenesulfonyl chloride (970 mg, 3.2 mmol) in dry pyridine (10 mL) was stirred at 20 °C for 16 h. The solvent was evaporated and the residue stirred in water (20 mL) for 1 h. The precipitate was filtered, washed with water (5 mL) and dried. The crude solid was purified by column chromatography, eluting with a gradient (0–20%) of MeOH/EtOAc, to give benzamide 28 (1.47 g, 93%) as a cream powder: mp (MeOH/EtOAc) 249–251 °C; 1H NMR δ 10.36 (s, 1H, NHCO), 8.93 (d, J = 2.3 Hz, 1H, H-2′), 8.34 (br s, 1H, NHSO2), 8.31 (dd, J = 4.7, 1.5 Hz, 1H, H-6′), 8.18 (ddd, J = 8.3, 2.5, 1.5 Hz, 1H, H-4′), 7.97 (ddd, J = 8.6, 2.2, 1.9 Hz, 2H, H-2″, H-6″), 7.90 (br d, J = 8.3 Hz, 2H, H-2, H-6), 7.56 (ddd, J = 8.6, 2.2, 1.9 Hz, 2H, H-3″, H-5″), 7.37–7.42 (m, 3H, H-3, H-5, H-5′), 4.09 (s, 2H, CH2N); 13C NMR δ 165.5, 144.5, 142.0, 141.6, 140.3, 138.1 (2), 135.8, 133.1, 128.2 (2), 127.7 (2), 127.5 (2), 127.3, 123.5, 100.3, 45.7; MS m/z 494.6 (MH+, 100%). Anal. Calcd for C19-H16IN3O3S: C, 46.26; H, 3.27; N, 8.52. Found: C, 46.43; H, 3.30; N, 8.52.
5.1.17. tert-Butyl 21-{4-[({4-[(3-pyridinylamino)carbonyl] benzyl}amino)sulfonyl]phenyl}-3,6,9,12,15,18-hexaoxahenicos-20-yn-1-ylcarbamate (29)
PdCl2(PPh3)2 (36 mg, 51 µmol) was added to a stirred, degassed solution of iodide 29 (250 mg, 510 µmol), acetylene 24 (320 mg, 770 µmol) and CuI (10 mg, 51 µmol) in Et3N (3 mL) and DMF (3 mL), and the mixture was stirred in a sealed pressure vessel at 50 °C for 3 h. The mixture was cooled to 20 °C, diluted with EtOAc (150 mL) and washed with water (3 × 50 mL), washed with brine (50 mL) and dried. The solvent was evaporated and the residue purified by column chromatography, eluting with a gradient (0–5%) of MeOH/EtOAc, to give carbamate 29 (367 mg, 92%) as a tan oil: 1H NMR (CDCl3) δ 8.61 (br s, 1H, H-2′), 8.41 (br s, 1H, NHSO2), 8.31 (d, J = 4.6 Hz, 1H, H-6′), 8.25 (ddd, J = 8.3, 2.4, 1.4 Hz, 1H, H-4′), 7.79–7.84 (m, 4H, H-2, H-6, H-2″, H-6″), 7.55 (dd, J = 8.6, 1.8 Hz, 2H, H-3″, H-5″), 7.28–7.34 (m, 3H, H-3, H-5, H-5′), 5.78 (br s, 1H, NHCO), 5.07 (br s, 1H, NHCO2), 4.42 (s, 2H, CH2O), 4.22 (br d, J = 6.0 Hz, 2H, CH2N), 3.74–3.77 (m, 2H CH2O), 3.68–3.71 (m, 2H CH2O), 3.55–3.66 (m, 18H, 9 × CH2O), 3.25 (br dd, J = 5.4, 5.2 Hz, 2H, CH2N), 1.43 [s, 9 H, C(CH3)3]; MS m/z 786.0 (MH+, 100%); HRMS (ESI) Calcd for C39H53N4O11S (MH+) m/z 785.3426, Found 785.3410 (2.5 ppm).
5.1.18. 4-[({[4-(21-Amino-4,7,10,13,16,19-hexaoxahenicos-1-yn-1-yl)phenyl]sulfonyl}amino)methyl]-N-(3-pyridinyl) benzamide (30)
A solution of carbamate 29 (360 mg, 0.46 mmol) in HCl saturated MeOH (10 mL) was stood at 20 °C for 16 h. The solvent was evaporated and the crude oil purified by preparative HPLC [gradient elution 5–55% of (90%MeCN/H2O)/(0.02% v/v aqueous CF3CO2- H)] to give the amine 30 as the trifluoracetate salt (270 mg, 73%) as a brown gum: 1H NMR δ 11.34 (s, 1H, NHCO), 9.42 (d, J = 2.2 Hz, 1H, H-2′), 8.88 (d, J = 8.8 Hz, 1H, H-4′), 8.64 (d, J = 4.9 Hz, 1H, H-4′), 8.47 (br s, 1H, NHSO2), 8.04 (br d, J = 8.3 Hz, 2H, H-2, H-6), 7.96 (m, 4H, NH2·CF3CO2H, H-5′), 7.82 (dd, J = 8.5, 1.8 Hz, 2H, H-2″, H-6″), 7.65 (dd, J = 8.5, 1.8 Hz, 2H, H-3″, H-5″), 7.44 (br d, J = 8.3 Hz, 2H, H-3, H-5), 4.43 (s, 2H, CH2O), 4.12 (br d, J = 6.2 Hz, 2H, CH2N), 3.51–3.65 (m, 22H, 11 × CH2O), 2.95 (br q, J = 5.5 Hz, 2H, CH2N); 13C NMR δ 165.9, 142.5, 140.5, 138.6, 137.0, 134.7, 133.5, 132.0 (2), 131.9, 129.1, 128.1 (2), 127.7, 127.5 (2), 127.1, 126.8 (2), 125.8, 89.2, 84.3, 69.7 (3), 69.6 (2), 69.5, 68.8, 66.6, 58.0, 48.5, 45.7, 38.8; HRMS (ESI) Calcd for C34H45-N4O9S (MH+) m/z 685.2902, Found 685.2898 (1.2 ppm).
5.1.19. tert-Butyl 21-{4-[({4-[(3-pyridinylamino)carbonyl] benzyl}amino)sulfonyl]phenyl}-3,6,9,12,15,18-hexaoxahenicos-1-ylcarbamate (31)
A mixture of alkyne 29 (743 mg, 0.95 mmol) and 10% Pd/C (250 mg, 0.1 mmol) in absolute EtOH (30 mL) was stirred at 20 °C under of H2 (60 psi) for 16 h. The mixture was filtered through diatomaceous earth, washed with EtOH (100 mL), and the solvent was evaporated. The residue was purified by column chromatography, eluting with a gradient (3–5%) of MeOH/DCM, to give carbamate 31 (650 mg, 87%) as a pale yellow oil: 1H NMR δ 10.35 (s, 1H, CONH), 8.92 (d, J = 2.4 Hz, 1H, H-2′), 8.31 (dd, J = 4.7, 1.4 Hz, 1H, H-6′), 8.17–8.20 (m, 2H, NHSO2, H-4′), 7.88 (br d, J = 8.3 Hz, 2H, H-2, H-6), 7.88 (br d, J = 8.3 Hz, 2H, H-2″, H-6″), 7.37–7.40 (m, 5 H, H-5′, H-3, H-5, H-3″, H-5″), 6.69 (br s, 1H, NHCO2), 4.08 (d, J = 5.0 Hz, 2H, CH2N), 3.45–3.52 (m, 20 H, 10 × CH2O), 3.37 (t, J = 6.2 Hz, 4H, H-3‴, H-20‴), 3.05 (q, J = 6.0 Hz, 2H, H-21‴), 2.69 (t, J = 7.7 Hz, 2H, H-1‴), 1.77–1.84 (m, 2H, H-2‴), 1.37 [s, 9 H, C(CH3)3]; HRMS (ESI) Calcd for C39H56N4O11S (MH+) m/z 789.3739; found 789.3723 (1.6 ppm).
5.1.20. 4-[({[4-(21-Amino-4,7,10,13,16,19-hexaoxahenicos-1-yl)phenyl]sulfonyl}amino) methyl]-N-(3-pyridinyl)benzamide (32)
Trifluoroacetic acid (0.74 mL, 10 mmol) was added to a solution of carbamate 31 (313 mg, 0.4 mmol) in anhydrous DCM (5 mL) and the reaction mixture was stirred at 20 °C for 2 h. The solvent was evaporated and the residue was purified by column chromatography, eluting with 8% MeOH/DCM containing 1% aqueous NH3, to give the amine 32 (195 mg, 71%) as a colourless oil: 1H NMR δ 10.35 (br s, 1H, CONH), 8.92 (d, J = 2.2 Hz, 1H, H-2′), 8.31 (dd, J = 4.7, 1.4 Hz, 1H, H-6′), 8.18 (ddd, J = 8.3, 2.5, 1.5 Hz, 1H, H-4′), 7.88 (br d, J = 8.3 Hz, 2H, H-2, H-6), 7.70 (br d, J = 8.3 Hz, 2H, H-2″, H-6″), 7.37–7.40 (m, 5 H, H-5′, H-3, H-5, H-3″, H-5″), 4.08 (s, 2H, CH2NHSO2), 3.45–3.52 (m, 20 H, 10 × CH2O), 3.37 (t, J = 6.3 Hz, 2H, H-3‴), 3.34 (t, J = 5.8 Hz, 2H, H-20‴), 2.70 (t, J = 7.9 Hz, 2H, H-1‴), 2.63 (t, J = 5.7 Hz, 2H, H-21‴), 1.80 (tt, J = 6.4, 7.9 Hz, 2H, H-2‴), NHSO2 and NH2 not observed; 13C NMR δ 165.4, 146.6, 144.4, 141.9, 141.7, 138.0, 135.7, 132.8, 128.9 (2), 127.5 (2), 127.3 (2), 127.2, 126.4 (2), 123.3, 72.2, 69.69 (2), 69.66 (4), 69.62 (2), 69.4, 69.3, 69.1, 45.6, 40.9, 31.3, 30.4; HRMS (ESI) Calcd for C34H48N4O9S (MH+) m/z 689.3215; Found 389.3224 (−1.0 ppm).
5.1.21. 4-(Aminomethyl)-N-phenylbenzamide hydrobromide (34)
Oxalyl chloride (0.92 mL, 10.5 mmol) was added dropwise to a stirred suspension of benzoic acid 2520 (2.0 g, 7.0 mmol) and DMF (3 drops) in dry THF (50 mL) and the solution was stirred at 20 °C for 4 h. The solvent was evaporated and the residue dissolved in pyridine (10 mL). Aniline (0.70 mL, 7.7 mmol) was added and the solution stirred at 20 °C for 16 h. The solvent was evaporated and the residue suspended in ice/water (100 mL) for 1 h. The precipitate was filtered, washed with water (5 mL) and dried to give crude benzyl 4-(anilinocarbonyl)benzyl carbamate (33) (1.45 g, 57%) as a white powder: mp (H2O) 276–279 °C; 1H NMR δ 10.16 (s, 1H, CONH), 7.86–7.93 (m, 3H, NHCO2, Haryl), 7.77 (d, J = 8.6 Hz, 2H, Haryl), 7.30–7.41 (m, 9H, Haryl), 7.10 (br t, J = 7.3 Hz, 1Haryl), 4.14 (br q, J = 5.4 Hz, 2H, CH2N). A suspension of carbamate 33 (1.45 g, 4.0 mmol) in HBr/AcOH (30%, 30 mL) was stirred at 20 °C for 6 h. Et2O (150 mL) was added, the mixture was stirred at 5 °C for 30 min, the precipitate filtered off, washed with Et2O and dried to give benzamide hydrobromide 34 (1.15 g, 93%) as a white solid: mp (Et2O) 276–279 °C; 1H NMR δ 10.25 (s, 1H, CONH), 8.27 (br s, 3H, NH2·HBr), 8.30 (br d, J = 8.2 Hz, 2H, H-2, H-6), 7.79 (dd, J = 8.5, 7.5 Hz, 2H, H-2′, H-6′), 7.61 (d, J = 8.4 Hz, 2H, H-3, H-5), 7.36 (dd, J = 8.5, 7.5 Hz, 2H, H-3′, H-5′), 7.11 (br t, J = 7.5 Hz, 1H, H-4′), 4.14 (q, J = 5.4 Hz, 2H, CH2N). Anal. Calcd for C14H15BrN2O: C, 54.74; H, 4.92; N, 9.12. Found: C, 54.73; H, 4.76; N, 8.96.
5.1.22. 4-({[(4-Iodophenyl)sulfonyl]amino}methyl)-N-phenylbenzamide (35)
A mixture of amine 34 (408 mg, 1.8 mmol) and 4-iodobenzenesulfonyl chloride (551 mg, 1.8 mmol) in dry pyridine (10 mL) was stirred at 20 °C for 16 h. The solvent was evaporated and the residue stirred in water (20 mL) for 1 h. The precipitate was filtered, washed with water (5 mL) and dried, to give benzamide 35 (710 g, 80%) as a white powder: mp (EtOAc) 252–254 °C; 1H NMR δ 10.15 (s, 1H, CONH), 8.32 (br t, J = 6.2 Hz, 1H, NHSO2), 7.97(ddd, J = 8.6, 2.2, 1.8 Hz, 2H, H-3″, H-5″), 7.88 (d, J = 8.3 Hz, 2H, H-2, H-6), 7.77 (dd, J = 8.4, 1.0 Hz, 2H, H-2′, H-6′), 7.56 (ddd, J = 8.6, 2.2, 1.8 Hz, 2H, H-2″, H-6″), 7.38–7.40 (m, 4H, H-3, H-5, H-3′, H-5′), 7.10 (tt, J = 7.4, 1.0 Hz, 1H, H-4’), 4.08 (d, J = 6.3 Hz, 2H, CH2N); 13C NMR δ 165.1, 141.2, 140.3, 139.1, 138.0 (2), 133.7, 128.5 (2), 128.2 (2), 127.6 (2), 127.4 (2), 123.6, 120.4 (2), 100.3, 45.7; MS m/z 493.6 (MH+, 100%). Anal. Calcd for C20H17IN2O3S: C, 48.79; H, 3.48; N, 5.69. Found: C, 49.06; H, 3.56; N, 5.84.
5.1.23. tert-Butyl 21-[4-({[4-(anilinocarbonyl)benzyl]amino}sulfonyl)phenyl]-3,6,9,12,15,18-hexaoxahenicos-20-yn-1-ylcarbamate (36)
PdCl2(PPh3)2 (85 mg, 0.12 mmol) was added to a stirred, degassed solution of iodide 35 (600 mg, 1.22 mmol), acetylene 19 (765 mg, 1.82 mmol) and CuI (23 mg, 0.12 mmol) in Et3N (5 mL) and DMF (5 mL), and the mixture was stirred in a sealed pressure vessel at 50 °C for 2 h. It was cooled to 20 °C, diluted with EtOAc (200 mL), washed with water (3 × 50 mL), washed with brine (50 mL) and dried. The solvent was evaporated and the residue purified by column chromatography, eluting with a gradient (0–5%) of MeOH/DCM, to give carbamate 36 (1.0 g, 100%) as a tan oil: 1H NMR δ 10.15 (s, 1H, NHCO), 8.34 (br s, 1H, NHSO2), 7.88 (br d, J = 8.3 Hz, 2H, H-3″, H-5″), 7.80 (br d, J = 8.5 Hz, 2H, H-3′, H-5′), 7.76 (br d, J = 7.6 Hz, 2H, H-2‴, H-6‴), 7.65 (br d, J = 8.5 Hz, 2H, H-2′, H-6′), 7.38 (br d, J = 8.5 Hz, 2H, H-2″, H-6″), 7.34 (br d, J = 8.3 Hz, 2H, H-3‴, H-5‴), 7.10 (br t, J = 7.4 Hz, 1H, H-4‴), 6.70 (br s, 1H, NHCO2), 4.43 (s, 2H, C≡CCH2O), 4.10 (s, 2H, CH2NHSO2), 3.62–3.65 (m, 2H, CH2O), 3.56–3.59 (m, 2H, CH2O), 3.49–3.53 (m, 16 H, 8 × CH2O), 3.37 (br t, J = 6.1 Hz, 2H, H-20), 3.06 (br q, J = 5.9 Hz, 2H, H-21), 1.37 [s, 9H, C(CH3)3]; HRMS (ESI) Calcd for C40H54N3O11S (MH+) m/z 784.3474; Found 784.3460.
5.1.24. tert-Butyl 21-[4-({[4-(anilinocarbonyl)benzyl] amino}sulfonyl)phenyl]-3,6,9,12,15,18-hexaoxahenicos-1-ylcarbamate (37)
A mixture of carbamate 36 (923 mg, 1.17 mmol) and 10% Pd/C (300 mg, 0.12 mmol) in absolute EtOH (50 mL) was stirred at 20 °C under H2 (60 psi) for 16 h. The mixture was filtered through a pad of diatomaceous earth, washed with EtOH (200 mL), the solvent was evaporated and the residue was purified by column chromatography, eluting with a gradient (0–5%) of MeOH/DCM to give carbamate 37 (742 mg, 80%) as a tan oil: 1H NMR δ 10.14 (s, 1H, NHCO), 7.86 (br d, J = 8.3 Hz, 2H, H-3″, H-5″), 7.76 (br dd, J = 8.7, 1.0 Hz, 2H, H-3′, H-5′), 7.71 (br d, J = 8.3 Hz, 2H, H-2‴, H-6‴), 7.32–7.40 (m, 6 H, H-2′, H-6′, H-2″, H-6″, H-3‴, H-5‴), 7.16 (t, J = 6.2 Hz, 1H, NHSO2), 7.09 (br t, J = 7.4 Hz, 1H, H-4‴), 6.69 [br s, 1H, NHC(CH3)3], 4.07 (d, J = 5.9 Hz, 2H, CH2NHSO2), 3.45–3.52 (m, 20 H, 10 × CH2O), 3.37 (br t, J = 6.2 Hz, 4H, H-19, H-2), 3.05 (br q, J = 5.9 Hz, 2H, H-1), 2.69 (br t, J = 7.9 Hz, 2H, H-21), 1.80 (tt, J = 7.9, 6.3 Hz, 2H, H-20), 1.37 [s, 9 H, C(CH3)3]; HRMS (ESI) Calcd for C40H58N3O11S (MH+) m/z: 788.3787; Found 788.3796.
5.1.25. 4-[({[4-(21-Amino-4,7,10,13,16,19-hexaoxahenicos-1-yl)phenyl]sulfonyl}amino)methyl]-N-phenylbenzamide (38)
A solution of carbamate 37 (245 mg, 0.31 mmol) in anhydrous DCM (5 mL) was treated with TFA (0.6 mL, 7.8 mmol) and the reaction mixture was stirred at 20 °C for 2 h. The solvent was evaporated and the residue was purified by column chromatography, eluting with a gradient (92:7:0.8–92:8.5:1) of DCM/MeOH/aq NH3 to give the amine 38 (164 mg, 77%) as a pale yellow oil: 1H NMR δ 10.14 (s, 1H, NHCO), 7.86 (br d, J = 8.3 Hz, 2H, H-2, H-6), 7.76 (br dd, J = 8.6, 1.0 Hz, 2H, H-2″, H-6″), 7.71 (br d, J = 8.3 Hz, 2H, H-2′, H-6′), 7.32–7.41 (m, 6 H, H-3, H-5, H-3′, H-5′, H3″, H-5″), 7.10 (br t, J = 7.4 Hz, 1H, H-4′), 4.07 (s, 2H, CH2NHSO2), 3.46–3.52 (m, 20H, 10 × CH2O), 3.37 (t, J = 6.3 Hz, 2H, H-3‴), 3.35 (t, J = 5.8 Hz, 2H, H-20‴), 2.69 (t, J = 8.0 Hz, 2H, H-1‴), 2.64 (br s, 2H, H-21‴), 1.80 (tt, J = 7.9, 6.3 Hz, 2H, H-2‴), NHSO2 and NH2 not observed; 13C NMR δ 165.0, 146.6, 141.3, 139.0, 138.0, 133.5, 128.9 (2), 128.4 (2), 127.4 (2), 127.2 (2), 126.4 (2), 123.5, 120.2 (2), 69.7 (9), 69.6, 69.4, 69.3, 69.1, 45.6, 31.3, 30.4; HRMS (ESI) Calcd for C35H50N3O9S (MH+) m/z 688.3262; Found: 688.3252.
5.1.26. Preparation of affinity reagents
A slurry of Affi-Gel 10 (5 mL) was washed with cold iPrOH (5 × 5 mL) in a sintered funnel and was transferred to a sealed pressure vessel. A solution of amine 32 or 38 (61 mg, 0.08 mmol) in MeOH (5 mL) was added, followed by iPr2OEt (0.14 mL, 0.8 mmol). The sealed tube was placed on a rotary shaker and gently agitated, monitoring the reaction by HPLC. After 6 h, ethanolamine (9 µL, 0.15 mmol) was added and the mixture was agitated for another 16 h. The mixture was filtered, and the gel washed with MeOH (5 × 5 mL) and iPrOH (5 × 5 mL), to give an off-white slurry which was used for affinity chromatography experiments.
5.2. Molecular modeling
5.2.1. Molecular modeling for PAT chemotype
A base CoMFA 3D-QSAR model was created as previously described.14
5.2.2. Molecular modeling for PPB chemotype
The likely protonation state of the test compounds at pH 7.4 was predicted using Filter (OpeneEye Scientific Software, Santa Fe, NM; http://www.eyesopen.com/), followed by conformer generation using OMEGA2 (version 3.2 OpenEye Scientific Software, Santa Fe, NM; http://www.eyesopen.com/). The MMFF94s forcefield was used for model construction and the dielectric set at 80. Strain energies were calculated during conformer generation using the MMFF94s forcefield and the conformer energy window was set at 20 kcal/mol, with the RMS between conformations set at 0.8, only one conformer was kept. All other settings were left as default. GOLD30 was then used to dock the lowest energy conformer into a 20 Å cavity that covered the internal channel of a GLUT1 homology model (PDB entry 1SUK).17 The Goldscore scoring function was used at maximum search efficiency, and 20 poses separated by a minimum RMSD of 2 Å were kept, all other conditions were kept at default settings. Predicted binding poses were subsequently refined by energy minimization using the Tripos force field with Gastieger–Huckel charge set. A minimisation protocol was used that included 1000 iterations of Steep Descents method followed by the Conjugate Gradient method until convergence with the cutoff set at 0.05 kcal/mol. The ligand and all protein atoms within 8 Å were allowed to move, while an additional 8 Å was immobile but still considered in the calculation. A set of ‘active’ compounds that represented small and large substitutions on the terminal benzene (S1–S6: RCC4 IC50 <1 µM) (Table S1, Supplementary data) were used in docking studies.
5.3. Cell viability assays
RCC4 parental cells and RCC4 cells with VHL reintroduced (RCC4/VHL),2 were maintained in DMEM supplemented with 10% FCS. Compounds were evaluated in a XTT growth inhibition assay (as IC50: the drug concentration required to inhibit cell growth by 50%). Five thousand cells were plated in 96-well plates. The following day vehicle (DMSO) or drug was added by serial dilution from a top concentration of 40 µM in triplicate. Four days later the media was aspirated, 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide (XTT) solution (0.3 mg/mL) (Sigma), 2.65 mg/ml N-methyl dibenxopyrazine methyl sulfate (Sigma) in phenol red-free media was added, and the plates were incubated at 37 °C for 1–2 h. Metabolism of XTT was quantified by measuring the absorbance at 450 nm. IC50 values were calculated using interpolation.
Supplementary Material
Acknowledgments
The authors thank Drs. Shannon Black and Sisira Kumara for technical assistance and acknowledge the Association for International Cancer Research 10-0042 (M.B.), the Maurice Wilkins Centre for Biodiscovery (M.P.H., J.U.F.), US NCI-CA-82566 (A.J.G., M.P.H.), and NCI-CA-123823 (D.A.C.).
Abbreviations
- BOC
tert-butyloxycarbonyl
- DCM
dichloromethane
- DMF
dimethylformamide
- HTS
high throughput screening
- PAT
pyridylanilino thiazole
- PPB
pyridylphenylsulfonyl benzamides
- PEG
polyethylene glycol
- RCC
renal cell carcinoma
- SAR
structure–activity relationship
- TBTA
tris-(benzyltriazolyl)amine
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- TMS
trimethylsilyl
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.2013.12.028.
References
- 1.Kaelin WG., Jr Nat. Rev. Cancer. 2005;5:689. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 2.Sutphin PD, Chan DA, Li JM, Turcotte S, Krieg AJ, Giaccia AJ. Cancer. Res. 2007;67:5896. doi: 10.1158/0008-5472.CAN-07-0604. [DOI] [PubMed] [Google Scholar]
- 3.Chan DA, Giaccia AJ. Cell Cycle. 2008;7:2987. doi: 10.4161/cc.7.19.6776. [DOI] [PubMed] [Google Scholar]
- 4.Turcotte S, Sutphin PD, Chan DA, Hay MP, Denny WA, Giaccia AJ. Cancer Cell. 2008;14:90. doi: 10.1016/j.ccr.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi J-T, Wu J, Solow-Cordero DE, Bonnet M, Flanagan JU, Bouley DM, Graves EE, Denny WA, Hay MP, Giaccia AJ. Sci. Trans. Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. CA-Cancer. J. Clin. 2007;7:43. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Jonash E, Futreal AP, Davis IA, Bailey ST, Kim WY, Brugarolas J, Giaccia AJ, Kurban G, Pause A, Frydman J, Zurita AJ, Rini BI, Sharma P, Atkins MB, Walker CL, Rathmell WK. Mol. Cancer. Res. 2012;10:859. doi: 10.1158/1541-7786.MCR-12-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frew IJ, Krek W. Sci. Signal. 2008;1:30. doi: 10.1126/scisignal.124pe30. [DOI] [PubMed] [Google Scholar]
- 9.Kaelin WG., Jr Nat. Rev. Cancer. 2008;8:865. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 10.Wilson WR, Hay MP. Nat. Rev. Cancer. 2011;11:393. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 11.Kim WY, Kaelin WG., Jr J. Clin. Oncol. 2004;22:4991. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 12.Sato S, Murata A, Shirakawa T, Uesugi M. Chem. Biol. 2010;17:616. doi: 10.1016/j.chembiol.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Hay MP, Turcotte S, Flanagan JU, Bonnet M, Chan DA, Sutphin PD, Nguyen P, Giaccia AJ, Denny WA. J. Med. Chem. 2010;53:787. doi: 10.1021/jm901457w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnet M, Flanagan JU, Chan DA, Lai EW, Nguyen P, Giaccia AJ, Denny WA. Bioorg. Med. Chem. 2011;19:3347. doi: 10.1016/j.bmc.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolb HC, Finn MG, Sharpless KB. Angew. Chem., Int. Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Sutphin PD, Chan DA, Turcotte S, Denny WA, Hay MP, Giddens AC, Bonnet M, Giaccia AJ. 2,011,011,514 A1. PCT Patent Appl. WO. 2011 Jan 27;
- 17.Salas-Burgos A, Iserovich P, Zuniga F, Vera JC, Fischbarg J. Biophys. J. 2004;87:2990. doi: 10.1529/biophysj.104.047886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen TR, Hilgraf R, Sharpless BK, Fokin V. Org. Lett. 2004;6:2853. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 19.Larsson A, Angbrant J, Ekeroth J, Mansson P, Liedberg B. Sens. Activators, B. 2006;13:730. [Google Scholar]
- 20.Loge C, Wallez V, Scalbert E, Cario-Tourmaniantz C, Loirand G, Pacaud P, Lesieur DJ. Enzyme Inhib. Med. Chem. 2002;17:381. doi: 10.1080/1475636021000005659. [DOI] [PubMed] [Google Scholar]
- 21.Wood TE, Dalili S, Simpson CD, Hurren R, Mao X, Saiz FS, Gronda M, Eberhard Y, Minden MD, Bilan PJ, Klip A, Batey RA, Schimmer AD. Mol. Cancer Ther. 2008;7:3546. doi: 10.1158/1535-7163.MCT-08-0569. [DOI] [PubMed] [Google Scholar]
- 22.Brockmann K, Wang D, Korenke CG, von Moers A, Ho YY, Pascual JM, Kuang K, Yang H, Ma L, Kranz-Eble P, Fischbarg J, Hanefeld F, De Vivo DC. Ann. Neurol. 2001;50:476. doi: 10.1002/ana.1222. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J, Chen X. Mol. Cancer Ther. 2012;11:1672. doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- 24.Garcia JC, Strube M, Leingang K, Keller K, Mueckler MM. J. Biol. Chem. 1992;267:7770. [PubMed] [Google Scholar]
- 25.Liu Y, Zhang W, Cao Y, Bergmeier S, Chen X. Cancer Lett. 2010;298:176. doi: 10.1016/j.canlet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Liu Y, Chen X, Bergmeier SC. Bioorg. Med. Chem. Lett. 2010;20:2191. doi: 10.1016/j.bmcl.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Chu PC, Yang C-N, Yan R, Chuang Y-C, Kulp SK, Chen C-S. J. Med. Chem. 2012;55:3827. doi: 10.1021/jm300015m. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Teicher BA, Linehan WM, Helman LJ. Cancer. Res. 2012;18:5537. doi: 10.1158/1078-0432.CCR-12-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaelin WG, Jr, Thompson CB. Nature. 2010:465–562. doi: 10.1038/465562a. [DOI] [PubMed] [Google Scholar]
- 30.Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD. Proteins. 2003;52:609. doi: 10.1002/prot.10465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.